Abstract
The interest in lentiviral vectors (LVs) has increased prominently for gene therapy applications, but few have reached the later stages of clinical trials. The main challenge has remained in scaling up the manufacturing process for the fragile vector to obtain high titers for in vivo usage. We have previously scaled up the LV production to iCELLis 500, being able to produce up to 180 L of harvest material in one run with perfusion. The following challenge considers the purification and concentration of the product to meet titer and purity requirements for clinical use. We have developed a downstream process, beginning with clarification, buffer exchange, and concentration, by tangential flow filtration. This is followed by a purification step using single membrane-based anion exchange chromatography and final formulation with tangential flow filtration. Different materials and conditions were compared to optimize the process, especially for the chromatography step that has been the bottleneck in lentiviral vector purification scale-up. The final infectious titer of the lentiviral vector product manufactured using the optimized scale-up process was determined to be 1.97 × 109 transducing units (TU)/mL, which can be considered as a high titer for lentiviral vectors.
Keywords: lentiviral vector, process, scale-up, tangential flow filtration, anion exchange, chromatography, purification
The concentration and purification of the LVs were optimized and scaled up to process a 180-L harvest, with the main optimization concentrating on anion exchange chromatography. The process yielded a high-titer LV product, with the majority of the impurities removed.
Introduction
Lentiviral vectors (LVs) are an increasingly popular1 and promising gene therapy tool, due to their ability to transduce both dividing and nondividing cells. Two LV-based gene therapy products have recently been commercialized: Kymriah (Novartis) for treating large B cell lymphoma and B cell precursor acute lymphoblastic leukemia,2,3 and Zynteglo (bluebird bio) for transfusion-dependent beta-thalassemia.4 Other applications assessed in clinical trials include treatments for cerebral adrenoleukodystrophy,5 metachromatic leukodystrophy,6 Wiskott-Aldrich syndrome,7 and Parkinson’s disease.8 The efficacy of LV-mediated gene therapy with herpes simplex virus 1 thymidine kinase (HSV-1-TK) in treatment of glioblastoma has previously been confirmed with animal models.9 In our concurrent study,10 we have examined the effect of the lentiviral vector encoding thymidine kinase/ganciclovir (LV-TK/GCV) treatment on glioblastoma in vivo using rat models. In addition, we optimized the upstream process and did two large-scale LV production runs (LV encoding green fluorescent protein [GFP] and LV-TK) using iCELLis adherent cell-culture technology. We provided the proof of concept of LV-TK/GCV functionality and successfully scaled up the LV upstream production toward a commercial scale with a harvest volume of 180 L.
Large-scale manufacturing has been the bottleneck in the transition from clinical trials to commercial use.11 The main obstacles in LV downstream (DS) have been the handling of large volumes and loss of functionality during processing. Because of the fragile nature of LV, short process times and a limited number of process steps are essential for maintaining the activity of the vector. The processing units and methods used for small-scale concentration and purification are generally not applicable to large scale. Scalable methods include depth microfiltration for clarification,12 tangential flow filtration (TFF) with either membrane,13 or hollow-fiber14 filters for concentration and buffer exchange and chromatography for purification. Anion exchange (AEX)15 and affinity chromatography16 have been suggested for the main purification step and size-exclusion chromatography for a polishing step.14 However, the recoveries have remained low, especially for the main chromatography purification step. Despite extensive studies on DS methods, only few publications show DS scale-up for starting volumes of over 50 L.14,17 One of the reasons for slow DS development and optimization rate is the need for large amounts of LV material with consistent characteristics, which means that the upstream production needs to be scaled up first to provide the material for DS.
In this work, we present the DS optimization for LVs. The steps include clarification, concentration, and buffer exchange with tangential flow filtration, AEX chromatography purification, as well as final formulation. For product formulation, several final formulation buffers (FFBs) were screened and virus stability studied.
Results
Downstream Development Overview
The production of the LV material is described in more detail in Leinonen et al.10 The upstream production of the LVs was optimized prior to and partly simultaneously with the downstream development. Vector with a GFP transgene was used in the first production runs, including the first scale-up run, to facilitate functional titering using flow cytometry. Clarification, TFF and AEX support selection (Table 1), and prescreening of main parameters (Tables 2 and 3) were mostly performed using LV material from these runs. In the later runs, we transferred to LV-TK production and used a qPCR-based titering method. Functional recoveries are essential to be able to value the success of the purification steps; however, p24 ELISA-based total viral particle (vp) recovery remains a sufficient method for a quick and noncell-based check for the overall LV recovery of a process step.18 A comparison between vp concentration and transducing units (TUs) also reveals the activity loss in a processing step. Analytics for the total amount of physical lentivirus particles by p24 ELISA were not performed in all experiments during small-scale process development, in which case, only the TUs were driving the study. The full DS process is depicted in Figure 1.
Table 1.
List of Materials Used during Downstream Process Development of Lentivirus Vector Production
| Process Step | Support | Material/MWCO | Manufacturer | Notes |
|---|---|---|---|---|
| Clarification/filtration | Millistak+ Pod depth filter CE50, 0.027 m2, ref. MCE50027H1 | cellulose | Merck Millipore | standard in small-scale runs |
| Sartopore 2 midcap size 7: 0.8 + 0.45 μm, 0.05 m2, ref. 5445306G7 | polyethersulfone (PES) | Sartorius Stedim Biotech | tested | |
| Sartoclean CA midcap size 7: 0.45 μm, 0.08 m2, ref. 5625306A7 | cellulose | Sartorius Stedim Biotech | tested | |
| Millistak+ Pod depth filter CE50, 0.77 m2, ref. MCE5007FS1 | cellulose | Merck Millipore | first and second scale-up run (single filter) | |
| TFF concentration and buffer exchange | Sartocon Slice Hydrosart 0.11 m2, 100 kDa, ref. 305 144 6801E-SG | cellulose/hydrosart | Sartorius Stedim Biotech | used in TFF1a development and in the scale-up runs TFF2b (single cassette) |
| Sartocon Hydrosart 0.6 m2, 100 kDa, ref. 302 144 6806E-SG | cellulose/hydrosart | Sartorius Stedim Biotech | 5 × 0.6 m2 in first scale-up run TFF1 | |
| Sartocube Hydrosart 3 m2, 100 kDa, ref. 302 144 6830E-BSW | cellulose/hydrosart | Sartorius Stedim Biotech | 1 Sartocube + 2 × Sartocon (total 4.2 m2) in second scale-up run TFF1 | |
| Pellicon 2 Mini Ultracel type V 0.1 m2, 100 kDa, ref. P2C100V01 | cellulose/Ultracel | Merck Millipore | tested for TFF1 | |
| Pellicon 2 Mini Ultracel type V 0.1 m2, 300 kDa, ref. P2C300V01 | cellulose/Ultracel | Merck Millipore | tested for TFF1 | |
| Pellicon 2 Mini Biomax type V 0.1 m2, 100 kDa, ref. P2B100V01 | PES/Biomax | Merck Millipore | tested for TFF1 | |
| Chromatography | CIMmultus QA 1 mL (2 μm), ref. 311.5113-2 | CIM monolithic advanced composite column | BIASeparations | tested for AEXc |
| CIMmultus QA 1 mL (6 μm), ref. 311.5113-6 | CIM monolithic advanced composite column | BIASeparations | tested for AEX | |
| CIMmultus DEAE 1 mL (2 μm), ref. 311.5114-2 | CIM monolithic advanced composite column | BIASeparations | tested for AEX | |
| Sartobind Q nano 3 mL, 8 mm, ref. 96IEXQ42EUC11-A | reinforced cellulose | Sartorius Stedim Biotech | standard in small-scale runs | |
| Sartobind Phenyl nano 3 mL, 8 mm, ref. 96HICP42EUC11-A | reinforced cellulose | Sartorius Stedim Biotech | tested for HIC | |
| Mustang Q XT Acrodisc 0.86 mL, ref. MSTGXT25Q16 | hydrophilic PES | Pall | tested for AEX | |
| Sartobind Q 150 mL, 8 mm, ref. 96IEXQ42E9BFF | reinforced cellulose | Sartorius Stedim Biotech | AEX/first scale-up run | |
| Sartobind Q 400 mL, 8 mm, ref. 96IEXQ42E1HSS | reinforced cellulose | Sartorius Stedim Biotech | AEX/second scale-up run |
The list includes clarification, tangential flow filtration, and chromatographic supports. DEAE, diethylaminoethyl; HIC, hydrophobic intercation chromatography; MWCO, molecular weight cutoff.
Concentration and buffer change after clarification using tangential flow filtration.
Final buffer change using tangential flow filtration.
Anion exchange chromatography.
Table 2.
