Abstract
Background
An epidemic of Coronavirus Disease 2019 (COVID-19) began in December 2019 and triggered a Public Health Emergency of International Concern (PHEIC). We aimed to find risk factors for the progression of COVID-19 to help reducing the risk of critical illness and death for clinical help.
Methods
The data of COVID-19 patients until March 20, 2020 were retrieved from four databases. We statistically analyzed the risk factors of critical/mortal and non-critical COVID-19 patients with meta-analysis.
Results
Thirteen studies were included in Meta-analysis, including a total number of 3027 patients with SARS-CoV-2 infection. Male, older than 65, and smoking were risk factors for disease progression in patients with COVID-19 (male: OR = 1.76, 95% CI (1.41, 2.18), P < 0.00001; age over 65 years old: OR =6.06, 95% CI(3.98, 9.22), P < 0.00001; current smoking: OR =2.51, 95% CI(1.39, 3.32), P = 0.0006). The proportion of underlying diseases such as hypertension, diabetes, cardiovascular disease, and respiratory disease were statistically significant higher in critical/mortal patients compared to the non-critical patients (diabetes: OR=3.68, 95% CI (2.68, 5.03), P < 0.00001; hypertension: OR = 2.72, 95% CI (1.60,4.64), P = 0.0002; cardiovascular disease: OR = 5.19, 95% CI(3.25, 8.29), P < 0.00001; respiratory disease: OR = 5.15, 95% CI(2.51, 10.57), P < 0.00001). Clinical manifestations such as fever, shortness of breath or dyspnea were associated with the progression of disease [fever: 0R = 0.56, 95% CI (0.38, 0.82), P = 0.003;shortness of breath or dyspnea: 0R=4.16, 95% CI (3.13, 5.53), P < 0.00001]. Laboratory examination such as aspartate amino transferase(AST) > 40U/L, creatinine(Cr) ≥ 133mol/L, hypersensitive cardiac troponin I(hs-cTnI) > 28pg/mL, procalcitonin(PCT) > 0.5ng/mL, lactatede hydrogenase(LDH) > 245U/L, and D-dimer > 0.5mg/L predicted the deterioration of disease while white blood cells(WBC)<4 × 109/L meant a better clinical status[AST > 40U/L:OR=4.00, 95% CI (2.46, 6.52), P < 0.00001; Cr ≥ 133μmol/L: OR = 5.30, 95% CI (2.19, 12.83), P = 0.0002; hs-cTnI > 28 pg/mL: OR = 43.24, 95% CI (9.92, 188.49), P < 0.00001; PCT > 0.5 ng/mL: OR = 43.24, 95% CI (9.92, 188.49), P < 0.00001;LDH > 245U/L: OR = 43.24, 95% CI (9.92, 188.49), P < 0.00001; D-dimer > 0.5mg/L: OR = 43.24, 95% CI (9.92, 188.49), P < 0.00001; WBC < 4 × 109/L: OR = 0.30, 95% CI (0.17, 0.51), P < 0.00001].
Conclusion
Male, aged over 65, smoking patients might face a greater risk of developing into the critical or mortal condition and the comorbidities such as hypertension, diabetes, cardiovascular disease, and respiratory diseases could also greatly affect the prognosis of the COVID-19. Clinical manifestation such as fever, shortness of breath or dyspnea and laboratory examination such as WBC, AST, Cr, PCT, LDH, hs-cTnI and D-dimer could imply the progression of COVID-19.
Keywords: COVID-19, Risk factor, Comorbidity, Clinical manifestation, Laboratory examination
Introduction
Since December 2019, a cluster of pneumonia have attacked all human beings.1 The pathogen was designated as SARS-CoV-2 by the International Committee on Taxonomy of Viruses, and this pneumonia was named as Coronavirus Disease2019 (COVID-19) by World Health Organization (WHO).2 Nowadays, there were more than one million confirmed cases and over 100000 deaths occurred in 208 countries/territories according to the report of WHO until April 12th, 2020. The rapidly increasing of patients, especially the critical or mortal patients, brought a big challenge to the public health. Lai et al.3 found that mortality was correlated with country health care resources. However, in many countries, the invasive ventilator and intensive care unit (ICU) were far from adequate for the treatment of critical patients. Clinical workers should pay attention to the risk factors of COVID-19 critical disease and death, identify critical patients early, allocate medical resources rationally and timely adjust the treatment plan to enhance the efficacy and reduce the risk of death. In this article, we analyzed the clinical characteristics of COVID-19 patients with critical/mortal illness and non-critical illness in 13 literatures with 3027 patients, to identify the risk factors for COVID-19 patients to develop critical disease or death, in order to effectively predict the progression of the disease, make early treatment response and allocate medical resources in a better way.
Data and Methods
Search strategy and selection criteria
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)Statement.
We selected relevant studies published between Jan 1, 2020 and Mar 20, 2020, by searching Pubmed, Embase, Web of Science, and CNKI. We applied no language restrictions. The search terms and relative variants were as follows: severe acute respiratory syndrome coronavirus 2 OR Wuhan coronavirus OR Wuhan seafood market pneumonia virus OR COVID-19 OR COVID19 OR coronavirus disease 2019 virus OR SARS-CoV-2 OR SARS2 OR 2019-nCoV OR 2019 novel coronavirus AND Mortalities OR Mortality OR Fatality OR Death OR acute respiratory distress syndrome (ARDS) OR ICU. We also reviewed the references of included articles to guarantee the comprehensiveness and accuracy of our research.
