Although the neural substrates of auditory word recognition have been a topic of inquiry since the heyday of classical neurology (Wernicke, 1874), they remain poorly understood (Bogen & Bogen, 1976; DeWitt & Rauschecker, 2013). The arc of development in classical models was increasingly to propose Wernicke’s area, the site of auditory word-form recognition, to lie along the posterior aspect of the superior temporal gyrus (STG) (DeWitt & Rauschecker, 2013). Appreciation of the dual-stream organization of primate auditory cortex (Rauschecker & Scott, 2009; Rauschecker & Tian, 2000) initiated a challenge to this perspective, resulting in a reformulation of models of language organization in humans (Binder et al., 2000; Hickok & Poeppel, 2007; Wise et al., 2001). The preponderance of evidence from human functional imaging studies now sites auditory word recognition in anterior STG, near the anterior-lateral aspect of Heschl’s gyrus, part of the auditory ventral stream (DeWitt & Rauschecker, 2012). Although findings from lesion studies generally support this conclusion, results from key experiments, namely, those specifically relating lesion site to auditory single-word comprehension, have been lacking (DeWitt & Rauschecker, 2013). A pair of recent studies contributes toward filling this gap (Mesulam, Thompson, Weintraub, & Rogalski, 2015; Roux et al., 2015).
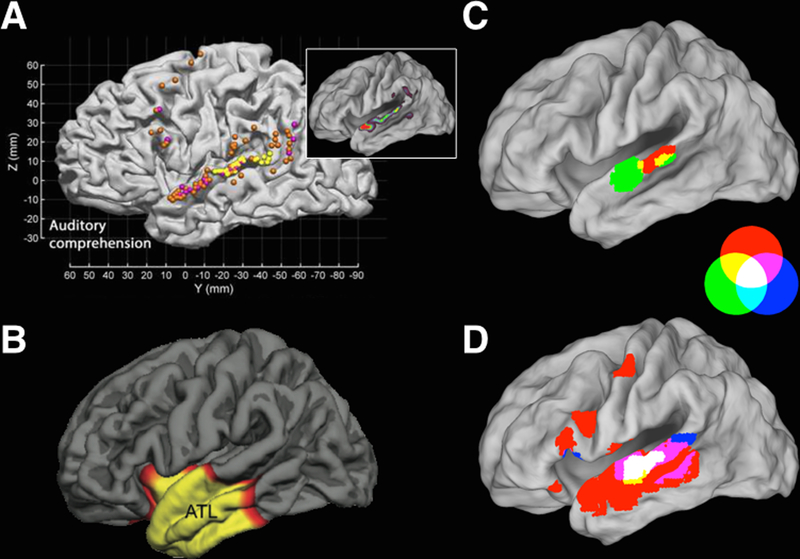
Roux et al. (2015) report findings from intraoperative stimulation mapping in 90 patients at 2754 cortical sites, extensively sampling STG, supramarginal gyrus (SMG), middle temporal gyrus (MTG), and angular gyrus (AG). Three striking findings emerge from the study (see Figure 1A): First, although MTG was extensively surveyed, it was found to be uninvolved in auditory single-word comprehension, as indexed by performance on auditory word-to-picture matching. Second, although stimulation of several SMG/AG sites impaired comprehension, the dominant finding was for comprehension to be impaired by STG stimulation (see Figure 1A, inset).
Figure 1.
Semantic retrieval in auditory single-word comprehension is associated with the integrity of anterior STG function, as found in stimulation mapping (A, orange markers) and lesion-symptom mapping (B). Auditory word-form recognition is associated with mid-to-anterior STG, as found in stimulation mapping (A, yellow markers) and meta-analysis of functional imaging (C, D). The meta-analytic results depicted are RGB overlap maps for effects of repetition suppression for phonemes (C, red shading) and auditory words (C, green shading), as well as for tests of speech-related combination sensitivity (D, red shading), invariant representation (D, green shading) and areal specialization (D, blue shading). Notably, when a probability density function is estimated for the reported stimulation mapping foci (A), peak density is found along STG, excluding the most posterior aspect of STG, with the global peak in anterior STG (A, inset). Together, these results imply a processing hierarchy for phonemes, word-forms and semantic retrieval that extends along STG in an anterior-oriented gradient. Adapted from (DeWitt & Rauschecker, 2012; Mesulam et al., 2015; Roux et al., 2015).
Third, analysis of error types shows an anterior-oriented processing hierarchy within STG. Performance on auditory word-to-picture matching involves (i) auditory word-form recognition, (ii) retrieval of the word-form’s semantic referents, and (iii) association of one of the referents to a visual depiction thereof. Roux et al. (2015) classified errors as either “speech discrimination/word deafness errors,” indicating a putative failure to resolve an auditory word-form from sensory input, or “lexical-semantic errors,” indicating putative failure to associate an accurately perceived word-form with a visual depiction of its semantic referent. Word-form analysis (transient word deafness) was associated with mid-to-anterior STG (Figure 1A, yellow markers) while semantic retrieval (matching error) was associated with anterior STG (Figure 1A, orange markers), indicating an anterior orientation in the major processing pathway.
Roux et al.’s (2015) findings complement a recent report from Mesulam et al. (2015). Mesulam et al. (2015) quantified cortical atrophy in 67 patients with primary progressive aphasia (PPA) and related that atrophy to auditory single-word comprehension, again via performance on auditory word-to-picture matching. Despite atrophy being present throughout the frontal lobe, temporal lobe and inferior parietal lobule (IPL) in the patient sample, only anterior temporal lobe (ATL) atrophy, in toto, was correlated with auditory single-word comprehension (see Figure 1B). The correlation of auditory single-word comprehension with ATL atrophy in toto, however, may be an artifact of high local spatial correlation in individual patient’s atrophy patterns or an artifact of a common pattern of progression (e.g., involving polar sites early in the progression). A conservative inference would assume a more circumscribed area within the ATL atrophy region to be driving the mapped correlation.
