Abstract
Background
Since its discovery, SARS-CoV-2 has been spread throughout China before becoming a global pandemic. In Beijing, family clusters are the main mode of human-human transmission accounting for 57.6% of the total confirmed cases.
Method
We present the epidemiological and clinical features of the clusters of three large and one small families.
Result
Our results revealed that SARS-CoV-2 is transmitted quickly through contact with index case, and a total of 22/24 infections were observed. Among those infected, 20/22 had mild symptoms and only two had moderate to severe clinical manifestations. Children in the families generally showed milder symptoms. The incubation period varied from 2 to 13 days, and the shedding of virus from the upper respiratory tract lasted from 5 to over 30 days. A prolonged period of virus shedding (>30 days) in upper respiratory tract was observed in 6/24 cases.
Conclusion
SARS-CoV-2 is transmitted quickly in the form of family clusters. While the infection rate is high within the cluster, the disease manifestations, latent period, and virus shedding period varied greatly. We therefore recommend rigorously testing contacts even during the no-symptom phase and consider whether viral shedding has ceased before stopping isolation measures for an individual.
Keywords: Coronavirus disease-19, COVID-19, SARS-CoV-2, Epidemiological features, Family clusters
As of Apr 13, 2020, the WHO reported a total of 1,739,007 confirmed cases of COVID-19 and 108,432 deaths caused by SARS-CoV-2 infections,1 and the case number has now grown significantly. The disease has now spread to most countries and has become a global pandemic.2 The new virus is highly infectious and has strong ability to transmit from human to human.3 On Feb 10th, 2020, 73 clusters of COVID-19 transmission had been identified in Beijing, of which 66 were family clusters in Beijing.4 The family clusters involved 197 confirmed cases, comprising 57.6% of the total confirmed cases in Beijing (Feb 10th). Thus, family clusters became the main mode of human-human transmission.5 In Beijing, the Beijing Ditan Hospital is the designated hospital for the COVID-19 patients. Here we report 4 family clusters of COVID-19 infection cases in Beijing, which highlight the epidemiological features of COVID-19 circulating in China.
Methods
Cluster investigation
We interviewed four families recruited from Jan 16th to Jan 29th, 2020 at Beijing Ditan Hospital, one of the designated hospitals for COVID-19 treatment in Beijing. All patients were diagnosed as SARS-CoV-2 positive according to “Guidelines for the Diagnosis and Treatment of Novel Coronavirus (SARS-CoV-2) Infection by the National Health Commission (Trial Version 5)”.6 The study was approved by the ethics committee of Beijing Ditan Hospital. Informed consent to the therapeutic regimen was obtained from each patient prior to the study.
Epidemiological and clinical characteristics and laboratory testing
Epidemiological, demographic, clinical, and laboratory testing data were collected through a review of medical records. Clinical data and laboratory testing results were closely monitored until March 6, 2020. Laboratory confirmation of SARS-CoV-2 was carried out by Beijing CDC as well as Beijing Ditan Hospital. Briefly, throat-swab specimens from the upper respiratory tract that were obtained from all patients at admission were maintained in viral-transport medium. SARS-CoV-2 was confirmed by RT-PCR using the protocol described previously.7 All patients had chest radiography at admission.
