“Le mal dans le monde vient presque toujours de l'ignorance, et les bonnes intentions peuvent faire autant de mal que la malveillance si elles manquent de compréhension”
- Albert Camus, The Plague, 1947
In this issue of Transfusion Medicine Reviews, we are pleased to offer readers an assessment as of April 2020 of COVID-19 convalescent plasma (CCP) by H Cliff Sullivan and John Roback of Emory University in Atlanta. The authors have brought together in one document those relevant background studies which have been used as support for implementation of CCP in the pandemic [1]. Readers will note how few high-quality randomized trials actually exist.
Although the absence of randomized controlled data is to be expected for rare and orphan diseases, there is no excuse for their absence in illnesses with thousands of patients. In fact, the sheer numbers of individuals afflicted with and dying from COVID-19 present a clear ethical as well as scientific requirement that the health care system seek truth regarding treatments. We all hope that CCP will be a beneficial treatment, and a preliminary report by Duan et al of its uncontrolled use in 10 patients in China might be seen as encouraging [2]. Although bypassing randomized controlled investigation of CCP may be tempting given the sense of urgency to “just do something,” a mistake repeated is a decision taken. Failure to “study first before wide-scale implementation” risks doing harm to both patients and the health care system. Consideration of the possible harms is important for research equipoise and informed consent and serves to remind us of the ultimate importance of good scientific method.
Potential Harm to Patients From CCP
Controlled trials bring the opportunity to address 4 areas of potential concern.
Transfusion-Associated Circulatory Overload in the Critically Ill
Transfusion-associated circulatory overload (TACO) is now recognized as the most common serious adverse effect of transfusion. Hemovigilance systems—known to grossly underestimate the true incidence of adverse events—suggest that TACO may occur in as many as 12% of at-risk patient populations [3]. The incidence of TACO would be expected to be even higher in elderly COVID-19 patients with acute lung injury who are being supported with mechanical ventilation. This may be especially relevant in the setting of the pulmonary inflammation and increased vascular permeability that characterize SARS-CoV-2 infection. TACO may be particularly relevant for those patients with viral myocarditis [4]. Recent data indicate that myocardial injury is present in some patients with COVID-19 and is associated with adult respiratory distress syndrome and increased mortality [5]. The “dose” of plasma being considered for CCP is well within the range known to be associated with TACO [[6], [7], [8]] in non–COVID-19 patients.
Complement and Coagulation
CCP will result in the direct infusion of a substantial amount of complement proteins and coagulation factors not found in purified immunoglobulin preparations. Synergy between these 2 ancient inflammatory protein systems has recently been reviewed in our journal [9]. COVID-19 is both highly inflammatory and prothrombotic. The precise role of complement-mediated tissue damage is uncertain. An additional concern regarding infusions of complement arises from research in other infections such as HIV [10] and Ebola [11] where complement-dependent antibody enhancement has been demonstrated. In the case of Ebola, antiviral antibody triggered complement binding to virus which then enhanced infection of cells bearing complement receptors. Whether or not such a mechanism enhances viral infection of lymphocytes (rich in complement receptors) causing the lymphopenia now being observed in COVID-19 patients is not yet known.
Antibody-Dependent Enhancement of COVID-19 Disease
Antibody-dependent enhancement (ADE) is a well-recognized effect in many viral illnesses [12,13]. It is characterized either as the facilitation of viral entry into cells by antibody or the enhancement by antibody of viral toxicity.
ADE is traditionally said to occur when antibody levels are insufficient to fully block viral entry but are sufficient to opsonize virus. Antibody-coated virus is then drawn into cells bearing Fcγ receptors, including monocytes and macrophages. Pulmonary macrophages are central to the inflammatory response in COVID-19 infection, and so considerations of ADE are relevant. The concept of ADE for SARS-CoV-2 is shown in Figure 1 .
Fig 1.
