Abstract
Background
Prenatal stress (PRS) is considered a risk factor for depressive disorder. Adult hippocampal neurogenesis is believed to play a role in the regulation of affective behaviors. GABAergic interneuron is a key modulator in adult hippocampal neurogenesis. Growing evidence indicates that PRS has adverse effects on adult hippocampal neurogenesis and DNA epigenetic modifications of the GABAergic system. The aim of this study was to investigate whether epigenetic GABAergic dysfunction participates in the negative impact of PRS on adult hippocampal neurogenesis and related emotional behaviors.
Methods
Behavioral tests were used to explore PRS-induced depression-like behaviors of adult female mice. Immunohistochemistry staining, real-time reverse transcription-polymerase chain reaction, western blot, and chromatin immunoprecipitation were employed to detect adult neurogenesis and epigenetic changes of the GABAergic system in the hippocampus of PRS mice.
Results
PRS mice developed a depression phenotype accompanied by the inhibited maturation of hippocampal newborn neurons. Compared with control mice, PRS mice showed decreased expression of glutamic acid decarboxylase 67 at the mRNA and protein levels. GABAA receptor agonist phenobarbital could rectify the decrease of 5-bromo-2-deoxyuridine/neuronal nuclei double-positive (BrdU+/NeuN+) cells in PRS mice. PRS mice also showed increased expression of DNA methyltransferase 1 and increased binding of DNA methyltransferase 1 to glutamic acid decarboxylase 67 promoter region. The treatment with DNA methyltransferase 1 inhibitor 5-aza-deoxycytidine restored the decrease of BrdU+/NeuN+ cells and depression-like behaviors in PRS mice via improving GABAergic system.
Conclusions
The present results indicate that epigenetic changes of the GABAergic system are responsible for adult hippocampus neurogenesis and depression-like behaviors in PRS mice.
Keywords: Adult neurogenesis, depression, DNMT1, GAD67, prenatal stress
Significance Statement.
The current work depicts that DNMT1-mediated epigenetic modifications of GABAergic interneurons is one of the important mechanisms underlying the adverse effect of prenatal stress (PRS) on adult hippocampal neurogenesis and depression-like behaviors. The discovery of our work can help to find the pathologic molecular mechanism of PRS-induced depression and new therapeutic targets. In addition, our study indicates that PRS, as the most common adverse factor in early life, induces neurobiological and behavioral changes, providing evidence for elucidating the relationship between early-life environment and diseases such as depression. We believe that PRS mice can be used as one of the representative animal models of depression induced by early adverse environment to illustrate the interaction between environment and related genes.
Introduction
Accumulated evidence suggests that exposure to stressful life events during pregnancy leads to depression disorder in adulthood (Alonso et al., 2000; Sickmann et al., 2015). Generation of new neurons in the hippocampus throughout adult life (adult hippocampal neurogenesis) plays a vital role in the regulation of affective behaviors (Lemaire et al., 2000; Abrous et al., 2005; Hill et al., 2015; Zhang et al., 2016). Prenatal stress (PRS) has been continuously proved to have an adverse effect on adult hippocampal neurogenesis (Schmitz et al., 2002; Lemaire et al., 2006; Rayen et al., 2011). Additionally, some studies have pointed out that neurogenesis-deficient mice display a depressive behavioral phenotype (Snyder et al., 2011; Wang et al., 2015). It is therefore believed that the abnormality of adult hippocampal neurogenesis is closely associated with PRS-induced depression. In the present study, we further focus on the molecular mechanisms underlying PRS-induced alterations in hippocampal neurogenesis and depression-related behaviors.
Although the mechanisms underlying such long-lasting impact of PRS on adult hippocampal neurogenesis and related behaviors are not clear, epigenetic mechanisms are regarded as the most plausible targets through which PRS could confer its enduring effects. Indeed, accumulating evidence has proved PRS-induced DNA epigenetic alterations (Matrisciano et al., 2012; Zheng et al., 2016; Papale et al., 2017). PRS has been reported to influence the expression of DNA methyltransferase (DNMT1 and 3a) in the hippocampus of postnatal brain (Matrisciano et al., 2013; Dong et al., 2015). DNMT1 and DNMT3a, which are the key enzymes for establishing and maintaining DNA methylation, have been found to selectively localize in GABAergic neurons (Veldic et al., 2004; Zhou et al., 2013). Glutamic acid decarboxylase 67 (GAD67), used to synthesize GABA transmitters (Bulfone et al., 1998; Gheusi et al., 2000), is one of the GABAergic neuronal markers. The promoter of GAD67 has been found to be embedded in large CpG islands and express methylation consensuses (Grayson et al., 2005; Costa et al., 2007). Decreased GAD67 expression, increased DNMTs expression, and enhanced methylation of GAD67 promoter have recently been reported in the hippocampus and cortex of adult mice prenatally exposed to stress (Matrisciano et al., 2013).
Increasing evidence supports the notion that GABAergic interneuron is a key player in adult hippocampal neurogenesis. This effect has been shown to be directly mediated through GABAA receptor (GABAAR) on progenitor cells in the hippocampal dentate gyrus (DG) and due to GABA’s excitatory action on neuronal differentiation and maturation (Ganguly et al., 2001; Liu et al., 2005; Tozuka et al., 2005; Quadrato et al., 2014). In genetic and pharmacological studies in mice, the inhibition of GABAergic interneuron functions greatly reduces the maturation and functional integration of adult-born hippocampal granule neurons (Tozuka et al., 2005; Ge et al., 2007). The activation of GABAAR on neural progenitor cells stimulates the differentiation and maturation of these cells into the neuronal phenotype (Tozuka et al., 2005; Ge et al., 2006). Considering that PRS has adverse effects on the expression of DNMTs and the methylation of GAD67 promoter in the hippocampal GABAergic system, we investigated whether epigenetic GABAergic dysfunction is involved in the negative impact of PRS on adult hippocampal neurogenesis and related affective behaviors. The goal of the present study is to increase the understanding of epigenetic mechanisms underlying depression disorder by recapitulating interactions between genes and the environment in animal models.
MATERIALS AND METHODS
The present studies were approved by the Animal Care and Use Committee of Nanjing Medical University. The protocols used here were in accordance with the guidelines published in the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals and their suffering.
Animal Model Preparation
Pregnant C57BL/6J mice (Oriental Bio Service Inc., Nanjing, Jiangsu, China) were individually housed and provided food and water ad libitum. Control dams were left undisturbed throughout gestation on a 12-hour-light/-dark cycle (lights on at 7:00 am and off at 7:00 pm), whereas stressed dams were subjected to the restraint stress as described previously (Zheng et al., 2016) with slight modification. From the tenth day of pregnancy until delivery, the pregnant dams were restrained in a transparent tube (12 cm × 3 cm) for 45 minutes 3 times per day and exposed to 24-hour constant light. The day of birth was referred to as postnatal day 0 (PND 0). Weaning occurred at PND 21–22, after which the offspring of control and stressed dams (control and PRS mice) were group-housed by litter and sex. To minimize the effect of parent-to-offspring interaction per litter, 1 female offspring was randomly selected from each litter as the object of study. Male offspring were not taken into experiments because it has been reported that PRS increases the risk of depression in adult female offspring, not male offspring (Alonso et al., 2000; Sickmann et al., 2015). Then, the following experiments were performed at PNDs 60–90.