Results
Epidemiologic and demographic characteristics
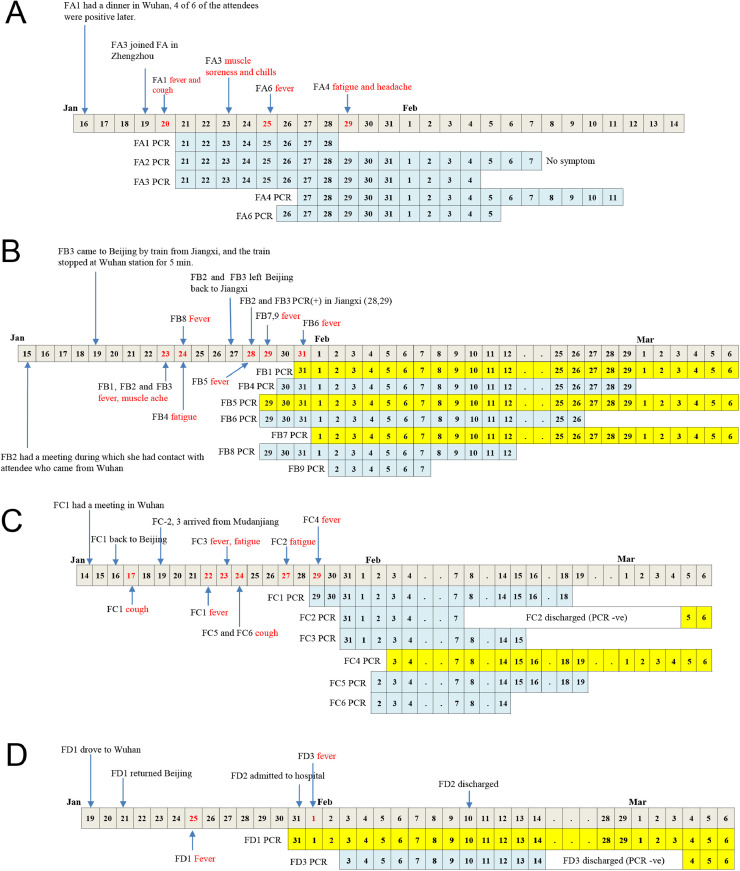
Four families were included in this study; altogether there were twenty-four family members. Each family had one index case, who had direct or indirect contact to Wuhan, which was where the COVID-19 outbreak started. To reveal the transmission of disease within these families, the relationship, contact timeline, and duration of PCR positive period are shown in Table 1 and Fig. 1 .
Table 1.
The epidemiological and demographic characteristics of the four family clusters.
| Family member# | Age (years) | Gender | Relation to index case | Date of nucleic acid test positive | Incubation period& (days) | Date of nucleic acid test negative | positive PCR duration (days)* | generations of case |
|---|---|---|---|---|---|---|---|---|
| FA1 | 36 | M | Index case | 21-Jan | 5 | 28-Jan | 7 | 1st |
| FA2 | 36 | F | Wife | 21-Jan | 5 | 7-Feb | 17 | 2nd |
| FA3 | 60 | F | Mother | 21-Jan | 2 | 4-Feb | 14 | 2nd |
| FA4 | 10 | F | Daughter | 27-Jan | 11 | 11-Feb | 15 | 2nd |
| FA5 | 7 | M | Son | ND | N/A | ND | ND | Negative |
| FA6 | 9m | F | Daughter | 26-Jan | 10 | 5-Feb | 10 | 2nd |
| FB2 | 60 | F | Index case | 28-Jan | 7 | 20-Feb | 23 | 2nd |
| FB3 | 58 | M | Husband | 29-Jan | 4 | 14-Feb | 16 | 3rd |
| FB1 | 33 | F | Daughter | 31-Jan | 7 | Still positive | >35 | 3rd |
| FB4 | 86 | F | Son-in-law's Grandma | 30-Jan | 8 | 29-Feb | 30 | 3rd |
| FB5 | 64 | F | Son-in-law's Mother | 29-Jan | 12 | Still positive | >37 | 3rd |
| FB6 | 65 | M | Son-in-law's Father | 29-Jan | 13 | 26-Feb | 28 | 3rd |
| FB7 | 37 | M | Son in law | 1-Feb | 13 | Still positive | >34 | 3rd |
| FB8 | 6 | F | Granddaughter | 29-Jan | 8 | 12-Feb | 14 | 3rd |
| FB9 | 10m | M | Grandson | 2-Feb | 13 | 7-Feb | 5 | 3rd |
| FC1 | 33 | M | Index case | 29-Jan | 3 | 18-Feb | 20 | 1st |
| FC2 | 59 | M | Father-in-law | 31-Jan | 8 | Returned to positive | 10 | 2nd |
| FC3 | 56 | F | Mother-in -law | 31-Jan | 4 | 15-Feb | 15 | 2nd |
| FC4 | 32 | F | Wife | 3-Feb | 13 | Still positive | >32 | 2nd |
| FC5 | 5 | F | Senior daughter | 2-Feb | 8 | 19-Feb | 17 | 2nd |
| FC6 | 3 | F | Junior daughter | 2-Feb | 8 | 14-Feb | 12 | 2nd |
| FD1 | 52 | M | Index case | 31-Jan | 6 | Still positive | >36 | 1st |
| FD2 | 46 | F | Wife | ND | NA | NA | ND | Negative |
| FD3 | 2 | F | Daughter | 3-Feb | 13 | Returned to positive | 15 | 2nd |
FA: Family A; FB: Family B; FC: Family C; FD: Family D; NS: no symptom; N/A: not applicable; ND: not detected.
Incubation period: from the contact with the index case to viral detection or symptom onset (whichever occurred first).