Tangential Flow Filtration Optimization Parameters
| Phase | System | Feed (kg/m2) | Flux (LMHa) | Crossflow (%) | TMP Maxb (Bar) |
|---|---|---|---|---|---|
| Optimization | ÄKTA crossflow | 10–70 | 10 | 64 | 0.06 |
| Optimization | Cogent M | 50–120 | 24–100 | 88–90 | 0.2–0.5 |
| First scale-up run TFF1 | Mobius | 42 | 14 | 88 | 0.1 |
| First scale-up run TFF2 | Cogent M | 8.4 | 12 | 94 | 0.2 |
| Second scale-up run TFF1 | Mobius | 43 | 13 | 89 | 0.2 |
| Second scale-up run TFF2 | Cogent M | 8.6 | 8.4–12 | 94–97 | NA |
NA, not applicable.
Liters per square meter per hour.
Maximum transmembrane pressure during the run.
Table 3.
Main Parameters Studied to Optimize the Chromatographic Capture Step
| Parameter | Conditions Tested | Chromatographic Step |
|---|---|---|
| Starting material for capture | TFF1a stock | AEXb |
| clarified stock | ||
| fresh and frozen stocks | ||
| Additives | sucrose 10% | AEX |
| Flow rate | 1 to 5 CV/minc | AEX |
| Conductivity | diluted (150 mM NaCl) and standard (300 mM NaCl) TFF1 stocks | AEX |
| Type of salt | 0.9 M NaCl and 1 M (NH4)2SO4 | HICd |
Concentration and buffer change after clarification using tangential flow filtration.
Anion exchange chromatography.
Column volumes per minute.
Hydrophobic intercation chromatography.
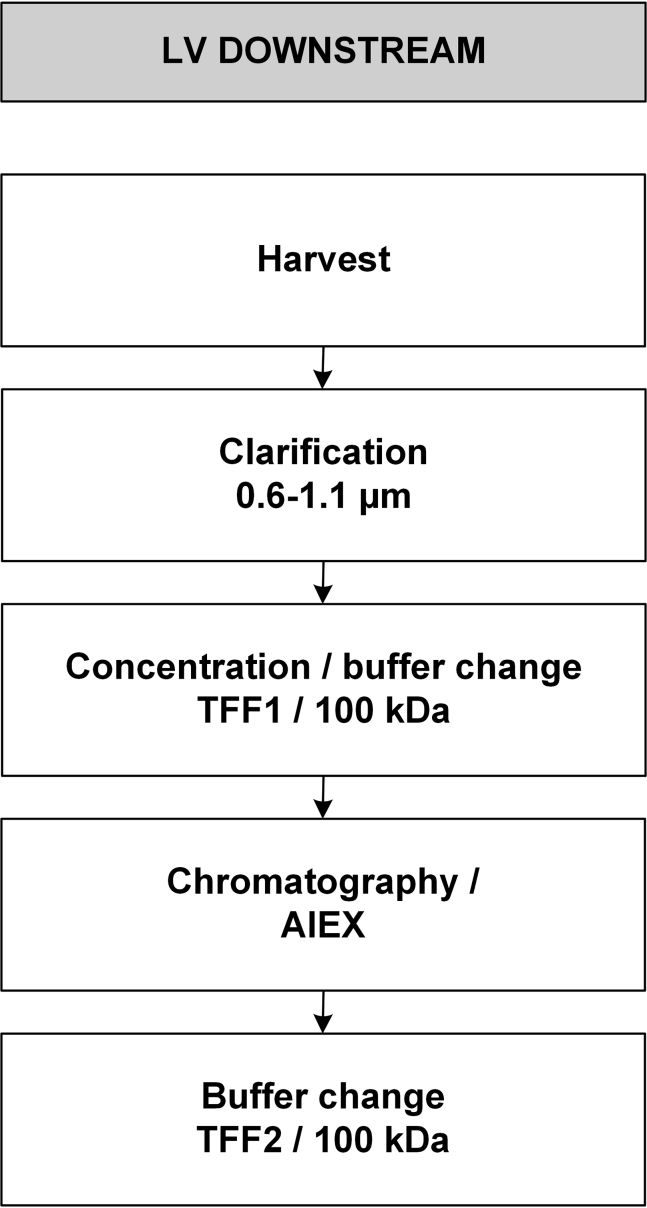
Figure 1.
Process Flow Chart for Scalable LV-TK Downstream Purification from Harvested Material
The first large-scale run (iCELLis 500/100 m2, LV-GFP) was performed to obtain uniform and concentrated upstream LV stock to further optimize the DS process. The second large-scale run (iCELLis 500/333 m2, LV-TK) was performed as a complete process from upstream production to final product. Main DS development results are described step by step in each corresponding section, whereas large-scale runs are presented as separate processes to evaluate the success of the scale-up.
Clarification
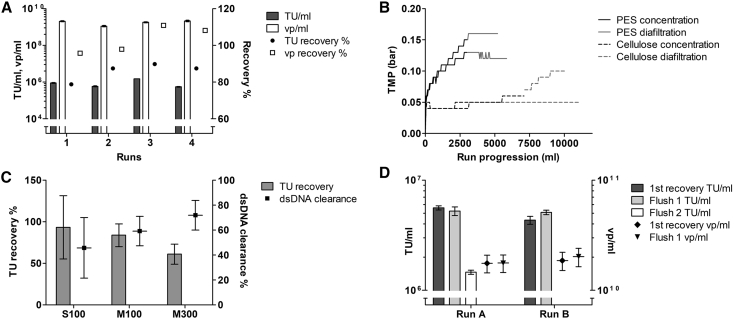
Two different depth filters were tested for clarification: Sartopore 2 and Millistak+ Pod (Table 1). Double-stranded DNA (dsDNA) clearance with the larger pore size Millistak+ was not as good as with the smaller pore size Sartopore. However, the recovery of the product through Millistak+ was higher both in functional (TUs) and total vps (determined by p24 ng/mL data; data not shown). Therefore, the larger pore-size filter retaining better infectivity was chosen for clarification. The importance of filter flushing after harvest collection was also assessed. Flushing the filter once with buffer, with approximately the filter void volume, resulted in a 60% increase in the vp recovery (data not shown). The clarification with Millistak+ filter was tested in several small-scale LV clarifications (220–440 L/m2) with flushing, the functional particle recovery was repeatedly 75%–90%, and total particle recovery approximately 100% (Figure 2A). Due to the fragile nature of the LVs, the functional particle recovery may decrease during processing. However, generally, the ratio of total physical particles compared to functional particles decreases toward the final product, which indicates increased quality after the purification process.
Figure 2.
Clarification and the Following Concentration/Diafiltration Optimization for Lentiviral Vectors (LVs)
(A) Clarification titers from four small-scale runs in TU/mL (±standard deviation [SD]) and total particle concentration (vp/mL ± SD), as well as recovery percentages. (B) Tangential flow filtration (TFF) transmembrane pressure (TMP) with 100 kDa polyethersulfone (feed 30–35 L/m2; solid lines) and cellulose (feed 59–74 L/m2; dashed lines) cassettes. Graphs of four independent runs are shown. (C) TU recovery percentage (±SD, gray bars) and dsDNA clearance percentage (±SD, dots) for clarified LV concentration and buffer change with three cassettes (S100 = Sartocon 100 kDa, M100 = Millipore 100 kDa, M300 = Millipore 300 kDa; all 0.1 m2). The results are averages over three runs with LV-GFP (2 runs, titering with flow cytometry) and LV-TK (one run, titering with qPCR). (D) TFF titer (TU/mL ± SD) and vp/mL (±SD) in two small-scale runs (Run A and Run B) in the first recovery of the product (dark gray bars and diamond dots), the 1st flush (light gray bars and triangle dots), and the 2nd flush (white bar, only for run A and measured in TU/mL). TUs, transducing units measured with flow cytometry if not stated otherwise; GFP, green fluorescent protein; TK, thymidine kinase. The total viral particles (vps) were calculated from p24 ELISA results.
Concentration and Buffer Exchange by TFF1
The clarified material was concentrated and diafiltrated into suitable buffer for chromatography in a tangential flow filtration step, denoted as TFF1. Enveloped vectors are highly susceptible to functionality loss due to envelope rupture during processing caused by foaming, shear forces, pressure changes, and aggregation. We have optimized the parameters of the concentration and buffer change step, keeping a suitably high crossflow to try to minimize clogging and product aggregation.
Choice of Ultrafiltration Membrane Material and Cutoff
Flat sheet cassette format was chosen for ultrafiltration in TFF, as previous data19 on hollow fibers have shown faster transmembrane pressure (TMP) increase and lower flux-regaining capability than with comparable flat sheets. Cellulose and polyethersulfone (PES) were considered for ultrafiltration membranes. PES membranes have a more open structure than cellulose-based membranes, which leads to higher permeability compared to cellulose-based membranes with same cutoff; however, the hydrophilicity of the cellulose membrane leads to a lower tendency to bind hydrophobic foulants.20 Small-scale tests revealed faster increasing TMP on the PES membrane compared to cellulose-based membrane of same cutoff (Figure 2B). Recoveries did not differ significantly between the two membrane materials (data not shown); however, with the consideration of the effects of the increase in TMP in large-scale processing, the cellulose-based membrane was chosen for scale-up.