The inclusion criterions for the 13 articles are as follows: (1) groups involving critical illness or death and non-critical illness; (2) patients should be confirmed to have been infected by 2019 novel coronavirus; (3) study designs included randomized controlled trials, nonrandomized controlled trials, case‐control studies, cohort studies, cross‐sectional studies, and also case reports; (4) at least one outcome reported among demographical characteristics, comorbidities, clinical manifestations or laboratory examinations; (5) study sample was larger than 20. We excluded duplicate reports, abstracts from conferences and commentary articles.
Data Extraction and Quality Assessment
Data extraction and the evaluation of literature quality were conducted independently by 2 investigators (Z.Z.H&T.W.L). Microsoft Excel database was used to record all available information, including baseline details, comorbidities, clinical manifestations and laboratory examinations. Any disagreement was resolved by another investigator (P.F). The MINORS4 was used to assess bias risk.
Statistical Analysis of Data
All analyses were performed using Microsoft Excel, State software version 15.0 and RevMan software version 5.3. The results of the included studies were performed with fixed-effect models (Mantel–Haenszel method) or random-effect models in cases of significant heterogeneity between studies. We used the I2 statistics to assess the magnitude of heterogeneity: 25%, 50%, and 75% represented low, moderate, and high degrees of heterogeneity, respectively. The chosen of the proper effect model was based on the analysis results: the fixed effect model was used if I2 ≤ 50% and the random effect model was used if I2>50%. If there was statistical heterogeneity among the results, a further sensitivity analysis was conducted to determine the source of heterogeneity. After the significant clinical heterogeneity was excluded, the randomized effects model was used for meta-analysis. P < 0.05 was considered as statistical significance.
Results
Research Selection and Quality Assessment
Based on the previous search strategy, 523 studies were searched from the online database. After deleting duplicate records, a total of 343 records were retained. Then, 311 articles were excluded by the titles and abstracts, and 19 of the remaining 32 articles were deleted for various reasons. The last 13 articles were included in the meta-analysis. Finally, a total of 13 studies with 3027 patients were included5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 ( Fig. 1 ).All of the selected studies were published in 2020 with different sample patient sizes that ranged from 27 to 1099 patients. Clinical outcome was defined as ICU admission in 3 studies,6 , 8 , 12 refractory in 1 study,7 severity in 2 studies,9 , 11 Sp02 < 90%in 1 study,13 onset of ARDS in 1 study14 and death in the remaining investigation.5 , 10 , 15, 16, 17 The risk of bias and applicability concerns included studies are showed in Table 1 . Over all, none of the studies was considered to be seriously flawed according to the MINORS assessment. The 13 included studies scored between 18 and 21. All studies were considered to have a low risk of bias for selection.
Fig. 1.
Flow diagram of the study selection process.
Table 1.
MINORS rating scale: ①A clearly stated aim;②Inclusion of consecutive patients; ③Prospective collection of data;④ Endpoints appropriate to the aim of the study;⑤Unbiased assessment of the study endpoint;⑥Follow-up period appropriate to the aim of the study;⑦Loss to follow up less than 5%;⑧ Prospective calculation of the study size.⑨Appropriate selection of control group;⑩Synchronization of control group; ⑪Baseline comparable between groups ⑫Appropriately statistical analysis. The global ideal score being 24 for comparative studies.
| Study | ① | ② | ③ | ④ | ⑤ | ⑥ | ⑦ | ⑧ | ⑨ | ⑩ | ⑪ | ⑫ | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Guan WJ | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 18 |
| Huang C | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 21 |
| Mo P | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 20 |
| Peng YD | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 2 | 2 | 2 | 2 | 19 |
| Shi Y | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 21 |
| Tang N | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 19 |
| Tian S | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 2 | 2 | 2 | 2 | 19 |
| Wang D | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 21 |
| Wang Z | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 21 |
| Wu C | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 19 |
| Yang X | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 2 | 2 | 2 | 2 | 19 |
| Yuan ML | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 21 |
| Zhou F | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 18 |
Demographical characteristics
The demographical characteristics of the included studies are shown in Table 2 . The results from the 13 included studies (with a total amount of 3027 patients) showed that the proportion of male was significant higher in critical/death group compared to the non-critical group [male: OR = 1.77, 95% CI (1.43, 2.19), P < 0.00001] (Fig. 2 ).
Table 2.