Drug Treatment
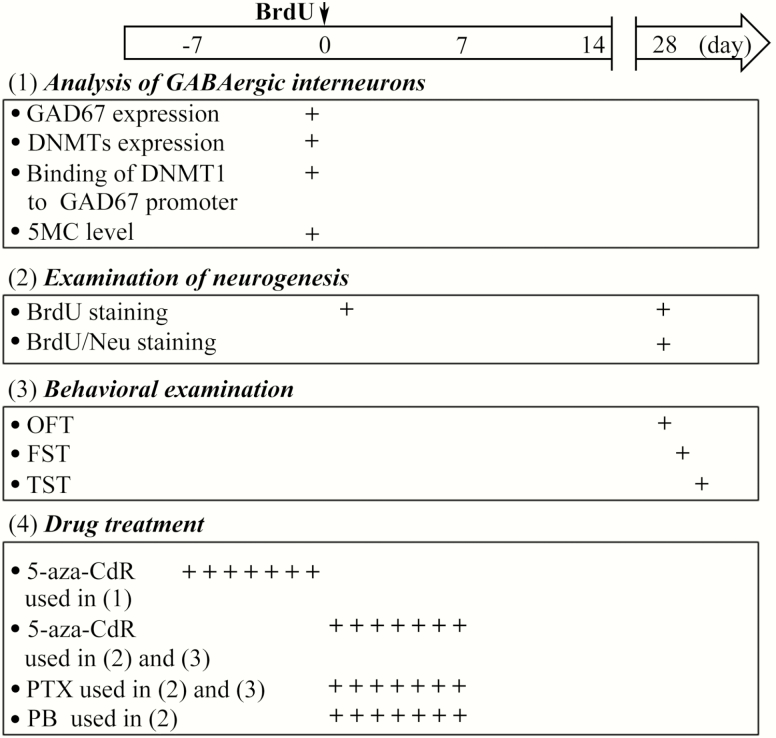
DNA synthesis marker 5-bromo-2-deoxyuridine (BrdU), GABAAR agonist phenobarbital (PB), GABAAR antagonist picrotoxin (PTX), and DNMT1 inhibitor 5-aza-deoxycytidine (5-aza-CdR) were purchased from Sigma-Aldrich. As shown in Figure 1, to detect the effect of PRS on hippocampal neurogenesis, 50 mg/kg BrdU was administered i.p. into mice 3 times at intervals of 8 hours. To investigate the specific effect of GABAAR on neuronal maturation, the mice received the i.p. administration of PB (50 mg/kg) or the intracerebroventricular (i.c.v.) injection of 5 μL of PTX (10 μM) once a day for 7 days after the last BrdU administration. To investigate the role of DNMT1 in the alterations of GABAergic interneurons, 5 μL of 5-aza-CdR (10 μM) was administered i.c.v. once a day for 7 days before the BrdU administration. To investigate the role of DNA epigenetic modifications of GABAergic interneurons in hippocampal neurogenesis and emotional behaviors, 5 μL of 5-aza-CdR (10 μM) and 5 μL of PTX (10 μM) were i.c.v. injected separately or in combination for 7 days after BrdU injection. For repeated i.c.v. injection, a 26-G stainless-steel guide cannula (Plastics One) was implanted into the right lateral ventricle (0.3 mm posterior to bregma, 1.0 mm lateral, and 2.3 mm ventral) and anchored to the skull with 3 stainless steel screws and dental cement (Zhou et al., 2014).
Figure 1.
Time course of the experimental procedure. “↓” Indicates the time of the injection of 5-bromo-2-deoxyuridine (BrdU). “+” Indicates the times when the drugs were administered and various tests (the analysis of GABAergic interneurons, the examination of depression-like behaviors, and the investigation of neurogenesis) were performed.
Behavior Analysis
As previously described (D’Adamo et al., 2004; Snyder et al., 2011; Wang et al., 2015), open field test (OFT), forced swimming test (FST), and tail suspension test (TST) were used to examine spontaneous activity and depression-like behaviors in mice on day 28–30 post BrdU injection following OFT→FST→TST sequence (Figure 1). The room was dimly lit during the tests. Animals’ behaviors were videotaped and quantified via Ethovision 3.0 software (Noldus Information Technology Inc.).
FST
Each mouse was placed in a glass cylinder (300 mm high, 280 mm diameter) filled with water to a height of 20 cm (25°C ± 1°C). The mice were subjected to a 6-minute swimming test (Sha et al., 2016). Each mouse was tested only once. The total immobility time (minimal movements to keep the head above water) was calculated.
TST
The mice were suspended by the tail from a rod 60 cm above the floor using adhesive tape, as previously described (Zhang et al., 2016). The trials were conducted for 6 minutes, during which the duration of immobility was recorded.
OFT
The novel environment was a 35 cm × 35 cm × 25 cm white Plexiglas arena. Mice were placed on a corner and were allowed to freely explore the field for 6 minutes (Zhang et al., 2016). Total traveled distance was recorded.
Immunohistochemistry Staining and Analysis
Mice were anesthetized with chloral hydrate (400 mg/kg, i.p.) and transcardially perfused with phosphate buffer saline and 4% paraformaldehyde sequentially. Brains were post-fixed overnight in 4% paraformaldehyde at 4°C, and 40-μm-thick or 5-μm-thick coronal brain sections were obtained from the whole hippocampus using a sliding microtome. For nonfluorescent staining, the sections were incubated with mouse monoclonal anti-BrdU (1:1000; Millipore) or mouse anti-GAD67 (1:1000; Millipore) primary antibody. The sections were incubated with the appropriate biotinylated secondary antibody in blocking buffer, followed by the avidin-biotin-peroxidase complex (Vector Laboratories), which was used according to the manufacturer’s protocol. Sections were developed in 0.05% 3,3′-diaminobenzidine tetrahydrochloride. Nuclear staining with hematoxylin was finally performed in GAD67 nonfluorescent staining. Rat monoclonal anti-BrdU antibody (1:200; Abcam) and mouse monoclonal anti-neuronal nuclei (NeuN) were used for immunofluorescence double staining of BrdU and NeuN. The appropriate fluorescent secondary antibodies were used, followed by 3 washes, after which sections were mounted using gelvatol fluorescence mounting media. 3,3′-diaminobenzidine tetrahydrochloride-labeled cells were observed using a conventional light microscope (Olympus DP70, × 60). Immunofluorescence double-labeled cells were observed by a confocal laser-scanning microscope (Leica). The number of BrdU+ or BrdU+/NeuN+ cells in the granule cell and subgranular layers of the DG per section (40 μm thick, 200 μm apart) was counted and multiplied by 5 to obtain a total number for each mouse (Zhou et al., 2014). The number of GAD67+ cells in the hippocampus per section (5 μm thick, 100 μm apart) was counted and multiplied by 20 to obtain a total number for each mouse.
Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Mice were killed and whole brains were extracted and immediately stored at −80°C until assayed. Brains sections (50 μm thick) were cut in the coronal plane using a cryostat. The hippocampus was dissected from the frozen slices on dry ice. Total RNA was extracted using TRIzol (Invitrogen) following the manufacturer’s instructions. Possible contamination with genomic DNA was removed by an on-column DNase I (Qiagen) treatment. mRNA was reverse transcribed using the high-capacity cDNA Reverse Transcription kit (Applied Biosystems) following the manufacturer’s instructions. The primer sequences of DNMT1, DNMT3a, GAD67, and GAPDH are exhibited in Table 1. RT-PCR was performed using a LightCycler FastStart DNA Master SYBR Green I kit (Roche) and an ABI Prism 7300 Sequence Detection System (Applied Biosystems). To improve the accuracy of the real-time PCR for quantification, amplifications were performed in triplicate for each RNA sample. The relative expression of genes was determined using the 2−ΔΔCt method with normalization to GAPDH expression.
