Abstract
Background
Transwomen have an increased risk of HIV acquisition compared with other adults. Drug–drug interactions between pre-exposure prophylaxis (PrEP) and gender-affirming therapy are cited as a reason for poor PrEP uptake among transwomen. We evaluated plasma tenofovir and emtricitabine pharmacokinetics and their active intracellular anabolites, tenofovir-diphosphate and emtricitabine-triphosphate, in transwomen receiving feminizing hormones.
Methods
We enrolled HIV-negative transwomen (≥19 years) not receiving PrEP. Participants took oral tenofovir disoproxil fumarate/emtricitabine 300/200 mg daily for 14 days. Plasma was collected at 0 h (pre-dose), 0.5, 1, 2, 3, 4, 6, 8 and 12 h on day 14 post-tenofovir disoproxil fumarate/emtricitabine dose. The plasma AUC0–24 was calculated using the trapezoidal rule and compared with historical HIV-negative cisgender adults as geometric mean ratios (GMRs, 90% CI). Secondarily, tenofovir-diphosphate and emtricitabine-triphosphate from PBMCs collected at 0 h and 12 h were reported descriptively as geometric means (90% CI). Clinical trials registration: NCT03270969.
Results
Among 15 transwomen (mean age 32 years), geometric mean tenofovir and emtricitabine plasma AUC0–24 were lower compared with controls: tenofovir, 2.10 versus 2.76 mg·h/L, GMR 0.76 (0.65–0.90), P = 0.01; emtricitabine, 9.15 versus 10.64 mg·h/L, GMR 0.86 (0.75–0.98), P = 0.07. Tenofovir-diphosphate and emtricitabine-triphosphate concentrations were higher than previously reported in the literature: 167.1 (146.6–190.5) fmol/106 cells and 15.4 (13.8–17.3) pmol/106 cells, respectively.
Conclusions
We observed lower plasma tenofovir and emtricitabine concentrations in transwomen compared with historical cisgender adults, yet intracellular tenofovir-diphosphate and emtricitabine-triphosphate concentrations were higher than previously reported in PBMCs. Understanding the differences of PrEP pharmacokinetics in plasma and tissue compartments and the resultant impact on efficacy remains important for transwomen.
Introduction
Transgender individuals have a gender identity that is incongruent with their sex assigned at birth. Based on estimates from US population-based surveys collected between 2007 and 2016, approximately 1 million adults were identified as transgender.1,2 Transgender women (transwomen) are assigned male at birth and have a female gender identity. Globally, transwomen have 49 times higher odds of acquiring HIV compared with other adults of reproductive age.3 Between 2009 and 2014 in the USA, nearly 2400 transgender adults were diagnosed with HIV; more than 80% were transwomen.4
Tenofovir disoproxil fumarate and emtricitabine are NRTIs that are phosphorylated to antivirally active intracellular moieties and inhibit proviral DNA formation.5 Daily tenofovir disoproxil fumarate/emtricitabine as oral HIV pre-exposure prophylaxis (PrEP) was shown to decrease HIV incidence among MSM and transwomen by 44% compared with placebo in the multinational iPrEx study.6 Despite this, a similar number of transwomen seroconverted in the treatment arm (n = 11) compared with the placebo arm [n = 10; HR (95% CI) 1.1 (0.5–2.7)].7 The active tenofovir anabolite, tenofovir-diphosphate (DP), was not detected in the cells of transwomen who seroconverted while taking tenofovir disoproxil fumarate/emtricitabine in the trial.7,8 In contrast, transwomen with tenofovir-DP concentrations suggesting at least four doses per week of oral tenofovir disoproxil fumarate/emtricitabine did not seroconvert, which indicated the importance of adherence among transwomen.7 In other studies, transwomen reported concerns about the influence of antiretrovirals on their gender transition,9 or that drug–drug interactions may occur between PrEP and concurrent gender-affirming hormonal therapy.10–12 These concerns contribute to lower acceptability of PrEP among transgender women.7,11 Evidence-based strategies to inform the use of PrEP in combination with feminizing hormones in transwomen is a critical gap that needs further investigation.13,14
Our primary objective was to characterize the disposition of tenofovir and emtricitabine in plasma among transwomen receiving feminizing hormonal therapy. Secondarily, we described steady-state active intracellular anabolites, tenofovir-DP and emtricitabine-triphosphate (TP) in PBMCs.
Participants and methods
Study design
We enrolled healthy volunteer transwomen receiving feminizing hormonal therapy in a 14-day, single-arm, open-label, pharmacokinetic (PK) study. Participants received a fixed-dose oral tenofovir disoproxil fumarate/emtricitabine (300/200 mg) tablet once daily for 14 days and returned for an intensive PK sampling visit on study day 14 in conjunction with the final tenofovir disoproxil fumarate/emtricitabine dose. Adherence to tenofovir disoproxil fumarate/emtricitabine and feminizing hormones during the study was evaluated by a patient questionnaire that assessed dosing schedules and missed or late doses (defined as >12 h after the usual dosage time), and by tenofovir disoproxil fumarate/emtricitabine pill counts on day 14. One hundred percent adherence was defined as no missed tenofovir disoproxil fumarate/emtricitabine doses for the duration of the study and no late doses within 3 days of the PK sampling visit. Data were compared with those from healthy, historical cisgender adults taking oral tenofovir disoproxil fumarate/emtricitabine daily without other antiretroviral medications.15 (ClinicalTrials.gov database: NCT03270969.)