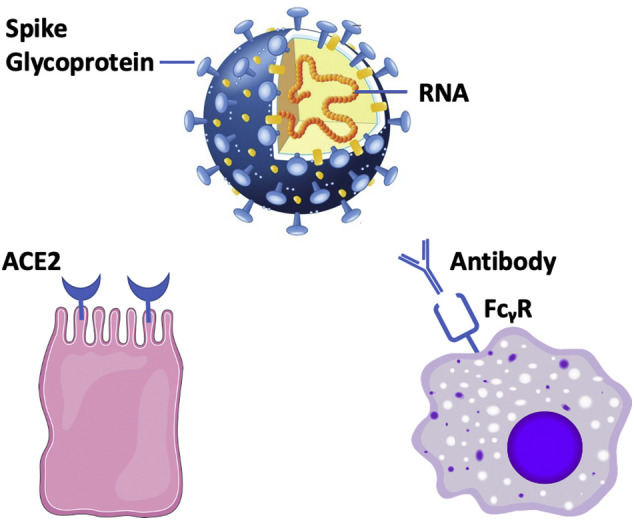
Two putative pathways for SARS-CoV-2 entry into human cells.
Viral Spike glycoproteins attach to receptors on host tissue (bottom left). In the case of SARS-CoV-2, the host receptor is considered to be ACE2 expressed on mucosal cells. A second pathway of viral entry (right) depends on antibody to virus and is referred to as antibody-dependent enhancement (ADE). In ADE, virus with bound antibody is drawn into cells that express the immunoglobulin Fcγ receptor (FcγR).
ADE has been suggested as one explanation (among several others) for the observation that older adults, who may have seen prior strains of a given virus, can have worse infection than young children without prior exposure—a clinical observation seen in COVID-19. ADE has been demonstrated to occur in human clinical dengue virus infection [14], is a well-recognized concern in vaccine research, and may be relevant for studies using CCP as prophylaxis in uninfected high-risk groups such as health care workers. We do not know yet whether ADE plays a role in COVID-19, but the following studies on ADE are relevant to concerns regarding the safety of patients infected with SARS-CoV-2 being considered for CCP infusions.
In a clinical study of human infection with SARS-CoV-1 in Hong Kong, Zhang et al reported that 80% of patients with acute lung injury developed ARDS after 12 days of infection. They observed that early presence of neutralizing anti-Spike antibody was associated with worse outcomes: among those who recovered, antibody peaked at day 20, whereas for those who died, antibody peaked at day 14. Deceased patients also appeared to have a higher titer of anti-Spike neutralizing antibody [15]. This study in SARS-CoV-1 suggested a temporal relationship between emergence of antibody and severe pulmonary toxicity.
Recently, a similar finding was seen by To et al who reported the temporal pattern of antibody response in 108 serum specimens from 23 patients with SARS-CoV-2 infection [16]. Both IgM and IgG antibodies to the viral Spike receptor binding domain (RBD) and to nucleoprotein were present in most patients at 10 days after the onset of symptoms. As was seen in SARS-CoV-1, patients with severe COVID-19 had earlier and higher levels of antibody. Similar findings were reported by Zhao [17]. Whether higher antibody levels are a response to more severe disease or are an ADE trigger resulting in more severe disease is completely unresolved at this time. Either way, it is noteworthy that most patients who are ill with COVID-19 already have an established antibody response, which raises questions regarding the rationale of antibody infusion with CCP.
An important 2019 preclinical study suggesting a causal relationship between transfusion of SARS-CoV-1 antibody and worsening lung pathology was reported by Liu et al in a macaque monkey model of SARS-CoV-1 [18]. This study used a Chinese rhesus monkey (Macaca mulatta) model of experimental SARS-CoV-1–induced acute lung injury to evaluate the effect of transfusion of anti-Spike immunoglobulin. The investigators transfused low-dose (5 mg) purified anti-Spike IgG into group A (n = 6), high-dose (200 mg) purified anti-Spike IgG into group B (n = 6 /group), and 200 mg of control IgG into group C (n = 4). All animals were then challenged with intranasal SARS-CoV-1. Half of the animals in each of the 3 groups were killed at day +2, and the other half were killed at day +21. At day +2, the investigators documented that the transfusion recipients had the expected levels of neutralizing antibody, which was not present in recipients of control Ig. The results were quite surprising: At both day +2 and day +21, histologic examination of the lungs of both the low-dose transfusion and the high-dose transfusion showed clear evidence of worse inflammation compared with the animals who were transfused with nonimmune control Ig. In addition, the investigators found that, at day +2 after transfusion, animals who had been transfused with high-dose antibody showed higher rates of monocyte infiltration and higher levels of IL-8 compared with controls. Overall, the authors concluded that in an experimental model of SARS-CoV-1 infection, transfusion of anti-Spike immunoglobulin caused worsened acute lung injury by provoking a greater inflammatory immune response. Readers will find that this study is well conducted and rich in data. However, the work is in an animal model, and recipients were transfused with purified anti-Spike immunoglobulin rather than polyvalent recovered plasma. Nevertheless, the study establishes important concerns regarding the potential adverse effect of CCP.
