Abstract
Neural tube defects (NTDs) result in prenatal mortality and lifelong morbidity, and available treatments have limited efficacy. We previously suggested that prenatal bone marrow-derived mesenchymal stem cell (BMSC) transplantation could treat neuron deficiency in NTD rats; however, BMSC-based therapy is limited by the low survival rate of BMSCs when used to treat severe NTDs. Herein, a new therapy using combined BMSC transplantation and small interfering RNA of collapsin response mediator protein 4 (CRMP4 siRNA), which was identified as a novel potential target for the NTD treatment, is proposed. The intra-amniotic CRMP4 siRNA, BMSC, and CRMP4 siRNA + BMSC injections repaired skin lesions, improved motor neural function, reduced neuronal apoptosis, and promoted expression of neural differentiation-related molecules and neurotrophic factors in the spinal cord of spina bifida rat fetuses. Therapeutic effects in the CRMP4 siRNA + BMSC injection group were superior to those of the CRMP4 siRNA only or BMSC only injection groups. CRMP4 siRNA + BMSC injection resulted in a 45.38% reduction in the skin lesion area and significantly shorter latency and higher amplitude of motor-evoked potentials (MEPs) in spina bifida fetuses. Our results suggest that intrauterine Ad-CRMP4 siRNA delivery with BMSCs is an innovative platform for developing fetal therapeutics to safely and efficaciously treat NTDs.
Keywords: neural tube defects, CRMP4 siRNA, mesenchymal stem cell transplantation, prenatal therapy, neurological repair, gene transfer, neural differentiatio, intra-amniotic injection
Graphical Abstract
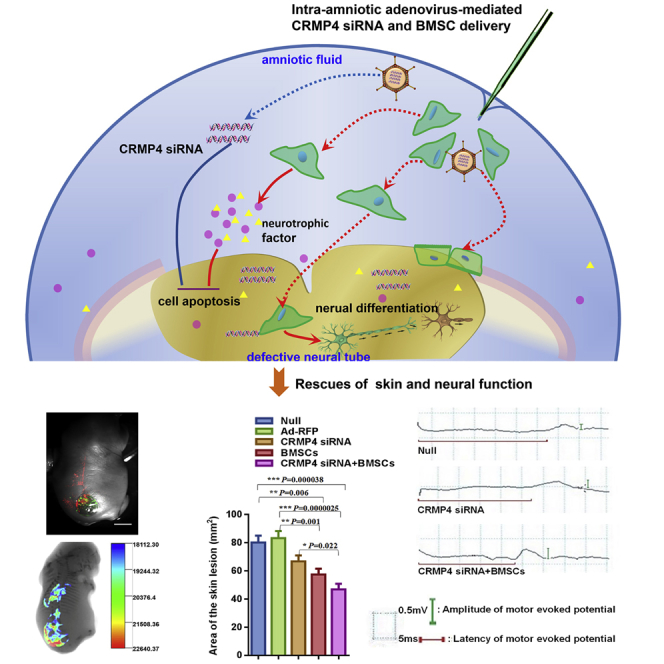
Yuan and colleagues identify CRMP4 as a novel therapeutic target in the treatment of spina bifida. Reducing CRMP4 expression by intra-amniotic delivery of adenovirus-mediated CRMP4 siRNA enhances the effects of bone mesenchymal stem cells (BMSCs) on skin lesion repair and neural function improvement in a rat fetal model of spina bifida.
Introduction
The most common types of severe congenital anomalies without effective treatments are congenital heart disease, genitourinary defects, and neural tube defects (NTDs).1 The most common and severe NTD phenotypes are anencephaly/exencephaly and spina bifida, which affect approximately 0.6–6 in every 1,000 pregnancies.2 Annual cost of medical and surgical treatment for all subjects with NTDs exceeded $200 million in the United States,3 and total lifetime direct cost of care for each person born with NTDs is estimated at $791,900.4 The neurological damage in NTDs is a two-stage process of failed neural tube closure followed by neurodegeneration in the uterine microenvironment. Prenatal open surgery aims to prevent further neurodegeneration by covering the persistently open neural tube and has been performed during fetal development in several centers during the last two decades.5 The Management of Myelomeningocele Study (MOMS), a multicenter randomized controlled clinical trial of prenatal surgery versus postnatal repair of myelomeningocele, demonstrated that although prenatal closure decreased hydrocephalus shunting by 50% and showed a degree of improvement in distal motor function postnatally compared to postnatal surgery, the recovery of neurological outcomes was limited, and individuals with NTDs continued to experience sensory and motor weakness in the leg as well as fecal or urinary incontinence after birth.6, 7, 8 In addition, an increased risk of pregnancy complications, such as preterm rupture and early preterm birth, were reported in the MOMS.6,7 Even with the recently developed endoscopic techniques and mini-hysterotomy interventions, which have shown a reduced incidence of premature delivery and uterine rupture, the neurological impairment remains a major complication of intrauterine endoscopic surgery for spina bifida.5,9, 10, 11 Most children who underwent in utero repair continued to suffer from life-long disabilities. Our previous results showed that deficiencies of sensory, motor, and parasympathetic neurons are primary anomalies in the malformed spinal cord of fetal rats with spina bifida aperta.12, 13, 14 Thus, neuron replacement therapy is likely a promising approach for achieving a better functional outcome in spina bifida.
Bone marrow-derived mesenchymal stem cells (BMSCs) originating from the mesodermal germ layer were discovered not only to give rise to different mesenchymal cell lineages,15 but also to transdifferentiate into non-mesenchymal lineages, including neurons, epithelial cells, and endothelial cells both in vitro and in vivo.16, 17, 18, 19 BMSCs have been shown to differentiate into various cell lineages in response to different microenvironments in numerous studies.20 Currently, BMSC-based therapy is being investigated for the treatment of neurodegenerative diseases and is shown to improve neural function and recovery of damaged neural tissues in animal studies and clinical trials.21, 22, 23, 24 In our previous studies, prenatal BMSC transplantation was successfully used to treat neuronal deficiency in NTDs. We showed that after transplantation into fetuses, BMSCs migrated and survived in the fetal neural tube, expressed markers of various neuron types, secreted neurotrophic factors, and reduced the neural apoptosis in surrounding neural tissue.25, 26, 27, 28 These results indicate that BMSC transplantation in utero may serve as a potential treatment option to repair NTD-associated neurological damage. However, BMSC-based therapy is limited by the low survival rate of BMSCs when used to treat severe types of NTDs. The neural tissue in NTD fetuses is severely damaged and usually accompanied by other anomalies. The survival rate of transplanted BMSCs in the defective spinal cord is approximately only 21.95% (intra-amniotic transplantation) to 24% (intra-spinal transplantation), and only 20% of engrafted BMSCs differentiate into various neural lineages.25,28 The survival and differentiation of BMSCs are mainly determined by the surrounding microenvironment. After transplantation, BMSCs are engrafted in specialized microenvironments that regulate their self-renewal and differentiation activities. The spinal cord microenvironment contains cells, extracellular matrix, spinal fluid, and cytokines, which play an important role in migration, survival, and differentiation of transplanted BMSCs. Harmful factors can reduce the efficacy of BMSC-based therapies.29,30 Reportedly, protecting the BMSCs from the poor microenvironment at defective sites by incubation with cytokines, or natural or chemical compounds, and the application of supporting materials could improve their function.30,31 Therefore, we hypothesized that several harmful factors may be present in the spinal cord tissue of NTD fetuses, which could not only cause neuronal injury in the spinal cord, but also lead to a low BMSC survival rate and thus reduce their effects. In our previous study, a proteomic approach was used to screen and identify the differentially expressed proteins in a rat model of spina bifida and found that collapsin response mediator protein 4 (CRMP4) was significantly upregulated in the early NTD embryos and might contribute to the pathogenesis of spina bifida by regulating neuronal apoptosis and neurite growth.32 In many recent studies, the CRMP4 expression was suggested to significantly change in some pathological conditions (Parkinson’s disease, spinal cord injury).33, 34, 35 Furthermore, CRMP4 can mediate inhibition of neurite outgrowth by interacting with RhoA36 and initiate neuronal apoptosis to promote neural degeneration.37,38 Reportedly, CRMP4 accelerates axonal degeneration, dopaminergic neuron loss, and inflammatory response after spinal cord injury or in a Parkinson’s disease mouse model.33,34 Duplan et al.38 suggested that CRMP4 overexpression in motor neurons of SOD1 mutant mice in vitro and in vivo leads to inhibition of neurite outgrowth followed by triggering cell apoptosis. In contrast, downregulated CRMP4 expression in motor neurons using small interfering RNA (siRNA) prevents nitric oxide (NO)-triggered neuronal death and promotes synaptic regeneration. CRMP4 suppressed apical dendrite bifurcation of CA1 pyramidal neurons and dendritic branching in cultured hippocampal neurons. However, the inhibitions were compromised in the CRMP4-deleted mice or CRMP4-deleted hippocampal neurons.39 Using a CRMP4-deleted mice model, Nagai et al.34 demonstrated that CRMP4 contributes to the limited recovery after adult central neural system injury, and CRMP4 deletion promotes neural axonal growth and functional recovery. These data indicate that CRMP4 could be a candidate target in the development of neuroregenerative medicine. However, the role of CRMP4 in the treatment of congenital neural diseases in vivo has not been examined to date.
In the present study, the therapeutic potentials of in utero BMSC transplantation and reduction of CRMP4 expression by intra-amniotic or intra-spinal injection of adenovirus-mediated CRMP4 siRNA (Ad-CRMP4 siRNA) in the treatment of spina bifida were investigated in a rat model. The etiological treatment of spina bifida using CRMP4 siRNA was evaluated. In addition, the spinal cord microenvironment could be improved to increase the survival rate of BMSCs and further enhance the therapeutic effects of BMSC repair and reconstruction to achieve a “double-edged sword” therapeutic effect. For the first time, the therapeutic effects of different treatment groups (CRMP4 siRNA injection, BMSC injection, or CRMP4 siRNA + BMSC injection) as well as different injection methods (intra-amniotic and intra-spinal injections) on rat fetuses with spina bifida were compared. The in utero CRMP4 siRNA, BMSC, and CRMP4 siRNA + BMSC injections effectively repaired the skin lesions and motor neural function, reduced neuronal apoptosis, and promoted the expression of neural differentiation-related molecules and neurotrophic factors in rat fetuses with spina bifida. The therapeutic effects in the CRMP4 siRNA + BMSC injection group were superior to those in the CRMP4 siRNA only or BMSC only injection groups.