Following the membrane material decision, two different cutoffs (100 kDa and 300 kDa) were tested with the cellulose-based cassettes. Cassettes from two different brands (Sartocon Slice, Sartorius; and Pellicon 2, Milllipore) were compared for the 100-kDa cassette, whereas size 300 kDa was only available as Pellicon 2. A 300-kDa cutoff resulted in higher dsDNA clearance than 100 kDa; however, the titer recoveries were lower than with the smaller cutoff cassette (Figure 2C). The results suggested that Sartocon resulted, on average, in slightly higher functional particle recovery of the two, 100-kDa cassettes, although the standard deviation (SD) of the results was higher. Therefore, finally, the Sartocon Slice 100-kDa cassette was finally chosen for scale-up.
Optimization of the TFF Parameters
The parameters for the TFF1 step were determined in small scale on ÄKTA crossflow (GE Healthcare) and Cogent M (Millipore) with the 100-kDa, 0.1 m2 Sartocon cassette. The Cogent M parameters at 24 L/m2/h (LMH) flux and 88% crossflow with permeate flow control resulted in the lowest TMP values. Fluxes as high as 100 LMH were tested, and the higher flux resulted in increasing TMP (Table 2). Generally, the feed load in TFF1 was approximately 60 kg/m2; however, up to 100 kg/m2 was applied as well with decent recoveries (approximately 75% recovery in functional units) (flow cytometry; data not shown).
In the end of the TFF process, the product is recirculated over the membrane with permeate closed (100% crossflow) to detach the layer formed on the membrane and to flush it into the retained product. The effect of flushing the TFF1 membrane after recovery of the retentate (Figure 2D) was tested. It was noticed that the first flush adds approximately 55% in TU to the recovery of the product. However, a second flush did not meaningfully improve the recovery.
Capture Chromatography
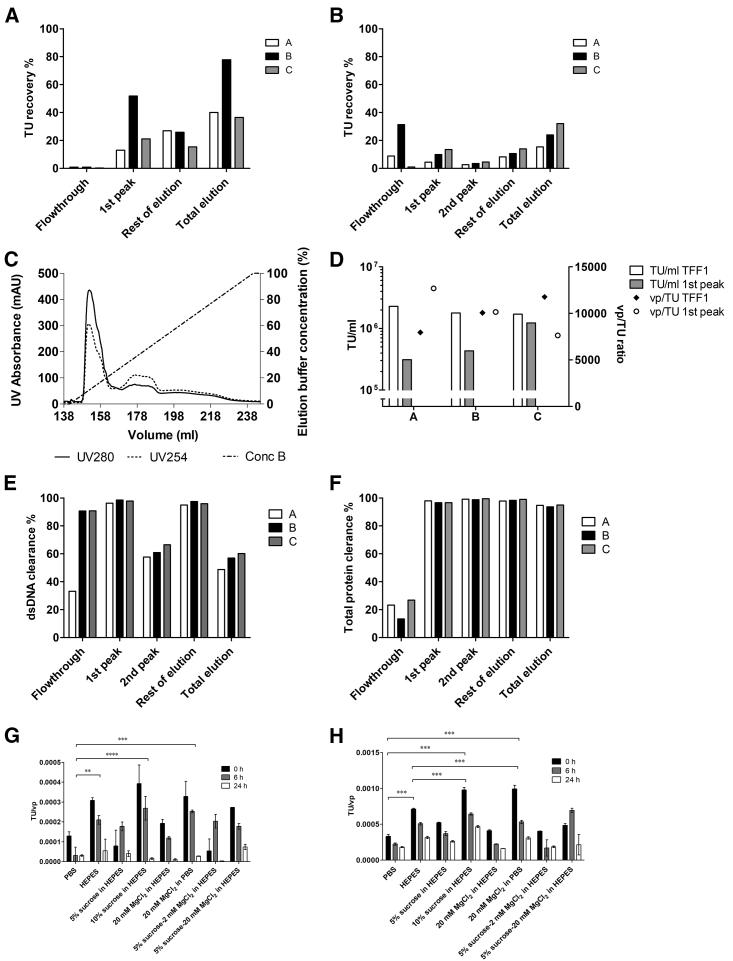
AEX chromatography development started focusing on two main support types (monoliths and membranes). A number of chromatographic runs were carried out to screen for the best supports and conditions (Tables 1 and 3). Weak and strong anion exchangers were compared for monoliths. Although better resolution could potentially be obtained with weak exchangers, recoveries using them were lower, so only strong exchangers were chosen for further evaluation. Both membranes and monoliths bind lentivirus particles efficiently from clarified harvest, as well as TFF1 product, with the exception of Mustang Q XT Acrodiscs that seem to lead to higher flowthrough with TFF1 material (Figures 3A and 3B). In linear gradients with TFF1 material, the column elution phase generally shows two main peaks with different UV profiles (280 milli-absorbance units [mAU] versus 254 mAU, elution profile from Sartobind shown in Figure 3C). However, the division into two peaks was not always clear with different columns, which is why it was important to report also the rest of elution. With clarified material, the resolution into two peaks was especially difficult, and thus, only the peak corresponding to the TFF1 material first peak was analyzed separately (Figure 3A). The first peak collection was 16%–22% of the total elution volume in Figures 3A and 3B. Figures 3D–3F show further analysis of the chromatographic peaks and impurity clearance obtained using TFF1 stocks.
Figure 3.
Preliminary Chromatography Optimization and Stability Studies for Lentiviral Vectors Expressing the Green Fluorescent Protein (LV-GFP)
(A) Functional particle TU recoveries from clarified feedstock for three different chromatography columns (A = CIMmultus QA 1 mL, B = Mustang Q XT Acrodisc 0.86 mL, C = Sartobind Q nano 3 mL). (B) Functional particle TU recoveries from small scale clarified and concentrated with TFF1 feedstock for three different chromatography columns determined with flow cytometry. (C) Typical chromatogram elution with Sartobind Q support showing the absorbance profile (UV280 and UV254) and the elution buffer concentration percentage. (D) Titers (TU/mL) and the vector quality as the ratio of total vps compared to TU for chromatography runs on three different columns with the frozen TFF1 stock of the first scale-up run. (E) Clearance of dsDNA in the fractions from frozen TFF1 stock of the first scale-up run for three different chromatography columns. (F) Clearance of total proteins in the fractions from frozen TFF1 stock of the first scale-up run for three different chromatography columns. (G) LV-GFP stability in room temperature in different buffers. The TU values are normalized against vp values for comparability (±SD). (H) LV-GFP stability in +4°C in different buffers. The TU values are normalized against vp values for comparability (±SD). ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001. All percentages obtained from average titer/concentration. TUs, transducing units determined with flow cytometry. The total vps were calculated from p24 ELISA results.
Similar feedstock volume per support volume was loaded at flow rates of 1 column/membrane volume (MV) per minute. Higher flow rates in ÄKTA Avant 150 equipment (GE Healthcare) had shown that the infectivity of up to 20% of the vps can be lost in the system in column bypass mode at 5 mL/min (data not shown), which relates to the fragility and sensitivity of the LV. The first elution peak, which eluted immediately after conductivity started increasing, was the fraction that contained most of the intact virus (Figures 3A and 3B). TU recoveries during elution were particularly low, and only up to 30% of the column-bound functional LVs eluted with gradients up to 1.5 M NaCl with the TFF1 material. The rest of the vector is putatively removed during the cleaning-in-place step with 0.5 M NaOH, a fraction that cannot be tested for viral activity. We also compared chromatography with and without sucrose added to the feedstock (TFF1 products). Sucrose has been shown to increase the stability of lentiviral vectors upon freezing.21 However, no improvements were observed in TU recoveries, so no additives were used in further runs (data not shown).
The dsDNA is mostly found in the second peak, where also some functional particles are eluted (Figure 3E). Both dsDNA and total protein clearances are high for the first peak (Figures 3E and 3F). Highest quality vector preparation after chromatography was eluted from Sartobind Q membranes (Figure 3D). Vector quality is inversely proportional to the ratio of the total vps to TUs: the lower the value of vp/TU, the higher the vector quality.
Increased precolumn pressure at higher flow rates during chromatography was observed. The problem was worse in the case of monoliths. A larger pore-size monolith column (6 μm versus 2 μm) was also tested, but the lower binding capacity still yielded lower recoveries than the Sartobind Q membrane. High dsDNA levels in the concentrated TFF1 stocks might be partially responsible for these problems, which point to the importance of an optimized endonuclease step prior to further DS processing. To minimize pressure increases, prefiltration of the TFF1 product (0.45 μm Sartoclean cellulose acetate [CA] and Sartopore 2 filters) (Table 1) was tried prior to chromatography. However, the functional particle recovery was low (49.6% with Sartoclean CA and 66.2% with Sartopore 2) and the dsDNA clearance poor (18.3% with Sartoclean CA and 18.5% with Sartopore 2), making this step unsuitable for our goals. Clarified viral stocks without preconcentration and buffer exchange seem to yield somewhat better recoveries (particularly for Mustang Q XT Acrodiscs; Figure 3A). However, the time required to load all of the clarified harvest material from large-scale runs to batch chromatography renders this option impractical for the fragile vector. Based on these results, the Sartobind Q membrane supports were selected for further process development and scale-up, since they consistently provided the best recoveries with minimal technical problems.
