Abstract
Doxorubicin (DOXO), a potent and widely used chemotherapeutic agent, causes irreversible heart failure by increasing oxidative stress, which limits its clinical utility. Nuclear factor erythroid-derived 2 -like 2 (Nrf2) is a prominent central regulator of cellular impenetrable to oxidants. The purpose of the study is to assess the ameliorative outcome of quercetin in cardiomyopathic rats induced by doxorubicin. Cardiomyopathy was produced in rats by single intraperitoneal weekly with DOXO (2 mg/kg) for 4 weeks. The rats were divided into five groups: (I) control group; (II) DOXO (2 mg/kg, i.p.) group; (III–V) DOXO + quercetin (10 mg/kg, 25 mg/kg and 50 mg/kg, orally), and were treated for 7 weeks. At the end of the treatment duration, cardiac function and biochemical parameters were assessed. Quercetin (10 mg/kg, 25 mg/kg and 50 mg/kg, orally) treatment reduced the raised blood pressure (BP) and left ventricular dysfunction. Withal, it prevented the rise in CKMB and LDH, suggesting the effect of quercetin in the maintaining the integrity of the cell membrane Besides, it also prevented the alteration in electrolyte levels, the activity of ATPase, and antioxidant status. Quercetin increased Nrf2 mRNA expression and reduced histological abnormalities compared 1to the DOXO control group. In conclusion, quercetin protected against DOXO- induced cardiomyopathy, by increasing expression of NRF2, and thereby increasing antioxidant defense and restoring biochemical and histological abnormalities.
Keywords: Doxorubicin, Cardiomyopathy, Quercetin, NRF2, SD rats, Antioxidant parameters, Langendroff apparatus, Histopathology, Serum cardiac marker, Gene expression, Food science, Agricultural science, Biological sciences, Veterinary medicine, Health sciences
Doxorubicin; Cardiomyopathy; Quercetin; NRF2; SD rats; Antioxidant parameters; Langendroff apparatus; Histopathology; Serum cardiac marker; Gene expression; Food science; Agricultural science; Biological sciences; Veterinary medicine; Health sciences
1. Introduction
Cardiomyopathy is a myocardial disorder that affects the structural as well as the functional integrity of myocardium (Sisakian, 2014). As stated by the World Health Organization (WHO), cardiomyopathy affects normal electrical rhythm and thus the ability to pump blood is reduced. Progresses to the development of heart failure or arrhythmias (Sisakian, 2014).
Chemotherapeutic medicines are used to treat various malignancies and types of cancer. Clinical adverse effects on heart restrict the use of chemotherapeutic agents (Nafees et al., 2015). One of the anthracycline antibiotics (doxorubicin) is used for cancer treatment. Doxorubicin (DOXO) treatment is associated with the apoptosis of cardiomyocyte, fibrosis of myocardium, cardiomyopathy, arrhythmias and congestive heart failure (CHF) (Benzer et al., 2018; Putt et al., 2015). DOXO derived metabolite induces cardiac injury via reactive oxygen species (ROS) generation leads to oxidative stress, further leading to mitochondrial damage, breaks in DNA strands, sarcomere structural alteration, and altered gene expression (Rashid et al., 2013; Schunke et al., 2013; Wu et al., 2016).
Nrf2 a master regulator of the oxidative stress signaling. It is a transcription factor that controls basal and inducible expression of antioxidant genes and other cytoprotective phase II detoxifying enzymes that are ubiquitously expressed in the cardiovascular system. Some recent shreds of evidence have revealed that cardiovascular homeostasis is regulated by Nrf2 via suppression of oxidative stress (Abo-Salem, 2012; Barancik et al., 2016). Thus, targeting NRF2 may prevent oxidative stress-induced cardiac injury (Han et al., 2008; Li et al., 2015; Yu et al., 2013; Zhou et al., 2014).
Over the last 20 years, polyphenols especially flavonoids have gained interest to be used as a new dietary intervention (Suen et al., 2016). Certain natural bioactive compounds rich in flavonoids reported for the suppression of oxidative stress and reduces the inflammatory responses in-vitro and inhibit atherosclerotic lesion development in-vivo (Giampieri et al., 2017; Goya et al., 2016; Loke et al., 2010; Pavlica and Gebhardt, 2010). Of the large family of flavonoids, quercetin is most widely distributed and commonly present in plant-based diets. Quercetin is an antioxidant bioflavonoid, actively obtained from broccoli, berries, grapes, citrus fruits, onions and cherries (Anand David et al., 2016). Quercetin possesses antihypertensive, anti-inflammatory, anti-obesity, vasodilator effects, anti-atherosclerotic and anti-hypercholesterolemia activities (Barteková et al., 2015; Salvamani et al., 2014; Sultana and Anwar, 2008). Many reports have shown the protective effect of quercetin on heart-related disorders or cardiac injuries (Egert et al., 2009; Jing et al., 2016; Perez-Vizcaino et al., 2009; Yamamoto and Oue, 2006). Various reported the beneficial effects of quercetin in chemical-induced hepatotoxicity (Abo-Salem et al., 2011; de David et al., 2011). Quercetin improved metabolic changes in diet-induced metabolic syndrome in rats (Panchal et al., 2012).
The purpose of the study to clarify the Nrf2 role in doxorubicin-induced myocardial disorder or cardiomyopathy. Additionally, the quercetin effect on Nrf2 targeted gene in DOXO-induced cardiomyopathy has yet not been investigated. Hence, our present study delineates the modulation of Nrf2 signaling by quercetin in DOXO-induced cardiomyopathy.
2. Materials and methods
2.1. Drugs, chemicals, and kits
All the chemicals used in this project were of analytical grade and were obtained from SD fine chemicals, (Mumbai, India). Doxorubicin Hydrochloride injection i.p. (DUXOCIN® INJECTION 10MG, Mfg. Lic. No- KD/2966-A, Batch No.:- (LDXAKI601) was used in the experiment. The standard reagent kits were purchased from Span Diagnostics Pvt. Ltd., India for the estimation of serum cardiac biomarkers and serum electrolyte estimation kits were purchased from Tulip diagnostic Ltd, Goa, India. For gene expression study: RNAlater (Qiagen), TRI (Sigma), DNase I (Qiagen), First-strand cDNA synthesis (Thermo scientific) and SYBR Green PCR kit (KAPA) were used. The primers were first designed by using the NCBI BLAST primer tool and then commercially synthesized (Eurofins Genomics). NIBP 200A small animal tail noninvasive blood pressure system (BIOPAC System, Inc.) was used to measure blood pressure and left ventricular function. Quercetin pure powder (Catalog no.: 117-39-5) obtained from Sigma Aldrich (Mumbai Co. St Louis, MO, USA). Quercetin was prepared freshly every day by suspending the drug in 0.5% Carboxymethyl Cellulose (CMC) suspension.
2.2. Animals
Male healthy Sprague-Dawley rats of 7–9 weeks of age and weight 250 ± 30 g were used for the study. The housing of animals was done in a standard cage under well-maintained conditions of temperature (24 ± 2 °C), humidity (55 ± 5%) and 12h/12h light-dark cycle. Free access to conventional laboratory diet (purchased from Pranav Argo Pvt. Ltd) and water ad libitum.
The experiment was conducted according to ethical norms approved by Institutional Animal Ethical Committee as per the guidance of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India (Protocol No: APC/2016-IAEC/1622).