Earlier laboratory work by Wang et al had investigated ADE in SARS-CoV-1. They used a human promonocyte cell line that expresses the viral receptor angiotensin-converting enzyme 2 (ACE2) and the FcγRII receptor. Infection results in release of interleukins and an observable cytopathic effect. Using this model, they observed that high concentrations of antibody against SARS-CoV-1 could neutralize infection in vitro but that diluted antisera enhanced infection of the human promonocyte cells, presumably as a result of ADE [19].
Laboratory evidence for ADE has also been demonstrated in Middle East Respiratory Syndrome (MERS). Wan et al prepared a neutralizing monoclonal antibody (called mersmab1) against the RBD of the spike protein of MERS-CoV [20]. The natural viral receptor for MERS-CoV is dipeptidyl peptidase 4 (DPP4). DPP4 is analogous to ACE2 in SARS-CoV-2 disease. Using a laboratory cell infection assay, the investigators could examine the effect of mersmab1 on cell targets that were DPP4 positive and Fcγ negative and on other cell targets that were DPP4 negative and Fcγ positive. As expected, they found that the neutralizing monoclonal antibody could block infection of DPP4+ cells. However, they also found that, compared with controls, the presence of the antibody significantly increased the ability to infect T cells and macrophages that were DPP4 negative and Fcγ positive. Antibody-enhanced infection was observed regardless of the subtype of Fc receptor (CD16A, CD32A, or CD64A). Of interest, they also found that antibody-enhanced infection did not occur if only the Fab or the Fc portion of the antibody was used, suggesting that ADE requires an intact Ig that joins the virus to the Fcγ receptor on host cells. Furthermore, using the same study design, the investigators repeated the experiments in a SARS-CoV-1 model. For these experiments, they used another neutralizing monoclonal antibody, named 33G4, which binds to the SARS-CoV-1 RBD. Antibody enhancement was again observed. Specifically, the antibody could neutralize infection of cells that expressed ACE2 but not Fcγ receptors and enhanced infection of cells that were ACE2 negative but expressed Fcγ receptors.
Although pulmonary inflammation and acute lung injury characterize a high proportion of hospitalized patients with COVID-19 infection, the mechanisms of lung damage remain unclear. Recently, Fu et al reviewed 3 possible mechanisms resulting in the severe pulmonary inflammation in COVID-19 infection: inflammation caused by rapid viral replication and cellular damage, inflammation caused by virus-induced ACE2 downregulation and shedding, and ADE [21]. One of the themes of the many studies on ADE is that an antibody may be classified as “neutralizing” but can still result in adverse clinical effects.
Unexpected Findings Are Best Understood by Randomized Trials
Unexpected findings are well known to be revealed in randomized trials. For example, studies originally designed to examine whether or not erythropoietin could improve the symptoms of anemia in cancer patients encountered the unexpected observation that erythropoietin worsened cancer prognosis [22]. In the setting of CCP and COVID-19, ABO blood groups may play an unexpected role. Non–peer-reviewed data from China demonstrate that group O individuals had lower rates of infection and lower mortality compared with non-O individuals [23]. Although the statistics surrounding this observation are strong, the reason for the observation is not known. Although the findings may relate to virus binding to cell surface glycoproteins with A- or B-like residues, ABO antibodies may also be relevant. Epidemiologic studies from the prior SARS-CoV-1 outbreak in Hong Kong showed that group O individuals were also favored in that outbreak. In 2008, Guillon et al used a laboratory adhesion assay to study the role of ABO antibodies and infection with SARS-CoV-1 [24]. The assay involves measuring the adhesion of CHO cells to Vero cells. The CHO cells were transfected to express both the SARS-CoV-1 Spike protein and A-antigen. The Vero cells expressed ACE2, the binding site for Spike protein, and no ABO antigen. As expected, adhesion of the 2 cells could be blocked using either a monoclonal antibody against viral Spike protein or a monoclonal antibody against ACE2. Using this adhesion model, the investigators found that anti-A antibodies blocked adhesion of the CHO cells to the Vero cells. Blockade of adhesion was specific for anti-A. Adhesion was blocked by normal human group O plasma in a dose-dependent fashion, with decreasing effect at higher dilutions of group O plasma. Although the assay system used is quite artificial and did not involve intact viruses, this line of investigation raises the completely unexpected possibility the ABO antibodies may play some role in SARS-CoV-2 infection. Although this seems unlikely, the real point is that unexpected outcomes are, by definition, unforeseen and are best revealed and analyzed by randomized controlled trials.
