Sir—Data from the World Health Organization (WHO) and China indicate significant higher mortality rates in male patients with coronavirus disease 2019 (COVID-19)1 , 2; however, this gender gap is far less noticeable when it comes to the prevalence of COVID-19 infection, indicating that women are as likely as men to contract the virus but are less likely to die. The goal of the present study was therefore to assess whether male patients with COVID-19 infection show more extensive lung involvement than female patients.
Data were collected from the Imelda Hospital, Bonheiden, Belgium. A total of 216 reverse transcription polymerase chain reaction (RT-PCR)-confirmed COVID-19 patients who underwent chest computed tomography (CT) at admission were retrospectively enrolled from 14 March to 5 April. A semi-quantitative scoring system was used to estimate the extent of pulmonary involvement as reported previously.3 In short, each lobe was scored from 0 to 5 with a total score ranging from 0 to 25: score 0, 0% involvement; score 1, <5% involvement; score 2, 5–25% involvement; score 3, 26–50% involvement; score 4, 51–75% involvement, score 5, 76–100% involvement. Patients were divided into four groups based on time since symptom onset: early stage (0–4 days), progressive stage (5–8 days), peak stage (10–13 days), and absorption stage (≥14 days).3 Data were analysed using R v.3.5.2. (Foundation for Statistical Computing, Vienna, Austria). This study was approved by the institutional review board and informed consent was waived. This study followed the reporting guidelines for cohort studies (STROBE statement). Summary statistics for continuous variables are reported as means ± standard deviations (SD) or as medians with interquartile ranges (IQR), as appropriate. Student's t-test for independent samples and the Mann–Whitney U-test were used to compare continuous variables between groups. Categorical variables are reported absolute numbers and percentages, and were compared by using the chi-squared test. A two-tailed p-value of <0.05 was considered to indicate statistical significance.
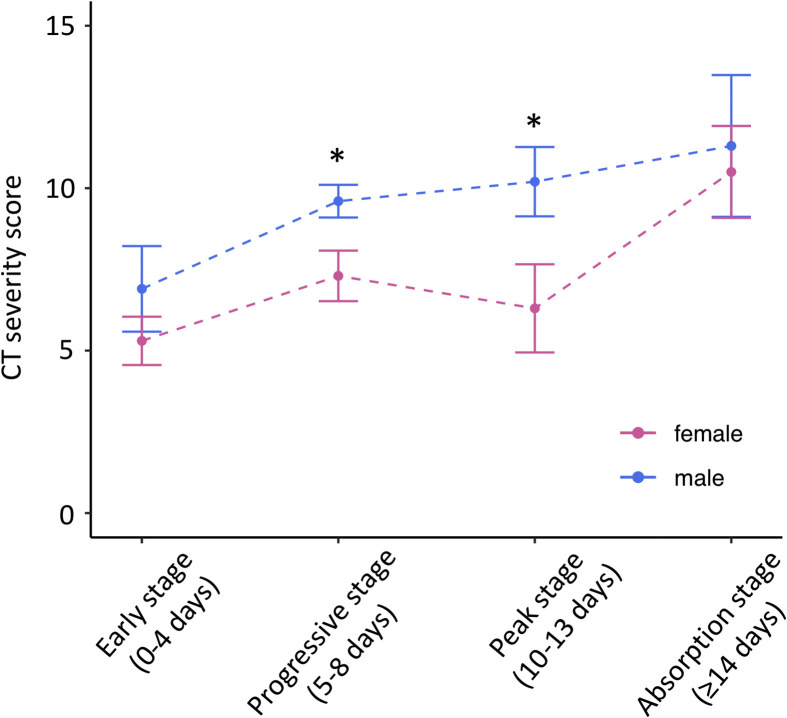
Patient demographics and CT findings are summarised in Table 1 . There were no significant differences between male and female patients in age or time since symptom onset. Heart disease was more prevalent in men (27.2% versus 13.3%, p=0.02). CT severity score was significantly greater in men (9.2±5 versus 7.0±4.8, p=0.001) with a trend toward more bilateral lung involvement (89.3% versus 78.8%, p=0.06). The differences in lung involvement scores were most pronounced during the progressive and peak stages of disease (Fig 1 ).