Demographics of the included studies.
| Study | Year | Research type | Country | Number of patients n |
Age median, y |
Male n (%) |
Current Smoking n (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | ||||
| Guan WJ et al. 5 | 2020 | Retrospective study | China | 67 | 1032 | 63 | 46 | 45(67.2%) | 592(57.5%) | 17(25.4%) | 120(11.7%) |
| Huang C et al.6 | 2020 | Retrospective study | China | 13 | 28 | 49 | 49 | 11(84.6%) | 19(67.9%) | 0 | 3(10.7%) |
| Mo P et al.7 | 2020 | Retrospective study | China | 85 | 70 | 61 | 46 | 55(64.7%) | 31(44.3%) | 4(4.7%) | 2(2.9%) |
| Peng YD et al.8 | 2020 | Retrospective study | China | 16 | 96 | 57.5 | 62 | 9(56.3%) | 44(45.8%) | — | — |
| Shi Y et al.9 | 2020 | Retrospective study | China | 49 | 438 | 56 | 45 | 36(73.5%) | 223(50.9%) | 6(12.2%) | 34(7.8%) |
| Tang N et al.10 | 2020 | Retrospective study | China | 21 | 162 | 64 | 52.4 | 16(76.2%) | 82(50.6%) | — | — |
| Tian S et al.11 | 2020 | Retrospective study | China | 46 | 216 | 61.4 | 44.5 | 26(56.5%) | 101(46.8%) | — | — |
| Wang D et al.12 | 2020 | Retrospective study | China | 36 | 102 | 66 | 51 | 22(61.1%) | 53(52.0%) | — | — |
| Wang Z et al.13 | 2020 | Retrospective study | China | 14 | 55 | 70.5 | 37.0 | 7(50.0%) | 25(45.5%) | — | — |
| Wu C et al.14 | 2020 | Retrospective study | China | 84 | 117 | 58.5 | 48 | 60(71.4%) | 68(58.1%) | — | — |
| Yang X et al.15 | 2020 | Retrospective study | China | 32 | 20 | 64.6 | 51.9 | 21(65.6%) | 14(70.0%) | — | — |
| Yuan ML et al.16 | 2020 | Retrospective study | China | 10 | 17 | 68 | 55 | 4(40.0%) | 8(47.1%) | — | — |
| Zhou F et al.17 | 2020 | Retrospective study | China | 54 | 147 | 69 | 52 | 38(70.4%) | 81(55.1%) | 5(9.3%) | 6(4.1%) |
Fig. 2.
Meta-analysis for male, age>65 years old and current smoking in COVID-19 cases. Heterogeneity analysis was carried out using Q test, the among studies variation (I2 index). Forest plots depict the comparison of the incidences of male, age>65 years old and current smoking in critical/mortal and non-critical patients.
The median ages ranged from 49 to 70.5 years old in the critical/mortal group across the enrolled studies. The median ages ranged from 37 to 62 years old in the non-critical group (Table1). The median ages were generally higher in critical/death group compared to the non-critical group. Furthermore, age over 65 years was analyzed as a subgroup by Guan8 and Tian11 (with a total amount of 1273 patients). Meta-analysis showed that the proportion of patients older than 65 years was higher in critical/death group compared to the non-critical group. [age over 65 years old: OR = 6.01, 95% CI (3.95, 9.16), P < 0.00001] (Fig. 2).
Five studies showed that the proportion of current smoker was statistically significant higher in critical/mortal group compared to the non-critical group [current smoking: OR = 2.04, 95% CI (1.32, 3.15), P = 0.0006] (Fig. 2).
There was no heterogeneity in the estimates of male, age over 65 years old and current smoking among the identified studies with I2 = 0.
Comorbidities
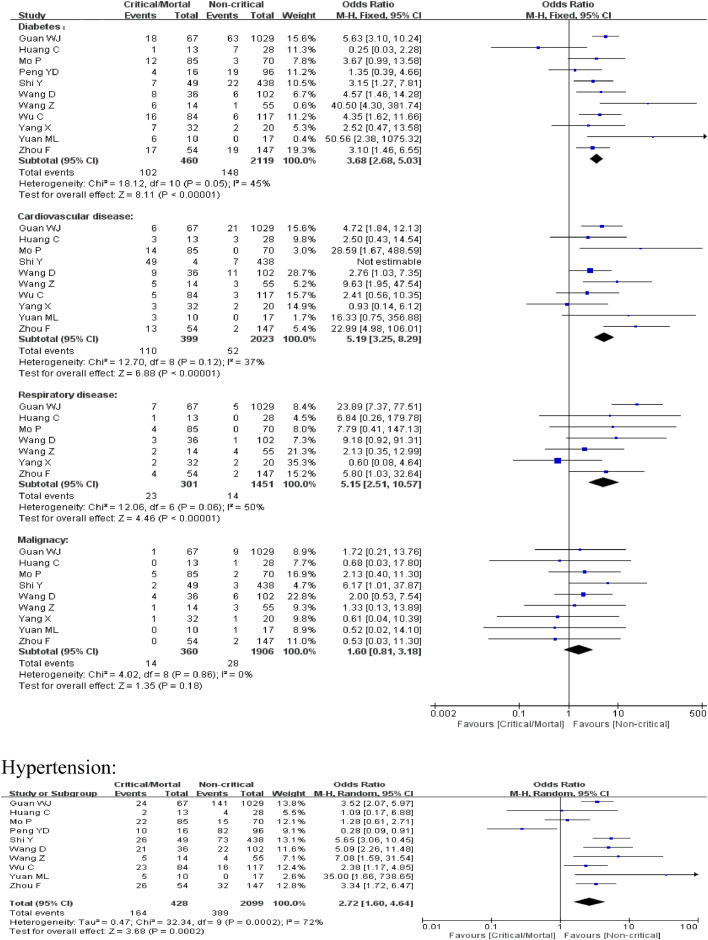