2.3. Experimental design of induction of doxorubicin in rats
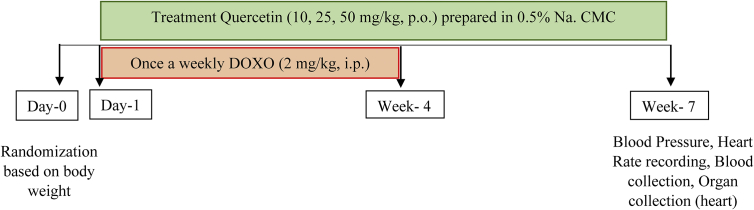
Male Sprague-Dawley rats (n = 50) were randomized based on their body weight into the following groups (Figure 1):
-
(I)
Control group (n=10): Normal saline was injected by intraperitoneal route once a week for 4 weeks along with vehicle (0.5% Na. CMC) orally for seven weeks.
-
(II)
DOXO group (n=10): Weekly single intraperitoneal injection of DOXO (2 mg/kg) was injected for 4 weeks along with daily administration of vehicle (0.5% Na. CMC) orally for seven weeks.
-
(III)
DOXO + Quercetin (10 mg/kg) group (n=10): Weekly single intraperitoneal injection of DOXO (2 mg/kg) was injected for 4 weeks along with daily administration of quercetin (10 mg/kg, p.o.) for seven weeks.
-
(IV)
DOXO + Quercetin (25 mg/kg) group (n=10): Weekly single intraperitoneal injection of DOXO (2 mg/kg) was injected for 4 weeks along with daily administration of quercetin (25 mg/kg, p.o.) for seven weeks.
-
(V)
DOXO + Quercetin (50 mg/kg) group (n=10): Weekly single intraperitoneal injection of DOXO (2 mg/kg) was injected for 4 weeks along with daily administration of quercetin (50 mg/kg, p.o.) for seven weeks.
Figure 1.
Experimental design.
2.4. Blood pressure and heart rate
At the time of termination, noninvasive BP was measured after animals were placed in restrainers by using the tail-cuff method. Recording of the BP was done by using a computerized MP35 data acquisition system (BIOPAC, Santa Barbara, California). By inserting needle electrodes under light ether anesthesia under the skin of the animals in the lead II position, heart rate recording was made using a computerized MP35 data acquisition system (BIOPAC, Santa Barbara, California).
2.5. Langendorff isolated perfused heart preparation (Patel et al., 2016)
The animal was kept free from stressful stimuli and they were anesthetized by intraperitoneal injection of 50–80 mg/kg pentobarbital. Before excision, the rat was intraperitoneally injected with 500 IU heparin to prevent the formation of thrombi in the excised heart. After losing pedal reflex activity and becomes unconscious, then animals were placed in the supine position to open the chest cavity by midsternal thoracotomy. After, the heart was scampered to excise and to transfer into oxygenated ice-cold Krebs-Henseleit buffer (KHB). The oxygenated KHB buffered cannula tied to the aorta. Then, the Langendorff perfusion apparatus started by connecting the heart attached cannula with the apparatus. The apparatus was maintained with a constant flow rate (20.0 ml/min) with carbogen (95% O2 and 5% CO2) saturated KHB at 37 °C temperature. To measure pharmacodynamic response, a latex balloon tied to the end of a polyethylene tube connected with pressure transducer was inserted into the left ventricle of the isolated heart. The balloon was inflated with 50% methanol to create a diastolic pressure of 5–6 mmHg. For 30 min the Langendorf assembly was allowed to stabilize before measurement of pharmacodynamics (heart rate (HR), coronary flow rate, left ventricular end-diastolic pressure (LVEDP), + (dp/dt) max and – (dp/dt) max. Reading was measured for 5 min after stabilization. The total number of reading were averaged.
2.6. Biochemical analysis
After BP and Heart rate was recorded, blood collection was done before the termination of the study. For the measurement of left ventricular function, the excision of heart tissue was done in the ice-cold condition. The heart tissue samples were made free of blood and tissue fluids by blotting. Then the weight of the heart was measured for the calculation of heart weight to body weight ratio and heart weight to tibia length ratio. Then they were stored at −80 °C for further analysis (CryoScientific, India). Heart weight to body weight ratio was calculated by dividing heart weight (g) by body weight (g) and heart weight to tibia length (mg/cm) ratio was calculated by dividing heart weight (g) by tibia length (cm). For the analysis of biochemical parameters, the serum sample was used.
2.7. Biochemical parameters in serum
2.7.1. Cardiac biomarkers
The estimation of cardiac marker enzymes creatinine kinase-MB (CK-MB), lactate dehydrogenase (LDH) was performed in serum samples by using commercially available standard enzymatic kits (Span Diagnostics Pvt. Ltd., India).
2.7.2. Electrolytes
Na+ and K+ concentrations were estimated by using commercially available kits (Tulip diagnostic Ltd, Goa, India).
2.8. Biochemical parameters (heart tissue)
2.8.1. Lipid peroxidation, glutathione (GSH) and antioxidant enzymes
Homogenate of excised heart tissue was prepared in chilled Tris–HCl buffer (0.1 M) of pH 7.4. Then it was centrifuged (10,000 rpm, 0 °C) using the Remi C-24 (high-speed cooling) centrifuge. The supernatant was then obtained used for the lipid peroxidation (MDA content) assay, superoxide dismutase (SOD) and catalase glutathione (GSH) assay (endogenous antiperoxidative enzymes). By the method of (Slater and Sawyer, 1971) lipid peroxidation (MDA content) formation was estimated. SOD estimation by the method of (Misra and Fridovich, 1972). Catalase based on the method of (Aebi, 1984) and GSH by the method of (Moron et al., 1979) was estimated.
2.8.2. Membrane-bound ATPase's
The leftover (after centrifuge) was again resuspended in ice-cold Tris–HCl buffer (0.1M) of pH 7.4 and was used for the estimations of membrane-bound enzymes. The amount of phosphorus liberated from the mixture containing homogenate (heart tissue), ATP and chloride salts of electrolyte for the estimation of membrane-bound enzymes activity (Na+/K+-ATPase, Ca+2-ATPase, and Mg+2-ATPase activity) (Bonting, 1970; Hjertén and Pan, 1983; Ohnishi et al., 1982).
2.9. Gene expression (quantitative reverse transcription-polymerase chain reaction)
TRIzol reagent was used to extract out total RNA from the heart sample as instructed in the kit insert. From each sample, 1 μg (μg) total RNA was taken for first-strand cDNA synthesis using the High Capacity cDNA Archive Kit. Quantitative real-time PCR was estimated with the ABI prism-7300 system by taking an equal amount of cDNA for each sample. Gene expression of rat Nrf2 (Nrf2, F: TTGTAGATGACCATGAGTCGC -21 and R: CAGGGGTGGTGAAGACTGAG -20) was determined using SYBR Green quantitative real-time PCR and QIAGEN QuantiFast SYBR Green kit. Normalization against the reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was made for the calculation of relative expression levels for each sample, using the delta-delta Ct method for comparing relative fold-expression differences.
2.10. Histopathology
The heart after dissection was fixed in 10% buffered formalin. The fixed tissues were embedded in paraffin and serial sections (5 μm thick) were cut. Each section was stained with hematoxylin and eosin (H&E) and they were examined for histological changes under the light microscope (Olympus BX10, Tokyo, Japan). Photomicrographs were taken under an Olympus DP12 camera, (Japan).
2.11. Statistical analysis
Research data are expressed as mean ± SD. Statistical significance was calculated using one-way ANOVA followed by Dunnett's post hoc analysis using Graph pad prism software. Differences were statistically significant when P < 0.05.
