Abstract
Background
Resistant bipolar disorder is a major mental health problem related to significant disability and overall cost. The aim of the current study was to perform a systematic review of the literature concerning (1) the definition of treatment resistance in bipolar disorder, (2) its clinical and (3) neurobiological correlates, and (4) the evidence-based treatment options for treatment-resistant bipolar disorder and for eventually developing guidelines for the treatment of this condition
Materials and Methods
The PRISMA method was used to identify all published papers relevant to the definition of treatment resistance in bipolar disorder and the associated evidence-based treatment options. The MEDLINE was searched to April 22, 2018.
Results
Criteria were developed for the identification of resistance in bipolar disorder concerning all phases. The search of the literature identified all published studies concerning treatment options. The data were classified according to strength, and separate guidelines regarding resistant acute mania, acute bipolar depression, and the maintenance phase were developed.
Discussion
The definition of resistance in bipolar disorder is by itself difficult due to the complexity of the clinical picture, course, and treatment options. The current guidelines are the first, to our knowledge, developed specifically for the treatment of resistant bipolar disorder patients, and they also include an operationalized definition of treatment resistance. They were based on a thorough and deep search of the literature and utilize as much as possible an evidence-based approach.
Keywords: treatment resistant, refractoriness, anticonvulsants, antidepressants, antipsychotics, bipolar disorder, evidence-based guidelines, lithium, mania, mood stabilizers, treatment
Introduction
Bipolar disorder (BD) has been described since the times of Hippocrates and Areteus, but recently the subtypes BD-I and BD-II were proposed with a combined prevalence rate of up to 2.4% (Merikangas et al., 2011). The treatment of BD is probably the most challenging of all mental disorders with the unique characteristic that each phase needs a different treatment approach (Vieta and Goikolea, 2005).
Such complex mental disorders are those that are expected to benefit more by the development of treatment guidelines, which during the last few decades are becoming an ever more important part of medical reality. This is especially since the translation of research findings to everyday clinical practice is becoming increasingly difficult with the accumulation of complex and often conflicting research findings, which are thereafter also included in meta-analysis. Guidelines aim to assist clinicians but also policymakers to arrive at decisions concerning the treatment and care of patients. They set the standard of care and training for health professionals, and they identify priority areas for further research since they are based primarily on the available evidence, but also, in areas where evidence is not available, on expert opinion.
The literature suggests that depression rather than mania is the most challenging phase (Manning, 2005). If subsyndromal, the presence of residual symptoms impose a greater risk of relapse (Keller et al., 1992), greater disability, and poorer overall outcome (Tohen et al., 1990a, 1990b, 2006). Therefore, full remission and recovery should be the ultimate treatment goal. A significant proportion of patients, however, do not fully respond to treatment, and their long-term course is characterized by frequent relapses and residual symptoms, causing significant disability and functional impairment (Esan et al., 2017).
To fulfil this need for expert translation of research findings into clinical practice and for the benefit of patients, the International College of Neuropsychopharmacology (CINP) launched an effort to critically appraise the literature and provide guidance to clinicians in the form of a treatment algorithm and guidelines as precise as the data allow. It is hoped that they will help the clinician to follow the state-of-the-art evidence, thus enabling their clinical practice to be based on informed decision-making process. They have been commissioned by the CINP, and the working group consisted of experts with extensive research and clinical experience in the field of BD. There was no funding from any source for the development of the guidelines and the activities of the working group.
All the members of the working group were psychiatrists who are in active clinical practice and were selected according to their expertise with the aim to cover a multitude of different cultures. All of them were involved in research and other academic activities, and therefore it is possible that through such activities some contributors have received income related to medicines discussed in this guideline. All conflicts of interest are mentioned at the end of this paper, which is the introductory paper to the CINP BD guidelines. It should also be noted that some drugs recommended in the guideline may not be available in all countries, and labeling and dosing might vary.
This project has already developed treatment algorithms and guidelines for BD (Fountoulakis et al., 2017a, 2017b, 2017c, 2017d), and the next step would be to search for data in the area of BD resistant to treatment.
Aim of the Current Study
The aim of the current study was to perform a PRISMA systematic review of the literature concerning (1) the definition of treatment resistance in BD, (2) its clinical and (3) neurobiological correlates, and (4) the evidence-based treatment options for treatment-resistant BD.
Materials and Methods
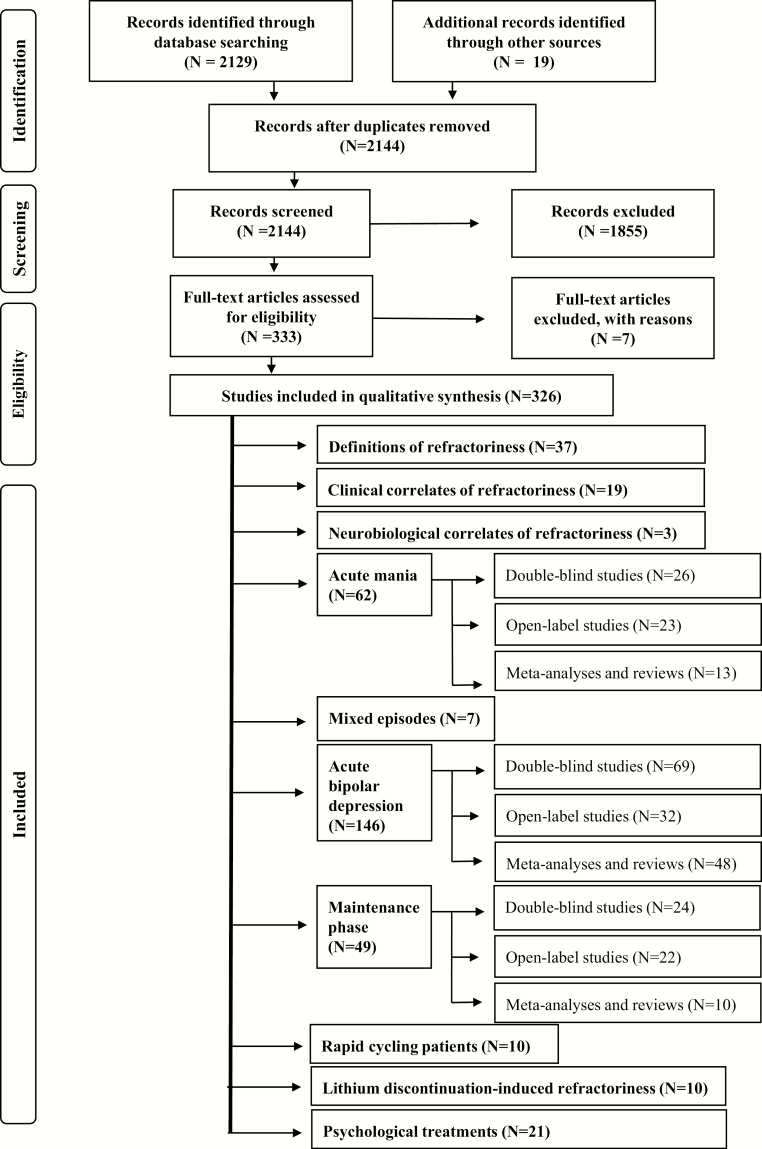
The PRISMA method (Hopewell et al., 2008; Liberati et al., 2009; Moher et al., 2009) was followed in the search of the literature. We searched MEDLINE to April 22, 2018 with the combination of keywords “refractory” or “refractoriness” or “resistant” with “mania,” “manic,” “bipolar,” “manic-depressive,” or “manic-depression.” The PRISMA flowchart is shown in Figure 1.
Figure 1.
The PRISMA flowchart.
The following inclusion criteria were utilized:
Papers in English language
Papers reporting specifically on BD, not on affective disorders in general. If the paper concerned affective disorders in general then there should be a specific elaboration on BD.
Concerning the definition of treatment resistance in BD: any paper that included a description or any kind of definition.
Papers considering resistant bipolar depression on the basis of failure of antidepressant treatment were not included. An exception was made for those specific antidepressants with a proven efficacy in the treatment of BD according to the CINP Bipolar Disorder Treatment Guidelines.
For clinical and neurobiological correlates we considered only papers with original data.
Concerning treatment options, all papers with original data, systematic reviews, and meta-analyses as well as post-hoc analyses were included.
Case reports and case series (including retrospective chart reviews) were not included (either referenced as such or according to the author’s judgement).
Relevant review papers were scanned to locate additional studies (Poon et al., 2012; Hui Poon et al., 2015; Fountoulakis et al., 2017a; Grunze et al., 2018). The data concerning the treatment of resistant BD were ranked according to the method previously developed by the authors (Fountoulakis et al., 2017d) and shown in Table 1.
Table 1.
Summary of the method for grading data and recommendation on the basis of both efficacy and safety/tolerability
| Grading on basis of efficacy | |
|---|---|
| Level 1 | Good research-based evidence, supported by at least 2 placebo controlled studies of sufficient magnitude and good quality. In case of the presence of negative RCTs, positive RCTs should outnumber negative ones |
| Level 2 | Fair research-based evidence, from 1 randomized, double-blind placebo controlled trial Also in case 1 or more trials exist, however, they fail to fulfil all the criteria above (e.g., very small sample size or no placebo control) as well as in case of positive meta-analysis alone |
| Level 3 | Some evidence from comparative studies without placebo arm or from post-hoc analyses |
| Level 4 | Inconclusive data or poor-quality RCTs |
| Level 5 | Negative data |
| Grading on the basis of safety and tolerability | |
| Level 1 | Very good tolerability, few side effects that are not enduring, do not cause significant distress, and are not life-threatening and do not compromise the overall somatic health of patient |
| Level 2 | Moderate tolerability, many side effects that could be enduring, and cause significant distress but are not life-threatening, although they could compromise the overall somatic health of the patient Agents with very good overall tolerability but with rare life-threatening adverse events, could be classified here only if the lethality risk can be essentially considered to be negligible with application of procedures and protocols (e.g., laboratory testing, titration schedules, etc.) |
| Level 3 | Poor tolerability, many side effects that are enduring, cause significant distress, compromise the overall somatic health of patient, or are life-threatening Agents with moderate overall tolerability and rare, life-threatening adverse events should be classified here even in case the lethality risk can be essentially considered to be negligible with the application of procedures and protocols (e.g., laboratory testing, titration schedules, etc.) |
| Recommendations for treatment (combination of efficacy and safety/tolerability) | |
| Level 1 | Level 1 or 2 for efficacy and 1 for safety/tolerability |
| Level 2 | Level 1 or 2 for efficacy and 2 for safety/tolerability |
| Level 3 | Level 3 for efficacy and 1 or 2 for safety/tolerability |
| Level 4 | Level 4 for efficacy or 3 for safety/tolerability |
| Level 5 | Level 5 for efficacy (not recommended) |
Abbreviation: RCT, randomized controlled trial.
Results
Definitions of Treatment Resistance in BD (n = 37)
There are several approaches to define “response,” “remission,” “recovery,” “relapse,” and “recurrence” in mental disorders. Although these definitions have been used in observational studies (Tohen et al., 1990a, 1990b, 2003a, 2003b), a common starting point is that these definitions apply to patients who received treatment with an adequate dosage of an effective treatment modality for a sufficient duration of time. Patients unable to tolerate such an adequate therapeutic trial for any reason as well as noncompliant patients are usually considered as “pseudorefractory” (Table 2).
Table 2.
Issues to be addressed in order to label a patient as “resistant”
| 1. Correct diagnosis |
| 2. Disorder not secondary to an organic disorder |
| 3. Poor response to treatment not due to somatic or mental comorbidity |
| 4. Poor response to treatment not due to a somatic condition that might not constitute a disorder by itself (e.g., genetic factors, smoking, alcohol use, gender, race, etc.) |
| 5. Failure of therapy not due to nontolerability |
| 6. Patient complies with recommended treatment and poor response not a consequence of lack of adherence |
Some authors suggest that the basis is an inadequate response to a therapeutic trial of lithium or an inability to tolerate lithium’s side effects (Barton and Gitlin, 1987; Aronson et al., 1989; Kramlinger and Post, 1989; Bauer, 1990; Pope, 1991; Schaff et al., 1993; McElroy et al., 1998; Altshuler et al., 1999; Calabrese et al., 1999; Green et al., 2000). Others consider a different definition on the basis of nonresponse to carbamazepine (Kramlinger and Post, 1989; Bauer, 1990; Schaff et al., 1993) or valproate (Calabrese et al., 1999), and their definition of failure also included intolerance. A more restrictive definition of treatment resistance demands failure to respond to at least 2 agents (McElroy et al., 1991; Kimmel et al., 1994; Calabrese et al., 1996), while other authors defined wider degrees of treatment nonresponse (tertiary resistance) (Vieta et al., 1998; Ciapparelli et al., 2000; Green et al., 2000; Suppes et al., 2003; Sajatovic et al., 2006).
The first comprehensive attempt defined treatment-resistant mania as mania without remission despite adequate therapy with at least 2 antimanic agents (lithium, antipsychotic, anticonvulsant, etc.) for at least 6 weeks on each agent in the absence of antidepressants or other mood-elevating agents (Sachs, 1996). For resistant bipolar depression, an extrapolation of the definition for unipolar to bipolar depression would be appropriate, which means no remission despite 2 adequate trials of standard antidepressant agents (6 weeks each), with or without augmentation strategies (Sachs, 1996). However, this definition is currently not supported by the data in a fundamental way since antidepressants are not considered to be efficacious in the treatment of bipolar depression, although they are among the most commonly used agents (Baek et al., 2014; Bjorklund et al., 2016; Kessing et al., 2016). Treatment-resistant mood cycling (resistance in the long term) was defined as continued cycling despite maximal tolerated lithium in combination with valproate or carbamazepine for a period of 3 times the average cycle length, or 6 months, whichever is longer, in the absence of antidepressants or other cycle-promoting agents (Sachs, 1996). This definition is not in accord with the data concerning the efficacious treatment of these conditions (Sachs, 1996).
The only attempt to grade treatment resistance of a stratified response/refractoriness graduation suggested the clustering of resistant cases into 3 groups: (1) primary resistance, that is, inadequate response to a therapeutic trial of lithium (>0.7 mmol/L), valproate, or carbamazepine; (2) secondary resistance, that is, inadequate response to sequential therapeutic trials of 2 mood stabilizers or an antipsychotic and a mood stabilizer; and (3) tertiary resistance, that is, inadequate response to sequential therapeutic trials of 3 agents: any antipsychotics, 2 mood stabilizers and an antipsychotic, 2 antipsychotics and a mood stabilizer, or 3 mood stabilizers (Keck and McElroy, 2001).
Another definition was based on the assumption that lithium at serum levels of 0.8 mmol/L or greater for at least 6 weeks should be the first line of treatment for bipolar depression if a patient is not already on a mood stabilizer and if lithium fails, the addition of lamotrigine, carbamazepine, valproate, antidepressants (not tricyclic antidepressants), or an atypical antipsychotic such as olanzapine might be a reasonable second-line options (Yatham et al., 2003a; Tohen et al., 2004; Taylor et al., 2014). This is in partial agreement with the data currently available. In the STEP-BD study, treatment-resistant depression was defined as no response to treatment after 12 weeks of treatment or a well-documented failure to respond to at least 2 trials of antidepressants or an antidepressant and a mood stabilizer (Nierenberg et al., 2006).
A more sophisticated 4-level system for the staging of resistance from resistance to a single agent to resistant to 3 agents plus neurostimulation has been proposed, but its clinical relevance is unclear since it puts the emphasis on the number of treatment options used but ignores other important details, including duration. It includes the following stages: Stage I: failed monotherapy trial of lithium, anticonvulsant, or atypical antipsychotic of adequate dose and for adequate duration (FDA-approved treatment based on mood episode); Stage II: Stage I plus failed trial of combination of 2 medications, lithium, or anticonvulsant and atypical antipsychotic; Stage III: Stage II plus failed trial of several different evidence-based adjunctive pharmacological compounds; and Stage IV: Stage III plus neurostimulation (Gajwani, 2009). Resistant BD-I or BD-II depression was defined in a stepped way as failure to reach remission with adequately dosed lithium (0.8 mEq/L) or to other adequate ongoing mood-stabilizing treatment, plus lamotrigine (50–200 mg/d) or with full dose (600 mg/d or more) of quetiapine as monotherapy (Pacchiarotti et al., 2009)
To date, the International Society for Bipolar Disorders nomenclature and definitions are the most comprehensive and up to date and utilize both a syndromal (on the basis of DSM criteria) and symptomatic (on the basis of rating scales) approach. These definitions recommend the use of incremental steps for symptom improvement (<25%, 25–49%, 50–74%, 75–100%) to define response. They propose multiple cut-off points for the definition of remission, with the most stringent being <6 for Hamilton Depression Rating Scale (HDRS)-17 and Montgomery-Asberg Depression Rating Scale (MADRS) and <5 for the Young Mania Rating Scale (YMRS) in the case of depression and mania, respectively. These stringent criteria allow for inclusion of subsyndromal states that are very important for failing functional recovery in BD (7–14 in HDRS or MADRS and 8–14 in YMRS). In essence, the definition of subsyndromal states is utilized also for the definition of Treatment Emergent Affective Switch. Non-criterion symptoms that are commonly associated with BD (usually during the depressive phase) such as anxiety, panic attacks, irritability, hopelessness, avoidance, or cognitive dysfunction should not be included in the definitions. These authors defined “recovery” as sustained remission after at least 8 weeks (Tohen et al., 2009), which is similar to the approach of the AMA (American Psychiatric Association, 2000). A modified version was proposed several years later (Fountoulakis, 2012).
Another proposal was that treatment-resistant BD should be conceptualized as failure to respond to at least 2 trials of dissimilar medication with presumably adequate doses and durations within a specific phase of bipolar illness (manic, depressive, or mixed) or for “breakthrough”; symptoms. These symptoms should emerge despite previous apparently effective maintenance treatment. There do not refer to patients who are intolerant (In table 4, the grading of treatment options according to safety issues is shown) of a treatment regimen and because of this they refrain from treatment and also to the extent possible, they do not refer to those patients who are not adherent to recommended treatment (Poon et al., 2012).
Τhe most recent attempt was to define treatment resistance in terms of failure to reach sustained symptomatic remission for 8 consecutive weeks after 2 different treatment trials, at adequate therapeutic doses, with at least 2 recommended monotherapy treatments or at least 1 monotherapy treatment and another combination treatment. It also introduced the term “multi-treatment-resistant BD” after failure of additional trials with at least 1 trial with an antidepressant, a psychological treatment, and a course of electroconvulsive therapy (ECT) (Hidalgo-Mazzei et al., 2019).
Considering the above, the International Society for Bipolar Disorders definition is a good starting point, but the authors suggest that additional considerations are necessary. The key points the therapist needs to consider before suggesting a patient might be treatment-resistant are summarized in Table 2. The proposed CINP definitions of response, remission, recovery, and resistance for BD are shown in Table 3. According to the authors of the current paper, a key issue is that nonresponse should be considered only after treatment according to the best evidence available. At this time, the options recommended by the CINP guidelines for BD (Fountoulakis et al., 2017a, 2017b, 2017c, 2017d) and the CANMAT Guidelines (Yatham et al., 2018) provide the most up-to-date and comprehensive overview of treatment options.
Table 3.
The CINP definitions of response, remission, recovery, and resistance for BD
| Phase | Scale scores | Treatment duration according to CINP guidelines | |
|---|---|---|---|
| Response | Acute mania | <25%, 25–49%, 50–74%, 75–100% reduction in YMRS or MRS scores No significant increase in MADRS or HDRS scores and MADRS and HDRS scores stay below 6 | 8–10 weeks |
| Acute Bipolar depression | <25%, 25–49%, 50–74%, 75–100% reduction in MADRS or HDRS scores No significant increase in YMRS or MRS scores and YMRS and MRS scores stay below 5 | 10–12 weeks | |
| Maintenance | Significant change in the frequency of episodes | 1 year | |
| Remission | Acute mania | YMRS and MRS scores stay below 5 No significant increase in MADRS or HDRS scores and MADRS and HDRS scores stay below 6 | 8 weeks |
| Acute Bipolar depression | MADRS and HDRS scores stay below 6 No significant increase in YMRS or MRS scores and YMRS and MRS scores stay below 5 | 8 weeks | |
| Maintenance | Very rare new episodes, and MADRS/HDRS scores <6 and YMRS/MRS scores <7 between episodes | 2–3 years? | |
| Recovery | Acute mania | YMRS and MRS scores stay below 5 No significant increase in MADRS or HDRS scores and MADRS and HDRS scores stay below 6 | 8 weeks |
| Acute Bipolar depression | MADRS and HDRS scores stay below 6 No significant increase in YMRS or MRS scores and YMRS and MRS scores stay below 5 | 8 weeks | |
| Maintenance | No new mood episodes and MADRS/HDRS scores <6 and YMRS/MRS scores <7 between episodes | 3–5 years | |
| Resistance | Acute mania | No significant reduction in YMRS or MRS scores, or significant increase in MADRS or HDRS scores or MADRS and HDRS scores exceed 6 | 8–10 weeks |
| Acute Bipolar depression | No significant reduction in in MADRS or HDRS scores or significant increase in YMRS or MRS scores or YMRS and MRS scores exceed 5 | 10–12 weeks | |
| Maintenance | No change in the frequency of episodes, or MADRS/HDRS scores >6 or YMRS/MRS scores >7 between episodes | 1 year |
Abbreviations: BD, bipolar disorder; HDRS, Hamilton Depression Rating Scale; MADRS, Mondgomery-Asberg Depression Rating Scale; MRS, Mania Rating Scale; YMRS, Young Mania Rating Scale.
Clinical Correlates of Treatment Resistance (n = 19)
A number of studies suggest that some kind of progression occurs over the long-term course of the illness, and this, in turn, contributes to treatment resistance (Berk et al., 2011b; Fries et al., 2012) with an association to number of mood episodes (Swann et al., 1999; Obrocea et al., 2002; Scott et al., 2006; Reinares et al., 2010; Berk et al., 2011a; da Costa et al., 2016) and hospitalizations (Nunez et al., 2018). This progression is not uniform (Swann et al., 1999; Scott et al., 2006).