HEPES-buffered solution was chosen for the TFF1 and chromatography buffer due to a higher buffering capacity in physiological pH compared to a Tris-based buffer (HEPES negative base-10 logarithm of the acid dissociation constant [pKa] 7.6 and Tris pKa 8.1 at 25°C), and the high temperature-dependent pH variability of Tris-based buffers.22 To minimize aggregation and also to retain column capacity by excluding part of the protein binding during loading, buffer containing 300 mM NaCl was chosen for the chromatography column equilibration/loading and washing. The effect of buffer conductivity was tested on the Sartobind Q column by comparing the standard TFF1 feedstock (29.2 mS/cm) with the diluted TFF1 stock (14.6 mS/cm). In addition, we tested the effect of adding 10% sucrose to the TFF1 buffer for increased stability during the chromatography step. No significant vector recovery improvements were obtained at low conductivity (23.4% TU at 29.2 mS/cm and 21.2% TU at 14.6 mS/cm), and the sucrose addition resulted in decreasing recovery (10.4% TU), most probably due to increased viscosity during the runs. The standard TFF1 buffer (300 mM NaCl) was chosen for further development to try to minimize impurity binding.
Final Formulation Buffer Study and Stability
Final formulation of the viral vector preparation can affect the stability of the product remarkably, especially with fragile LVs, and the formulation should not only support the preservation of the product but also be applicable in vivo. Both sucrose and magnesium chloride have been used in final formulation of LVs to increase stability of the product during storage.21,23,24 The stability of the LVs was studied in several HEPES and PBS-based buffers for product storage. Short-term storing results were consistent; the most efficient buffers to maintain short-term stability of LV at −80°C were 10% sucrose in 50 mM HEPES, 20 mM MgCl2 in PBS, 5% sucrose-20 mM MgCl2 in 50 mM HEPES, and 50 mM HEPES. These buffers performed significantly better than standardly used PBS (Figures 3G and 3H; 0 h samples). In addition, 10% sucrose in 50 mM HEPES and 20 mM MgCl2 in PBS could be considered better compared to 50 mM HEPES only.
None of the buffers was able to prevent a significant decrease of the titers during incubation at room temperature (Figure 3G; 6 h and 24 h samples). All of the samples were statistically different between 0 h and 24 h time points. However, the FFBs that were best to maintain the stability at room temperature were 5% sucrose-20 mM MgCl2 in 50 mM HEPES, 50 mM HEPES, and 20 mM MgCl2 in PBS. After a 6-h incubation, 65%, 68%, and 77% of the vector activity remained, respectively. After 24 h, only 27%, 18%, and 8% remained (Figure 3G).
When the LV was incubated at +4°C, the smallest decrease in titer was observed with 5% sucrose-20 mM MgCl2 in 50 mM HEPES, PBS, and 5% sucrose in 50 mM HEPES (Figure 3H; 6 h and 24 h samples). After a 6-h incubation period, there was 82%, 67%, and 71% of the activity remaining, respectively. After 24 h, there was 59%, 55%, and 50% activity remaining. However, there was a significant decrease in titer from the initial 0-h titering point to the 24-h titering point, indicating that none of the FFBs could maintain stability at +4°C.
The final formulation step with TFF2 with uniform virus stocks from the AEX capture requires a minimum amount of material, which was not obtained here during process development in iCELLis nano scale. Therefore, this unit operation was not optimized prior to the first large-scale run (described in the following section). Accordingly, the same parameters as for the TFF1 step were implemented, with the exception of a larger crossflow. For the scale-up runs, we combined the prominent cryopreservative effects observed with 10% sucrose in 50 mM HEPES and 20 mM MgCl2 in PBS into the final formulation buffer, 50 mM HEPES + 20 mM MgCl2, with 10% sucrose (pH 7.5). The second scale-up run final product (LV-TK) was reanalyzed after 1 year and 2 years storage in −80°C, and the functional units were normalized against total vps (vp/TU). The vp/TU ratio seemed to remain similar during the storage, changing from 3.46 × 103 vp/TU at 0 time point to 4.29 × 103 vp/TU at a 1-year time point and to 2.81 × 103 vp/TU at a 2-year time point.
First Scale-Up Run Clarification and TFF1
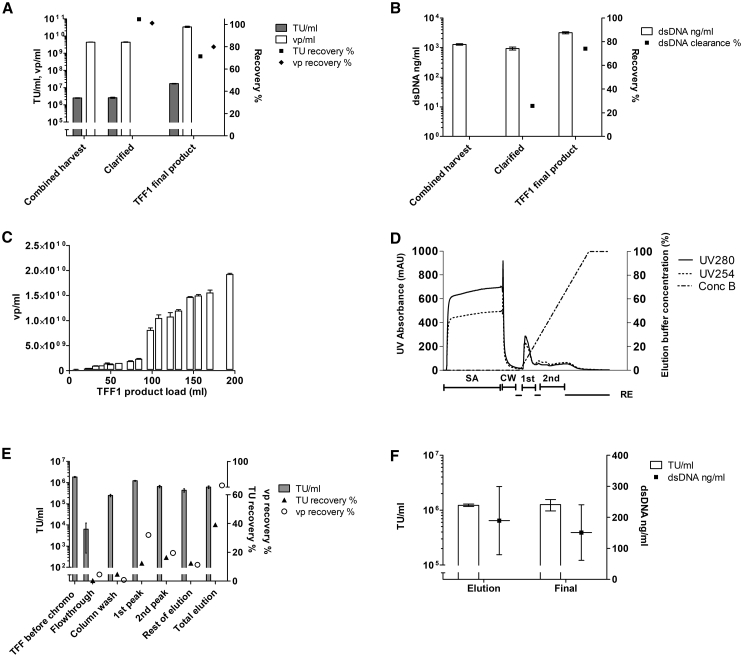
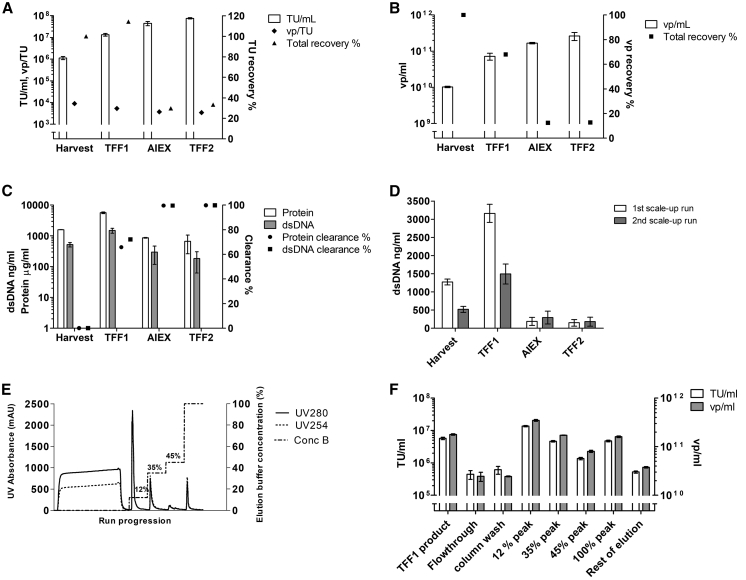
In the first large-scale clarification, the feed and flux were reduced to 215 kg/m2 and 146 LMH, respectively, to ensure effortless scale-up. 126.8 kg of the total of 168.8 kg clarified product was processed further in TFF1. TFF1 was performed using the small-scale optimized parameters as guidelines. A lower feed load (42 kg/m2) than in the small-scale experiments was used to ensure operability in the scale-up. TMP increased up to 0.1 bar during the run (Table 2). In this first large-scale run, the product was not recirculated before recovery. However, the system was flushed as in the small-scale experiments, and the first flush was combined with the initial recovery to form the final TFF1 product. A second and third flush did not improve the recovery, confirming the results from small-scale experiments (data not shown). Both functional particle and vp recoveries were close to 100% for the clarification step, and 70%–80% for the TFF1 step (Figure 4A). The clarification dsDNA clearance was 25%, and over 65% of the remaining dsDNA was removed in the TFF1 step (Figure 4B). However, due to volumetric concentration in the TFF1 step, the dsDNA concentration in the TFF1 product was higher than in the harvest.
Figure 4.
First Large-Scale Run and Further Chromatography Optimization for Lentiviral Vectors Expressing the Green Fluorescent Protein (LV-GFP)
(A) Clarification and the following TFF1: functional particle titer in TU/mL (±SD) and total particle concentration vp/mL (±SD), as well as recoveries. (B) dsDNA concentration (±SD) and clearance (%) after clarification and TFF1. (C) Breakthrough curve based on total vp concentration (vp/mL ± SD) for Sartobind Q with frozen TFF1 stocks of the first large-scale run. (D) First scale-up run chromatogram showing the absorbance profile (UV280 and UV254) and the elution buffer concentration percentage with the collected fractions marked below the x axis (SA, sample application; CW, column wash; 1st, first elution peak; 2nd, second elution peak; RE [lower lines], rest of elution). (E) First large-scale run chromatography functional particle titer (TU/mL ± SD), as well as functional particle and vp recoveries. (F) Titer in functional particles (TU/mL ± SD, bars) and dsDNA concentration (with mean and ±SD, dots) for chromatography elution and for elution processed with TFF for final buffer change (TFF2, “Final”). TUs, transducing units determined with flow cytometry. The total vps were calculated from p24 ELISA results.