3. Results
3.1. Effect of chronic quercetin treatment on physical parameters in doxorubicin treated rats
After DOXO administration, the mortality rate was found to be high mostly in DOXO group animals alone however, the reduction in mortality was observed in quercetin treatment groups. Also, no significant change in body weight and tibia length in either group was observed (Table 1). Moreover, the increase in heart weight was significant in the DOXO group compared with the control group (1240 ± 57.7 versus 770 ± 35.4 mg, respectively). Furthermore, the DOXO group showed a significant increase in heart weight to body weight ratio than that of the control group (4.49 ± 0.56 versus 2.50 ± 0.29 mg/g, respectively, Table 1). Compared with DOXO group, quercetin (10, 25 and 50 mg/kg) treated group lowered heart weight (990 ± 20.7, 920 ± 16.9 and 960 ± 9.5 mg, respectively, Table 1) and the ratio of heart to body weight (3.47 ± 0.34, 3.15 ± 0.42 and 3.35 ± 0.34 mg/g, respectively, Table 1). Similarly, the DOXO group increased the ratio of heart weight to tibia length (244.25 ± 18.3 versus 146.11 ± 8.3 to mg/cm, respectively) compared with control (Table 1). Treatment groups showed a significant reduction in the heart weight to tibia length ratio (192.34 ± 8.5, 178.41 ± 6.5 and 188.14 ± 6.9 mg/cm, respectively, Table 1) when compared with DOXO group.
Table 1.
Effect of chronic quercetin treatment on physical parameters in doxorubicin treated rats.
| Groups | Body weight(g) | Heart wt. (mg) | Heart wt./Body wt. (mg/g) | Tibia length (cm) | Heart wt./Tibia length (mg/cm) |
|---|---|---|---|---|---|
| Control | 307.53 ± 21.41 | 770 ± 35.4 | 2.50 ± 0.29 | 5.27 ± 0.16 | 146.11 ± 8.3 |
| DOXO (2 mg/kg, i.p.) | 274.89 ± 25.34 | 1240 ± 57.7# | 4.49 ± 0.56# | 5.07 ± 0.15 | 244.25 ± 18.3# |
| Quercetin (10 mg/kg) + DOXO | 283.64 ± 28.45 | 990 ± 20.7∗ | 3.47 ± 0.34∗ | 5.14 ± 0.24 | 192.34 ± 8.5∗ |
| Quercetin (25 mg/kg) + DOXO | 291.94 ± 19.87 | 920 ± 16.9∗ | 3.15 ± 0.42∗ | 5.16 ± 0.17 | 178.41 ± 6.5∗ |
| Quercetin (50 mg/kg) + DOXO | 286.77 ± 18.74 | 960 ± 9.5∗ | 3.35 ± 0.34∗ | 5.09 ± 0.19 | 188.14 ± 6.9∗ |
All values are presented as mean ± SD.
#P < 0.05 as compared to the control group.
∗P < 0.05 as compared to doxorubicin (DOXO) group.
Table 2.
Effect of chronic quercetin treatment on left ventricular function in doxorubicin treated rats.
| Groups | Coronary flow rate (ml/min) | +(dp/dt) max (mmHg/s) | -(dp/dt) max (mmHg/s) |
|---|---|---|---|
| Control | 18.33 ± 0.81 | 3186.91 ± 194.16 | 2319.78 ± 184.14 |
| DOXO | 12.33 ± 0.58# | 1550.17 ± 125.17# | 1112.63 ± 112.82# |
| Quercetin (10 mg/kg) + DOXO | 14.87 ± 0.82∗ | 1988.13 ± 73.24∗ | 1611.27 ± 67.31∗ |
| Quercetin (25 mg/kg) + DOXO | 15.88 ± 0.83∗ | 2161.76 ± 99.38∗ | 1785.23 ± 54.95∗ |
| Quercetin (50 mg/kg) + DOXO | 15.71 ± 1.21∗ | 2077.57 ± 118.55∗ | 1654.31 ± 54.11∗ |
All values are presented as mean ± SD.
#P < 0.05 as compared to the control group.
∗P < 0.05 as compared to doxorubicin (DOXO) group.
3.2. Effect of chronic quercetin treatment on blood pressure, heart rate and left ventricular function in doxorubicin treated rats
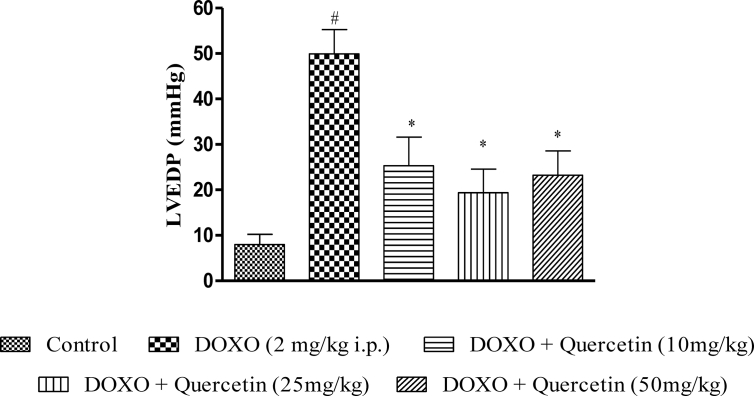
Heart rate was significantly higher in DOXO treated rats (Figure 2B), LVEDP was 7.24 ± 1.24 mmHg in the control group, and it was 49.94 ± 5.32 mmHg in DOXO treated group, respectively (Figure 3), BP increased from (147.18 ± 27.21 versus 93.56 ± 9.25 mmHg, respectively, Figure 2A), indicating LV overload. On the other hand, a significant decrease in coronary flow rate and dp/dt max was observed in the DOXO group with that of the control group (Table 2). Treatment with quercetin (10, 25 and 50 mg/kg) improved the functioning of heart, HR, coronary flow rate, dp/dt max, LVEDP (23.52 ± 6.32, 19.35 ± 5.21 and 23.24 ± 5.32 mmHg, respectively, Figure 3) and BP (128.99 ± 18.21, 114.13 ± 17.24 and 121.49 ± 16.24 mmHg, respectively, Figure 2A) against DOXO group.
Figure 2.
Effect of chronic quercetin treatment in doxorubicin treated rats. (A) Blood pressure and (B) Heart rate. #P < 0.05 as compared to control group. ∗P < 0.05 as compared to doxorubicin (DOXO) group.
Figure 3.
Effect of chronic quercetin treatement on LVEDP in doxorubicin treated rats. #P < 0.05 as compared to control group, ∗P < 0.05 as compared to doxorubicin (DOXO) group.
3.3. Effect of chronic quercetin treatment on cardiac marker enzymes and other biochemical parameters in doxorubicin treated rats
Serum CK-MB level (56.35 ± 5.32 U/L versus 20.60 ± 2.95, respectively, Figure 4A) and serum LDH level (352.24 ± 12.35 U/L versus 185.70 ± 9.32, respectively, Figure 4B) were significantly greater in DOXO group as compared with the control animals. Different quercetin treatment groups significantly prevented a rise in serum CK-MB level (35.57 ± 3.25, 27.11 ± 3.45 and 33.37 ± 6.32, respectively, Figure 4A) and serum LDH level (262.03 ± 15.32, 242.15 ± 11.35 and 261.09 ± 14.36, respectively, Figure 4B) as compared to DOXO animals.
Figure 4.