The comorbidities of patients of the included studies are shown in Table 3 . We then compared the difference of the prevalence of the comorbidities between critical/mortal patients and non-critical patients. For diabetes, cardiovascular disease and respiratory disease, the heterogeneity test results were calculated as I2 = 45%, 37% and 50%. Thus, the fixed-effect model was used for further analyses. The proportion of diabetes, cardiovascular disease and respiratory disease was statistically significant higher in critical/mortal group compared to the non-critical group [diabetes:0R = 3.68, 95% CI (2.68, 5.03), P < 0.00001;cardiovascular disease: OR = 5.19, 95% CI (3.25, 8.29), P < 0.00001; respiratory disease: OR = 5.15, 95% CI (2.51, 10.57), P < 0.00001](Fig. 3 ). For hypertension, the heterogeneity test showed that I2 = 72%. Given that the severity of illness and severity of epidemic might contribute to the heterogeneity, we classified the studies into two subgroups according to whether the study site was located in Wuhan. However, heterogeneity still exists. So the random effect model was used. The result indicated a higher proportion of hypertension in critical/mortal group[OR = 2.72, 95% CI (1.60,4.64), P = 0.0002] (Fig. 3).For malignancy, the fixed-effect model(I2 = 0) meta-analysis showed that the proportion of malignancy was higher in critical/death group yet without statistical significance[OR = 1.60, 95% CI (0.81, 3.18), P = 0.18] (Fig. 3).
Table 3.
Comorbidities of patients of the included studies.
| Study | Diabetes n (%) |
Hypertensionn (%) |
Cardiovascular disease n (%) |
Respiratory disease n (%) |
Malignancy n (%) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | Critical | Non-critical | |
| Guan WJ | 67 (26.9%) | 63 (6.1%) | 24 (35.8%) | 141 (13.7%) | 6 (9.0%) | 21 (2.0%) | 7 (10.4%) | 5 (0.5%) | 1 (1.5%) | 9 (0.9%) |
| Huang C | 13 (7.7%) | 7 (25.0%) | 2 (15.4%) | 4 (14.3%) | 3 (23.1%) | 3 (10.7%) | 1 (7.7%) | 0 | 0 | 1 (3.6%) |
| Mo P | 85 (14.1%) | 3 (4.3%) | 22 (25.9%) | 15 (21.4%) | 14 (16.5%) | 0 | 4 (4.7%) | 0 | 5 (5.9%) | 2 (2.9%) |
| Peng YD | 16 (25.0%) | 19 (19.8%) | 10 (62.5%) | 82 (85.4%) | — | — | — | — | — | — |
| Shi Y | 49 (14.3%) | 22 (5.0%) | 26 (53.1%) | 73 (16.7%) | 4 (8.2%) | 7 (1.6%) | — | — | 2 (4.1%) | 3 (0.7%) |
| Wang D | 36 (22.2%) | 6 (5.9%) | 21 (58.3%) | 22 (21.6%) | 9 (25.0%) | 11 (10.8%) | 3 (8.3%) | 1 (1.0%) | 4 (11.1%) | 6 (5.9%) |
| Wang Z | 14 (42.9%) | 1 (1.8%) | 5 (35.7%) | 4 (7.3%) | 5 (35.7%) | 3 (5.5%) | 2 (14.3%) | 4 (7.3%) | 1 (7.1%) | 3 (5.5%) |
| Wu C | 84 (19.0%) | 6 (5.1%) | 23 (27.4%) | 16 (13.7%) | 5 (6.0%) | 3 (2.6%) | — | — | — | — |
| Yang X | 32 (21.9%) | 2 (10.0%) | — | — | 3 (9.4%) | 2 (10.0%) | 2 (6.3%) | 2 (10.0%) | 1 (3.1%) | 1 (5.0%) |
| Yuan ML | 10 (60.0%) | 0 | 5 (50.0%) | 0 | 3 (30.0%) | 0 | — | — | 0 | 1 (5.9%) |
| Zhou F | 54 (31.5%) | 19 (12.9%) | 26 (48.1%) | 32 (21.8%) | 13 (24.1%) | 2 (1.4%) | 4 (7.4%) | 2 (1.4%) | 0 | 2 (1.4%) |
Fig. 3.
Meta-analysis for comorbidities in COVID-19 cases. Fix-effect model for diabetes, cardiovascular disease, respiratory disease and malignancy. Random-effect model for hypertension. Heterogeneity analysis was carried out using Q test, the among studies variation (I2 index). Forest plots depict the comparison of the incidences of the 5 diseases in critical/mortal and non-critical patients.