Clinical characteristics of treatment-resistant patients are the frequent presence of rapid cycling (37%), other forms of cycling (32%), chronic depression (26%), mixed states (6%) (Cole et al., 1993), and anxiety (Wooderson et al., 2014; Parker and Graham, 2017; Nunez et al., 2018). Other features include melancholia, comorbidity with social phobia, current suicidal risk and severe intensity of current depressive episode (Mendlewicz et al., 2010), cognitive difficulties, and sleep disturbance (Kessler et al., 2013; Wooderson et al., 2014). However, some studies failed to identify such clinical markers of resistance (Vo and Dunner, 2003; Amsterdam et al., 2016a; Sandu et al., 2017)
Other features that might distinguish between resistant and nonresistant BD include being female, older, older age at illness onset, a higher incidences of family depression, lower likelihood of being gainfully employed, a higher number of lifetime stressors, medical conditions, a different personality and temperament profile, and more regular use of benzodiazepines (Parker and Graham, 2017). It is believed that substance use and unhealthy lifestyle might contribute to treatment resistance, although data are lacking (Schaffer et al., 2017).
It is important that those clinical variables with supposed value in predicting response or resistance to lithium treatment and including the presence of elation, grandiosity, paranoia, irritability, delusions, and hallucinations did not predict treatment response to lithium (Miller et al., 1991).
Overall, the data are of low quality and it is unknown whether the clinical picture is useful in predicting treatment response.
Neurobiological Correlates of Treatment Resistance (n = 3)
Only 3 studies reported on possible neurobiological correlations of treatment resistance and identified family history of affective disorder (Cole et al., 1993; Parker and Graham, 2017) and electroencephalographic abnormalities (Cole et al., 1993) as risk factors, while neuroinflammation mechanisms seem to promote the progression towards treatment resistance (Bauer et al., 2017)
Treatment of Resistant BD
Resistant Acute Mania (n = 62)
Double Blind Studies in the Treatment of Resistant Acute Mania (n = 26)
Valproate monotherapy (serum levels 50 and 100 mg/L), in 36 lithium-resistant manic patients (including patients who could not tolerate lithium), produced a 54% decrease in scores on the YMRS in the valproate arm vs a 5% decrease in the placebo arm (Pope et al., 1991)
In 1 underpowered study, 13 BD-I and 15 BD-II patients received 900–4800 mg/d gabapentin, lamotrigine, or placebo in a crossover design for 6 weeks, and at endpoint there was no difference between the 3 arms (Frye et al., 2000). In another study, 114 BD-I outpatients resistant to lithium, valproate, or their combination were randomized to adjunctive gabapentin (600–3600 mg/d) or placebo for up to 10 weeks, with placebo performing better (Pande et al., 2000).
There are positive data on add-on phenytoin in patients resistant to haloperidol treatment (Mishory et al., 2000) and of add-on 600–1200 mg/d carbamazepine or oxcarbazepine in patients resistant to lithium (Juruena et al., 2009). The second study was of poor quality. A study adding lovastatin to lithium was negative (Ghanizadeh et al., 2014).
In patients resistant to lithium, valproate, or carbamazepine, it is beneficial to add olanzapine, quetiapine, aripiprazole, or asenapine (Tohen et al., 2002; Sachs et al., 2004; Yatham et al., 2007b; Vieta et al., 2008; Szegedi et al., 2012) but not ziprasidone, topiramate, or paliperidone (Roy Chengappa et al., 2006; Berwaerts et al., 2011; Sachs et al., 2012a, 2012b). For patients resistant to both lithium and carbamazepine monotherapy, their combination was reported to be beneficial (at dosages corresponding to lithium levels 0.7–1.2 mmol/L and up to 1600 mg/d of carbamazepine) (Kramlinger and Post, 1989).
One study provided inconclusive data for risperidone (Yatham et al., 2003b) as the results were likely confounded by the effects of carbamazepine on serum levels of risperidone.
There was only 1 sham-controlled trial of ECT as adjunctive treatment to chlorpromazine (600 mg/d) in 30 acutely manic patients and supported the efficacy of ECT with a faster rate of improvement (Sikdar et al., 1994).
One placebo-controlled 4-week random controlled trial in 180 acutely manic patients supported the efficacy and safety of allopurinol (600 mg/d) and dipyridamole (200 mg/d) as adjunctive to lithium (Machado-Vieira et al., 2008). However, a previous study was negative in patients resistant to lithium, valproic acid, carbamazepine, or atypical antipsychotic medications (Fan et al., 2012). Findings for valproate are inconclusive (Fan et al., 2012; Jahangard et al., 2014).
Folic acid was reported useful as an adjunct to valproate; however, the study is problematic concerning its methods of analysis and the reporting of results (Behzadi et al., 2009). Valnoctamide (the valproic acid precursor) plus risperidone combination was more effective than risperidone plus placebo (Bersudsky et al., 2010), while a pilot 8-week study in 21 acutely manic outpatients on the usefulness of adjunctive ramelteon states failed (McElroy et al., 2011) and another 2 on donepezil were negative (Eden Evins et al., 2006; Chen et al., 2013).
Open Label Studies in the Treatment of Resistant Acute Mania (n = 23)
Treating resistant manic patients with olanzapine 5–40 mg/d monotherapy resulted in remission in three-quarters of patients (McElroy et al., 1998; Chen et al., 2011), and several small studies support the efficacy of up to 550 mg/d clozapine with three-quarters of the patients responding after prolonged treatment (Kimmel et al., 1994; Calabrese et al., 1996; Ciapparelli et al., 2000; Green et al., 2000). The combination of aripiprazole with clozapine in BD patients that failed to respond to other atypical antipsychotics returned a positive result in psychotic manic patients (Benedetti et al., 2010). Results concerning risperidone are equivocal (Sajatovic et al., 1996; Vieta et al., 1998)
Adding gabapentin or pregabalin (600–3600 mg/d) resulted in response in three-quarters of resistant manic patients (McElroy et al., 1997; Altshuler et al., 1999; Schaffer et al., 2013). Only one-quarter of rapid cycling patients responded (Altshuler et al., 1999). Adding 100–300 mg/d topiramate resulted in a response rate of almost 60% (Chengappa et al., 1999; Vieta et al., 2002b) but titrating up to 1300 mg/d did not increase the response (Calabrese et al., 2001). Adding leviracetam (500–1000 mg/d) produced response or remission in one-half of patients (Post et al., 2005); data concerning verapamil (up to 240 mg/d) were negative (Barton and Gitlin, 1987) while for nifedipine 120 mg/d were unimpressive (De Beaurepaire, 1992). Some positive findings exist for diltiazem (Silverstone and Birkett, 2000). Adding l-thyroxine to lithium or carbamazepine resulted in a 50% response rate (Bauer and Whybrow, 1990).
Three-quarters of resistant manic patients respond to ECT (Perugi et al., 2017) with equal efficacy for unilateral vs bilateral ECT (Mukherjee et al., 1988), and bifrontal ECT was as efficacious as bitemporal ECT and better tolerated (Barekatain et al., 2008; Hiremani et al., 2008).
Meta-Analytic and Review Studies in the Treatment of Resistant Acute Mania (n = 13)
Meta-analytic studies also suggest that combination treatment is superior to monotherapy at the cost of more frequent adverse events; however, these meta-analyses do not distinguish between add-on studies (which utilize patients resistant to monotherapy) and combination studies (which utilize general patient populations) (Scherk et al., 2007; Smith et al., 2007; Tarr et al., 2011; Ogawa et al., 2014). Due to the insufficient evidence base, earlier reviews provide only vague conclusions (Gitlin, 2001, 2006; Keck and McElroy, 2001). Overall, there is a striking paucity of research concerning resistant cases, and existing studies are small and insufficiently controlled and findings remain preliminary. ECT is generally considered as an option for resistant patients. In acute manic patients who are partial responders to lithium/valproate/carbamazepine, adding an antipsychotic is a reasonable choice. Encouraging results have been reported by adding aripiprazole, clozapine, and pregabalin in resistant mania (Hui Poon et al., 2015). The analysis of the data for the cholinesterase inhibitors galantamine and donepezil as well as the glutamate receptor antagonist memantine was negative (Veronese et al., 2016). One meta-analysis of combination studies confirmed the higher rate of adverse events compared with monotherapy (Galling et al., 2015). One review of open studies supported the usefulness of clozapine (Li et al., 2015).
There are a few randomized controlled trials of ECT in mania, and they consistently report clinically meaningful efficacy, with a majority of pharmacotherapy-resistant patients responding to ECT. Evidence for the use of other brain stimulation therapies in treating bipolar mood states is preliminary and limited (Loo et al., 2011; Versiani et al., 2011).
Conclusion Concerning the Treatment of Resistant Acute Mania
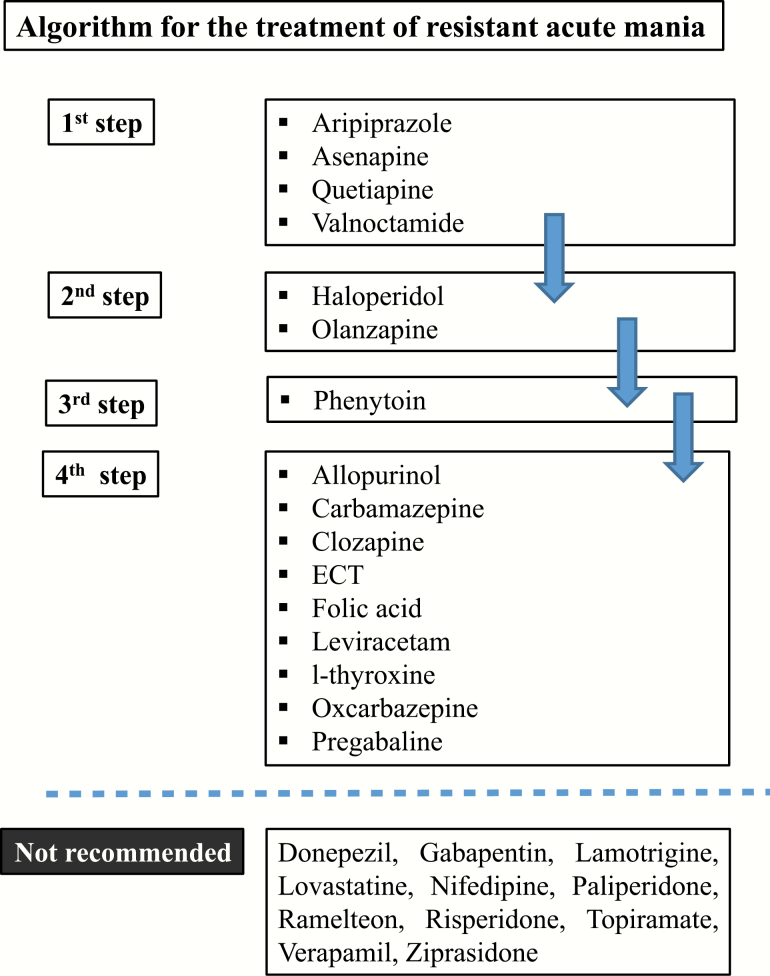
Controlled data suggest that in patients resistant (principally) to lithium, valproate, or carbamazepine, it is beneficial to add aripiprazole, asenapine, folic acid, quetiapine, or valnoctamide. The next choice should be adding haloperidol, olanzapine, or phenytoin on lithium, valproate, or carbamazepine. The data are inconclusive concerning allopurinol, carbamazepine, clozapine, ECT, leviracetam, l-thyroxine, oxcarbazepine, and pregabalin.
According to controlled data, the agents not recommended include donepezil, gabapentin, lamotrigine, lovastatin, paliperidone, ramelteon, risperidone, topiramate, and ziprasidone, while additionally, on the basis of open data, nifedipine and verapamil are also not recommended (Table 5; Figure 2).
Table 5.
Levels of recommendation concerning adjunctive treatment for resistant acute mania and recommended dosages for medication options
| Treatment modality to add | Grading | ||
|---|---|---|---|
| In terms of efficacy | In terms of recommendation | Dosage | |
| Aripiprazole | 2 | 1 | Up to 30 mg/d |
| Asenapine | 2 | 1 | Up to 20 mg/d |
| Quetiapine | 1 | 1 | Up to 800 mg/d |
| Valnoctamide | 2 | 1 | 1200 mg/d |
| Haloperidol | 2 | 2 | Up to 12 mg/d |
| Olanzapine | 2 | 2 | Up to 40 mg/d |
| Phenytoin | 3 | 3 | 400 mg/d |
| Allopurinol | 4 | 4 | 600 mg/d |
| Carbamazepine | 4 | 4 | Up to 1200 mg/d |
| Clozapine | 4 | 4 | Up to 550 mg/d |
| ECT | 4 | 4 | – |
| Folic acid | 4 | 4 | 3 mg/d |
| Leviracetam | 4 | 4 | 2000–3000 mg/d |
| l-Thyroxine | 4 | 4 | Until FT4 higher than upper limit |
| Oxcarbazepine | 4 | 4 | Up to 1200 mg/d |
| Pregabaline | 4 | 4 | 75–150 mg/d |
| Donepezil | NR | NR | |
| Gabapentin | NR | NR | |
| Lamotrigine | NR | NR | |
| Lovastatin | NR | NR | |
| Nifedipine | NR | NR | |
| Paliperidone | NR | NR | |
| Ramelteon | NR | NR | |
| Risperidone | NR | NR | |
| Topiramate | NR | NR | |
| Verapamil | NR | NR | |
| Ziprasidone | NR | NR |
Abbreviations: ECT, electroconvulsive therapy; FT4, free-T4; NR, not recommended.
Figure 2.
Algorithm for the treatment of resistant acute mania.
Table 4.
Grading of treatment options according to safety issues following system shown in Table 1
| Agent/modality | Grade | Comments |
|---|---|---|
| Agomelatine | 2 | Elevation of liver enzymes |
| Allopurinol | 2 | Swelling of mouth and lips, severe skin rashes, infections, eye irritation, hepatitis, appetite and weight loss, and painful or bloody urination |
| Amitriptyline | 2 | Many adverse effects, some risk for cardiovascular events |
| Aripiprazole | 1 | |
| Armodafinil/modafinil | 2 | Stimulant, risk for abuse |
| Asenapine | 1 | |
| Bupropion | 1 | |
| Carbamazepine | 2 | Hepatic enzymes induction, many adverse effects |
| Cariprazine | 1 | |
| Celecoxib | 1 | |
| Choline | Food supplement | |
| Chromium | Food supplement | |
| Clozapine | 3 | Potentially lethal agranulocytosis, metabolic syndrome |
| DBS | 1 | |
| Diltiazem | 1 | |
| Donepezil | 1 | |
| ECT | 2 | Not preferred by patients, mild cognitive problems |
| Folic acid | Food supplement | |
| Gabapentin | 1 | |
| Galantamine | ||
| Haloperidol | 2 | Extra-pyramidal symptoms, tardive dyskinesia, neuroleptic malignant syndrome, switch risk |
| Imipramine | 2 | Cardiac side effects, many adverse effects, switch risk |
| Inositol | ||
| Ketamine | 3 | Transient dissociation and elevation of blood pressure |
| Lamotrigine | 2 | Good overall tolerability but potentially lethal skin reaction that can be avoided by slow titration |
| Levetiracetam | 3 | Induction of suicidality |
| Light therapy | ||
| Lisdexamfetamine | 3 | High risk for abuse and dependence |
| Lithium | 2 | Many adverse effects, weight gain, toxicity |
| Lovastatin | ||
| L-sulpiride | 1 | |
| l-thyroxine | 2 | Mild cardiovascular, skin and bone adverse effects |
| Lurasidone | 1 | |
| Magnesium | Food supplement | |
| Memantine | 1 | |
| Modafinil | 2 | Stimulant, risk for abuse |
| n-3 fatty acids | Food supplement | |
| N-acetyl cysteine | 1 | |
| Nimodipine | 1 | |
| Olanzapine | 2 | Metabolic syndrome |
| Omega 3 fatty acids | 1 | |
| Oxcarbazepine | 1 | |
| Paliperidone | 1 | |
| Paroxetine | 1 | Weight gain |
| Phenytoin | 2 | Many adverse effects |
| Pioglitazone | 2 | Not recommended in patients with diabetes mellitus type I and in liver disease Absolute contraindication in heart failure patients |
| Pramipexole | 2 | Adverse effects include the induction of compulsive behaviors and psychotic symptoms |
| Pregabaline | 2 | Risk of abuse, weight gain |
| Pregnenolone | 2 | Not well studied |
| Primidone | ||
| Quetiapine | 1 | Weight gain |
| Ramelteon | 1 | |
| Risperidone/RLAI | 1 | Increased prolactin, weight gain |
| S-adenosyl-L-methione | 2 | Some adverse effects; long-term effects unknown |
| Sleep deprivation | 1 | |
| TMS | 1 | |
| Topiramate | 3 | Induction of depression and suicidality |
| Tranylcypromine | 2 | Many adverse effects |
| Tryptophan | Food supplement | |
| Valnoctamide | 1 | |
| Valproate | 1 | Cautious use in women of childbearing age |
| Venlafaxine | 2 | Switch risk |
| Verapamil | 1 | |
| Ziprasidone | 2 | QTc prolongation, patient ECG recommended when used in combination |
Abbreviations: DBS, deep brain stimulation; ECG, electrocardiogram; ECT, electroconvulsive therapy; EPS, extrapyramidal signs; RLAI, risperidon long acting injection; TMS, transcranial magnetic stimulation.
Mixed Episodes (n = 7)
Adding olanzapine or placebo to divalproex-resistant mixed patients for 6 weeks returned positive results both for the manic as well as for the depressive component (Houston et al., 2009).
One open study of adjunct gabapentin (300–2000 mg/d) reported an almost 50% response rate but this concerned exclusively the depressive component (Perugi et al., 1999).
There were no randomized trials of ECT in mixed episodes (Loo et al., 2011). The results of open trials concerning the usefulness of ECT suggest that approximately 40% to two-thirds of patients responded and 30% remitted, and the response concerned both the manic and the depressive components (Medda et al., 2010, 2015; Perugi et al., 2017). One study concerning repetitive transcranial stimulation (rTMS) reported a lower response rate (around 40%) and response restricted to the depressive component (Pallanti et al., 2014).
Treatment-Resistant BD (n = 145)
Double Blind Studies in the Treatment of Resistant Acute BD (n = 69)
In bipolar depressed patients who experience depression while under lithium treatment, it is appropriate to add lamotrigine (van der Loos et al., 2009, 2010, 2011), the D2 antagonist L-sulpiride (Bocchetta et al., 1993), pramipexole (Goldberg et al., 2004), or possibly oxcarbazepine (Juruena et al., 2009) but not imipramine (Nemeroff et al., 2001). The data on adding paroxetine and amitriptyline are equivocal (Bocchetta et al., 1993; Bauer et al., 1999; Young et al., 2000; Pilhatsch et al., 2010; van der Loos et al., 2010). Imipramine and venlafaxine might pose the patients at an increased risk of switching without a superior benefit compared with other antidepressants (Nemeroff et al., 2001; Vieta et al., 2002a); however, 1 study suggests quite the opposite (Amsterdam et al., 2016b). One study suggested that the addition of lamotrigine to quetiapine treatment improved outcomes, but folic acid seems to nullify the effect of lamotrigine (Geddes et al., 2016).
In BD patients experiencing depression during treatment with lithium or valproate, ketamine or lurasidone could be added. Lurasidone also improves anxiety (Loebel et al., 2014) and ketamine improves suicidality. Response to a single ketamine infusion may appear within hours but does not last more than 3 to 4 days (Diazgranados et al., 2010; Zarate et al., 2012; Lally et al., 2014; Loebel et al., 2014; Xu et al., 2015). One study reported negative results (Xu et al., 2015). Besides reducing suicidality, ketamine has been reported to be efficacious especially against anhedonia (Lally et al., 2014) and fatigue (Saligan et al., 2016). Repeated administration of ketamine studies have not been carried in BD (Murrough et al., 2013; Phillips et al., 2019). There is 1 failed study with lurasidone as add-on to lithium or valproate (Suppes et al., 2013, 2016).
An underpowered placebo-controlled adjunctive study of aripiprazole to lithium and citalopram was negative (Quante et al., 2010).
For a depressed episode during treatment with mood stabilizers, it is not beneficial to add ziprasidone (Sachs et al., 2011; Patkar et al., 2015). Topiramate and levetiracetam should be avoided because of a risk of worsening depression and inducing suicidality (CIT0371). Imipramine and venlafaxine increased the risk of switching without superior benefits compared with other antidepressants (Sachs et al., 1994, 2011; Post et al., 2001, 2006; Shelton and Stahl, 2004; Schaffer et al., 2006; Altshuler et al., 2009; Saricicek et al., 2011).
The data are negative concerning the addition of memantine on lamotrigine (Anand et al., 2012) or valproate (Lee et al., 2014a, 2014b), ketamine on ECT (Abdallah et al., 2012), lisdexamfetamine to treatment as usual (TAU) (McElroy et al., 2015), and agomelatine to lithium or valproate (Yatham et al., 2016).
A study in 85 patients with adjunctive modafinil (mean dosage 177 mg/d) was positive without switching to mania or hypomania. Response and remission rates were higher in the modafinil group (44% and 39%) compared with the placebo group (23% and 18%) (Frye et al., 2007). However, modafinil could cause subclinical switches (Fountoulakis et al., 2008b). One published study for the treatment of acute BD-I depression with adjunct armodafinil (dosage 150 mg/d; n = 128) to lithium, valproate, or olanzapine was positive (Calabrese et al., 2010, 2014). However, 2 other studies were negative (Ostacher, 2014; Ketter et al., 2015).
One small study on pioglitazone as add-on to lithium in bipolar patients without diabetes mellitus was positive (Zeinoddini et al., 2015). A trial of celecoxib (400 mg/d) was negative in the treatment of depressive or mixed episodes (Nery et al., 2008). One study with add-on pregnenolone (titrated to 500 mg/d) was negative (Brown et al., 2014). While a very small report without an a priori defined primary outcome suggested that adding supraphysiologic doses of levothyroxine (L-T4) to a mood stabilizer improves the outcome (Bauer et al., 2016), a previous, more complete report on the same placebo-controlled dataset was negative (Stamm et al., 2014). That study reported positive findings in females but not in males. A small quasi-placebo-controlled study was positive concerning the addition of inositol (Chengappa et al., 2000).