Study participants
We invited self-identified transwomen (≥19 years of age) attending routine clinic appointments for gender-affirming care at The Nebraska Medical Center to participate in this study. Eligible participants were receiving feminizing hormone therapy with 17β-estradiol (daily oral/sublingual tablets or weekly transdermal patches) plus spironolactone tablets as part of recommended gender-affirming care16 for at least 3 months prior to enrolment. Exclusion criteria included current use of tenofovir disoproxil fumarate/emtricitabine, use of injectable estradiol due to substantial PK variability over the dosing interval after intramuscular administration,17 serum estradiol concentration less than 100 pg/mL at screening, serological evidence of HIV infection or estimated CLCR less than 60 mL/min. An estradiol threshold of 100 pg/mL was selected as this is the lower end of the target range for feminizing hormone therapy.16 Demographic information and medical history were abstracted from participants’ medical records. The historical control group was selected based on similar eligibility criteria that included cisgender men and cisgender women.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the University of Nebraska Medical Center (UNMC) Institutional Review Board (491-17-FB). All participants provided written informed consent.
PK evaluations
Blood samples for determination of tenofovir and emtricitabine PK parameters in plasma and PBMCs were collected during the day 14 outpatient visit at the Clinical Research Center. Participants received the final tenofovir disoproxil fumarate/emtricitabine dose as an observed dose within 30 min of a light meal. Blood samples were drawn at 0 h (pre-dose) and 0.5, 1, 2, 3, 4, 6, 8 and 12 h after the observed tenofovir disoproxil fumarate/emtricitabine dose. For determination of tenofovir and emtricitabine concentrations in plasma, 6 mL of whole blood was collected into K2EDTA tubes (BD Vacutainer, Franklin Lakes, NJ, USA) at each timepoint. Within 30 min of collection, plasma was separated by centrifugation and aliquots were stored at −80°C until analysis.
Intracellular tenofovir-DP and emtricitabine-TP concentrations were measured from whole blood collected at 0 h and 12 h into two 8 mL sodium heparin cell preparation tubes (CPTs; BD Vacutainer) for PBMC isolation. CPTs were mixed by inversion and centrifuged within 30 min of collection at room temperature (18°C–25°C) for 30 min at 1800 g. The buffy coat was removed and cells were pelleted by centrifugation at room temperature for 10 min at 400 g. Isolated mononuclear cells were washed once with 1× PBS and lysed with 70% cold methanol. Lysate was stored at −80°C until analysis. PBMC enumeration was performed by quantification of RNase P (RPP30) gene copy numbers using a highly sensitive droplet digital PCR assay (ddPCR) as previously described.18 Plasma tenofovir and emtricitabine and intracellular tenofovir-DP and emtricitabine-TP concentrations were measured by validated LC-MS/MS procedures.19,20 The analytical range for the tenofovir and emtricitabine plasma assays was 10–1500 ng/mL. The tenofovir-DP and emtricitabine-TP intracellular analytical ranges were 2.5–2000 fmol/sample and 0.1–200 pmol/sample, respectively.
Serum estradiol and testosterone measurement
Serum estradiol was quantified from blood samples collected at screening, day 0 and day 14; testosterone was measured on days 0 and 14. Estradiol was measured by a chemiluminescent immunoassay method (Beckman Coulter DXI 800, Beckman Coulter, Inc., Brea, CA, USA). The intra-assay coefficient of variation (CV) was <10.4% and the lower limit of quantification (LLOQ) was 20 pg/mL. Total testosterone was quantified by LC-MS/MS (AB Sciex 5500 QTRAP, Applied Biosystems, Foster City, CA, USA). Between- and within-run CVs were <11.3% and the LLOQ was 1.0 ng/dL. The free testosterone concentration was derived from measured sex hormone-binding globulin using the Vermeulen equation.21
Safety and tolerability
Participants were questioned directly about suspected adverse events by phone on day 7 and in person during the day 14 PK visit. Safety labs were performed at study entry and on study day 14. Clinical and laboratory adverse events were graded according to the 2014 NIAID Division of AIDS toxicity tables.22
PK and statistical analyses
Plasma tenofovir and emtricitabine concentration–time curves were analysed by non-compartmental methods (WinNonlin version 8.0, Pharsight Corporation, Mountain View, CA, USA). The concentration at 24 h post-dose was imputed from the pre-dose concentration (0 h). The concentration at 0 h, Cmax and Tmax were determined by direct inspection of the plasma concentration–time curve. The plasma AUC0–24 was estimated using the log-linear trapezoidal method. Individual estimates of the terminal elimination rate constant (λz) were determined from the log-linear portion of the plasma AUC curve. The plasma elimination t½ was calculated by 0.693/λz. Apparent oral clearance was calculated as dose/AUC0–24. Intracellular tenofovir-DP and emtricitabine-TP concentrations were divided by the total number of cells per assayed sample volume and reported per million cells. The average intracellular concentration at steady-state (Cavg) was reported descriptively as the geometric mean of individual tenofovir-DP and emtricitabine-TP concentrations measured at 0 h and 12 h post-dose on day 14.