Results
The siRNA RSH049252-4 Effectively Downregulated CRMP4 Expression Both In Vitro and In Vivo
Immunoblot analysis was performed to investigate the effects of recombinant adenovirus plasmids that expressed siRNAs targeting CRMP4 expression in neural stem cells (NSCs). The results showed that compared with control, the siRNAs RSH049252-1, RSH049252-2, RSH049252-3, and RSH049252-4 all significantly reduced the CRMP4 protein expression in NSCs. The downregulation of CRMP4 expression induced by RSH049252-4 was more apparent than by other siRNAs, thus, the RSH049252-4 sequence was used for the recombinant adenovirus CRMP4 siRNA expression vector (Figure 1A).
Figure 1.
The siRNA RSH049252-4 Effectively Downregulated the CRMP4 Expression Both In Vitro and In Vivo
(A) Upper: immunoblot analysis of protein expression of CRMP4 in NSCs treated by different siRNA sequences. Lower: quantification of relative protein levels determined from immunoblots. (B) Relative mRNA expression of CRMP4 in the E21 spinal cord of fetuses treated by intra-amniotic phosphate-buffered saline (PBS, null), Ad-RFP, CRMP4 siRNA, BMSC, or CRMP4 siRNA + BMSC injection. (C) Upper: immunoblot analysis of protein expression of CRMP4 in the spinal cord of fetuses treated by intra-amniotic PBS (null), Ad-RFP, CRMP4 siRNA, BMSC, or CRMP4 siRNA + BMSC injection. Lower: quantification of immunoblot result. Relative density of CRMP4 in different groups. siRNA, small interfering RNA. Data are presented as the mean ± SEM. Statistical significance according to a one-way ANOVA with Bonferroni’s multiple comparison test is indicated. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.49053751752599004905375175259900
To assess the effects of Ad-red fluorescent protein (RFP), Ad-CRMP4 siRNA, BMSCs, or CRMP4 siRNA + BMSCs on CRMP4 expression in the spinal cord of rat fetuses in vivo, AdRFPCRMP4 siRNA only, BMSCs only, or CRMP4 siRNA + BSMCs were injected into the amniotic cavity of fetuses and the CRMP4 expression was evaluated. Both the CRMP4 mRNA and protein levels were significantly decreased in the intra-amniotic CRMP4 siRNA-injected and CRMP4 siRNA + BMSC-injected fetuses (Figures 1B and 1C); the mRNA expression decreased by 31.79% and 33.97% respectively, and the protein decreased by 24.78% and 33.27%, respectively. In addition, the Crmp4 mRNA expression in the BMSC-injected fetuses was slightly downregulated, but it did not reach statistical significance (Figure 1B).
Skin Repair and Motor Neurological Function in Spina Bifida Aperta Rat Fetuses after Intra-Amniotic CRMP4 siRNA, BMSC, or CRMP4 + BMSC Injections
To observe whether CRMP4 siRNA-RFP+ve cells and/or GFP+ve BMSCs successfully survived in the defective spinal cords of fetuses with spina bifida aperta, whole-fetus images were taken using a stereomicroscopic fluorescent microscope. The results showed typical images of GFP+ve BMSCs surviving and RFP+ve cell populations in the spina bifida fetuses with intra-amniotic Ad-RFP, CRMP4 siRNA, BMSC, or CRMP4 siRNA + BSMC injections. The transplanted GFP+ve BMSCs spontaneously migrated to the dorsal region of defective spinal columns, and the CRMP4 siRNA-RFP+ve cells were clearly observed along the dorsal spinal cord and especially at the defective spinal cord site (Figure 2A). To determine the repair effect of CRMP4 siRNA or BMSC injection on skin tissue in vivo, the skin lesion area of spina bifida rat fetuses was measured. Using an in vivo imaging system, the GFP/RFP+ve cells were found distributed in the defective tissue sites with skeletal dysplasia of spina bifida fetuses injected with CRMP4 siRNA, BMSCs, or CRMP4 siRNA + BMSCs (Figure 2B). Compared with the null control group, a 16.62% reduction of the skin lesion areas was observed in the spina bifida fetuses injected with CRMP4 siRNA, a 28.39% decrease in the fetuses with BMSC transplantation, and a 45.38% decrease in the fetuses injected with CRMP4 siRNA + BMSCs (Figure 2C).
Figure 2.
Skin Repair and Neural Function Recovery in NTD Fetuses after Intra-Amniotic CRMP4 siRNA, BMSC, or CRMP4 siRNA + BMSC Injection
(A) Representative images of E21 rat fetuses with spina bifida after Ad-RFP, CRMP4 siRNA, and/or BMSC injection using fluorescence stereomicroscopy. Scale bar, 2.5 mm. (B) Representative overlays of X-ray and fluorescence images show a large number of Ad-CRMP4 siRNA RFP+ve cells or GFP+ve BMSCs at the defective lumbosacral region using an in vivo imaging system. (C) Quantitative analysis of the skin lesion area comparing the PBS-injected (null, n = 28), Ad-RFP-injected (n = 26), CRMP4 siRNA-injected (n = 29), BMSC-injected (n = 32), and CRMP4 + BMSC-injected (n = 25) groups. (D) Left: representative MEP waveforms collected using a multi-channel electrophysiology recorder in the spina bifida fetuses injected with PBS (null), CRMP4 siRNA, or CRMP4 siRNA + BMSCs. Right: analysis of MEP latency and amplitude in the null control (n = 6), CRMP4 siRNA-injected (n = 10), and CRMP4 siRNA + BMSC-injected groups (n = 10). Data are presented as the mean ± SEM. Statistical significance according to a one-way ANOVA with a Bonferroni’s multiple comparison test is indicated. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.49053751752599004905375175259900
To determine whether reducing the CRMP4 expression improved neurological function in NTD fetuses, the motor-evoked potentials (MEPs) elicited using transcranial electrical stimulation were monitored in embryonic day 21 (E21) fetuses. Compared with the null control and Ad-RFP injection group, a significantly shorter latency and significantly higher MEP amplitude were observed in the CRMP4 siRNA + BMSC injection group; however, in the CRMP4 siRNA injection group, only the MEP amplitude was significantly increased. The results indicated that the fetuses injected with CRMP4 siRNA, or CRMP4 siRNA + BMSCs, had a significantly improved motor neurological outcome (Figure 2D).
Reduction of Apoptosis after Intra-Amniotic CRMP4 siRNA, BMSC, or CRMP4 siRNA + BMSC Injections
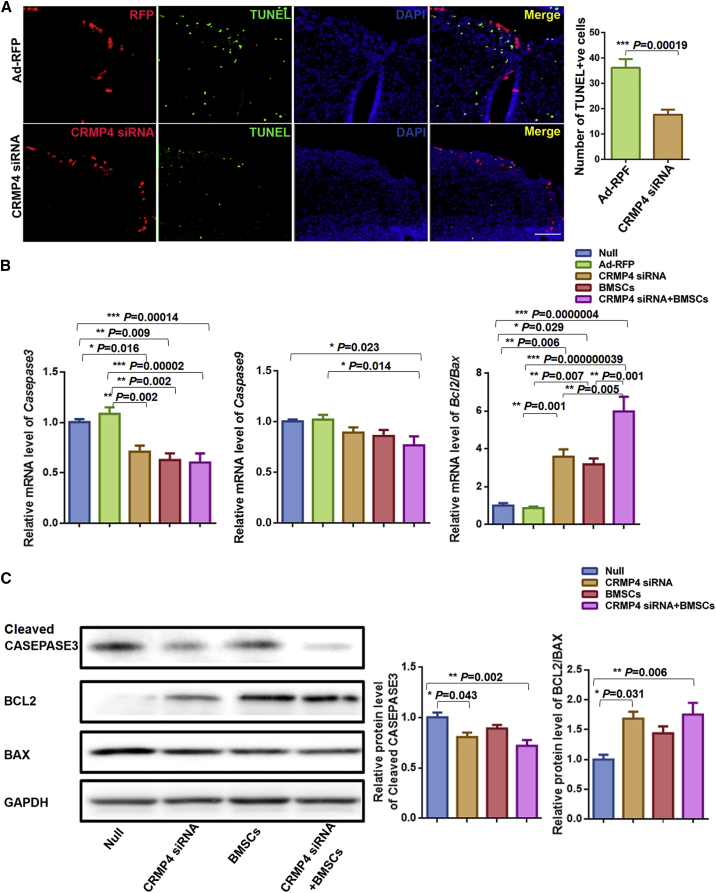
To clarify whether intra-amniotic CRMP4 siRNA injection might ameliorate the apoptosis in the defective spinal neural tissue, cell apoptosis in the spinal cord was evaluated using TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling) analysis. The apoptotic cells were frequently localized at the region of defective spinal cord in untreated fetuses. In contrast, the apoptotic cells were rarely found at the defective spinal cord region around the region of CRMP4 siRNA expression (Figure 3A).
Figure 3.