Additional Chromo Optimization and the First Scale-up Run Chromatography
Prior to scale-up of chromatography and final formulation, the obtained large-scale TFF1 product was used to optimize the chromatography step further. First, elution was improved, converting a 0% to 100% linear gradient into a shallower 0% to 60%, aiming to resolve better the nucleic acid from the viral-containing fraction. Breakthrough curves, based on total vps, were obtained for Sartobind Q, with the first scale-up run frozen TFF1 stocks. A 20% breakthrough point was determined at 2.06 × 1012 vp (Figure 4C).
The AEX chromatography step was planned as a 50-fold intermediate scale-up from the Sartobind Q nano (3 mL) to the 150-mL version of the same membrane support and bed height. The total particle concentration of the large-scale run TFF1 product was determined at 2.23 × 1010 vp/mL, and to keep the total particle load below the 20% breakthrough point, 4.3 kg of frozen and thawed TFF1 product was loaded into the column.
The full chromatogram is shown in Figure 4D with the collected fractions underlined. Figure 4E summarizes the obtained results. When total particles are considered, the vp recoveries show the expected pattern, with the first peak containing the biggest amount (32.0%). However, the recovered amount of functional virus was only 12.4% in the same fraction. The reason for this result could not be determined. Mass balances show that as in small scale, a big proportion of the viral vector is not recovered out from the column during elution (total vp recovery 62.8%), and total infectious LV-GFP vector eluted was 39.2%. The second peak, which comprises most of the eluted dsDNA (25.7% versus 1.6% in 1st peak; data not shown), also contained 16.4% of the recovered TUs. This, together with the vector recovered in the rest of the elution fraction, points to difficulties in accurate manual fractionating of virus peaks from this linear gradient. In the next large scale-up run, the elution was planned as gradient steps instead. Altogether, this process step provided the main dsDNA impurity clearance (over 98%) but did not help to concentrate the LV-GFP vector.
First Scale-Up Run TFF2
Buffer change to the FFB was performed with another TFF step (TFF2) after the chromatography purification. The main parameters used in TFF1 were applied for the technically similar TFF2 process (Table 2). The flux was decreased slightly to increase the crossflow over the retentate side of the membrane and consequently, to prevent aggregation or pressure increase. TU recovery was 59.98%, based on average TU/mL titer (Figure 4F). The final volume of LV-GFP-purified vector in FFB (50 mM HEPES, 20 mM MgCl2, pH 7.5, with 10% sucrose) was 560 mL at 1.26 × 106 ± 2.94 × 105 TU/mL (±SD) with additional dsDNA clearance (Figure 4F).
Second Large-Scale Run Using Further Optimized Parameters
In the second large-scale run (LV-TK), downstream was performed continually from clarification to final formulation (Figure 1). The main downstream modifications were the conversion of the AEX linear gradient elution into three discrete steps (12%, 30%, and 45% elution buffer concentration) and use of a larger Sartobind Q column (400 mL). A 178.4-kg harvest was obtained, clarified, and conditioned into TFF1 buffer to recover 15.59 kg of concentrated LV-TK vector. TFF1 recoveries based on functional titer and physical particles were excellent (Figures 5A and 5B). Clarified stock results are not shown due to a sampling error. Approximately 70% of both proteins and dsDNA were removed during the TFF1 step (Figure 5C). The overall dsDNA concentration of the harvest was lower in the second scale-up run compared to the first (Figure 5D); however, the clearance during the TFF1 step was similar between the two runs.
Figure 5.
Second Downstream Scale-up Run with Lentiviral Vectors Expressing Thymidine Kinase (LV-TK) with Optimized Parameters
(A) Functional titers (TU/mL ±SD) and vector quality in terms of total vps compared to functional transducing units (TUs), as well as TU recoveries from harvest for downstream processing. (B) Total particle concentration vp/mL and vp recovery from harvest for downstream processing. (C) Total protein and dsDNA concentrations (±SD) and percentage clearances for downstream processing. (D) Comparison of dsDNA clearance in the two scale-up runs measured as the concentrations of dsDNA (±SD) during downstream processing. (E) The chromatogram of the second scale-up run showing the absorbance profile (UV280 and UV254) and the elution buffer concentration percentage. (F) Chromatography functional particle titers (TU/mL) and total particle concentration vp/mL in the fractions collected. Functional titer was determined with qPCR. The total vps were calculated from p24 ELISA results.
To avoid potentially harmful holding times, chromatography started immediately after TFF1, and hence, titration was not performed between the two steps. A total of 11 kg was loaded into the AEX column (Figure 5E). Breakthrough and maximal load for chromatography (initially estimated breakthrough at 2 × 1012 vp/mL membrane) were determined based on expected productivity and chromatography scale-up factor (30 mL of TFF1 per milliliter of membrane support). Due to a higher vp productivity in this large-scale run compared to the first scale-up run, an excess of virus was loaded per milliliter of membrane (5 × 1012 vp/mL membrane), resulting in a higher loss than expected in the flowthrough (13.8%). However, virus recovery was significantly higher in this second large-scale run, and 22.4% of the functional LV vector was present on the product fraction (12% step peak). As expected, other elution fractions also did contain some functional virus, resulting in total elution recovery of 33.1% of the loaded vector (Figure 5F).
The purification factor (measured as total protein and dsDNA clearance) was obtained during the TFF1 and chromatography steps (Figure 5C). The dsDNA levels in the final product were similar to the first scale-up run LV-GFP product, 150.8 ng/mL and 182.5 ng/mL in the first and second scale-up run, respectively. However, the optimized endonuclease treatment implemented prior to clarification in the second scale-up run was clearly more efficient (Figure 5D).
In the TFF2 step, similarly to the first run, the main virus fraction (12% step) was processed to formulate the product into our final buffer. The total volume of LV-TK vector obtained was 570 mL with a titer based on HeLa cell transduction and subsequent qPCR at 7.60 × 107 ± 4.98 × 106 TU/mL (±SD) (Figure 5A). External analysis results in C8166 cells showed a functional titer of 1.97 × 109 TU/mL and consequently, a total production of 1.12 × 1012 TUs. The external analysis also confirmed the final product free of replication-competent lentiviruses.
Discussion
Lentiviral vectors are an increasingly popular alternative in gene therapy.1 However, large-scale manufacturing methods for LV, required for clinical trials and commercial use, are still few.11 In our concurrent study,10 we have scaled up the production of LVs into the iCELLis 500 bioreactors, being able to produce up to 180 L of LV stock with perfusion. Upstream process optimization and scale-up are required prior to downstream optimization to provide consistent feedstock availability for downstream development, ensuring repeatability. In this study, we developed and optimized the purification and concentration protocol for LVs to obtain a high titer-purified LV product. The majority of the scalable LV DS protocols follow a standard outline with filtrations and chromatography.25 We start the downstream processing with microfiltration-scale clarification, followed by concentration and buffer change into suitable chromatography buffer with TFF, anion exchange chromatography for the purification, and final formulation into the storage buffer with TFF. The DNA digestion step in our protocol takes place in the upstream harvest.
The scale-up of clarification was effortless, and the functional particle recoveries were consistently 75%–90%. We find that a TFF step before the purification with chromatography is necessary to decrease preliminarily the impurity load of the feedstock and to change the buffer into a more suitable solution for anion exchange chromatography. With sufficient concentration and diafiltration factors, the overall process time can also be decreased. For the concentration and diafiltration with TFF, we chose a 100-kDa cassette over the 300-kDa cassette, due to slightly higher functional particle recoveries. Our findings are in line with other publications that have reported either similar recoveries between the two cutoffs or higher recovery with the smaller cutoff.13,26 However, the higher cutoff cassette could be an alternative when higher impurity clearance in the TFF step is required. The key parameter in the LV TFF seems to be the crossflow percentage compared to the feed flow that is used to flush the retentate side of the membrane and consequently to keep the membrane from clogging.
In chromatography, we screened through several membrane- and monolith-based supports.18 After prescreening, Sartobind Q membrane-based columns were chosen for further optimization, as they resulted in the highest recovery in functional units from the concentrated material. Mustang Q XT Acrodisc columns resulted in markedly better recoveries from the clarified material; however, the chromatography loading without a preconcentration step would be lengthy and thus possibly destructive for the LV. In Sartobind Q, the impurity reduction of the product in the 12% step (444 mM NaCl) was efficient, but vector particles, both functional and p24 protein, were detected in other fractions as well, although in smaller amounts. The elution with higher salt concentration increases the vector recovery but with higher impurity concentration. Marino et al.27 determined in their 2015 publication that 65% of the LVs eluted between 200 and 400 mM NaCl. Bandeira et al.21 eluted the LV in 650 mM NaCl, resulting in higher recovery; however, more DNA was left in the elution, and a dilution step was required to decrease the salt concentration. Finally, the tradeoff between recovery and impurity clearance has to be carefully selected depending on the additional downstream steps included in the process to reach a high-quality final product.