A 24-week trial on the efficacy of N-acetyl cysteine (NAC, 1 g twice daily) adjunctive to usual medication in subsyndromal but treatment-resistant depressive symptoms was positive (Berk et al., 2008); however, a more recent study was negative (Berk et al., 2019a, 2019b). The data on omega-3 fatty acids are conflicting and inconclusive (Stoll et al., 1999; Frangou et al., 2006, 2007; Keck et al., 2006; Murphy et al., 2012). One negative study exists concerning S-adenosyl-L-methione up to 1400 mg/d (Murphy et al., 2014).
ECT may be more effective than pharmacotherapy for treatment-resistant bipolar depression (Schoeyen et al., 2015) but TMS is poorly investigated in bipolar depression (Dell’Osso et al., 2009). Active deep TMS was superior to sham at end point (P = .03) but not at follow-up (Tavares et al., 2017). One study on transcranial direct current stimulation reported that the cumulative response rates were higher in the active vs sham groups (67.6% vs 30.4%; P = .01) but not remission rates (37.4% vs 19.1%; P = .18) (Sampaio-Junior et al., 2018).
Sleep deprivation and other noninvasive circadian-related interventions could be useful add-on treatments to accelerate and sustain the antidepressant response (Wu et al., 2009). A study on bright light therapy in bipolar depression was negative (Dauphinais et al., 2012), whereas other controlled studies were positive (Sit et al., 2018; Yorguner Kupeli et al., 2018; Zhou et al., 2018).
Open Label Studies in Treatment of Resistant Acute Bipolar Depression (n = 32)
One study reported a 31% response rate with add-on levetiracetam titrated to a target dose of 2000 mg/d (Post et al., 2005). Another study supported the adjunctive therapy with diltiazem (Silverstone and Birkett, 2000).
Adding bupropion to ongoing treatment after 4 weeks yielded a 60% response (Erfurth et al., 2002) while adding tranylcypromine in imipramine-resistant patients resulted in a 75% response (Thase et al., 1992).
Resistant depressive patients from the STEP-BD trial were randomly assigned to open-label adjunctive treatment with lamotrigine, inositol, or risperidone for up to 16 weeks without any significant between-group differences. However, the recovery rate with lamotrigine was 23.8% vs 17.4% with inositol and 4.6% with risperidone (Nierenberg et al., 2006). Another STEP-BD subgroup received adjunctive aripiprazole, but the response rate was as low as 27% (Ketter et al., 2006).
Adjunctive gabapentin for 12 weeks (mean dose 1725 mg/d) resulted in a 55% response (Wang et al., 2002) and for 8 weeks with mean dosage 1270 ± 561 mg in a 42% response rate (Perugi et al., 2002). A third very small trial (n = 5) reported that all patients responded to adjunctive gabapentin (Altshuler et al., 1999).
Adding lamotrigine 75–100 mg/d resulted in an approximately 50–70% response (Kusumakar and Yatham, 1997; Nierenberg et al., 2006; Kagawa et al., 2014).
Adding levetiracetam up to 3000 mg/d resulted in 31% remission (Post et al., 2005). Approximately 23% of patients from the Stanley Foundation Bipolar Network long-term follow-up study responded after adding 8.7 mg/d of tiagabine (Suppes et al., 2002) and one-half of patients responded to topiramate (Vieta et al., 2002b). Two-thirds of patients responded to 0.95 mg/d pramipexole (Lattanzi et al., 2002).
There were 3 positive papers (2 on the same dataset) on the utilization of a single infusion of ketamine (0.5 mg/kg) over 40 min resulting in a 50% response (Rybakowski et al., 2013, 2017; Ionescu et al., 2015). One small study was positive for omega-3 fatty acids (Chiu et al., 2005).
More than one-half of patients and two-thirds of those with BD-I are reported to respond to ECT (Medda et al., 2009, 2010; Perugi et al., 2012, 2017; Schoeyen et al., 2015) while one-fourth manifest remission (Medda et al., 2010). One study, however, reported similar remission rates with an algorithm-based pharmacological treatment (34.8% vs 30.0%) (Schoeyen et al., 2015). Another study with nonconvulsive electrotherapy reported 73% response and 55% remission rates (Regenold et al., 2015).
Early rTMS studies reported a 60% response rate (Dell’Osso et al., 2009; Wozniak-Kwasniewska et al., 2015). One study of sequential bilateral rTMS vs sham treatment was negative with both arms having a 10% response rate (Fitzgerald et al., 2016), but another with high-frequency repetitive transcranial magnetic stimulation reported 35% response and 30% remission rates (Poleszczyk et al., 2018).
The results with deep brain stimulation in a very small study were rather poor (<20% acute response and remission rates) (Holtzheimer et al., 2012), and similarly a study with deep (H1-coil) transcranial magnetic stimulation (deep TMS) vs sham was superior at week-4 in HDRS but not at follow-up at week-8. There was no difference in response and remission rates (Tavares et al., 2017). An earlier H1-Coil rTMS study reported that two-thirds of patients responded and one-half of them remitted (Harel et al., 2011).
Total sleep deprivation plus light therapy for 1 week resulted in a 44% response in resistant patients (Benedetti et al., 2005).
Post-hoc, Review, and Meta-Analytic Studies (n = 48)
The problems in the literature concerning the treatment of resistant bipolar depression are described in several reviews and meta-analytical studies. The overall conclusion is that the available hard data are extremely scarce and most of the strategies remain essentially experimental; however, there seem to be some that are potentially efficacious and promising (Aan Het Rot et al., 2012; Poon et al., 2012; Sienaert et al., 2013; Hui Poon et al., 2015). In addition, combination studies confirmed the higher rate of adverse events compared with monotherapy (Galling et al., 2015).
Existing papers suggest minimal effects of lamotrigine, risperidone, inositol (Parikh et al., 2010), or lurasidone (Sanford and Dhillon, 2015) and that the addition of an antidepressant does not increase efficacy (Parikh et al., 2010; Van Lieshout and MacQueen, 2010). The combination with best data in resistant acute bipolar depression is lithium plus lamotrigine (Fountoulakis et al., 2012c). Antidepressant reviews provide conflicting conclusions (Gijsman et al., 2004; Sidor and Macqueen, 2011; Vazquez et al., 2013; Zhang et al., 2013); 1 meta-analysis was negative regarding the usefulness of galantamine, donepezil, and memantine (Veronese et al., 2016) while another concluded that second-generation antidepressants produced a significant but small score change but had no effect in response and remission rates. There was also no increased risk of treatment-emergent mania or hypomania during the acute phase while there was some risk in the long term (McGirr et al., 2016a).
Some studies support the efficacy of stimulants, especially modafinil and armodafinil (Corp et al., 2014), ketamine (Corp et al., 2014; Fond et al., 2014; McGirr et al., 2015), and antiinflammatory agents (Rosenblat et al., 2016). A meta-analytic study supported the efficacy of dopaminergic drugs (Szmulewicz et al., 2017). The review of pramipexole data suggested that two-thirds of patients respond (Dell’Osso and Ketter, 2013; Tondo et al., 2014).
The meta-analysis of ketamine studies supported its efficacy but also suggested the data are conflicting as to whether the therapeutic effect extends beyond day 4 and up to day 7 (Sienaert et al., 2013; Caddy et al., 2014; Fond and Boyer, 2014; Fond et al., 2014; Tondo et al., 2014; Coyle and Laws, 2015; Lee et al., 2015; McGirr et al., 2015; Newport et al., 2015; Parsaik et al., 2015; Romeo et al., 2015; Bobo et al., 2016; Kishimoto et al., 2016; Saligan et al., 2016; Xu et al., 2016; Kraus et al., 2017).
One meta-analysis was positive concerning the usefulness of light therapy (Tseng et al., 2016).
Reviews and meta-analyses are positive concerning omega-fatty acids, but they do not include all trials (Sarris et al., 2012; Sylvia et al., 2013; Grosso et al., 2014; Ciappolino et al., 2017). Another meta-analysis was negative for adjunctive inositol (Mukai et al., 2014) and another one supported the usefulness of clozapine (Li et al., 2015).
There are no studies with adequate methodology on ECT (Loo et al., 2011; Versiani et al., 2011). One meta-analysis compared the efficacy of ECT in unipolar vs bipolar depression and identified 6 relevant studies. It reported a similar rate of response in both disorders (50.9% vs 53.2%) (Dierckx et al., 2012).
A meta-analysis of rTMS in bipolar depression, although based overall on a smaller number of participants, supported efficacy (McGirr et al., 2016b).
One post-hoc study pooled the data from transcranial direct current stimulation (transcranial direct current stimulation) trials and reported significant positive results (D’Urso et al., 2017) while a review suggested that Vagus nerve stimulation is promising for bipolar depression (Cimpianu et al., 2017). Another review focused on surgical interventions but without any conclusion (Lipsman et al., 2010).
Conclusion Concerning the Treatment of Resistant Acute Bipolar Depression
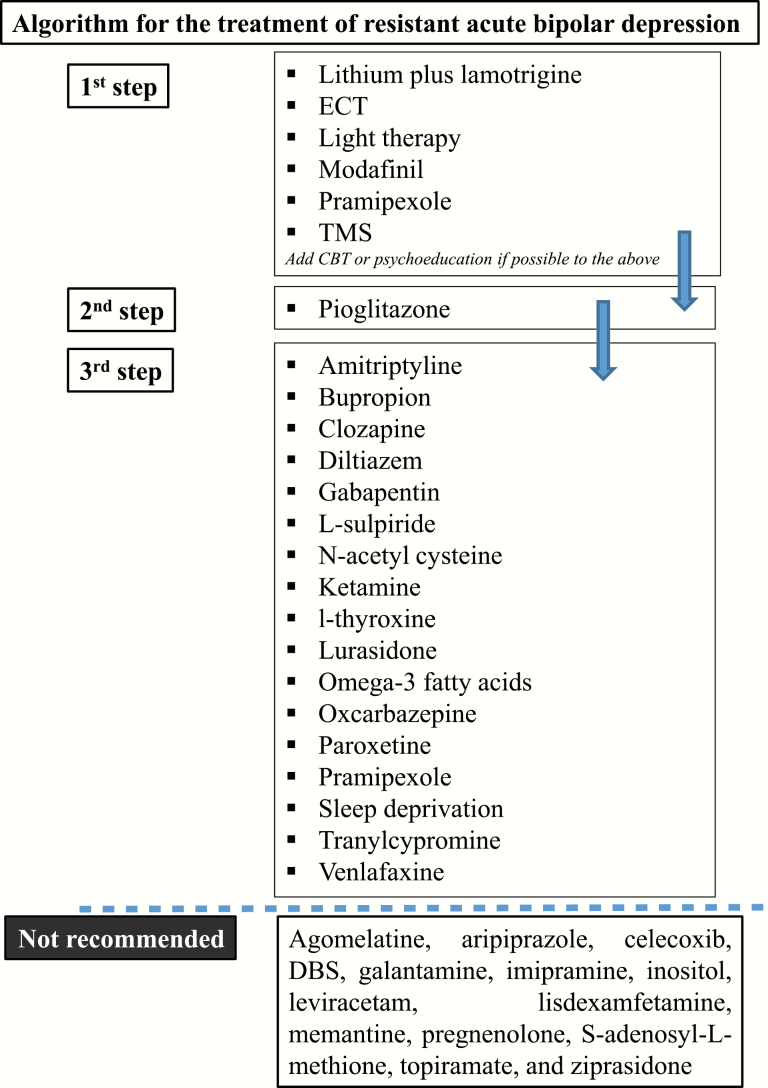
Controlled data suggest that in resistant bipolar depressive patients, it is beneficial to use lithium plus lamotrigine or adding lamotrigine, modafinil, or pramipexole. Ketamine is also another option but carries the risk of transient dissociation and increased blood pressure. The data are inconclusive concerning L-sulpiride, amitriptyline, bupropion, clozapine, diltiazem, ECT, gabapentin, l-thyroxine, lurasidone, NAC, omega-3 fatty acids, oxcarbazepine, paroxetine, pramipexole, sleep deprivation, TMS, tranylcypromine, and venlafaxine.
According to either controlled or open data, the interventions not recommended include agomelatine, aripiprazole, celecoxib, deep brain stimulation, galantamine, imipramine, inositol, leviracetam, lisdexamfetamine, memantine, pregnenolone, S-adenosyl-L-methione, topiramate, and ziprasidone (Table 6; Figure 3).
Table 6.
Levels of recommendation concerning adjunctive treatment for resistant acute bipolar depression and recommended dosages for medication options
| Treatment modality to add | Grading | ||
|---|---|---|---|
| In terms of efficacy | In terms of recommendation | Dosage | |
| Lamotrigine (on lithium) | 1 | 2 | Up to 200 mg/d |
| Light therapy | 1 | 1 | |
| ECT | 2 | 2 | |
| Modafinil | 2 | 2 | Up to 200 mg/d |
| Pramipexole | 2 | 2 | Up to 2.5 mg/d |
| TMS | 2 | 2 | |
| Pioglitazone | 3 | 3 | 30 mg/d |
| Amitriptyline | 4 | 4 | Up to 150 mg/d |
| Bupropion | 4 | 4 | Up to 375 mg/d |
| Clozapine | 4 | 4 | Up to 600 mg/d |
| Diltiazem | 4 | 4 | Up to 240 mg/d |
| Gabapentin | 4 | 4 | 600–2400 mg/d |
| L-sulpiride | 4 | 4 | 50–75 mg/d |
| N-acetyl cysteine | 4 | 4 | 2000 mg/d |
| Ketamine | 1 | 4 | Intravenous infusion 0.5 mg/kg |
| l-Thyroxine | 4 | 4 | 300 mcg/d |
| Lurasidone | 4 | 4 | Up to 120 mg/d |
| Omega-3 fatty acids | 4 | 4 | Various |
| Oxcarbazepine | 4 | 4 | Up to 1200 mg/d |
| Paroxetine | 4 | 4 | Up to 40 mg/d |
| Pramipexole | 4 | 4 | 3 mg/d |
| Sleep deprivation | 4 | 4 | – |
| Tranylcypromine | 4 | 4 | 30–60 mg/d |
| Venlafaxine | 4 | 4 | 75–225 mg/d |
| Agomelatine | NR | NR | |
| Aripiprazole | NR | NR | |
| Celecoxib | NR | NR | |
| DBS | NR | NR | |
| Galantamine | NR | NR | |
| Imipramine | NR | NR | |
| Inositol | NR | NR | |
| Leviracetam | NR | NR | |
| Lisdexamfetamine | NR | NR | |
| Memantine | NR | NR | |
| Pregnenolone | NR | NR | |
| S-adenosyl-L-methione | NR | NR | |
| Topiramate | NR | NR | |
| Ziprasidone | NR | NR |
Abbreviations: DBS, deep brain stimulation; ECT, electroconvulsive therapy; NR, not recommended; TMS, transcranial magnetic stimulation.
Figure 3.
Algorithm for the treatment of resistant acute bipolar depression.
BD Resistant to Maintenance Treatment (n = 49)
Double-Blind Studies in Maintenance Treatment of Resistant BD (n = 24)
A small study supported the adding of phenytoin to TAU (Mishory et al., 2003) as did another small one for gabapentin (but not on top of antipsychotics) (Vieta et al., 2006). One study suggested a beneficial effect of clozapine on a small subsample of nonpsychotic BD (Suppes et al., 1999). Data are equivocal for the addition of lamotrigine to lithium (van der Loos et al., 2011) and negative for adjunctive pramipexole to TAU in stabilized BD patients with the aim to improve neurocognition (Burdick et al., 2012). Two studies suggest that Risperidone Long Acting Injectable (RLAI) on TAU significantly prolongs the time to relapse (Macfadden et al., 2009; Quiroz et al., 2010), as did adding aripiprazole (Marcus et al., 2011) or ziprasidone (Citrome, 2010) to lithium or valproate. Adding aripiprazole to lamotrigine did not improve long-term outcome (Carlson et al., 2012). Patients who responded to treatment with lithium, valproate, or carbamazepine plus antidepressants were more likely to maintain response with continuation of the combined treatment; however, those patients who manifested only a partial acute response were unlikely to further improve when the same treatment was continued (Altshuler et al., 2009). Adjunctive asenapine to lithium or valproate was well tolerated for up to 52 weeks, but no efficacy data were reported from that trial due to lack of statistical power (Szegedi et al., 2012).
One trial in 75 BD patients reported that NAC treatment caused a significant improvement on the MADRS score compared with placebo (P = .002) during maintenance. There was no effect of NAC on time to a mood episode and no significant between-group differences in adverse events (Berk et al., 2008). Another study in 14 BD-II patients from the previous study reported a superiority of the NAC group vs placebo in terms of remission (P = .031) (Magalhaes et al., 2011a). Data were conflicting concerning ramelteon (Norris et al., 2013; Mahableshwarkar et al., 2017) and negative results for memantine in patients on valproate treatment (Lee et al., 2014b).
There are some studies suggesting that there is a role for various nutritional supplements such as n-3 fatty acids (eicosapentaenoic acid and docosahexaenoic acid), chromium, choline, magnesium, and tryptophan alone or in combination with pharmacotherapies for the treatment of BD, but the data are of low quality (Sylvia et al., 2013).
Open-Label Studies in Maintenance Treatment of Resistant BD (n = 22)
Open studies support the usefulness of adding clozapine (Suppes et al., 1999; Ciapparelli et al., 2000, 2003), gabapentin (Schaffer and Schaffer, 1999), lamotrigine (Calabrese et al., 1999), levetiacetam (Post et al., 2005), RLAI (Yatham et al., 2007a), and the anticonvulsant primidone (Schaffer et al., 1999) with approximately 30–40% of patients responding, while a higher than 40% response rate was reported for olanzapine (McElroy et al., 1998; Vieta et al., 2001), higher than 50% with L-thyroxine T(4) (Bauer et al., 2002), and higher than 70% for the N-methyl-D-aspartate antagonist memantine (Koukopoulos et al., 2010).
One-third of participants responded well to the combination of lithium plus valproate (Denicoff et al., 1997), but the BALANCE study neither supported nor refuted the superiority of the combination of lithium plus valproate over monotherapy (Geddes et al., 2002; Rendell et al., 2004; Investigators et al., 2010). Results were somewhat positive also for the combination of carbamazepine plus nimodipine, but negative for adding the calcium channel blocker verapamil (Pazzaglia et al., 1998). For overweight patients, adding topiramate (McElroy et al., 2000; Lykouras and Hatzimanolis, 2004; Gabriel, 2007)or zonisamide (Wang et al., 2008) could be an option. However, mood destabilization was observed in another study with the addition of zonisamide.
Using maintenance ECT for more than 18 months, with a treatment at approximately monthly intervals, resulted in an up to 80% response rate (Vanelle et al., 1994).
Post-hoc, Review, and Meta-Analytic Studies (n = 10)
Meta-analyses support the efficacy of antidepressants added to mood stabilizers in the long-term treatment of bipolar patients without increasing risk of new manic/hypomanic episodes (Liu et al., 2017). Ziprasidone plus lithium or valproate treatment showed modest to moderate remission rates at week 24 based on 4 different remission criteria in terms of symptomatic and sustained remission (Pae et al., 2012).
The addition of an atypical antipsychotic-antimanic agent in some BD patients might help to reduce suicidal ideation (Houston et al., 2006). Efficacy of NAC was also supported by 2 post-hoc analyses (Magalhaes et al., 2011b, 2013), which, however did not include more recent negative data. Two other studies supported the usefulness of RLAI (Bobo and Shelton, 2010) for the maintenance treatment of BD-I disorder in adults as an adjunct to lithium or valproate. One paper supported the usefulness of the dopamine agonist pramipexole (Dell’Osso and Ketter, 2013). Overall, the review papers support the usefulness of ECT (Vaidya et al., 2003; Fountoulakis et al., 2012c), the combination of antiepileptics with antipsychotics (Fountoulakis et al., 2012c), and clozapine (Li et al., 2015) for the maintenance treatment of resistant patients.
Conclusion Concerning Treatment for Resistant Patients During Maintenance Phase
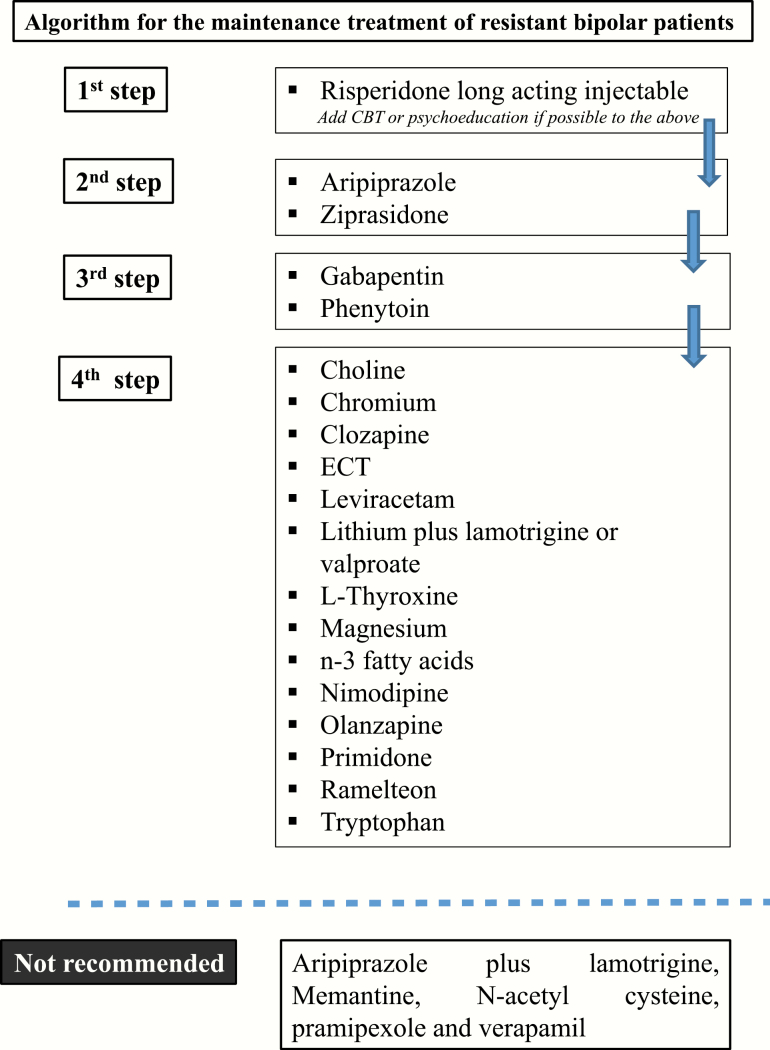
Controlled data suggest that in resistant patients (principally to lithium, valproate, or carbamazepine), it is beneficial to add antidepressants, RLAI, aripiprazole, or ziprasidone to the ongoing treatment. The next choice should be adding gabapentin or phenytoin. The data are inconclusive concerning clozapine, ECT, leviracetam, lithium plus lamotrigine or valproate, L-thyroxine, the calcium blocker nimodipine, olanzapine, primidone, ramelteon, and the food supplements choline, chromium, magnesium, n-3 fatty acids, and tryptophan.