Effect of chronic quercetin treatment on cardiac biomarker in doxorubicin treated rats. (A) Serum CKMB and (B) Serum LDH. #P < 0.05 as compared to control group, ∗P < 0.05 as compared to doxorubicin (DOXO) group.
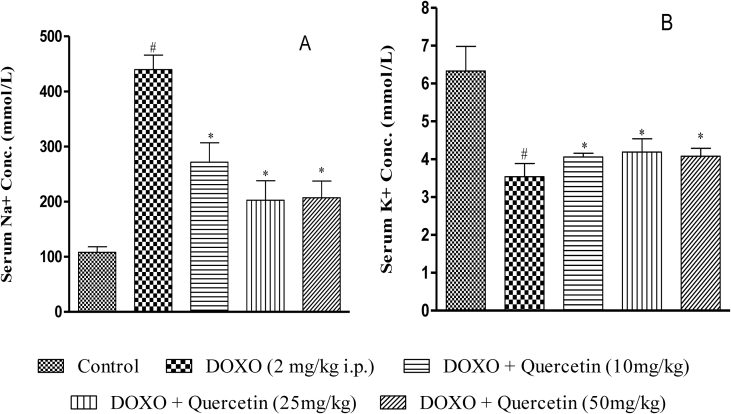
DOXO treatment increased serum Na+ conc. by 4.1 fold and reduced serum K+ conc. by 44.08 %, against the control group (Figure 5A-B). Quercetin (10 mg/kg, 25 mg/kg and 50 mg/kg) treatment showed a significant reduction in serum sodium level by (1.62 fold, 2.17 fold, and 2.1 fold, respectively, Figure 5A) and increased serum potassium level by (14.86 %, 18.36 %, and 15.25 %, respectively, Figure 5B) compared to DOXO treated animals.
Figure 5.
Effect of chronic quercetin treatment on electrolytes in doxorubicin treated rats. (A) Serum Na + concentration (B) Serum K+ concentration. #P < 0.05 as compared to control group, ∗P < 0.05 as compared to doxorubicin (DOXO) group.
3.4. Effect of chronic quercetin treatment on ATPase activity and antioxidant enzymes in doxorubicin treated rats
Na+/K+ ATPase was significantly lowered from that of control group (65.12 ± 13.19 versus 146.25 ± 16.74 U/ml, respectively) while the activity of Mg+2 ATPase (106.03 ± 21.32 versus 42.25 ± 10.01 U/ml, respectively) and Ca+2 ATPase (207.82 ± 27.32 versus 105.04 ± 15.10 U/ml, respectively) was significantly raised in DOXO group animals as compared with the control animals. Quercetin (10, 25 and 50 mg/kg) treatment significantly improved Na+/K+ ATPase activity (96.49 ± 15.02, 116.03 ± 21.11 and 108.14 ± 14.28 U/ml, respectively), Mg+2 ATPase (90.15 ± 11.66, 79.71 ± 13.46 and 78.46 ± 10.13 U/ml, respectively) and Ca+2 ATPase (174.59 ± 16.45 to 144.96 ± 20.19 and 154.07 ± 18.44 U/ml, respectively) (Table 3).
Table 3.
Effect of chronic quercetin treatment on membrane-bound ATPase enzymes in doxorubicin treated rats.
| Groups | Na + K + ATPase (U/ml) | Ca2+ ATPase (U/ml) | Mg2+ ATPase (U/ml) |
|---|---|---|---|
| Control | 146.25 ± 16.74 | 105.04 ± 15.10 | 42.28 ± 10.01 |
| DOXO | 65.12 ± 13.19# | 207.82 ± 27.32# | 106.03 ± 21.32# |
| Quercetin (10 mg/kg) + DOXO | 96.49 ± 15.02∗ | 174.59 ± 16.45∗ | 90.15 ± 11.66∗ |
| Quercetin (25 mg/kg) + DOXO | 116.03 ± 21.11∗ | 144.96 ± 20.19∗ | 79.71 ± 13.46∗ |
| Quercetin (50 mg/kg) + DOXO | 108.14 ± 14.28∗ | 154.07 ± 18.44∗ | 78.46 ± 10.13∗ |
All values are presented as mean ± SD.
#P < 0.05 as compared to the control group.
∗P < 0.05 as compared to doxorubicin (DOXO) group.
Significantly increased levels of MDA which is the end product of lipid peroxidation as well as a marker for oxidative stress (0.334 ± 0.03 versus 0.068 ± 0.009 μg/ml, respectively) and a significant decrease in GSH (3.82 ± 0.29 versus 8.19 ± 0.61 μg/ml, respectively), SOD (291.83 ± 19.27 versus 449.07 ± 36.14 μg/ml, respectively) and catalase (19.21 ± 2.14 versus 32.05 ± 3.01 to mmol/g of tissue, respectively) observed in DOXO group as compared with the control group. Different doses of quercetin treatment improved the altered antioxidant levels MDA (0.232 ± 0.023, 0.19 ± 0.027 and 0.212 ± 0.021 μg/ml), GSH (5.47 ± 0.38, 6.04 ± 0.14 and 5.92 ± 0.13 μg/ml, respectively), SOD (347.52 ± 34.87, 375.58 ± 29.19 and 337.12 ± 32.13 μg/ml, respectively) and catalase (23.30 ± 3.09, 26.28 ± 1.99 and 23.36 ± 2.11 mmol/g of tissue) compared to DOXO group (Table 4).
Table 4.
Effect of chronic quercetin treatment on lipid peroxidation, glutathione and antioxidant enzymes in doxorubicin treated rats.
| Groups | MDA (ug/ml) | SOD (ug/ml) | Catalase (mmol/g of tissue) | GSH (ug/ml) |
|---|---|---|---|---|
| Control | 0.0675 ± 0.009 | 449.07 ± 36.14 | 32.05 ± 3.01 | 8.19 ± 0.61 |
| DOXO | 0.334 ± 0.030# | 291.83 ± 19.27# | 19.21 ± 2.14# | 3.82 ± 0.29# |
| Quercetin (10 mg/kg) + DOXO | 0.232 ± 0.023∗ | 347.52 ± 34.87∗ | 23.30 ± 3.09∗ | 5.47 ± 0.38∗ |
| Quercetin (25 mg/kg) + DOXO | 0.190 ± 0.027∗ | 375.58 ± 29.19∗ | 26.28 ± 1.99∗ | 6.04 ± 0.14∗ |
| Quercetin (50 mg/kg) + DOXO | 0.212 ± 0.021∗ | 337.12 ± 32.13∗ | 23.36 ± 2.11∗ | 5.92 ± 0.13∗ |
All values are presented as mean ± SD.
#P < 0.05 as compared to the control group.
∗P < 0.05 as compared to doxorubicin (DOXO) group.
3.5. Effect of chronic quercetin treatment on Nrf2 gene expression in the heart of doxorubicin treated rats
NRF2 mRNA expression was significantly down-regulated after administration of doxorubicin by 2.85 ± 0.03 fold in heart tissue, compared to control group animals (Figure 6). Whereas, treatment with quercetin (10 mg/kg, 25 mg/kg and 50 mg/kg) avert the DOXO effect by change in 3.92 ± .04, 6.29 ± 0.09 and 3.29 ± 0.05 fold, respectively (Figure 6).
Figure 6.
Effect of chronic quercetin treatment on fold change of Nrf2 expression in doxorubicin treated rats. #P < 0.05 as compared to control group, ∗P < 0.05 as compared to doxorubicin (DOXO) group.
