Abstract
Background
Different effectiveness profiles among antipsychotics may be a key point to optimize treatment in patients suffering a first episode of psychosis to impact on long-term outcome. The aim of this study is to compare the clinical effectiveness of olanzapine, risperidone, haloperidol, aripiprazole, ziprasidone, and quetiapine in the treatment of first episode of psychosis at 3-year follow-up.
Method
From February 2001 to January 2011, 2 phases of a prospective, randomized, open-label study were undertaken. A total of 376 first-episode drug-naïve patients were randomly assigned to olanzapine (n = 55), risperidone (n = 63), haloperidol (n = 56), aripiprazole (n = 78), ziprasidone (n = 62), or quetiapine (n = 62) and followed up for 3 years. The primary effectiveness measure was all cause of treatment discontinuation. In addition, an analysis based on intention-to-treat principle was conducted in the analysis for clinical efficacy.
Results
The overall dropout rate at 3 years reached 20.75%. Treatment discontinuation rates were significantly different among treatment groups (olanzapine = 69.09, risperidone = 71.43, aripiprazole = 73.08%, ziprasidone = 79.03%, haloperidol = 89.28%, and quetiapine = 95.53%) (χ2 = 79.86; P = .000). Statistically significant differences in terms of lack of efficacy, adherence, and tolerability were observed among treatment groups along the 3-year follow-up, determining significant differences in time to all-cause discontinuation (log-rank = 92.240; P = .000). Significant differences between treatments were found in the categories of sleepiness/sedation, increased sleep duration, akinesia, weight gain, ejaculatory dysfunction, extrapyramidal-symptoms, and amenorrhea.
Conclusions
Olanzapine, risperidone, and aripiprazole presented advantages for the first-line treatment of first episode of psychosis in terms of effectiveness. Identifying different discontinuation patterns may contribute to optimize treatment selection after first episode of psychosis.
ClinicalTrials.gov Identifier: NCT02526030 https://clinicaltrials.gov/show/NCT02526030
Keywords: schizophrenia, antipsychotics, first-episode-psychosis
Significance Statement.
Despite the fact that antipsychotics are recommended for the maintenance treatment in schizophrenia, comparative long-term effectiveness among antipsychotics is unclear, as long-term head-to-head comparisons among SGAs are limited in clinical practice and, especially, in first-episode-psychosis. The present paper supplies evidence on the differences in terms of long-term effectiveness among 6 widely used SGAs (olanzapine, risperidone, haloperidol, aripiprazole, quetiapine, and ziprasidone). Results showed remarkable effectiveness, efficacy, and safety differences between the 6 antipsychotics included in the study. We distinguished 2 treatment groups according to their effectiveness performance: olanzapine, risperidone, and aripiprazole presented clear advantages for the first-line treatment of FEP. Multiple tolerability profiles and different efficacy measures were identified and may lead to fit the best antipsychotic choice after FEP.
Introduction
Implementing the most suitable treatment strategies and making appropriate clinical decisions about individuals with a first episode of psychosis (FEP) is a complex and crucial task with relevant impact in illness outcome (Crespo-Facorro et al., 2016). Antipsychotics have demonstrated over the last 4 decades that they work both to treat acute psychotic episodes and to reduce relapse rates over the short to medium term (Correll et al., 2018). Prevention of recurrent episodes is likely to be critical to prevent disease progression (Emsley et al., 2013; Pelayo-Terán et al., 2017). Relapses involve an increased risk for a poorer and delayed response in the subsequent episode with higher treatment doses, potential side-effects emergence, and longer duration of active psychosis determining neurotoxicity (Pelayo-Terán et al., 2018; Samara et al., 2015; Takeuchi et al., 2019). Nonadherence to medication seems to represent the highest risk for relapse after a FEP. Lack of efficacy, poor tolerability, and acceptability are the other main causes that increase the risk for discontinuation during early phases after a FEP. Hence, higher treatment discontinuation rates are linked to higher relapse rates and a worsened prognosis (Leucht et al., 2013). That is why FEP first-line antipsychotic choice is so critical. A recent meta-analysis focused on long-term patients with schizophrenia suggested that, although there are some significant differences in effectiveness, efficacy, and tolerability among second-generation antipsychotics (SGAs) in the long-term treatment of schizophrenia due to the limited number of head-to-head clinical trials available so far, the comparative effectiveness of some SGAs is unclear (Kishimoto et al., 2019). Therefore, knowledge about the comparative clinical characteristics of antipsychotics in the long-term treatment (in which the magnitude of benefits and risks of medications may be different from acute phase treatment) of schizophrenia is important albeit insufficient so far (Correll and Hert, 2013).
Thus, with this pool analysis, comparing head-to-head 6 antipsychotics widely used in routine clinical settings, we aim to add detailed information on effectiveness, efficacy, and tolerability outcomes to contribute to further guide the evidence-based long-term treatment of patients with schizophrenia (Crespo-Facorro et al., 2012; Gómez-Revuelta et al., 2018). This may lead to identify predictors of beneficial outcomes with specific antipsychotics, which would further enhance the ability to personalize treatments.
We hypothesize that differences regarding the efficacy, tolerability, and adherence would result in differential effectiveness to lead such a critical election as first antipsychotic choice may be for the outcome of FEP patients.
Experimental Procedures
Study Setting
Data for the present investigation were obtained from an ongoing 3-year longitudinal intervention program of first-episode psychosis called PAFIP (Programa de Atención a las Fases Iniciales de Psicosis) conducted at the outpatient clinic and the inpatient unit of the University Hospital Marqués de Valdecilla, Spain (Pelayo-Terán et al., 2008). Conforming to international standards for research ethics, this program was approved by the local institutional review board. Patients meeting inclusion criteria and their families provided written informed consent prior to their inclusion to the program.
Participants
From February 2001 to January 2011, all referrals to PAFIP were screened for patients who met the following criteria: (1) 15–60 years old; (2) living in the catchment area; (3) experiencing their first episode of psychosis; (4) no prior treatment with antipsychotic medication or, if previously treated, a total lifetime of adequate antipsychotic treatment of less than 6 weeks; (5) DSM-IV criteria for brief psychotic disorder, schizophreniform disorder, schizophrenia, psychotic disorder not otherwise specified, or schizoaffective disorder. Patients were excluded for any of the following reasons: (1) meeting DSM-IV criteria for drug dependence, (2) meeting DSM-IV criteria for mental retardation, (3) having a history of neurological disease or head injury. The diagnoses were confirmed using the Structured Clinical Interview for DSM-IV (First et al., 2002) carried out by an experienced psychiatrist 6 months on from the baseline visit. Our operational definition for a “first episode of psychosis” included individuals with a nonaffective psychosis (meeting the inclusion criteria defined above) regardless of the duration of untreated psychosis.
Study Design
Patients included in this study were assigned in 2 different albeit consecutive phases of the PAFIP (2001–2004 and 2005–2011), encompassing 2 randomized, flexible-dose, and open-label clinical trials. During the first phase, from February 2001 to September 2005, patients were randomly assigned to treatment with olanzapine, risperidone, or haloperidol. Consecutively, between October 2005 and January 2011, patients were randomly assigned to treatment with aripiprazole, ziprasidone, or quetiapine.
We used a simple randomization procedure. An automated randomization list was drawn up. At study intake, all patients but 11 (2.92%) were antipsychotic naïve (mean duration of prior treatment = 2.75 weeks, SD = 1.8, range = 0.4–4.0). Before starting on the assigned drug, these participants underwent a 2- to 4-day washout period. Mean antipsychotic doses expressed as chlorpromazine equivalents (CPZeq; mg/d) (Woods, 2003) were as follows: olanzapine 5–20 mg/d (100–400 CPZeq), risperidone 3–6 mg/d (150– 300 CPZeq), haloperidol 3–9 mg/d (150–450 CPZeq), quetiapine 100–600 mg/d (133.33–800 CPZeq), ziprasidone 40–160 mg/d (66.67–266.67 CPZeq), and aripiprazole 5–30 mg/d (66,67–400 CPZeq). Rapid titration schedule (5 days), until optimal dose was reached, was used as a rule unless severe side effects occurred.
At the treating psychiatrist´s discretion, the dose and type of antipsychotic medication could be changed based on clinical efficacy and the profile of side effects during the follow-up period. Anticholinergic medication, lormetazepam, and clonazepam were permitted for clinical reasons. No anticholinergic agents were administered prophylactically. Antidepressants and mood stabilizers were permitted if clinically needed. The severity scale of the Clinical Global Impression (CGI) scale (Guy, 1976), the Brief Psychiatric Rating Scale (BPRS) (expanded version of 24 items) (Overall and Gorham, 1962), the Scale for the Assessment of Positive symptoms (SAPS) (Andreasen, 1984), the Scale for the Assessment of Negative symptoms (SANS) (Andreasen, 1989), the Calgary Depression Scale for Schizophrenia (CDSS) (Addington et al., 1993), and the Young Mania Rating Scale (YMRS) (Young et al., 1978) were used to evaluate clinical symptomatology. The scale of the Udvalg for Kliniske Undersogelser (UKU) (Committee of Clinical Trials) (Lingjærde et al., 1987), the Simpson-Angus Rating Scale (SARS) (Simpson and Angus, 1970), and the Barnes Akathisia Scale (BAS) (Barnes, 1989) were used to assess side effects. Clinical assessments and measurements were completed at baseline, 3 weeks, 6 weeks, 3 months, 6 months, 12 months, 18 months, 24 months, 30 months, and 36 months. All patients included in the analysis had at least the baseline and 3-year assessments, and they were considered for drop-out in those cases in which they did not attend 2 consecutive check-point assessments. The same trained psychiatrist (B.C.-F.) completed all clinical assessments.