According to either controlled or open data, the nonrecommended agents include memantine, NAC, pramipexole, and verapamil. Aripiprazole plus lamotrigine was also not effective (Table 7; Figure 4).
Table 7.
Levels of recommendation concerning adjunctive treatment for resistant patients during maintenance phase and recommended dosages for medication options
| Treatment modality to add | Grading | ||
|---|---|---|---|
| In terms of efficacy | In terms of recommendation | Dosage | |
| RLAI | 1 | 1 | Up to 100 mg/mo |
| Aripiprazole | 2 | 2 | Up to 30 mg/d |
| Ziprasidone | 2 | 2 | Up to 160 mg/d |
| Gabapentin | 3 | 3 | Up to >2500 mg/d |
| Phenytoin | 3 | 3 | 380 mg/d |
| Choline | 4 | 4 | – |
| Chromium | 4 | 4 | – |
| Clozapine | 4 | 4 | Up to 600 mg/d |
| ECT | 4 | 4 | – |
| Leviracetam | 4 | 4 | Up to 3000 mg/d |
| Lithium plus lamotrigine or valproate | 4 | 4 | Usual recommended dosages |
| L-Thyroxine | 4 | 4 | 500 μg/d |
| Magnesium | 4 | 4 | – |
| n-3 fatty acids | 4 | 4 | – |
| Nimodipine | 4 | 4 | Up to 360 mg/d |
| Olanzapine | 4 | 4 | Up to 30 mg/d |
| Primidone | 4 | 4 | Up to 250 mg/d |
| Ramelteon | 4 | 4 | 8 mg/d |
| Tryptophan | 4 | 4 | |
| Aripiprazole plus lamotrigine | NR | NR | |
| Memantine | NR | NR | |
| N-acetyl cysteine | NR | NR | |
| Pramipexole | NR | NR | |
| Verapamil | NR | NR |
Abbreviations: DBS, deep brain stimulation; ECT, electroconvulsive therapy; NR, not recommended; TMS, transcranial magnetic stimulation.
Figure 4.
Algorithm for the treatment of resistant cases of bipolar disorder (BD) during the maintenance phase.
It is important to note that the scarcity of the data does not permit a differential choice between agents and treatment options to prevent the relapse into a manic or depressive episode preferentially.
Resistant Rapid Cycling Cases (n = 10)
The data on resistant patients with rapid cycling course are very few. Overall, they suggest that the combination of lithium plus divalproex for up to 16 weeks leads to only 14% stabilization with no additional value of adjunct lamotrigine (Kemp et al., 2012). One small trial of clorgyline 2.5–10.0 mg/d, alone or in combination with lithium carbonate, was positive (Potter et al., 1982), while clozapine was found to be less efficacious in resistant rapid cycling patients (Suppes et al., 2004; Li et al., 2015). On the contrary, lamotrigine as an add-on therapy exerted a similar effect in rapid and non-rapid cycling patients (Bowden et al., 1999). A small cross-over, double blind study was positive for adjunctive nimodipine (Pazzaglia et al., 1993).
Adding mexiletine 200–1200 mg/d led to 46% remission and 15% partial response (Schaffer et al., 2000) while levothyroxine improved only depressive symptoms (Bauer and Whybrow, 1990). Vagus nerve stimulation was associated with a 38.1% mean improvement in overall illness over a 12-month study period (Marangell et al., 2008). Adding chromium resulted in an acute response in one-third of patients, but only regarding depression. The high drop-out rate made it impossible to test for maintenance efficacy (Amann et al., 2007).
Lithium Discontinuation-Induced Treatment Resistance (n = 10)
Treatment resistance possibly induced by lithium discontinuation was suggested for the first time by Post et al following a systematic life-chart methodology in the study of 4 patients with BD in whom long periods (6–15 years) of effective lithium prophylaxis were followed by relapses on lithium discontinuation. Once the drug was reinstituted, it was no longer effective (Post et al., 1992). However, the reason for discontinuation in these 4 cases was not reported, and the discussion of these cases clearly leaves room for a progression of the illness rather than a specific lithium-related cause as the most probable explanation.
Case reports and selected reviews support this (Post et al., 1993; Bauer, 1994; Murray, 1994; Koukopoulos et al., 1995; Maj et al., 1995; Tondo et al., 1997; Post and Leverich, 2008; Post, 2012) but the arguments are scientifically weak.
The only existing systematic review and meta-analysis of the literature identified the existence of 212 patient data relevant to this question and the meta-analysis returned negative results, suggesting there is no convincing evidence that lithium is less effective when treatment is discontinued and restarted compared with uninterrupted treatment (de Vries et al., 2013).
Psychological Treatments (n = 21)
There are some but overall limited data of problematic quality concerning the usefulness of specific adjunctive psychotherapies (Reinares et al., 2014; Miziou et al., 2015).
Although the overall data for the long-term efficacy of cognitive-behavioral therapy (CBT) either as monotherapy or as add on to TAU are negative concerning relapse prevention, there are some positive results for the acute depressive phase in BD (Ball et al., 2006; Scott et al., 2006; Zaretsky et al., 2008; Costa et al., 2011; Gomes et al., 2011; Meyer and Hautzinger, 2012; Gonzalez Isasi et al., 2014). The effectiveness of psychotherapy for resistant patients was reported to increase with time, and this improvement was not significant until 12 months of follow-up (Gonzalez-Isasi et al., 2010, 2012; Isasi et al., 2010). A post-hoc analysis suggested that CBT could be more effective than TAU in patients with less than 12 previous episodes but less effective in those with more episodes (Scott et al., 2006). In BD patients with insomnia, CBT for insomnia was superior to psychoeducation concerning manic relapses (Harvey et al., 2015).
The data on adjunctive psychoeducation suggest that compared with TAU or nonspecific intervention, it prevents relapse to both poles if administered to patients in clinical remission (Perry et al., 1999; Colom et al., 2003; Colom et al., 2009; Lobban et al., 2010; de Barros Pellegrinelli et al., 2013), but it has no effect on biological rhythms (Cardoso Tde et al., 2015). Again, a post-hoc analysis suggested that patients with more than 7 episodes did not show significant improvement with group psychoeducation for time to recurrence, and those with more than 14 episodes did not benefit from the treatment in terms of time spent ill (Colom et al., 2010). A systematic review confirmed the above (Bond and Anderson, 2015).
There are no data to support the usefulness of interpersonal and social rhythm therapy, family focus treatment, intensive psychosocial intervention, cognitive remediation and functional remediation, mindfulness-based interventions (MBCT), or internet-based interventions in the treatment of resistant BD patients.
Overall, there are limited data to suggest that any kind of psychotherapy is useful in resistant BD patients; some data suggest that it could be useful in resistant patients at the early stages of the disorder.
Algorithm for Treatment of Resistant BD
A visual representation of the recommended steps in the treatment of resistant cases during each phase is shown in Figures 2–4. As already mentioned, this specific algorithm is based on the materials collected to develop a general algorithm for BD by the CINP (Fountoulakis et al., 2017a, 2017b, 2017c, 2017d).
The authors decided that although mixed episodes are not included in DSM-5, it would be important to include a guidance option for them since some relevant data do exist. Also, they decided to include a recommendation for adding CBT and/or psychoeducation at a level higher than the evidence suggests, because, if available, they could be added without problems to existing pharmacotherapy. However, the recommendation is that this addition should not interfere with the application of the algorithm itself, that is, the first step of the algorithm concerning pharmacotherapy should always be initialized and pseudo-resistance should have been ruled out (Schaffer et al., 2017).
Discussion
The current guidelines are the first to our knowledge developed specifically for the treatment of resistant bipolar patients, and they also include an operationalized definition of treatment resistance.
The key issue is the definition of “response,” since this is a prerequisite for the definition of “resistant.” There are several papers defining response, remission, and resistance in mental disorders, and it seems that all the definitions are characterized by a rather narrow and vague approach. With BD, these definitions face a particular problem. They perform well with disorders with a predominantly linear course, characterized by exacerbations and remissions and with a single major factor or constellation of symptoms (e.g., unipolar melancholic depression). They also perform relatively well with complex disorders like schizophrenia that, in spite of the large variability of the clinical picture (to the extent it is possible 2 patients with schizophrenia not sharing a single symptom), their treatment is more or less unimodal (antipsychotics) and the course of the disease is monotonous. In BD, however, the course is characterized by episodes of distinct clusters of symptoms while the resulting accumulated stress has a profound adverse effect on neurocognition and general functioning with the neurobiology of the patient to change under the pressure of what is called “allostatic load,” leading eventually to treatment resistance, disability, high comorbidity, and preterm mortality (Vieta et al., 2013).
When it comes to BD, things are quite different from other mental disorders. The reliability and validity of usual approaches are questionable because both the clinical picture and the treatment are complex and interrelated. Also, the course is not monotonous but, on the contrary, it manifests with unpredictable switches with a complex and unique relationship between clinical symptoms and impairment. Another issue is the problematic interplay of the clinical picture with psychometric scale scores when the longitudinal course of the disorder is complex, as in the case of BD. For example, the use of the MADRS scale to assess response to treatment with a tricyclic antidepressant could lead to erroneous conclusions in case this depressive BD patient becomes mixed or rapid cycling emerges. The MADRS will probably classify him as being a “responder,” but whether this is true is a matter of debate. Accepting simplistic approaches cannot serve as a real solution.
The current study developed a definition of treatment resistance in BD and an algorithm for its treatment. These were based on a thorough and deep search of the literature. During the last couple of decades, our knowledge concerning the treatment of BD has changed radically (Grande et al., 2016; Vieta et al., 2018), and a rather narrow therapeutic effect for most agents is accepted, so narrow that the very existence of the term “mood stabilizer” is under question. Further, the collapse of the “class effect” approach to BD treatment (Rosa et al., 2011) raises important questions as to which patients are truly resistant and which were simply treated in a suboptimal way.
The review of the literature suggested that there are some evidence-based options for the treatment of resistant acute mania but much fewer for the treatment of resistant depression, mixed states, and rapid cycling cases. Thus, the relative shortage of hard data (Poon et al., 2012) leaves the clinician in many cases with the heavy burden to decide on the basis of clinical experience and wisdom. The current treatment guidelines on one hand rely on hard data; however, they provide a limited number of options for the treatment of a variety of cases, and without the ability to tailor treatment to the clinical picture and the specific needs of the individual patient. Future specific and targeted research is essential and necessary to test possible treatment approaches for resistant patients of all kinds.
Most of the problems concerning the interplay of clinical features with definitions of response and resistance, especially for the maintenance phase, have been discussed elsewhere (Ghaemi et al., 2004; Fountoulakis, 2010; Vieta and Garriga, 2016; Fountoulakis et al., 2017b, 2017c, 2017d). Clinical wisdom is also of high importance in the planning of long-term treatment of BD patients. In this frame, the concept of “predominant polarity” and the “polarity index” of a given agent are of great importance since it guides the clinician to tailor treatment to the specific needs of the specific patient (Nivoli et al., 2011; Popovic et al., 2012, 2013, 2014; Sentissi et al., 2019).
Better knowledge of the underlying neurobiological substrate of treatment resistance in BD would be important, since the failure of physiological compensatory mechanisms over time, accompanied by neuroprogression and cross-sensitization of episode recurrence, trauma exposure, and substance use could constitute modifiable factors both in the prevention but also in the treatment of such cases (da Costa et al., 2016). So far, however, our knowledge does not permit a reliable prediction or assessment of resistant cases since no neurobiological or clinical variables have proven reliable (Gonzalez-Isasi et al., 2012). However, the observation that psychoeducation is preferentially efficacious in patients with less than 7 episodes, while it has no effect in those with more than 14 episodes, provides us with a first clue of the stages and the timetable of the development of resistance (Colom et al., 2010; Bond and Anderson, 2015; van der Markt et al., 2019). On the other hand, while the stage of the progression of the disorder has been proposed as a factor that should be taken into consideration in the planning of treatment especially in resistant cases (Passos and Kapczinski, 2017), in essence this might reflect a cyclical logic with treatment resistance defining the stage and vice versa.
Statement of Interest
K.N.F. has received grants and served as consultant, advisor, or CME speaker for the following entities: AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Ferrer, Gedeon Richter, Janssen, Lundbeck, Otsuka, Pfizer, the Pfizer Foundation, Sanofi-Aventis, Servier, Shire, and others. E.V. has received grants and served as consultant, advisor, or CME speaker for the following entities: AB-Biotics, Abbott, Allergan, Angelini, AstraZeneca, Bristol-Myers Squibb, Dainippon Sumitomo Pharma, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo-Smith-Kline, Janssen, Lilly, Lundbeck, Otsuka, Pfizer, Roche, SAGE, Sanofi-Aventis, Servier, Shire, Sunovion, Takeda, the Brain and Behaviour Foundation, the Generalitat de Catalunya (AGAUR and PERIS), the Spanish Ministry of Science, Innovation and Universities (AES and CIBERSAM), the Seventh European Framework Programme and Horizon 2020, and the Stanley Medical Research Institute. A.H.Y. is employed by King’s College London; is Honorary Consultant SLaM (NHS UK); has paid lectures by and participated in advisory boards for all major pharmaceutical companies with drugs used in affective and related disorders; no shareholdings in pharmaceutical companies. He was lead investigator for Embolden Study (AZ), BCI Neuroplasticity study, and Aripiprazole Mania Study; investigator-initiated studies from AZ, Eli Lilly, Lundbeck, and Wyeth; and has received grant funding (past and present) from: NIHR-BRC (UK); NIMH (USA); CIHR (Canada); NARSAD (USA); Stanley Medical Research Institute (USA); MRC (UK); Wellcome Trust (UK); Royal College of Physicians (Edin); BMA (UK); UBC-VGH Foundation (Canada); WEDC (Canada); CCS Depression Research Fund (Canada); MSFHR (Canada); NIHR (UK). H.G. within the last 3 years received honoraria or consultation fees from: Desitin, Gedeon-Richter, Lundbeck, and Pfizer. L.Y. has been on speaker/advisory boards for, or has received research grants from Alkermes, Allergan, AstraZeneca, Bristol Myers Squibb, CANMAT, CIHR, Eli Lilly, Forest, GlaxoSmithKline, Intas, Janssen, the Michael Smith Foundation for Health Research, Pfizer, Servier, Sumitomo Dainippon, Sunovion, and the Stanley Foundation. S.K. within the last 3 years received grants/research support, consulting fees, and honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, AstraZeneca, Eli Lilly, Janssen, KRKA-Pharma, Lundbeck, Neuraxpharm, Pfizer, Pierre Fabre, Schwabe, and Servier. H.J.M. received honoraria for lectures or for advisory activities or received grants by the following pharmaceutical companies: Lundbeck, Servier, Schwabe, and Bayer. He was president or on the executive board of the following organizations: CINP, ECNP, WFSBP, and EPA and chairman of the WPA-section on Pharmacopsychiatry. P.B. has received research grants from, honoraria for participation in advisory boards from, and/or gave presentations for: Allergan, Astra Zeneca, Bristol Myers Squibb, Canadian Institute for Health Research, Eli Lilly, Lundbeck, Janssen, Ontario Brain Institute, Meda-Valeant, Merck, Otsuka, Pierre Fabre Medicaments, Pfizer, Shire, Sunovion, and Takeda. M.T. has been a consultant for AstraZeneca, Abbott, BMS, Lilly, GSK, J&J, Otsuka, Roche, Lundbeck, Elan, Allergan, Alkermes, Merck, Minerva, Neuroscience, Pamlab, Alexza, Forest, Teva, Sunovion, Gedeon Richter, and Wyeth. He was a full-time employee at Lilly (1997–2008). His spouse is a former employee at Lilly (1998–2013).
