Graphical abstract
Dear Editor,
As discussed in the Journal recently1 the SARS-CoV-2, a new β-Coronavirus, uses the Angiotensin Converting Enzyme-2 Receptor to enter airway cells. Viral endocytosis is mediated by several factors, including clathrin, the adaptor protein-2 complex (AP2) and the adaptor-associated kinase-1 (AAK1).2
According to a recent report,3 COVID-19, the disease caused by SARS-CoV-2, is characterized by three clinical patterns: no symptoms, mild to moderate disease, severe pneumonia requiring admission to Intensive Care Unit (ICU) in up to 31% of the patients.3
Thus far, there is no specific therapy for COVID-19 infection. No benefit of lopinavir-ritonavir treatment resulted in a recent trial.4 Hydroxychloroquine, currently used in view of its “in vitro” observed effect of reduction of viral replication, seems unsatisfactory.5
Elevated proinflammatory cytokine/chemokine responses seem associated with respiratory failure.3 Recently, tocilizumab, an interleukin-6 inhibitor, was reported as effective in patients with severe COVID-19 pneumonia.6
Baricitinib, another inhibitor of cytokine-release, seems an interesting anti-inflammatory drug. It is a Janus kinase inhibitor (anti-JAK) licensed for the treatment of rheumatoid arthritis (RA) with good efficacy and safety records.7 Moreover it seems to have anti-viral effects by its affinity for AP2-associated protein AAK1, reducing SARS-CoV-2 endocytosis.8
On this basis, we assessed the safety of baricitinib therapy combined with lopinavir-ritonavir in moderate COVID-19 pneumonia patients and we evaluated its clinical impact.
All consecutive hospitalized patients (March 16th −30th) with moderate COVID-19 pneumonia, older than 18 years, were treated for 2 weeks with baricitinib tablets 4 mg/day added to ritonavir-lopinavir therapy. The last consecutive patients with moderate COVID-19 pneumonia receiving standard of care therapy (lopinavir/ritonavir tablets 250 mg/bid and hydroxychloroquine 400 mg/day/orally for 2 weeks) admitted before the date of the first baricitinib-treated patient served as controls. Antibiotics were scheduled only in the case of suspected bacterial infection.
Inclusion criteria were: a. SARS-Co-V2 positivity in the nasal/oral swabs; b. presence of at least 3 of the following symptoms: fever, cough, myalgia, fatigue; c. evidence of radiological pneumonia. After discharge, patients treated with baricitinib were planned to be followed for additional 6 weeks. Exclusion criteria: history of thrombophlebitis (TP), latent tuberculosis infection (QuantiFERON Plus-test positivity, Qiagen, Germany9), pregnancy and lactation.
Mild to moderate COVID-19 disease definition: presence of bilateral pneumonia with or without ground glass opacity and in absence of consolidation, not requiring intubation at enrollment; arterial oxygen saturation (SpO2) >92% at room-air, and ratio arterial oxygen partial pressure/fractional inspired oxygen (PaO2/FiO2) 100–300 mmHg. Parameters daily accessed were: fever, pulmonary function, Modified Early Warning Score (MEWS),10 pulse rate, blood pressure. After the initial execution, radiology imaging was performed on demand. Laboratory investigations included blood cell counts with differential counts, tests for liver and kidney functions, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and procalcitonin.
The trial was approved by the Azienda-USL Toscana Centro Committee for off-label use of drugs. All patients signed a written informed consent to participate to the study.
Descriptive statistics, presented as median and interquartile range (IQR), were calculated using Microsoft ® Office Excel for Windows and ©2019 Minitab, LLC for Windows. Mann-Whitney U test was used for pairwise comparisons, Wilcoxon-test for paired data, Fisher's exact test for categorical variables comparisons. P values ≤ 0.05 were considered statistically significant.
Twelve patients, 10 males and 2 females, with a median age of 63.5 (IQR: 57.7–72.2) years were enrolled in the hospitals in Prato and Alessandria. Clinical characteristics are shown in Table 1 . Comorbidities were similar in the two groups.
Table 1.