Power calculation found that 15 participants provided 80% power to test our primary hypothesis based on the tenofovir plasma geometric mean AUC (90% CI) at a significance level of 0.05. Participants were included in the analysis if they completed the day 14 PK sampling. Tenofovir and emtricitabine plasma Cmax, Cmin, AUC0–24, t½ and CL/F were summarized as geometric means with 90% CI. PK parameters were compared with a historical control group of healthy cisgender adults who received tenofovir disoproxil fumarate/emtricitabine (300/200 mg) daily with food and intensive PK sampling on day 7.15 Statistical analysis was performed using independent two-sample t-test after log transformation. Within-group comparisons of hormone concentrations at day 0 and day 14 were performed using Wilcoxon signed-rank test. The proportion of participants with serum estradiol >100 pg/mL at day 0 and day 14 was compared using McNemar’s test. A two-sided P value <0.05 was considered to be statistically significant.
Results
Study participants
Sixteen adult transwomen (12 white, 1 black, 2 Hispanic and 1 other) were enrolled and 15 completed the PK sampling visit (day 14). One participant withdrew from the study after enrolment for personal reasons prior to the day 14 visit and was excluded from primary endpoint analysis but is included in the demographics of the participants and safety analysis. Among all enrolled participants, the mean (range) age was 32 (19–59) years and BMI at screening was 28.7 (17.3–49.8) kg/m2. The median duration of hormone therapy at entry was 16.5 months (range 3–112 months). The majority of participants (81.3%) were taking sublingual estradiol tablets as feminizing hormone therapy, and the median (range) total daily dose was 4 mg (2–6 mg) daily, divided into twice-daily dosing. Participants using transdermal estradiol patches (n = 3) had a median (range) estradiol dose of 0.15 mg per day (0.1–0.2 mg daily). For all participants, the median (range) total daily spironolactone dose was 100 mg (100–300 mg) daily, divided into twice-daily dosing. Eight (53%) participants also received progestogen as either 100 mg progesterone daily (n = 7) or 5 mg medroxyprogesterone acetate daily (n = 1). The majority had high (100%) tenofovir disoproxil fumarate/emtricitabine adherence during the study; one participant reported 93% adherence.
The historical control group (n = 17) included HIV-negative cisgender men and women with mean (range) ages of 26 (19–41) years.15 Baseline demographics for all participants are included in Table 1.
Table 1.
Baseline participant demographics
| Characteristic | Transwomen, n = 16 | Historical control,15n = 19 |
|---|---|---|
| Mean age, years (range) | 32 (19–59) | 26 (19–41) |
| Gender, n (%) | 16 (100), transwomen | 15 (79), male |
| Race/ethnicity, n (%) | ||
| white, non-Hispanic | 12 (75) | 19 (100) |
| Hispanic | 2 (12.5) | 0 (0) |
| black, non-Hispanic | 1 (6.3) | 0 (0) |
| more than one race | 1 (6.3) | 0 (0) |
| Mean BMI, kg/m2 (range) | 28.7 (17.3–49.8) | not reported |
| Mean body weight, kg (range) | 90 (55–167) | 74 (62–94) |
| Mean CLCR, mL/min (range) | 140 (96–246) | 114 (94–157) |
PK of tenofovir and emtricitabine
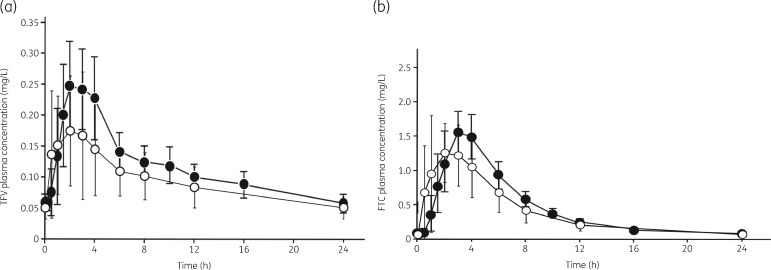
Steady-state plasma tenofovir and emtricitabine PK parameters are described in Table 2, and the time-dependent concentration curves are shown in Figure 1(a and b), respectively. The tenofovir AUC0–24 was 24% lower among transwomen compared with cisgender adults [geometric mean ratio (GMR) 0.76 (0.65–0.90)], reflecting a lower Cmax and a faster apparent oral clearance. The emtricitabine AUC0–24 was 14% lower [GMR 0.86 (0.75–0.98)] among transwomen compared with cisgender adults, with a faster apparent oral clearance reflected in a shorter estimated half-life.
Table 2.