Reduction of Apoptosis after Intra-Amniotic CRMP4 siRNA, BMSC, or CRMP4 siRNA + BMSC Injection
(A) Left: representative confocal image showing decreased apoptosis (green dots) around the CRMP4 siRNA-RFP+ve cells (red). Apoptotic cells (green dots) were frequently localized in the defective spinal cord region in the Ad-RFP-injected rat fetuses (upper). In contrast, apoptotic cells were rarely found in the regions adjacent to the CRMP4 siRNA-RFP+ve region. Sections were counterstained with DAPI (blue) for nuclei. Right: the number of TUNEL+ve cells in the spinal cord of rat fetuses with spina bifida after Ad-RFP injection (n = 10) or CRMP4 siRNA injection (n = 10). Scale bar, 100 μm. (B) Relative mRNA expression of Casapase3, Caspase9, and Bcl2 and Bax in the spinal cord of E21 rat fetuses injected with PBS (null, n = 10), Ad-RFP (n = 9), CRMP4 siRNA (n = 9), BMSCs (n = 9), or CRMP4 siRNA + BMSCs (n = 9). Values represent fold expression differences compared with the PBS-injected group (null). (C) Immunoblot analysis of protein expression of cleaved CASPASE3, BCL2, and BAX in the spinal cord of E21 fetuses injected with PBS (null, n = 6), Ad-RFP (n = 6), CRMP4 siRNA (n = 6), BMSC (n = 6), or CRMP4 siRNA + BMSC (n = 6) injection. Relative protein levels were quantified using immunoblots. Values represent fold expression differences compared with the PBS-injected group (null). Data are presented as the mean ± SEM. Statistical significance according to a Student’s t test (A) or a one-way ANOVA with Bonferroni’s multiple comparison test (B and C) is indicated. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.49053751752599004905375175259900
Various mRNA expressions of apoptosis-related genes were observed in whole spinal cords from different intra-amniotic injection groups. The Caspase3 mRNA expression was significantly decreased in fetuses treated with CRMP4 siRNA and CRMP4 siRNA + BMSCs. Caspase9 expression was only downregulated in the CRMP4-siRNA + BMSC injection group. The Bcl2/Bax was significantly upregulated in all treatment groups compared with the null and Ad-RFP injection groups (Figure 3B).
Immunoblot analysis further confirmed decreased CASPASE3 expression and upregulated BCL2/BAX in the spinal cord of fetuses injected with intra-amniotic CRMP4 siRNA and CRMP4-siRNA + BMSCs compared with the null-injected fetuses (Figure 3C).
Improved mRNA and Protein Expression of Neural Differentiation-Related Molecules in the Spinal Cord After Intra-Amniotic CRMP4 siRNA, BMSC, or CRMP4 siRNA + BMSC Injection
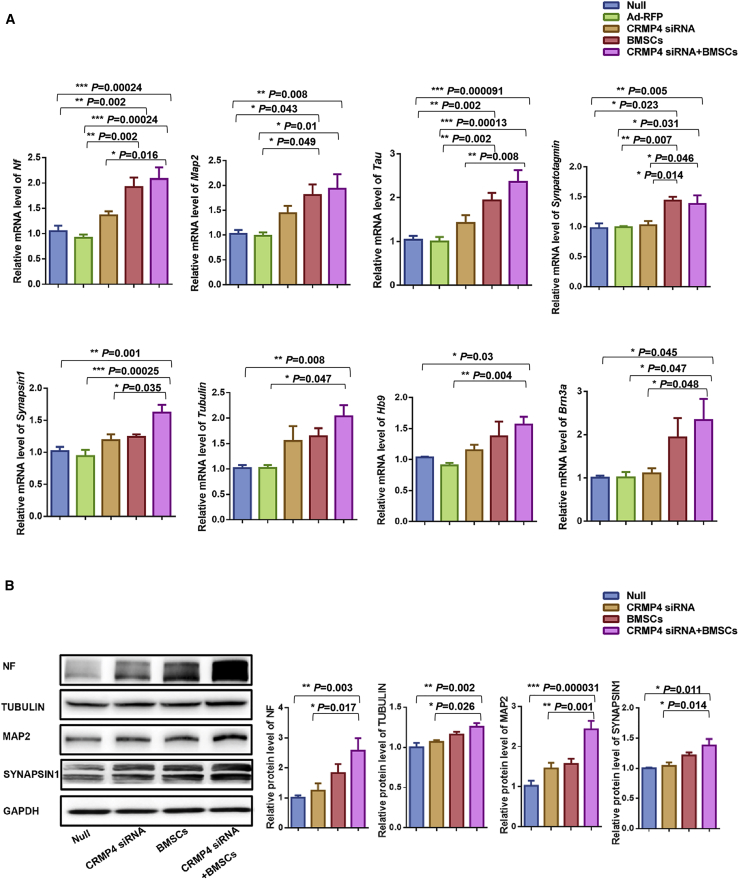
To clarify whether the neural differentiation was improved in the fetuses after intra-amniotic CRMP4 siRNA, BMSC, or CRMP4 siRNA + BMSC injection, various expressions of neural differentiation-related molecules were observed in spinal cords from different injection groups. Compared with null control and Ad-RFP injection groups, the mRNA expressions of Nf, Map2, Tau, and Synaptotagmin were significantly upregulated in the spinal cords injected with BMSCs and CRMP4 siRNA + BMSCs, whereas the upregulated expression of Synapsin1, Tubulin, Hb9, and Brn3a were only observed in the CRMP4 siRNA + BMSC injection group. Compared with the CRMP4 siRNA injection group, the Synaptotagmin mRNA expression in the BMSC injection group and CRMP4 siRNA +BMSC injection group, and the expressions of Nf, Tau, Synapsin1, and Brn3a in the CRMP4 siRNA + BMSC injection group, were significantly increased. However, the significant difference was not observed in the expression of these neural differentiation-related genes between the CRMP4 siRNA injection group and the null control or Ad-RFP groups (Figure 4A).
Figure 4.
Improved mRNA and Protein Expressions of Neural Differentiation-Related Molecules in the Spinal Cord after Intra-Amniotic CRMP4 siRNA, BMSC, or CRMP4 siRNA + BMSC Injection
(A) Relative mRNA expression of Nf, Map2, Tau, Synaptotagmin, Synapsin1, Tubulin, Hb9, and Brn3a in the spinal cord of E21 rat fetuses injected with PBS (null, n = 10), Ad-RFP (n = 9), CRMP4 siRNA (n = 9), BMSCs (n = 9), or CRMP4 siRNA + BMSCs (n = 9). Values represent fold expression differences compared with the no injection group (null). (B) Immunoblot analysis of protein expression of NF, TUBULIN, MAP2, and SYNAPSIN1 in the spinal cord of E21 fetuses injected with PBS (null, n = 6), Ad-RFP (n = 6), CRMP4 siRNA (n = 6), BMSCs (n = 6), or CRMP4 siRNA + BMSCs (n = 6). Quantification of relative protein levels was determined from immunoblots. Values represent fold expression differences compared with the PBS injection group (null). Data are presented as the mean ± SEM. Statistical significance according to a one-way ANOVA with Bonferroni’s multiple comparison test is indicated. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Immunoblot analysis further confirmed that the protein expression of NF, TUBULIN, MAP2, and SYNAPSIN1 were significantly upregulated in the spinal cord of fetuses injected with intra-amniotic CRMP4 siRNA + BMSCs compared with the null control and CRMP4 siRNA injection groups. In addition, the expression of the neural differentiation-related proteins in the CRMP4 siRNA + BMSC injection group was slightly higher than in the BMSC injection groups (Figure 4B).
Intra-Amniotic BMSC or CRMP4 siRNA Injections Promoted Secretion of Growth Factors in the Spinal Cords of Spina Bifida Fetuses
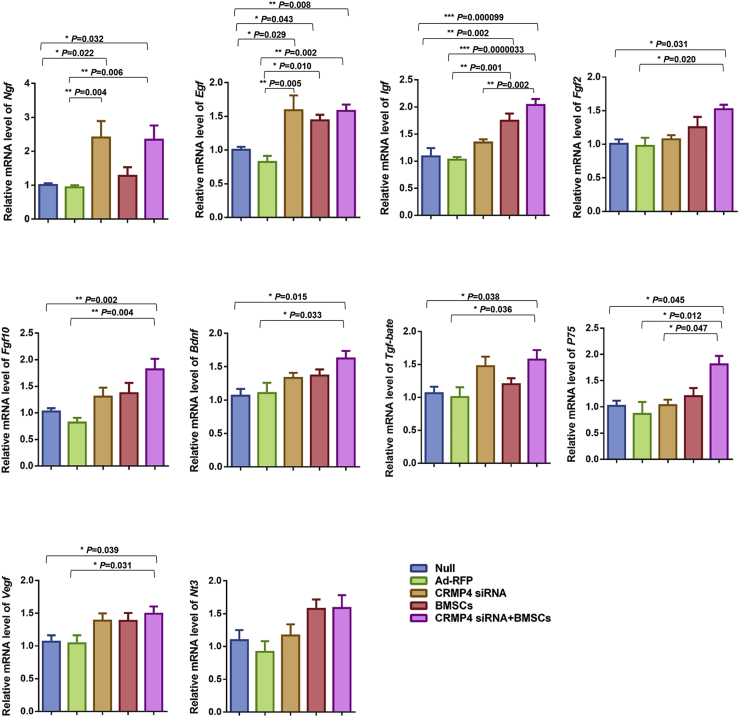
To investigate whether the expression of neurotrophic factors was changed after intra-amniotic CRMP4 siRNA or BMSC injection, expression of various growth factors in spinal cords from different transplant groups was evaluated. Compared with the null control and Ad-RFP injection groups, the mRNA expression of Ngf, Egf, Igf, Fgf2, Fgf10, Bdnf, Tgf-beta, P75, and Vegf were significantly increased in the CRMP4 siRNA + BMSC injection group. The mRNA level of Igf and P75 in the CRMP4 siRNA + BMSC-injected spinal cords was higher than that in the CRMP4 siRNA injection group. The Nt3 expression was not significantly changed between different treatment groups (Figure 5).
Figure 5.