3.6. Effect of chronic quercetin treatment on histopathology changes in the heart tissue
DOXO treated animals revealed interstitial fibrosis spread widely into the surrounding myocardium. Hypertrophic or atrophic myocardial fibers were observed in and around the lesions. Some cardiomyocytes showed degeneration and an increased amount of inflammatory cell infiltration. The arrangement of the myocardial fibers in the lesions was disturbed. However, myocardial fibers existing far distant from the lesions showed normal appearance. Treatment with quercetin resulted in a decrease amount of inflammatory cells and improved cardiac muscle fibers morphology and no evidence of focal necrosis (Figure 7).
Figure 7.
Effect of chronic quercetin treatment on histopathological changes in heart tissue in doxorubicin treated rats (Heart tissues were stained with hematoxylin and eosin an visualized under the light microscope at 20× magnification).
4. Discussion
This present study evaluates the quercetin effect in the animal model of cardiomyopathy induced by doxorubicin. Doxorubicin cardiomyopathy is a fatal disease. The mortality rate increased to 50% during congestive heart failure. Unfortunately, treatment for established cardiomyopathy induced by doxorubicin has no effective treatment presently available (Chatterjee et al., 2010; dos Santos and dos Santos Goldenberg, 2018). Many reported doxorubicin to cause cardiomyopathy in-vivo in different animal species (Chen et al., 2015; O'Connell et al., 2017; Rodynskii et al., 2018) and also confirmed induction cardiotoxicity and oxidative stress in experimental models by doxorubicin. In cardiac tissue, a toxic and short-lived converted metabolite of doxorubicin that is its semiquinone form interacts with molecular oxygen and initiates a prominent role of ROS generation cascade including hydroxyl radical production in doxorubicin-induced cardiotoxicity (Asensio-López et al., 2017). The reported mechanism for the doxorubicin-induced stress is by the formation of an anthracycline-iron (Fe2+) free radical complex (Gammella et al., 2014).
Myocardium contains a plentiful concentration of diagnostic markers and its contents are released into the extracellular fluid once metabolically damaged (Elberry et al., 2010; Mantawy et al., 2014). During tissue damage or injury, macromolecules leak out from that damaged tissue, and because of tissue specificity and catalytic activity enzymes are the best markers. CK-MB and LDH are specific and sensitive diagnostic cardiac marker enzymes, widely used as diagnostic markers in cardiac toxicity (Shah et al., 2012). Many reports showed necrotic damage of the myocardium and plasma membrane leakiness and our present study also confirms the significant elevation of these diagnostic marker enzymes in serum in animals injected with doxorubicin (Omóbòwálé et al., 2018). Treatment with quercetin lowered the activity of these marker enzymes in the serum, but quercetin (25 mg/kg) showed more effect compared to quercetin (10 and 50 mg/kg). It demonstrated that quercetin could restrict the enzymes' leakage by maintaining membrane integrity.
After prolonged administration of doxorubicin, altered cardiac injury marker levels and changes in cardiac muscle by histological examination manifest cardiac dysfunction along with rising LVEDP and decreased systolic function represented by +(dP/dt) max and diastolic function represented by -(dP/dt) max and mechanical dysfunction of the heart (Ghigo et al., 2016). Earlier studies hypothesized that the generation of ROS leads to a change heart rate after extended doxorubicin treatment. Furthermore, ion pumps are more potently inhibited by its metabolite than doxorubicin. Hence, the direct indicator of cardiac systolic and diastolic function are LVEDP, +(dP/dt) max, and -(dP/dt) max (Mathias et al., 2019). In the present study, treatment with quercetin (25 mg/kg) compares with the other two doses showed a more significant attenuation of the adverse effect of doxorubicin by reversing the BP and HR as was shown in the previous report (Barteková et al., 2015). Treatment with quercetin preserved left ventricular function, by a significant decrease in LVEDP, rise in +(dP/dt) max and -(dP/dt) max which indicates that the cardiac function increased significantly.
Following doxorubicin administration, a significant rise in heart weight, with relatively unchanged bodyweight resulting rise in heart to body-weight ratio. Edematous intramuscular space and increased content of water may attribute to a significant rise in heart weight (Patel et al., 2010). Treatment with all three doses of quercetin brings down the heart weight, heart to body-weight ratio and heart weight to tibia length but quercetin (25 mg/kg) indicates more myocardium protection against infiltration and also be due to the decrease myocardium water content as was align with the earlier report (Barteková et al., 2015).
As discussed earlier, the semiquinone form of doxorubicin leads to the generation of enormous amounts of ROS which form free radicals that cause an increase in the reaction of lipid peroxidation by attacking adjacent fatty acids within the membranes (Zilinyi et al., 2018). The lipid hydroperoxide accumulation reflects damage to the cardiac constituents which is an important pathogenic event in myocardial necrosis. The major end product of lipid peroxidation is MDA; the increased free radical generation and/or decreased antioxidant defense activities may be contributed due to increased MDA content (Zhao et al., 2018). Doxorubicin administration leads to an elevation in the lipid peroxidation which is expressed as MDA content as reported in previous studies (Zhao et al., 2018; Zilinyi et al., 2018) which is in line with the present work. Quercetin treatment at 25 mg/kg dose showed a more significant decrease compared to other doses in the level of MDA content in the myocardium can be attributed against free radicals generated by doxorubicin which shows the potent antioxidant activity of quercetin.
In the heart tissue of doxorubicin-injected animals, the activities of antiperoxidative enzymes (SOD and catalase) were found to be significantly decreased. Hydrogen peroxide and molecular oxygen are the end product forms after the dismutation of 2 superoxide radicals catalyzed by SOD. Thus, the catalase or GSH redox system inactivates the generated hydrogen peroxide (Ramachandran et al., 2006). An increase in ROS generation such as hydrogen peroxide and superoxide in doxorubicin-induced cardiomyopathy may lead to inhibition of these enzymes. Furthermore, the decrease in the removal of superoxide radicals and hydrogen peroxide radicals is the result of due to a decrease in these antiperoxidative enzyme activities. The decreased levels of SOD and catalase in doxorubicin heart tissue is more comparably gets restored with quercetin (25 mg/kg) treatment. This could be due to the direct effect of quercetin by scavenging free radical, indirect effect of its reinforcing and protective ability of antioxidative enzymes from oxidative damage (Murakami et al., 2015).
In the current study, the concentration of GSH which is the most abundant non-enzymatic antioxidant biomolecules was observed to be lowered in the heart of doxorubicin-treated rats. By reacting with superoxide radicals, peroxy radicals, and singlet oxygen, GSH shows direct antioxidant function by forming oxidized GSH and other disulfides (Meister, 1988). It several enzymes involved in free radical scavenging by forming important substrates like GPx, GST, and others (Meister, 1988). Decreased activity of these enzymes leads to the accumulation of oxidants and make myocardial cell membranes more susceptible to oxidative damage. The present study observed a significant rise in the concentration of GSH in the heart of quercetin (10 mg/kg, 25 mg/kg and 50 mg/kg) treated groups. Quercetin may attribute to the stimulatory activity on antioxidant enzymes like GSH by stimulating a rate-limiting enzyme γ-glutamylcysteine synthase (Li et al., 2016).