Potential Harm by CCP to the Overall Worldwide Health Care Response to COVID-19
Estimates of the number of individuals who will be hospitalized for COVID-19 infection worldwide are astronomical. Given the failure of hyperimmune globulin in randomized controlled trials to improve outcomes in influenza A [25,26] or in respiratory syncytial virus [27] and given the absence of any randomized trials of convalescent plasma for any other viral disease, it is reasonable to imagine that 1-2 U of CCP in COVID-19 will have no meaningful benefit. Establishing lack of efficacy through controlled clinical trials takes an immense importance. In addition to offering false hope to patients, widespread application of CCP therapy prior to establishing a clear and objective measure of its efficacy through randomized trials risks diverting an enormous amount of resources away from other priorities in the pandemic. Test kits for infection and assays for neutralizing antibody levels which will likely be used to screen CCP donors are critical resources that will be diverted away from patient care or public health testing. Resources and equipment of blood collection agencies tasked with production of CCP stocks are resources that will not be used for the collection of the volunteer blood supply. The size of the potential diversion is worthy of investigation by mathematical modeling, as the overall number of CCP donors evaluated and bled could be extremely high. If CCP is not harmful but is also found to be of no clinical value, mass donations of an experimental biologic outside the context of trials will have been an unforgiveable waste of resources. On the other hand, if passive immunoglobulin infusions are shown to have benefit, such information would provide valuable evidence to support the development of hyperimmune globulin preparations either derived from pools of CCP or prepared from engineered antibody produced in vitro. Such preparations are more likely to administer an effective dose than individual doses of CCP.
COVID-19 represents a historic challenge to health care worldwide. In wealthy nations, COVID-19 patients will be receiving multiple therapeutic interventions simultaneously including other experimental therapies in addition to CCP. This fact makes randomization essential because only randomized trials will balance the very large number of confounders that exist in such complex care settings. Without the benefit of randomized controlled trials, it will be nearly impossible to understand the benefit, lack of benefit, risk, or comparative value of CCP. This is indeed a critical moment for all of us. On behalf of patients and their families, devoted health care workers, unselfish blood donors worldwide, and society at large, we rise to applaud those investigators from around the world who are showing leadership through the conduct of randomized trials. Some examples of emerging CCP research trials are presented in a companion paper in this issue of Transfusion Medicine Reviews. For those trials of sufficiently similar design, there would be an outstanding opportunity for researchers to work together to combine outcome analyses. We wish the investigators great success in their work. This is not the moment to abandon primum non nocere. Indeed, there has never been a more urgent or important time for the highest-quality scientific research to lead the way.
Conflict of Interest
The author declares no conflict of interest related to this document.