PK parameters of tenofovir and emtricitabine in plasma and tenofovir-DP and emtricitabine-TP in PBMCs during daily oral tenofovir disoproxil fumarate/emtricitabine
| Analyte | Parameter | Transwomen, n = 15, GM (90% CI) | Historical control,15n = 17, GM (90% CI) | Transwomen: historical control, GMR (90% CI) | P value |
|---|---|---|---|---|---|
| Plasma | |||||
| TFV | C max, mg/L | 0.23 (0.19–0.26) | 0.28 (0.26–0.31) | 0.80 (0.67–0.96) | 0.06 |
| C min, mg/L | 0.05 (0.04–0.05) | 0.05 (0.05–0.06) | 0.89 (0.75–1.05) | 0.26 | |
| T max, h | 1.45 (1.26–1.68) | 2.24 (1.81–2.78) | 0.65 (0.50–0.84) | 0.02 | |
| AUC0–24, mg·h/L | 2.10 (1.81–2.43) | 2.76 (2.57–2.96) | 0.76 (0.65–0.90) | 0.01 | |
| t ½, h | 16.83 (15.32–18.50) | 15.46 (12.87–18.58) | 1.09 (0.89–1.34) | 0.52 | |
| CL/F, mL/min | 1072.61 (998.72–1151.97) | 815.89 (705.03–944.17) | 1.32 (1.12–1.55) | 0.01 | |
| FTC | C max, mg/L | 1.55 (1.35–1.78) | 1.66 (1.55–1.79) | 0.93 (0.80–1.09) | 0.46 |
| C min, mg/L | 0.07 (0.06–0.08) | 0.07 (0.07–0.08) | 0.89 (0.75–1.07) | 0.31 | |
| T max, h | 1.74 (1.61–1.88) | 2.92 (2.40–3.55) | 0.60 (0.48–0.74) | 0.001 | |
| AUC0–24, mg·h/L | 9.15 (8.04–10.30) | 10.64 (10.18–11.11) | 0.86 (0.75–0.98) | 0.07 | |
| t ½, h | 6.00 (5.63–6.39) | 10.57 (10.13–11.02) | 0.57 (0.53–0.61) | <0.001 | |
| CL/F, mL/min | 364.42 (347.40–382.28) | 313.75 (281.52–349.67) | 1.16 (1.03–1.31) | 0.05 | |
| PBMCs | |||||
| TFV-DP | C avg, fmol/106 cells | 167.1 (146.6–190.5) | N/A | ||
| FTC-TP | C avg, pmol/106 cells | 15.4 (13.8–17.3) | N/A | ||
Duration of tenofovir/emtricitabine for each group was as follows: transwomen, 14 days; historical control (Blum et al.15), 7 days. P < 0.05 in bold.
C avg, geometric mean of intracellular concentration at steady-state; TFV, tenofovir; FTC, emtricitabine; TFV-DP, tenofovir-DP; FTC-TP, emtricitabine-TP.
Figure 1.
(a) Mean (± SD) tenofovir (TFV) plasma concentration–time curves at steady-state following tenofovir disoproxil fumarate/emtricitabine 300/200 mg daily for transwomen (open circles, n = 15) versus cisgender adults (filled circles, n = 17). (b) Mean (± SD) emtricitabine (FTC) plasma concentration–time curves at steady-state following tenofovir disoproxil fumarate/emtricitabine 300/200 mg daily for transwomen (open circles, n = 15) versus cisgender adults (filled circles, n = 17). Cisgender data are from Blum et al. (2007).15 The figures are adapted from Blum MR, Chittick GE, Begley JA et al. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J Clin Pharmacol 2007; 47: 751–9 by permission of John Wiley & Sons, Inc.
The geometric mean (90% CI) Cavg tenofovir-DP and emtricitabine-TP concentrations in transwomen were 167.1 (146.6–190.5) fmol/106 cells and 15.4 (13.8–17.3) pmol/106 cells, respectively (Table 2). Concentrations of tenofovir-DP and emtricitabine-TP ranged from 64.8 to 417.8 fmol/106 cells and from 8.0 to 29.3 pmol/106 cells, respectively.
Serum estradiol and testosterone concentrations
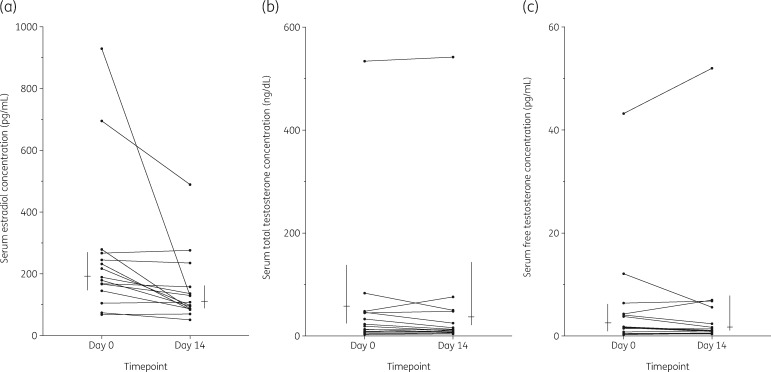
At study entry, the median (IQR) serum estradiol was 189 (145–267) pg/mL, compared with 108 (87–158) pg/mL at day 14 (P < 0.001) (Figure 2a). The proportion of participants with serum estradiol concentration greater than 100 pg/mL decreased from 86.7% to 53.3% from entry to day 14 (P = 0.03). Median (IQR) serum total testosterone concentrations at day 0 were 19 (8–46) ng/dL and 12 (7–78) ng/dL at day 14 (P = 0.38) (Figure 2b). Serum free testosterone concentrations were similar at day 0 and day 14 (P = 0.68) (Figure 2c).
Figure 2.