In the final TFF step, we performed buffer change into the final formulation buffer for storage. We combined the cryopreservative effects of sucrose and MgCl2 into a 50-mM HEPES, 20-mM MgCl2 with 10% sucrose formulation. Final product sterility is commonly achieved with a final sterile filtration step; however, we have experienced approximately 30%–50% loss in final sterile filtration of LVs (data not shown). Loss in sterile filtration has been reported in other publications as well,14,21 and it is a cost that has to be accepted in good manufacturing practice (GMP) production.9 Overall recovery in large-scale LV downstream processes has been 20%–40%25. The cooling of the processing units or the flowpaths would likely increase the recoveries compared to operating in room temperature.28 In our case, we used in TFF1 and TFF2 buffers that had been cooled to +4°C before starting the process. In addition, if an intermediate titer is needed for chromatography loading, then an on-hold step for the product in +4°C is required before the chromatography step. For processing without intermediate analytics, the process should be highly standardized to avoid large fluctuations in the titer and impurity load.
In some of the results, we reached over 100% recoveries in TUs. The impurity level has been shown to affect the transducibility and cryopreservance of retroviral vectors12 and LVs.29 It must be noted that the analytical methods for functional lentiviral vector titering remain complicated. Unfortunately, the cell type used for cell-based titration causes variability for functional lentiviral vector titers. Differing results can be obtained even with the same cell type in different laboratories.18,30 Similarly, the level of variability between independent titrations of the same sample can be significant, making the analysis of multistep processes challenging. When advancing toward GMP production, the comparability should be based on both transducibility and genome copies, and the analytics should be based on an international standardized reference material.30 Standardized LV analytics are already available, and a study aiming at releasing LV reference material is ongoing.31,32
The second optimized scale-up of downstream processing for 180 L of LV-TK resulted in a final product of 570 mL with an in-house qPCR-based functional titer of 7.60 × 107 ± 4.98 × 106 TU/mL (±SD, HeLa cells). However, the external qPCR analysis resulted in a titer of 1.97 × 109 TU/mL (C8166 cells) and a total production of 1.12 × 1012 TUs. The quality in physical particles compared to functional particles of the product was 3.5 × 103 vp/TU, which is comparable to LV qualities obtained in other large-scale productions.14,17 The dsDNA removal during downstream processing was 99.8% from the upstream harvest. In addition, the final titer and dsDNA concentration in the two scale-up runs did not differ remarkably, which confirms that the transgene does not seem to alter the downstream requirements. The LV titer currently required for clinical trials is approximately 1–5 × 109 TU/mL.33,34 If the final product obtained here would be subjected to sterile filtration with 50% losses in functional units, then the product (approximately 1 × 109 TU/mL) would be sufficient for approximately 950 doses of 600 μL with 5.91 × 108 TU per dose.
Future studies considering the downstream development should concentrate on improving the chromatography step that remains a bottleneck, with the lowest step recoveries of the downstream process. However, to obtain higher titers overall in the LV process, the upstream development is the key, as the concentration capacity of the downstream process is limited. In conclusion, we show here that the large-scale purification and concentration of LV product are feasible, and with relatively small modifications, the methods described here could be transferred to a current good manufacturing practice-compliant environment to produce clinical-grade LVs.
Materials and Methods
Viral Vector Production
Viral vectors were produced in 293T cells (ATCC, Manassas, VA, USA). In transfection, third-generation, self-inactivating LV, expressing GFP or TK under the human phosphoglycerate kinase (PGK) promoter, was produced using a four-plasmid system (pVSVg, pGag-Pol, pRev, and LV plasmid expressing GFP or HSV-TK). Plasmids were manufactured by PlasmidFactory (Bielefeld, Germany). 200 ng/cm2 or 300 ng/cm2 of plasmids was used for transfection with PEIpro (Polyplus, Illkirch, France)-mediated transfection with a 1:1 DNA:polyethylenimine (PEI) ratio in iCELLis bioreactors (iCELLis nano for small scale35 and iCELLis 500 for scale-up; Pall, Brussels, Belgium). The production is described in Leinonen et al.10
Virus Titration
In-house functional particle titration was performed with HeLa cells (LGC Standards; CCL-2, lot 57818419). For LV-GFP, functional particle titer was analyzed in duplicates by determining the percentage GFP-positive cells with flow cytometry36 (FACSCalibur, Becton Dickinson; FACSCanto II, Becton Dickinson; or CytoFLEX S, Beckman Coulter). For LV-TK functional particle titration, we used the qPCR method on LV-transduced cells. On the 3rd day after transduction, the cells were detached, and cell pellets were stored in −80°C until DNA extraction. An extraction kit (NucleoSpin DNA RapidLyse) was used for DNA extraction, according to the kit’s instructions. The qPCR was run with TaqMan Genotyping Master Mix (Thermo Fisher Scientific/Life Technologies), TaqMan Copy Number Reference Assay (human, RNaseP; Thermo Fisher Scientific/Life Technologies, Bleiswijk, the Netherlands), and Woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) forward and reverse primers and probe: WPRE forward 5′-GGCACTGACAATTCCGTGGT-3′, WPRE reverse 5′-AGGGACGTAGCAGAAGGACG-3′, and WPRE probe 5′-FAM-CGTCCTTTCCATGGCTGCTCGC-OQA-3′ (OQA = Onyx Quencher A, Merck). The results were determined by using a plasmid-generated standard curve.
External functional titer was analyzed by GenoSafe on C8166 cells (qPCR, dGAG, E = 95.35%, r2 = 0.999).
For physical particle analysis, we used the Alliance HIV-1 p24 antigen ELISA kit NEK050B (PerkinElmer, Waltham, MA, USA), which measures the concentration of the p24 capsid protein. p24 concentrations were transformed to vp titers by assuming 12,500 LV particles per 1 pg of p24 (2,000 molecules of p24 per 1 LV particle37).
Total Protein and dsDNA Quantitation
Total protein quantification was performed with Pierce BCA (bicinchoninic acid) Protein Assay Kit (ref. 23225; Thermo Scientific), according to the manufacturer’s instructions. dsDNA concentration was determined with a Quant-iT PicoGreen dsDNA assay kit (ref. P11496; Invitrogen), according to the manufacturer’s instructions.
Clarification
Harvested material was clarified using a peristaltic pump (Watson Marlow 520S with 5 mm inner diameter [ID] tubing in small scale and Watson Marlow 620 with 12.7 mm ID tubing in large scale) with either a Millistak+ filter or Sartopore 2 filter (Table 1) at a flux of 120 LMH (filter tests), 220 LMH (small-scale batch clarification), or 146 LMH (large scale). Small-scale clarification tests were performed with feed up to 290 kg/m2, and the large-scale runs were performed with feed of 215 kg/m2 (first scale-up run) and 232 kg/m2 (second scale-up run). The Millistak+ filters were preconditioned as instructed by the manufacturer, with final equilibration with the TFF1 buffer (50 mM HEPES + 300 mM NaCl, pH 7.5). In addition, the filters were sanitized with 0.5 M NaOH, with 30 min incubation time. Sartopore 2 filters were delivered sterile, and consequently, they were only flushed with TFF1 buffer before filtration. After filtration, both filters were treated similarly. The filters were flushed with minimum filter void volume of TFF1 buffer and emptied to product collection. The flowpath of clarification was of single-use materials.
Prefiltration before Chromatography
The prefiltration tests for TFF1 material were conducted using a peristaltic pump (Watson Marlow 520S) with Sartopore 2 and Sartoclean CA filters (Table 1) at a flux of 120 LMH (Sartopore 2) or 75 LMH (Sartoclean CA). The filters were equilibrated with TFF1 buffer before filtration. After filtration, the filters were flushed with 100 mL of TFF1 buffer and emptied to product collection.
Concentration and Diafiltration, TFF1
Optimization
Small-scale TFF1 was performed with Pellicon 2 Biomax, Pellicon 2 Ultracel, and Sartocon Slice Hydrosart, with cassette areas 0.1 m2 in 100 kDa or 300 kDa (Table 1). The material comparison runs, two per material, were performed with the same filters, with sanitation and water flux test between runs. The cassettes in the other runs were used only once. TFF1 buffer 50 mM HEPES + 300 mM NaCl (pH 7.5) was prepared in-house for the small-scale tests.
TFF1 was performed in small scale, either with ÄKTA crossflow with UNICORN 5 software (GE Healthcare Life Sciences) or with Cogent M (Millipore). Before processing the material, the system was sanitized together with the cassette using 0.5 M NaOH with 30 min incubation. Following the sanitation, the system was flushed with water and equilibrated with cold-stored (buffer temperature +4°C in the beginning of the TFF1 step) TFF1 buffer. The permeate line was equipped with a conductivity sensor: ÄKTA crossflow built in with Cogent M; we used a SciLog flow cell (080-599PSX-5-G; Parker) with a SciCon monitor (Parker). The conductivity reading was used to monitor sanitation and equilibration of the system, as well as efficiency of diafiltration. The process was controlled manually by adjusting the permeate flux with a permeate valve to reach the target flux with the target crossflow percentage of the feed flow. TMP was not limited but monitored during the process. The process parameters are shown in Table 2.