Intra-Amniotic BMSC or CRMP4 siRNA Injections Promoted Secretion of Growth Factors in the Spinal Cords of Spina Bifida Fetuses
Relative mRNA expression of Ngf, Egf, Igf, Fgf2, Fgf10, Bdnf, Tgf-beta, P75, Vegf, and Nt3 in the E21 spinal cord of fetuses injected with PBS (null, n = 10), Ad-RFP (n = 9), CRMP4 siRNA (n = 9), BMSCs (n = 9), and CRMP4 siRNA + BMSCs (n = 9). Values present fold expression differences compared with the no injection group (null). Data are presented as the mean ± SEM. Statistical significance according to a one-way ANOVA with Bonferroni’s multiple comparison test is indicated. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.4905375175259900
Comparison of Therapeutic Effects Between Intra-Spinal CRMP4 siRNA + BMSC Injection and Intra-Amniotic CRMP4 siRNA + BMSC Injection for Spina Bifida
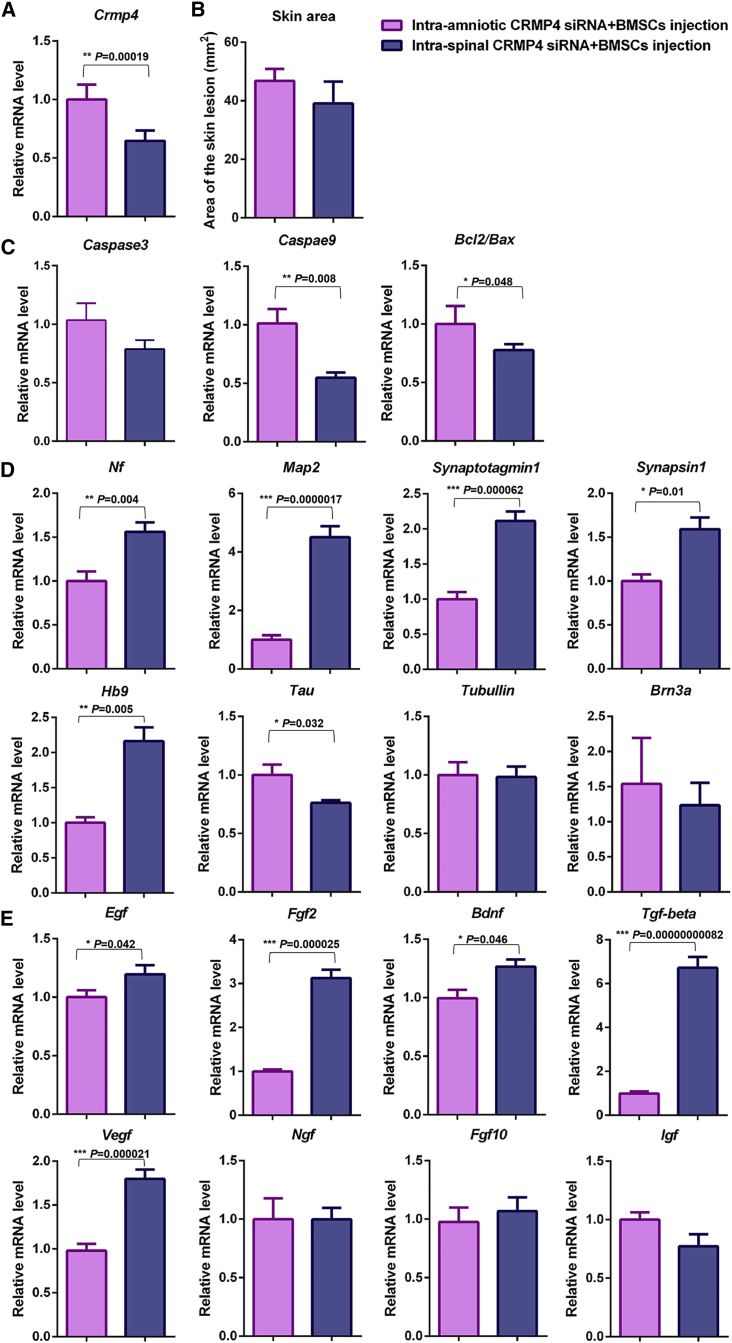
The intra-amniotic CRMP4 siRNA, BMSC, and CRMP4 siRNA + BMSC injections during the embryonic developmental stage were shown to effectively treat spina bifida; the effects of skin repair, anti-apoptosis, neural differentiation improvement, and growth factor secretion in the CRMP4 siRNA + BMSC injection group were superior to those in the CRMP4 siRNA only or BMSC only injection groups. The effects of intra-amniotic injection of CRMP4 siRNA + BMSCs in rat fetuses with spina bifida were compared with those of intra-spinal injection. The pregnant rats and fetuses that received intra-amniotic injection were all alive until euthanized on E21; however, the survival rates of pregnant rats and fetuses that received intra-spinal injection were 95.19% and 79.62%, respectively. The reduction of Crmp4 mRNA expression induced by a local spinal injection of CRMP4 siRNA + BMSCs was more effective than an intra-amniotic injection (Figure 6A). The skin lesion was slightly decreased in the intra-spinal siRNA + BMSC-injected fetuses compared with the intra-amniotic-injected fetuses; however, the statistical significance was not reached (Figure 6B). The mRNA expression of Caspase3, Caspase9, and Bcl2/Bax in the spinal cords of rat fetuses injected with intra-spinal siRNA + BMSCs was all reduced compared with the expression changes induced by intra-amniotic injection; however, the difference of Casepase3 expression did not reach statistical significance (Figure 6C). The expression of Nf, Map2, Synaptotagmin, Synaspin1, and Hb9 in the fetuses injected with intra-spinal CRMP4 siRNA + BMSCs was higher than that in the intra-amniotic CRMP4 siRNA + BMSC-injected fetuses, and the upregulated Tau expression was more apparent in the intra-amniotic-injected fetuses (Figure 6D). The increased expression of Egf, Fgf2, Bdnf, Tgf-beta, and Vegf in the CRMP4 siRNA + BMSC-injected fetuses was more evident when induced by intra-spinal injection than by intra-amniotic injection (Figure 6E).
Figure 6.
Comparison of Therapeutic Effects between Intra-Amniotic CRMP4 siRNA + BMSC Injection and Intra-Spinal CRMP4 siRNA + BMSC Injection for Spina Bifida
(A) Relative mRNA expression of Crmp4 in the spinal cord of fetuses of E21 spina bifida with CRMP4 siRNA + BMSC treatment through intra-amniotic (n = 9) and intra-spinal (n = 9) injection. (B) Quantitative analysis of the skin lesion area comparing the intra-amniotic CRMP4 siRNA + BMSC-injected fetuses (n = 25) and intra-spinal CRMP4 siRNA + BMSC-injected groups (n = 27). (C) Relative mRNA expression of Casepase3, Casepase9, and Bcl2 and Bax in the spinal cord of E21 rat fetuses in the intra-amniotic CRMP4 siRNA + BMSC injection group (n = 9) and intra-spinal CRMP4 siRNA + BMSC injection group (n = 9). (D) Relative mRNA expression of Nf, Map2, Tau, Synaptotagmin, Synapsin1, Tubulin, Hb9, and Brn3a in the spinal cord of E21 rat fetuses in the intra-amniotic CRMP4 siRNA + BMSC injection group (n = 9) and intra-spinal CRMP4 siRNA + BMSC injection group (n = 9). (E) Relative mRNA expression of Ngf, Egf, Igf, Fgf2, Fgf10, Bdnf, Tgf-beta, and Vegf in the spinal cord of E21 rat fetuses in the intra-amniotic CRMP4 siRNA + BMSC injection group (n = 9) and intra-spinal CRMP4 siRNA + BMSC injection group (n = 9). Values present fold expression differences compared with the intra-amniotic CRMP4 siRNA + BMSC injection group. Data are presented as the mean ± SEM. Statistical significance according to a Student’s t test is indicated. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.490537517525990049053751752599004905375175259900
Overall, direct injection of CRMP4 siRNA + BMSCs into the spinal column of rat fetuses on E16 showed obvious advantages in reducing the CRMP4 expression and enhancing the expression of several neurotrophic factors and neural differentiation-related genes. However, significant difference in repairing skin defects and attenuating the expression of pro-apoptotic genes was not observed between intra-spinal and intra-amniotic injections.
Discussion
Although folic acid supplementation has reduced the prevalence of NTDs, they remain one of the most common congenital malformations. Globally, 300,000–400,000 babies are born with NTDs each year,40 resulting in approximately 88,000 deaths and 8.6 million disability-adjusted life years (DALYs).41 Current therapeutics for NTDs have limited efficacy, with neurological complications as the main therapeutic issue, which have seriously affected children’s psychological development and quality of life as well as imposing a significant burden on families and society. The in utero BMSC transplantation in cell replacement strategies for NTDs has been reported in our previous studies; however, therapeutic efficacy was limited due to the low survival rate of transplanted BMSCs and low efficiency of neural differentiation of BMSCs induced by numerous obstacles present in the surrounding environment, such as secondary tissue damage and the activation of pro-apoptotic factors, which need to be overcome. Herein, CRMP4 was identified as a novel potential therapeutic target to improve the surrounding environment of transplanted BMSCs in the defective neural tissue, and reducing CRMP4 expression enhanced the therapeutic effects of BMSC transplantation to treat spina bifida in a rat fetal model. The in utero injection of CRMP4 siRNA, BMSCs, or CRMP4 siRNA + BMSCs repaired the skin lesions, improved neural function, reduced neuronal apoptosis, and promoted the expression of neural differentiation-related molecules and neurotrophic factors in the spinal cord of spina bifida rat fetuses. Compared with the CRMP4 siRNA only or BMSC only injection groups, the CRMP4 siRNA + BMSC injection group showed a more effective promotion of neural differentiation and secretion of neurotrophic factors to improve the spinal cord microenvironment, resulting in inhibited cell apoptosis and reduced skin lesion area in the NTD fetuses. Neural differentiation is critical for the recovery of neural function. The neurotrophic factors are essential for survival and neurodifferentiation of transplanted BMSCs and neural cells. Repaired skin can cover the exposed spinal tissue and prevent further damage of the neural tube. Thus, if these aspects are improved, to infer better neural repair effects on NTD fetuses is reasonable.