(a) Participant serum estradiol concentration before (day 0) and during steady-state tenofovir/emtricitabine 300/200 mg (day 14), n = 15. Wilcoxon signed-rank paired comparison: P < 0.001. Vertical lines represent median (IQR) concentrations on day 0 and day 14. (b) Participant serum total testosterone concentration before (day 0) and during steady-state tenofovir and emtricitabine concentrations on day 14 post administration of tenofovir disoproxil fumarate/emtricitabine (300/200 mg), n = 15. Wilcoxon signed-rank paired comparison: P = 0.38. Vertical lines represent median (IQR) concentrations on day 0 and day 14. (c) Serum free testosterone concentration on day 0 and day 14; Wilcoxon signed-rank paired comparison: P = 0.68. Vertical lines represent median (IQR) concentrations on day 0 and day 14.
Safety and tolerability
Seven participants (44%) reported some adverse effects associated with tenofovir disoproxil fumarate/emtricitabine use; all were mild or moderate (grade 1 or 2) severity. Nausea was the most commonly reported adverse effect (n = 5, 31%) during the study period, followed by one report (6%) of headache and one report (6%) of anorexia. None of the 16 participants discontinued the study due to a reported adverse event.
Discussion
We investigated the PK of tenofovir and emtricitabine in plasma and their active phosphorylated intracellular moieties in PBMCs, in transwomen who were taking oral tenofovir disoproxil fumarate/emtricitabine with feminizing hormones. Tenofovir and emtricitabine plasma AUCs were 24% and 14% lower, respectively, among transwomen compared with a historical control group of cisgender adults. In our study, there were five transwomen who had a BMI >30 (median, range: 39, 33–50 kg/m2). Given that body weight is associated with decreased tenofovir plasma exposure and increased oral clearance in special patient populations,23,24 we conducted a post hoc comparison of tenofovir plasma AUC0–24 between non-obese (BMI ≤30, n = 10) transwomen in our study compared with the historical control group. There was a 15% decrease in tenofovir plasma AUC among non-obese transwomen (GMR, 90% CI: 0.85, 0.73–0.98) compared with the 24% decrease in tenofovir AUC observed for all (n = 15) participants. Hiransuthikul et al.25 enrolled only participants with a BMI in a non-obese range (18.5–24.9 kg/m2) and observed a 12% decrease in plasma tenofovir AUC (P = 0.03). Intracellular NRTI concentrations measured in the current study exceed those previously reported to be associated with protection from HIV acquisition among MSM, but high variability was observed.26,27
These findings are similar to tenofovir and emtricitabine PK data for HIV-negative transwomen reported in the literature.25,28 A single-arm study of 20 transwomen observed 12% lower tenofovir plasma AUC with versus without feminizing hormones (P = 0.03),25 and another observed a 27% decrease in tenofovir plasma AUC in transwomen (n = 8) compared with cisgender men (P = 0.065).28 When considering intracellular anabolite exposure, one study found a 24% decrease in tenofovir-DP exposure in PBMCs among transwomen (n = 8) versus cisgender men (P = 0.121).28 A small study of four transwomen living with HIV taking tenofovir disoproxil fumarate/emtricitabine as part of suppressive antiretroviral therapy found no difference in plasma tenofovir and emtricitabine concentrations, or tenofovir-DP and emtricitabine-TP from PBMCs, when compared with postmenopausal cisgender women (n = 4 HIV-positive) and cisgender men (n = 2 HIV-positive; n = 2 HIV-negative).29
Given the outcomes observed among transwomen from HIV prevention studies, 7 and variable NRTI pharmacology in different tissue sites,30 it was important to identify whether adequate NRTI concentrations were reached in blood compartments. We observed steady-state intracellular tenofovir-DP and emtricitabine-TP concentrations that were within or above the concentration ranges reported in the literature for HIV-positive and negative adults receiving daily tenofovir disoproxil fumarate/emtricitabine (approximately 36–350 fmol/106 cells and 2.2–6.6 pmol/106 cells, respectively).5,26,27,30–35 Haaland et al.33 reported steady-state intracellular tenofovir-DP and emtricitabine-TP concentrations in PBMCs of healthy cisgender men and women that reflected the intracellular NRTI ranges quantified in our study [median (range) tenofovir-DP: 64 (20–387) fmol/106 cells; emtricitabine-TP: 6.5 (1.4–30.3) pmol/106 cells]. Direct comparison across studies is limited, however, by substantial interindividual variability among intracellular NRTI concentrations and differences in cell quantification methodology. In the present study, we determined cell counts with a method based on detection of a single-copy RPP30 gene in the genomic DNA extracted from viable PBMCs. Conventional cell count methods may report lower intracellular NRTI concentrations because of the loss of cells during a series of washing steps after counting and lower accuracy of haemocytometers.18,36 Additionally, intracellular NRTI concentrations between men and women have been noted to be different, with women trending toward higher concentrations than men,5,37 and genetic polymorphisms of transporter proteins are also associated with changes in intracellular NRTI concentrations in cisgender adults.5,38 Thus, these findings should be interpreted as hypothesis generating for future PK studies in transgender individuals.9,39
There is no clear mechanistic explanation for decreased NRTI plasma concentrations in transwomen. Estradiol undergoes hepatic metabolism, and tenofovir and emtricitabine are renally eliminated,5,40 thus overlapping metabolic pathways are avoided. Exogenous estradiol may reduce tenofovir and emtricitabine exposure by modulating expression of transporter proteins. In vitro, the efflux transporter P-glycoprotein is induced 4-fold by estrone, the predominant metabolite of estradiol.41 Tenofovir disoproxil fumarate is a substrate of P-glycoprotein,5 and its efflux from the systemic circulation may be a mechanism of decreased tenofovir concentrations in plasma. Elevated renal clearance is a proposed mechanism of decreased plasma NRTI concentrations in transwomen.28 In a small study, increased renal function was observed among transwomen taking tenofovir disoproxil fumarate/emtricitabine compared with cisgender men [median (IQR) estimated CLCR: transwomen, n = 8: 204 (120–218) mL/min; cisgender men, n = 8: 108 (83–125), P = 0.01].28 In the present study, the median (IQR) estimated CLCR among transwomen was 133 (118–148) mL/min, and the observed oral clearance rates of tenofovir and emtricitabine increased 32% and 16%, respectively, compared with cisgender adults. Further work is needed to clarify the potential role of elevated renal function in transwomen and its link with altered plasma NRTI concentrations.