Table 1.
Primers for Reference and Target Genes
| Gene | Forward primer (5’ to 3’) | Reverse primer (5’ to 3’) |
|---|---|---|
| DNMT1 | TGACAGTGGTGCTGAAGAAGCCAT | AGAATGGAGCCTCGAATTCTGAGA |
| DNMT3a | AACAACAACTGCTGCAGGTGCTTT | ACTCCTGGATATGCTTCTGTGTGA |
| GAD67 | CTTTGGAGCTCTTCCTGATTGA | TTGTTTGAGGGCTGTCTCTG |
| GAPDH | ACCAGGGAGGGCTGCAGTCC | TCAGTTCGGAGCCCACACGC |
| CpG-richGAD67 promoter | GAGGAGAGCGGGCCAAGA | GTGCCGCTCCACACGCC |
Western-Blot Analysis
For protein quantification, we conducted measurements as described in detail elsewhere (Matrisciano et al., 2012). The hippocampus was dissected on ice, and the protein was extracted using a total protein extraction kit (KeyGEN Biotech). Mouse anti-GAD67 (1:5000; Millipore) and rabbit polyclonal anti-DNMT1 antibodies (1:1000; Novusbio) were used to detect GAD67 and DNMT1 protein. Anti-β-actin monoclonal antibody (1:5000; Millipore) was used as an internal antibody. The IMAGEQUANT analysis software was used to perform the densitometric analysis of interest bands. The values were presented as an optical density ratio with respect to β-actin.
Chromatin Immunoprecipitation (ChIP) Assays
ChIP assays were used to detect DNMT1 binding to CpG-rich regions of the GAD67 promoter. The Chip procedure was carried out using a ChIP kit (Upstate). Briefly, approximately 10 mg of the hippocampus was used for this procedure. Tissue was incubated at 37°C for 10 minutes with 500 μL of phosphate buffer saline containing 1% formaldehyde and a cocktail of protease inhibitors (Sigma). Tissue was homogenized in 300 μL of SDS lysis buffer (supplied by ChIP kit, Upstate), and the lysate was sonicated for 15 minutes on ice. Immunoprecipitation was performed overnight at 4°C by the addition of 10 μg of ChIP grade DNMT1 (Abcam) to the sonicated solution. An aliquot of the sonicated lysate without antibody was used as input to quantify the total amount of DNA in sample extracts. Protein-free DNA was extracted and used for detection and quantification of CpG-rich regions of GAD67 promoter by quantitative PCR. The primers of CpG-rich GAD67 promoter were decided by the report of Matrisciano et al. (2013) and shown in Table 1. The level of immunoprecipitated GAD67 promoter by the DNMT1 antibody was expressed as a percentage of the input DNA using the following equation: %(DNA − IP/total input) = 2[(Ct(10%input) – 3.32) – Ct (DNA-IP)] × 100%.
Methylated DNA Immunoprecipitation
The enrichment of 5-methylcytosine (5MC) on GAD67 promoter were assessed using MeDIP (Diagenode) followed by quantitative PCR. The procedures for sample treatment and immunoprecipitation are described in the kit instruction manuals. The following quantitative PCR, the primers of GAD67 promoter, and the calculation of the level of immunoprecipitated GAD67 promoter by the 5MC antibody were the same as those in the ChIP assays.
Data Analysis/Statistics
Data were retrieved and processed with the Origin 6.1 software (Micro-Cal Software Inc.). The group data were expressed as the means ± SEM. Two-tailed student’s t test was used for comparisons between 2 groups. ANOVAs followed by Fisher’s protected least significant difference post hoc test was employed if more than 2 groups were compared. Statistical analysis was performed using Stata 7 software (Stata Corp). P < .05 was considered statistically significant.
RESULTS
PRS Mice Express Depression-Like Behaviors in Behavioral Experiments
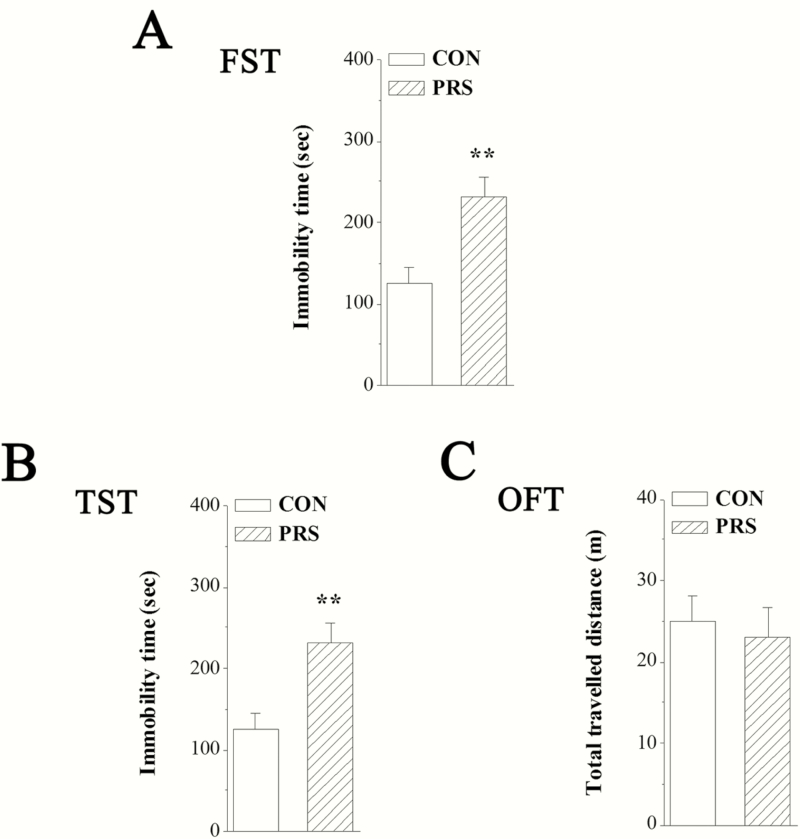
Depression-like behavior was examined using the FST and TST, which are well-established behavioral paradigms. Increased immobility in both the TST and FST has been defined as a behavioral measurement for depression. The mean immobility time in the FST and TST are illustrated in Figure 2A,B. Compared with control mice, PRS mice exhibited a significant increase in the immobility in the FST (t[26] = 3.39, P < .01) and TST (t[26] = 3.50, P < .01). To exclude the influence of locomotion abnormality in the FST and TST, the total traveled distance in the OFT was also measured. No significant difference in total traveled distance between control and PRS groups was observed in the present study (t[26] = 0.39, P > .05; Figure 2C).
Figure 2.
Mice exposed to prenatal stress (PRS) show obvious depression-like behaviors. CON and PRS represent control and PRS mice, respectively. (A and B) The bars represent the immobility time observed in the forced swimming test (FST) and tail suspension test (TST). (C) The bar graph represents the total traveled distance in the open field test. Student’s t test; **P < .01 vs control mice; n = 14 per group.