Sodium, potassium, calcium, and magnesium translocation requires energy from ATPases and also closely associated with the plasma membrane (Wang et al., 2017). Our study showed a decrease in the activities of Na + -K + -ATPase and an increase in activity of Ca+2-ATPase and Mg+2-ATPase in the DOXO treated group, as same reported previously by other authors (Dodd et al., 1993; Wang et al., 2017). The rise in lipid peroxidation leads to protein oxidation activates Na + -K + -ATPase activity which is a lipid-dependant enzyme-containing –SH group (Jayachandran et al., 2009). The Na+ and Ca+2 ion-exchange mechanisms get activated by the inhibition of Na + -K + -ATPase in the myocardium. The adenylate cyclase may activate due to the increased activity of Ca+2-ATPase. All the quercetin treatment groups showed an increase in activity of Na + -K + -ATPase and decreased activity of Ca+2-ATPase and Mg+2-ATPase, which is novel finding as through Nrf2 it may attribute to the antioxidant activity directly and thereby protects the –SH group from oxidative damage. Sodium pump inhibition may give accelerates the increased levels of intracellular sodium (Murry et al., 1986). The FFAs increased levels may have caused in non-competitive inhibition of Na + -K + -ATPase, thus leads to an increase in Na + ions accumulation and a decrease in K+ ion in doxorubicin-induced rats. And the quercetin treated rats at (25 mg/kg) compared to the other two doses shows more effects on serum Na+ and K+ levels.
Histopathological observation of myocardial tissue in the control group exemplifies the normal myocardial cell membrane integrity and no inflammatory cell infiltration. The doxorubicin-treated group showed necrosis, muscle fibers separation of cardiac muscle and inflammatory cell infiltration. The cardioprotective effect of quercetin was confirmed by the reduction of the infiltration of inflammatory cells and normal muscle fibers architecture.
Nrf2 controls oxidative and electrophilic stress by the cellular response and also manages the activity of gene detoxification. As Nrf2 is a redox-sensitive transcription factor, in the response of stress (oxidative or electrophilic), it gets translocated into the nucleus from the cytosol by Kelch-like ECH-associated protein 1 (Keap1). After getting into the nucleus several antioxidant genes such as GPx, SOD, and HO-1 get actuated in the response of Nrf2. In normal conditions, in cytosol Nrf2 is suppressed by Keap1 (Barančík et al., 2016). Nrf2 pathway inhibition may lead to the cardiac protective enzymes suppression and one of the studies reported that in the heart of DOXO administrated animals the active nuclear Nrf2 gets reduced and leads to the cardiac damage (Wang et al., 2015). Cytoprotective antioxidant target genes induction by the Nrf2 upregulation conferred protection against DOXO-induced cardiotoxicity (Sonawane et al., 2018). Our results also show agreement with a decrease in the expression of Nrf2 in the DOXO-administered group. In contrast, the administration of quercetin prevents these deleterious effects of DOXO and surprisingly quercetin at (25 mg/kg) which is mid-dose shows more upregulation of Nrf2 expression compared to quercetin (10 and 50 mg/kg) groups which are very interesting to see for the first time. Our study provides presumption that the antioxidant capacity of the heart enhancement via Nrf2 pathway activation shows cardioprotective activity of quercetin (El-Agamy et al., 2019; Ma, 2013; Wang et al., 2015). In future, futher study is needed to check the effect of quercetin on different genes as a cell marker which are associated with oxidative stress response on Nrf2 signalling pathway.
In conclusion, the uncovering of the present study suggests cardioprotective effects of quercetin by preventing cellular damage which leads to normalizing BP. Interestingly, quercetin (25 mg/kg) treatment shows more upregulation of the Nrf2 target gene compared with 10 mg/kg and 50 mg/kg dose of quercetin and simultaneously affects ATPase activity. Quercetin (25 mg/kg) restores altered biochemical, histopathological, hemodynamic, physical and physiological parameters by maintaining antioxidant status and appeared to be promising in reducing the mortality caused by doxorubicin-induced cardiomyopathy.
Declarations
Author contribution statement
A. Sharma: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
M. Parkih: Conceived and designed the experiments.
H. Shah: Analyzed and interpreted the data.
T. Gandhi: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No Additional Information is available for this paper.
Footnotes
Anish Sharma et al., MODULATION OF NRF2 BY QUERCETIN IN DOXORUBICIN TREATED RATS.
References
- Abo-Salem O.M. The protective effect of aminoguanidine on doxorubicin-induced nephropathy in rats. J. Biochem. Mol. Toxicol. 2012;26:1–9. doi: 10.1002/jbt.20422. [DOI] [PubMed] [Google Scholar]
- Abo-Salem O.M., Abd-Ellah M.F., Ghonaim M.M. Hepatoprotective activity of quercetin against acrylonitrile-induced hepatotoxicity in rats. J. Biochem. Mol. Toxicol. 2011;25:386–392. doi: 10.1002/jbt.20406. [DOI] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Anand David A.V., Arulmoli R., Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Phcog. Rev. 2016;10:84–89. doi: 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio-López M.C., Soler F., Pascual-Figal D., Fernández-Belda F., Lax A. Doxorubicin-induced oxidative stress: the protective effect of nicorandil on HL-1 cardiomyocytes. PLoS One. 2017;12:e0172803. doi: 10.1371/journal.pone.0172803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barancik M., Gresova L., Bartekova M., Dovinova I. Nrf2 as a key player of redox regulation in cardiovascular diseases. Physiol. Res. 2016;65(Suppl 1):S1–s10. doi: 10.33549/physiolres.933403. [DOI] [PubMed] [Google Scholar]
- Barančík M., Grešová L., Barteková M., Dovinová I. Nrf2 as a key player of redox regulation in cardiovascular diseases. Physiol. Res. 2016;65(Suppl 1):S1–S10. doi: 10.33549/physiolres.933403. [DOI] [PubMed] [Google Scholar]
- Barteková M., Šimončíková P., Fogarassyová M., Ivanová M., Okruhlicová Ľ., Tribulová N., Dovinová I., Barančík M. Quercetin improves postischemic recovery of heart function in doxorubicin-treated rats and prevents doxorubicin-induced matrix metalloproteinase-2 activation and apoptosis induction. Int. J. Mol. Sci. 2015;16:8168–8185. doi: 10.3390/ijms16048168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer F., Kandemir F.M., Ozkaraca M., Kucukler S., Caglayan C. 2018. Curcumin Ameliorates Doxorubicin-Induced Cardiotoxicity by Abrogation of Inflammation, Apoptosis, Oxidative DNA Damage, and Protein Oxidation in Rats; p. 32. [DOI] [PubMed] [Google Scholar]
- Bonting S. Wiley Interscience; London: 1970. Membrane and Ion Transport, Presence of Enzyme Systems in Mammalian Tissues. [Google Scholar]