Outcome Measures
Primary Outcome Measures: Effectiveness
The main outcome of effectiveness was the all-cause treatment discontinuation rate, which is the percentage of all-cause discontinuation of the initially assigned treatment (patients who completed the 3-year follow-up assessment and their initial antipsychotic treatment were switched during follow-up) and the mean time to all-cause medication discontinuation. Four reasons for the discontinuation were recorded: (1) nonsufficient or insufficient efficacy, (2) significant side effects, (3) nonadherence, and (4) other causes. Insufficient efficacy was established at the treating physician’s judgment only after at least 3 weeks of treatment. Adherence to antipsychotic drugs was assessed by the information obtained from patients, close relatives, and staff (nurse, social worker, and psychiatrists) involved in the follow-up. According to previous definition (Gómez-Revuelta et al., 2018), patients were consensually dichotomized into having a good (defined as patients regularly taking at least 90% of prescribed medication) and a poor adherence (medium or poor compliance). If more than 1 reason for discontinuation was present, the most important reason according to the above ranking was selected.
Secondary Outcome Measures: Efficacy and Safety
The efficacy outcomes were the mean change from baseline to 3 years in BPRS, SAPS, and SANS total scores. Additional analyses included changes from baseline to 3 years in CGI, YMRS, and CDSS total scores. Patients were defined as responders to the optimum dose of antipsychotic if they had a ≥50% reduction of BPRS total score and a CGI severity-score ≤4 after 6 months since the beginning of the treatment. Side effects were evaluated using the UKU side effects rating scale. Only side effects rated as moderate or severe and with a possible causal relationship to medication were recorded. Treatment-emergent akathisia and extrapyramidal symptoms were assessed using BAS and SARS scales.
Statistical Analyses
All data were tested for normality (using Kolmogorov-Smirnov test) and equality of variances (using Levene test). To ensure group comparability, baseline sociodemographic and clinical characteristics were tested by 1-way ANOVA or Kruskal-Wallis tests for continuous variables or by chi-squared tests for qualitative variables.
Kaplan-Meier survival curves and log-rank tests were used to assess time to all-cause medication discontinuation. Concerning these 2 analyses, patients were followed-up from the inclusion in the study until discontinuation of the initial treatment or censoring. Survival time could be censored by the end of the observation period or by lost to follow-up.
For efficacy and safety measures, we performed both intention-to-treat analyses and per-protocol analyses. Differences between groups in the degree of change in clinical scores from baseline were evaluated with ANCOVA after baseline scores were controlled. Finally, comparisons of the discontinuation rates and the prevalence of side effects as well as the use of concomitant treatment between the 6 antipsychotics were carried out, performing chi-squared tests with Bonferroni correction for multiple comparisons when necessary.
STATA 15.1 was used for statistical analysis. Statistical tests were 2-tailed with a 95% confidence interval.
Results
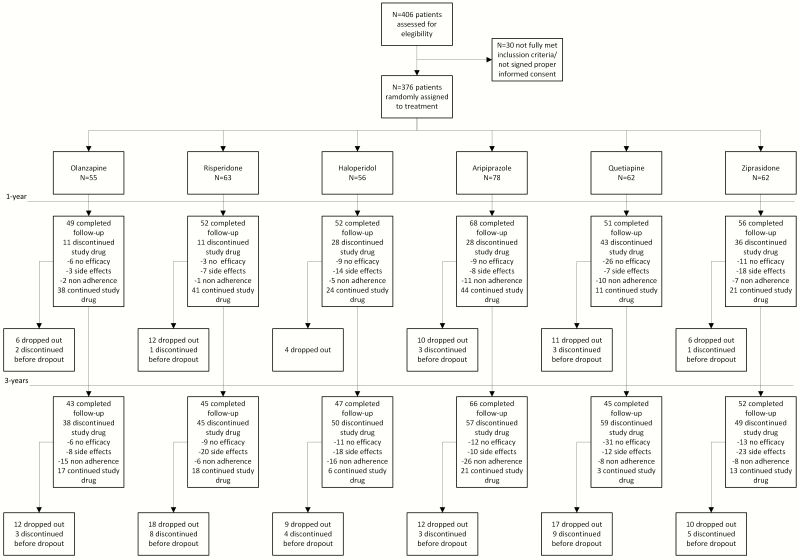
Of 406 individuals who were initially randomized to treatments, 30 were finally removed from the dataset after verifying they did not fully meet inclusion criteria or removed proper written consent during the first week. Thus, the final sample consisted of 376 participants who were randomly assigned to 6 different antipsychotic treatments: 55 patients were randomly assigned to the olanzapine group, 63 to the risperidone group, 56 to the haloperidol group, 78 patients to the aripiprazole group, 62 were assigned to the quetiapine group, and 62 to the ziprasidone group (Figure 1). The overall dropout rate at 3 years was small (n = 78; 20.74%); 46 patients dropped out prior to treatment discontinuation and were censored for the survival analysis (5-haloperidol, 9-olanzapine, 10-risperidone, 9-aripiprazole, 8-quetiapine, and 5- ziprasidone). Our study gave the option to patients who were stable (symptom free for at least 12 months) and functionally recovered (at least 6 months under sustained recovery) to discontinue use of antipsychotic treatment while continuing to be followed-up by study clinicians (Mayoral-van Son et al., 2016). Seventeen patients entered this antipsychotic discontinuation program and were carefully monitored during the following 18 months after discontinuation. Sixteen of these 17 patients completed the 3-year assessment but were censored for the primary outcome analysis.
Figure 1.
Consort flow chart.
Five patients committed suicide during the 3-year follow-up (1 olanzapine, 1 aripiprazole, 1 ziprasidone, and 2 quetiapine) and there was 1 sudden death (aripiprazole; heart attack).
All but 11 individuals in the study were white Caucasian (no significant differences were found between treatment groups after Bonferroni correction). Instead, we found significant differences between treatment groups concerning some demographic variables (living with parents, single status, age at admission, age at FEP onset, and duration of illness), which were adjusted by Bonferroni correction later. Demographic and clinical characteristics of patients are shown in Table 1.
Table 1.
Demographic and clinical characteristics of 376 drug-naïve patients with a FEP randomly assigned to treatment with olanzapine, risperidone, haloperidol, aripiprazole, ziprasidone, or quetiapine
| Aripiprazole (n = 78) | Risperidone (n = 63) | Olanzapine (n = 55) | Quetiapine (n = 62) | Ziprasidone (n = 62) | Haloperidole (n = 56) | Total (n = 376) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Statistics | P |
| Age at admission (y) | 32.1 | 11.1 | 26.3 | 11.6 | 27.5 | 6.9 | 31.0 | 9.2 | 32.1 | 10.5 | 28.3 | 8.7 | 29.55 | 2.61 | F = 5.19 | .00 |
| Age at psychosis onset (y) | 30.9 | 9.9 | 25.2 | 7.2 | 26.5 | 6.8 | 30.3 | 9.2 | 31.1 | 10.6 | 36.8 | 7.5 | 28.47 | 2.36 | F = 5.61 | .00 |
| Duration of illnessa (mo) | 31.1 | 56.4 | 29.5 | 39.6 | 21.2 | 33.2 | 22.1 | 42.8 | 16.4 | 29.4 | 32.3 | 39.7 | 25.43 | 5.86 | z = 13.85 | .017 |
| Duration of psychosis (mo) | 2.25 | 17.1 | 13.4 | 21.8 | 12.1 | 19.1 | 3.0 | 11.0 | 2.25 | 9.0 | 17.8 | 37.2 | 8.46 | 6.21 | z = 5.33 | .377 |
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | χ2 | P | |
| Schizophrenia diagnosis | 39 | 50.0 | 36 | 57.14 | 29 | 52.73 | 38 | 61.29 | 36 | 58.06 | 42 | 75.0 | 220 | 58.51 | 9.608 | .087 |
| Gender (male) | 38 | 48.72 | 39 | 61.90 | 33 | 60.0 | 41 | 66.13 | 29 | 46.77 | 36 | 64.29 | 216 | 57.45 | 8.9622 | .111 |
| Race (Caucasian) | 76 | 97.44 | 63 | 100.0 | 55 | 100.0 | 58 | 93.55 | 58 | 93.55 | 56 | 100.0 | 366 | 97.34 | 11.6443 | .040 |
| Education level (elementary) | 31 | 39.74 | 34 | 53.97 | 27 | 49.09 | 38 | 61.29 | 27 | 43.55 | 28 | 50.0 | 185 | 49.20 | 7.7968 | .168 |
| Socioeconomic status of parents (not/less qualified) | 34 | 44.16 | 36 | 60.0 | 32 | 58.18 | 30 | 48.39 | 28 | 45.16 | 36 | 64.29 | 196 | 52.69 | 9.0923 | .105 |
| Urban area (yes) | 55 | 71.43 | 50 | 79.37 | 41 | 74.55 | 48 | 77.42 | 46 | 74.19 | 41 | 73.21 | 281 | 74.93 | 1.4769 | .916 |
| Living with parents (yes) | 31 | 40.26 | 45 | 71.43 | 31 | 56.36 | 33 | 53.23 | 29 | 46.77 | 34 | 60.71 | 203 | 54.13 | 16.018 | .007 |
| Student (yes) | 16 | 20.8 | 11 | 17.5 | 10 | 18.0 | 8 | 12.9 | 15 | 24.2 | 14 | 25.0 | 74 | 19.68 | 3,340 | 0,007 |
| Single (yes) | 50 | 64.94 | 56 | 88.89 | 43 | 78.18 | 41 | 66.13 | 44 | 70.97 | 47 | 83.93 | 281 | 74.93 | 16.429 | .006 |
| Unemployed (yes) | 35 | 45.45 | 32 | 50.79 | 23 | 41.82 | 32 | 51.61 | 23 | 37.1 | 24 | 42.86 | 169 | 45.07 | 3.848 | .571 |
| Family psychiatric history (yes) | 17 | 21.8 | 7 | 11.1 | 15 | 27.3 | 14 | 22.6 | 17 | 27.4 | 13 | 23.2 | 83 | 22.07 | 1,866 | 0,100 |
| Hospital status inpatient (yes) | 26 | 33.33 | 30 | 47.62 | 20 | 36.36 | 21 | 33.87 | 21 | 33.87 | 15 | 26.79 | 133 | 35.37 | 6.227 | 0 |
| Tobacco use (yes) | 47 | 60.26 | 38 | 60.32 | 32 | 58.18 | 39 | 62.90 | 33 | 53.23 | 31 | 55.36 | 220 | 58.51 | 1.620 | .899 |
| Cannabis use (yes) | 30 | 38.46 | 31 | 49.21 | 26 | 47.27 | 29 | 46.77 | 20 | 32.26 | 25 | 44.64 | 161 | 42.82 | 5.3967 | .369 |
| Alcohol use (yes) | 40 | 51.28 | 37 | 58.73 | 25 | 46.3 | 39 | 62.9 | 29 | 46.77 | 34 | 60.71 | 204 | 54.4 | 6.3721 | .272 |
| Other drugs (yes) | 15 | 19.5 | 17 | 27.0 | 17 | 30.9 | 18 | 29.0 | 6 | 9.7 | 13 | 23.2 | 86 | 22.87 | 0,699 | .624 |
Abbreviation: FEP, first episode of psychosis.