PRS Inhibits Maturation of Newborn Neurons in Hippocampal DG
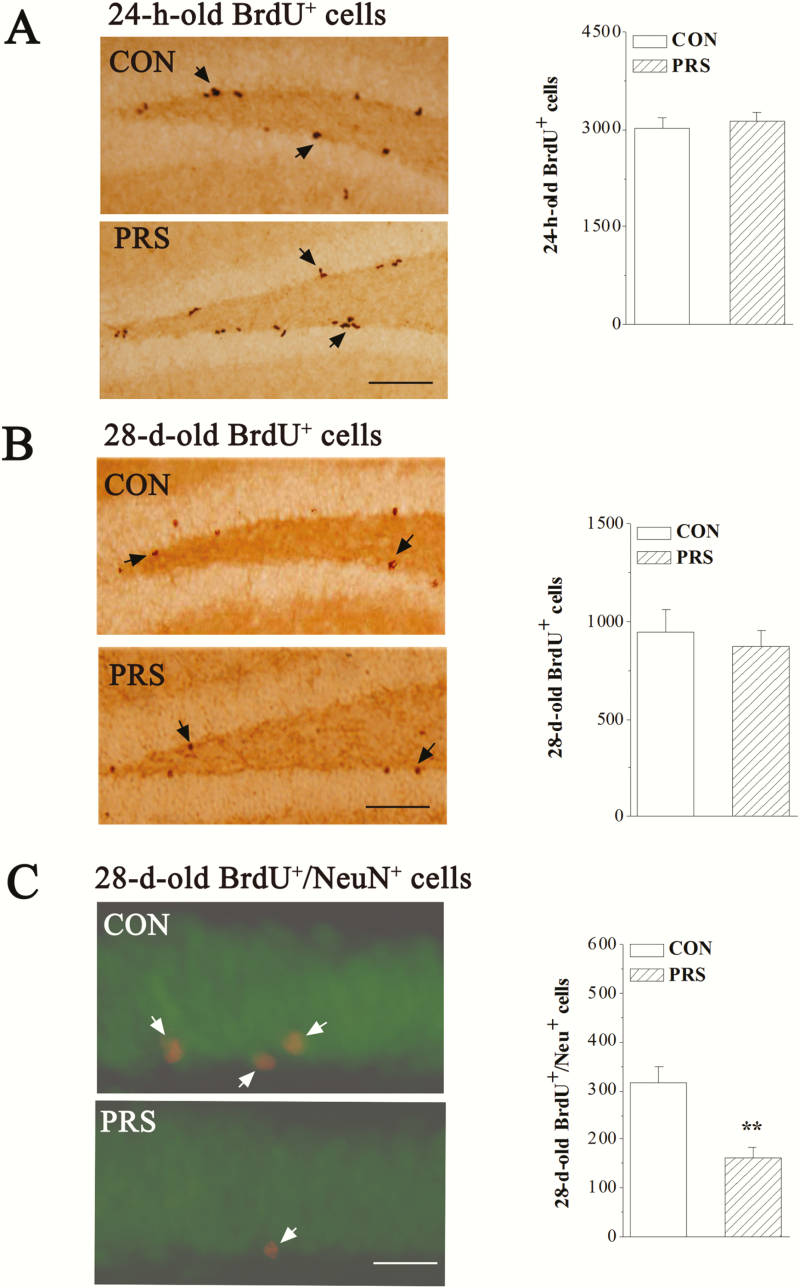
To examine the influence of PRS on the adult neurogenesis in hippocampal DG, we examined the proliferation of stem cells and the survival and maturation of newborn neurons in control mice and PRS mice after the BrdU injection. Firstly, the proliferation was examined at 24 hours after the last BrdU injection (24-hour-old BrdU+ cells). Compared with control mice, the number of 24-hour-old BrdU+ cells was not altered in PRS mice (t[14] = 0.49, P > .05; Figure 3A). Secondly, the survival and maturation of newborn neurons was examined on day 28 after BrdU injection (28-day-old BrdU+ cells). The number of 28-day-old BrdU+ cells in PRS mice did not differ from that in control mice (t[14] = 0.44, P > .05; Figure 3B). The number of 28-day-old BrdU+ cells expressing neuron-specific nuclear protein NeuN (BrdU+/NeuN+) in PRS mice was lower than that in control mice (t[10] = 4.06, P < .01; Figure 3C).
Figure 3.
PRS mice show a deficit in the maturation of newborn neurons. CON and PRS represent control and PRS mice, respectively. (A and B) Representative images of BrdU immunostaining (left panels). Black arrows indicate BrdU+ cells. Scale bar = 100 μm. The bar graphs show the mean numbers of 24-hour-old BrdU+ cells and 28-day-old BrdU+ cells in hippocampal dentate gyrus (DG) of control and PRS mice. Student’s t test; P > .05 between the data of control and PRS mice (n = 8 per group). (C) Representative image of BrdU/neuronal nuclei (NeuN) immunostaining (left panel). NeuN is shown in green and BrdU is shown in red, BrdU+/NeuN+ newborn cells are shown in yellow (white arrows). Scale bar = 25 μm. The bar graph shows the mean number of 28-day-old BrdU+/NeuN+ cells in hippocampal DG of control and PRS mice. Student’s t test; **P < .01 vs control mice; n = 6 per group.
PRS Decreases Hippocampal GAD67 Protein Expression to Impair Maturation of Newborn Neurons
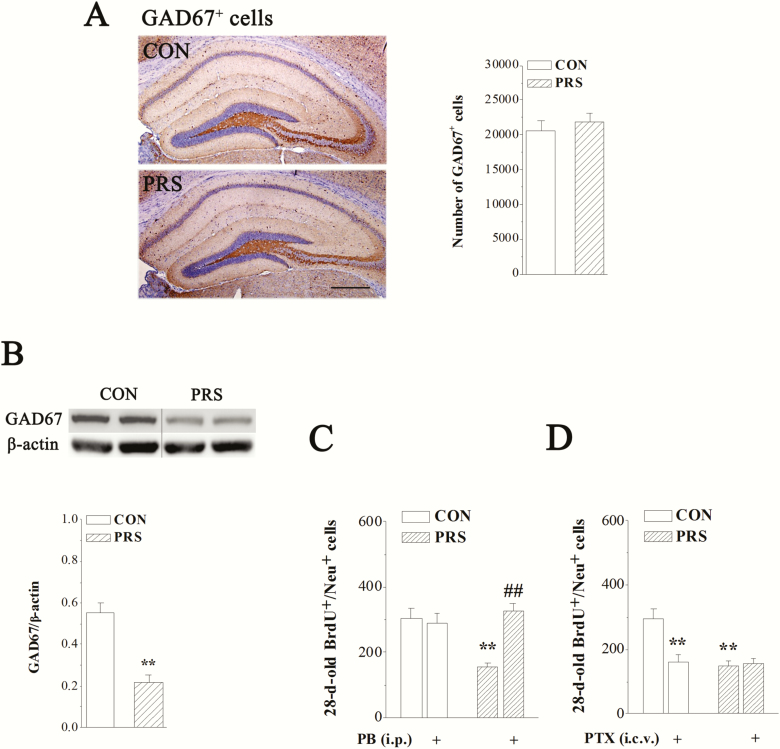
The phenotype of abnormal hippocampal neurogenesis in PRS mice—impaired neuronal maturation—suggests the possibility of hippocampal GABAergic deficits (Tozuka et al., 2005; Ge et al., 2006; Earnheart et al., 2007). To determine whether PRS impairs GABAergic interneurons in the hippocampus, we analyzed the number of GAD67+ GABAergic interneurons and the level of GAD67 protein in the hippocampus of control and PRS mice. There was no significant difference in the number of GAD67+ interneurons between control and PRS mice (t[14] = 0.60, P > .05; Figure 4A). However, a marked decrease in GAD67 protein was observed in the hippocampus of PRS mice (t[10] = 5.69, P < .01; Figure 4B). GABAAR agonist PB and antagonist PTX were used to analyze the correlation between GABAergic deficits and impaired maturation of newborn neurons in PRS mice. Two-way ANOVA displayed main effects of group and drug treatment and their interaction on the number of BrdU+/NeuN+ cells (Figure 4C: group: F(1,32) = 5.83, P < .05; drug treatment: F(1,32) = 11.15, P < .01; interaction: F(1,32) = 15.65, P < .001; Figure 4D: group: F(1,32) = 11.67, P < .01; drug treatment: F(1,32) = 7.91, P < .01; interaction: F(1,32) = 10.55, P < .01). The repeated i.p. injection of PB could rectify the reduction of BrdU+/NeuN+ cells in PRS mice (P < .01) without affecting that in control mice (P > .05; Figure 4C).Whereas the repeated i.c.v. administration of PTX decreased the number of BrdU+/NeuN+ cells in control mice (P < .01), it had no influence on that in PRS mice (P > .05; Figure 4D).