Table 1.
Patient characteristics and CT findings.
| Physical examination and demographics | All (n=216) | Male (n=103) | Female (n=113) | p-Value |
|---|---|---|---|---|
| Age (years)a | 65.4±17.1 | 67.6±14.6 | 63.3±19.0 | 0.09 |
| BMI (kg/m2)a | 28.7±3.7 | 29.1±3.6 | 28.3±4.3 | 0.80 |
| Time since symptom onset (days)b | 7 (4–10) | 7 (5–9) | 6 (3–10) | 0.22 |
| Clinical symptoms | ||||
| Fever | 117 (54.2) | 62 (60.2) | 55 (48.7) | 0.12 |
| Cough | 118 (54.6) | 58 (56.3) | 60 (53.1) | 0.74 |
| Dyspnoea | 105 (48.6) | 47 (45.6) | 58 (51.3) | 0.48 |
| Chest pain | 22 (10.2) | 9 (8.7) | 13 (11.5) | 0.66 |
| Current smoker | 19 (8.8) | 11 (10.7) | 8 (7.1) | 0.49 |
| Arterial hypertension | 70 (32.4) | 31 (30.1) | 39 (34.5) | 0.58 |
| Diabetes mellitus | 36 (16.7) | 21 (20.4) | 15 (13.3) | 0.22 |
| Heart disease | 43 (19.9) | 28 (27.2) | 15 (13.3) | 0.02 |
| Chest CT findings | ||||
| Ground-glass opacities | 188 (87.0) | 94 (91.3) | 94 (83.2) | 0.12 |
| Consolidation | 109 (50.5) | 47 (45.6) | 62 (54.9) | 0.22 |
| Bilateral involvement | 181 (83.8) | 92 (89.3) | 89 (78.8) | 0.06 |
| Lymphadenopathy | 34 (15.7) | 13 (12.6) | 21 (18.6) | 0.31 |
| Pleural effusion | 14 (6.5) | 6 (5.8) | 8 (7.1) | 0.92 |
| CT severity scorea | 8.0±5.0 | 9.2±5.0 | 7.0±4.8 | 0.001 |
Unless otherwise specified, data are numbers of patients, with percentages in parentheses.
CT, computed tomography.
Data are means ± SD.
Data are medians, with interquartile ranges in parentheses.
Figure 1.
CT severity scores for male and female patients. Patients were grouped based on time from symptom onset. ∗p<0.05 between male and female patients.
This study is the first to use CT to demonstrate more extensive lung disease in male patients with COVID-19, despite similar age and time from symptom onset for both gender groups. Male vulnerability to COVID-19 may, in part, be explained by a gender disparity in behaviour with men more likely than women to engage in unhealthy habits such as smoking and their poorer and less timely use of medical advice.4 Additionally, biological differences in the immune response may result in differential susceptibility of males and females to infectious diseases (e.g., animal studies have suggested a protective effect of oestrogen against severe acute respiratory syndrome coronavirus [SARS-CoV], a virus closely related to SARS-CoV 2).5 , 6 These results may advance our understanding of the epidemiological differences in patient outcome.
This study was limited by a lack of information on whether this more extensive lung involvement on chest CT correlated with a more adverse clinical outcome during follow-up.
Conflicts of interest
The authors declare no conflict of interest
References
- 1.World Health Organization . 2020. Coronavirus disease (COVID-19) outbreak.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available at: [accessed 15 April 2020] [Google Scholar]
- 2.Jin J.M., Bai P., He W. Gender differences in patients with COVID-19: focus on severity and mortality. medRxiv. 2020:20026864. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dangis A., Gieraerts C., Bruecker Y.D. Accuracy and reproducibility of low-dose submillisievert chest CT for the diagnosis of COVID-19. Radiol: Cardiothorac Imaging. Apr 21 2020 doi: 10.1148/ryct.2020200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yousaf O., Grunfeld E.A., Hunter M.S. A systematic review of the factors associated with delays in medical and psychological help-seeking among men. Health Psychol Rev. 2015;9:264–276. doi: 10.1080/17437199.2013.840954. [DOI] [PubMed] [Google Scholar]
- 5.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 6.Channappanavar R., Fett C., Mack M. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]