Clinical Manifestation
The study of clinical manifestation included 13 studies, a total of 3025 cases. The clinical features are showed in Table 4 and the results of meta-analysis are showed in Table 5 . For fever (temperature ≥ 37.3°C), the proportion of fever was statistically lower in critical/mortal group [0R = 0.56, 95% CI (0.38, 0.82), P = 0.003]. The proportion of headache and myalgia/arthralgia were lower in critical/mortal group compared to the non-critical group [headache: OR = 0.82, 95% CI (0.50, 1.36), P = 0.45; myalgia/arthralgia: OR = 0.77, 95% CI (0.58, 1.04), P = 0.09] but there was no statistical significance. For cough, sputum production, fatigue, diarrhea and nausea/ vomiting, the proportion of them was higher in critical/mortal patients[cough: OR = 1.08, 95% CI (0.85,1.38), P = 0.52; sputum production: OR = 1.14, 95% CI (0.84, 1.54), P = 0.39; fatigue: OR = 1.13, 95% CI (0.88, 1.44), P = 0.34;diarrhea: OR = 1.41, 95% CI (0.82, 2.43), P = 0.22;nausea/ vomiting: OR = 1.32, 95% CI (0.72, 2.42), P = 0.37], however, without statistical significance. For shortness of breath/ dyspnea, the proportion of this clinical manifestation was statistically significant higher in critical/mortal group[0R = 4.16, 95% CI (3.13, 5.53), P < 0.00001].
Table 4.
Clinical manifestation of patients of the included studies.
| Study | Fever (%) |
Cough (%) |
Sputum production (%) |
Dyspnea (%) |
Headache (%) |
Nausea or vomiting (%) |
Fatigue (%) |
Myalgia or arthralgia (%) |
Diarrhea (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | Critical | Non-critical | |
| Guan WJ | 88.1% | 89.0% | 68.7% | 67.9% | 29.9% | 34.0% | 53.7% | 16.4% | 11.9% | 13.8% | 4.5% | 5.1% | 32.8% | 38.6% | 9.0% | 15.4% | 6.0% | 3.7% |
| Huang C | 100.0% | 96.4% | 84.6% | 71.4% | 38.5% | 23.1% | 92.3% | 37.0% | 0.0% | 12.0% | — | — | 53.8% | 39.3% | 53.8% | 39.3% | 0.0% | 3.6% |
| Mo P | 74.1% | 90.0% | 63.5% | 61.4% | 0.0% | 0.0% | 41.2% | 21.4% | 5.9% | 4.3% | 2.4% | 2.9% | 38.8% | 38.6% | 25.9% | 40.0% | 5.9% | 2.9% |
| Peng YD | 100.0% | 88.5% | 75.0% | 66.7% | 0.0% | 0.0% | 18.8% | 10.4% | — | — | — | — | 56.3% | 64.6% | 56.3% | 64.6% | 12.5% | 13.5% |
| Tian S | 80.4% | 82.4% | 54.3% | 44.0% | — | — | 32.6% | 1.4% | 6.5% | 6.5% | — | — | 32.6% | 25.0% | — | — | — | — |
| Wang D | 100.0% | 98.0% | 58.3% | 59.8% | 22.2% | 28.4% | 63.9% | 19.6% | 8.3% | 5.9% | 19.4% | 11.8% | 80.6% | 65.7% | 33.3% | 35.3% | 16.7% | 7.8% |
| Wang Z | — | — | 57.1% | 54.5% | 28.6% | 29.1% | 50.0% | 23.6% | 0.0% | 18.2% | 7.1% | 3.6% | 50.0% | 40.0% | 14.3% | 34.5% | 14.3% | 14.5% |
| Wu C | 92.9% | 94.0% | 81.0% | 81.2% | 48.8% | 35.9% | 59.5% | 25.6% | — | — | — | — | 32.1% | 32.5% | 32.1% | 32.5% | — | — |
| Yang X | 96.9% | 100.0% | 78.1% | 75.0% | — | — | 65.6% | 60.0% | 6.3% | 5.0% | 3.1% | 5.0% | — | — | 12.5% | 10.0% | — | — |
| Yuan ML | 60.0% | 88.2% | 50.0% | 64.7% | — | — | 100.0% | 5.9% | — | — | — | — | — | — | 10.0% | 11.8% | — | — |
| Zhou F | 94.4% | 87.8% | 72.2% | 76.2% | 25.9% | 20.4% | 63.0% | 15.0% | — | — | 5.6% | 2.7% | 27.8% | 19.7% | 14.8% | 14.3% | 3.7% | 4.8% |
Table 5.
Results of meta-analysis of the clinical manifestation.