Baseline demographic, clinical, and laboratory characteristics of COVID-19 patients treated with either baricitinib or with standard COVID-19 therapy.
| Feature | Baricitinib group | Standard COVID-19 therapy | P value |
|---|---|---|---|
| Patient number, N (%) | 12 (100) | 12 (100) | |
| Male/female, N (%) | 10/2 (83/17) | 10/2 (83/17) | 1 |
| Age years, median (IQR) | 63.5 (57.7–72.2) | 63 (55.5–69.5) | 0.707 |
| Days interval from symptoms onset and therapy starting, days N | 6 (4–6.25) | 4.5 (4–5.25) | 0.204 |
| Cough N (%) | 10 (83) | 12 (100) | 0.478 |
| Dyspnea N (%) | 10 (83) | 9 (75) | 1.000 |
| Sputum production N (%) | 4 (33) | 3 (25) | 1.000 |
| Headache N (%) | 5 (42) | 4 (33) | 1.000 |
| Diarrhea N (%) | 2 (17) | 1 (8) | 1.000 |
| Ageusia/Anosmia N (%) | 6 (50) | 5 (42) | 1.000 |
| Hypertension N (%) | 3 (25) | 2 (17) | 1.000 |
| Diabetes N (%) | 3 (25) | 4 (33) | 1.000 |
| COPD N (%) | 2 (17) | 3 (25) | 1.000 |
| CVD N (%) | 2 (17) | 2 17) | 1.000 |
| Malignancy | 0 (0) | 1 (8) | 1.000 |
| Fever °C, median (IQR) | 38 (37.4–38.2) | 38.1 (37.7–38.7) | 0.356 |
| Breath rate N/min, median (IQR), | 23 (19.5–24.2) | 22 (19.7–24) | 0.665 |
| SpO2 (%),median (IQR) | 91 (90–92.5) | 92 (91.2–93) | 0.157 |
| PaO2/FiO2, median (IQR) | 290 (199.2–292.2) | 268.6 (264.4–295) | 0.603 |
| Pulse rate, median (IQR) | 82 (73–88.3) | 90 (87.2–94.5) | 0.069 |
| SBP mm/Hg, median (IQR) | 120 (110–131.2) | 105 (100–111.25) | 0.003 |
| DBP mm/Hg, median (IQR) | 70 (60–80) | 62.5 (60–66.25) | 0.094 |
| WBC (x109/L), median (IQR) | 7.8 (5.8–10.8) | 8.2 (7.3–8.8) | 0.908 |
| Neutrophils (x109/L), median (IQR) | 6,5 (4.5–7.7) | 6.9 (6.4–7.6) | 0.707 |
| Lymphocytes (x109/L), median (IQR) | 0.7 (0.7–1.2) | 0.89 (0.7–0.9) | 1.000 |
| Hemoglobin (g/L), median (IQR) | 118 (102–134.2) | 125 (108–134) | 0.568 |
| Platelets (x109/L), median (IQR) | 203 (174–227) | 366 (340–407) | 0.000 |
| ALT (U/L), median (IQR) | 28.5 (23.5–52) | 44 (37–50) | 0.157 |
| AST (U/L), median (IQR) | 34 (26.2–48) | 44 (34.7–47) | 0.525 |
| Creatinine (mg/dl), median (IQR) | 1.0 (0.9–1.1) | 1.00 (0.9–1) | 0.583 |
| CRP (mg/dl), median (IQR) | 8.2 (5.8–14.5) | 3 (1.5–3.2) | 0.002 |
| Procalcitonin ng/ml, median (IQR) | 0.7 (0.4–1.1) | 1.2 (0.8–2.1) | 0.902 |
| MEWS, median (IQR) | 3 (2–3.25) | 3 (3–4) | 0.544 |
Abbreviations and symbols:N = number;% = percentage; °C: grade Celsius; min = minute; SpO2 = peripheral capillary oxygen saturation; PaO2/FiO2 = ratio of arterial oxygen partial pressure to fractional inspired oxygen; SBP = systolic blood pressure; DBP = diastolic blood pressure; WBC = white blood cells; AST = serum glutamic oxaloacetic transaminase; ALT = serum alanine aminotransferase; MEWS = Modified Early Warning Score; IQR: Interquartile range.
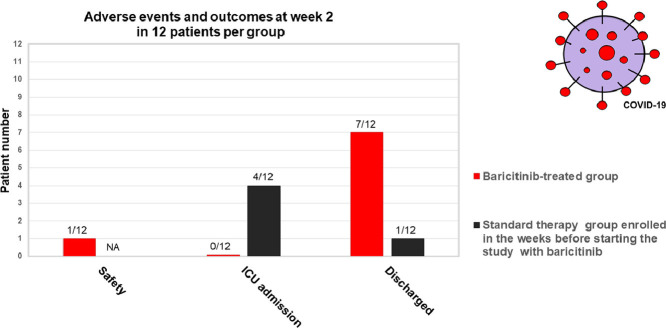
Baricitinib-treatment was well tolerated with no serious adverse events (AEs). Therapy was withdrawn in 1 patient after 10 days of treatment due to consistent transaminases elevation (AST: 267 U/L; ALT: 298 U/L), probably due to the antiviral therapy rather than to the baricitinib treatment, which is mainly renal-metabolized. In addition, no bacterial or opportunistic infections, trombo-flebitis or hematologic toxicity were observed.