Among transwomen, gender-affirming hormones are often prioritized over other medications.11,12 This highlights the importance of ensuring that antiretroviral therapies will not interfere with feminizing hormones. We observed median serum estradiol concentrations that were significantly lower between entry and day 14 (P < 0.001); however, this should be interpreted with caution. There was substantial interindividual estradiol variability on both study days [median (range) day 0: 189 (68–929) pg/mL; day 14: 108 (51–489) pg/mL]. Intraindividual estradiol variability (CV%) was approximately 40% between screening and study entry (data not shown). Further, three transwomen had serum estradiol levels >100 pg/mL at screening yet fell below this threshold by entry. There are well-known analytical challenges in serum sex hormone quantification,42 which may contribute to variable estradiol measurement. Hiransuthikul et al.25 described an estradiol PK profile with a distinct peak and trough over a 24 h sampling period, yet clinical guidelines recommend monitoring random estradiol concentrations without regard to the time since last dose.16,43 Though we measured hormones at approximately the same time at each visit, the estradiol samples in our study were not adjusted relative to the time of last hormone dose, which may add variability to these measurements. Finally, non-adherence to hormones may have been a contributory factor, as barriers to taking hormones as prescribed have been reported in up to 31% of transwomen.12 Hiransuthikul et al.25 performed intensive estradiol PK sampling with and without tenofovir disoproxil fumarate/emtricitabine in transwomen and found no difference in estradiol concentrations. In the present study, total and free testosterone remained suppressed in all participants (total testosterone: <55 ng/dL),16 with the exception of one participant whose total testosterone was slightly elevated at day 14 (76 ng/dL), and another participant who maintained concentrations in the physiological male range (320–1000 ng/dL)16 for the study duration. Collectively, these findings suggest no clinically significant effect of tenofovir disoproxil fumarate/emtricitabine on gender-affirming hormone therapy.
This study has certain limitations. Participants received feminizing hormones as part of ongoing gender-affirming care, and our study group varied in terms of exposure and duration of feminizing hormones at entry. This reduced our ability to reliably evaluate a bidirectional drug interaction of tenofovir and emtricitabine on the disposition of feminizing hormones. Second, to avoid interruption of gender-affirming care for participants, a non-contemporaneous control group of adults who were not receiving feminizing hormones was used for comparison. There is debate around selection of appropriate control groups in transgender health research,44 thus we selected a historical control group from a PK study that enrolled both cisgender men and cisgender women and permitted cisgender women to continue hormonal contraception use during the study period.15 In addition, our participants had a higher observed BMI compared with the control group. There were fewer PK samples drawn during the elimination phase of tenofovir and emtricitabine in plasma compared with the historical control, which may have impacted estimates of plasma half-life and clearance rate. Participant medication adherence during the study period may have been overestimated by self-report methodology,26,45 although tenofovir/emtricitabine and phosphorylated moieties were detected from plasma and PBMCs collected from all participants at the day 14 pre-dose blood draw.
We describe modestly lower plasma tenofovir and emtricitabine exposure in HIV-negative transwomen; however, we did not observe lower PBMC concentrations of tenofovir-DP and emtricitabine-TP relative to published concentrations from cisgender adults. This offers reassurance that NRTIs are reaching blood compartments in transwomen, despite the modest decrease of the parent drug in plasma. A previous study observed increased intracellular NRTI concentrations in PBMCs with decreased systemic parent drug exposure,46 thus an actual increase in phosphorylated moieties cannot be ruled out. Given that observed plasma tenofovir concentrations are apparently similar to concentrations associated with PrEP efficacy in cisgender adults,26,47 we do not advise any alterations to daily PrEP administration in transwomen on hormone therapy. This study adds to current data by estimating intracellular tenofovir-DP and emtricitabine-TP concentrations in healthy transwomen taking transdermal estradiol, in addition to sublingual/oral estradiol.