That Mesulam et al. (2015) did not find atrophy associated with comprehension deficits to extend through mid STG, as found in Roux et al. (2015), may reflect behavioral inclusion criteria that intentionally biased the sample toward high-functioning patients (i.e., few cases exhibiting word deafness). Consistent with this, none of the patients performed at floor level on the auditory comprehension task. Therefore, although definitive conclusion cannot be reached, the atrophy reported by Mesulam et al. (2015) more likely reflects errors in semantic retrieval. These considerations place the findings of Mesulam et al. (2015) and Roux et al. (2015) in good agreement.
Mesulam et al.’s (2015) report further dissociates cortical areas supporting syntactic aspects of sentence comprehension from areas involved in auditory single-word comprehension, finding syntactic processing deficits to be associated with IPL lesions in PPA patients, as has been similarly documented in stroke patients (Thothathiri, Kimberg, & Schwartz, 2012). This affirms a role for the dorsal stream in syntactic processing and language evolution (Bornkessel-Schlesewsky & Schlesewsky, 2013; Bornkessel-Schlesewsky et al., 2015; DeWitt & Rauschecker, 2013; Friederici, 2012) and clarifies the role of anterior STG as being chiefly involved in word recognition.
Together, Roux et al. (2015) and Mesulam et al. (2015) provide compelling evidence for the causal involvement of anterior STG in auditory single-word comprehension. Specifically, semantic retrieval appears to depend upon the integrity of STG in a region immediately anterior to the anterior-lateral aspect of Heschl’s gyrus. This comports with results from functional imaging (see Figure 1C,D, this commentary) (DeWitt & Rauschecker, 2012) and with an anterior-oriented model of the auditory ventral stream, where auditory word-form recognition occurs in mid-to-anterior STG and semantic retrieval occurs in anterior STG. Further, these regions are dissociated from higher-order aspects of comprehension, namely, syntactic processing.
Acknowledgements
This work was supported by the National Institute of Deafness and Communication Disorders Intramural Research Program (I.D.), the National Science Foundation (grants BCS-0519127 and OISE-0730255, J.P.R.), the National Institutes of Health (grants R01DC03489 and R01NS052494, J.P.R.) and the Technische Universität München–Institute for Advanced Study (funded by the German Excellence Initiative and the European Union Seventh Framework Programme agreement n° 291763, J.P.R.).
References
- Binder J J A R, Frost, T A Hammeke, P S Bellgowan, J A Springer, J N Kaufman, & E T. Possing (2000). Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex, 10, 512–528. [DOI] [PubMed] [Google Scholar]
- J E Bogen, & G M Bogen. (1976). Wernicke’s region, where is it? Ann New York Acad Sci, 280, 834–843. doi: 10.1111/j.1749-6632.1976.tb25546.x [DOI] [PubMed] [Google Scholar]
- Bornkessel-Schlesewsky Ina, & Schlesewsky Matthias. (2013). Reconciling time, space and function: A new dorsal-ventral stream model of sentence comprehension. Brain Lang, 125, 60–76. doi: 10.1016/j.bandl.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Bornkessel-Schlesewsky I, Schlesewsky M, Small SL, Rauschecker JP (2015) Neurobiological roots of language in primate audition: common computational properties. Trends Cogn Sci, 19,142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt Iain, & Rauschecker Josef P. (2012). Phoneme and word recognition in the auditory ventral stream. Proc Nat Acad Sci USA, 109, E505–514. doi: 10.1073/pnas.1113427109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt Iain, & Rauschecker Josef P. (2013). Wernicke’s area revisited: Parallel streams and word processing. Brain Lang, 127, 181–191. doi: 10.1016/j.bandl.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici Angela D. (2012). The cortical language circuit: from auditory perception to sentence comprehension. Trends Cogn Sci, 16, 262–268. doi: 10.1016/j.tics.2012.04.001 [DOI] [PubMed] [Google Scholar]
- Hickok Gregory, & Poeppel David. (2007). The cortical organization of speech processing. Nat Rev Neurosci, 8, 393–402. doi: 10.1038/nrn2113 [DOI] [PubMed] [Google Scholar]
- Mesulam M Marsel, Thompson Cynthia K, Weintraub Sandra, & Rogalski Emily J. (2015). The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain, in press. doi: 10.1093/brain/awv154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker Josef P, & Scott Sophie K. (2009). Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci, 12, 718–724. doi: doi: 10.1038/nn.2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker Josef P, & Tian Biao. (2000). Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci USA, 97, 11800–11806. doi: 10.1073/pnas.97.22.11800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux Franck-Emmanuel, Miskin Krassimir, Durand Jean-Baptiste, Sacko Oumar, Rehault Emilie, Tanova Rositsa, & Demonet Jean Francois. (2015). Electrostimulation mapping of comprehension of auditory and visual words. Cortex, in press. [DOI] [PubMed] [Google Scholar]
- Thothathiri Malathi, Kimberg Daniel Y, & Schwartz Myrna F. (2012). The neural basis of reversible sentence comprehension: evidence from voxel-based lesion symptom mapping in aphasia. J Cogn Neurosci, 24, 212–222. doi: 10.1162/jocn_a_00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke C. (1874). Der aphasische Symptomencomplex. Breslau: Max Cohn & Weigert. [Google Scholar]
- Wise Richard J S, Scott SK, Blank SC, Mummery CJ, Murphy K, & Warburton EA (2001). Separate neural subsystems within “Wernicke’s area”. Brain, 124, 83–95. [DOI] [PubMed] [Google Scholar]