Results are summarized in Table 2 . Overall, in the baricitinib-treated group, all clinical characteristics and respiratory function parameters significantly improved both at week 1 and week 2 compared to baseline. CRP values significantly decreased at the same timeframes. In the control-group, no significant changes were recorded at week 2 compared to baseline.
Table 2.
Clinical, laboratory and respiratory parameters of COVID-19 patients after 1- or 2-week treatment in the baricitinib-treated group and in the standard-treated group: comparison within the same treatment group and between the 2 different treatment groups.
|
Baricitinib-treated |
Standard therapy |
Baricitinib vs Standard therapy |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical, laboratory, respiratory parameters | Baseline | Week 1 | Week 2 | P value | Baseline | Week 1 | Week 2 | P value | P value |
| Baseline values vs Week 1* or vs Week 2† values | Baseline values vs Week 1* or vs Week 2† values | Week 1* comparisons or Week 2† comparisons | |||||||
| Cough N (%) | 10 (83) | 8 (66) | 0 | 0.640 * | 12 (100) | 10 (83) | 9 (75) | 0.478 * | 0.640 * |
| 0.000 † | 0.217 † | 0.000 † | |||||||
| Dyspnea N (%) | 10 (83) | 1 (8) | 0 | 0.001 * | 9 (75) | 8 (67) | 8 (67) | 1.000 * | 0.001 * |
| 0.000 † | 1.000 † | 0.001 † | |||||||
| Sputum production N (%) | 4 (33) | 1 (8) | 1 (8) | 0.317 * | 3 (25) | 3 (25) | 3 (25) | 1.000 * | 0.590 * |
| 0.317 † | 1.000 † | 0.217 † | |||||||
| Headache N (%) | 5 (42) | 0 | 0 | 0.037 * | 4 (33) | 2 (17) | 3 (25) | 0.640 * | 0.478 * |
| 0.037 † | 1.000 † | 0.217 † | |||||||
| Diarrhea N (%) | 2 (17) | 0 | 0 | 0.478 * | 1 (8) | 1 (8) | 0 | 1.000 * | 1.000 * |
| 0.478 † | 1.000 † | NA † | |||||||
| Ageusia/Anosmia N (%) | 6 (50) | 3 (25) | 2 (17) | 0.400 * | 5 (42) | 4 (33) | 3 (25) | 1.000 * | 1.000 * |
| 0.193 † | 0.667 † | 1.000 † | |||||||
| Fever, °C median (IQR) | 38 | 36.1 | 36 | 0.001 * | 38.1 | 37.7 | 37.8 | 0.123 * | 0.000 * |
| (37.4–38.2) | (36–36.4) | (36–36.1) | 0.001 † | (37.7–38.7) | (37.1–38.2) | (37.4–38.1) | 0.285 † | 0.000 † | |
| Breath, N/min median (IQR) | 23 | 18 | 16 | 0•004 * | 22 | 19 | 18 | 0.063 * | 0.603 * |
| (19.5–24.2) | (14–20.2) | (16–18) | 0.010 † | (19.7–24) | (17.5–21.7) | (16–22.7) | 0.885 † | 0.094 † | |
| SpO2,% median (IQR) | 91 | 96 | 97 | 0.000 * | 92 | 93.6 | 93.1 | 0.289 * | 0.000 * |
| (90–92.5) | (96–98.2) | (95.7–98) | 0.002 † | (91.2–93) | (90.8–94.1) | (86.5–94.2) | 0.544 † | 0.000 † | |
| PaO2/FiO2 value median (IQR) | 290 | 410 | 421.5 | 0•000 * | 268.6 | 302.2 | 267.6 | 0.106 * | 0.237 * |
| (199.2–292.2) | (315.7–452) | (308.6–456) | 0.001 † | (264.4–295) | (240.1–405.7) | (144.2–350.5) | 0.862 † | 0.017 † | |
| Pulse rate, N/minute median (IQR) | 82 | 70 | 67 | 0•050 * | 90 | 85.5 | 89 | 0.433 * | 0.002 * |
| (73–88.3) | (68–76) | (63.2–71.7) | 0.077 † | (87.2–94.5) | (77.5–93.5) | (84.5–104.5) | 1.000 † | 0.000 † | |
| WBC, x109/L median (IQR) | 7.8 | 7.3 | 7.2 | 0•581 * | 8.2 | 6.9 | 8 | 0.013 * | 0.389 * |
| (5.8–10.8) | (6.2–9) | (6.3–9.3) | 0.122 † | (7.3–8.8) | (6.5–7.4) | (7.4–8.3) | 0.931 † | 0.634 † | |