Figure 4.
The deficiency of glutamic acid decarboxylase 67 (GAD67) protein is involved in the impaired maturation of newborn neurons in PRS mice. CON and PRS represent control and PRS mice, respectively. (A) Representative images of GAD67 immunostaining (left panels). Scale bar = 200 μm. The bar graph shows the mean numbers of GAD67+ cells in the hippocampus of control and PRS mice. Student’s t test; P > .05 between the data of control and PRS mice (n = 8 per group). (B) Representative image of GAD67 protein in the hippocampus of control and PRS mice (upper panel). The bars represent the mean level of GAD67 protein in control and PRS mice. Immunoblot data of GAD67 are normalized by β-actin protein level. Student’s t test; **P < .01 vs control mice; n = 6 per group. (C and D) The bar graphs show the mean numbers of 28-day-old BrdU+/NeuN+ cells in no drug-treated control and PRS mice or drug-treated control and PRS mice. Two-way ANOVA followed by Fisher’s protected least significant difference post hoc test; **P < .01 vs control mice and ##P < .01 vs PRS mice; n = 8 per group.
GAD67 Promoter DNMT1-Induced Hypermethylation Leads to GAD67 Expression Downregulation in Hippocampus of PRS Mice
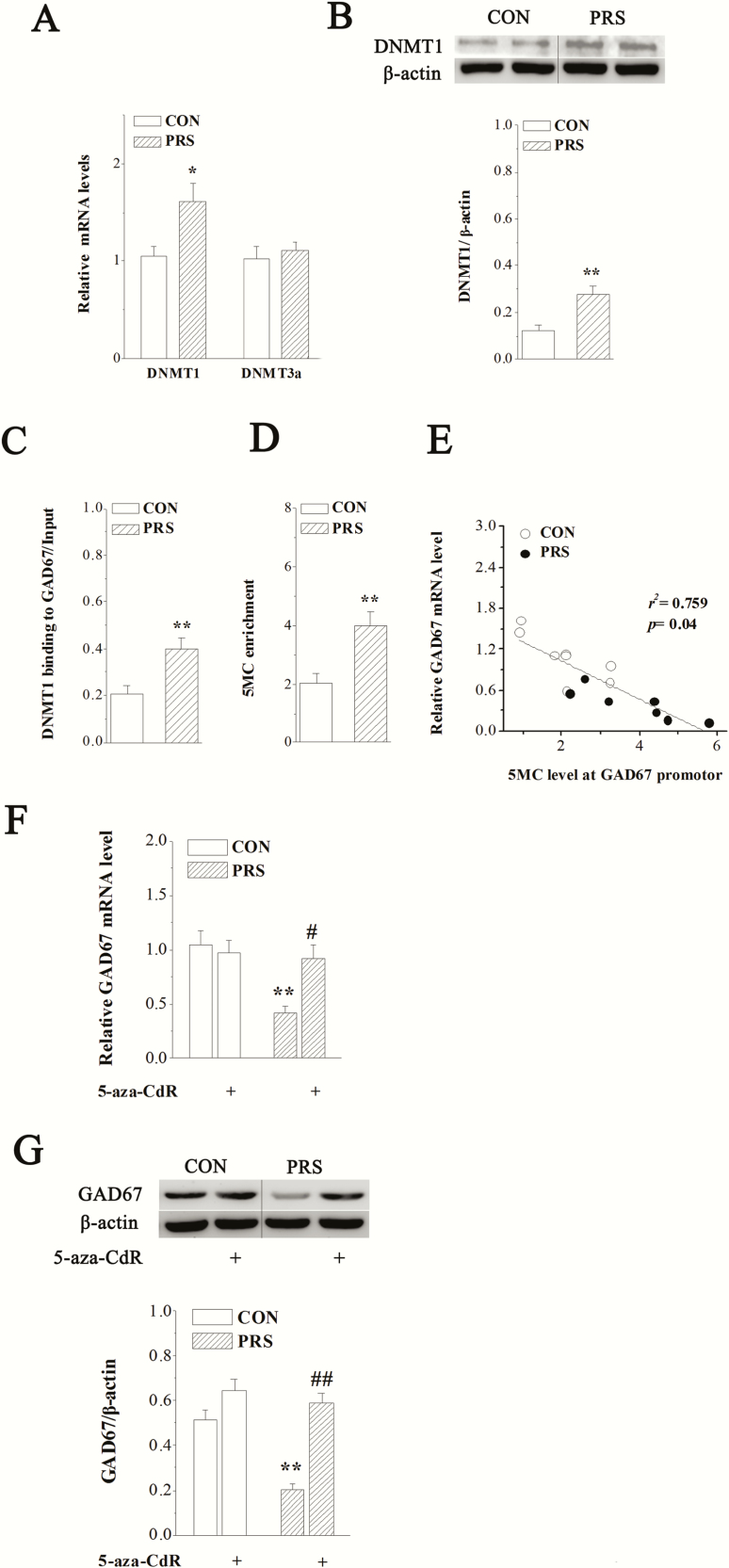
Earlier reports suggest that an increase in DNMT levels is associated with a downregulation of GAD67 in the postmortem brain tissues from patients with bipolar disorders (Guidotti et al., 2011) and in the brains of early postnatal stressed rats (Zhang et al., 2010). DNA epigenetic alterations were therefore investigated to determine the mechanisms underlying the detrimental effects of PRS on hippocampal GABAergic interneurons. The results of real-time RT-PCR in Figure 5A showed that there was no significant difference in DNMT3a mRNA expression between control and PRS mice (t(10) = 0.50, P > .05). However, the expression of DNMT1 mRNA was significant higher in the hippocampus of PRS mice compared with control mice (t(10) = 2.61, P < .05). Consistently, the excess expression of DNMT1 protein was also found in PRS mice (t(10) = 3.83, P < .01; Figure 5B). To test whether the overexpression of DNMT1 in PRS mice leads to the changes of binding of DNMT1 to GAD67 CpG-rich promoter, we measured (1) the binding of DNMT1 to GAD67 promoter and (2) the enrichment of 5MC at GAD67 promoter, which is an important epigenetic mark. The results showed that the binding of DNMT1 to GAD67 promoter region was increased in PRS mice (t(10) = 4.15, P < .01; Figure 5C). Consistently, 5MC was enriched at the GAD67 promoter in PRS mice compared with control mice (t(10) = 3.64, P < .01; Figure 5D). These findings suggest that PRS leads to CpG hypermethylation at the GAD67 promoter. To investigate whether DNMT1-induced hypermethylation at the GAD67 promoter leads to the downregulation of GAD67 expression in PRS mice, we analyzed the relation between 5MC level at GAD67 promoter and GAD67 transcript, and the effect of DNMT1 inhibitor 5-aza-CdR on GAD67 mRNA and protein expressions. The results showed that the enrichment of 5MC at the GAD67 promoter was negatively correlated with the level of the corresponding GAD67 transcript (r2 = 0.759, P = .04; Figure 5E). Additionally, the repeated i.c.v. injection of 5-aza-CdR abolished the alterations of GAD67 mRNA (P < .05; Figure 5F) and protein expressions (P < .01; Figure 5G) in PRS mice. Two-way ANOVA displayed main effects of group and drug treatment and their interaction on GAD67 mRNA (group: F[1,24] = 8.56, P < .01; drug treatment: F[1,24] = 5.53, P < .05; interaction: F[1,24] = 6.23, P < .05) and GAD67 protein (group: F[1,24] = 20.79, P < .01; drug treatment: F[1,24] = 41.59, P < .01; interaction: F[1,24] = 10.20, P < .01).