| Clinical manifestation | OR | 95%CI | P value |
|---|---|---|---|
| Fever | 0.56 | 0.38-0.82 | P=0.003 |
| Headache | 0.82 | 0.50-1.36 | 0.45 |
| Myalgia or arthralgia | 0.77 | 0.58-1.04 | 0.09 |
| Cough | 1.08 | 0.85-1.38 | 0.52 |
| Sputum production | 1.14 | 0.84-1.54 | 0.39 |
| Fatigue | 1.13 | 0.88-1.44 | 0.34 |
| Diarrhea | 1.41 | 0.82-2.43 | 0.22 |
| Nausea or vomiting | 1.32 | 0.72-2.42 | 0.37 |
| Shortness of breath/Dyspnea | 4.16 | 3.1- 5.53 | <0.00001 |
Laboratory examination
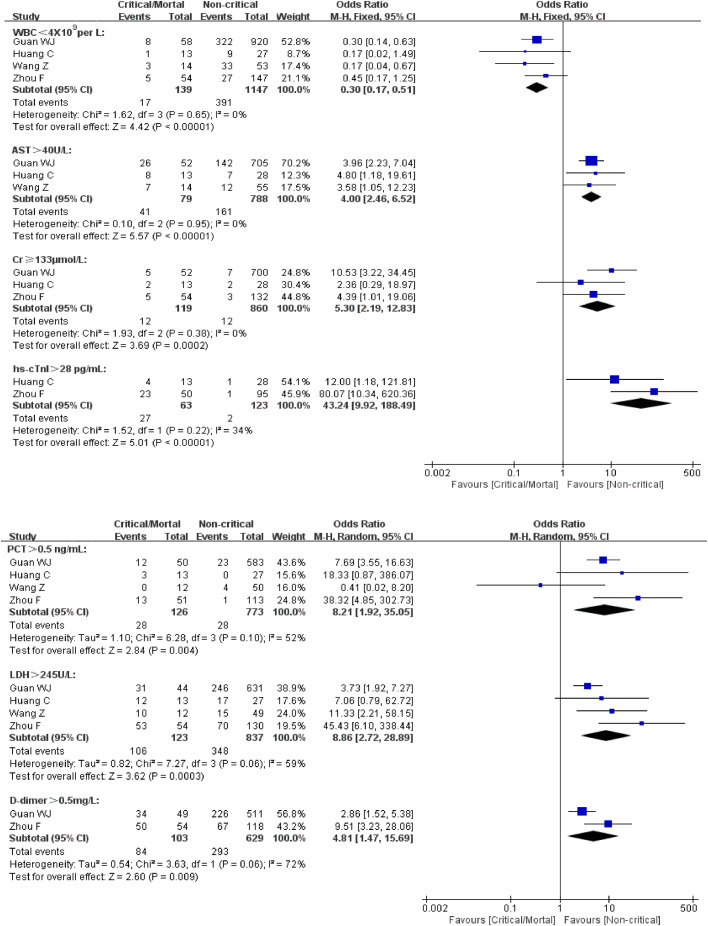
The laboratory examination of the included studies (4 studies, a total of 1286 cases) are shown in Table 6 . For “WBC < 4 × 109per L”, the analysis results of the fixed effect-model (I2 = 0)showed that the proportion of “WBC < 4 × 109per L” was statistically lower in critical/mortal group[0R = 0.30, 95% CI (0.17, 0.51), P < 0.00001] (Fig. 4 ). For “AST > 40U/L”, “Cr ≥ 133μmol/L” and “hs-cTnI > 28 pg/mL”, the heterogeneity test results were calculated as I2 = 0%, 0% and 34%. We used the fixed-effect model for further analyses. The proportion of “AST > 40U/L”, “Cr ≥ 133μmol/L” and “hs-cTnI > 28 pg/mL” was statistically significant higher in critical/death group compared to the non-critical group [“AST > 40U/L”:0R = 4.00, 95% CI (2.46, 6.52), P < 0.00001;“Cr ≥ 133μmol/L”:0R = 5.30, 95% CI (2.19, 12.83), P = 0.0002; “hs-cTnI > 28 pg/mL”:0R = 43.24, 95% CI (9.92, 188.49), P < 0.00001] (Figure 5). The I2 value of “PCT > 0.5 ng/mL”, “LDH > 245U/L” and “D-dimer > 0.5mg/L” was, respectively, 52%, 59% and 72%. So the random effect model was used. The results indicated a higher proportion of “PCT > 0.5 ng/mL”, “LDH > 245U/L” and “D-dimer > 0.5mg/L” in critical/mortal patients with statistical significance [“PCT > 0.5 ng/mL”: OR = 43.24, 95% CI (9.92, 188.49), P< 0.00001; “LDH > 245U/L”:OR=43.24, 95% CI (9.92, 188.49), P < 0.00001;“D-dimer0.5mg/L”: OR = 43.24, 95% CI (9.92, 188.49), P < 0.00001] (Figure 5).
Table 6.
Laboratory examination of patients of the included studies.
| Study | White blood cells <4 × 109 per L n (%) |
Aspartate aminotransferase>40U/L n (%) |
Creatinine ≥133μmol/L n (%) |
Hypersensitive troponin I>28 pg/mL n (%) |
Procalcitonin >0.5 ng/mL n (%) |
Lactate dehydrogenase >245U/L n (%) |
D-dimer>0.5mg/L n (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Critical/Mortal | Non-critical | Critical/Mortal | Non-Critical | Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | Critical/Mortal | Non-critical | |
| Guan WJ | 8 (13.8%) | 322 (35.0%) | 26 (50.0%) | 26 (50.0%) | 5 (9.6%) | 7 (1.0%) | — | — | 12 (24.0%) | 23 (3.9%) | 31 (70.5%) | 246 (39.0%) | 34 (69.4%) | 226 (44.2%) |
| Huang C | 1 (7.7%) | 9 (33.3%) | 8 (61.5%) | 8 (61.5%) | 2 (15.4%) | 2 (7.1%) | 4 (30.8%) | 1 (3.6%) | 3 (23.1%) | 0 | 1 (92.3%) | 17 (63.0%) | — | — |
| Wang Z | 3 (21.4%) | 33 (62.3%) | 7 (50.0%) | 7 (50.0%) | — | — | — | — | 0 | 4 (8.0%) | 2 (83.3%) | 15 (30.6%) | — | — |
| Zhou F | 5 (9.3%) | 27 (18.4%) | — | — | 5 (9.3%) | 3 (2.3%) | 23 (46.0%) | 1 (1.1%) | 13 (25.5%) | 1 (0.9%) | 1 (98.1%) | 70 (53.8%) | 50 (92.6%) | 67 (56.8%) |
Fig. 4.