Primary Outcome Measures
Treatment Discontinuation Rate and Time to Discontinuation
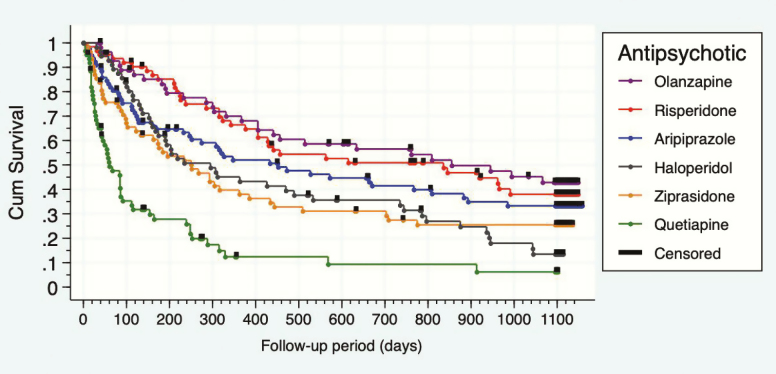
An impressive 79.25 % of the initial sample completed follow-up. Overall treatment discontinuation rate (the cumulative percentage of discontinuation considering the 6 arms of the study) also reached 79.25% by 3 years, which is in line with other medium and long-term (52 weeks or more) follow-up studies (Lieberman et al., 2005; Kahn et al., 2008a). It differed significantly between treatment groups (χ 2 = 79.860; P = .000) (Table 2). Patients on quetiapine showed a higher (95.16 %) treatment discontinuation rate than those on olanzapine (69.09%), risperidone (71.43%), aripiprazole (73.08 %), ziprasidone (79.03 %), or haloperidol (89.28%). The mean time (days) until discontinuation was 855 days for olanzapine, 786 days for risperidone, 452 days for aripiprazole, 295 days for haloperidol, 251 days for ziprasidone, and 60 days for quetiapine. There was a significant difference between groups (log rank = 90.240; P = .000) (see Figure 2). Log-rank pairwise comparisons showed no statistical differences between olanzapine, risperidone, and aripiprazole. However, results differed significantly favoring any antipsychotic when compared with quetiapine: aripiprazole (χ 2 = 28.95; P < .001), ziprasidone (χ 2 = 14.29; P < .001), olanzapine (χ 2 = 45.56; P = .156), haloperidol (χ 2 = 18.84; P < .001), and risperidone (χ 2 = 46.52; P < .001). Results also highlighted higher effectiveness of risperidone (χ 2 = 9.44; P = .002) and aripiprazole (χ 2 = 7.657; P = .022) over haloperidol. Olanzapine also took significant advantage over haloperidol (χ 2 = 11.23; P = .001) and ziprasidone (χ 2 = 8.81; P = .003). We only found a trend towards nondiscontinuation favoring aripiprazole and risperidone over ziprasidone and no differences were found between ziprasidone and haloperidol. Non- or insufficient efficacy in the group of quetiapine was the main reason for discontinuation rate differences (χ 2 = 87.43; P = .000). Patients under quetiapine treatment were significantly more likely to discontinue due to non- or insufficient efficacy compared with aripiprazole (χ 2 = 32.15; P = .000), ziprasidone (χ 2 = 19.35; P = .000), olanzapine (χ 2 = 39.91; P = .000), risperidone (χ 2 = 41.17; P = .000), or haloperidol patients (χ 2 = 27.83; P = .000). No significant differences were found between the remaining treatment groups. Mean (SD) doses (adjusted by chlorpromazine equivalents) prior to discontinuation due to non- or insufficient efficacy were aripiprazole-520 CPZeq (SD = 131.7), ziprasidone-386.5 CPZeq (SD = 149.5), haloperidol-351.8 CPZeq (SD = 164.9), olanzapine-510 CPZeq (SD = 292.4), risperidone-550 CPZeq (SD = 229.1), and quetiapine-586.2 CPZeq (SD = 251.1). Analysis of treatment discontinuation due to side effects revealed significant differences between groups (quetiapine 12.9%, ziprasidone 37.1%, risperidone 31.7%, olanzapine 14.5%, haloperidol 31.1%, and aripiprazole 12.82 %; χ 2 = 19.800; P = .001). Patients on ziprasidone discontinued treatment due to side effects significantly more frequently than those on aripiprazole (χ² = 11.490; P = .001), olanzapine (χ² = 12.400; P = .000), or quetiapine (χ² = 9.677; P = .006). We did not find significant differences in the direct comparisons between the remaining groups. Finally, there was a remarkable difference in terms of treatment adherence as individuals in the risperidone group showed better adherence than those individuals in the aripiprazole (χ² = 12.13; P = .001), quetiapine (χ² = 19.43; P = .000), and haloperidol groups (χ² = 10.59; P = .001); aripiprazole patients also demonstrated worse adherence than those under treatment with ziprasidone (χ² = 8.45; P = .003). Mean chlorpromazine equivalent daily doses at 3 years were: aripiprazole 16.3 mg = 216.8 (SD = 164.1) CPZeq; ziprasidone 121.3 mg = 202.2 (SD = 136.5) CPZeq; quetiapine 195.9 mg = 261.1 (SD = 136.5) CPZeq; risperidone 2.9 mg = 145 (95) (SD = 136.5) CPZeq; olanzapine 8.74 mg = 174.8 (SD = 92) CPZeq; and haloperidol 3.2 mg = 160 (SD = 60) CPZeq. No significant differences were observed (P = .279).
Table 2.
Any-Cause Discontinuation Rate and Discontinuation Rates by Allocated Causes
| Aripiprazole (n = 78) | Risperidone (n = 63) | Olanzapine (n = 55) | Quetiapine (n = 62) | Ziprasidone (n = 62) | Haloperidol (n = 56) | Total (n = 376) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | % | Total | % | Total | % | Total | % | Total | % | Total | % | Total | % | χ² | P | |
| Any cause discontinuation | 57 | 73.08 | 45 | 71.43 | 38 | 69.09 | 59 | 95.53 | 49 | 79.03 | 50 | 89.28 | 298 | 79.25 | 79.86 | .000 |
| No efficacy | 12 | 15.38 | 9 | 14.28 | 6 | 10.91 | 31 | 50,0 | 13 | 20.97 | 11 | 19.64 | 82 | 21.81 | 87.43 | .000 |
| No adherence | 26 | 33.3 | 6 | 9.53 | 15 | 27.27 | 12 | 19.3 | 8 | 12.9 | 16 | 28.57 | 83 | 22.07 | 25.87 | .000 |
| Side effects | 10 | 12.83 | 20 | 31.75 | 8 | 14.55 | 8 | 12.9 | 23 | 37.1 | 18 | 32.14 | 87 | 23.14 | 19.80 | .001 |
| Dropout | 9 | 11.54 | 10 | 15.87 | 9 | 16.36 | 8 | 13.33 | 5 | 8.06 | 5 | 8.93 | 46 | 12.23 | 15.13 | .010 |
Figure 2.
Kaplan-Meyer survival graph: any cause discontinuation.