Figure 5.
The hypermethylation of GAD67 promoter induced by DNA methyltransferase 1 (DNMT1) overexpression is responsible for the downregulation of GAD67 in PRS mice. CON and PRS represent control and PRS mice, respectively. (A) The bar graph shows the mean level of DNMT1 or DNMT3a mRNA in control and PRS mice. Student’s t test; *P < .05 vs control mice; n = 6 per group. (B) Representative image of DNMT1 protein in the hippocampus of control and PRS mice (upper panel). The bars represent the mean level of DNMT1 protein in control and PRS mice. Immunoblot data of DNMT1 are normalized by β-actin protein level. Student’s t test; **P < .01 vs control mice; n = 6 per group. (C and D) The bar graphs show the mean data of binding of DNMT1 to GAD67 promoter and 5MC enrichment at GAD67 promoter region. Student’s t test; **P < .01 vs control mice; n = 6 per group. (E) The scatter plot shows the correlation between GAD67 mRNA level and 5-methylcytosine enrichment at GAD67 promoter region (n = 14). (F and G) The influence of 5-aza-CdR on hippocampal GAD67 expression. The bar graphs show the levels of GAD67 mRNA and protein in no drug-treated control and PRS mice or drug-treated control and PRS mice. Two-way ANOVA followed by Fisher’s protected least significant difference post hoc test; **P < .01 vs control mice; #P < .05 and ##P < .01 vs PRS mice; n = 6 per group.
5-aza-CdR Corrects Impaired Maturation of Hippocampal Newborn Neurons and Depression-Like Behaviors in PRS Mice via GABAergic System
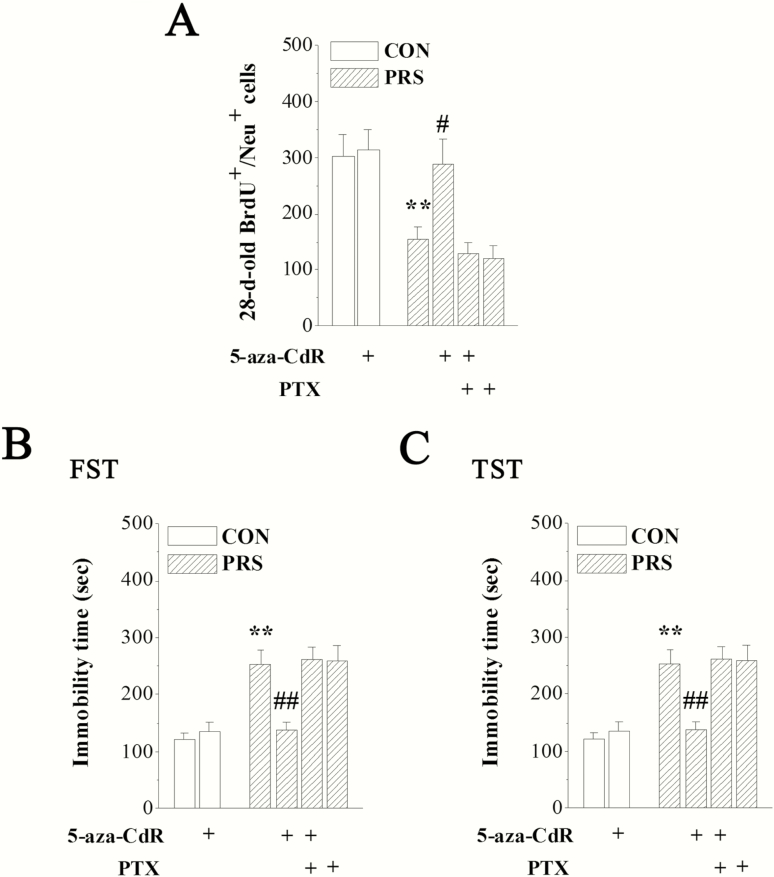
To test whether the impaired neuronal maturation and increased depression-like behaviors in PRS mice were mediated by DNA epigenetic modifications of hippocampal GABAergic interneurons, PRS mice and control mice received the i.c.v. administration of 5-aza-CdR alone or combined with PTX for 7 consecutive days after BrdU injection (Figure 1). Two-way ANOVA displayed main effects of group and drug treatment and their interaction on BrdU+/NeuN+ cells (group: F[1,48] = 6.97, P < .05; drug treatment: F[3,48] = 4.74, P < .01; interaction: F[1,48] = 3.56, P > .05), the immobility time in the FST (group: F[1,72] = 12.22, P < .01; drug treatment: F[3,72] = 6.28, P < .01; interaction: F[1,72] = 11.10, P < .01), and the immobility time in the TST (group: F[1,72] = 9.80, P < .01; drug treatment: F[3,72] = 4.13, P < .05; interaction: F[1,72] = 2.83, P > .05). The repeated i.c.v. treatment with 5-aza-CdR reversed the loss of 28-day-old BrdU+/NeuN+ cells in PRS mice (P < .05) without affecting that in control mice (P > .05; Figure 6A). Additionally, the prolonged immobility time in the FST or TST was also rectified by 5-aza-CdR in PRS mice (P < .01 in Figure 6B,C). Notably, the repeated i.c.v. treatment with PTX abolished the improvement effect of 5-aza-CdR on neurogenesis deficits or depression-like behaviors in PRS mice compared with PRS mice (P > .05).
Figure 6.
DNMT1 inhibitor 5-aza-deoxycytidine corrects the impaired maturation of hippocampal newborn neurons and depression-like behaviors in PRS mice through GABAergic system. CON and PRS represent control and PRS mice respectively. (A–C) The bar graphs show the mean data of BrdU+/NeuN+ cells in hippocampal DG and the immobility time in the FST and TST in no drug-treated control and PRS mice or drug-treated control and PRS mice. Two-way ANOVA followed by Fisher’s protected least significant difference post hoc test; **P < .01 vs control mice; #P < .05 and ##P < .01 vs PRS mice; n = 8 per group in (A) and n = 12 per group in (B and C).
Discussion
The present study provides, for the first time to our knowledge, evidence that PRS obviously inhibits maturation of newborn neurons in hippocampal DG in mice via DNMT1-related epigenetic reprogramming of GABAergic system, which causes depression-like behaviors. In addition, the present data also suggest the long-term neurobehavioral effects of PRS are reversible in the adult period.