References
- 1.Sullivan H.C., Roback J.D. Convalescent plasma: therapeutic hope or hopeless strategy in the SARS-CoV-2 pandemic. Transf Med Rev. 2020 doi: 10.1016/j.tmrv.2020.04.001. This Issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duan K., Liu B., Li C., Zhang H., Yu T. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. PNAS. 2020 doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosboom J.J., Klanderman R.B., Zijp M., Hollmann M.W., Veelo D.P., Binnekade J.M. Transfusion-associated circulatory overload: a clinical perspective. Transf Med Rev. 2019;33:69–77. doi: 10.1016/j.tmrv.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y.Y., Ma Y.-T., Zhang J.-Y., Xiang X. COVID-19 and the cardiovascular system. Nature Rev Cardiology. 2020 doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan. China JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liberman L., Maskens C., Cserti-Gazdewich C., Hansen M., Lin Y., Pendergrast J. A retrospective review of patient factors, transfusion practices, and outcomes in patients with transfusion-associated circulatory overload. Transf Med Rev. 2013;27:206–212. doi: 10.1016/j.tmrv.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Piccin A., Cronin M., Brady R., Sweeney J., Marcheselli L., Lawlor E. Transfusion-associated circulatory overload in Ireland: a review of cases reported to the National Haemovigilance Office 2000 to 2010. Transfusion. 2015;55:1223–1230. doi: 10.1111/trf.12965. [DOI] [PubMed] [Google Scholar]
- 8.Wiersum-Osselton J., Whitaker B., Grey S., Land K., Perez G., Rajbhanday S. Revised international surveillance case definition of transfusion-associated circulatory overload: a classification agreement validation study. Lancet Haematol. 2019;6:e350–e358. doi: 10.1016/S2352-3026(19)30080-8. [DOI] [PubMed] [Google Scholar]
- 9.Dzik S. Complement and coagulation: cross talk through time. Transf Med Rev. 2019 doi: 10.1016/j.tmrv.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Willey S., Aasa-Chapman M.M., O’Farrell S., Pellegrino P., Williams I., Weiss R.A. Extensive complement-dependent enhancement of HIV-1 by autologous non-neutralizing antibodies at early stages of infection. Retrovirology. 2011 doi: 10.1186/1742-4690-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takada A., Ebihara H., Feldmann H., Geisbert T.W., Kawaoka Y. Epitopes required for antibody-dependent enhancement of Ebola virus infection. J Infect Dis. 2007;196(Suppl. 2):S347–S356. doi: 10.1086/520581. [DOI] [PubMed] [Google Scholar]
- 12.Smatti M.K., Al Thani A.S., Yassine H.M. Viral-induced enhanced disease illness. Front Microbiol. 2018 doi: 10.3389/fmicb.2018.02991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor A., Foo S.S., Bruzzone R., Dinh L.V., King N.J.C., Mahalingam S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol Rev. 2015;268:340–364. doi: 10.1111/imr.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katzelnick L.C., Gresh L., Holloran E., Mercado J.C., Kuan G., Gordon A. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358:929–932. doi: 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L., Zhang F., Yu W., He T., Yu J., Yi C.E. Antibody responses against SARS coronavirus are correlated with disease outcome of infected individuals. J Med Viol. 2006;78:1–8. doi: 10.1002/jmv.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.To KK, Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L., Wei Q., Lin Q., Fang J., Wang H., Kwok H. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2018 doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S.F., Tseng S.P., Yen C.H., Yang J.Y., Tsao C.H., Shen C.W. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Comm. 2014;451:208–214. doi: 10.1016/j.bbrc.2014.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan Y., Shang J., Sun S., Tai W., Chen J., Geng Q. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J Virology. 2020 doi: 10.1128/JVI.02015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2 mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sinica. 2020 doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blau C.A. Erythropoietin in cancer: presumption of innocence? Stem Cells. 2007;25:2094–2097. doi: 10.1634/stemcells.2007-0229. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J, Yang Y, Huang H, Li D, Gu D, Lu X, et al. Relationship between the ABO blood group and the COVID-19 susceptibility. medRxiv 2020.03.11.20031096. 10.1101/2020.03.11.20031096. [DOI]
- 24.Guillon P., Clément M., Sébille V., Rivain J.G., Chou C.F., Ruvoën-Clouet N. Inhibition of the interaction between the SARS-CoV Spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology. 2008;18:1085–1093. doi: 10.1093/glycob/cwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davey R.T., Fernandez-Cruz E., Markowitz N., Pett S., Babiker A.G., Wentworth D. Anti-influenza hyperimmune intravenous immunoglobulin for adults with influenza A or B infection (FLU-IVIG): a double-blind, randomized, placebo-controlled trial. Lancet Resp Med. 2019;7:951–963. doi: 10.1016/S2213-2600(19)30253-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung I.F.N., KKW To, Lee C.K., Lee K.L., Yan W.W., Chan K. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A (H1N1) infection. Chest. 2013;144:464–473. doi: 10.1378/chest.12-2907. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez W.J., Gruber W.C., Groothuis J.R., Simoes E.A., Rosas A., Lepow M. Respiratory syncytial virus immune globulin treatment of RSV lower respiratory tract infection in previously healthy children. Pediatrics. 1997;100:937–942. doi: 10.1542/peds.100.6.937. [DOI] [PubMed] [Google Scholar]