Secondary Outcome Measures
Clinical Efficacy
ANCOVA analyses showed multiple differences (Table 3) in the changes of the total scores of the clinical scales between treatments after controlling by baseline measurements except for the SANS score (P = .265) and the negative dimension score (P = .314). Thereby, significant differences on clinical scales showed CGI improvement: significantly larger for aripiprazole vs haloperidol and ziprasidone vs haloperidol, and olanzapine; SAPS score: olanzapine improvement significantly lower compared with aripiprazole and ziprasidone; positive dimension: aripiprazole and ziprasidone scores were significantly lower than those proceeding from haloperidol and olanzapine patients; disorganized dimension: patients on aripiprazole, quetiapine, and ziprasidone got significant larger improvements than olanzapine patients; CDSS score: ziprasidone and quetiapine patients got significant improvements in depressive symptoms compared with haloperidol patients; YMRS score: significant improvement for aripiprazole compared with olanzapine. Per protocol analyses solely unveiled significant differences concerning CGI (P = .008) and YMRS (P = .001) baseline scores. Bonferroni post-hoc analyses showed significantly higher baseline scores for aripiprazole compared with olanzapine and risperidone for both clinical scales (data available upon request).
Table 3.
Intention-to-Treat Sample: Psychopathological Characteristics at Baseline and 3 Years and Clinical Changes During Follow-Up Period
| Aripiprazole (n = 66) | Risperidone (n = 45) | Olanzapine (n = 43) | Quetiapine (n = 45) | Ziprasidone (n = 52) | Haloperidole (n = 47) | Total (n = 298) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Statistic | Value | P | |
| CGI - Baseline | 6.6 | 0.6 | 6.1 | 0.7 | 6.0 | 0.8 | 6.5 | 0.6 | 6.3 | 0.6 | 6.2 | 0.6 | 6.3 | 0.7 | F | 6.553 | .000 |
| CGI - 3Y | 2.5 | 1.7 | 3.1 | 1.6 | 3.1 | 1.6 | 2.5 | 1.8 | 2.1 | 1.4 | 3.4 | 1.6 | 2.7 | 1.7 | F | 4.440 | .001 |
| CGI - 3Y-Baseline | −4.2 | 1.8 | −3.0 | 1.8 | −2.9 | 1.7 | −4.1 | 1.9 | −4.2 | 1.5 | −2.8 | 1.8 | −3.6 | 1.8 | F | 7.757 | .000 |
| (Covariated) CGI - 3Y-Baseline | −3.9 | 0.2 | −3.2 | 0.2 | −3.2 | 0.3 | −3.9 | 0.2 | −4.2 | 0.2 | −2.9 | 0.2 | F | 4.602 | .000 | ||
| CDSS - Baseline | 2.8 | 3.6 | 1.3 | 2.3 | 2.6 | 3.4 | 2.7 | 3.3 | 2.0 | 3.5 | 2.0 | 2.6 | 2.3 | 3.2 | F | 1.514 | .185 |
| CDSS - 3Y | 0.7 | 1.8 | 0.8 | 1.7 | 0.7 | 1.6 | 0.1 | 0.3 | 0.3 | 0.9 | 1.5 | 2.8 | 0.7 | 1.7 | F-w | 6.822 | .000 |
| CDSS - 3Y-Baseline | −2.1 | 4.1 | −0.6 | 2.7 | −1.9 | 3.7 | −2.6 | 3.3 | −1.8 | 3.5 | −0.5 | 3.7 | −1.6 | 3.6 | F | 2.713 | .021 |
| (Covariated) CDSS - 3Y-Baseline | −1.6 | 0.2 | −1.5 | 0.3 | −1.6 | 0.3 | −2.2 | 0.2 | −2.0 | 0.2 | −0.7 | 0.2 | F | 4.240 | .001 | ||
| Total BPRS - Baseline | 67.7 | 12.6 | 57.4 | 10.3 | 59.4 | 11.9 | 63.9 | 12.0 | 61.7 | 12.6 | 61.8 | 11.2 | 62.4 | 12.2 | F | 4.951 | .000 |
| Total BPRS - 3Y | 29.6 | 10.0 | 33.3 | 12.2 | 35.6 | 14.4 | 30.6 | 9.8 | 29.1 | 7.3 | 35.7 | 13.7 | 32.1 | 11.5 | F-w | 3.242 | .009 |
| Total BPRS - 3Y-Baseline | −38.0 | 15.1 | −24.1 | 16.9 | −23.8 | 20.3 | −33.3 | 13.7 | −32.6 | 12.0 | −26.0 | 18.4 | −30.3 | 16.9 | F-w | 6.382 | .000 |
| (Covariated) Total BPRS - 3Y-Baseline | −32.9 | 1.4 | −28.9 | 1.7 | −26.7 | 1.7 | −31.9 | 1.7 | −33.3 | 1.6 | −26.6 | 1.6 | F | 3.476 | .005 | ||
| Total SAPS - Baseline | 14.6 | 4.4 | 13.3 | 4.2 | 12.2 | 4.5 | 13.9 | 4.1 | 13.9 | 4.3 | 13.4 | 3.5 | 13.6 | 4.2 | F | 1.780 | .117 |
| Total SAPS - 3Y | 1.0 | 2.8 | 2.6 | 4.2 | 3.5 | 5.5 | 1.4 | 3.0 | 1.1 | 2.7 | 2.6 | 3.9 | 1.9 | 3.8 | F-w | 3.166 | .010 |
| Total SAPS - 3Y-Baseline | −13.6 | 4.7 | −10.7 | 5.6 | −8.7 | 6.3 | −12.5 | 5.3 | −12.8 | 4.6 | −10.7 | 4.9 | −11.7 | 5.4 | F | 5.791 | .000 |
| (Covariated) Total SAPS - 3Y-Baseline | −12.7 | 0.5 | −11.0 | 0.5 | −10.0 | 0.6 | −12.2 | 0.5 | −12.6 | 0.5 | −11.0 | 0.5 | F | 4.351 | .001 | ||
| Total SANS - Baseline | 7.5 | 6.8 | 7.4 | 6.5 | 7.4 | 6.0 | 6.3 | 6.0 | 6.1 | 5.4 | 7.0 | 6.2 | 6.9 | 6.2 | F | 0.497 | .778 |
| Total SANS - 3Y | 3.9 | 5.7 | 4.0 | 4.9 | 3.9 | 5.4 | 3.8 | 5.8 | 3.1 | 4.8 | 5.7 | 5.3 | 4.2 | 5.4 | F | 1.379 | .232 |
| Total SANS - 3Y-Baseline | −3.6 | 8.3 | −3.3 | 8.3 | −2.6 | 7.5 | −2.5 | 5.7 | −3.0 | 7.5 | −1.3 | 7.5 | −2.8 | 7.5 | F | 0.577 | .717 |
| (Covariated) Total SANS - 3Y-Baseline | −3.1 | 0.7 | −3.0 | 0.8 | −2.1 | 0.8 | −3.1 | 0.8 | −3.8 | 0.7 | −1.3 | 0.8 | F | 1.298 | .265 | ||
| Positive dim - Baseline | 7.6 | 2.6 | 7.4 | 2.5 | 7.1 | 2.4 | 7.6 | 2.4 | 7.3 | 2.3 | 7.8 | 2.1 | 7.5 | 2.4 | F | 0.445 | .816 |
| Positive dim - 3Y | 0.5 | 1.8 | 1.5 | 2.2 | 1.8 | 2.6 | 1.0 | 1.9 | 0.5 | 1.3 | 1.9 | 3.1 | 1.1 | 2.2 | F-w | 4.064 | .002 |
| Positive dim - 3Y-Baseline | −7.0 | 2.8 | −6.0 | 3.0 | −5.3 | 3.5 | −6.6 | 3.0 | −6.8 | 2.6 | −5.9 | 3.4 | −6.3 | 3.1 | F | 2.317 | .044 |
| (Covariated) Positive dim - 3Y-Baseline | −7.0 | 0.3 | −6.0 | 0.3 | −5.6 | 0.3 | −6.5 | 0.3 | −6.9 | 0.3 | −5.6 | 0.3 | F | 4.112 | .001 | ||
| Disorganized dim - Baseline | 7.0 | 3.2 | 5.9 | 3.4 | 5.1 | 3.6 | 6.3 | 3.2 | 6.6 | 3.5 | 5.6 | 2.8 | 6.2 | 3.4 | F | 2.205 | .054 |
| Disorganized dim - 3Y | 0.5 | 1.5 | 1.2 | 2.5 | 1.7 | 3.4 | 0.4 | 1.4 | 0.6 | 1.8 | 0.7 | 1.3 | 0.8 | 2.1 | F-w | 1.650 | .151 |
| Disorganized dim - 3Y-Baseline | −6.5 | 3.6 | −4.7 | 4.1 | −3.4 | 4.0 | −5.9 | 3.6 | −6.0 | 3.6 | −4.9 | 3.0 | −5.4 | 3.8 | F | 4.691 | .000 |
| (Covariated) Disorganized dim - 3Y-Bas | −5.8 | 0.3 | −5.0 | 0.3 | −4.4 | 0.3 | −5.8 | 0.3 | −5.6 | 0.3 | −5.4 | 0.3 | F | 3.282 | .007 | ||
| Negative dim - Baseline | 5.0 | 6.0 | 6.1 | 6.0 | 6.2 | 5.8 | 4.3 | 5.4 | 3.9 | 4.7 | 5.7 | 5.7 | 5.2 | 5.6 | F | 1.332 | .251 |
| Negative dim - 3Y | 3.5 | 5.1 | 3.3 | 4.2 | 4.3 | 5.3 | 3.3 | 5.1 | 2.8 | 4.4 | 5.1 | 4.9 | 3.7 | 4.9 | F | 1.448 | .207 |
| Negative dim - 3Y-Baseline | −1.6 | 7.2 | −2.8 | 6.9 | −1.9 | 7.1 | −1.0 | 5.0 | −1.2 | 6.5 | −0.6 | 6.8 | −1.5 | 6.6 | F | 0.650 | .662 |
| (Covariated) Negative dim - 3Y-Baseline | −1.7 | 0.6 | −2.0 | 0.7 | −1.1 | 0.7 | −1.7 | 0.7 | −2.2 | 0.7 | −0.1 | 0.7 | F | 1.189 | .314 | ||
| YMRS - Baseline | 11.8 | 5.0 | 9.0 | 4.1 | 8.8 | 4.3 | 12.3 | 5.5 | 1.7 | 4.6 | 9.2 | 4.6 | 10.6 | 4.9 | F | 5.693 | .000 |
| YMRS - 3Y | 1.0 | 2.2 | 2.1 | 4.2 | 3.1 | 5.0 | 1.9 | 3.6 | 1.5 | 4.1 | 2.2 | 3.3 | 1.9 | ||||
| 3.8 | F | 1.844 | 0.104 | ||||||||||||||
| YMRS - 3Y-Baseline | −10.8 | 5.0 | −6.9 | 5.6 | −5.7 | 6.3 | −10.4 | 6.8 | −10.1 | 4.7 | −7.0 | 5.7 | −8.7 | 5.9 | F | 7.506 | .000 |
| (Covariated) YMRS - 3Y-Baseline | −9.7 | 0.5 | −8.3 | 0.6 | −7.3 | 0.6 | −8.8 | 0.6 | −9.2 | 0.5 | −8.2 | 0.6 | F | 2.408 | .037 |
Abbreviations: BPRS, Brief Psychiatric Rating Scale; CDSS, Calgary Depression Rating Scale for Schizophrenia; CGI, Clinical Global Impression; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; YMRS, Young Mania Rating Scale.