Meta-analysis for laboratory examination in COVID-19 cases. Fix-effect model for “WBC < 4 × 109per L” “AST > 40U/L” “Cr ≥ 133μmol/L” and “hs-cTnI > 28 pg/mL”. Random-effect model for “PCT > 0.5 ng/mL” “LDH > 245U/L” and “D-dimer > 0.5mg/L”. Heterogeneity analysis was carried out using Q test, the among studies variation (I2 index). Forest plots depict the comparison of the incidences of the laboratory examination in critical/mortal and non-critical patients.
Discussion
The results of meta-analysis showed that male, aged over 65 and smoking patients might face a greater risk of developing into the critical or mortal condition and the comorbidities such as hypertension, diabetes, cardiovascular disease or respiratory diseases could also greatly affect the prognosis of the COVID-19. We found that patients with shortness of breath/dyspnea were more likely to develop into critical illness or even die, but patients with fever progressed better than those without fever. Laboratory examinations such as WBC, AST, Cr, hs-cTnI, PCT, LDH and D-dimer could imply the progression of COVID-19.
Coronavirus is an enveloped, non-segmented, single-stranded RNA virus.18 At present, six human coronaviruses have been identified. And the SARS-CoV-2, which isolated from the lower respiratory tract of pneumonia patients with unknown causes in Wuhan, is identified as the seventh human coronaviruses.19 SARS-CoV-2 attacks the alveolar epithelial cells via angiotensin-converting enzyme 2 (ACE2). ACE2 is the ACE of isozyme, mainly distributed in cardiovascular, kidneys, testes, lung and colon, and other organizations.20 The main role of ACE2 is to incise Ang II to generate Ang 1-7, which mediates the protective effects of vasodilation, anti-inflammatory and anti-proliferation, to antagonize Ang II-induced vascular smooth muscle contraction, cell proliferation, fibrosis promotion and vascular inflammation.21, 22, 23 When SARS-CoV-2 binds to ACE2 receptor on the surface of alveolar epithelial cells, The expression of ACE2 in alveolar epithelial cells is down-regulated by mechanisms such as internalization, shedding and viral replication.24 Then the increased concentration of Ang II leads to inflammatory response, and exudation of neutrophils, macrophages and fibrinous, resulting in loss of pulmonary ventilation function and difficulty in maintaining oxygenation.25 At the same time, viral infection will cause the imbalance of T helper-1 and T helper-2 responses, and induce an inflammatory storm by increasing the levels of inflammatory factors such as interleukin-4, interleukin-10 and interleukin-6.26 Inflammatory storm in critical patients releases cytokines, causing systemic immune injury, which may be an important cause of multiple organ failure and even death.27
Studies have found that women are less susceptible to viral infection than men, possibly because of the protection of X chromosome and sex hormones, which play an important role in innate and adaptive immunity.28 At the same time, men tend to be associated with bad lifestyle habits such as smoking and underlying diseases. As a result, the majority of critical or mortal patients are male. As the body's immunity declines with age, elderly patients are more likely to develop critical illness or even die. Therefore, when the patient is male, over 65 years old and smoking, the patient has a higher risk of developing critical illness or death.
When patients are combined with basic diseases such as diabetes and hypertension, the body is in a state of stress for a long time and the immunity tends to be low. Moreover, the long-term history of diabetes and hypertension will damage the vascular structure, and it is more likely to develop into critical disease in infection. Patients with chronic heart disease are more likely to be infected due to their weakened heart function and low immunity. When infected with SARS-CoV-2, they are more likely to have acute cardiovascular events and develop into severe diseases. When the patient has previous respiratory diseases such as chronic obstructive pulmonary disease, the patient's lung function is damaged. They have lower resistance to the virus and are prone to developing ARDS. Thus, underlying diseases such as diabetes, hypertension, cardiovascular disease or respiratory disease are risk factors for disease progression. This is consistent with the analytical results in this paper.
The common clinical manifestations of COVID-19 patients are fever, cough and sputum.6 When the patient's immune response is low, it may manifest as normal body temperature. Shortness of breath or dyspnea suggests poor lung function and lacking of oxygen. Therefore, when the patient is found to have difficulty in breathing or no fever, it is necessary to be alert for further deterioration of the patient's condition.