Neurogenesis in the hippocampal DG region is mainly divided into 4 stages: proliferation of stem cells into neuronal phenotypes, differentiation of neuronal characteristics, neurite growth and migration of young neurons, and functional maturation of newborn neurons (Li et al., 2009; Aimone et al., 2014). In the immunostaining, the number of 24-hour-old BrdU+ cells, the number of 28-day-old BrdU+ cells, and the number of 28-day-old BrdU+/NeuN+ cells represents the proliferation of stem cells, the survival of new cells, and the maturation of differentiated nerve cells, respectively. It was found in the present study that there was no difference in the number of 24-hour-old BrdU+ cells and the number of 28-day-old BrdU+ cells between control and PRS offspring, but the number of 28-day-old BrdU+/NeuN+ cells significantly decreased in PRS offspring. The group of data indicates that the damage of PRS to hippocampal neurogenesis occurs in the stage of the differentiation/maturation of newborn neurons. Newborn neurons integrate into hippocampal circuits at 3 to 4 weeks after birth (Toni et al., 2008). Compared with mature synapses (Schmidt-Hieber et al., 2004), newborn synapses have lower activation thresholds and higher resting potentials. Newly formed synapses in adulthood are thought to be critical for hippocampal function and output (Ming and Song, 2011). Based on the reports about the adverse effect of stress on neurogenesis and the promoting effect of neurogenesis of a variety of antidepressants, the deficit in adult neurogenesis is considered as an important pathophysiological mechanism of mood disorders (Mirescu and Gould, 2006; Boldrini et al., 2009), and this viewpoint has been supported by the animal result that neurogenesis-deficient mice displayed a depressive phenotype in FST and TST (Snyder et al., 2011; Wang et al., 2015). Hence, it is conceivable that in PRS mice, the potentiation of depression-like behaviors is the consequence of the impaired differentiation/maturation of hippocampal newborn neurons.
There is increasing evidence that prenatal stress-induced DNA epigenetic changes play an important role in the complex phenotype of neuropsychiatric disorders (Matrisciano et al., 2012; Zheng et al., 2016; Papale et al., 2017). DNA epigenetic mechanisms are therefore considered as the potential targets through which PRS could exert long-lasting effects on adult hippocampal neurogenesis. DNMTs are the important components of DNA methylation that dynamically regulate the expression of key molecules involved in brain function. Here, we focused on DNMT1 and DNMT3a because they have been shown to be involved in the pathophysiological mechanisms of a variety of neurodevelopmental disorders, including depression. Our results showed that the expression of DNMT1 but not DNMT3a was found to be elevated in PRS mice. DNMT1 overexpression has been reported to account for the downregulation of GABAergic interneurons, which are the key regulator of hippocampal neurogenesis (Matrisciano et al., 2013). Due to the specific location of DNMT1 in GABA intermediate neurons and the enrichment of CpG in the promoter region of GAD67 gene, it is believed that the abnormality of DNMT1-mediated methylation regulation is responsible for the detected decrease of GAD67 mRNA and protein in the present study. To prove this possibility, relevant tests were performed. The results showed that the binding level of DNMT1 to GAD67 gene promoter region was significantly increased in the PRS mice. Consistently, there was significant methylation (high levels of 5mC) at the GAD67 promoter in PRS mice. The methylation level in the GAD67 promoter region was also found to be negatively correlated with GAD67 mRNA expression. Moreover, the repeated injection of DNMT1 inhibitor 5-aza-CdR could correct the decrease of GAD67 mRNA or protein expression in PRS mice. These data clearly indicate that the decrease of GAD67 expression is related to DNMT1-regulated hypermethylation of GAD67 promotor in PRS mice.
GABAergic signaling plays an essential regulatory role in adult hippocampus neurogenesis (Tozuka et al., 2005; Ge et al., 2006). It is therefore inferred that the altered neurogenesis in PRS mice is the consequence of DNMT1-induced epigenetic alterations of GABAergic neurons. Based on the reports that DNMT is positively correlated with the proliferative state of cells and significantly decreases in differentiated cells (Singer-Sam et al., 1990; Yen et al., 1992; Goto et al., 1994). DNA methylation has been thought to be in an unregulated static state after cell differentiation. Our present study and others have found that the high level of DNMT1 mRNA and protein is detected in the hippocampus, amygdala, cortex, and other brain regions of adult mice (Levenson et al., 2006; Kadriu et al., 2012). These results suggest a possibility that DNMT stasis is absent in the mature brain. Due to the high expression of DNMT1, it is possible to analyze the role of DNMT1-mediated GABAergic abnormality in adult hippocampus neurogenesis using pharmacological methods. In fact, we have found that the blockade of DNMT1 with 5-aza-CdR not only recovered the decrease of GAD67 expression but also corrects the impairment in maturation of hippocampal newborn neurons and depression-like behaviors in PRS mice. And the improvement effect of 5-aza-CdR on neurogenesis deficits or depression-like behaviors in PRS mice could be blocked by GABAAR antagonist PTX. These findings strongly suggest the involvement of epigenetic GABAergic dysfunction in PRS-induced alteration of hippocampus neurogenesis and related depression-like behaviors.
A report by Matrisciano has found the sustained overexpression of DNMT1 in the hippocampus of PRS mice from birth to adulthood (Matrisciano et al., 2013). Thus, it is speculated that the increase of DNMT1 in the hippocampus of adult PRS mice may be the result of embryonic changes. Further, maternal stress exposure during pregnancy was found to lead to the decreased maternal care towards their pups (Pardon et al., 2000). Previous studies suggest that the variations of maternal cares have a long-term adverse effect on the expression of emotion-related genes and emotional behaviors through DNA methylation epigenetic mechanisms (Weaver et al., 2006). Therefore, the change of maternal care could be another factor involved in DNMT1 upregulation in PRS mice.
Accumulating studies report that early-life stress leads to persistent alterations in neuroplasticity, increasing the vulnerability to mood disorders in later life (Meaney et al., 2007; Darnaudery and Maccari, 2008; Lupien et al., 2009). The current data suggest that PRS leads to the development of depression-like behaviors with hippocampal neurogenesis deficits by affecting DNMT1-mediated epigenetic regulation of GABAergic interneurons, providing further support for the above notion. We believe that PRS mice can be used as one of the representative animal models of depression induced by early adverse environment to illustrate the interaction between environment and related genes. Additionally, the present study indicates that a DNMT1 inhibitor is useful in correcting decreased GAD67 expression in GABAergic interneurons, impaired neurogenesis, and increased depression-like behaviors in PRS mice. This means that DNMT1 may represent possible new molecular targets to treat with the long-term neurobehavioral effects of developmental stress exposure.
Interest Statement:
The authors have declared that no conflict of interest exists.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81471385) and the Natural Science Foundation of Jiangsu Province of China (BK20151552).