Initially, adenovirus-mediated siRNA targeting CRMP4 effectively reduced the mRNA and protein expressions of CRMP4 in NSCs and fetal spinal cords. CRMP4 was found to mediate various aspects of neural development, play important roles in the developing mouse brain,3,8 and be involved in neurite outgrowth inhibition and neural degeneration. The CRMP4 level was closely associated with neural repair, Parkinson’s disease,33 central nervous system injury,34 and peripheral nerve injury.35 CRMP4 deletion attenuated myelin-associated glycoprotein-induced inhibition of neurite outgrowth.42 CRMP4 knockout mice showed enhanced axonal growth and a reduced inflammation response against spinal cord injury.34 In the present study, decreased CRMP4 expression in the spinal cord of spina bifida rat fetuses injected with CRMP4 siRNA was accompanied by slightly reduced skin lesion area and shortened MEP latency. When CRMP4 siRNA + BMSCs was injected into the amniotic cavity of fetuses, a more effective repair of defective skin and neural function was achieved than with CRMP4 siRNA only or BMSC only injections. The secondary injury of spina bifida is mainly due to the exposure of neural tissue to amniotic fluid and sustained chemical stimulation by amniotic fluid components. Thus, if skin repair is improved, the exposed neural tissue can be better covered and promote neural repair. In addition, we previously reported that BMSC transplantation during embryonic development could repair the spinal neuron deficiency by promoting neural differentiation and regeneration of neurons.25, 26, 27, 28 CRMP4 was also reported to be involved in neural differentiation and influence neurite outgrowth by bundling F-actin.36 In the present study, the neural differentiation was promoted in the whole spinal cord of CRMP4 siRNA-injected rat fetuses. The results indicated that reducing CRMP4 expression in the spinal cords when transplanting BMSCs into the amniotic cavity may have a better effect on promoting neural differentiation in defective spinal cords, which is beneficial for the recovery of neural function.
In previous studies, excessive apoptosis was shown to be an important cause of NTDs.43,44 We previously demonstrated that BMSC transplantation rescued neuronal apoptosis in the defective spinal cord of rat fetuses with spina bifida by secreting neurotrophic factors and growth factors.25,26 CRMP4 has been shown to be associated with neuronal apoptosis. Reportedly, calpain-mediated truncation of CRMP4 induces neuronal apoptosis after neurotoxin treatment. CRMP4 deletion reduced cell apoptosis at the lesion site in the mouse model of spinal cord injury, indicating a neuroprotective effect (suppressing depolymerization of microtubules, apoptosis, and demyelination) on neurons and axonal growth.34,38,39 In the present study, intra-amniotic CRMP4 siRNA injection inhibited cell apoptosis. Similarly, CRMP4 siRNA + BMSC injection had an inhibitory effect on cell apoptosis in the defective spinal cord. Reduction of apoptosis in spinal tissue of rat fetuses injected with CRMP4 siRNA can partly be explained by inhibition of pro-apoptotic factors (caspase3, caspase9, and Bax) and activation of Bcl2. In addition, neurotrophic factors reportedly have a significant effect on anti-apoptosis in neural tissue.45 In our previous studies, the intrauterine intra-spinal BMSC transplantation promoted the secretion of neurotrophic factors, thus improving the spinal cord microenvironment,31 which is essential for the survival and differentiation of transplanted BMSCs and nerve tissue cells. In the present study, several growth factors in the spinal cord of spina bifida fetuses were induced after CRMP4 siRNA, BMSC, or CRMP4 siRNA + BMSC injection. Nine of the growth factors detected were upregulated in the CRMP4 siRNA + BMSC-injected fetuses; however, only Ngf and Egf were upregulated in the CRMP4 siRNA-injected group, and only Egf and Igf were upregulated in the BMSC-injected group. Therefore, the CRMP4 siRNA + BMSC injection might create a more permissive environment for neuron survival and regrowth of axons in the defective spinal cord of spina bifida fetuses.
In our previous studies, BMSCs were transplanted into the rat fetuses with spina bifida via intra-spinal injection. Comparison of the therapeutic effects of intra-amniotic versus intra-spinal CRMP4 siRNA + BMSC injection is important for exploring more effective NTD treatments in the future. In the present study, intra-spinal injection of CRMP4 siRNA + BMSC on E16 reduced the CRMP4 expression more effectively than did intra-amniotic injection. Therefore, achieving improved expression of several neurodifferentiation-related genes and neurotrophic factors is important and more conducive to promote the differentiation and survival of neurons and transplanted BMSCs in the spinal cord of spina bifida fetuses. Possibly, a higher transfection rate of Ad-CRMP4 siRNA in the spinal cord can be achieved with intra-spinal rather than intra-amniotic injection on E16. However, intra-spinal injection is a more technically demanding procedure than intra-amniotic injection and is a challenge regarding managing the risk of trauma to the fetus and abortion. In addition, the prenatal cell transplantation surgery is typically performed during late pregnancy, when pathology is already evident and the neural damage caused by NTDs may be irreversible. The intra-amniotic injection approach would avoid mechanical engagement of the embryo and mother, and allow delivery of stem cells and/or CRMP4 siRNA at the early stage of embryonic development before irreversible damages are formed.
Collectively, the results in the present study demonstrated the therapeutic potential of intra-amniotic adenovirus-mediated siRNA targeting CRMP4 combined with BMSC delivery in a rat fetal model of spina bifida. The efficacy of the treatment was predominantly attributed to the reduction of CRMP4, the promotion of neural differentiation, the inhibition of cell apoptosis, and the repair of skin lesion in the rat fetuses with spina bifida. In our previous study, in utero BMSC transplantation in rat fetuses with spina bifida indicated neuron replacement potential and neuroprotective functions of BMSCs. We propose that reducing CRMP4 expression in defective spinal cords may improve the therapeutic effects of BMSC transplantation on NTDs by attenuating cell apoptosis, weakening the axonal outgrowth inhibition, improving the microenvironment of spinal cord, and increasing the survival and neural differentiation of BMSCs. Although the exact mechanisms by which injected CRMP4 siRNA + BMSCs elicited tissue repair and/or neural recovery need to be elucidated, the data provided in the present study indicate in utero reduction of CRMP4 expression in the spinal cords via intra-amniotic injection of Ad-CRMP4 siRNA + BMSCs is beneficial for treating spina bifida fetuses, and it could be used in the future as a therapeutic strategy for fetuses with NTDs. Postnatal long-term functional outcome of prenatal CRMP4 siRNA + BMSC injection, particularly in the recovery of sensorimotor functions, has not been determined in the present study because the rat fetuses with spina bifida aperta died after birth. Further investigations are required to assess the long-term functional outcome of the proposed novel treatment. Based on the present study results, we suggest that BMSCs with CRMP4 siRNA treatment can enhance the therapeutic potential for spina bifida. Intra-amniotic injection of CRMP4 siRNA + BMSCs is an innovative platform for developing fetal therapeutics to safely and efficaciously treat congenital diseases. In the future, the application of intra-amniotic BMSC transplantation and gene transfer for treating NTDs and other congenital malformations should be performed in large animals to explore the optimal dosage of BMSCs and transplantation time.
Materials and Methods
Experiment Animals
Outbred Wistar rats 10–12 weeks old (250–300 g) and 4 weeks old (about 100 g) were purchased from the animal center of China Medical University. All rats were supplied with food and water ad libitum and kept under pathogen-free conditions with a 12-h light/12-dark cycle. Female rats were mated with male rats overnight. The morning that the vaginal plug was observed was considered as E0. All of the animal experiments were approved by the Committee for Animal Care at China Medical University.
Construction of Recombinant Adenovirus Encoding CRMP4 siRNA (pHBAd-CRMP4 siRNA)
The sequence of the full-length Crmp4 gene was obtained from GenBank. Four recombinant adenovirus plasmids that expressed siRNAs targeting CRMP4 were designed (Table 1) and synthesized by GeneCopoeia (USA). A BLAST search on the NCBI website was used to confirm that the sequences did not have homology to any other rat genes. The most suitable siRNA was selected as the target sequence.
Table 1.
Sequences of CRMP4 siRNA used in this study
| Clone Name | Symbol | Location | Length | Target Sequence |
|---|---|---|---|---|
| RSH049252-1 | CRMP4 | 281 | 19 | GGAGGCATTGATGTCCATA |
| RSH049252-2 | CRMP4 | 121 | 19 | TGACCGTCTTCTAATCAAG |
| RSH049252-3 | CRMP4 | 656 | 19 | GCCATTGCTCAAGTTCATG |
| RSH049252-4 | CRMP4 | 859 | 19 | GGCTGATCTCATCTCACAA |
| Control | 21 | TTCTCCGAACGTGTCACGTAA |
RSH049252-4-HIVU6 (OS371208) was selected as the target sequence.
The gene fragments of siRNA containing EcoRI and BamIII restriction enzyme cutting sites were synthesized and inserted into a pHBAd-U6-RFP vector, transfected with Escherichia coli DH5a, and detected using sequencing. The gene fragments were observed using a fluorescence microscope (Nikon, Japan). The adenoviruses containing the siRNA targeting CRMP4 were generated by homologous recombination via co-transfection of plasmids pHBAd-U6-CRMP4 siRNA-RFP and pHBAd-BHG in HEK293 cells using Lipofectmaine 2000 (Invitrogen, Carlsbad, CA, USA). After several rounds of plaque purification, the adenoviruses containing the CRMP4 siRNA gene were amplified and purified from cell lysates by banding twice in CsCl density gradients. Viral products were desalted and stored at −80°C in phosphate-buffered saline (PBS) containing 10% glycerol (v∕v). The infectious titer was determined using a standard plaque assay and averaged 1 × 1011 plaque-forming units (PFU)/mL.