References
- Aan Het Rot M, Zarate CA Jr., Charney DS, Mathew SJ (2012) Ketamine for depression: where do we go from here? Biol Psychiatry 72:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Fasula M, Kelmendi B, Sanacora G, Ostroff R (2012) Rapid antidepressant effect of ketamine in the electroconvulsive therapy setting. J ECT 28:157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler LL, Keck PE Jr, McElroy SL, Suppes T, Brown ES, Denicoff K, Frye M, Gitlin M, Hwang S, Goodman R, Leverich G, Nolen W, Kupka R, Post R (1999) Gabapentin in the acute treatment of refractory bipolar disorder. Bipolar Disord 1:61–65. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Post RM, Hellemann G, Leverich GS, Nolen WA, Frye MA, Keck PE Jr, Kupka RW, Grunze H, McElroy SL, Sugar CA, Suppes T(2009) Impact of antidepressant continuation after acute positive or partial treatment response for bipolar depression: a blinded, randomized study. J Clin Psychiatry 70:450–457. [DOI] [PubMed] [Google Scholar]
- Amann BL, Mergl R, Vieta E, Born C, Hermisson I, Seemueller F, Dittmann S, Grunze H (2007) A 2-year, open-label pilot study of adjunctive chromium in patients with treatment-resistant rapid-cycling bipolar disorder. J Clin Psychopharmacol 27:104–106. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders 4th Edition, Text Revision, DSM-IV-TR. Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Amsterdam JD, Lorenzo-Luaces L, DeRubeis RJ (2016a) Step-wise loss of antidepressant effectiveness with repeated antidepressant trials in bipolar II depression. Bipolar Disord 18:563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam JD, Lorenzo-Luaces L, Soeller I, Li SQ, Mao JJ, DeRubeis RJ(2016b) Short-term venlafaxine v. lithium monotherapy for bipolar type II major depressive episodes: effectiveness and mood conversion rate. Br J Psychiatry 208:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Gunn AD, Barkay G, Karne HS, Nurnberger JI, Mathew SJ, Ghosh S (2012) Early antidepressant effect of memantine during augmentation of lamotrigine inadequate response in bipolar depression: a double-blind, randomized, placebo-controlled trial. Bipolar Disord 14:64–70. [DOI] [PubMed] [Google Scholar]
- Aronson TA, Shukla S, Hirschowitz J (1989) Clonazepam treatment of five lithium-refractory patients with bipolar disorder. Am J Psychiatry 146:77–80. [DOI] [PubMed] [Google Scholar]
- Baek JH, Ha K, Yatham LN, Chang JS, Ha TH, Jeon HJ, Hong KS, Chang SM, Ahn YM, Cho HS, Moon E, Cha B, Choi JE, Joo YH, Joo EJ, Lee SY, Park Y (2014) Pattern of pharmacotherapy by episode types for patients with bipolar disorders and its concordance with treatment guidelines. J Clin Psychopharmacol 34:577–587. [DOI] [PubMed] [Google Scholar]
- Ball JR, Mitchell PB, Corry JC, Skillecorn A, Smith M, Malhi GS (2006) A randomized controlled trial of cognitive therapy for bipolar disorder: focus on long-term change. J Clin Psychiatry 67:277–286. [DOI] [PubMed] [Google Scholar]
- Barekatain M, Jahangard L, Haghighi M, Ranjkesh F (2008) Bifrontal versus bitemporal electroconvulsive therapy in severe manic patients. J ECT 24:199–202. [DOI] [PubMed] [Google Scholar]
- Barton BM, Gitlin MJ (1987) Verapamil in treatment-resistant mania: an open trial. J Clin Psychopharmacol 7:101–103. [PubMed] [Google Scholar]
- Bauer IE, Soares JC, Selek S, Meyer TD (2017) The Link between refractoriness and neuroprogression in treatment-resistant bipolar disorder. Mod Trends Pharmacopsychiatry 31:10–26. [DOI] [PubMed] [Google Scholar]
- Bauer M. (1994) Refractoriness induced by lithium discontinuation despite adequate serum lithium levels. Am J Psychiatry 151:1522. [DOI] [PubMed] [Google Scholar]
- Bauer M, Zaninelli R, Müller-Oerlinghausen B, Meister W (1999) Paroxetine and amitriptyline augmentation of lithium in the treatment of major depression: a double-blind study. J Clin Psychopharmacol 19:164–171. [DOI] [PubMed] [Google Scholar]
- Bauer M, Berghöfer A, Bschor T, Baumgartner A, Kiesslinger U, Hellweg R, Adli M, Baethge C, Müller-Oerlinghausen B (2002) Supraphysiological doses of L-thyroxine in the maintenance treatment of prophylaxis-resistant affective disorders. Neuropsychopharmacology 27:620–628. [DOI] [PubMed] [Google Scholar]
- Bauer M, Berman S, Stamm T, Plotkin M, Adli M, Pilhatsch M, London ED, Hellemann GS, Whybrow PC, Schlagenhauf F (2016) Levothyroxine effects on depressive symptoms and limbic glucose metabolism in bipolar disorder: a randomized, placebo-controlled positron emission tomography study. Mol Psychiatry 21:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer MS. (1990) Rapid cycling bipolar affective disorder. Archives of General Psychiatry 47:435. [DOI] [PubMed] [Google Scholar]
- Bauer MS, Whybrow PC (1990) Rapid cycling bipolar affective disorder. II. Treatment of refractory rapid cycling with high-dose levothyroxine: a preliminary study. Arch Gen Psychiatry 47:435–440. [DOI] [PubMed] [Google Scholar]
- Behzadi AH, Omrani Z, Chalian M, Asadi S, Ghadiri M (2009) Folic acid efficacy as an alternative drug added to sodium valproate in the treatment of acute phase of mania in bipolar disorder: a double-blind randomized controlled trial. Acta Psychiatr Scand 120:441–445. [DOI] [PubMed] [Google Scholar]
- Benedetti A, Di Paolo A, Lastella M, Casamassima F, Candiracci C, Litta A, Ciofi L, Danesi R, Lattanzi L, Del Tacca M, Cassano GB(2010) Augmentation of clozapine with aripiprazole in severe psychotic bipolar and schizoaffective disorders: a pilot study. Clinical practice and epidemiology in mental health: CP & EMH 6:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Barbini B, Fulgosi MC, Colombo C, Dallaspezia S, Pontiggia A, Smeraldi E (2005) Combined total sleep deprivation and light therapy in the treatment of drug-resistant bipolar depression: acute response and long-term remission rates. J Clin Psychiatry 66:1535–1540. [DOI] [PubMed] [Google Scholar]
- Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Bush AI(2008) N-acetyl cysteine for depressive symptoms in bipolar disorder--a double-blind randomized placebo-controlled trial. Biol Psychiatry 64:468–475. [DOI] [PubMed] [Google Scholar]
- Berk M, Brnabic A, Dodd S, Kelin K, Tohen M, Malhi GS, Berk L, Conus P, McGorry PD(2011a) Does stage of illness impact treatment response in bipolar disorder? Empirical treatment data and their implication for the staging model and early intervention. Bipolar Disord 13:87–98. [DOI] [PubMed] [Google Scholar]
- Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Yücel M, Gama CS, Dodd S, Dean B, Magalhães PV, Amminger P, McGorry P, Malhi GS (2011b) Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev 35:804–817. [DOI] [PubMed] [Google Scholar]
- Berk M, Turner A, Malhi GS, Ng CH, Cotton SM, Dodd S, Samuni Y, Tanious M, McAulay C, Dowling N, Sarris J, Owen L, Waterdrinker A, Smith D, Dean OM (2019a) A randomised controlled trial of a mitochondrial therapeutic target for bipolar depression: mitochondrial agents, N-acetylcysteine, and placebo. BMC Med 17:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Turner A, Malhi GS, Ng CH, Cotton SM, Dodd S, Samuni Y, Tanious M, McAulay C, Dowling N, Sarris J, Owen L, Waterdrinker A, Smith D, Dean OM (2019b) Correction to: a randomised controlled trial of a mitochondrial therapeutic target for bipolar depression: mitochondrial agents, N-acetylcysteine, and placebo. BMC Med 17:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersudsky Y, Applebaum J, Gaiduk Y, Sharony L, Mishory A, Podberezsky A, Agam G, Belmaker RH (2010) Valnoctamide as a valproate substitute with low teratogenic potential in mania: a double-blind, controlled, add-on clinical trial. Bipolar Disord 12:376–382. [DOI] [PubMed] [Google Scholar]
- Berwaerts J, Lane R, Nuamah IF, Lim P, Remmerie B, Hough DW (2011) Paliperidone extended-release as adjunctive therapy to lithium or valproate in the treatment of acute mania: a randomized, placebo-controlled study. J Affect Disord 129:252–260. [DOI] [PubMed] [Google Scholar]
- Bjørklund L, Horsdal HT, Mors O, Østergaard SD, Gasse C (2016) Trends in the psychopharmacological treatment of bipolar disorder: a nationwide register-based study. Acta Neuropsychiatr 28:75–84. [DOI] [PubMed] [Google Scholar]
- Bobo WV, Shelton RC (2010) Risperidone long-acting injectable (Risperdal Consta®) for maintenance treatment in patients with bipolar disorder. Expert Rev Neurother 10:1637–1658. [DOI] [PubMed] [Google Scholar]
- Bobo WV, Vande Voort JL, Croarkin PE, Leung JG, Tye SJ, Frye MA (2016) Ketamine for treatment-resistant unipolar and bipolar major depression: critical review and implications for clinical practice. Depress Anxiety 33:698–710. [DOI] [PubMed] [Google Scholar]
- Bocchetta A, Bernardi F, Burrai C, Pedditzi M, Del Zompo M (1993) A double-blind study of L-sulpiride versus amitriptyline in lithium-maintained bipolar depressives. Acta Psychiatr Scand 88:434–439. [DOI] [PubMed] [Google Scholar]
- Bond K, Anderson IM (2015) Psychoeducation for relapse prevention in bipolar disorder: a systematic review of efficacy in randomized controlled trials. Bipolar Disord 17:349–362. [DOI] [PubMed] [Google Scholar]
- Bowden CL, Calabrese JR, McElroy SL, Rhodes LJ, Keck PE Jr, Cookson J, Anderson J, Bolden-Watson C, Ascher J, Monaghan E, Zhou J (1999) The efficacy of lamotrigine in rapid cycling and non-rapid cycling patients with bipolar disorder. Biol Psychiatry 45:953–958. [DOI] [PubMed] [Google Scholar]
- Brown ES, Park J, Marx CE, Hynan LS, Gardner C, Davila D, Nakamura A, Sunderajan P, Lo A, Holmes T (2014) A randomized, double-blind, placebo-controlled trial of pregnenolone for bipolar depression. Neuropsychopharmacology 39:2867–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Braga RJ, Nnadi CU, Shaya Y, Stearns WH, Malhotra AK (2012) Placebo-controlled adjunctive trial of pramipexole in patients with bipolar disorder: targeting cognitive dysfunction. J Clin Psychiatry 73:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddy C, Giaroli G, White TP, Shergill SS, Tracy DK (2014) Ketamine as the prototype glutamatergic antidepressant: pharmacodynamic actions, and a systematic review and meta-analysis of efficacy. Ther Adv Psychopharmacol 4:75–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese JR, Kimmel SE, Woyshville MJ, Rapport DJ, Faust CJ, Thompson PA, Meltzer HY (1996) Clozapine for treatment-refractory mania. Am J Psychiatry 153:759–764. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Bowden CL, McElroy SL, Cookson J, Andersen J, Keck PE Jr, Rhodes L, Bolden-Watson C, Zhou J, Ascher JA (1999) Spectrum of activity of lamotrigine in treatment-refractory bipolar disorder. Am J Psychiatry 156:1019–1023. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Keck PE Jr, McElroy SL, Shelton MD (2001) A pilot study of topiramate as monotherapy in the treatment of acute mania. J Clin Psychopharmacol 21:340–342. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Ketter TA, Youakim JM, Tiller JM, Yang R, Frye MA (2010) Adjunctive armodafinil for major depressive episodes associated with bipolar I disorder: a randomized, multicenter, double-blind, placebo-controlled, proof-of-concept study. J Clin Psychiatry 71:1363–1370. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Frye MA, Yang R, Ketter TA; Armodafinil Treatment Trial Study Network (2014) Efficacy and safety of adjunctive armodafinil in adults with major depressive episodes associated with bipolar I disorder: a randomized, double-blind, placebo-controlled, multicenter trial. J Clin Psychiatry 75:1054–1061. [DOI] [PubMed] [Google Scholar]
- Cardoso T de A, Campos Mondin T, Reyes AN, Zeni CP, Souza LD, da Silva RA, Jansen K (2015) Biological rhythm and bipolar disorder: twelve-month follow-up of a randomized clinical trial. J Nerv Ment Dis 203:792–797. [DOI] [PubMed] [Google Scholar]
- Carlson BX, Ketter TA, Sun W, Timko K, McQuade RD, Sanchez R, Vester-Blokland E, Marcus R (2012) Aripiprazole in combination with lamotrigine for the long-term treatment of patients with bipolar I disorder (manic or mixed): a randomized, multicenter, double-blind study (CN138-392). Bipolar Disord 14:41–53. [DOI] [PubMed] [Google Scholar]
- Chen J, Muzina DJ, Kemp DE, Conroy C, Chan P, Serrano MB, Ganocy SJ, Fang Y, Calabrese JR, Gao K (2011) Safety and efficacy of olanzapine monotherapy in treatment-resistant bipolar mania: a 12-week open-label study. Hum Psychopharmacol 26:588–595. [DOI] [PubMed] [Google Scholar]
- Chen J, Lu Z, Zhang M, Zhang J, Ni X, Jiang X, Xu H, Heeramun-Aubeeluck A, Hu Q, Jin H, Davis JM (2013) A randomized, 4-week double-blind placebo control study on the efficacy of donepezil augmentation of lithium for treatment of acute mania. Neuropsychiatr Dis Treat 9:839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chengappa KN, Rathore D, Levine J, Atzert R, Solai L, Parepally H, Levin H, Moffa N, Delaney J, Brar JS (1999) Topiramate as add-on treatment for patients with bipolar mania. Bipolar Disord 1:42–53. [DOI] [PubMed] [Google Scholar]
- Chengappa KN, Levine J, Gershon S, Mallinger AG, Hardan A, Vagnucci A, Pollock B, Luther J, Buttenfield J, Verfaille S, Kupfer DJ (2000) Inositol as an add-on treatment for bipolar depression. Bipolar Disord 2:47–55. [DOI] [PubMed] [Google Scholar]
- Chiu CC, Huang SY, Chen CC, Su KP (2005) Omega-3 fatty acids are more beneficial in the depressive phase than in the manic phase in patients with bipolar I disorder. J Clin Psychiatry 66:1613–1614. [DOI] [PubMed] [Google Scholar]
- Ciapparelli A, Dell’Osso L, Pini S, Chiavacci MC, Fenzi M, Cassano GB (2000) Clozapine for treatment-refractory schizophrenia, schizoaffective disorder, and psychotic bipolar disorder: a 24-month naturalistic study. J Clin Psychiatry 61:329–334. [DOI] [PubMed] [Google Scholar]
- Ciapparelli A, Dell’Osso L, Bandettini di Poggio A, Carmassi C, Cecconi D, Fenzi M, Chiavacci MC, Bottai M, Ramacciotti CE, Cassano GB (2003) Clozapine in treatment-resistant patients with schizophrenia, schizoaffective disorder, or psychotic bipolar disorder: a naturalistic 48-month follow-up study. J Clin Psychiatry 64:451–458. [DOI] [PubMed] [Google Scholar]
- Ciappolino V, Delvecchio G, Agostoni C, Mazzocchi A, Altamura AC, Brambilla P (2017) The role of n-3 polyunsaturated fatty acids (n-3PUFAs) in affective disorders. J Affect Disord 224:32–47. [DOI] [PubMed] [Google Scholar]
- Cimpianu CL, Strube W, Falkai P, Palm U, Hasan A (2017) Vagus nerve stimulation in psychiatry: a systematic review of the available evidence. J Neural Transm (Vienna) 124:145–158. [DOI] [PubMed] [Google Scholar]
- Citrome L. (2010) Ziprasidone HCl capsules for the adjunctive maintenance treatment of bipolar disorder in adults. Expert Rev Neurother 10:1031–1037. [DOI] [PubMed] [Google Scholar]
- Cole AJ, Scott J, Ferrier IN, Eccleston D (1993) Patterns of treatment resistance in bipolar affective disorder. Acta Psychiatr Scand 88:121–123. [DOI] [PubMed] [Google Scholar]
- Colom F, Vieta E, Martinez-Aran A, Reinares M, Goikolea JM, Benabarre A, Torrent C, Comes M, Corbella B, Parramon G, Corominas J (2003) A randomized trial on the efficacy of group psychoeducation in the prophylaxis of recurrences in bipolar patients whose disease is in remission. Arch Gen Psychiatry 60:402–407. [DOI] [PubMed] [Google Scholar]
- Colom F, Vieta E, Sánchez-Moreno J, Palomino-Otiniano R, Reinares M, Goikolea JM, Benabarre A, Martínez-Arán A (2009) Group psychoeducation for stabilised bipolar disorders: 5-year outcome of a randomised clinical trial. Br J Psychiatry 194:260–265. [DOI] [PubMed] [Google Scholar]
- Colom F, Reinares M, Pacchiarotti I, Popovic D, Mazzarini L, Martínez-Arán A, Torrent C, Rosa A, Palomino-Otiniano R, Franco C, Bonnin CM, Vieta E (2010) Has number of previous episodes any effect on response to group psychoeducation in bipolar patients? A 5-year follow-up post hoc analysis. Acta Neuropsychiatr 22:50–53. [DOI] [PubMed] [Google Scholar]
- Corp SA, Gitlin MJ, Altshuler LL (2014) A review of the use of stimulants and stimulant alternatives in treating bipolar depression and major depressive disorder. J Clin Psychiatry 75:1010–1018. [DOI] [PubMed] [Google Scholar]
- Costa RT, Cheniaux E, Rosaes PA, Carvalho MR, Freire RC, Versiani M, Rangé BP, Nardi AE (2011) The effectiveness of cognitive behavioral group therapy in treating bipolar disorder: a randomized controlled study. Braz J Psychiatry 33:144–149. [DOI] [PubMed] [Google Scholar]
- Coyle CM, Laws KR (2015) The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol 30:152–163. [DOI] [PubMed] [Google Scholar]
- da Costa SC, Passos IC, Lowri C, Soares JC, Kapczinski F (2016) Refractory bipolar disorder and neuroprogression. Prog Neuropsychopharmacol Biol Psychiatry 70:103–110. [DOI] [PubMed] [Google Scholar]
- Dauphinais DR, Rosenthal JZ, Terman M, DiFebo HM, Tuggle C, Rosenthal NE (2012) Controlled trial of safety and efficacy of bright light therapy vs. negative air ions in patients with bipolar depression. Psychiatry Res 196:57–61. [DOI] [PubMed] [Google Scholar]
- de Barros Pellegrinelli K, de O Costa LF, Silval KI, Dias VV, Roso MC, Bandeira M, Colom F, Moreno RA (2013) Efficacy of psychoeducation on symptomatic and functional recovery in bipolar disorder. Acta Psychiatr Scand 127:153–158. [DOI] [PubMed] [Google Scholar]
- De Beaurepaire R. (1992) Treatment of neuroleptic-resistant mania and schizoaffective disorders. Am J Psychiatry 149:1614–1615. [DOI] [PubMed] [Google Scholar]
- Dell’Osso B, Ketter TA (2013) Assessing efficacy/effectiveness and safety/tolerability profiles of adjunctive pramipexole in bipolar depression: acute versus long-term data. Int Clin Psychopharmacol 28:297–304. [DOI] [PubMed] [Google Scholar]
- Dell’Osso B, Mundo E, D’Urso N, Pozzoli S, Buoli M, Ciabatti M, Rosanova M, Massimini M, Bellina V, Mariotti M, Altamura AC (2009) Augmentative repetitive navigated transcranial magnetic stimulation (rTMS) in drug-resistant bipolar depression. Bipolar Disord 11:76–81. [DOI] [PubMed] [Google Scholar]
- Denicoff KD, Smith-Jackson EE, Bryan AL, Ali SO, Post RM (1997) Valproate prophylaxis in a prospective clinical trial of refractory bipolar disorder. Am J Psychiatry 154:1456–1458. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr (2010) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierckx B, Heijnen WT, van den Broek WW, Birkenhäger TK (2012) Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: a meta-analysis. Bipolar Disord 14:146–150. [DOI] [PubMed] [Google Scholar]
- D’Urso G, Dell’Osso B, Rossi R, Brunoni AR, Bortolomasi M, Ferrucci R, Priori A, de Bartolomeis A, Altamura AC(2017) Clinical predictors of acute response to transcranial direct current stimulation (tDCS) in major depression. J Affect Disord 219:25–30. [DOI] [PubMed] [Google Scholar]
- Eden Evins A, Demopulos C, Nierenberg A, Culhane MA, Eisner L, Sachs G (2006) A double-blind, placebo-controlled trial of adjunctive donepezil in treatment-resistant mania. Bipolar Disord 8:75–80. [DOI] [PubMed] [Google Scholar]
- Erfurth A, Michael N, Stadtland C, Arolt V (2002) Bupropion as add-on strategy in difficult-to-treat bipolar depressive patients. Neuropsychobiology 45 (Suppl 1):33–36. [DOI] [PubMed] [Google Scholar]
- Esan O, Osunbote C, Oladele O, Fakunle S, Ehindero C, Fountoulakis KN (2017) Bipolar I disorder in remission vs. schizophrenia in remission: is there a difference in burden? Compr Psychiatry 72:130–135. [DOI] [PubMed] [Google Scholar]
- Fan A, Berg A, Bresee C, Glassman LH, Rapaport MH (2012) Allopurinol augmentation in the outpatient treatment of bipolar mania: a pilot study. Bipolar Disord 14:206–210. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Hoy KE, Elliot D, McQueen S, Wambeek LE, Daskalakis ZJ (2016) A negative double-blind controlled trial of sequential bilateral rTMS in the treatment of bipolar depression. J Affect Disord 198:158–162. [DOI] [PubMed] [Google Scholar]
- Fond G, Boyer L (2014) Ketamine’s effectiveness in unipolar versus bipolar depression. Psychopharmacology (Berl) 231:4417–4418. [DOI] [PubMed] [Google Scholar]