The clarified product was first concentrated 20-fold in a fed-batch mode and diafiltered into the TFF1 buffer with a 10-fold buffer change, also in a fed-batch mode. After the process, the product on the retentate side was recirculated with permeate closed over the retentate side of the cassette and recovered without emptying the filter. Following the first recovery, the retentate side was flushed with the minimum operable void volume of the system of TFF1 buffer. The flush was combined with the previously recovered product by emptying the retentate side of the system, including the filter.
First and Second Scale-Up Run
In the first large-scale run, we used five Sartocon Hydrosart 0.6 m2, 100 kDa cassettes in TFF1 (total area 3 m2). In the second large-scale run, we used two Sartocon Hydrosart 0.6 m2, 100 kDa cassettes, together with one Sartocube Hydrosart 3 m2, 100 kDa, increasing the total area to 4.2 m2 (Table 1). TFF1 buffer 50 mM HEPES + 300 mM NaCl (pH 7.5) was purchased for the large-scale runs as a custom-made product (L9242-200; Merck).
The first and second scale-up TFF1 runs were performed with the Mobius FlexReady TFF system (Merck) with the Smart Flexware assembly kit (Merck). The sanitation and system equilibration were performed similarly to small scale. The system was operated with preprogrammed recipes, and the permeate flow was controlled with a valve, similarly to the small-scale experiments. TMP was limited to 0.4 bar. The system had a built-in conductivity sensor for permeate monitoring. The process parameters are shown in Table 2.
The clarified product was first concentrated 13-fold (first scale-up run) or 18-fold (second scale-up run) in a fed-batch mode and diafiltered into the TFF1 buffer with a 10-fold (first scale-up run) or 9-fold (second scale-up run) buffer change, also in a fed-batch mode. The concentration target was 10 kg product weight before recovery. In the first large-scale run, the product was not recirculated before the first recovery, and in the second large-scale run, the product was recirculated. Following the first recovery, the retentate side was flushed with the minimum operable void volume of the system of TFF1 buffer. The flush was combined with the previously recovered product by emptying the retentate side of the system, including the filter.
AEX Chromatography
Three different types of chromatographic equipment were used for this work. Development was carried out using the ÄKTA Avant 25 and 150 versions with UNICORN 6.1 and 7.2, respectively (GE Healthcare). Scale-up chromatography runs were performed using the ÄKTA Pilot v06 equipment with UNICORN 5.3 (GE Healthcare). Irrespective of the run, equipment and columns were always sanitized with 0.5 M NaOH with 30 min contact time prior to and after each run. All columns were generally used according to the manufacturer’s instructions, and they are listed in Table 1. Buffers used are referred to as A (elution gradient 0%, column wash and equilibration, 50 mM HEPES, 300 mM NaCl, pH 7.5, TFF1 buffer) and B (elution gradient 100%, 50 mM HEPES, 1,500 mM NaCl, pH 7.5). When tested, diluted TFF1 buffer was used as buffer A (50 mM HEPES, 150 mM NaCl, pH 7.5). Buffers at different pH were used for pH effect evaluation.
Development runs were carried out with variable amounts of feed depending on availability (generally 30 to 50 MVs). All columns were always sequentially conditioned first with B buffer, followed by corresponding A buffer, until stable conductivity. After loading, column wash was done with 5 MVs. Elution was carried out generally with 30 to 40 MVs, whether linear or step gradients. A 100% B buffer strip was always added to the methods. Automatic fractionation was used in small-scale runs, and selected fractions comprising each peak were pooled after the run. In ÄKTA Pilot, fractions were collected by manually selecting the outlets.
In the first scale-up run, the TFF1 product was stored in −80°C for 2.5 months to perform preliminary tests on the TFF1 material before the chromatography step. In the second scale-up run, the chromatography step was performed directly after TFF1. Feed load was 30 MV in the first scale-up run and 29 MV in the second scale-up run. The flow rate was 1 MV/min in the first scale-up run; however, the flow rate was decreased to 0.5 MV/min in the second scale-up run, due to system limitations. The first scale-up run elution was a linear gradient from 0% to 60% of B buffer over 35 MVs, followed with a strip phase with 100% B for 5 MV. In the second scale-up run, step gradient elutions were implemented (12%, 35%, 45%, and 100% of B buffer, each for 8 MV).
Final Formulation, TFF2
TFF2 was performed only in the large-scale runs. For this final step, we used one Sartocon Slice Hydrosart 0.1 m2, 100 kDa (Table 1) cassette. The final formulation buffer for TFF2, 50 mM + 20 mM MgCl2 (pH 7.5) with 10% sucrose, was prepared in-house. The conditions were similar in both large-scale runs, with a volumetric buffer exchange factor of 7. In addition, a nominal volumetric concentration factor of 1.5 was achieved, although the focus was on final formulation rather than concentration. TFF2 was performed with Cogent M (Millipore). The system was sanitized and equilibrated before the process, similarly to TFF1, except with TFF2 buffer (buffer temperature +4°C in the beginning of the TFF2 step). The process was controlled similarly to the TFF1 process, targeting the parameters shown in Table 2. In the second scale-up run TFF2, the feed flow rate was increased during the process to reach the target filtrate flux.
Stability Studies
For the stability studies, the TFF1-purified LV-GFPs were ultracentrifuged at 68,566 g for 2 h, 10 min at 16°C to further concentrate the material. The buffers used for screening were the following: PBS (ref. 14190; Gibco); PBS supplied with 20 mM MgCl2; 50 mM HEPES; and 50 mM HEPES supplied with 5% sucrose, 10% sucrose-20 mM MgCl2, 5% sucrose-2 mM MgCl2, or 5% sucrose-20 mM MgCl2. LV pellets were suspended with the different buffers overnight at 4°C. Final products were frozen, stored at −80°C, and thawed for buffer studies. LVs in different buffers were incubated up to 24 h at +4°C or at room temperature, and transductions of 293T cells were conducted at three different time points (after 0 h, 6 h, and 24 h of incubation). The results are expressed as normalized values (TU/vp), due to large variation in the flow cytometry results. Significance was determined with two-way ANOVA in GraphPad Prism 5.04 (GraphPad Software).
Author Contributions
A.J.V., I.O., J.M., and T.M. performed the downstream runs. P.K. performed the final formulation stability tests. H.M.L. supervised the stability tests and took part in analytics. H.H. and V.T. took part in analytics. E.M.L., T.H., N.P., S.Y.-H., and H.P.L. supervised the process. A.J.V., I.O., and E.M.L. wrote the manuscript. Figures were done by A.J.V., I.O., and P.K.
Conflicts of Interest
A.J.V., E.M.L., I.O., H.M.L., H.H., V.T., J.M., and H.P.L. are employees of Kuopio Center for Gene and Cell Therapy and FinVector. P.K., T.M., T.H., N.P., and S.Y.-H. declare no competing interests.
Acknowledgments
We thank Iina Laaksonen, Riikka Kärnä, Sonja Kotoneva, and Siiri Valjakka for help and support. We thank Marjut Köylijärvi for the critical review of the manuscript. In addition, we thank Genosafe.