NSC Culture and Transfection of Crmp4 siRNA Plasmids
Primary dissociated NSCs were obtained from the hippocampus of two to three Wistar rat embryos on E14 under sterile conditions. Briefly, the hippocampus was isolated and the blood vessels and meninges were removed. The hippocampal tissues were dispersed and added to NSC culture medium (Cyagen) containing B27 (2%), basic fibroblast growth factor (bFGF, 20 ng/mL), epithelial growth factor (EGF, 20 ng/mL), and 1% penicillin-streptomycin supplement and dispersed into pieces with a sterile pipette. Then, the mixture was filtered through a 200-mesh filter. Cells were cultured in an incubator with 5% CO2 at 37°C. The medium was replaced once every 2 days and passages were performed once a week using light mechanical dissociation of the formed spheres. When 80% confluent, the NSCs were transfected with Lipofectamine 2000 (Invitrogen, USA) and assigned to the control (with 0.1 mol/L PBS added, 100 μL), empty vector (transfected with 100 mol of empty vector plasmid), and test groups (transfected with 100 mol of CRMP4 siRNA recombinant adenovirus plasmids) to detect the silencing efficiency. The transfection efficiency was evaluated by observing the RFP expression at 2 days post-transfection. In addition, 4 days after transfection, total cell protein was extracted and CRMP4 protein expression was detected using immunoblot blot analysis to select the most suitable siRNA.
BMSC Culture and Transfection
BMSCs were isolated from the bone marrow of 4-week-old Wister rats and were cultured in Dulbecco’s modified Eagle’s Medium: nutrient mixture F-12 (DMEM/F12; HyClone, USA) supplemented with 10% fetal bovine serum (FBS; Corning Life Sciences/Cellgro, USA) in 25-cm2 tissue culture flasks (Nunc, USA). Primary isolated BMSCs were defined as P0. At confluency, cells were passaged (1:2) with fresh medium, and P3–P4 were used for transplantation. To visualize the BMSCs after transplantation into rat fetus, cells were transfected with enhanced green fluorescent protein (EGFP)-expressing adeno-5 vector (Ad-GEP, 100 PFU/cell; Hanbio, China) before transplantation (24 h). The Ad-GFP-transfected BMSCs were trypsinized, centrifuged, and resuspended in PBS before in utero injection. GFP+ve BMSCs were counted and adjusted with PBS to yield concentrations ranging from 2 × 109 to 3 × 109 cells/μL.
In Utero Intra-Amniotic Mircoinjections
Spina bifida aperta was induced by a single intra-gastric gavage of all-trans retinoic acid (atRA) (Sigma-Aldrich; 4% w/v in olive oil; 140 mg/kg body weight) to pregnant rats on E10 as previously described.46
In utero intra-amniotic injection was performed on E16 fetuses. Under pentobarbital sodium (40 mg/kg body weight) anesthesia, an incision was made in the abdominal wall of pregnant rats, and the uterus was exteriorized. The fetuses with uniform position and size of spinal bifida were randomly divided into PBS-injected (null), RFP-expressing adeno-5 vector-injected (Ad-RFP injected; 100 PFU/cell; Hanbio, China), Ad-CRMP4 siRNA-RFP-injected (CRMP4 siRNA injected), GFP-BMSC-injected (BMSC injected), and Ad-CRMP4 siRNA-RFP combined with GFP-BMSC-injected (CRMP4 siRNA + BMSC injected) groups under an operation microscope (Möller, Germany). Microinjection was performed by transuterine injection of 2 μL of solution using a glass micropipette (internal tip diameter 100 μm) connected to a Hamilton syringe. Micropipettes for injection were made from borosilicate glass capillaries (model GD-1; Narishige Scientific Instruments, Japan) by using a micropipette puller (model PB-7; Narishige Scientific Instruments, Japan). After injection, the uterus was returned to the abdomen, and laparotomy was closed in two layers. The pregnant rats recovered from the anesthesia within 1 h and were returned to their home cage. In average, six to eight fetuses could be injected in one dam without compromising the survival of the fetuses. The pregnant rats were euthanized at E21 by an overdose injection of pentobarbital sodium, and the injected fetuses were harvested for analysis.
Fetal Surgery and Intra-Spinal Microinjection
We injected Ad-CRMP4 siRNA or BMSCs into defective spinal cord of fetuses with combined techniques of fetal surgery and microinjection. The pregnant rats of E16 with atRA treatment were anaesthetized with pentobarbital sodium (40 mg/kg body weight). An incision was made in the abdominal wall, and the uterine horn was exteriorized. To alleviate uterine spasm, atropine (0.1 mg/kg body weight) was injected intraperitoneally, and the uterus was covered with wet gauze immersed with warm physiologic saline. Under the microscope, the fetuses with uniform position and size of spinal bifida were chosen, and were randomly divided into Null-injected and CRMP4 siRNA +BMSCs-injected groups. The 7-0-nylon purse-string suture and a small incision were made on the wall of the uterus. Then, the amniotic sac was opened and the defective region of the spinal cord was exposed. A suspension (0.2 μL/injection site) was injected into the defective region of the spinal cord with a micropipette connected to a Hamilton syringe (intra-spinal injection). After injection, the fetuses were returned to the uterus, and the wound of the uterus was closed and returned to the abdomen, then the abdominal cavity was closed. The pregnant rats recovered from the anesthesia within 1 h and were returned to their home cage. On E21, the pregnant rats were euthanized by an overdose injection of pentobarbital sodium, and the fetuses that received intra-spinal injection were harvested by cesarean section.
In Vivo Imaging and Fluorescence Imaging
The fetuses were anesthetized on E21, the distribution of RFP/GFP+ve cells in the surface of fetuses was observed under a fluorescence stereomicroscope (M165FC, Leica, Germany), and the dorsal images of fetuses were taken with a DS-Qi2 CCD camera (NY-1S35, Nikon, Japan). The skin lesion areas of fetus were measured by NIS-Elements BR analysis software.
The E21 fetuses under anesthesia were also imaged on the dorsal sides using an in vivo image system (MS FX PRO, Carestream Health, USA), which enables visualization of RFP/GFP marked cells throughout the whole body of fetuses (including the surface tissues and deep tissues). The parameters for in vivo imaging were as follows: GFP signal: excitation, 470 nm; emission, 535 nm; exposure time, 5 s; RFP signal: excitation, 540 nm; emission, 600 nm; exposure time, 5 s; X-ray exposure time, 3 min; X-ray filter, 0.4 mm.
Quantitative Real-Time Reverse Transcriptase PCR (RT-PCR) Analysis
On E21, the spinal cords of fetuses with spinal bifida in different treatment groups were dissected. Quantitative real-time RT-PCR analysis was performed according to standard procedures. Briefly, total RNA was isolated with an RNeasy mini kit (QIAGEN, Hamburg, Germany) according to the manufacturer’s instructions. The RNA (2 μg) was reverse transcribed at 37°C for 15 min, 85°C for 5 s, and 4°C with random 6-mer oligonucleotides (50 pmol), oligo(dT) primer (25 pmol), and the PrimeScript RT reagent kit (Takara, Tokyo, Japan) in 20 μL of reaction solution. Diluted RT-PCR products (1:10) (2 μL) were subjected to quantitative real-time PCR using a LightCycler 480 SYBR Green I Master (Roche, USA). The reaction solution (20 μL) contained 2 μL of the cDNA templates, 0.4 μM of each primer, and 10 μL of 2× SYBR Green Master Mix and was brought to final volume with RNase-free water. Reactions were performed in triplicate on the LightCycler 480 Instrument (Roche, Mannheim, Germany). PCR was performed as follows: pre-denaturation at 95°C for 30 s, 45 cycles of denaturation at 95°C for 5 s, and 20 s of annealing at 60°C–62°C. The negative control (without reverse transcriptase) gave no signal. The relative mRNA levels of each sample were calculated using the 2−ΔΔCt method and following glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression normalization, and they were expressed as fold induction compared with control fetuses. Details of the primers are reported in Table 2.
Table 2.
