To the Editor
Since December 2019, an increasing number of coronavirus disease-19 (COVID-19) cases have been reported all around the globe following the outbreak of the pandemic acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1]. To date, COVID-19 lacks a specific treatment to counteract the onset of SARS and subsequent multiorgan failure, whose evolution is usually rapid and severe. Liang and collaborators recently described an increased risk of COVID-19 among patients with cancer, which is also associated with a poorer COVID-19 prognosis [2]. Thus, international panels have recommended to delay or suspend anticancer treatments, when feasible, raising the issue of potential cancer progressions [3,4]. Concerning oncogene-addicted non-small cell lung cancer (NSCLC) patients, outcomes are strongly influenced by the continuous administration of targeted tyrosine-kinase inhibitors (TKIs). Thus, the withdrawal of TKI might be detrimental in this subgroup of patients. This becomes even more relevant when a pulmonary infection by SARS-CoV-2 is diagnosed accidently in asymptomatic patients. Herein, we present two cases of oncogene-driven NSCLC patients suspected infected by SARS-CoV-2 who maintained targeted therapy with ALK/ROS1 TKIs in the presence of SARS-CoV-2 interstitial pneumonia and recovered from infection without specific antiviral treatments.
1. Case 1
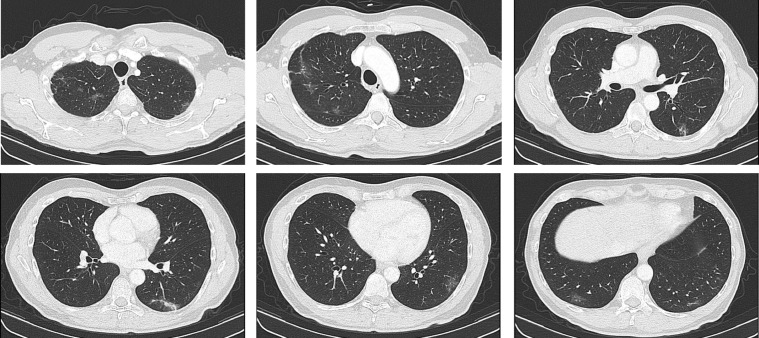
A former smoker 62-years old man was diagnosed with ALK-rearranged NSCLC in January 2020. A computed tomography (CT) scan performed in late December 2019 documented a tumor lesion in the right hilum and right lower paratracheal lymphadenopathy, associated with bilateral lung metastases. On January 31st, the patient started targeted therapy with alectinib for advanced disease. On March 16th the patient reported asthenia and dry cough, in absence of fever, which was treated with beclometasone/formoterol inhalations twice daily. Six days later (March 22nd), the patient developed ageusia, anosmia and night sweats, with concomitant resolution of cough. All the symptoms resolved on March 29th and did not require further medications. CT scan performed on March 30th, after two cycles of alectinib, showed partial response of disease and, collaterally, the onset of slight bilateral subpleuric ground-glass opacities (Fig. 1 ). Due to the lack of symptoms the patient was discharged without any specific medication and alectinib was continued. The test for SARS-CoV-2 was performed on RT-PCR on April 4th, and the result was negative. At the time of writing the manuscript, the patient was completely asymptomatic and was continuing targeted therapy.
Fig. 1.
CT scan of Case 1 (alectinib for ALK-positive NSCLC) showed the onset of multiple bilateral subpleuric ground glass opacities in upper and lower lobes, suspicious for SARS-CoV-2 pneumonia.
2. Case 2
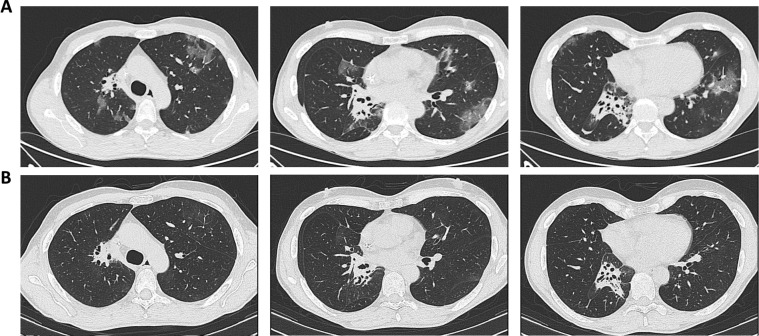
A never smoker 52 years-old man was diagnosed with advanced ROS1-positive NSCLC in September 2015. The patient was currently undergoing lorlatinib from November 2019, following brain progression of disease after failure of previous crizotinib. Since the beginning, the treatment was well tolerated and the patient achieved metabolic complete response of thoracic disease with decrease of brain lesions in January 2019, which was still documented at the last radiological assessment performed in February 2020. On the 9th of March 2020, the patient reported fever (until 39 °C) and dry cough, which worsened in the subsequent three days. After the admission to Emergency Service, a High-Resolution CT (HRCT) scan of the thorax (March 12th) showed the onset of multiple bilateral ground glass opacities, with a crazy paving pattern (Fig. 2 A). RT-PCR performed on March 13th was negative for SARS-CoV-2. The patient was treated with antibiotic treatment with azithromycin and ceftriaxone for 10 days since March 12th, and lorlatinib treatment was not discontinued. Six days after the beginning of antibiotic therapy, the symptoms were improved and the patient was weaned completely off oxygen supply. C-reactive protein values, which were high at the time of admission to the hospital (49 mg/L), were normalized on March 20th (3 mg/L). Due to remission of symptoms, the patient was discharged on March 23rd. A new CT scan performed on April 9th showed complete resolution of interstitial pneumonia (Fig. 2B).