| Neutrophils, x109/L median (IQR) | 6.5 | 5.2 | 4.8 | 0•351 * | 6.9 | 5.9 | 6.8 | 0.026 * | 0.436 * |
| (4.5–7.7) | (4.8–6.9) | (4–7.1) | 0.201 † | (6.4–7.6) | (5.2–6.4) | (6.1–7.2) | 1.000 † | 0.201 † | |
| Lymphocytes, x109/L median (IQR) | 0.7 | 1.14 | 1.3 | 0•147 * | 0.89 | 0.9 | 0.9 | 0.624 * | 0.187 * |
| (0.7–1.2) | (0.9–1.6) | (1.1–1.6) | 0.038 † | (0.7–0.9) | (0.7–1) | (0.7–0.9) | 0.817 † | 0.092 † | |
| Hb, g/L median (IQR) | 118 | 122 | 128 | 0•411 * | 125 | 123 | 121 | 0.702 * | 0.607 * |
| (102–134.2) | (104–128.1) | (119–129) | 0.451 † | (108–134) | (112–127.2) | (114–130) | 0.689 † | 0.191 † | |
| Platelets, x109/L median (IQR) | 203 | 312 | 354 | 0•121 * | 366 | 358 (321- | 389 | 0.468 * | 0.315 * |
| (174–227) | (233–358) | (65–512) | 0.018 † | (340–407) | 461) | (315–430) | 0.624 † | 0.468 † | |
| ALT, U/L median (IQR) | 28.5 | 49 | 78 | 0•712 * | 44 | 56.5 | 44.5 | 0.094 * | 0.624 * |
| (23.5–52) | (43.2–57.2) | (43–83.7) | 0.838 † | (37–50) | (36–67.2) | (39.5–54.5) | 0.419 | 0.141 † | |
| AST, U/L median (IQR) | 34 | 41 | 48.5 | 0•542 * | 44 | 44.5 | 42.5 | 0.624 † | 0.665 * |
| (26.2–48) | (37–54) | (38–58.5) | 0.080 † | (34.7–47) | (41.7–51.5) | (35–55.2) | 0.453 † | ||
| Creatinine, mg/dl median (IQR) | 1.0 | 0•92 | 0.88 | 0•478 * | 1.00 | 1.0 | 1.1 | 0.347 * | 0.572 * |
| (0.9–1.1) | (0.8–1) | (0.7–0.9) | 0.238 † | (0.9–1) | (0.9–1.1) | (1–1.2) | 0.214 † | 0.165 † | |
| CRP, mg/dl median (IQR) | 8.2 | 2.26 | 1.07 | 0•003 * | 3 | 1.7 | 3.2 | 0.171 * | 0.862 * |
| (5.8–14.5) | (0.9–4.8) | (0.7–3.4) | 0.001 † | (1.5–3.2) | (1.1–8.5) | (2.1–9.2) | 0.225 † | 0.023 † | |
| Procalcitonine, ng/ml median (IQR) | 0.7 | 0.8 | 0.8 (0.7- | 0.625 * | 1.2 | 1.4 | 1.2 | 0.268 * | 0.256 * |
| (0.4–1.1) | (0.6–1.3) | 1.5) | 0.567 † | (0.8–2.1) | (07–1.6) | (0.6–1.3) | 0.782 † | 0.189 † | |
| MEWS median (IQR) | 3 | 1 | 0 | 0.000 * | 3 | 2 | 2 | 0.643 * | 0.177 * |
| (2–3.25) | (1–1) | (0–1) | 0.000 † | (3–4) | (2–3) | (1–6.2) | 0.063 † | 0.016 † | |
| ICU transfer N (%) | 0 | 0 (0) | 0 (0) | NA * | 0 | 3 (25) | 4 (33) | 0.217 * | 0.217 * |
| NA † | 0.093 † | 0.093 † | |||||||
| Discharged N (%) | 0 | 3 (25) | 7 (58)ì | 0.217 * | 0 | 0 (0) | 1 (8) | NA * | 0.217 * |
| 0.005 † | 1.000 † | 0.027 † | |||||||
Abbreviations and symbols: N: number;%: percentage; °C: grade Celsius; min: minute; SpO2: peripheral capillary oxygen saturation; PaO2/FiO2: ratio of arterial oxygen partial pressure to fractional inspired oxygen; SBP: systolic blood pressure; DBP: diastolic blood pressure; WBC: white blood cells; AST: serum glutamic oxaloacetic transaminase; ALT: serum alanine aminotransferase; IU: international unit; MEWS: Modified Early Warning Score; IQR: Interquartile range. Statistical analysis was performed using the Wilcoxon test (for paired comparisons) or the Mann-Whitney test. P value was considered significant if <0.05. *Differences between the values at baseline and after 1 week. † Differences between the values at baseline and after 2 weeks. Standard therapy group: COVID-19 patients under standard respiratory therapy and antiretrovirals and hydroxychloroquine treatment that were admitted in the hospital the week before starting the therapy with baricitinib.