Safety
Adverse Events
Intention-to-treat analyses of moderate and severe side effects that were frequent (in at least 5% of patients in any of the treatment groups) are displayed in Table 4. Significant differences between treatments were found in the categories of sleepiness/sedation, increased sleep duration, akinesia, weight gain, ejaculatory dysfunction, and amenorrhea. After adjustment by Bonferroni correction, aripiprazole was shown to cause less increased sleep duration than quetiapine (P = .015) and ziprasidone (P = .05) and risperidone was also less likely to do so than olanzapine, quetiapine, haloperidol, and ziprasidone (all P < .05). Quetiapine was associated with increased somnolence compared with aripiprazole (P = .034), but amenorrhea was significantly less likely to emerge in this group compared with haloperidol (P = .037) and risperidone (P = .022). Risperidone produced an greater frequency of ejaculatory dysfunction than aripiprazole (P = .034). Aripiprazole patients were more likely to suffer akinesia compared with olanzapine (P = .004) and ziprasidone patients (P = .033). Finally, olanzapine caused a significantly higher weight gain rate than ziprasidone (P = .001). Per-protocol analysis showed rather similar results (data available in supplementary Material). In this sense, there were only 3 side-effect categories that showed statistically significant differences (weight gain, sialorrhea, and ejaculatory dysfunction). None of them resulted in statistical significance after post-hoc analyses.
Table 4.
Intention-to-Treat Sample: Moderate or Severe Treatment-Emergent Adverse Effects That Occurred at a Rate of at Least 5% in Either Treatment Group
| Aripiprazole (n = 66) | Risperidone (n = 45) | Olanzapine (n = 43) | Quetiapine (n = 45) | Ziprasidone (n = 52) | Haloperidole (n = 47) | Total (n = 298) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 wk to 3 y | n | % | n | % | n | % | n | % | n | % | n | % | n | % | Statistic | value | P |
| Concentration difficulties | 13 | 19.7 | 5 | 11.1 | 8 | 18.6 | 6 | 13.3 | 10 | 19.2 | 12 | 25.5 | 54 | 18.1 | χ 2 | 4.086 | .537 |
| Asthenia/increased fatigability | 26 | 39.4 | 15 | 33.3 | 19 | 44.2 | 24 | 53.3 | 24 | 46.2 | 26 | 55.3 | 134 | 45.0 | χ 2 | 6.638 | .249 |
| Sleepiness/sedation | 26 | 39.4 | 19 | 42.2 | 25 | 58.1 | 31 | 68.9 | 29 | 55.8 | 25 | 53.2 | 155 | 52.0 | χ 2 | 12.040 | .034 |
| Memory impairment | 3 | 4.5 | 0 | 0.0 | 0 | 0.0 | 1 | 2.2 | 3 | 5.8 | 1 | 2.1 | 8 | 2.7 | F | 5.289 | .461 |
| Depression | 3 | 4.5 | 0 | 0.0 | 1 | 2.3 | 1 | 2.2 | 3 | 5.8 | 0 | 0.0 | 8 | 2.7 | F | 5.365 | .402 |
| Restlessness | 3 | 4.5 | 2 | 4.4 | 1 | 2.3 | 1 | 2.2 | 3 | 5.8 | 4 | 8.5 | 14 | 4.7 | F | 2.826 | .774 |
| Increased sleep duration | 14 | 21.2 | 7 | 15.6 | 14 | 32.6 | 19 | 42.2 | 19 | 36.5 | 18 | 38.3 | 91 | 30.5 | χ 2 | 12.664 | .027 |
| Decreased sleep duration | 2 | 3.0 | 2 | 4.4 | 4 | 9.3 | 0 | 0.0 | 0 | 0.0 | 3 | 6.4 | 11 | 3.7 | F | 8..637 | .087 |
| Rigidity | 2 | 3.0 | 1 | 2.2 | 0 | 0.0 | 1 | 2.2 | 6 | 11.5 | 2 | 4.3 | 12 | 4.0 | F | 10.331 | .116 |
| Akinesia | 23 | 34.8 | 5 | 11.1 | 2 | 4.7 | 8 | 17.8 | 15 | 28.8 | 12 | 25.5 | 65 | 21.8 | χ 2 | 19.343 | .002 |
| Tremor | 9 | 13.6 | 5 | 11.1 | 2 | 4.7 | 2 | 4.4 | 3 | 5.8 | 5 | 10.6 | 26 | 8.7 | F | 5.039 | .448 |
| Akathisia | 15 | 22.7 | 8 | 17.8 | 2 | 4.7 | 8 | 17.8 | 16 | 30.8 | 13 | 27.7 | 62 | 20.8 | χ 2 | 11.932 | .036 |
| Increased salivation | 11 | 16.7 | 13 | 28.9 | 2 | 4.7 | 9 | 20.0 | 9 | 17.3 | 11 | 23.4 | 55 | 18.5 | χ 2 | 9.721 | .084 |
| Decreased salivation | 5 | 7.6 | 6 | 13.3 | 9 | 20.9 | 8 | 17.8 | 9 | 17.3 | 9 | 19.1 | 46 | 15.4 | χ 2 | 5.096 | .404 |
| Constipation | 7 | 10.6 | 4 | 8.9 | 5 | 11.6 | 7 | 15.6 | 5 | 9.6 | 3 | 6.4 | 31 | 10.4 | F | 2.314 | .820 |
| Miction impairment | 4 | 6.1 | 1 | 2.2 | 0 | 0.0 | 1 | 2.2 | 1 | 1.9 | 1 | 2.1 | 8 | 2.7 | F | 4.310 | .665 |
| Vertigo | 3 | 4.5 | 2 | 4.4 | 0 | 0.0 | 0 | 0.0 | 4 | 7.7 | 0 | 0.0 | 9 | 3.0 | F | 8.916 | .098 |
| Weight gain | 33 | 50.0 | 26 | 57.8 | 31 | 72.1 | 20 | 44.4 | 16 | 30.8 | 22 | 46.8 | 148 | 49.7 | χ 2 | 17.911 | .003 |
| Diminished sexual desire | 6 | 9.1 | 7 | 15.6 | 4 | 9.3 | 6 | 13.3 | 4 | 7.7 | 5 | 10.6 | 32 | 10.7 | F | 2.189 | .829 |
| Orgasmic dysfunction | 1 | 1.5 | 3 | 6.7 | 2 | 4.7 | 2 | 4.4 | 3 | 5.8 | 1 | 2.1 | 12 | 4.0 | F | 2.800 | .707 |
| Headache | 1 | 1.5 | 1 | 2.2 | 3 | 7.0 | 0 | 0.0 | 1 | 1.9 | 0 | 0.0 | 6 | 2.0 | F | 7.355 | .223 |
| Male | |||||||||||||||||
| Erectile dysfunction | 2 | 6.5 | 9 | 34.6 | 4 | 14.8 | 7 | 24.1 | 5 | 22.7 | 7 | 22.6 | 34 | 20.5 | χ 2 | 7.858 | .164 |
| Ejaculatory dysfunction | 2 | 6.5 | 10 | 38.5 | 2 | 7.4 | 6 | 20.7 | 5 | 22.7 | 4 | 12.9 | 29 | 17.5 | F | 13.531 | .025 |
| Female | |||||||||||||||||
| Amenorrhea | 5 | 14.3 | 9 | 47.4 | 2 | 12.5 | 0 | 0.0 | 7 | 23.3 | 8 | 50.0 | 31 | 23.5 | F | 19.925 | .001 |
| Galactorrhea | 0 | 0.0 | 2 | 10.5 | 1 | 6.3 | 0 | 0.0 | 2 | 6.7 | 1 | 6.3 | 6 | 4.5 | F | 4.520 | .319 |
Extrapyramidal Symptoms
Significant differences in the SARS total score changes at 3 years between treatments were found (F = 13.441; P = .020). The percentage of patients with treatment-emergent extrapyramidal symptoms (EPS) was statistically different between treatments (aripiprazole = 23.8%, ziprasidone = 23.5%, quetiapine 20%, risperidone 40%, olanzapine 30%, haloperidol 48.8%; χ 2 = 13.441; P = .020). Haloperidol supplied a significant burden of extrapyramidal side effects compared with aripiprazole, quetiapine, and ziprasidone (all P´s < .05) and so did risperidone compared with quetiapine (P = .043). There was no significant difference between treatments in the severity of akathisia (BAS total score) (F = 0.939; P = .456). Although the difference did not reach significance, more individuals in the aripiprazole (22.6 %), haloperidol (27.7%), and ziprasidone groups (32.7 %) experienced treatment emergent akathisia compared with the other groups (quetiapine 17.8 %, olanzapine 4.7%, and risperidone 17.8%). These differences were significant for the UKU scale akathisia item (χ² = 11.932; P = .036), but after Bonferroni correction only the differences between ziprasidone and olanzapine groups remained significant (P = .001). Per protocol analyses showed no statistically significant differences.