References
- Abrous DN, Koehl M, Le Moal M (2005) Adult neurogenesis: from precursors to network and physiology. Physiol Rev 85:523–569. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH (2014) Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev 94:991–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso SJ, Damas C, Navarro E (2000) Behavioral despair in mice after prenatal stress. J Physiol Biochem 56:77–82. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V (2009) Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 34:2376–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfone A, Wang F, Hevner R, Anderson S, Cutforth T, Chen S, Meneses J, Pedersen R, Axel R, Rubenstein JL (1998) An olfactory sensory map develops in the absence of normal projection neurons or GABAergic interneurons. Neuron 21:1273–1282. [DOI] [PubMed] [Google Scholar]
- Costa E, Dong E, Grayson DR, Guidotti A, Ruzicka W, Veldic M (2007) Reviewing the role of DNA (cytosine-5) methyltransferase overexpression in the cortical GABAergic dysfunction associated with psychosis vulnerability. Epigenetics 2:29–36. [DOI] [PubMed] [Google Scholar]
- D’Adamo P, Wolfer DP, Kopp C, Tobler I, Toniolo D, Lipp HP (2004) Mice deficient for the synaptic vesicle protein Rab3a show impaired spatial reversal learning and increased explorative activity but none of the behavioral changes shown by mice deficient for the Rab3a regulator Gdi1. Eur J Neurosci 19:1895–1905. [DOI] [PubMed] [Google Scholar]
- Darnaudéry M, Maccari S (2008) Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev 57:571–585. [DOI] [PubMed] [Google Scholar]
- Dong E, Dzitoyeva SG, Matrisciano F, Tueting P, Grayson DR, Guidotti A (2015) Brain-derived neurotrophic factor epigenetic modifications associated with schizophrenia-like phenotype induced by prenatal stress in mice. Biol Psychiatry 77:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnheart JC, Schweizer C, Crestani F, Iwasato T, Itohara S, Mohler H, Lüscher B (2007) GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J Neurosci 27:3845–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M (2001) GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell 105:521–532. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H (2006) GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439:589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H (2007) GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci 30:1–8. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM (2000) Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci U S A 97:1823–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K, Numata M, Komura JI, Ono T, Bestor TH, Kondo H (1994) Expression of DNA methyltransferase gene in mature and immature neurons as well as proliferating cells in mice. Differentiation 56:39–44. [DOI] [PubMed] [Google Scholar]
- Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A, Costa E (2005) Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci U S A 102:9341–9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, Grayson DR, Matrisciano F, Pinna G, Satta R, Sharma RP, Tremolizzo L, Tueting P (2011) Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology 60:1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AS, Sahay A, Hen R (2015) Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology 40:2368–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadriu B, Guidotti A, Chen Y, Grayson DR (2012) DNA methyltransferases1 (DNMT1) and 3a (DNMT3a) colocalize with GAD67-positive neurons in the GAD67-GFP mouse brain. J Comp Neurol 520:1951–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN (2000) Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A 97:11032–11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Lamarque S, Le Moal M, Piazza PV, Abrous DN (2006) Postnatal stimulation of the pups counteracts prenatal stress-induced deficits in hippocampal neurogenesis. Biol Psychiatry 59:786–792. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, Malone LM, Sweatt JD (2006) Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem 281:15763–15773. [DOI] [PubMed] [Google Scholar]
- Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K, Halabisky B, Deng C, Mahley RW, Huang Y (2009) GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell 5:634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A (2005) Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci 8:1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–445. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Tueting P, Maccari S, Nicoletti F, Guidotti A (2012) Pharmacological activation of group-II metabotropic glutamate receptors corrects a schizophrenia-like phenotype induced by prenatal stress in mice. Neuropsychopharmacology 37:929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisciano F, Tueting P, Dalal I, Kadriu B, Grayson DR, Davis JM, Nicoletti F, Guidotti A (2013) Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology 68:184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M, Seckl JR (2007) Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med 13:269–277. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70:687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Gould E (2006) Stress and adult neurogenesis. Hippocampus 16:233–238. [DOI] [PubMed] [Google Scholar]
- Papale LA, Madrid A, Li S, Alisch RS (2017) Early-life stress links 5-hydroxymethylcytosine to anxiety-related behaviors. Epigenetics 12:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon M, Gérardin P, Joubert C, Pérez-Diaz F, Cohen-Salmon C (2000) Influence of prepartum chronic ultramild stress on maternal pup care behavior in mice. Biol Psychiatry 47:858–863. [DOI] [PubMed] [Google Scholar]
- Quadrato G, Elnaggar MY, Duman C, Sabino A, Forsberg K, Di Giovanni S (2014) Modulation of GABAA receptor signaling increases neurogenesis and suppresses anxiety through NFATc4. J Neurosci 34:8630–8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayen I, van den Hove DL, Prickaerts J, Steinbusch HW, Pawluski JL (2011) Fluoxetine during development reverses the effects of prenatal stress on depressive-like behavior and hippocampal neurogenesis in adolescence. Plos One 6:e24003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J (2004) Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429:184–187. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Rhodes ME, Bludau M, Kaplan S, Ong P, Ueffing I, Vehoff J, Korr H, Frye CA (2002) Depression: reduced number of granule cells in the hippocampus of female, but not male, rats due to prenatal restraint stress. Mol Psychiatry 7:810–813. [DOI] [PubMed] [Google Scholar]
- Sha S, Xu J, Lu ZH, Hong J, Qu WJ, Zhou JW, Chen L (2016) Lack of JWA enhances neurogenesis and long-term potentiation in hippocampal dentate gyrus leading to spatial cognitive potentiation. Mol Neurobiol 53:355–368. [DOI] [PubMed] [Google Scholar]
- Sickmann HM, Arentzen TS, Dyrby TB, Plath N, Kristensen MP (2015) Prenatal stress produces sex-specific changes in depression-like behavior in rats: implications for increased vulnerability in females. J Dev Orig Health Dis 6:462–474. [DOI] [PubMed] [Google Scholar]
- Singer-Sam J, Robinson MO, Bellvé AR, Simon MI, Riggs AD (1990) Measurement by quantitative PCR of changes in HPRT, PGK-1, PGK-2, APRT, MTase, and Zfy gene transcripts during mouse spermatogenesis. Nucleic Acids Res 18:1255–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA (2011) Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476:458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF (2008) Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci 11:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T (2005) GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 47:803–815. [DOI] [PubMed] [Google Scholar]
- Veldic M, Caruncho HJ, Liu WS, Davis J, Satta R, Grayson DR, Guidotti A, Costa E (2004) DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci U S A 101:348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Warner-Schmidt J, Varela S, Enikolopov G, Greengard P, Flajolet M (2015) Norbin ablation results in defective adult hippocampal neurogenesis and depressive-like behavior in mice. Proc Natl Acad Sci U S A 112:9745–9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Meaney MJ, Szyf M (2006) Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A 103:3480–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen RW, Vertino PM, Nelkin BD, Yu JJ, el-Deiry W, Cumaraswamy A, Lennon GG, Trask BJ, Celano P, Baylin SB (1992) Isolation and characterization of the cDNA encoding human DNA methyltransferase. Nucleic Acids Res 20:2287–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TY, Hellstrom IC, Bagot RC, Wen X, Diorio J, Meaney MJ (2010) Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. J Neurosci 30:13130–13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hong J, Zhang S, Zhang T, Sha S, Yang R, Qian Y, Chen L (2016) Postpartum estrogen withdrawal impairs hippocampal neurogenesis and causes depression- and anxiety-like behaviors in mice. Psychoneuroendocrinology 66:138–149. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Fan W, Zhang X, Dong E (2016) Gestational stress induces depressive-like and anxiety-like phenotypes through epigenetic regulation of BDNF expression in offspring hippocampus. Epigenetics 11:150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lu Z, Li L, Chen L, Qi J, Chen L (2014) Pro-neurogenesis and anti-dementia properties of tetradecyl 2,3-dihydroxybenzoate through TrkA receptor-mediated signalling pathways. Int J Neuropsychopharmacol 17:1847–1861. [DOI] [PubMed] [Google Scholar]
- Zhou R, Chen F, Chang F, Bai Y, Chen L (2013) Persistent overexpression of DNA methyltransferase 1 attenuating GABAergic inhibition in basolateral amygdala accounts for anxiety in rat offspring exposed perinatally to low-dose bisphenol A. J Psychiatr Res 47:1535–1544. [DOI] [PubMed] [Google Scholar]