- Fond G, Loundou A, Rabu C, Macgregor A, Lançon C, Brittner M, Micoulaud-Franchi JA, Richieri R, Courtet P, Abbar M, Roger M, Leboyer M, Boyer L (2014) Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl) 231:3663–3676. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN. (2010) An update of evidence-based treatment of bipolar depression: where do we stand? Curr Opin Psychiatry 23:19–24. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN. (2012) Refractoriness in bipolar disorder: definitions and evidence-based treatment. CNS Neurosci Ther 18:227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountoulakis KN, Magiria S, Siamouli M, Panagiotidis P, Nimatoudis I, Iacovides A, Kaprinis GS (2007a) A seven- year follow-up of an extremely refractory bipolar I patient. CNS Spectr 12:733–734. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, Siamouli M, Panagiotidis P, Magiria S, Kantartzis S, Iacovides A, Kaprinis GS (2008) Ultra short manic-like episodes after antidepressant augmentation with modafinil. Prog Neuropsychopharmacol Biol Psychiatry 32:891–892. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, Yatham L, Grunze H, Vieta E, Young A, Yatham L, Blier P, Kasper S, Moeller HJ (2017a) The International College of Neuro-Psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), part 2: review, grading of the evidence, and a precise algorithm. Int J Neuropsychopharmacol 20:121–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountoulakis KN, Grunze H, Vieta E, Young A, Yatham L, Blier P, Kasper S, Moeller HJ (2017b) The International College of Neuro-Psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), part 3: the clinical guidelines. Int J Neuropsychopharmacol 20:180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountoulakis KN, Vieta E, Young A, Yatham L, Grunze H, Blier P, Moeller HJ, Kasper S (2017c) The International College of Neuropsychopharmacology (CINP) Treatment Guidelines for Bipolar Disorder in Adults (CINP-BD-2017), part 4: unmet needs in the treatment of bipolar disorder and recommendations for future research. Int J Neuropsychopharmacol 20:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountoulakis KN, Young A, Yatham L, Grunze H, Vieta E, Blier P, Moeller HJ, Kasper S (2017d) The International College of Neuropsychopharmacology (CINP) Treatment Guidelines for Bipolar Disorder in Adults (CINP-BD-2017), part 1: background and methods of the development of guidelines. Int J Neuropsychopharmacol 20:98–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountoulakis KN, Kasper S, Andreassen O, Blier P, Okasha A, Severus E, Versiani M, Tandon R, Moller HJ, Vieta E (2012) Efficacy of pharmacotherapy in bipolar disorder: a report by the WPA section on pharmacopsychiatry. Eur Arch Psychiatry Clin Neurosci 262(Suppl 1):1–48. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, Gonda X, Baghai TC, Baldwin DS, Bauer M, Blier P, Gattaz W, Hasler G, Moller HJ, Tandon R, Vieta E, Kasper S (2015) Report of the WPA section of pharmacopsychiatry on the relationship of antiepileptic drugs with suicidality in epilepsy. Int J Psychiatry Clin Pract 19:158–167. [DOI] [PubMed] [Google Scholar]
- Frangou S, Lewis M, McCrone P (2006) Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry 188:46–50. [DOI] [PubMed] [Google Scholar]
- Frangou S, Lewis M, Wollard J, Simmons A (2007) Preliminary in vivo evidence of increased N-acetyl-aspartate following eicosapentanoic acid treatment in patients with bipolar disorder. J Psychopharmacol 21:435–439. [DOI] [PubMed] [Google Scholar]
- Fries GR, Pfaffenseller B, Stertz L, Paz AV, Dargél AA, Kunz M, Kapczinski F (2012) Staging and neuroprogression in bipolar disorder. Curr Psychiatry Rep 14:667–675. [DOI] [PubMed] [Google Scholar]
- Frye MA, Ketter TA, Kimbrell TA, Dunn RT, Speer AM, Osuch EA, Luckenbaugh DA, Cora-Ocatelli G, Leverich GS, Post RM (2000) A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 20:607–614. [DOI] [PubMed] [Google Scholar]
- Frye MA, Grunze H, Suppes T, McElroy SL, Keck PE Jr, Walden J, Leverich GS, Altshuler LL, Nakelsky S, Hwang S, Mintz J, Post RM (2007) A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry 164:1242–1249. [DOI] [PubMed] [Google Scholar]
- Gabriel A. (2007) Adjunctive topiramate treatment in refractory obese bipolar patients: a descriptive open label study. Eat Weight Disord 12:48–53. [DOI] [PubMed] [Google Scholar]
- Gajwani P. (2009) Treatment-refractory bipolar disorder: classification to aid in clinical management. Expert Opin Pharmacother 10:1907–1915. [DOI] [PubMed] [Google Scholar]
- Galling B, Garcia MA, Osuchukwu U, Hagi K, Correll CU (2015) Safety and tolerability of antipsychotic-mood stabilizer co-treatment in the management of acute bipolar disorder: results from a systematic review and exploratory meta-analysis. Expert Opin Drug Saf 14:1181–1199. [DOI] [PubMed] [Google Scholar]
- Geddes JR, Rendell JM, Goodwin GM(2002) BALANCE: a large simple trial of maintenance treatment for bipolar disorder. World Psychiatry 1:48–51. [PMC free article] [PubMed] [Google Scholar]
- Geddes JR, Gardiner A, Rendell J, Voysey M, Tunbridge E, Hinds C, Yu LM, Hainsworth J, Attenburrow MJ, Simon J, Goodwin GM, Harrison PJ; CEQUEL Investigators and Collaborators (2016) Comparative evaluation of quetiapine plus lamotrigine combination versus quetiapine monotherapy (and folic acid versus placebo) in bipolar depression (CEQUEL): a 2 × 2 factorial randomised trial. Lancet Psychiatry 3:31–39. [DOI] [PubMed] [Google Scholar]
- Ghaemi SN, Pardo TB, Hsu DJ (2004) Strategies for preventing the recurrence of bipolar disorder. J Clin Psychiatry 65 (Suppl 10):16–23. [PubMed] [Google Scholar]
- Ghanizadeh A, OmraniSigaroodi M, Javadpour A, Dabbaghmanesh MH, Shafiee S (2014) Lovastatin as an adjuvant to lithium for treating manic phase of bipolar disorder: a 4-week, randomized, double-blind, placebo-controlled clinical trial. Depress Res Treat 2014:730505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijsman HJ, Geddes JR, Rendell JM, Nolen WA, Goodwin GM (2004) Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry 161:1537–1547. [DOI] [PubMed] [Google Scholar]
- Gitlin M. (2006) Treatment-resistant bipolar disorder. Mol Psychiatry 11:227–240. [DOI] [PubMed] [Google Scholar]
- Gitlin MJ. (2001) Treatment-resistant bipolar disorder. Bull Menninger Clin 65:26–40. [DOI] [PubMed] [Google Scholar]
- Goldberg JF, Burdick KE, Endick CJ (2004) Preliminary randomized, double-blind, placebo-controlled trial of pramipexole added to mood stabilizers for treatment-resistant bipolar depression. Am J Psychiatry 161:564–566. [DOI] [PubMed] [Google Scholar]
- Gomes BC, Abreu LN, Brietzke E, Caetano SC, Kleinman A, Nery FG, Lafer B (2011) A randomized controlled trial of cognitive behavioral group therapy for bipolar disorder. Psychother Psychosom 80:144–150. [DOI] [PubMed] [Google Scholar]
- González-Isasi A, Echeburúa E, Mosquera F, Ibáñez B, Aizpuru F, González-Pinto A (2010) Long-term efficacy of a psychological intervention program for patients with refractory bipolar disorder: a pilot study. Psychiatry Res 176:161–165. [DOI] [PubMed] [Google Scholar]
- González-Isasi A, Echeburúa E, Limiñana JM, González-Pinto A (2012) Predictors of good outcome in patients with refractory bipolar disorder after a drug or a drug and cognitive-behavioral treatment. Compr Psychiatry 53:224–229. [DOI] [PubMed] [Google Scholar]
- González Isasi A, Echeburúa E, Limiñana JM, González-Pinto A (2014) Psychoeducation and cognitive-behavioral therapy for patients with refractory bipolar disorder: a 5-year controlled clinical trial. Eur Psychiatry 29:134–141. [DOI] [PubMed] [Google Scholar]
- Grande I, Berk M, Birmaher B, Vieta E (2016) Bipolar disorder. Lancet 387:1561–1572. [DOI] [PubMed] [Google Scholar]
- Green AI, Tohen M, Patel JK, Banov M, DuRand C, Berman I, Chang H, Zarate C Jr, Posener J, Lee H, Dawson R, Richards C, Cole JO, Schatzberg AF (2000) Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry 157:982–986. [DOI] [PubMed] [Google Scholar]
- Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C, Drago F, Caraci F (2014) Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. Plos One 9:e96905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunze H, Vieta E, Goodwin GM, Bowden C, Licht RW, Azorin JM, Yatham L, Mosolov S, Möller HJ, Kasper S; Members of the WFSBP Task Force on Bipolar Affective Disorders Working on this topic (2018) The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: acute and long-term treatment of mixed states in bipolar disorder. World J Biol Psychiatry 19:2–58. [DOI] [PubMed] [Google Scholar]
- Harel EV, Zangen A, Roth Y, Reti I, Braw Y, Levkovitz Y (2011) H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry 12:119–126. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Soehner AM, Kaplan KA, Hein K, Lee J, Kanady J, Li D, Rabe-Hesketh S, Ketter TA, Neylan TC, Buysse DJ (2015) Treating insomnia improves mood state, sleep, and functioning in bipolar disorder: a pilot randomized controlled trial. J Consult Clin Psychol 83:564–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo-Mazzei D, et al. (2019) Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry 214:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiremani RM, Thirthalli J, Tharayil BS, Gangadhar BN (2008) Double-blind randomized controlled study comparing short-term efficacy of bifrontal and bitemporal electroconvulsive therapy in acute mania. Bipolar Disord 10:701–707. [DOI] [PubMed] [Google Scholar]
- Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, Wint D, Craighead MC, Kozarsky J, Chismar R, Moreines JL, Mewes K, Posse PR, Gutman DA, Mayberg HS (2012) Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry 69:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, Schulz KF; CONSORT Group (2008) CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. Plos Med 5:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston JP, Ahl J, Meyers AL, Kaiser CJ, Tohen M, Baldessarini RJ (2006) Reduced suicidal ideation in bipolar I disorder mixed-episode patients in a placebo-controlled trial of olanzapine combined with lithium or divalproex. J Clin Psychiatry 67:1246–1252. [DOI] [PubMed] [Google Scholar]
- Houston JP, Tohen M, Degenhardt EK, Jamal HH, Liu LL, Ketter TA (2009) Olanzapine-divalproex combination versus divalproex monotherapy in the treatment of bipolar mixed episodes: a double-blind, placebo-controlled study. J Clin Psychiatry 70:1540–1547. [DOI] [PubMed] [Google Scholar]
- Hui Poon S, Sim K, Baldessarini RJ (2015) Pharmacological approaches for treatment-resistant bipolar disorder. Curr Neuropharmacol 13:592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Investigators B, collaborators; Geddes JR, Goodwin GM, Rendell J, Azorin JM, Cipriani A, Ostacher MJ, Morriss R, Alder N, Juszczak E(2010) Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet 375:385–395. [DOI] [PubMed] [Google Scholar]
- Ionescu DF, Luckenbaugh DA, Niciu MJ, Richards EM, Zarate CA Jr (2015) A single infusion of ketamine improves depression scores in patients with anxious bipolar depression. Bipolar Disord 17:438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isasi AG, Echeburúa E, Limiñana JM, González-Pinto A (2010) How effective is a psychological intervention program for patients with refractory bipolar disorder? A randomized controlled trial. J Affect Disord 126:80–87. [DOI] [PubMed] [Google Scholar]
- Jahangard L, Soroush S, Haghighi M, Ghaleiha A, Bajoghli H, Holsboer-Trachsler E, Brand S (2014) In a double-blind, randomized and placebo-controlled trial, adjuvant allopurinol improved symptoms of mania in in-patients suffering from bipolar disorder. Eur Neuropsychopharmacol 24:1210–1221. [DOI] [PubMed] [Google Scholar]
- Juruena MF, Ottoni GL, Machado-Vieira R, Carneiro RM, Weingarthner N, Marquardt AR, Fleig SS, Broilo L, Busnello EA (2009) Bipolar I and II disorder residual symptoms: oxcarbazepine and carbamazepine as add-on treatment to lithium in a double-blind, randomized trial. Prog Neuropsychopharmacol Biol Psychiatry 33:94–99. [DOI] [PubMed] [Google Scholar]
- Kagawa S, Mihara K, Nakamura A, Nemoto K, Suzuki T, Nagai G, Kondo T (2014) Relationship between plasma concentrations of lamotrigine and its early therapeutic effect of lamotrigine augmentation therapy in treatment-resistant depressive disorder. Ther Drug Monit 36:730–733. [DOI] [PubMed] [Google Scholar]
- Keck PE Jr, McElroy SL (2001) Definition, evaluation, and management of treatment refractory mania. Psychopharmacol Bull 35:130–148. [PubMed] [Google Scholar]
- Keck PE Jr, Mintz J, McElroy SL, Freeman MP, Suppes T, Frye MA, Altshuler LL, Kupka R, Nolen WA, Leverich GS, Denicoff KD, Grunze H, Duan N, Post RM (2006) Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry 60:1020–1022. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Kane JM, Gelenberg AJ, Rosenbaum JF, Walzer EA, Baker LA (1992) Subsyndromal symptoms in bipolar disorder. A comparison of standard and low serum levels of lithium. Arch Gen Psychiatry 49:371–376. [DOI] [PubMed] [Google Scholar]
- Kemp DE, Gao K, Fein EB, Chan PK, Conroy C, Obral S, Ganocy SJ, Calabrese JR (2012) Lamotrigine as add-on treatment to lithium and divalproex: lessons learned from a double-blind, placebo-controlled trial in rapid-cycling bipolar disorder. Bipolar Disord 14:780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessing LV, Vradi E, Andersen PK (2016) Nationwide and population-based prescription patterns in bipolar disorder. Bipolar Disord 18:174–182. [DOI] [PubMed] [Google Scholar]
- Kessler U, Schoeyen HK, Andreassen OA, Eide GE, Hammar Å, Malt UF, Oedegaard KJ, Morken G, Sundet K, Vaaler AE (2013) Neurocognitive profiles in treatment-resistant bipolar I and bipolar II disorder depression. BMC Psychiatry 13:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketter TA, Wang PW, Chandler RA, Culver JL, Alarcon AM (2006) Adjunctive aripiprazole in treatment-resistant bipolar depression. Ann Clin Psychiatry 18:169–172. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Yang R, Frye MA (2015) Adjunctive armodafinil for major depressive episodes associated with bipolar I disorder. J Affect Disord 181:87–91. [DOI] [PubMed] [Google Scholar]
- Kimmel SE, Calabrese JR, Woyshville MJ, Meltzer HY (1994) Clozapine in treatment-refractory mood disorders. J Clin Psychiatry 55(Suppl B):91–93. [PubMed] [Google Scholar]
- Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, Correll CU (2016) Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med 46:1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukopoulos A, Reginaldi D, Minnai G, Serra G, Pani L, Johnson FN (1995) The long term prophylaxis of affective disorders. Adv Biochem Psychopharmacol 49:127–147. [PubMed] [Google Scholar]
- Koukopoulos A, Reginaldi D, Serra G, Koukopoulos A, Sani G, Serra G (2010) Antimanic and mood-stabilizing effect of memantine as an augmenting agent in treatment-resistant bipolar disorder. Bipolar Disord 12:348–349. [DOI] [PubMed] [Google Scholar]
- Kramlinger KG, Post RM (1989) Adding lithium carbonate to carbamazepine: antimanic efficacy in treatment-resistant mania. Acta Psychiatr Scand 79:378–385. [DOI] [PubMed] [Google Scholar]
- Kraus C, Rabl U, Vanicek T, Carlberg L, Popovic A, Spies M, Bartova L, Gryglewski G, Papageorgiou K, Lanzenberger R, Willeit M, Winkler D, Rybakowski JK, Kasper S (2017) Administration of ketamine for unipolar and bipolar depression. Int J Psychiatry Clin Pract 21:2–12. [DOI] [PubMed] [Google Scholar]
- Kusumakar V, Yatham LN (1997) An open study of lamotrigine in refractory bipolar depression. Psychiatry Res 72:145–148. [DOI] [PubMed] [Google Scholar]
- Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA (2014) Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry 4:e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzi L, Dell’Osso L, Cassano P, Pini S, Rucci P, Houck PR, Gemignani A, Battistini G, Bassi A, Abelli M, Cassano GB (2002) Pramipexole in treatment-resistant depression: a 16-week naturalistic study. Bipolar Disord 4:307–314. [DOI] [PubMed] [Google Scholar]
- Lee EE, Della Selva MP, Liu A, Himelhoch S (2015) Ketamine as a novel treatment for major depressive disorder and bipolar depression: a systematic review and quantitative meta-analysis. Gen Hosp Psychiatry 37:178–184. [DOI] [PubMed] [Google Scholar]
- Lee SY, Chen SL, Chang YH, Chen PS, Huang SY, Tzeng NS, Wang YS, Wang LJ, Lee IH, Wang TY, Yeh TL, Yang YK, Hong JS, Lu RB (2014a) The effects of add-on low-dose memantine on cytokine levels in bipolar II depression: a 12-week double-blind, randomized controlled trial. J Clin Psychopharmacol 34:337–343. [DOI] [PubMed] [Google Scholar]
- Lee SY, Chen SL, Chang YH, Chen SH, Chu CH, Huang SY, Tzeng NS, Wang CL, Wang LJ, Lee IH, Yeh TL, Yang YK, Hong JS, Lu RB (2014b) Genotype variant associated with add-on memantine in bipolar II disorder. Int J Neuropsychopharmacol 17:189–197. [DOI] [PubMed] [Google Scholar]
- Li XB, Tang YL, Wang CY, de Leon J (2015) Clozapine for treatment-resistant bipolar disorder: a systematic review. Bipolar Disord 17:235–247. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsman N, McIntyre RS, Giacobbe P, Torres C, Kennedy SH, Lozano AM (2010) Neurosurgical treatment of bipolar depression: defining treatment resistance and identifying surgical targets. Bipolar Disord 12:691–701. [DOI] [PubMed] [Google Scholar]
- Liu B, Zhang Y, Fang H, Liu J, Liu T, Li L (2017) Efficacy and safety of long-term antidepressant treatment for bipolar disorders - A meta-analysis of randomized controlled trials. J Affect Disord 223:41–48. [DOI] [PubMed] [Google Scholar]
- Lobban F, Taylor L, Chandler C, Tyler E, Kinderman P, Kolamunnage-Dona R, Gamble C, Peters S, Pontin E, Sellwood W, Morriss RK (2010) Enhanced relapse prevention for bipolar disorder by community mental health teams: cluster feasibility randomised trial. Br J Psychiatry 196:59–63. [DOI] [PubMed] [Google Scholar]
- Loebel A, Cucchiaro J, Silva R, Kroger H, Sarma K, Xu J, Calabrese JR (2014) Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry 171:169–177. [DOI] [PubMed] [Google Scholar]
- Loo C, Katalinic N, Mitchell PB, Greenberg B (2011) Physical treatments for bipolar disorder: a review of electroconvulsive therapy, stereotactic surgery and other brain stimulation techniques. J Affect Disord 132:1–13. [DOI] [PubMed] [Google Scholar]
- van der Loos ML, Mulder PG, Hartong EG, Blom MB, Vergouwen AC, de Keyzer HJ, Notten PJ, Luteijn ML, Timmermans MA, Vieta E, Nolen WA; LamLit Study Group (2009) Efficacy and safety of lamotrigine as add-on treatment to lithium in bipolar depression: a multicenter, double-blind, placebo-controlled trial. J Clin Psychiatry 70:223–231. [DOI] [PubMed] [Google Scholar]
- van der Loos ML, Mulder P, Hartong EG, Blom MB, Vergouwen AC, van Noorden MS, Timmermans MA, Vieta E, Nolen WA; LamLit Study Group (2010) Efficacy and safety of two treatment algorithms in bipolar depression consisting of a combination of lithium, lamotrigine or placebo and paroxetine. Acta Psychiatr Scand 122:246–254. [DOI] [PubMed] [Google Scholar]
- van der Loos ML, Mulder P, Hartong EG, Blom MB, Vergouwen AC, van Noorden MS, Timmermans MA, Vieta E, Nolen WA; LamLit Study Group (2011) Long-term outcome of bipolar depressed patients receiving lamotrigine as add-on to lithium with the possibility of the addition of paroxetine in nonresponders: a randomized, placebo-controlled trial with a novel design. Bipolar Disord 13:111–117. [DOI] [PubMed] [Google Scholar]
- Lykouras L, Hatzimanolis J (2004) Adjunctive topiramate in the maintenance treatment of bipolar disorders: an open-label study. Curr Med Res Opin 20:843–847. [DOI] [PubMed] [Google Scholar]
- Macfadden W, Alphs L, Haskins JT, Turner N, Turkoz I, Bossie C, Kujawa M, Mahmoud R (2009) A randomized, double-blind, placebo-controlled study of maintenance treatment with adjunctive risperidone long-acting therapy in patients with bipolar I disorder who relapse frequently. Bipolar Disord 11:827–839. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Soares JC, Lara DR, Luckenbaugh DA, Busnello JV, Marca G, Cunha A, Souza DO, Zarate CA Jr, Kapczinski F (2008) A double-blind, randomized, placebo-controlled 4-week study on the efficacy and safety of the purinergic agents allopurinol and dipyridamole adjunctive to lithium in acute bipolar mania. J Clin Psychiatry 69:1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes PV, Dean OM, Bush AI, Copolov DL, Malhi GS, Kohlmann K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Berk M (2011a) N-acetylcysteine for major depressive episodes in bipolar disorder. Braz J Psychiatry 33:374–378. [DOI] [PubMed] [Google Scholar]
- Magalhaes PV, Dean OM, Bush AI, Copolov DL, Malhi GS, Kohlmann K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Berk M (2011b) N-acetyl cysteine add-on treatment for bipolar II disorder: a subgroup analysis of a randomized placebocontrolled trial. J Affect Disord 129:317–320. [DOI] [PubMed] [Google Scholar]
- Magalhães PV, Dean OM, Bush AI, Copolov DL, Malhi GS, Kohlmann K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Berk M (2013) A preliminary investigation on the efficacy of N-acetyl cysteine for mania or hypomania. Aust N Z J Psychiatry 47:564–568. [DOI] [PubMed] [Google Scholar]
- Mahableshwarkar AR, Calabrese JR, Macek TA, Budur K, Adefuye A, Dong X, Hanson E, Sachs GS (2017) Efficacy and safety of sublingual ramelteon as an adjunctive therapy in the maintenance treatment of bipolar I disorder in adults: a phase 3, randomized controlled trial. J Affect Disord 221:275–282. [DOI] [PubMed] [Google Scholar]
- Maj M, Pirozzi R, Magliano L (1995) Nonresponse to reinstituted lithium prophylaxis in previously responsive bipolar patients: prevalence and predictors. Am J Psychiatry 152:1810–1811. [DOI] [PubMed] [Google Scholar]
- Manning JS. (2005) Burden of illness in bipolar depression. Prim Care Companion J Clin Psychiatry 7:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangell LB, Suppes T, Zboyan HA, Prashad SJ, Fischer G, Snow D, Sureddi S, Allen JC (2008) A 1-year pilot study of vagus nerve stimulation in treatment-resistant rapid-cycling bipolar disorder. J Clin Psychiatry 69:183–189. [DOI] [PubMed] [Google Scholar]
- Marcus R, Khan A, Rollin L, Morris B, Timko K, Carson W, Sanchez R (2011) Efficacy of aripiprazole adjunctive to lithium or valproate in the long-term treatment of patients with bipolar I disorder with an inadequate response to lithium or valproate monotherapy: a multicenter, double-blind, randomized study. Bipolar Disord 13:133–144. [DOI] [PubMed] [Google Scholar]