Concomitant Medication Use
Significant differences were found between treatment groups regarding the use of benzodiazepines (χ² = 21.52, P = .001), hypnotics (χ² = 47.76, P = .004), and anticholinergics (χ² = 43.76, P = .004). After adjustment by Bonferroni correction, we found that the benzodiazepine use in the aripiprazole group was significantly higher than in the quetiapine, haloperidol, olanzapine, and risperidone groups (all P < .05); similarly, aripiprazole, quetiapine, and ziprasidone patients needed hypnotics more frequently than olanzapine, risperidone, and haloperidol patients (all P < .05 except for the comparison between quetiapine and haloperidol). Almost one-half of the sample required treatment with anticholinergics at any time of the follow-up. Olanzapine (27.27%) stood out for being the treatment group that least frequently needed this sort of treatment (Table 5).
Table 5.
Concomitant Psychiatric Medication Used During Follow-Up
| Aripiprazole n = 78 | Risperidone n = 63 | Olanzapine n = 55 | Ziprasidone n = 62 | Quetiapine n = 62 | Haloperidol n = 56 | Total n = 376 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | χ² | P | |
| Benzodiacepines | 72 | 92.31 | 42 | 66.67 | 38 | 69.09 | 54 | 87.1 | 45 | 72.58 | 40 | 71.43 | 291 | 77.39 | 21.523 | .001 |
| Hypnotics | 53 | 67.95 | 20 | 31.75 | 15 | 27.27 | 42 | 67.74 | 36 | 58.86 | 20 | 35.71 | 186 | 49.47 | 47.765 | .004 |
| Antidepressants | 24 | 30.77 | 16 | 25.40 | 15 | 27.27 | 26 | 42.94 | 16 | 25.81 | 11 | 19.64 | 108 | 28.72 | 8.356 | .138 |
| Anticholinergics | 53 | 67.95 | 20 | 31.75 | 15 | 27.27 | 42 | 67.74 | 36 | 58.86 | 20 | 35.71 | 186 | 49.47 | 43.765 | .004 |
| Mood stabilizers | 6 | 7.69 | 4 | 6.35 | 5 | 9.09 | 4 | 6.45 | 6 | 9.68 | 5 | 8.93 | 30 | 7.98 | 0.838 | .975 |
Discussion
Our aim with this research was to supply information from our experience to guide the choice of the first-line treatment for an FEP. Results show remarkable effectiveness, efficacy, and safety differences between the 6 antipsychotics included in the study. We distinguished 2 treatment groups according to their effectiveness performance, multiple tolerability profiles, and different efficacy measures.
Given that almost all the SGAs have reduced risk of EPS and tardive dyskinesia than FGAs, not all lead to important weight gain, and many are now available both in oral and long-acting intramuscular forms, treatment with an SGA would be the first choice in most cases (Smith et al., 2019).
Primary Outcome Measures
Treatment Discontinuation Rate and Mean Time to Discontinuation
Different patterns for discontinuation emerged at different phases during follow-up. This circumstance may determine different intensity interventions focused on efficacy, compliance, and tolerability adapted to each specific phase of the follow-up: over one-half of the patients who discontinued treatment did so during the first year after the outbreak (52.68% by the end of 1 year), of whom 40.71% discontinued treatment due to lack of efficacy. This compelling information is remarkable in that mean-time until all-cause discontinuation was after more than 2 years in the olanzapine and risperidone groups and slightly after 1 year in the aripiprazole group. Instead, mean time until all-cause discontinuation appeared for patients on quetiapine barely reached 2 months follow-up. Mean time until all-cause discontinuation occurred after half a year follow-up for patients on haloperidol and ziprasidone treatment. Side effects (36.3%) were the following cause for discontinuation during the first year of follow-up, while nonadherence (30.54%) was the main cause for discontinuation between 1- and 3-year follow-up. Concerning the all-cause discontinuation rate and the mean time to discontinuation variables, we may split the antipsychotic treatments used for this research into 2 different groups according to their performance on preventing patients from discontinuation due to any cause: a leading group composed by olanzapine, risperidone, and aripiprazole and an underperformer group, including ziprasidone, quetiapine, and haloperidol. Therefore, olanzapine, risperidone, and aripiprazole proved to be significantly superior to quetiapine and haloperidol. Meanwhile, though ziprasidone was superior to quetiapine and haloperidol as well, it was significantly defeated by olanzapine and both aripiprazole and risperidone showed a trend towards superiority compared with ziprasidone. There were no significant differences between olanzapine, risperidone, and aripiprazole concerning any-cause discontinuation risk and mean time to discontinuation, though olanzapine performed slightly better, especially in terms of mean time to discontinuation, which was almost 2-fold longer compared with aripiprazole. Regarding these data aripiprazole effectiveness approaches that for other well-stablished effective antipsychotics have not been seen in other effectiveness studies (Leucht et al., 2013). Higher risk of all-cause treatment discontinuation and shorter mean time to discontinuation in quetiapine patients had already been described during early phases of treatment (McEvoy et al., 2007); in our sample, 50% of patients on quetiapine discontinued treatment due to non- or insufficient efficacy. Effectiveness studies using standard dosage ranges pointed out that quetiapine may be less effective than some other widely used SGAs (Tiihonen et al., 2017) and FGAs (Vanasse et al., 2016). Our results are consistent with the notion that most of the patients who start quetiapine stop taking it within a few weeks (Asmal et al., 2013). Inadequate and transient dopamine-2 receptor occupancy has been proposed as a possible mechanism underlying quetiapine lack of efficacy. On the other hand, its weak dopamine antagonism allows low maximal occupancy values and relatively little variability in occupancy values, suggesting that high doses of this antipsychotic are not likely to exceed thresholds of more than 80% occupancy, indicative of D2-receptor–mediated side effects (Lako et al., 2013). This supports the idea that higher doses may be tolerable and more efficient (Wang et al., 2017). We did not find significant differences between groups concerning treatment dosage adjusted by chlorpromazine equivalents prior to discontinuation due to non- or insufficient efficacy. The quetiapine group presented with the second highest relative dosage compared with the other groups. According to other reports (Tiihonen et al., 2017), it is difficult to discern whether the poorer performance of quetiapine is due to the product itself or to nonoptimal dosage. The main reasons for discontinuation in the haloperidol group were nonadherence (28.57%) and side effects (32.14%). Ziprasidone (37.1%) and risperidone (31.75%) patients were also more likely to discontinue treatment due to side effects. Most discontinuations due to side effects accrued during the 1-year follow-up. This difficulty arose during antipsychotic titration in these groups, explaining why patients on ziprasidone and haloperidol tolerated lower relative doses than the remaining quartet. According to these results, EPS, sexual adverse events, and somnolence (the most frequently reported secondary effects for discontinuation for risperidone, ziprasidone, and haloperidol) seem to represent the worst tolerated side effects. At the next stage of follow-up (from 18 months on), we find an accumulation of treatment discontinuation due to nonadherence basically affecting aripiprazole (33.3%) and olanzapine (27.27%) patients, for whom this was the main reason for discontinuation. The increase in discontinuations due to nonadherence observed during the last year of follow-up in these groups might be explained by a natural decrease in the acceptability mediated by long-term exposure to treatment (Lieberman et al., 2005). Improvements in educational and other prophylactical measures like the use of long acting injectable formulations (Kishimoto et al., 2014; Jann and Penzak, 2018) may be of interest to deal with this preventable issue.