Fig. 2.
A) CT scan of Case 2 (lorlatinib for ROS1-positive NSCLC) performed on March 12th documented multiple bilateral subpleuric ground-glass opacities in a crazy paving pattern, highly suggestive for SARS-CoV-2 pneumonia; B) CT scan after recovery of symptoms (April 9th) showed complete resolution of interstitial pneumonia.
3. Discussion
To our knowledge, this is the first report of advanced NSCLC patients who carried on targeted therapy with ALK/ROS1 TKIs during SARS-CoV-2 pneumonia. In both our cases, the young age and overall good performance status were two important factors which influenced the decision of continuing the therapy. However, we should note that the severity of COVID-19 in our reports was mild, and both patients did not require antivirals or (hydroxy)chloroquine.
Both of our cases were negative for SARS-CoV-2 tested on nasopharyngeal swab. In both cases nevertheless, symptoms, HRCT and laboratory findings were highly evocative for SARS-CoV-2 infection. While in Case 1 the SARS-CoV-2 test was performed once the infection was in remission, hence the negative result, we speculate that the SARS-CoV-2 test of the Case 2 was a false negative. Indeed, a not negligible rate of false negative results has been documented when assessing by RT-PCR, considering that this method heavily rely on the presence of viral genome at the site of sample collection and is operator-dependent [5].
In a recent paper, Zhang and collaborators described a case of EGFR-mutated NSCLC patient who continued osimertinib during COVID-19. Similarly to our cases, the patient had a slight discomfort during the infection which not required intensive care, and he recovered from pneumonia following antiviral treatment with lopinavir/ritonavir [6].
Considering that interstitial pneumonia is a rare albeit potentially severe adverse events in patients receiving ALK-TKIs, a differential diagnosis between SARS-CoV-2 manifestation and a TKI-induced side effect must be taken into account [7]. Since the radiological patterns of COVID-19 are evocative but not always diriment, a multidisciplinary discussion with radiologists is advisable in this subgroup of patients, and CT scan findings must be necessarily correlated with clinical and laboratory features. Even though the COVID-19 outcome in our patients who continued TKIs was favorable, we could not draw definitive conclusions. Large scale studies are urgently needed to assess whether or not TKIs should be maintained during SARS-CoV-2 pneumonia, especially when not severe, in order to avoid potentially-dangerous withdrawal of effective anticancer drugs.
Declaration of Competing Interest
Francesco Facchinetti declares he has attended editorial activities sponsored by Roche and BMS.
Marcello Tiseo declares advisory boards and speakers’ fees from AstraZeneca, Pfizer, Eli Lilly, BMS, Novartis, Roche, MSD, Boehringer Ingelheim, Otsuka, Takeda, Pierre Fabre; research grants from AstraZeneca and Boehringer Ingelheim.
All other authors declare they have no conflict of interest to disclose.
References
- 1.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Q&A on COVID-19 | ESMO. https://www.esmo.org/newsroom/covid-19-and-cancer/q-a-on-covid-19. Accessed April 2, 2020.
- 4.Hanna T.P., Evans G.A., Booth C.M. Cancer, COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat Rev Clin Oncol. 2020;17(5):268–270. doi: 10.1038/s41571-020-0362-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo L., Ren L., Yang S., Xiao M., Chang D., Yang F. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin Infect Dis. 2020 March 21 doi: 10.1093/cid/ciaa310. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H., Huang Y., Xie C. The Treatment and outcome of a lung cancer patient infected with SARS-CoV-2. J Thorac Oncol. 2020 March 5 doi: 10.1016/j.jtho.2020.02.025. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pellegrino B., Facchinetti F., Bordi P., Silva M., Gnetti L., Tiseo M. Lung toxicity in non–small-cell lung cancer patients exposed to ALK inhibitors: report of a peculiar case and systematic review of the literature. Clin Lung Canc. 2018;19 doi: 10.1016/j.cllc.2017.10.008. e151-1. [DOI] [PubMed] [Google Scholar]