- Chatterjee K., Zhang J., Honbo N., Karliner J.S. Doxorubicin cardiomyopathy. Cardiology. 2010;115:155–162. doi: 10.1159/000265166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Tang Y., Zhang Y.-C., Huang X.-H., Xie Y.-Q., Xiang Y. A metabolomic study of rats with doxorubicin-induced cardiomyopathy and Shengmai injection treatment. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de David C., Rodrigues G., Bona S., Meurer L., Gonzalez-Gallego J., Tunon M.J., Marroni N.P. Role of quercetin in preventing thioacetamide-induced liver injury in rats. Toxicol. Pathol. 2011;39:949–957. doi: 10.1177/0192623311418680. [DOI] [PubMed] [Google Scholar]
- Dodd D.A., Atkinson J.B., Olson R.D., Buck S., Cusack B.J., Fleischer S., Boucek R.J., Jr. Doxorubicin cardiomyopathy is associated with a decrease in calcium release channel of the sarcoplasmic reticulum in a chronic rabbit model. J. Clin. Invest. 1993;91:1697–1705. doi: 10.1172/JCI116379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos D.S., dos Santos Goldenberg R.C. IntechOpen; 2018. Doxorubicin-Induced Cardiotoxicity: from Mechanisms to Development of Efficient Therapy, Cardiotoxicity. [Google Scholar]
- Egert S., Bosy-Westphal A., Seiberl J., Kurbitz C., Settler U., Plachta-Danielzik S., Wagner A.E., Frank J., Schrezenmeir J., Rimbach G., Wolffram S., Muller M.J. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009;102:1065–1074. doi: 10.1017/S0007114509359127. [DOI] [PubMed] [Google Scholar]
- El-Agamy D.S., El-Harbi K.M., Khoshhal S., Ahmed N., Elkablawy M.A., Shaaban A.A., Abo-Haded H.M. Pristimerin protects against doxorubicin-induced cardiotoxicity and fibrosis through modulation of Nrf2 and MAPK/NF-kB signaling pathways. Canc. Manag. Res. 2019;11:47–61. doi: 10.2147/CMAR.S186696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elberry A.A., Abdel-Naim A.B., Abdel-Sattar E.A., Nagy A.A., Mosli H.A., Mohamadin A.M., Ashour O.M. Cranberry (Vaccinium macrocarpon) protects against doxorubicin-induced cardiotoxicity in rats. Food Chem. Toxicol. : Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010;48:1178–1184. doi: 10.1016/j.fct.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Gammella E., Maccarinelli F., Buratti P., Recalcati S., Cairo G. The role of iron in anthracycline cardiotoxicity. Front. Pharmacol. 2014;5:25. doi: 10.3389/fphar.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghigo A., Li M., Hirsch E. New signal transduction paradigms in anthracycline-induced cardiotoxicity. Biochim. Biophys. Acta. 2016;1863:1916–1925. doi: 10.1016/j.bbamcr.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Giampieri F., Alvarez-Suarez J.M., Cordero M.D., Gasparrini M., Forbes-Hernandez T.Y., Afrin S., Santos-Buelga C., González-Paramás A.M., Astolfi P., Rubini C., Zizzi A., Tulipani S., Quiles J.L., Mezzetti B., Battino M. Strawberry consumption improves aging-associated impairments, mitochondrial biogenesis and functionality through the AMP-activated protein kinase signaling cascade. Food Chem. 2017;234:464–471. doi: 10.1016/j.foodchem.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Goya L., Martín M., Sarriá B., Ramos S., Mateos R., Bravo L. Effect of cocoa and its flavonoids on biomarkers of inflammation: studies of cell culture, animals and humans. Nutrients. 2016;8:212. doi: 10.3390/nu8040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Pan J., Ren D., Cheng Y., Fan P., Lou H. Naringenin-7-O-glucoside protects against doxorubicin-induced toxicity in H9c2 cardiomyocytes by induction of endogenous antioxidant enzymes. Food Chem. Toxicol. : Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2008;46:3140–3146. doi: 10.1016/j.fct.2008.06.086. [DOI] [PubMed] [Google Scholar]
- Hjertén S., Pan H. Purification and characterization of two forms of a low-affinity Ca2+-ATPase from erythrocyte membranes. Biochim. Biophys. Acta Biomembr. 1983;728:281–288. doi: 10.1016/0005-2736(83)90480-7. [DOI] [PubMed] [Google Scholar]
- Jayachandran K.S., Vasanthi H.R., Rajamanickam G.V. Antilipoperoxidative and membrane stabilizing effect of diosgenin, in experimentally induced myocardial infarction. Mol. Cell. Biochem. 2009;327:203–210. doi: 10.1007/s11010-009-0058-9. [DOI] [PubMed] [Google Scholar]
- Jing Z., Wang Z., Li X., Li X., Cao T., Bi Y., Zhou J., Chen X., Yu D., Zhu L., Li S. Protective effect of quercetin on posttraumatic cardiac injury. Sci. Rep. 2016;6:30812. doi: 10.1038/srep30812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Kim D.S., Yadav R.K., Kim H.R., Chae H.J. Sulforaphane prevents doxorubicin-induced oxidative stress and cell death in rat H9c2 cells. Int. J. Mol. Med. 2015;36:53–64. doi: 10.3892/ijmm.2015.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Zhang W.J., Choi J., Frei B. Quercetin affects glutathione levels and redox ratio in human aortic endothelial cells not through oxidation but formation and cellular export of quercetin-glutathione conjugates and upregulation of glutamate-cysteine ligase. Redox Biol. 2016;9:220–228. doi: 10.1016/j.redox.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke W.M., Proudfoot J.M., Hodgson J.M., McKinley A.J., Hime N., Magat M., Stocker R., Croft K.D. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 2010;30:749–757. doi: 10.1161/ATVBAHA.109.199687. [DOI] [PubMed] [Google Scholar]
- Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantawy E.M., El-Bakly W.M., Esmat A., Badr A.M., El-Demerdash E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur. J. Pharmacol. 2014;728:107–118. doi: 10.1016/j.ejphar.2014.01.065. [DOI] [PubMed] [Google Scholar]
- Mathias L.M.B.S., Alegre P.H.C., Dos Santos I.d.O.F., Bachiega T., Figueiredo A.M., Chiuso-Minicucci F., Fernandes A.A., Bazan S.G.Z., Minicucci M.F., Azevedo P.S., Okoshi M.P., Zornoff L.A.M., Paiva S.A.R., Polegato B.F. Euterpe oleracea mart. (Açai) supplementation attenuates acute doxorubicin-induced cardiotoxicity in rats. Cell. Physiol. Biochem. 2019;53:388–399. doi: 10.33594/000000145. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Moron M.S., Depierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Kawata A., Ito S., Katayama T., Fujisawa S. Radical-scavenging and anti-inflammatory activity of quercetin and related compounds and their combinations against RAW264. 7 cells stimulated with porphyromonas gingivalis fimbriae. Relationships between anti-inflammatory activity and quantum chemical parameters. In Vivo. 2015;29:701–710. [PubMed] [Google Scholar]
- Murry C.E., Jennings R.B., Reimer K.A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Nafees S., Rashid S., Ali N., Hasan S.K., Sultana S. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: role of NFkappaB/MAPK pathway. Chem. Biol. Interact. 2015;231:98–107. doi: 10.1016/j.cbi.2015.02.021. [DOI] [PubMed] [Google Scholar]
- O'Connell J.L., Romano M.M., Campos Pulici E.C., Carvalho E.E., de Souza F.R., Tanaka D.M., Maciel B.C., Salgado H.C., Fazan-Junior R., Rossi M.A., Simoes M.V. Short-term and long-term models of doxorubicin-induced cardiomyopathy in rats: a comparison of functional and histopathological changes. Exp. Toxicol. Pathol. Offic. J. Gesellschaft fur Toxikologische Pathologie. 2017;69:213–219. doi: 10.1016/j.etp.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Suzuki T., Suzuki Y., Ozawa K. A comparative study of plasma membrane Mg2+-ATPase activities in normal, regenerating and malignant cells. Biochim. Biophys. Acta Biomembr. 1982;684:67–74. doi: 10.1016/0005-2736(82)90050-5. [DOI] [PubMed] [Google Scholar]