These findings are reassuring for transwomen who are concerned about interactions between PrEP and gender-affirming hormones. Given the unexpected nature of decreased plasma tenofovir and emtricitabine, the mechanism of this change and the potential impact on drug exposure in other tissue compartments that are important for PrEP efficacy (e.g. lymphatic, rectal and genital) require further investigation in transwomen. These mechanistic findings will help predict potential interactions between feminizing hormones and other emerging PrEP pharmacological agents, including tenofovir alafenamide, and other antiretroviral agents.
Acknowledgements
We acknowledge study participants, and the nurses and staff of the UNMC Clinical Research Center and Specialty Care Center; and the Antiviral Pharmacology Laboratory at the UNMC College of Pharmacy for analysing tenofovir and emtricitabine concentrations. We wish to thank Dr Peter Anderson, PharmD at the Colorado Antiviral Pharmacology Laboratory for his support with intracellular NRTI data analysis. At the time of this work, L.R.C. was an antiretroviral pharmacology fellow at the College of Pharmacy, University of Nebraska Medical Center, Omaha, NE, USA.
Funding
This work was supported by the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development under award number 1R01HD085887 to K.K.S., and National Institutes of Health, National Institute of Allergy and Infectious Diseases under award numbers RO1 AI124965 and UM1AI06701 to C.V.F. and 1K23AI134307 to A.T.P. In addition, the work was supported by the Research Support Fund grant from Nebraska Medicine, Omaha, NE, USA and a seed grant from the College of Pharmacy, University of Nebraska Medical Center, Omaha, NE, USA.
Transparency declarations
J.P.H. and S.H.B. disclose research funding to their institution from Gilead Sciences. All other authors: none to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Meerwijk EL, Sevelius JM.. Transgender population size in the United States: a meta-regression of population-based probability samples. Am J Public Health 2017; 107: e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Flores A, Herman J, Gates G. et al. How Many Adults Identify as Transgender in the United States? Williams Institute, 2016. https://williamsinstitute.law.ucla.edu/wp-content/uploads/How-Many-Adults-Identify-as-Transgender-in-the-United-States.pdf. [Google Scholar]
- 3. Baral SD, Poteat T, Stromdahl S. et al. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis 2013; 13: 214–22. [DOI] [PubMed] [Google Scholar]
- 4. Clark H, Babu AS, Wiewel EW. et al. Diagnosed HIV infection in transgender adults and adolescents: results from the National HIV Surveillance System, 2009–2014. AIDS Behav 2017; 21: 2774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson PL, Kiser JJ, Gardner EM. et al. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J Antimicrob Chemother 2011; 66: 240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grant RM, Lama JR, Anderson PL. et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363: 2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deutsch MB, Glidden DV, Sevelius J. et al. HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV 2015; 2: e512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grant RM, Anderson PL, McMahan V. et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14: 820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reisner SL, Radix A, Deutsch MB.. Integrated and gender-affirming transgender clinical care and research. J Acquir Immune Defic Syndr 2016; 72 Suppl 3: 235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sevelius JM, Deutsch MB, Grant R.. The future of PrEP among transgender women: the critical role of gender affirmation in research and clinical practices. J Int AIDS Soc 2016; 19 Suppl 6: 21105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sevelius JM, Patouhas E, Keatley JG. et al. Barriers and facilitators to engagement and retention in care among transgender women living with human immunodeficiency virus. Ann Behav Med 2014; 47: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Braun HM, Candelario J, Hanlon CL. et al. Transgender women living with HIV frequently take antiretroviral therapy and/or feminizing hormone therapy differently than prescribed due to drug–drug interaction concerns. LGBT Health 2017; 4: 371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grant RM, Sevelius JM, Guanira JV. et al. Transgender women in clinical trials of pre-exposure prophylaxis. J Acquir Immune Defic Syndr 2016; 72 Suppl 3: 226–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Radix A, Sevelius J, Deutsch MB.. Transgender women, hormonal therapy and HIV treatment: a comprehensive review of the literature and recommendations for best practices. J Int AIDS Soc 2016; 19 Suppl 2: 20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blum MR, Chittick GE, Begley JA. et al. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J Clin Pharmacol 2007; 47: 751–9. [DOI] [PubMed] [Google Scholar]
- 16. Hembree WC, Cohen-Kettenis PT, Gooren L. et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2017; 102: 3869–903. [DOI] [PubMed] [Google Scholar]
- 17. Driowo MA, Landgren BM, Stenström B. et al. A comparison of the pharmacokinetic properties of three estradiol esters. Contraception 1980; 21: 415–24. [DOI] [PubMed] [Google Scholar]
- 18. Dyavar SR, Ye Z, Byrareddy SN. et al. Normalization of cell associated antiretroviral drug concentrations with a novel RPP30 droplet digital PCR assay. Sci Rep 2018; 8: 3626.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delahunty T, Bushman L, Robbins B. et al. The simultaneous assay of tenofovir and emtricitabine in plasma using LC/MS/MS and isotopically labeled internal standards. J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877: 1907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. King T, Bushman L, Kiser J. et al. Liquid chromatography–tandem mass spectrometric determination of tenofovir-diphosphate in human peripheral blood mononuclear cells. J Chromatogr B Analyt Technol Biomed Life Sci 2006; 843: 147–56. [DOI] [PubMed] [Google Scholar]
- 21. Vermeulen A, Verdonck L, Kaufman JM.. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84: 3666–72. [DOI] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Corrected Version 2.1. 2017. https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf.