Primers Used in the Study
| Genes | Accession No. | Primer Sequences (5′→3′)/Exon Location (nt) | Annealing Temperature (°C) | PCR Product (bp) |
|---|---|---|---|---|
| Crmp4 | NM_012934.1 | sense: CCCAGGAGCAGGCACGAAT/696–715 | 60 | 109 |
| antisense: ACGGTGATGGCACGGAACA/804–785 | ||||
| Casepase3 | NM_012922.2 | sense: GGAACGAACGGACCTGTGG/441–459 | 60 | 220 |
| antisense: CGGGTGCGGTAGAGTAAGC/660–642 | ||||
| Caspase9 | NM_031632.1 | sense: GCTGGACGCAGTGTCAAGTTT/1147–1168 | 60 | 185 |
| antisense: CATTGGCGACCCTGAGAAGGAGG/1331–1309 | ||||
| Bcl2 | NM_016993.1 | sense: GAGCGTCAACAGGGAGATG/705–723 | 60 | 170 |
| antisense: GAGACAGCCAGGAGAAATCA/874–855 | ||||
| Bax | NM_017059.2 | sense: TGGAAGAAGATGGGCTGAGGC/651–671 | 60 | 139 |
| antisense: CATTCCCACCCCTCCCAATAAT/789–768 | ||||
| Nf | NM_031073.3 | sense: CGTCCCTGGAAATAGTCATA/255–274 | 60 | 110 |
| antisense: CACGGAGATAAGCAAGAAAT/364–345 | ||||
| Map2 | NM_013066.1 | sense: TTGGGCAGTGATTACTACGAG/1852–1872 | 60 | 152 |
| antisense: GTGCTGTACCCTGCTTCGTGT/2003–1983 | ||||
| Tau | NM_017212.2 | sense: TGGCTCCTTGGATAACATCACC/1005–1026 | 60 | 239 |
| antisense: CGGACACTTCATCGGCTAACG/1243–1223 | ||||
| Synapsin1 | NM_019133.2 | sense: TGGGCAAGGTCAAGGTAGA/928–946 | 60 | 121 |
| antisense: TGGACACGCACATCGTATTTA/1048–1028 | ||||
| Synaptotagmin | NM_001033680.2 | sense: CGCTGAGAAAGAAGAGCAAGA/1134–1154 | 60 | 131 |
| antisense: ATAAGCCACCCACATCCATC/1264–1245 | ||||
| Tubulin | NM_13924. 2 | sense: CAAGCGCATCTCCGAGCAGTTTAC/1183–1206 | 60 | 196 |
| antisense: CTCCTCGTCGTCATCTTCATACATC/1378–1354 | ||||
| Hb9 | NM_001271274.1 | sense: TCCAGAACCGCCGAATGAAATG/903–924 | 60 | 136 |
| antisense: AGCAGCTCCTCCTCCGTCTTCTCC/1038–1051 | ||||
| Brn3a | sense: ATCGCGGTGTCCCAGGGCAAGA/495–516 | 60 | 168 | |
| antisense: CGAGATGTGGTCCAGCAGGTCA/662–641 | ||||
| Ngf | NM_001277055.1 | sense: GCGTAATGTCCATGTTGTTC/178–197 | 58 | 123 |
| antisense: GTTTAGTCCAGTGGGCTTCA/300–281 | ||||
| Egf | NM_012842.1 | sense: CCACGGTTACATTCACTCC/1063–1081 | 60 | 144 |
| antisense: GCTATCCAAATCGCCTTC/1206–1189 | ||||
| Igf | NM_001082479.1 | sense: ATGGGGAAAATCAGCAGTC/281–299 | 60 | 130 |
| antisense: TGGTAAAGGTGAGCAAGCA/410–392 | ||||
| Fgf2 | NM_019305.2 | sense: CACGTCAAACTACAGCTCCAA/704–724 | 60 | 152 |
| antisense: GACTCCAGGCGTTCAAAGA/855–837 | ||||
| Fgf10 | NM_012951.1 | sense: GTTGTTGCCGTCAAAGCCATTA/379–400 | 60 | 163 |
| antisense: TGCCGTTGTGCTGCCAGTT/541–523 | ||||
| BDNF | NM_012513.4 | sense: GTCACAGTCCTGGAGAAAGTC/1156–1176 | 60 | 149 |
| antisense: GATTGGGTAGTTCGGCATT/1304–1286 | ||||
| Tgf-beta | NM_021578.2 | sense: TCAAGTCAACTGTGGAGCAA/624–643 | 60 | 108 |
| antisense: GCCACTCAGGCGTATCAG/731–714 | ||||
| P75 | NM_012610.2 | sense: CTGGGCTGATGCTGAATGCG/662–681 | 60 | 216 |
| antisense: ACAGGAATGAGGTTGTCGGTGGT/855–877 | ||||
| Vegf | NM_001110335.1 | sense: GCAGCATAGCAGATGTGAA/435–453 | 60 | 129 |
| antisense: TGAACGCTCCAGGATTTA/563-546 | ||||
| Nt3 | NM_031073.3 | sense: CGTCCCTGGAAATAGTCATA/255–274 | 60 | 110 |
| antisense: CACGGAGATAAGCAAGAAAT/364–345 | ||||
| GAPDH | NM_017008.4 | sense: TGCCGCCTGGAGAAACCTGC/808-827 | 60 | 168 |
| antisense:AGCAATGCCAGCCCCAGCAT/956–975 |
Immunoblots
Proteins were isolated from spinal cords of E21 fetuses by a lysis buffer (P0013B, Beyotime, China), and the protein concentration was quantified using the Bradford method. In total, 50 μg of protein extract was separated by 12.5% SDS-PAGE and then transferred with Tris-HCl methanol (20 mM Tris, 150 mM glycine, 20% methanol) onto polyvinylidene fluoride membranes (Millipore, USA) in a trans-blot electrophoresis transfer cell (Bio-Rad, USA). Blotting was probed with antibodies against cleaved CASEPASE3 (Cell Signaling Technology, USA), BCL2 (Sigma, USA), BAX (Cell Signaling Technology, USA), βШ-TUBULIN (Millipore, USA), SYNAPSIN11 (Millipore, USA), NF (Abcam, USA), or GAPDH (Proteintech, China). All immunoblots were run at least in triplicate. Immunopositive bands were visualized using enhanced chemiluminescence reagents (Millipore, USA). Detected bands were quantified by ImageJ software. The relative density of each protein was calculated by dividing the optical density value of each protein by that of the loading control (GAPDH).
TUNEL Analysis
TUNEL analysis was performed using an In Situ Cell Death Detection Kit (Roche, USA) and with mouse anti-RFP antibody (Abbkine, China) according to the manufacturer’s protocol. Briefly, the sections of fetal spinal cord at E21 were permeated with proteinase K and blocked with PBS containing 10% FBS and 0.1% Triton X-100. Then, the sections were incubated with TUNEL reaction mixture and TRITC (tetramethylrhodamine isothiocyanate)-anti-rabbit immunoglobulin G (IgG) at 37°C for 1.5 h. After washing, sections were stained with DAPI and mounted with anti-fade mounting medium. The images were taken with a C1 confocal microscope (Nikon, Japan). The number of total TUNEL+ve cells in the neuroepithelium of each section was counted.
Electrophysiological Tests
The E21 fetuses were anesthetized with pentobarbitone (40 mg/kg) and placed prone on a warmed plate to maintain skin temperature above 32°C. A polyphysiograph (PL3508/P, PowerLab, Australia) was used for the tests. MEPs were recorded with monopolar needle electrodes from the anterior tibialis muscles; the active recording needle electrode was inserted in the ventral side of the tibialis anterior muscles with a reference electrode at the fourth toe and a ground electrode at the base of the tail. While the contralateral sensorimotor cortex was stimulated by single electrical pulses of 3 mA intensity at 1 Hz and 100 μs duration, delivered by two needle electrodes inserted subcutaneously, the anode was placed over the skull overlying the sensorimotor cortex, and the cathode was placed on the nose. To ensure reproducibility of the MEP tests, stimulation and recording needles were placed in the same locations on all fetuses. The signals were amplified, filtered (band-pass 1–5,000 Hz), and displayed on the monitor to measure the latency to onset and the amplitude from peak-to-peak of the largest positive-negative deflection. To ensure reproducibility, 15 consecutive responses were recorded with a 3-s interval between stimuli. The recording with the highest amplitude was used for analysis.
Statistical Analysis
Statistical analyses were conducted using SPSS 16.0, and the details for each experiment are given in the figure legends. All data presented in this study are expressed as the mean ± SEM. The two-tailed Student’s t test was used for single comparisons. One-way analysis of variance (ANOVA) followed by the Bonferroni’ s post hoc test (equal variances assumed) was used to compare three or more groups. A p value <0.05 was considered statistically significant.
Author Contributions
X.W., S.C., and Z.Y. designed the study; X.W. wrote the paper; X.W., S.C., and C.Z. performed the experiments; W.M., H.G., D.L., and W.L. analyzed the data; Y.B. and W.W. helped to revise the manuscript; Z.Y. supervised the entire project.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the National Key Research and Development Program (2016YFC1000505); the National Natural Foundation of China (Grants 81871219, 81671469, and 81901565); the 345 Talent Project; the Specialized Research Fund for the Doctoral Program of Liaoning Province (201601136); and by the Scientific Research Fund of Liaoning Provincial Education Department (LQNK201710).
Contributor Information
Songying Cao, Email: csymn01@163.com.
Zhengwei Yuan, Email: yuanzw@hotmail.com.
References
- 1.Copp A.J., Stanier P., Greene N.D. Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol. 2013;12:799–810. doi: 10.1016/S1474-4422(13)70110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molloy A.M., Kappen C. Papers from the 7th International Neural Tube Defects Conference. Birth Defects Res. A Clin. Mol. Teratol. 2012;94:747–748. doi: 10.1002/bdra.23082. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control (CDC) Economic burden of spina bifida—United States, 1980–1990. MMWR Morb. Mortal. Wkly. Rep. 1989;38:264–267. [PubMed] [Google Scholar]
- 4.Grosse S.D., Berry R.J., Mick Tilford J., Kucik J.E., Waitzman N.J. Retrospective assessment of cost savings from prevention: folic acid fortification and spina bifida in the U.S. Am. J. Prev. Med. 2016;50(5, Suppl 1):S74–S80. doi: 10.1016/j.amepre.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruner J.P., Richards W.O., Tulipan N.B., Arney T.L. Endoscopic coverage of fetal myelomeningocele in utero. Am. J. Obstet. Gynecol. 1999;180:153–158. doi: 10.1016/s0002-9378(99)70167-5. [DOI] [PubMed] [Google Scholar]
- 6.Adzick N.S., Thom E.A., Spong C.Y., Brock J.W., 3rd, Burrows P.K., Johnson M.P., Howell L.J., Farrell J.A., Dabrowiak M.E., Sutton L.N., MOMS Investigators A randomized trial of prenatal versus postnatal repair of myelomeningocele. N. Engl. J. Med. 2011;364:993–1004. doi: 10.1056/NEJMoa1014379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson M.P., Bennett K.A., Rand L., Burrows P.K., Thom E.A., Howell L.J., Farrell J.A., Dabrowiak M.E., Brock J.W., 3rd, Farmer D.L. The Management of Myelomeningocele Study: obstetrical outcomes and risk factors for obstetrical complications following prenatal surgery. Am. J. Obstet. Gynecol. 2016;215:778.e1–778.e9. doi: 10.1016/j.ajog.2016.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joyeux L., Danzer E., Flake A.W., Deprest J. Fetal surgery for spina bifida aperta. Arch. Dis. Child. Fetal Neonatal Ed. 2018;103:F589–F595. doi: 10.1136/archdischild-2018-315143. [DOI] [PubMed] [Google Scholar]
- 9.Araujo Júnior E., Tonni G., Martins W.P. Outcomes of infants followed-up at least 12 months after fetal open and endoscopic surgery for meningomyelocele: a systematic review and meta-analysis. J. Evid. Based Med. 2016;9:125–135. doi: 10.1111/jebm.12207. [DOI] [PubMed] [Google Scholar]