The number with a “>” on the left show that these cases remained positive when the study ended on Mar 6th 2020. “Return to positive” suggests that PCR test had been negative before it was back to positive again.
Fig. 1.
Detailed epidemiological and clinical timelines for family clusters A (A), B (B), C (C), and D (D). PCR positive timelines for each family members are shown below the main timeline. And the timelines were highlighted yellow for those who remained PCR positive at the end of this study (Mar 6th).
Family A
On Jan 16th 2020, the index case (FA1) had a reunion dinner in Wuhan (4 out of 6 attendees at that dinner were later diagnosed with COVID-19) and returned home at midnight to join his wife (FA2) and three children (FA4, FA5 and FA6). FA1 shared the same bedroom with F2 and F6. On Jan 19th, the four members of the family joined the FA1’s mother (FA3) at Zhengzhou, Henan Province, and the entire family took the train and arrived at Beijing on Jan 20th at noon. On the afternoon of Jan 20th, FA1 started to show symptoms of a fever and dry cough, while other members of the family hadn't shown any symptoms. On Jan 21st, all the elders within the family (i.e. FA1, FA2, FA3) were tested positive for SARS-CoV-2. FA2 never showed any symptoms during the whole course of disease, but a CT chest scan suggested pathological changes in both lungs. FA3 had muscle soreness and chills between Jan 23rd and 31st, and a chest CT scan performed on Feb 3rd showed resolving inflammation in both lungs. One child (FA5) tested negative and remained symptom free for the whole period of this study. On the other hand, FA4 was PCR positive before the onset of disease (fatigue and headache), whereas FA-6 had a fever one day before she tested PCR positive. Neither FA4 nor FA6 showed any abnormality in their chest CT scans. All members of the family were released from hospital by Feb 14th.
Family B
Family B had 4 generations living in two different cities. FB1’s mother (FB2) is an employee at public health institutes in Nanchang who came to Beijing for a meeting on Jan15th 2020 during which she had contact with attendees from Wuhan. In Beijing, she stayed in her daughter's (FB1) house. FB1’s father (FB3) lived in Jiangxi province - he came to Beijing by train on Jan 19th to stay in FB1’s house, and the train had stopped at Wuhan station for 5 min. FB2 and FB3 left Beijing for Nanchang on Jan 27th. The rest of the family, which include FB1, FB4 (mother-in-law), FB5 (husband), FB6 (father-in-law), FB7 (husband's grandmother), FB8 (daughter), and FB9 (son), stayed in Beijing the whole time in the same apartment and had no contact history with people from Wuhan. All the above-mentioned family members contracted COVID-19. The earliest onset of disease occurred on Jan 23rd and included FB1 and her mother (FB2) and father (FB3), and the latest was FB6 on Jan 31st. Meanwhile, PCR assays were performed frequently after the index case and all family members became PCR positive between the period Jan 28th and Feb 2nd. Importantly, FB1 had been sick for 8 days before she was tested positive for SARS-CoV-2. On the other hand, FB6 (father-in-law) was first tested positive by PCR (Jan 29th) before he became sick on Jan 31st, indicating virus shedding before the onset of disease. FB6 became PCR negative on Feb 26th. From the incubation of the index case to becoming PCR negative, there was a period of just over one month. As of Mar 6th, three of the family members were still hospitalized.
Family C
Family C had 3 generations living together. The index case FC1 (husband) had a meeting in Wuhan: he arrived on Jan 14th 2020 and came back to Beijing on Jan 16th. He had cough the next day (Jan 17th), fever on Jan 22nd, and abnormality on his chest CT scan on Jan 27th. He had positive PCR results on Jan 29th when he clinically deteriorated and required critical care. FC2 and FC3 are parent-in-laws of FC1, who visited their home on Jan 19th, and later developed symptoms on Jan 27th and 23rd, respectively; however, they were both PCR confirmed only on Jan 31st. Similarly, the children (FC5 and FC6), who stayed with FC1 the whole time, developed symptoms on Jan 24nd, and were PCR confirmed on Feb 2nd. The wife (FC4), who stayed with her husband and daughters in the same bedroom, was the last one to develop symptoms, on Jan 29th and was PCR positive on Feb 3rd. She was still in hospital as of Mar 6th. In this family cluster, PCR results all became positive much later than the initial onset of disease symptoms, and FC2 was tested PCR negative on Feb 7th and discharged from hospital before it became positive again on Mar 5th. Furthermore, all family members had CT scans, but only the adults showed abnormalities.