References
- 1.Ginn S.L., Amaya A.K., Alexander I.E., Edelstein M., Abedi M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018;20:e3015. doi: 10.1002/jgm.3015. [DOI] [PubMed] [Google Scholar]
- 2.European Medicines Agency Kymriah (tisagenlecleucel): An overview of Kymriah and why it is authorised in the EU. 2018. https://www.ema.europa.eu/en/documents/overview/kymriah-epar-medicine-overview_en.pdf
- 3.U.S. Food and Drug Administration FDA News Release: FDA approval brings first gene therapy to the United States. 2017. https://www.fda.gov/news-events/press-announcements/fda-approval-brings-first-gene-therapy-united-states
- 4.European Medicines Agency Zynteglo: Autologous CD34+ cells encoding βA-T87Q-globin gene. 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/zynteglo
- 5.Eichler F., Duncan C., Musolino P.L., Orchard P.J., De Oliveira S., Thrasher A.J., Armant M., Dansereau C., Lund T.C., Miller W.P. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N. Engl. J. Med. 2017;377:1630–1638. doi: 10.1056/NEJMoa1700554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sessa M., Lorioli L., Fumagalli F., Acquati S., Redaelli D., Baldoli C., Canale S., Lopez I.D., Morena F., Calabria A. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476–487. doi: 10.1016/S0140-6736(16)30374-9. [DOI] [PubMed] [Google Scholar]
- 7.Ferrua F., Cicalese M.P., Galimberti S., Giannelli S., Dionisio F., Barzaghi F., Migliavacca M., Bernardo M.E., Calbi V., Assanelli A.A. Lentiviral haemopoietic stem/progenitor cell gene therapy for treatment of Wiskott-Aldrich syndrome: interim results of a non-randomised, open-label, phase 1/2 clinical study. Lancet Haematol. 2019;6:e239–e253. doi: 10.1016/S2352-3026(19)30021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palfi S., Gurruchaga J.M., Lepetit H., Howard K., Ralph G.S., Mason S., Gouello G., Domenech P., Buttery P.C., Hantraye P. Long-Term Follow-Up of a Phase I/II Study of ProSavin, a Lentiviral Vector Gene Therapy for Parkinson’s Disease. Hum. Gene Ther. Clin. Dev. 2018;29:148–155. doi: 10.1089/humc.2018.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huszthy P.C., Giroglou T., Tsinkalovsky O., Euskirchen P., Skaftnesmo K.O., Bjerkvig R., von Laer D., Miletic H. Remission of invasive, cancer stem-like glioblastoma xenografts using lentiviral vector-mediated suicide gene therapy. PLoS ONE. 2009;4:e6314. doi: 10.1371/journal.pone.0006314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leinonen H.M., Lipponen E.M., Valkama A.J., Hynynen H., Oruetxebarria I., Turkki V., Olsson V., Kurkipuro J., Samaranayake H., Määttä A.-M. Preclinical Proof-of-Concept, Analytical Development, and Commercial Scale Production of Lentiviral Vector in Adherent Cells. Mol. Ther. Methods Clin. Dev. 2019;15:63–71. doi: 10.1016/j.omtm.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarron A., Donnelley M., McIntyre C., Parsons D. Challenges of up-scaling lentivirus production and processing. J. Biotechnol. 2016;240:23–30. doi: 10.1016/j.jbiotec.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Segura M.M., Kamen A., Trudel P., Garnier A. A novel purification strategy for retrovirus gene therapy vectors using heparin affinity chromatography. Biotechnol. Bioeng. 2005;90:391–404. doi: 10.1002/bit.20301. [DOI] [PubMed] [Google Scholar]
- 13.Geraerts M., Michiels M., Baekelandt V., Debyser Z., Gijsbers R. Upscaling of lentiviral vector production by tangential flow filtration. J. Gene Med. 2005;7:1299–1310. doi: 10.1002/jgm.778. [DOI] [PubMed] [Google Scholar]
- 14.Merten O.-W., Charrier S., Laroudie N., Fauchille S., Dugué C., Jenny C., Audit M., Zanta-Boussif M.-A., Chautard H., Radrizzani M. Large-scale manufacture and characterization of a lentiviral vector produced for clinical ex vivo gene therapy application. Hum. Gene Ther. 2011;22:343–356. doi: 10.1089/hum.2010.060. [DOI] [PubMed] [Google Scholar]
- 15.Scherr M., Battmer K., Eder M., Schüle S., Hohenberg H., Ganser A., Grez M., Blömer U. Efficient gene transfer into the CNS by lentiviral vectors purified by anion exchange chromatography. Gene Ther. 2002;9:1708–1714. doi: 10.1038/sj.gt.3301848. [DOI] [PubMed] [Google Scholar]
- 16.Segura M.M., Garnier A., Durocher Y., Coelho H., Kamen A. Production of lentiviral vectors by large-scale transient transfection of suspension cultures and affinity chromatography purification. Biotechnol. Bioeng. 2007;98:789–799. doi: 10.1002/bit.21467. [DOI] [PubMed] [Google Scholar]
- 17.Ausubel L.J., Hall C., Sharma A., Shakeley R., Lopez P., Quezada V., Couture S., Laderman K., McMahon R., Huang P. Production of CGMP-Grade Lentiviral Vectors. Bioprocess Int. 2012;10:32–43. [PMC free article] [PubMed] [Google Scholar]
- 18.Logan A.C., Nightingale S.J., Haas D.L., Cho G.J., Pepper K.A., Kohn D.B. Factors influencing the titer and infectivity of lentiviral vectors. Hum. Gene Ther. 2004;15:976–988. doi: 10.1089/hum.2004.15.976. [DOI] [PubMed] [Google Scholar]
- 19.Nestola P., Martins D.L., Peixoto C., Roederstein S., Schleuss T., Alves P.M., Mota J.P.B., Carrondo M.J.T. Evaluation of novel large cut-off ultrafiltration membranes for adenovirus serotype 5 (Ad5) concentration. PLoS ONE. 2014;9:e115802. doi: 10.1371/journal.pone.0115802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeon S., Rajabzadeh S., Okamura R., Ishigami T., Hasegawa S., Kato N., Matsuyama H. The effect of membrane material and surface pore size on the fouling properties of submerged membranes. Water. 2016;8:602. [Google Scholar]
- 21.Bandeira V., Peixoto C., Rodrigues A.F., Cruz P.E., Alves P.M., Coroadinha A.S., Carrondo M.J.T. Downstream processing of lentiviral vectors: releasing bottlenecks. Hum. Gene Ther. Methods. 2012;23:255–263. doi: 10.1089/hgtb.2012.059. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J., Russell D.W., editors. Molecular Cloning: A Laboratory Manual, Third Edition. Cold Spring Harbor Laboratory Press; 2001. Preparation of reagents and buffers used in molecular cloning; pp. A1.2–A1.3. [Google Scholar]
- 23.Kumru O.S., Wang Y., Gombotz C.W.R., Kelley-Clarke B., Cieplak W., Kim T., Joshi S.B., Volkin D.B. Physical Characterization and Stabilization of a Lentiviral Vector Against Adsorption and Freeze-Thaw. J. Pharm. Sci. 2018;107:2764–2774. doi: 10.1016/j.xphs.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Boudefa D., Merten O.-W., Fenard D. 2017. Method for purifying enveloped viruses or viral vectors. US patent 20170002332 A1, filed December 17, 2014, and published January 5, 2017. [Google Scholar]
- 25.Merten O.-W., Hebben M., Bovolenta C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016;3:16017. doi: 10.1038/mtm.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmermann K., Scheibe O., Kocourek A., Muelich J., Jurkiewicz E., Pfeifer A. Highly efficient concentration of lenti- and retroviral vector preparations by membrane adsorbers and ultrafiltration. BMC Biotechnol. 2011;11:55. doi: 10.1186/1472-6750-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marino M.P., Panigaj M., Ou W., Manirarora J., Wei C.H., Reiser J. A scalable method to concentrate lentiviral vectors pseudotyped with measles virus glycoproteins. Gene Ther. 2015;22:280–285. doi: 10.1038/gt.2014.125. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues T., Carvalho A., Roldão A., Carrondo M.J.T., Alves P.M., Cruz P.E. Screening anion-exchange chromatographic matrices for isolation of onco-retroviral vectors. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006;837:59–68. doi: 10.1016/j.jchromb.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 29.Cooper A.R., Patel S., Senadheera S., Plath K., Kohn D.B., Hollis R.P. Highly efficient large-scale lentiviral vector concentration by tandem tangential flow filtration. J. Virol. Methods. 2011;177:1–9. doi: 10.1016/j.jviromet.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Medicines Agency Guideline on Development and Manufacture of Lentiviral Vectors. 2005. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-development-manufacture-lentiviral-vectors_en.pdf
- 31.ISBioTech Lentivirus project. 2019. https://isbiotech.org/ReferenceMaterials/lentivirus-home.html
- 32.Zhao Y., Stepto H., Schneider C.K. Development of the First World Health Organization Lentiviral Vector Standard: Toward the Production Control and Standardization of Lentivirus-Based Gene Therapy Products. Hum. Gene Ther. Methods. 2017;28:205–214. doi: 10.1089/hgtb.2017.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Humbert O., Gisch D.W., Wohlfahrt M.E., Adams A.B., Greenberg P.D., Schmitt T.M., Trobridge G.D., Kiem H.P. Development of third-generation cocal envelope producer cell lines for robust lentiviral gene transfer into hematopoietic stem cells and t-cells. Mol. Ther. 2016;24:1237–1246. doi: 10.1038/mt.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alton E.W.F.W., Beekman J.M., Boyd A.C., Brand J., Carlon M.S., Connolly M.M., Chan M., Conlon S., Davidson H.E., Davies J.C. Preparation for a first-in-man lentivirus trial in patients with cystic fibrosis. Thorax. 2017;72:137–147. doi: 10.1136/thoraxjnl-2016-208406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valkama A.J., Leinonen H.M., Lipponen E.M., Turkki V., Malinen J., Heikura T., Ylä-Herttuala S., Lesch H.P. Optimization of lentiviral vector production for scale-up in fixed-bed bioreactor. Gene Ther. 2018;25:39–46. doi: 10.1038/gt.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Follenzi A., Naldini L. Generation of HIV- 1 Derived Lentiviral Vectors. Methods Enzymol. 2002;346:454–465. doi: 10.1016/s0076-6879(02)46071-5. [DOI] [PubMed] [Google Scholar]
- 37.Piatak M., Saag M.S., Yang L.C., Clark S.J., Kappes J.C., Luk K.C., Hahn B.H., Shaw G.M., Lifson J.D. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993;259:1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]