Secondary Outcome Measures
Efficacy
Our first-episode patients showed a decrease in total BPRS, SAPS, SANS, and CGI scores during the 3-year follow-up. Previous studies using pair-wise meta-analytic (Leucht et al., 2009) comparisons have shown that SGAs like olanzapine, risperidone, amisulpride, or clozapine are more efficacious than FGAs. Our study shows clear advantages for SGAs except for quetiapine when compared with haloperidol. More recent network meta-analyses (Leucht et al., 2013) have been able to discern differences even between SGAs with the formerly mentioned SGAs leading the rankings in terms of efficacy for positive symptoms. Curiously, in our long-term study, we find that after controlling for baseline and sociodemographic measures, efficacy was superior for aripiprazole and ziprasidone compared with olanzapine for positive symptoms and CGI improvement after 3 years. In previous short and mid-term studies involving the same sample, we did not find significant differences by 1 year (Crespo-Facorro et al., 2011, 2014). Clinical differences for positive symptoms seem to establish from that point on. Despite that apparent inferior efficacy, olanzapine offered the best performance regarding long-term discontinuation due to non- or insufficient efficacy. It might be explained by the fact that most of non- or insufficient efficacy discontinuations accrue during the first 6 months of follow-up. No differences for remission or efficacy on positive symptoms were found during the 1-year follow-up period in our sample, but there was a decreasing trend towards inferiority for quetiapine. Optimal efficacy may critically impact long-term discontinuation outcome during this early phase. Nevertheless, long-term beneficious evolution concerning positive symptoms, especially for aripiprazole, should be acknowledged when choosing first-line treatment after FEP. Regarding depressive symptoms, quetiapine is acknowledged as a first-line treatment even in monotherapy in affective psychoses (Lindström et al., 2017), but in several previous first-episode nonaffective psychoses studies, no significant differences between SGAs (including quetiapine) were found in reducing depressive symptoms after mid-term follow-up (Kahn et al., 2008b). No notable changes on negative symptoms were found with any of the 6 antipsychotics. Per protocol analysis did not show statistically significant differences along follow-up.
Side Effects and Concomitant Medications
The differences in the percentage of patients with treatment-emergent parkinsonism may be of clinical interest. A higher percentage of extrapyramidal side effects was identified among patients treated with haloperidol and risperidone. As reported in previous studies, akathisia (Juncal-Ruiz et al., 2017) represented a challenge among aripiprazole- and ziprasidone-treated individuals. These circumstances may partially explain that significantly more patients on aripiprazole and ziprasidone needed hypnotics, benzodiazepines, and anticholinergics to relieve akathisia. In agreement with previous reports (Lee et al., 2011; Vázquez-Bourgon et al., 2018), no significant differences were found in the frequency of body weight increase between treatments (except for the comparison between ziprasidone and olanzapine), but a uniform trend to weight increase was appreciated with all of them. Intention-to-treat analysis revealed that over 70% of the individuals on olanzapine showed a rapid body weight gain (Table 4). This should be regarded as a possible limitation for its first-line use as it contributes to a higher risk of metabolic issues (Smith et al., 2008). Interestingly, discontinuation due to severe or intolerable side effects was the main cause for discontinuation in our study but remained relatively low (23.14 %). Nevertheless, it was significant in the case of ziprasidone and haloperidol as was their main cause of discontinuation. Sleepiness/sedation was the most prevalent reported secondary effect for discontinuation in the case of ziprasidone (n = 8; 34.7%) and haloperidol despite low doses prior to discontinuation due to side effects (58.5 mg [SD = 34.3] and 1.8 mg [SD = 1.2], respectively). In the case of risperidone, we distinguished 2 patterns for discontinuation mediated by side effects; during the acute phase, most complaints focused on somnolence and EPS, while during the second half of follow-up, discontinuations were due to sexual complaints and weight gain. Olanzapine and quetiapine patients demonstrated lower secondary effects rates and the lowest discontinuation rates due to side effects. However, they are linked with more incidence of metabolic impairment (Davy et al., 2016) and a higher risk for health events (Vanasse et al., 2016). Thus, their use as first-line treatments should be considered cautiously. Per protocol analyses replicated the results for weight gain, showing a trend towards significant weight gain in the olanzapine group consistent with extensive data available in research literature. Sialorrhea and ejaculatory dysfunction also presented statistically significant results under per protocol analyses, but no significant differences were identified between treatment groups.
Limitations and Strengths
Several limitations should be considered when interpreting our results. First, as a practical clinical trial, patients and observers (B.C.-F.) were not blinded to treatments in our study. The fact that the observers knew the medications prescribed may have involuntarily biased the outcomes. As a non-industry–funded study, the risk for systematic biased measuring study outcomes favoring any of the treatments is limited. Second, the mean doses of quetiapine used could be understood as somewhat low to treat first-episode individuals. However, controlled investigations have clearly confirmed that standard dosage range should be appropriate in everyday clinical practice (Johnsen et al., 2010). Optimal doses of antipsychotics within licensed range were chosen based on clinical efficacy and the presence of side effects and were adjusted according to the clinical situation of each patient. It is important to note that chlorpromazine equivalents are a proxy measure for the comparison of antipsychotic dosage in antipsychotic effectiveness studies. Thus, results emerging from these comparisons should be considered cautiously. Third, treatment adherence measures were collected from self-report and close observers (family members and social assistants) but not from antipsychotic blood levels. This could have an impact in the accuracy of discontinuation measures due to nonadherence.
On the other hand, this is one of the longest head-to-head antipsychotic effectiveness studies regarding follow-up (3 years). It was performed on a large (n = 376), well-characterized, and homogeneous sample, as most of patients (97.08%) were antipsychotic naïve prior to their inclusion and comprised some of the most widely used antipsychotic treatments.
Conclusions
Establishing risks and benefits of SGA treatments and identifying different discontinuation patterns may contribute to make better treatment selection after an FEP. In our research, 2 treatment groups were separated according to their performance: olanzapine, risperidone, and aripiprazole presented clear advantages for the first-line treatment of FEP in terms of effectiveness. In addition, guaranteeing a good adherence to effective antipsychotic treatments is one of the priority challenges in the treatment of FEP individuals to prevent a malignant course of the disease and has proven to be the main reason for discontinuation for the most effective treatments. Finally, balancing most frequent and severe side effects (metabolic risk and EPS) should be considered to fit the antipsychotic choice to each individual case.
Acknowledgments
This study was conducted as part of a clinical trial “ Comparative Study of Aripiprazole, Quetiapine and Ziprasidone in Treatment of First Episode Psychosis: 3-year Follow-up.” ClinicalTrials.gov Identifier: NCT02526030.
The authors thank the “Programa Asistencial de las Fases Iniciales de Psicosis” (PAFIP) research team, especially Víctor Ortiz-García de la Foz, Gema Pardo, and Obdulia Martínez and all patients and family members who participated in the study. The authors are entirely responsible for the scientific content of the paper.
The study was carried out at the Hospital Marqués de Valdecilla, University of Cantabria, Santander, Spain, under the following grant supports: Plan Nacional de Drogas Research (2005-Orden sco/3246/2004); SENY Fundació (CI 2005–0308007); and Fundación Marqués de Valdecilla (API07/011); Gerencia Regional de Salud de Castilla y León (INT/M/04/17).
Unrestricted educational and research grants from AstraZeneca, Pfizer, Bristol-Myers Squibb, and Johnson & Johnson provided support for PAFIP activities. No pharmaceutical industry or institutional sponsors participated in the study design, data collection, analysis, and interpretation of the results.
Statement of Interest
None.