Family D
Family D is a small family with three members. The father (FD1) drove to Wuhan on Jan 19th 2020, and stayed in his brother's home, who later was also diagnosed with COVID-19. FD1 returned to his family on Jan 21st, became sick on Jan 25th, and was tested PCR positive for SARS-CoV-2 on Jan 31st. The same day, his wife (FD2) was admitted to hospital for observation only, but she never developed any symptoms other than an occasional cough and remained PCR negative the whole time. On the other hand, their daughter (FD3) became ill on Feb 1st, and was PCR positive two days later. The child was released on Feb 14th, but returned again to hospital on Mar 4th because of the PCR test became positive again, although no clinical symptom was observed.
All families except Family B had an index case who was exposed in Wuhan. The index of Family B (FB1) didn't have direct exposure in Wuhan, but had a meeting with an individual who had come from Wuhan to Beijing. The index cases in the other three families were regarded as the 1st generation contact with the disease, while the other family members were regarded as the 2nd generation cases. The FB index case was the 2nd generation, and the FB family members were regarded as the 3rd generation.
From our study of these four families it is obvious that the period from the contact with the index case to viral detection or symptom onset was highly variable, ranging from 2 to 13 days (Fig. 2 ). Similarly, the shedding of virus in upper respiratory tract (i.e. viral nucleic acid detected in throat swabs) was also variable; it ranged from 5 to over 30 days (Fig. 2). There were 8 cases who remained PCR positive for over 3 weeks, including 5 cases in the family B, 2 cases in Family C and 1 case in Family D, regardless of symptoms. The virus shedding from the upper respiratory tract usually followed the onset of symptoms, but in some of the cases it preceded any symptoms (Fig. 1). Recently, some of the patients, FC2 and FD3, became PCR positive again without showing any symptoms, and this occurred after they were tested negative and released from hospital earlier.
Fig. 2.
The duration of incubation period (blue bar) and PCR positive days (orange bar) for each family members. Incubation period is defined as the period (days) from contacting the index case to the time the patient is PCR positive or shows any disease symptoms.
Clinical characteristics and laboratory assessment
There were 13 cases with fever at presentation but none of them developed fever after hospitalization. Most had a cough. Only two of the adults had moderate to severe symptoms but all stayed in hospital if they had positive PCR tests. Some had radiographic changes but none of them developed ARDS. Most of the blood test results were normal except that one patient had slightly low lymphocytes counts (Table 2 ).
Table 2.
A summary of clinical test results for patients examined in this study.
| Variable (Unit) | Normal Range | Children (Medium and range) | Adults (Medium and range) |
|---|---|---|---|
| WBC: 109/L | 4-10 | 6.685 (4.76-10.48) | 4.96 (3.36-10.55) |
| NE: 109/L | 2-8 | 1.945 (1.2-3.03) | 2.69 (2.30-7.57) |
| LY: 109/L | 1-5 | 4.53 (2.68-6.59) | 1.24 (0.90-3.06) |
| HGB: g/L | 120-160 | 130 (106-145) | 137 (129-150) |
| PLT: 109/L | 100-300 | 312.5 (223-406.1) | 181 (94-276) |
| CRP: mg/L | 0-5 | 1 (0.4-6.5) | 19.65 (3.4-39.6) |
| ALT: U/L | 15-40 | 13 (12-14.5) | 20.5 (10.8-36) |
| CREA: umol/L | 57-97 | 29.55 (14-37.1) | 61 (49.9-301) |
| CK: U/L | 50-310 | 92.5 (67.2-131) | 86.05 (61-301) |
| ALB: g/L | 40-55 | 50 (40.1-51.1) | 46 (41.2-47) |
| LDH: U/L | 120-250 | 262.5 (227-298.9) | 193.3 (192-236.3) |
| PT: s | 9.4-12.5 | 10.8 (10.5-12.3) | 11.75 (10-14.4) |
| APTT: s | 25.1-36.5 | 36 (34.8-42.9) | 35 (26.8-59.6) |
| TT: s | 10.3-16.6 | 18 (16-20.7) | 16.25 (14-17.2) |
| FIB: ug/ml | 200-400 | 204 (183-386) | 216.5 (178-453) |
Most of the children were asymptomatic or only mildly symptomatic. Only 2 of them had fever. However, all patients, including the oldest lady (84 years old) who had several co-morbidities, recovered in the end, although some remained hospitalized because their throat swabs were tested PCR positive. For these patients with no symptoms, daily examinations results were all within normal range. All patients received basic supportive care, along with alpha-interferon (atomization inhalation) and lopinavir/ritonavir (tablets) for antiviral therapy according to local guidelines.v
Discussion
This study documents how COVID-19 spreads within families and suggests adults are more likely to be symptomatic compared to children.8 Eight children were included in this study. The youngest was aged 9-months and the oldest was ten years old. The symptoms of the disease varied from asymptomatic to mild. One child didn't have symptoms and the PCR test for SARS-CoV-2 virus remained negative. Previous studies showed that children appear to have milder infections than adults.9 All the children with positive PCR results became negative after 5-17 days. For the adults, the PCR remained positive much longer, some for over 28 days. In addition, we have shown that viral shedding can precede the onset of symptoms, which may contribute to the spread of this virus. Another interesting observation is that there seemed to be differences in the onset and duration viral shedding between family clusters, suggesting the possible differences in virulence of the viruses, which merits further investigation with the inclusion of the virus genome data.