- 23. Kiser JJ, Fletcher CV, Flynn PM. et al. Pharmacokinetics of antiretroviral regimens containing tenofovir disoproxil fumarate and atazanavir–ritonavir in adolescents and young adults with human immunodeficiency virus infection. Antimicrob Agents Chemother 2008; 52: 631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Best BM, Burchett S, Li H. et al. Pharmacokinetics of tenofovir during pregnancy and postpartum. HIV Med 2015; 16: 502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hiransuthikul A, Janamnuaysook R, Himmad K. et al. Drug–drug interactions between feminizing hormone therapy and pre-exposure prophylaxis among transgender women: the iFACT study. J Int AIDS Soc 2019; 22: e25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hendrix CW, Andrade A, Bumpus NN. et al. Dose frequency ranging pharmacokinetic study of tenofovir–emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). AIDS Res Hum Retroviruses 2016; 32: 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anderson PL, Glidden DV, Liu A. et al. Emtricitabine–tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4: 151ra125.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shieh E, Marzinke MA, Fuchs EJ. et al. Transgender women on oral HIV pre-exposure prophylaxis have significantly lower tenofovir and emtricitabine concentrations when also taking oestrogen when compared to cisgender men. J Int AIDS Soc 2019; 22: e25405.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cottrell ML, Prince HMA, Schauer AP. et al. Decreased tenofovir diphosphate concentrations in a transgender female cohort: implications for human immunodeficiency virus preexposure prophylaxis. Clin Infect Dis 2019; 69: 2201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seifert SM, Chen X, Meditz AL. et al. Intracellular tenofovir and emtricitabine anabolites in genital, rectal, and blood compartments from first dose to steady-state. AIDS Res Hum Retroviruses 2016; 32: 981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adams JL, Sykes C, Menezes P. et al. Tenofovir diphosphate and emtricitabine triphosphate concentrations in blood cells compared with isolated peripheral blood mononuclear cells: a new measure of antiretroviral adherence? J Acquir Immune Defic Syndr 2013; 62: 260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seifert SM, Glidden DV, Meditz AL. et al. Dose response for starting and stopping HIV preexposure prophylaxis for men who have sex with men. Clin Infect Dis 2015; 60: 804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haaland RE, Holder A, Pau C-P. et al. Levels of intracellular phosphorylated tenofovir and emtricitabine correlate with natural substrate concentrations in peripheral blood mononuclear cells of persons prescribed daily oral Truvada for HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr 2017; 75: e86–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jackson A, Moyle G, Watson V. et al. Tenofovir, emtricitabine intracellular and plasma, and efavirenz plasma concentration decay following drug intake cessation: implications for HIV treatment and prevention. J Acquir Immune Defic Syndr 2013; 62: 275–81. [DOI] [PubMed] [Google Scholar]
- 35. Podany AT, Bares SH, Havens J. et al. Plasma and intracellular pharmacokinetics of tenofovir in patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. AIDS 2018; 32: 761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benech H, Theodoro F, Herbet A. et al. Peripheral blood mononuclear cell counting using a DNA-detection-based method. Anal Biochem 2004; 330: 172–4. [DOI] [PubMed] [Google Scholar]
- 37. Lahiri CD, Tao S, Jiang Y. et al. Impact of protease inhibitors on intracellular concentration of tenofovir-diphosphate among HIV-1 infected patients. AIDS 2015; 29: 1113–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kiser JJ, Aquilante CL, Anderson PL. et al. Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV-infected patients. J Acquir Immune Defic Syndr 2008; 47: 298–303. [DOI] [PubMed] [Google Scholar]
- 39. Reisner SL, Deutsch MB, Bhasin S. et al. Advancing methods for US transgender health research. Curr Opin Endocrinol Diabetes Obes 2016; 23: 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005; 8 Suppl 1: 3–63. [DOI] [PubMed] [Google Scholar]
- 41. Kim WY, Benet LZ.. P-glycoprotein (P-gp/MDR1)-mediated efflux of sex-steroid hormones and modulation of P-gp expression in vitro. Pharm Res 2004; 21: 1284–93. [DOI] [PubMed] [Google Scholar]
- 42. Stanczyk FZ, Cho MM, Endres DB. et al. Limitations of direct estradiol and testosterone immunoassay kits. Steroids 2003; 68: 1173–8. [DOI] [PubMed] [Google Scholar]
- 43. Coleman E, Bockting W, Botzer M. et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int J Transgend 2012; 13: 165–232. [Google Scholar]
- 44. Feldman J, Brown GR, Deutsch MB. et al. Priorities for transgender medical and healthcare research. Curr Opin Endocrinol Diabetes Obes 2016; 23: 180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Blumenthal J, Pasipanodya EC, Jain S. et al. Comparing self-report pre-exposure prophylaxis adherence questions to pharmacologic measures of recent and cumulative pre-exposure prophylaxis exposure. Front Pharmacol 2019; 10: 721.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baheti G, King JR, Acosta EP. et al. Age-related differences in plasma and intracellular tenofovir concentrations in HIV-1 infected children, adolescents and adults. AIDS 2013; 27: 221.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Donnell D, Baeten JM, Bumpus NN. et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr 2014; 66: 340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]