References
- Addington D, Addington J, Maticka-Tyndale E (1993) Assessing depression in schizophrenia: the Calgary depression scale. Br J Psychiatry 163(Suppl 22): 39–44. [PubMed] [Google Scholar]
- Andreasen N. (1984) The Scale for the Assessment of Positive Symptoms (SAPS). Iowa City: Univ Iowa. [Google Scholar]
- Andreasen NC. (1989) Scale for the assessment of negative symptoms (SANS). Br J Psychiatry 155:53–58. [PubMed] [Google Scholar]
- Asmal L, Flegar SJ, Wang J, Rummel-Kluge C, Komossa K, Leucht S (2013) Quetiapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev Advance online publication. http://doi.wiley.com/10.1002/14651858.CD006625.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes TR. (1989) A rating scale for drug-induced akathisia. Br J Psychiatry 154:672–676. [DOI] [PubMed] [Google Scholar]
- Correll CU, De Hert M (2013) Antipsychotics for acute schizophrenia: making choices. Lancet 382:919–920. [DOI] [PubMed] [Google Scholar]
- Correll CU, Rubio JM, Kane JM (2018) What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry 17:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Facorro B, Pérez-Iglesias R, Mata I, Caseiro O, Martínez-Garcia O, Pardo G, Ramirez-Bonilla M, Pelayo-Terán JM, Vázquez-Barquero JL (2011) Relapse prevention and remission attainment in first-episode non-affective psychosis. A randomized, controlled 1-year follow-up comparison of haloperidol, risperidone and olanzapine. J Psychiatr Res 45:763–769. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Pérez-Iglesias R, Mata I, Martínez-Garcia O, Ortiz V, Pelayo-Terán JM, Valdizan E, Vazquez-Barquero JL (2012) Long-term (3-year) effectiveness of haloperidol, risperidone and olanzapine: results of a randomized, flexible-dose, open-label comparison in first-episode nonaffective psychosis. Psychopharmacology (Berl) 219:225–233. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, de la Foz VO, Mata I, Ayesa-Arriola R, Suarez-Pinilla P, Valdizan EM, Martinez-Garcia O, Pérez-Iglesias R (2014) Treatment of first-episode non-affective psychosis: a randomized comparison of aripiprazole, quetiapine and ziprasidone over 1 year. Psychopharmacology (Berl) 231:357–366. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Pelayo-Teran JM, Mayoral-van Son J (2016) Current data on and clinical insights into the treatment of first episode nonaffective psychosis: a comprehensive review. Neurol Ther 5:105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy V, Correll CU, Britta G, Michel P, Marc DH, Ward PB, Simon R, Fiona G, John L, Brendon S (2016) Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta‐analysis. World Psychiatry 15:166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley R, Chiliza B, Asmal L, Harvey BH (2013) The nature of relapse in schizophrenia. BMC Psychiatry 13:50 Advance online publication. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3599855&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (2002) Structured clinical interview for DSM-IV-TR axis I disorders research version, patient edition with psychotic screen (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Gómez-Revuelta M, Pelayo-Terán JM, Juncal-Ruiz M, Ortiz-García de la Foz V, Vázquez-Bourgon J, González-Pinto A, Crespo-Facorro B (2018) Long-term antipsychotic effectiveness in first episode of psychosis: a 3-year follow-up randomized clinical trial comparing aripiprazole, quetiapine, and ziprasidone. Int J Neuropsychopharmacol 21:1090–1101. Advance online publication. https://academic.oup.com/ijnp/advance-article/doi/10.1093/ijnp/pyy082/5095916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. (1976) ECDEU Assessment Manual for Psychopharmacology: revised. Rockville: National Institute of Mental Health. [Google Scholar]
- Jann MW, Penzak SR (2018) Long-acting injectable second-generation antipsychotics: an update and comparison between agents. CNS Drugs 32:603. [DOI] [PubMed] [Google Scholar]
- Johnsen E, Kroken RA, Wentzel-Larsen T, Jørgensen HA (2010) Effectiveness of second-generation antipsychotics: a naturalistic, randomized comparison of olanzapine, quetiapine, risperidone, and ziprasidone. BMC Psychiatry 10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncal-Ruiz M, Ramirez-Bonilla M, Gomez-Arnau J, Ortiz-Garcia de la Foz V, Suarez-Pinilla P, Martinez-Garcia O, Neergaard KD, Tabares-Seisdedos R, Crespo-Facorro B (2017) Incidence and risk factors of acute akathisia in 493 individuals with first episode non-affective psychosis: a 6-week randomised study of antipsychotic treatment. Psychopharmacology (Berl) 234:2563–2570. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IP, Gheorghe MD, Rybakowski JK, Galderisi S, Libiger J, Hummer M, Dollfus S, López-Ibor JJ, Hranov LG, Gaebel W, Peuskens J, Lindefors N, Riecher-Rössler A, Grobbee DE (2008a) Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet 371:1085–1097. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Wolfgang Fleischhacker W, Boter H, Davidson M, Vergouwe Y, M Keet IP, Gheorghe MD, Rybakowski JK, Galderisi S, Libiger J, Hummer M, Dollfus S, López-Ibor JJ, Hranov LG, Gaebel W, Peuskens J, Lindefors N, Riecher-Rössler A, Grobbee DE (2008b) Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet 371:1085–1097. [DOUBLE REFERENCE (a and b)] [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Robenzadeh A, Leucht C, Leucht S, Watanabe K, Mimura M, Borenstein M, Kane JM, Correll CU (2014) Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull 40:192–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Hagi K, Nitta M, Kane JM, Correll CU (2019) Long-term effectiveness of oral second-generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta-analysis of direct head-to-head comparisons. World Psychiatry 18:208–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lako IM, van den Heuvel ER, Knegtering H, Bruggeman R, Taxis K (2013) Estimating dopamine D₂ receptor occupancy for doses of 8 antipsychotics: a meta-analysis. J Clin Psychopharmacol 33:675–681. [DOI] [PubMed] [Google Scholar]
- Lee SY, Park MH, Patkar AA, Pae CU (2011) A retrospective comparison of BMI changes and the potential risk factors among schizophrenic inpatients treated with aripiprazole, olanzapine, quetiapine or risperidone. Prog Neuropsychopharmacol Biol Psychiatry 35:490–496. [DOI] [PubMed] [Google Scholar]
- Leucht S, Komossa K, Rummel-Kluge C, Corves C, Hunger H, Schmid F, Asenjo Lobos C, Schwarz S, Davis JM (2009) A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry 166:152–163. [DOI] [PubMed] [Google Scholar]
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM (2013) Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382:951–962. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223. [DOI] [PubMed] [Google Scholar]
- Lindström L, Lindström E, Nilsson M, Höistad M(2017) Maintenance therapy with second generation antipsychotics for bipolar disorder–A systematic review and meta-analysis. J Affect Disord 213:138–150. [DOI] [PubMed] [Google Scholar]
- Lingjærde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K(1987) The UKU side effect rating scale: a new comprehensive rating scale for psychotropic drugs and a cross‐sectional study of side effects in neuroleptic‐treated patients. Acta Psychiatr Scand 76:1–100. [DOI] [PubMed] [Google Scholar]
- Mayoral-van Son J, de la Foz VO, Martinez-Garcia O, Moreno T, Parrilla-Escobar M, Valdizan EM, Crespo-Facorro B (2016) Clinical outcome after antipsychotic treatment discontinuation in functionally recovered first-episode nonaffective psychosis individuals: a 3-year naturalistic follow-up study. J Clin Psychiatry 77:492–500. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Lieberman JA, Perkins DO, Hamer RM, Gu H, Lazarus A, Sweitzer D, Olexy C, Weiden P, Strakowski SD (2007) Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry 164:1050–1060. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR (1962) The brief psychiatric rating scale. Psychol Rep 10:799–812. [Google Scholar]
- Pelayo-Terán JM, Pérez-Iglesias R, Ramírez-Bonilla M, González-Blanch C, Martínez-García O, Pardo-García G, Rodríguez-Sánchez JM, Roiz-Santiáñez R, Tordesillas-Gutiérrez D, Mata I, Vázquez-Barquero JL, Crespo-Facorro B (2008) Epidemiological factors associated with treated incidence of first-episode non-affective psychosis in Cantabria: insights from the clinical programme on early phases of psychosis. Early Interv Psychiatry 2:178–187. [DOI] [PubMed] [Google Scholar]
- Pelayo-Terán JM, Gajardo Galán VG, de la Ortiz-García de la Foz V, Martínez-García O, Tabarés-Seisdedos R, Crespo-Facorro B, Ayesa-Arriola R (2017) Rates and predictors of relapse in first-episode non-affective psychosis: a 3-year longitudinal study in a specialized intervention program (PAFIP). Eur Arch Psychiatry Clin Neurosci 267:315–323. [DOI] [PubMed] [Google Scholar]
- Pelayo-Terán JM, Gajardo-Galán V, Gómez-Revuelta M, Ortiz-García de la Foz V, Ayesa-Arriola R, Tabarés-Seisdedos R, Crespo-Facorro B (2018) Duration of active psychosis and functional outcomes in first-episode non-affective psychosis. Eur Psychiatry 52:29–37. [DOI] [PubMed] [Google Scholar]
- Samara MT, Leucht C, Leeflang MM, Anghelescu IG, Chung YC, Crespo-Facorro B, Elkis H, Hatta K, Giegling I, Kane JM, Kayo M, Lambert M, Lin CH, Möller HJ, Pelayo-Terán JM, Riedel M, Rujescu D, Schimmelmann BG, Serretti A, Correll CU, Leucht S (2015) Early improvement as a predictor of later response to antipsychotics in schizophrenia: a diagnostic test review. Am J Psychiatry 172:617–629. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JW (1970) A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 212:11–19. [DOI] [PubMed] [Google Scholar]
- Smith M, Hopkins D, Peveler RC, Holt RI, Woodward M, Ismail K (2008) First- v. second-generation antipsychotics and risk for diabetes in schizophrenia: systematic review and meta-analysis. Br J Psychiatry 192:406–411. [DOI] [PubMed] [Google Scholar]
- Smith RC, Leucht S, Davis JM (2019) Maximizing response to first-line antipsychotics in schizophrenia: a review focused on finding from meta-analysis. Psychopharmacology (Berl) 236:545–559. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Siu C, Remington G, Fervaha G, Zipursky RB, Foussias G, Agid O (2019) Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology 44:1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Mittendorfer-Rutz E, Majak M, Mehtälä J, Hoti F, Jedenius E, Enkusson D, Leval A, Sermon J, Tanskanen A, Taipale H (2017) Real-world effectiveness of antipsychotic treatments in a nationwide Cohort of 29 823 patients with schizophrenia. JAMA Psychiatry 74:686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanasse A, Blais L, Courteau J, Cohen AA, Roberge P, Larouche A, Grignon S, Fleury MJ, Lesage A, Demers MF, Roy MA, Carrier JD, Delorme A (2016) Comparative effectiveness and safety of antipsychotic drugs in schizophrenia treatment: a real-world observational study. Acta Psychiatr Scand 134:374–384. [DOI] [PubMed] [Google Scholar]
- Vázquez-Bourgon J, Pérez-Iglesias R, Ortiz-García de la Foz V, Suárez Pinilla P, Díaz Martínez Á, Crespo-Facorro B (2018) Long-term metabolic effects of aripiprazole, ziprasidone and quetiapine: a pragmatic clinical trial in drug-naïve patients with a first-episode of non-affective psychosis. Psychopharmacology (Berl) 235:245–255. [DOI] [PubMed] [Google Scholar]
- Wang C, Shi W, Huang C, Zhu J, Huang W, Chen G(2017) The efficacy, acceptability, and safety of five atypical antipsychotics in patients with first-episode drug-naïve schizophrenia: a randomized comparative trial. Ann Gen Psychiatry 16:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW. (2003) Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 64:663–667. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA (1978) A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 133:429–435. [DOI] [PubMed] [Google Scholar]