Viral infection will cause inflammation in human body. Various inflammatory factors produced by the inflammatory storm can cause systemic immune damage and even cause multi-organ failure. When the patient's laboratory indicator shows PCT > 0.5 ng/mL, there is a higher risk of progression to critical illness. Study showed that the total number of WBC in the early stage of the disease is normal or reduced.6 However, WBC < 4 × 109/L means a better clinical outcome in this paper. Therefore, when the total number of WBC is found to be higher than the previous one, the patient may be associated with other infections that aggravated the disease. At this moment, the clinicians might pay more attentions to these patients and improve treatment. When AST > 40U/L, LDH > 245U/L and Cr ≥ 133 mol/L, it indicates that the liver and kidney dysfunctions have been involved, and corresponding treatments should be taken in time to prevent further deterioration of the disease. Current studies have shown that up to 20% of covid-19 patients have abnormal coagulation function.6 Monocytes and tissue cells are activated after injury, causing the release of cytokines and the expression of tissue factors, and finally causing the hypercoagulability of blood. It will increase the risk of thrombosis and more likely to cause ischemia and hypoxia due to the embolization of the viscera, which leads to the progression of the disease to critical disease or death. When the D-dimer > 0.5mg/L, it indicates the hypercoagulability of blood and suggests the deterioration of patients. At the same time, SARS-CoV-2 can cause myocardial injury by direct and/or indirect action. The direct injury is to infect cardiomyocytes by identifying ACE2 receptor, while the indirect injury may be caused by inflammatory storm inducing by immune response and/or oxygen supply imbalance inducing by acute respiratory distress syndrome. When hs-cTnI > 28pg/mL, it strongly suggests the possibility of further deterioration of the patient's condition.
The quality of the literature included in this study is high, the analysis is rigorous, and the conclusions drawn by the study are highly credible. However, this meta‐analysis also has some limitations: (a) most of the studies included in this meta-analysis were cross-sectional studies with insufficient demonstration ability. (b) most of the patients in our meta-analysis were Chinese, and our aim was to use the findings of this study to predict the overall profile of patients, including other countries and races; (c) more detailed patient information, such as iconograph and oxygen at ion index, was not available in most studies at the time of analysis. The conclusions of this meta-analysis still need to be verified by more relevant studies with more careful design, more rigorous execution, and larger sample size.
This study analyzed the risk factors for progression to critical illness or death in COVID-19 patients to help assessing patient status and identify critical patients early. Pay close attention to these risk factors, and when relevant laboratory risk value appears, timely and personalized treatment regimens are needed to enhance the efficacy and reduce the risk of death.
References
- 1.Chan J F, Yuan S, Kok K H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster.[J] Lancet. 2020;395(10223):514–523. doi: 10.1016/s0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Yang X L, Wang X G. A pneumonia outbreak associated with a new coronavirus of probable bat origin.[J] Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai C C, Wang C Y, Wang Y H. Global epidemiology of coronavirus disease 2019: disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status.[J] Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slim K, Nini E, Forestier D. Methodological index for non-randomized studies (minors): development and validation of a new instrument.[J] ANZ J Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 5.Guan W J, Ni Z Y, Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China.[J] N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China.[J] Lancet. 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mo P, Xing Y, Xiao Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China.[J] Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng Y D, Meng K, Guan H Q. [Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV].[J] Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(0):E004. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Yu X, Zhao H. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan.[J] Crit Care. 2020;24(1):108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang N, Li D, Wang X. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia.[J] J Thromb Haemost. 2020 doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian S, Hu N, Lou J. Characteristics of COVID-19 infection in Beijing.[J] J Infect. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Hu B, Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China.[J] Jama. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Yang B, Li Q. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China.[J] Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C, Chen X, Cai Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China.[J] JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Yu Y, Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study.[J] Lancet Respir Med. 2020 doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan M, Yin W, Tao Z. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China.[J] PLoS One. 2020;15(3) doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study.[J] Lancet. 2020 doi: 10.1016/s0140-6736(20)30566-3. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fehr A R, Perlman S. Coronaviruses: an overview of their replication and pathogenesis.[J] Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding Q, Lu P, Fan Y. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China.[J] J Med Virol. 2020:10. doi: 10.1002/jmv.25781. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tipnis S R, Hooper N M, Hyde R. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase.[J] J Biol Chem. 2000;275(43):33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 21.Griendling K K, Sorescu D, Lassegue B. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology.[J] Arterioscler Thromb Vasc Biol. 2000;20(10):2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 22.Vickers C, Hales P, Kaushik V. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase.[J] J Biol Chem. 2002;277(17):14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 23.Santos R A, Simoes e Silva A.C, Maric C. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas.[J] Proc Natl Acad Sci U S A. 2003;100(14):8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glowacka I, Bertram S, Herzog P. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63.[J] J Virol. 2010;84(2):1198–1205. doi: 10.1128/jvi.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Z, Shi L, Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome.[J] Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/s2213-2600(20)30076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study.[J] Lancet. 2020;395(10223):507–513. doi: 10.1016/s0140-6736(20)30211-716/s2213-2600(20)30076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castrucci M R. Factors affecting immune responses to the influenza vaccine.[J] Hum Vaccin Immunother. 2018;14(3):637–646. doi: 10.1080/21645515.2017.1338547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gal-Oz S T, Maier B, Yoshida H. ImmGen report: sexual dimorphism in the immune system transcriptome.[J] Nat Commun. 2019;10(1):4295. doi: 10.1038/s41467-019-12348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]