Fever, SpO2, PaO2/FiO2, CRP, and MEWS significantly improved in the baricitinib-treated group compared with controls (p: 0.000; 0.000; 0.017; 0.023; 0.016, respectively). ICU transfer was requested in 33% (4/12) of controls and in none of the baricitinib-treated patients (p = 0.093). Discharge at week 2 occurred in 58% (7/12) of the baricitinib-treated patients vs 8% (1/12) of controls (p = 0.027). At discharge, 57% (4/7) had negative viral nasal/oral swabs.
These preliminary results on 12 patients with moderate COVID-19 pneumonia confirmed the safety of baricitinib therapy in a clinical context different from RA.7 No infections, cardiovascular and hematologic AEs occurred after 2 weeks treatment. The short-term drug exposure may probably explain the absence of the supposed AEs. To confirm the long-term safety, the patients will be followed-up for further 6 weeks, but the restricted time of treatment and the short half-life of the drug (12.5 h) suggest as unlikely the later AEs occurrence.
Remarkably, both at week 1 and week 2, baricitinib therapy significantly improved the clinical and laboratory parameters, none of the patients required ICU support, and the majority of the patients were discharged. These results were likely due to the rapid action of the drug and the short median interval of 6 days from symptoms-onset and therapy starting,
The major limitations of this pilot study were its open-label design with no randomization and the low number of treated patients. A proper control group was missing and this is indeed required to formally demonstrate the efficacy of the therapy.
The use of baritinicib therapy may limit the cytokine-release syndrome associated with COVID-19 and it may be useful because it acts against a wide-range of cytokines. Although our results cannot be generalized to all COVID-19 patients, we believe that these data are encouraging in terms of safety, improvement of clinical impact and reduction of severity progression, and it may be the first-step for future controlled, larger studies.
Funding
This work was partly supported by the Italian Ministry of Health “Ricerca Corrente” Linea 1.
Acknowledgments
The authors are grateful to all the patients, nurses and physicians who helped to perform this study.
References
- 1.Li R., Qiao S., Zhang G. Analysis of angiotensin-converting enzyme 2 (ACE2) from different species sheds some light on cross-species receptor usage of a novel coronavirus 2019-nCoV. J Infect. Apr 2020;80(4):469–496. doi: 10.1016/j.jinf.2020.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conner S.D., Schmid S.L. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J Cell Biol. 2002;156:921–929. doi: 10.1083/jcb.200108123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X. Clinical characteristics of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao B., Wang Y., Wen D. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020 Mar 18 doi: 10.1056/NEJMoa2001282. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferner R.E., Aronson J.K. Chloroquine and hydroxychloroquine in covid-19. BMJ. 2020;369:m1432. doi: 10.1136/bmj.m1432. [DOI] [PubMed] [Google Scholar]
- 6.Xu X., Han M., Li T., et al. Effective treatment of severe COVID-19 patients with tocilizumab, 2020. ChinaXiv: 202003.00026v1. [DOI] [PMC free article] [PubMed]
- 7.Bechman K., Subesinghe S., Norton S. A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatology. 2019;58:1755–1766. doi: 10.1093/rheumatology/kez087. [DOI] [PubMed] [Google Scholar]
- 8.Richardson P., Griffin I., Tucker C. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goletti D., Lee M.R., Wang J.Y., Walter N., Ottenhoff T.H.M. Update on tuberculosis biomarkers: from correlates of risk, to correlates of active disease and of cure from disease. Respirology. May 2018;23(5):455–466. doi: 10.1111/resp.13272. [DOI] [PubMed] [Google Scholar]
- 10.Subbe C.P., Kruger M., Rutherford P., Gemmel L. Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94:521–526. doi: 10.1093/qjmed/94.10.521. [DOI] [PubMed] [Google Scholar]