- van der Markt A, Klumpers UM, Draisma S, Dols A, Nolen WA, Post RM, Altshuler LL, Frye MA, Grunze H, Keck PE Jr, McElroy SL, Suppes T, Beekman AT, Kupka RW (2019) Testing a clinical staging model for bipolar disorder using longitudinal life chart data. Bipolar Disord 21:228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Dessain EC, Pope HG Jr, Cole JO, Keck PE Jr, Frankenberg FR, Aizley HG, O’Brien S (1991) Clozapine in the treatment of psychotic mood disorders, schizoaffective disorder, and schizophrenia. J Clin Psychiatry 52:411–414. [PubMed] [Google Scholar]
- McElroy SL, Soutullo CA, Keck PE Jr, Kmetz GF (1997) A pilot trial of adjunctive gabapentin in the treatment of bipolar disorder. Ann Clin Psychiatry 9:99–103. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Frye M, Denicoff K, Altshuler L, Nolen W, Kupka R, Suppes T, Keck PE Jr, Leverich GS, Kmetz GF, Post RM (1998) Olanzapine in treatment-resistant bipolar disorder. J Affect Disord 49:119–122. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Suppes T, Keck PE, Frye MA, Denicoff KD, Altshuler LL, Brown ES, Nolen WA, Kupka RW, Rochussen J, Leverich GS, Post RM (2000) Open-label adjunctive topiramate in the treatment of bipolar disorders. Biol Psychiatry 47:1025–1033. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Winstanley EL, Martens B, Patel NC, Mori N, Moeller D, McCoy J, Keck PE Jr (2011) A randomized, placebo-controlled study of adjunctive ramelteon in ambulatory bipolar I disorder with manic symptoms and sleep disturbance. Int Clin Psychopharmacol 26:48–53. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Martens BE, Mori N, Blom TJ, Casuto LS, Hawkins JM, Keck PE Jr (2015) Adjunctive lisdexamfetamine in bipolar depression: a preliminary randomized, placebo-controlled trial. Int Clin Psychopharmacol 30:6–13. [DOI] [PubMed] [Google Scholar]
- McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW (2015) A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med 45:693–704. [DOI] [PubMed] [Google Scholar]
- McGirr A, Vöhringer PA, Ghaemi SN, Lam RW, Yatham LN (2016a) Safety and efficacy of adjunctive second-generation antidepressant therapy with a mood stabiliser or an atypical antipsychotic in acute bipolar depression: a systematic review and meta-analysis of randomised placebo-controlled trials. Lancet Psychiatry 3:1138–1146. [DOI] [PubMed] [Google Scholar]
- McGirr A, Karmani S, Arsappa R, Berlim MT, Thirthalli J, Muralidharan K, Yatham LN (2016b) Clinical efficacy and safety of repetitive transcranial magnetic stimulation in acute bipolar depression. World Psychiatry 15:85–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medda P, Perugi G, Zanello S, Ciuffa M, Cassano GB (2009) Response to ECT in bipolar I, bipolar II and unipolar depression. J Affect Disord 118:55–59. [DOI] [PubMed] [Google Scholar]
- Medda P, Perugi G, Zanello S, Ciuffa M, Rizzato S, Cassano GB (2010) Comparative response to electroconvulsive therapy in medication-resistant bipolar I patients with depression and mixed state. J Ect 26:82–86. [DOI] [PubMed] [Google Scholar]
- Medda P, Toni C, Mariani MG, De Simone L, Mauri M, Perugi G (2015) Electroconvulsive therapy in 197 patients with a severe, drug-resistant bipolar mixed state: treatment outcome and predictors of response. J Clin Psychiatry 76:1168–1173. [DOI] [PubMed] [Google Scholar]
- Mendlewicz J, Massat I, Linotte S, Kasper S, Konstantinidis A, Lecrubier Y, Montgomery S, Serretti A, Zohar J, Souery D; Group for the Study of Resistant Depression (GSRD) (2010) Identification of clinical factors associated with resistance to antidepressants in bipolar depression: results from an European Multicentre Study. Int Clin Psychopharmacol 25:297–301. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, Viana MC, Andrade LH, Hu C, Karam EG, Ladea M, Medina-Mora ME, Ono Y, Posada-Villa J, Sagar R, Wells JE, Zarkov Z (2011) Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry 68:241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TD, Hautzinger M (2012) Cognitive behaviour therapy and supportive therapy for bipolar disorders: relapse rates for treatment period and 2-year follow-up. Psychol Med 42:1429–1439. [DOI] [PubMed] [Google Scholar]
- Miller F, Tanenbaum JH, Griffin A, Ritvo E (1991) Prediction of treatment response in bipolar, manic disorder. J Affect Disord 21:75–77. [DOI] [PubMed] [Google Scholar]
- Mishory A, Yaroslavsky Y, Bersudsky Y, Belmaker RH (2000) Phenytoin as an antimanic anticonvulsant: a controlled study. Am J Psychiatry 157:463–465. [DOI] [PubMed] [Google Scholar]
- Mishory A, Winokur M, Bersudsky Y (2003) Prophylactic effect of phenytoin in bipolar disorder: a controlled study. Bipolar Disord 5:464–467. [DOI] [PubMed] [Google Scholar]
- Miziou S, Tsitsipa E, Moysidou S, Karavelas V, Dimelis D, Polyzoidou V, Fountoulakis KN (2015) Psychosocial treatment and interventions for bipolar disorder: a systematic review. Ann Gen Psychiatry 14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai T, Kishi T, Matsuda Y, Iwata N (2014) A meta-analysis of inositol for depression and anxiety disorders. Hum Psychopharmacol 29:55–63. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Sackeim HA, Lee C (1988) Unilateral ECT in the treatment of manic episodes. Convuls Ther 4:74–80. [PubMed] [Google Scholar]
- Murphy BL, Stoll AL, Harris PQ, Ravichandran C, Babb SM, Carlezon WA Jr, Cohen BM (2012) Omega-3 fatty acid treatment, with or without cytidine, fails to show therapeutic properties in bipolar disorder: a double-blind, randomized add-on clinical trial. J Clin Psychopharmacol 32:699–703. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Babb SM, Ravichandran C, Cohen BM (2014) Oral SAMe in persistent treatment-refractory bipolar depression: a double-blind, randomized clinical trial. J Clin Psychopharmacol 34:413–416. [DOI] [PubMed] [Google Scholar]
- Murray JB. (1994) Lithium maintenance therapy for bipolar I patients: possible refractoriness to reinstitution after discontinuation. Psychol Rep 74:355–361. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV (2013) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Evans DL, Gyulai L, Sachs GS, Bowden CL, Gergel IP, Oakes R, Pitts CD (2001) Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry 158:906–912. [DOI] [PubMed] [Google Scholar]
- Nery FG, Monkul ES, Hatch JP, Fonseca M, Zunta-Soares GB, Frey BN, Bowden CL, Soares JC (2008) Celecoxib as an adjunct in the treatment of depressive or mixed episodes of bipolar disorder: a double-blind, randomized, placebo-controlled study. Hum Psychopharmacol 23:87–94. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB; APA Council of Research Task Force on Novel Biomarkers and Treatments (2015) Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 172:950–966. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Ostacher MJ, Calabrese JR, Ketter TA, Marangell LB, Miklowitz DJ, Miyahara S, Bauer MS, Thase ME, Wisniewski SR, Sachs GS (2006) Treatment-resistant bipolar depression: a STEP-BD equipoise randomized effectiveness trial of antidepressant augmentation with lamotrigine, inositol, or risperidone. Am J Psychiatry 163:210–216. [DOI] [PubMed] [Google Scholar]
- Nivoli AM, Pacchiarotti I, Rosa AR, Popovic D, Murru A, Valenti M, Bonnin CM, Grande I, Sanchez-Moreno J, Vieta E, Colom F (2011) Gender differences in a cohort study of 604 bipolar patients: the role of predominant polarity. J Affect Disord 133:443–449. [DOI] [PubMed] [Google Scholar]
- Norris ER, Burke K, Correll JR, Zemanek KJ, Lerman J, Primelo RA, Kaufmann MW (2013) A double-blind, randomized, placebo-controlled trial of adjunctive ramelteon for the treatment of insomnia and mood stability in patients with euthymic bipolar disorder. J Affect Disord 144:141–147. [DOI] [PubMed] [Google Scholar]
- Nuñez NA, Comai S, Dumitrescu E, Ghabrash MF, Tabaka J, Saint-Laurent M, Vida S, Kolivakis T, Fielding A, Low N, Cervantes P, Booij L, Gobbi G (2018) Psychopathological and sociodemographic features in treatment-resistant unipolar depression versus bipolar depression: a comparative study. BMC Psychiatry 18:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrocea GV, Dunn RM, Frye MA, Ketter TA, Luckenbaugh DA, Leverich GS, Speer AM, Osuch EA, Jajodia K, Post RM (2002) Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biol Psychiatry 51:253–260. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Tajika A, Takeshima N, Hayasaka Y, Furukawa TA (2014) Mood stabilizers and antipsychotics for acute mania: a systematic review and meta-analysis of combination/augmentation therapy versus monotherapy. CNS Drugs 28:989–1003. [DOI] [PubMed] [Google Scholar]
- Ostacher MJ. (2014) When positive isn’t positive: the hopes and disappointments of clinical trials. J Clin Psychiatry 75:e1186–e1187. [DOI] [PubMed] [Google Scholar]
- Pacchiarotti I, Mazzarini L, Colom F, Sanchez-Moreno J, Girardi P, Kotzalidis GD, Vieta E (2009) Treatment-resistant bipolar depression: towards a new definition. Acta Psychiatr Scand 120:429–440. [DOI] [PubMed] [Google Scholar]
- Pae CU, Masand PS, Mandel FS, O’Gorman C (2012) Achieving and sustaining remission in bipolar I disorder with ziprasidone: a post hoc analysis of a 24-week, double-blind, placebo-controlled study. Clin Drug Investig 32:747–754. [DOI] [PubMed] [Google Scholar]
- Pallanti S, Grassi G, Antonini S, Quercioli L, Salvadori E, Hollander E (2014) rTMS in resistant mixed states: an exploratory study. J Affect Disord 157:66–71. [DOI] [PubMed] [Google Scholar]
- Pande AC, Crockatt JG, Janney CA, Werth JL, Tsaroucha G (2000) Gabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Gabapentin Bipolar Disorder Study Group. Bipolar Disord 2:249–255. [DOI] [PubMed] [Google Scholar]
- Parikh SV, LeBlanc SR, Ovanessian MM (2010) Advancing bipolar disorder: key lessons from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Can J Psychiatry 55:136–143. [DOI] [PubMed] [Google Scholar]
- Parker GB, Graham RK (2017) Clinical characteristics associated with treatment-resistant bipolar disorder. J Nerv Ment Dis 205:188–191. [DOI] [PubMed] [Google Scholar]
- Parsaik AK, Singh B, Khosh-Chashm D, Mascarenhas SS (2015) Efficacy of ketamine in bipolar depression: systematic review and meta-analysis. J Psychiatr Pract 21:427–435. [DOI] [PubMed] [Google Scholar]
- Passos IC, Kapczinski F (2017) Should bipolar disorder treatment be modified depending on staging? Expert Rev Neurother 17:93–95. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Pae CU, Vöhringer PA, Mauer S, Narasimhan M, Dalley S, Loebel A, Masand PS, Ghaemi SN (2015) A 13-week, randomized double-blind, placebo-controlled, cross-over trial of ziprasidone in bipolar spectrum disorder. J Clin Psychopharmacol 35:319–323. [DOI] [PubMed] [Google Scholar]
- Pazzaglia PJ, Post RM, Ketter TA, George MS, Marangell LB (1993) Preliminary controlled trial of nimodipine in ultra-rapid cycling affective dysregulation. Psychiatry Res 49:257–272. [DOI] [PubMed] [Google Scholar]
- Pazzaglia PJ, Post RM, Ketter TA, Callahan AM, Marangell LB, Frye MA, George MS, Kimbrell TA, Leverich GS, Cora-Locatelli G, Luckenbaugh D (1998) Nimodipine monotherapy and carbamazepine augmentation in patients with refractory recurrent affective illness. J Clin Psychopharmacol 18:404–413. [DOI] [PubMed] [Google Scholar]
- Perry A, Tarrier N, Morriss R, McCarthy E, Limb K (1999) Randomised controlled trial of efficacy of teaching patients with bipolar disorder to identify early symptoms of relapse and obtain treatment. Bmj 318:149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perugi G, Toni C, Ruffolo G, Sartini S, Simonini E, Akiskal H (1999) Clinical experience using adjunctive gabapentin in treatment-resistant bipolar mixed states. Pharmacopsychiatry 32:136–141. [DOI] [PubMed] [Google Scholar]
- Perugi G, Toni C, Frare F, Ruffolo G, Moretti L, Torti C, Akiskal HS (2002) Effectiveness of adjunctive gabapentin in resistant bipolar disorder: is it due to anxious-alcohol abuse comorbidity? J Clin Psychopharmacol 22:584–591. [DOI] [PubMed] [Google Scholar]
- Perugi G, Medda P, Zanello S, Toni C, Cassano GB (2012) Episode length and mixed features as predictors of ECT nonresponse in patients with medication-resistant major depression. Brain Stimul 5:18–24. [DOI] [PubMed] [Google Scholar]
- Perugi G, Medda P, Toni C, Mariani MG, Socci C, Mauri M (2017) The role of electroconvulsive therapy (ECT) in bipolar disorder: effectiveness in 522 patients with bipolar depression, mixed-state, mania and catatonic features. Curr Neuropharmacol 15:359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, Owoeye O, Batten LA, Blier P (2019) Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry 176:401–409. [DOI] [PubMed] [Google Scholar]
- Pilhatsch M, Wolf R, Winter C, Lewitzka U, Bauer M (2010) Comparison of paroxetine and amitriptyline as adjunct to lithium maintenance therapy in bipolar depression: a reanalysis of a randomized, double-blind study. J Affect Disord 126:453–457. [DOI] [PubMed] [Google Scholar]
- Poleszczyk A, Rakowicz M, Parnowski T, Antczak J, Święcicki Ł (2018) Are there clinical and neurophysiologic predictive factors for a positive response to HF-rTMS in patients with treatment-resistant depression? Psychiatry Res 264:175–181. [DOI] [PubMed] [Google Scholar]
- Poon SH, Sim K, Sum MY, Kuswanto CN, Baldessarini RJ (2012) Evidence-based options for treatment-resistant adult bipolar disorder patients. Bipolar Disord 14:573–584. [DOI] [PubMed] [Google Scholar]
- Pope HG. (1991) Valproatereatment of Acute Mania. Arch Gen Psychiatry 48:62. [DOI] [PubMed] [Google Scholar]
- Pope HG Jr, McElroy SL, Keck PE Jr, Hudson JI (1991) Valproate in the treatment of acute mania. A placebo-controlled study. Arch Gen Psychiatry 48:62–68. [DOI] [PubMed] [Google Scholar]
- Popovic D, Reinares M, Goikolea JM, Bonnin CM, Gonzalez-Pinto A, Vieta E (2012) Polarity index of pharmacological agents used for maintenance treatment of bipolar disorder. Eur Neuropsychopharmacol 22:339–346. [DOI] [PubMed] [Google Scholar]
- Popovic D, Reinares M, Scott J, Nivoli A, Murru A, Pacchiarotti I, Vieta E, Colom F (2013) Polarity index of psychological interventions in maintenance treatment of bipolar disorder. Psychother Psychosom 82:292–298. [DOI] [PubMed] [Google Scholar]
- Popovic D, Torrent C, Goikolea JM, Cruz N, Sánchez-Moreno J, González-Pinto A, Vieta E (2014) Clinical implications of predominant polarity and the polarity index in bipolar disorder: a naturalistic study. Acta Psychiatr Scand 129:366–374. [DOI] [PubMed] [Google Scholar]
- Post RM. (2012) Acquired lithium resistance revisited: discontinuation-induced refractoriness versus tolerance. J Affect Disord 140:6–13. [DOI] [PubMed] [Google Scholar]
- Post RM, Altshuler LL, Frye MA, Suppes T, McElroy SL, Keck PE Jr, Leverich GS, Kupka R, Nolen WA, Luckenbaugh DA, Walden J, Grunze H (2005) Preliminary observations on the effectiveness of levetiracetam in the open adjunctive treatment of refractory bipolar disorder. J Clin Psychiatry 66:370–374. [DOI] [PubMed] [Google Scholar]
- Post RM, Altshuler LL, Frye MA, Suppes T, Rush AJ, Keck PE Jr, McElroy SL, Denicoff KD, Leverich GS, Kupka R, Nolen WA (2001) Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord 3:259–265. [PubMed] [Google Scholar]
- Post RM, Altshuler LL, Leverich GS, Frye MA, Nolen WA, Kupka RW, Suppes T, McElroy S, Keck PE, Denicoff KD, Grunze H, Walden J, Kitchen CM, Mintz J (2006) Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion and sertraline. Br J Psychiatry 189:124–131. [DOI] [PubMed] [Google Scholar]
- Post RM, Leverich G(2008) Loss of previous responsiveness to lithium following its discontinuation and subsequent episode recurrence. In: Treatment of Bipolar Illness: A Casebook for Clinicians and Patients (Post RM, Leverich G, eds), pp 123–130. New York: WW Norton, Inc. [Google Scholar]
- Post RM, Leverich GS, Altshuler L, Mikalauskas K (1992) Lithium-discontinuation-induced refractoriness: preliminary observations. Am J Psychiatry 149:1727–1729. [DOI] [PubMed] [Google Scholar]
- Post RM, Leverich GS, Pazzaglia P, Mikalauskas K, Denicoff K(1993) Lithium tolerance and discontinuation as pathways to refractoriness. In: Lithium in Medicine and Biology (Birch N, Padgham C, Hughes M, eds), pp 71–84. Lancashire, UK: Marius Press. [Google Scholar]
- Potter WZ, Murphy DL, Wehr TA, Linnoila M, Goodwin FK (1982) Clorgyline. A new treatment for patients with refractory rapid-cycling disorder. Arch Gen Psychiatry 39:505–510. [DOI] [PubMed] [Google Scholar]
- Quante A, Zeugmann S, Luborzewski A, Schommer N, Langosch J, Born C, Anghelescu I, Wolf J (2010) Aripiprazole as adjunct to a mood stabilizer and citalopram in bipolar depression: a randomized placebo-controlled pilot study. Hum Psychopharmacol 25:126–132. [DOI] [PubMed] [Google Scholar]
- Quiroz JA, Yatham LN, Palumbo JM, Karcher K, Kushner S, Kusumakar V (2010) Risperidone long-acting injectable monotherapy in the maintenance treatment of bipolar I disorder. Biol Psychiatry 68:156–162. [DOI] [PubMed] [Google Scholar]
- Regenold WT, Noorani RJ, Piez D, Patel P (2015) Nonconvulsive electrotherapy for treatment resistant unipolar and bipolar major depressive disorder: a proof-of-concept trial. Brain Stimul 8:855–861. [DOI] [PubMed] [Google Scholar]
- Reinares M, Colom F, Rosa AR, Bonnín CM, Franco C, Solé B, Kapczinski F, Vieta E (2010) The impact of staging bipolar disorder on treatment outcome of family psychoeducation. J Affect Disord 123:81–86. [DOI] [PubMed] [Google Scholar]
- Reinares M, Sánchez-Moreno J, Fountoulakis KN (2014) Psychosocial interventions in bipolar disorder: what, for whom, and when. J Affect Disord 156:46–55. [DOI] [PubMed] [Google Scholar]
- Rendell JM, Juszczak E, Hainsworth J, Gucht EV, Healey C, Morriss R, Ferrier N, Young AH, Young H, Goodwin GM, Geddes JR (2004) Developing the BALANCE trail–the role of the pilot study and start-up phase. Bipolar Disord 6:26–31. [DOI] [PubMed] [Google Scholar]
- Romeo B, Choucha W, Fossati P, Rotge JY (2015) Meta-analysis of short- and mid-term efficacy of ketamine in unipolar and bipolar depression. Psychiatry Res 230:682–688. [DOI] [PubMed] [Google Scholar]
- Rosa AR, Fountoulakis K, Siamouli M, Gonda X, Vieta E (2011) Is anticonvulsant treatment of mania a class effect? Data from randomized clinical trials. CNS Neurosci Ther 17:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblat JD, Kakar R, Berk M, Kessing LV, Vinberg M, Baune BT, Mansur RB, Brietzke E, Goldstein BI, McIntyre RS (2016) Anti-inflammatory agents in the treatment of bipolar depression: a systematic review and meta-analysis. Bipolar Disord 18:89–101. [DOI] [PubMed] [Google Scholar]
- Roy Chengappa KN, Schwarzman LK, Hulihan JF, Xiang J, Rosenthal NR; Clinical Affairs Product Support Study-168 Investigators (2006) Adjunctive topiramate therapy in patients receiving a mood stabilizer for bipolar I disorder: a randomized, placebo-controlled trial. J Clin Psychiatry 67:1698–1706. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Permoda-Osip A, Skibinska M, Adamski R, Bartkowska-Sniatkowska A (2013) Single ketamine infusion in bipolar depression resistant to antidepressants: are neurotrophins involved? Hum Psychopharmacol 28:87–90. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Permoda-Osip A, Bartkowska-Sniatkowska A (2017) Ketamine augmentation rapidly improves depression scores in inpatients with treatment-resistant bipolar depression. Int J Psychiatry Clin Pract 21:99–103. [DOI] [PubMed] [Google Scholar]
- Sachs G, Chengappa KN, Suppes T, Mullen JA, Brecher M, Devine NA, Sweitzer DE (2004) Quetiapine with lithium or divalproex for the treatment of bipolar mania: a randomized, double-blind, placebo-controlled study. Bipolar Disord 6:213–223. [DOI] [PubMed] [Google Scholar]
- Sachs GS. (1996) Treatment-resistant bipolar depression. Psychiatr Clin North Am 19:215–236. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Lafer B, Stoll AL, Banov M, Thibault AB, Tohen M, Rosenbaum JF (1994) A double-blind trial of bupropion versus desipramine for bipolar depression. J Clin Psychiatry 55:391–393. [PubMed] [Google Scholar]
- Sachs GS, Ice KS, Chappell PB, Schwartz JH, Gurtovaya O, Vanderburg DG, Kasuba B (2011) Efficacy and safety of adjunctive oral ziprasidone for acute treatment of depression in patients with bipolar I disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 72:1413–1422. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Vanderburg DG, Karayal ON, Kolluri S, Bachinsky M, Cavus I (2012a) Adjunctive oral ziprasidone in patients with acute mania treated with lithium or divalproex, part 1: results of a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 73:1412–1419. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Vanderburg DG, Edman S, Karayal ON, Kolluri S, Bachinsky M, Cavus I (2012b) Adjunctive oral ziprasidone in patients with acute mania treated with lithium or divalproex, part 2: influence of protocol-specific eligibility criteria on signal detection. J Clin Psychiatry 73:1420–1425. [DOI] [PubMed] [Google Scholar]
- Sajatovic M, DiGiovanni SK, Bastani B, Hattab H, Ramirez LF (1996) Risperidone therapy in treatment refractory acute bipolar and schizoaffective mania. Psychopharmacol Bull 32:55–61. [PubMed] [Google Scholar]