- Omóbòwálé T.O., Oyagbemi A.A., Folasire A.M., Ajibade T.O., Asenuga E.R., Adejumobi O.A., Ola-Davies O.E., Oyetola O., James G., Adedapo A.A. Ameliorative effect of gallic acid on doxorubicin-induced cardiac dysfunction in rats. J. Basic Clin. Physiol. Pharmacol. 2018;29:19–27. doi: 10.1515/jbcpp-2016-0194. [DOI] [PubMed] [Google Scholar]
- Panchal S.K., Poudyal H., Brown L. Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J. Nutr. 2012;142:1026–1032. doi: 10.3945/jn.111.157263. [DOI] [PubMed] [Google Scholar]
- Patel P., Parikh M., Shah H., Gandhi T. Inhibition of RhoA/Rho kinase by ibuprofen exerts cardioprotective effect on isoproterenol induced myocardial infarction in rats. Eur. J. Pharmacol. 2016;791:91–98. doi: 10.1016/j.ejphar.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Patel V., Upaganlawar A., Zalawadia R., Balaraman R. Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: a biochemical, electrocardiographic and histoarchitectural evaluation. Eur. J. Pharmacol. 2010;644:160–168. doi: 10.1016/j.ejphar.2010.06.065. [DOI] [PubMed] [Google Scholar]
- Pavlica S., Gebhardt R. Protective effects of flavonoids and two metabolites against oxidative stress in neuronal PC12 cells. Life Sci. 2010;86:79–86. doi: 10.1016/j.lfs.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Perez-Vizcaino F., Duarte J., Jimenez R., Santos-Buelga C., Osuna A. Antihypertensive effects of the flavonoid quercetin. Pharmacol. Rep. PR. 2009;61:67–75. doi: 10.1016/s1734-1140(09)70008-8. [DOI] [PubMed] [Google Scholar]
- Putt M., Hahn V.S., Januzzi J.L., Sawaya H., Sebag I.A., Plana J.C., Picard M.H., Carver J.R., Halpern E.F., Kuter I., Passeri J., Cohen V., Banchs J., Martin R.P., Gerszten R.E., Scherrer-Crosbie M., Ky B. Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. Clin. Chem. 2015;61:1164–1172. doi: 10.1373/clinchem.2015.241232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran J., Thirupurasundari C., Devaraj N. Pretreatment with alcoholic extract of Crataegus oxycantha (AEC) activates mitochondrial protection during isoprotere. Mol. Cell. Biochem. 2006;292:59–67. doi: 10.1007/s11010-006-9218-3. [DOI] [PubMed] [Google Scholar]
- Rashid S., Ali N., Nafees S., Ahmad S.T., Arjumand W., Hasan S.K., Sultana S. Alleviation of doxorubicin-induced nephrotoxicity and hepatotoxicity by chrysin in Wistar rats. Toxicol. Mech. Methods. 2013;23:337–345. doi: 10.3109/15376516.2012.759306. [DOI] [PubMed] [Google Scholar]
- Rodynskii O., Kozlova Y.V., Kozlov S., Rodynska G., Sapozhnychenko L. Doxorubicin-induced cardiomyopathy in rats: behavior of the animals in the open field. Neurophysiology. 2018;50:259–265. [Google Scholar]
- Salvamani S., Gunasekaran B., Shaharuddin N.A. 2014. Antiartherosclerotic Effects of Plant Flavonoids; p. 480258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunke K.J., Coyle L., Merrill G.F., Denhardt D.T. Acetaminophen attenuates doxorubicin-induced cardiac fibrosis via osteopontin and GATA4 regulation: reduction of oxidant levels. J. Cell. Physiol. 2013;228:2006–2014. doi: 10.1002/jcp.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S.L., Mali V.R., Zambare G.N., Bodhankar S.L. Cardioprotective activity of methanol extract of fruit of Trichosanthes cucumerina on doxorubicin-induced cardiotoxicity in Wistar rats. Toxicol. Int. 2012;19:167. doi: 10.4103/0971-6580.97218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisakian H. Cardiomyopathies: evolution of pathogenesis concepts and potential for new therapies. World J. Cardiol. 2014;6:478–494. doi: 10.4330/wjc.v6.i6.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T.F., Sawyer B.C. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. General features of the systems used. Biochem. J. 1971;123:805–814. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawane V.K., Mahajan U.B., Shinde S.D., Chatterjee S., Chaudhari S.S., Bhangale H.A., Ojha S., Goyal S.N., Kundu C.N., Patil C.R. A chemosensitizer drug: disulfiram prevents doxorubicin-induced cardiac dysfunction and oxidative stress in rats. Cardiovasc. Toxicol. 2018;18:459–470. doi: 10.1007/s12012-018-9458-y. [DOI] [PubMed] [Google Scholar]
- Suen J., Thomas J., Kranz A., Vun S., Miller M. Effect of flavonoids on oxidative stress and inflammation in adults at risk of cardiovascular disease: a systematic review. Healthcare (Basel, Switzerland) 2016;4 doi: 10.3390/healthcare4030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana B., Anwar F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008;108:879–884. doi: 10.1016/j.foodchem.2007.11.053. [DOI] [PubMed] [Google Scholar]
- Wang H.-L., Cui X.-H., Yu H.-L., Wu R., Xu X., Gao J.-P. Synergistic effects of polydatin and vitamin C in inhibiting cardiotoxicity induced by doxorubicin in rats. Fundam. Clin. Pharmacol. 2017;31:280–291. doi: 10.1111/fcp.12258. [DOI] [PubMed] [Google Scholar]
- Wang L.F., Su S.W., Wang L., Zhang G.Q., Zhang R., Niu Y.J., Guo Y.S., Li C.Y., Jiang W.B., Liu Y., Guo H.C. Tert-butylhydroquinone ameliorates doxorubicin-induced cardiotoxicity by activating Nrf2 and inducing the expression of its target genes. Am. J. Trans. Res. 2015;7:1724–1735. [PMC free article] [PubMed] [Google Scholar]
- Wu X., Qi X., Lu Y., Lin C., Yuan Y., Zhu Q., Yin Q., Li W., Li Y., Bian H. Liguzinediol protects against cardiac fibrosis in rats in vivo and in vitro. Biomed. Pharmacother. Biomed. Pharmacother. 2016;80:260–267. doi: 10.1016/j.biopha.2016.03.033. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Oue E. Antihypertensive effect of quercetin in rats fed with a high-fat high-sucrose diet. Biosc. Biotech. Biochem. 2006;70:933–939. doi: 10.1271/bbb.70.933. [DOI] [PubMed] [Google Scholar]
- Yu X., Cui L., Zhang Z., Zhao Q., Li S. alpha-Linolenic acid attenuates doxorubicin-induced cardiotoxicity in rats through suppression of oxidative stress and apoptosis. Acta Biochim. Biophys. Sin. 2013;45:817–826. doi: 10.1093/abbs/gmt082. [DOI] [PubMed] [Google Scholar]
- Zhao L., Qi Y., Xu L., Tao X., Han X., Yin L., Peng J. MicroRNA-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress via targeting Nrf2 and Sirt2. Redox Biol. 2018;15:284–296. doi: 10.1016/j.redox.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Sun W., Zhang Z., Zheng Y. The role of Nrf2-mediated pathway in cardiac remodeling and heart failure. Oxid. Med. Cell. Long. 2014;2014:260429. doi: 10.1155/2014/260429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilinyi R., Czompa A., Czegledi A., Gajtko A., Pituk D., Lekli I., Tosaki A. The cardioprotective effect of metformin in doxorubicin-induced cardiotoxicity: the role of autophagy. Molecules. 2018;23 doi: 10.3390/molecules23051184. [DOI] [PMC free article] [PubMed] [Google Scholar]