The index cases had no exposure to the Huanan Seafood Market. Not all family members had coughs so it is possible that much of the transmission was via saliva. Kelvin et al.10 have detected and cultured the COVID-19 in saliva. Our data indicate close contact (e.g., sleeping in the same room as an index patient) and even casual contact or simple proximity (e.g., eating in the same room as an index patient) increases the risk for transmission. This transmission route seems similar to the MERS,11 but more effective. As with MERS-CoV infection control, some authors advise stringent control standards to minimize the emergence of new generations of virus.12
We can see that some cases are PCR positive for prolonged periods after symptom resolution. It is not clear whether this represent viable virus. If so, it might be necessary to ensure a patient has a negative PCR result before stopping isolation measures. This, plus the recognition of subclinical infection, could potentially decrease the probability of fully controlling the outbreaks as both sorts of individuals could be important sources of infection.
Our study had several limitations. First, the small sample size did not enable multivariable risk factor analysis and collinearity could not be evaluated. Second, we need more virological data. We did not culture viruses in those who remained PCR positive. Thirdly, specimens were not available for detailed sequencing analysis, which might help us to clarify the transmission chains within the families.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgement
This study was supported by the Beijing Key Laboratory of Emerging infectious Diseases Grant (No. DTKF 201701) and Grant DTZLX201709. We thank all the medical staff who were involved in treating the patients.
References
- 1.Coronavirus disease (COVID-19) Situation dashboard – by WHO. Available at:https://who.sprinklr.com/. Accessed 13 April 2020.
- 2.Sohrabi C. World Health Organization declares Global Emergency: A review of the 2019 Novel Coronavirus (COVID-19) Int J Surg. 2020 doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahase E. Coronavirus covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. Bmj. 2020;368:m641. doi: 10.1136/bmj.m641. [DOI] [PubMed] [Google Scholar]
- 4.COVID-19 family clusters in Beijing (news report). Available at:https://news.sina.com.cn/c/2020-02-10/doc-iimxxstf0313909.shtml. Accessed 12 February 2020.
- 5.Chan J.F. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020 doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin L., Li T.S. Interpretation of "Guidelines for the Diagnosis and Treatment of Novel Coronavirus (2019-nCoV) Infection by the National Health Commission (Trial Version 5)". Zhonghua Yi Xue Za Zhi. 2020;100:E001. doi: 10.3760/cma.j.issn.0376-2491.2020.0001. (0) [DOI] [PubMed] [Google Scholar]
- 7.Epidemiology Working Group. N.E.R. Policy Working Group for COVID-19 Cluster investigation Technical Guidelines for the 2019 Novel Coronavirus Pneumonia (COVID-19), China (1st Trial Version) Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(3):293–295. doi: 10.3760/cma.j.issn.0254-6450.2020.0003. [DOI] [PubMed] [Google Scholar]
- 8.Guan W.J. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z.M., Fu J.F., Shu Q. New coronavirus: new challenges for pediatricians. World J Pediatr. 2020 doi: 10.1007/s12519-020-00346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.To K.K. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arwady M.A. Middle East Respiratory Syndrome Coronavirus Transmission in Extended Family, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22(8):1395–1402. doi: 10.3201/eid2208.152015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S.S., Wong N.S. Probable transmission chains of Middle East respiratory syndrome coronavirus and the multiple generations of secondary infection in South Korea. Int J Infect Dis. 2015;38:65–67. doi: 10.1016/j.ijid.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]