- Sajatovic M, Subramoniam M, Fuller MA (2006) Risperidone in the treatment of bipolar mania. Neuropsychiatr Dis Treat 2:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saligan LN, Luckenbaugh DA, Slonena EE, Machado-Vieira R, Zarate CA Jr (2016) An assessment of the anti-fatigue effects of ketamine from a double-blind, placebo-controlled, crossover study in bipolar disorder. J Affect Disord 194:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Junior B, Tortella G, Borrione L, Moffa AH, Machado-Vieira R, Cretaz E, Fernandes da Silva A, Fraguas R, Aparício LV, Klein I, Lafer B, Goerigk S, Benseñor IM, Lotufo PA, Gattaz WF, Brunoni AR (2018) Efficacy and safety of transcranial direct current stimulation as an add-on treatment for bipolar depression: a randomized clinical trial. JAMA Psychiatry 75:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandu AL, Artiges E, Galinowski A, Gallarda T, Bellivier F, Lemaitre H, Granger B, Ringuenet D, Tzavara ET, Martinot JL, Paillère Martinot ML (2017) Amygdala and regional volumes in treatment-resistant versus nontreatment-resistant depression patients. Depress Anxiety 34:1065–1071. [DOI] [PubMed] [Google Scholar]
- Sanford M, Dhillon S (2015) Lurasidone: a review of its use in adult patients with bipolar I depression. CNS Drugs 29:253–263. [DOI] [PubMed] [Google Scholar]
- Saricicek A, Maloney K, Muralidharan A, Ruf B, Blumberg HP, Sanacora G, Lorberg B, Pittman B, Bhagwagar Z (2011) Levetiracetam in the management of bipolar depression: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 72:744–750. [DOI] [PubMed] [Google Scholar]
- Sarris J, Mischoulon D, Schweitzer I (2012) Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry 73:81–86. [DOI] [PubMed] [Google Scholar]
- Schaff MR, Fawcett J, Zajecka JM (1993) Divalproex sodium in the treatment of refractory affective disorders. J Clin Psychiatry 54:380–384. [PubMed] [Google Scholar]
- Schaffer A, Levitt AJ, Joffe RT (2000) Mexiletine in treatment-resistant bipolar disorder. J Affect Disord 57:249–253. [DOI] [PubMed] [Google Scholar]
- Schaffer A, Zuker P, Levitt A (2006) Randomized, double-blind pilot trial comparing lamotrigine versus citalopram for the treatment of bipolar depression. J Affect Disord 96:95–99. [DOI] [PubMed] [Google Scholar]
- Schaffer C, Schaffer L, Howe J(2017) Treatment-resistant bipolar disorder. Psychiatric Times 34:33–35. [Google Scholar]
- Schaffer CB, Schaffer LC (1999) Open maintenance treatment of bipolar disorder spectrum patients who responded to gabapentin augmentation in the acute phase of treatment. J Affect Disord 55:237–240. [DOI] [PubMed] [Google Scholar]
- Schaffer LC, Schaffer CB, Caretto J (1999) The use of primidone in the treatment of refractory bipolar disorder. Ann Clin Psychiatry 11:61–66. [DOI] [PubMed] [Google Scholar]
- Schaffer LC, Schaffer CB, Miller AR, Manley JL, Piekut JA, Nordahl TE (2013) An open trial of pregabalin as an acute and maintenance adjunctive treatment for outpatients with treatment resistant bipolar disorder. J Affect Disord 147:407–410. [DOI] [PubMed] [Google Scholar]
- Scherk H, Pajonk FG, Leucht S (2007) Second-generation antipsychotic agents in the treatment of acute mania: a systematic review and meta-analysis of randomized controlled trials. Arch Gen Psychiatry 64:442–455. [DOI] [PubMed] [Google Scholar]
- Schoeyen HK, Kessler U, Andreassen OA, Auestad BH, Bergsholm P, Malt UF, Morken G, Oedegaard KJ, Vaaler A (2015) Treatment-resistant bipolar depression: a randomized controlled trial of electroconvulsive therapy versus algorithm-based pharmacological treatment. Am J Psychiatry 172:41–51. [DOI] [PubMed] [Google Scholar]
- Scott J, Paykel E, Morriss R, Bentall R, Kinderman P, Johnson T, Abbott R, Hayhurst H (2006) Cognitive-behavioural therapy for severe and recurrent bipolar disorders: randomised controlled trial. Br J Psychiatry 188:313–320. [DOI] [PubMed] [Google Scholar]
- Sentissi O, Popovic D, Moeglin C, Stukalin YB, Mosheva M, Vieta E, Serretti A, Souery D (2019) Predominant polarity in bipolar disorder patients: the COPE bipolar sample. J Affect Disord 250:43–50. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Stahl SM (2004) Risperidone and paroxetine given singly and in combination for bipolar depression. J Clin Psychiatry 65:1715–1719. [DOI] [PubMed] [Google Scholar]
- Sidor MM, Macqueen GM (2011) Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry 72:156–167. [DOI] [PubMed] [Google Scholar]
- Sienaert P, Lambrichts L, Dols A, De Fruyt J (2013) Evidence-based treatment strategies for treatment-resistant bipolar depression: a systematic review. Bipolar Disord 15:61–69. [DOI] [PubMed] [Google Scholar]
- Sikdar S, Kulhara P, Avasthi A, Singh H (1994) Combined chlorpromazine and electroconvulsive therapy in mania. Br J Psychiatry 164:806–810. [DOI] [PubMed] [Google Scholar]
- Silverstone PH, Birkett L (2000) Diltiazem as augmentation therapy in patients with treatment-resistant bipolar disorder: a retrospective study. J Psychiatry Neurosci 25:276–280. [PMC free article] [PubMed] [Google Scholar]
- Sit DK, McGowan J, Wiltrout C, Diler RS, Dills JJ, Luther J, Yang A, Ciolino JD, Seltman H, Wisniewski SR, Terman M, Wisner KL (2018) Adjunctive bright light therapy for bipolar depression: a randomized double-blind placebo-controlled trial. Am J Psychiatry 175:131–139. [DOI] [PubMed] [Google Scholar]
- Smith LA, Cornelius V, Warnock A, Tacchi MJ, Taylor D (2007) Acute bipolar mania: a systematic review and meta-analysis of co-therapy vs. monotherapy. Acta Psychiatr Scand 115:12–20. [DOI] [PubMed] [Google Scholar]
- Stamm TJ, Lewitzka U, Sauer C, Pilhatsch M, Smolka MN, Koeberle U, Adli M, Ricken R, Scherk H, Frye MA, Juckel G, Assion HJ, Gitlin M, Whybrow PC, Bauer M (2014) Supraphysiologic doses of levothyroxine as adjunctive therapy in bipolar depression: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 75:162–168. [DOI] [PubMed] [Google Scholar]
- Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, Cress KK, Marangell LB (1999) Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 56:407–412. [DOI] [PubMed] [Google Scholar]
- Suppes T, Webb A, Paul B, Carmody T, Kraemer H, Rush AJ (1999) Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry 156:1164–1169. [DOI] [PubMed] [Google Scholar]
- Suppes T, Chisholm KA, Dhavale D, Frye MA, Altshuler LL, McElroy SL, Keck PE, Nolen WA, Kupka R, Denicoff KD, Leverich GS, Rush AJ, Post RM (2002) Tiagabine in treatment refractory bipolar disorder: a clinical case series. Bipolar Disord 4:283–289. [DOI] [PubMed] [Google Scholar]
- Suppes T, Webb A, Paul B, Carmody T, Kraemer H, Rush AJ(2003) Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Focus 1:37–43. [DOI] [PubMed] [Google Scholar]
- Suppes T, Ozcan ME, Carmody T (2004) Response to clozapine of rapid cycling versus non-cycling patients with a history of mania. Bipolar Disord 6:329–332. [DOI] [PubMed] [Google Scholar]
- Suppes T, Calabrese JR, Silva R, Kroger H, Cucchiaro J, Pikalov A, Loebel A(2013) Lurasidone adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study (PREVAIL 3). Neuropsychopharmacology 38:S533–S534. [DOI] [PubMed] [Google Scholar]
- Suppes T, Kroger H, Pikalov A, Loebel A (2016) Lurasidone adjunctive with lithium or valproate for bipolar depression: a placebo-controlled trial utilizing prospective and retrospective enrolment cohorts. J Psychiatr Res 78:86–93. [DOI] [PubMed] [Google Scholar]
- Swann AC, Bowden CL, Calabrese JR, Dilsaver SC, Morris DD (1999) Differential effect of number of previous episodes of affective disorder on response to lithium or divalproex in acute mania. Am J Psychiatry 156:1264–1266. [DOI] [PubMed] [Google Scholar]
- Sylvia LG, Peters AT, Deckersbach T, Nierenberg AA (2013) Nutrient-based therapies for bipolar disorder: a systematic review. Psychother Psychosom 82:10–19. [DOI] [PubMed] [Google Scholar]
- Szegedi A, Calabrese JR, Stet L, Mackle M, Zhao J, Panagides J; Apollo Study Group (2012) Asenapine as adjunctive treatment for acute mania associated with bipolar disorder: results of a 12-week core study and 40-week extension. J Clin Psychopharmacol 32:46–55. [DOI] [PubMed] [Google Scholar]
- Szmulewicz AG, Angriman F, Samamé C, Ferraris A, Vigo D, Strejilevich SA (2017) Dopaminergic agents in the treatment of bipolar depression: a systematic review and meta-analysis. Acta Psychiatr Scand 135:527–538. [DOI] [PubMed] [Google Scholar]
- Tarr GP, Herbison P, de la Barra SL, Glue P (2011) Study design and patient characteristics and outcome in acute mania clinical trials. Bipolar Disord 13:125–132. [DOI] [PubMed] [Google Scholar]
- Tavares DF, Myczkowski ML, Alberto RL, Valiengo L, Rios RM, Gordon P, de Sampaio-Junior B, Klein I, Mansur CG, Marcolin MA, Lafer B, Moreno RA, Gattaz W, Daskalakis ZJ, Brunoni AR (2017) Treatment of bipolar depression with deep TMS: results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology 42:2593–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DM, Cornelius V, Smith L, Young AH (2014) Comparative efficacy and acceptability of drug treatments for bipolar depression: a multiple-treatments meta-analysis. Acta Psychiatr Scand 130:452–469. [DOI] [PubMed] [Google Scholar]
- Thase ME, Mallinger AG, McKnight D, Himmelhoch JM (1992) Treatment of imipramine-resistant recurrent depression, IV: a double-blind crossover study of tranylcypromine for anergic bipolar depression. Am J Psychiatry 149:195–198. [DOI] [PubMed] [Google Scholar]
- Tohen M, Waternaux CM, Tsuang MT (1990a) Outcome in Mania. A 4-year prospective follow-up of 75 patients utilizing survival analysis. Arch Gen Psychiatry 47:1106–1111. [DOI] [PubMed] [Google Scholar]
- Tohen M, Waternaux CM, Tsuang MT, Hunt AT (1990b) Four-year follow-up of twenty-four first-episode manic patients. J Affect Disord 19:79–86. [DOI] [PubMed] [Google Scholar]
- Tohen M, Chengappa KN, Suppes T, Zarate CA Jr, Calabrese JR, Bowden CL, Sachs GS, Kupfer DJ, Baker RW, Risser RC, Keeter EL, Feldman PD, Tollefson GD, Breier A (2002) Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 59:62–69. [DOI] [PubMed] [Google Scholar]
- Tohen M, Zarate CA Jr, Hennen J, Khalsa HM, Strakowski SM, Gebre-Medhin P, Salvatore P, Baldessarini RJ (2003a) The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry 160:2099–2107. [DOI] [PubMed] [Google Scholar]
- Tohen M, Ketter TA, Zarate CA, Suppes T, Frye M, Altshuler L, Zajecka J, Schuh LM, Risser RC, Brown E, Baker RW (2003b) Olanzapine versus divalproex sodium for the treatment of acute mania and maintenance of remission: a 47-week study. Am J Psychiatry 160:1263–1271. [DOI] [PubMed] [Google Scholar]
- Tohen M, Chengappa KN, Suppes T, Baker RW, Zarate CA, Bowden CL, Sachs GS, Kupfer DJ, Ghaemi SN, Feldman PD, Risser RC, Evans AR, Calabrese JR (2004) Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry 184:337–345. [DOI] [PubMed] [Google Scholar]
- Tohen M, Bowden CL, Calabrese JR, Lin D, Forrester TD, Sachs GS, Koukopoulos A, Yatham L, Grunze H (2006) Influence of sub-syndromal symptoms after remission from manic or mixed episodes. Br J Psychiatry 189:515–519. [DOI] [PubMed] [Google Scholar]
- Tohen M, Frank E, Bowden CL, Colom F, Ghaemi SN, Yatham LN, Malhi GS, Calabrese JR, Nolen WA, Vieta E, Kapczinski F, Goodwin GM, Suppes T, Sachs GS, Chengappa KR, Grunze H, Mitchell PB, Kanba S, Berk M (2009) The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord 11:453–473. [DOI] [PubMed] [Google Scholar]
- Tondo L, Baldessarini RJ, Floris G, Rudas N (1997) Effectiveness of restarting lithium treatment after its discontinuation in bipolar I and bipolar II disorders. Am J Psychiatry 154:548–550. [DOI] [PubMed] [Google Scholar]
- Tondo L, Vázquez GH, Baldessarini RJ (2014) Options for pharmacological treatment of refractory bipolar depression. Curr Psychiatry Rep 16:431. [DOI] [PubMed] [Google Scholar]
- Tseng PT, Chen YW, Tu KY, Chung W, Wang HY, Wu CK, Lin PY (2016) Light therapy in the treatment of patients with bipolar depression: a meta-analytic study. Eur Neuropsychopharmacol 26:1037–1047. [DOI] [PubMed] [Google Scholar]
- Vaidya NA, Mahableshwarkar AR, Shahid R (2003) Continuation and maintenance ECT in treatment-resistant bipolar disorder. J Ect 19:10–16. [DOI] [PubMed] [Google Scholar]
- Van Lieshout RJ, MacQueen GM (2010) Efficacy and acceptability of mood stabilisers in the treatment of acute bipolar depression: systematic review. Br J Psychiatry 196:266–273. [DOI] [PubMed] [Google Scholar]
- Vanelle JM, Loo H, Galinowski A, de Carvalho W, Bourdel MC, Brochier P, Bouvet O, Brochier T, Olie JP (1994) Maintenance ECT in intractable manic-depressive disorders. Convuls Ther 10:195–205. [PubMed] [Google Scholar]
- Vázquez GH, Tondo L, Undurraga J, Baldessarini RJ (2013) Overview of antidepressant treatment of bipolar depression. Int J Neuropsychopharmacol 16:1673–1685. [DOI] [PubMed] [Google Scholar]
- Veronese N, Solmi M, Luchini C, Lu RB, Stubbs B, Zaninotto L, Correll CU (2016) Acetylcholinesterase inhibitors and memantine in bipolar disorder: a systematic review and best evidence synthesis of the efficacy and safety for multiple disease dimensions. J Affect Disord 197:268–280. [DOI] [PubMed] [Google Scholar]
- Versiani M, Cheniaux E, Landeira-Fernandez J (2011) Efficacy and safety of electroconvulsive therapy in the treatment of bipolar disorder: a systematic review. J Ect 27:153–164. [DOI] [PubMed] [Google Scholar]
- Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, Calabrese JR, Gao K, Miskowiak KW, Grande I (2018) Bipolar disorders. Nat Rev Dis Primers 4:18008. [DOI] [PubMed] [Google Scholar]
- Vieta E, Garriga M (2016) Adjunctive antidepressants in bipolar depression. Lancet Psychiatry 3:1095–1096. [DOI] [PubMed] [Google Scholar]
- Vieta E, Gasto C, Colom F, Martinez A, Otero A, Vallejo J (1998) Treatment of refractory rapid cycling bipolar disorder with risperidone. J Clin Psychopharmacol 18:172–174. [DOI] [PubMed] [Google Scholar]
- Vieta E, Goikolea JM (2005) Atypical antipsychotics: newer options for mania and maintenance therapy. Bipolar Disord 7(Suppl 4):21–33. [DOI] [PubMed] [Google Scholar]
- Vieta E, Manuel Goikolea J, Martínez-Arán A, Comes M, Verger K, Masramon X, Sanchez-Moreno J, Colom F (2006) A double-blind, randomized, placebo-controlled, prophylaxis study of adjunctive gabapentin for bipolar disorder. J Clin Psychiatry 67:473–477. [DOI] [PubMed] [Google Scholar]
- Vieta E, Martinez-Arán A, Goikolea JM, Torrent C, Colom F, Benabarre A, Reinares M (2002a) A randomized trial comparing paroxetine and venlafaxine in the treatment of bipolar depressed patients taking mood stabilizers. J Clin Psychiatry 63:508–512. [DOI] [PubMed] [Google Scholar]
- Vieta E, Popovic D, Rosa AR, Solé B, Grande I, Frey BN, Martinez-Aran A, Sanchez-Moreno J, Balanzá-Martínez V, Tabarés-Seisdedos R, Kapczinski F (2013) The clinical implications of cognitive impairment and allostatic load in bipolar disorder. Eur Psychiatry 28:21–29. [DOI] [PubMed] [Google Scholar]
- Vieta E, Reinares M, Corbella B, Benabarre A, Gilaberte I, Colom F, Martínez-Arán A, Gastó C, Tohen M (2001) Olanzapine as long-term adjunctive therapy in treatment-resistant bipolar disorder. J Clin Psychopharmacol 21:469–473. [DOI] [PubMed] [Google Scholar]
- Vieta E, T’joen C, McQuade RD, Carson WH Jr, Marcus RN, Sanchez R, Owen R, Nameche L (2008) Efficacy of adjunctive aripiprazole to either valproate or lithium in bipolar mania patients partially nonresponsive to valproate/lithium monotherapy: a placebo-controlled study. Am J Psychiatry 165:1316–1325. [DOI] [PubMed] [Google Scholar]
- Vieta E, Torrent C, Garcia-Ribas G, Gilabert A, Garcia-Parés G, Rodriguez A, Cadevall J, García-Castrillón J, Lusilla P, Arrufat F (2002b) Use of topiramate in treatment-resistant bipolar spectrum disorders. J Clin Psychopharmacol 22:431–435. [DOI] [PubMed] [Google Scholar]
- de Vries C, van Bergen A, Regeer EJ, Benthem E, Kupka RW, Boks MP (2013) The effectiveness of restarted lithium treatment after discontinuation: reviewing the evidence for discontinuation-induced refractoriness. Bipolar Disord 15:645–649. [DOI] [PubMed] [Google Scholar]
- Vo D, Dunner DL (2003) Treatment-resistant bipolar disorder: a comparison of rapid cyclers and nonrapid cyclers. CNS Spectr 8:948–952. [DOI] [PubMed] [Google Scholar]
- Wang PW, Santosa C, Schumacher M, Winsberg ME, Strong C, Ketter TA (2002) Gabapentin augmentation therapy in bipolar depression. Bipolar Disord 4:296–301. [DOI] [PubMed] [Google Scholar]
- Wang PW, Yang YS, Chandler RA, Nowakowska C, Alarcon AM, Culver J, Ketter TA (2008) Adjunctive zonisamide for weight loss in euthymic bipolar disorder patients: a pilot study. J Psychiatr Res 42:451–457. [DOI] [PubMed] [Google Scholar]
- Wooderson SC, Fekadu A, Markopoulou K, Rane LJ, Poon L, Juruena MF, Strawbridge R, Cleare AJ (2014) Long-term symptomatic and functional outcome following an intensive inpatient multidisciplinary intervention for treatment-resistant affective disorders. J Affect Disord 166:334–342. [DOI] [PubMed] [Google Scholar]
- Woźniak-Kwaśniewska A, Szekely D, Harquel S, Bougerol T, David O (2015) Resting electroencephalographic correlates of the clinical response to repetitive transcranial magnetic stimulation: a preliminary comparison between unipolar and bipolar depression. J Affect Disord 183:15–21. [DOI] [PubMed] [Google Scholar]
- Wu JC, Kelsoe JR, Schachat C, Bunney BG, DeModena A, Golshan S, Gillin JC, Potkin SG, Bunney WE (2009) Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry 66:298–301. [DOI] [PubMed] [Google Scholar]
- Xu AJ, Niciu MJ, Lundin NB, Luckenbaugh DA, Ionescu DF, Richards EM, Vande Voort JL, Ballard ED, Brutsche NE, Machado-Vieira R, Zarate CA Jr (2015) Lithium and valproate levels do not correlate with ketamine’s antidepressant efficacy in treatment-resistant bipolar depression. Neural Plast 2015:858251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Hackett M, Carter G, Loo C, Galvez V, Glozier N, Glue P, Lapidus K, McGirr A, Somogyi AA, Mitchell PB, Rodgers A(2016) Effects of low-dose and very low-dose ketamine among patients with major depression: a systematic review and meta-analysis. Int J Neuropsychopharmacol 19. pii: pyv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatham LN, Calabrese JR, Kusumakar V (2003a) Bipolar depression: criteria for treatment selection, definition of refractoriness, and treatment options. Bipolar Disord 5:85–97. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Grossman F, Augustyns I, Vieta E, Ravindran A (2003b) Mood stabilisers plus risperidone or placebo in the treatment of acute mania. International, double-blind, randomised controlled trial. Br J Psychiatry 182:141–147. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Fallu A, Binder CE(2007a) A 6-month randomized open-label comparison of continuation of oral atypical antipsychotic therapy or switch to long acting injectable risperidone in patients with bipolar disorder. Acta Psychiatr Scand Suppl. 434:50–56. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Vieta E, Young AH, Möller HJ, Paulsson B, Vågerö M (2007b) A double blind, randomized, placebo-controlled trial of quetiapine as an add-on therapy to lithium or divalproex for the treatment of bipolar mania. Int Clin Psychopharmacol 22:212–220. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Vieta E, Goodwin GM, Bourin M, de Bodinat C, Laredo J, Calabrese J; Agomelatine Study Group (2016) Agomelatine or placebo as adjunctive therapy to a mood stabiliser in bipolar I depression: randomised double-blind placebo-controlled trial. Br J Psychiatry 208:78–86. [DOI] [PubMed] [Google Scholar]
- Yatham LN, et al. (2018) Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord 20:97–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorguner Kupeli N, Bulut NS, Carkaxhiu Bulut G, Kurt E, Kora K (2018) Efficacy of bright light therapy in bipolar depression. Psychiatry Res 260:432–438. [DOI] [PubMed] [Google Scholar]
- Young LT, Joffe RT, Robb JC, MacQueen GM, Marriott M, Patelis-Siotis I (2000) Double-blind comparison of addition of a second mood stabilizer versus an antidepressant to an initial mood stabilizer for treatment of patients with bipolar depression. Am J Psychiatry 157:124–126. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA (2012) Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky A, Lancee W, Miller C, Harris A, Parikh SV (2008) Is cognitive-behavioural therapy more effective than psychoeducation in bipolar disorder? Can J Psychiatry 53:441–448. [DOI] [PubMed] [Google Scholar]
- Zeinoddini A, Sorayani M, Hassanzadeh E, Arbabi M, Farokhnia M, Salimi S, Ghaleiha A, Akhondzadeh S (2015) Pioglitazone adjunctive therapy for depressive episode of bipolar disorder: a randomized, double-blind, placebo-controlled trial. Depress Anxiety 32:167–173. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yang H, Yang S, Liang W, Dai P, Wang C, Zhang Y (2013) Antidepressants for bipolar disorder: a meta-analysis of randomized, double-blind, controlled trials. Neural Regen Res 8:2962–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou TH, Dang WM, Ma YT, Hu CQ, Wang N, Zhang GY, Wang G, Shi C, Zhang H, Guo B, Zhou SZ, Feng L, Geng SX, Tong YZ, Tang GW, He ZK, Zhen L, Yu X (2018) Clinical efficacy, onset time and safety of bright light therapy in acute bipolar depression as an adjunctive therapy: a randomized controlled trial. J Affect Disord 227:90–96. [DOI] [PubMed] [Google Scholar]