- 10.Botelho R.D., Imada V., Rodrigues da Costa K.J., Watanabe L.C., Rossi Júnior R., De Salles A.A.F., Romano E., Peralta C.F.A. Fetal myelomeningocele repair through a mini-hysterotomy. Fetal Diagn. Ther. 2017;42:28–34. doi: 10.1159/000449382. [DOI] [PubMed] [Google Scholar]
- 11.Belfort M., Deprest J., Hecher K. Current controversies in prenatal diagnosis 1: in utero therapy for spina bifida is ready for endoscopic repair. Prenat. Diagn. 2016;36:1161–1166. doi: 10.1002/pd.4972. [DOI] [PubMed] [Google Scholar]
- 12.Yuan Z.W., Lui V.C., Tam P.K. Deficient motor innervation of the sphincter mechanism in fetal rats with anorectal malformation: a quantitative study by fluorogold retrograde tracing. J. Pediatr. Surg. 2003;38:1383–1388. doi: 10.1016/s0022-3468(03)00401-9. [DOI] [PubMed] [Google Scholar]
- 13.Guan K., Li H., Fan Y., Wang W., Yuan Z. Defective development of sensory neurons innervating the levator ani muscle in fetal rats with anorectal malformation. Birth Defects Res. A Clin. Mol. Teratol. 2009;85:583–587. doi: 10.1002/bdra.20576. [DOI] [PubMed] [Google Scholar]
- 14.Jia H., Zhang K., Zhang S., Yuan Z., Bai Y., Wang W. Quantitative analysis of sacral parasympathetic nucleus innervating the rectum in rats with anorectal malformation. J. Pediatr. Surg. 2007;42:1544–1548. doi: 10.1016/j.jpedsurg.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 15.Lee D.E., Ayoub N., Agrawal D.K. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res. Ther. 2016;7:37. doi: 10.1186/s13287-016-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian L., Saltzman W.M. Improving the expansion and neuronal differentiation of mesenchymal stem cells through culture surface modification. Biomaterials. 2004;25:1331–1337. doi: 10.1016/j.biomaterials.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz-Elias G., Marcus A.J., Coyne T.M., Woodbury D., Black I.B. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. J. Neurosci. 2004;24:4585–4595. doi: 10.1523/JNEUROSCI.5060-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mezey E., Chandross K.J., Harta G., Maki R.A., McKercher S.R. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa H., Akita S., Fukui M., Fujii T., Akino K. Human mesenchymal stem cells successfully improve skin-substitute wound healing. Br. J. Dermatol. 2005;153:29–36. doi: 10.1111/j.1365-2133.2005.06554.x. [DOI] [PubMed] [Google Scholar]
- 20.Phinney D.G., Prockop D.J. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 21.Venkataramana N.K., Kumar S.K., Balaraju S., Radhakrishnan R.C., Bansal A., Dixit A., Rao D.K., Das M., Jan M., Gupta P.K., Totey S.M. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl. Res. 2010;155:62–70. doi: 10.1016/j.trsl.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Bai L., Lennon D.P., Caplan A.I., DeChant A., Hecker J., Kranso J., Zaremba A., Miller R.H. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat. Neurosci. 2012;15:862–870. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honmou O., Houkin K., Matsunaga T., Niitsu Y., Ishiai S., Onodera R., Waxman S.G., Kocsis J.D. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parr A.M., Tator C.H., Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 25.Li H., Gao F., Ma L., Jiang J., Miao J., Jiang M., Fan Y., Wang L., Wu D., Liu B. Therapeutic potential of in utero mesenchymal stem cell (MSCs) transplantation in rat foetuses with spina bifida aperta. J. Cell. Mol. Med. 2012;16:1606–1617. doi: 10.1111/j.1582-4934.2011.01470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H., Miao J., Zhao G., Wu D., Liu B., Wei X., Cao S., Gu H., Zhang Y., Wang L. Different expression patterns of growth factors in rat fetuses with spina bifida aperta after in utero mesenchymal stromal cell transplantation. Cytotherapy. 2014;16:319–330. doi: 10.1016/j.jcyt.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Li X., Yuan Z., Wei X., Li H., Zhao G., Miao J., Wu D., Liu B., Cao S., An D. Application potential of bone marrow mesenchymal stem cell (BMSCs) based tissue-engineering for spinal cord defect repair in rat fetuses with spina bifida aperta. J. Mater. Sci. Mater. Med. 2016;27:77. doi: 10.1007/s10856-016-5684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma W., Wei X., Gu H., Li H., Guan K., Liu D., Chen L., Cao S., An D., Zhang H. Sensory neuron differentiation potential of in utero mesenchymal stem cell transplantation in rat fetuses with spina bifida aperta. Birth Defects Res. A Clin. Mol. Teratol. 2015;103:772–779. doi: 10.1002/bdra.23401. [DOI] [PubMed] [Google Scholar]
- 29.He N., Zhang L., Cui J., Li Z. Bone marrow vascular niche: home for hematopoietic stem cells. Bone Marrow Res. 2014;2014:128436. doi: 10.1155/2014/128436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mias C., Trouche E., Seguelas M.H., Calcagno F., Dignat-George F., Sabatier F., Piercecchi-Marti M.D., Daniel L., Bianchi P., Calise D. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells. 2008;26:1749–1757. doi: 10.1634/stemcells.2007-1000. [DOI] [PubMed] [Google Scholar]
- 31.Cao S., Wei X., Li H., Miao J., Zhao G., Wu D., Liu B., Zhang Y., Gu H., Wang L. Comparative study on the differentiation of mesenchymal stem cells between fetal and postnatal rat spinal cord niche. Cell Transplant. 2016;25:1115–1130. doi: 10.3727/096368915X689910. [DOI] [PubMed] [Google Scholar]
- 32.Zhou F., Fan Y., Wei X., Wang L., Miao J., Li H., Shan L., Yuan Z. Enhanced expression of collapsin response mediator protein 4 in spinal cords of rat fetuses with spina bifida aperta. Int. J. Clin. Exp. Med. 2016;9:1576–1584. [Google Scholar]
- 33.Tonouchi A., Nagai J., Togashi K., Goshima Y., Ohshima T. Loss of collapsin response mediator protein 4 suppresses dopaminergic neuron death in an 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. J. Neurochem. 2016;137:795–805. doi: 10.1111/jnc.13617. [DOI] [PubMed] [Google Scholar]
- 34.Nagai J., Kitamura Y., Owada K., Yamashita N., Takei K., Goshima Y., Ohshima T. Crmp4 deletion promotes recovery from spinal cord injury by neuroprotection and limited scar formation. Sci. Rep. 2015;5:8269. doi: 10.1038/srep08269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jang S.Y., Shin Y.K., Jung J., Lee S.H., Seo S.Y., Suh D.J., Park H.T. Injury-induced CRMP4 expression in adult sensory neurons; a possible target gene for ciliary neurotrophic factor. Neurosci. Lett. 2010;485:37–42. doi: 10.1016/j.neulet.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 36.Alabed Y.Z., Pool M., Ong Tone S., Fournier A.E. Identification of CRMP4 as a convergent regulator of axon outgrowth inhibition. J. Neurosci. 2007;27:1702–1711. doi: 10.1523/JNEUROSCI.5055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z., Ottens A.K., Sadasivan S., Kobeissy F.H., Fang T., Hayes R.L., Wang K.K. Calpain-mediated collapsin response mediator protein-1, -2, and -4 proteolysis after neurotoxic and traumatic brain injury. J. Neurotrauma. 2007;24:460–472. doi: 10.1089/neu.2006.0078. [DOI] [PubMed] [Google Scholar]
- 38.Duplan L., Bernard N., Casseron W., Dudley K., Thouvenot E., Honnorat J., Rogemond V., De Bovis B., Aebischer P., Marin P. Collapsin response mediator protein 4a (CRMP4a) is upregulated in motoneurons of mutant SOD1 mice and can trigger motoneuron axonal degeneration and cell death. J. Neurosci. 2010;30:785–796. doi: 10.1523/JNEUROSCI.5411-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niisato E., Nagai J., Yamashita N., Abe T., Kiyonari H., Goshima Y., Ohshima T. CRMP4 suppresses apical dendrite bifurcation of CA1 pyramidal neurons in the mouse hippocampus. Dev. Neurobiol. 2012;72:1447–1457. doi: 10.1002/dneu.22007. [DOI] [PubMed] [Google Scholar]
- 40.Bhandari S., Sayami J.T., K C R.R., Banjara M.R. Prevalence of congenital defects including selected neural tube defects in Nepal: results from a health survey. BMC Pediatr. 2015;15:133. doi: 10.1186/s12887-015-0453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaganjor I., Sekkarie A., Tsang B.L., Williams J., Razzaghi H., Mulinare J., Sniezek J.E., Cannon M.J., Rosenthal J. Describing the prevalence of neural tube defects worldwide: a systematic literature review. PLoS ONE. 2016;11:e0151586. doi: 10.1371/journal.pone.0151586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagai J., Goshima Y., Ohshima T. CRMP4 mediates MAG-induced inhibition of axonal outgrowth and protection against Vincristine-induced axonal degeneration. Neurosci. Lett. 2012;519:56–61. doi: 10.1016/j.neulet.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Wei X., Li H., Miao J., Zhou F., Liu B., Wu D., Li S., Wang L., Fan Y., Wang W., Yuan Z. Disturbed apoptosis and cell proliferation in developing neuroepithelium of lumbo-sacral neural tubes in retinoic acid-induced spina bifida aperta in rat. Int. J. Dev. Neurosci. 2012;30:375–381. doi: 10.1016/j.ijdevneu.2012.03.340. [DOI] [PubMed] [Google Scholar]
- 44.Alles A.J., Sulik K.K. Retinoic acid-induced spina bifida: evidence for a pathogenetic mechanism. Development. 1990;108:73–81. doi: 10.1242/dev.108.Supplement.73. [DOI] [PubMed] [Google Scholar]
- 45.Guillemot F., Zimmer C. From cradle to grave: the multiple roles of fibroblast growth factors in neural development. Neuron. 2011;71:574–588. doi: 10.1016/j.neuron.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Danzer E., Schwarz U., Wehrli S., Radu A., Adzick N.S., Flake A.W. Retinoic acid induced myelomeningocele in fetal rats: characterization by histopathological analysis and magnetic resonance imaging. Exp. Neurol. 2005;194:467–475. doi: 10.1016/j.expneurol.2005.03.011. [DOI] [PubMed] [Google Scholar]