Abstract
Aims
The chemotherapy drug doxorubicin (Dox) is commonly used for treating a variety of human cancers; however, it is highly cardiotoxic and induces heart failure. We previously reported that the Bcl-2 mitochondrial death protein Bcl-2/19kDa interaction protein 3 (Bnip3), is critical for provoking mitochondrial perturbations and necrotic cell death in response to Dox; however, the underlying mechanisms had not been elucidated. Herein, we investigated mechanism that drives Bnip3 gene activation and downstream effectors of Bnip3-mediated mitochondrial perturbations and cell death in cardiac myocytes treated with Dox.
Methods and results
Nuclear factor-κB (NF-κB) signalling, which transcriptionally silences Bnip3 activation under basal states in cardiac myocytes was dramatically reduced following Dox treatment. This was accompanied by Bnip3 gene activation, mitochondrial injury including calcium influx, permeability transition pore (mPTP) opening, loss of nuclear high mobility group protein 1, reactive oxygen species production, and cell death. Interestingly, impaired NF-κB signalling in cells treated with Dox was accompanied by protein complexes between Bnip3 and cyclophilin D (CypD). Notably, Bnip3-mediated mPTP opening was suppressed by inhibition of CypD—demonstrating that CypD functionally operates downstream of Bnip3. Moreover, restoring IKKβ–NF-κB activity in cardiac myocytes treated with Dox suppressed Bnip3 expression, mitochondrial perturbations, and necrotic cell death.
Conclusions
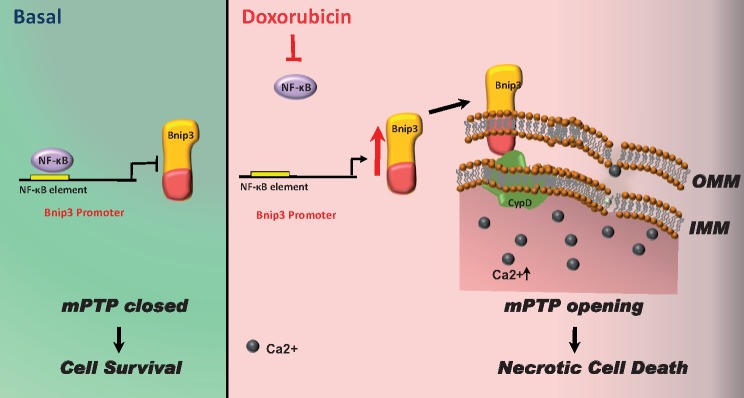
The findings of the present study reveal a novel signalling pathway that functionally couples NF-κB and Dox cardiomyopathy to a mechanism that is mutually dependent upon and obligatorily linked to the transcriptional control of Bnip3. Our findings further demonstrate that mitochondrial injury and necrotic cell death induced by Bnip3 is contingent upon CypD. Hence, maintaining NF-κB signalling may prove beneficial in reducing mitochondrial dysfunction and heart failure in cancer patients undergoing Dox chemotherapy.
Keywords: Cardiac myocytes, Cell death, Bnip3, NF-κB, Doxorubicin, Mitochondria
Graphical Abstract
Graphical Abstract.
1. Introduction
Nuclear factor-κB (NF-κB) is ubiquitously expressed transcription factor that is highly conserved throughout evolution. In cells, NF-κB exists as inactive dimer comprised of RelA/p65 and p50 subunits bound to its inhibitor protein IκBα.1–3 Activation of NF-κB involves the phosphorylation dependent degradation of IκBα by the IκB kinases (IKKα/IKKβ/NEMO).4,5 Notably, IKKβ is crucial for activating NF-κB in cardiac myocytes.6 A cytoprotective role for NF-κB has been suggested as many survival signalling pathways including PI3K/Akt-mTOR activate NF-κB.7,8 The importance of NF-κB signalling in cell survival is further illustrated by the lethality of the p65−/− and IKKβ−/− knock-out mice9–12 and our own data in cardiac myocytes.13 The cytoprotective properties of NF-κB have largely been attributed to its role in activation of survival factors such Bcl-2, c-IAP1/2, superoxide dismutase (SOD), and others.14,15 However, NF-κB can also suppress cell death by transcriptionally inhibiting certain genes known to promote cell death such as the inducible mitochondrial death protein Bcl-2/19kDa interaction protein 3 (Bnip3).16,17 Indeed, previous work by our laboratory demonstrated that NF-κB was critical for suppressing cell death of cardiac myocytes by a mechanism that transcriptionally repressed expression of Bnip3.16–19 Notably, Bnip3 is distinguished from other Bcl2 family members by its regulation by NF-κB and its ability to form homodimers, which is essential for mitochondrial integration and provoking cell death.17
Anthracyclines including doxorubicin (Dox) are used for treating human cancers.20,21 However, a well-established consequence of Dox and related anthracyclines is their propensity for inducing heart failure.22–24 The underlying mechanism for this property of Dox is poorly understood. The mitochondrion regulates vital cellular processes including oxidative metabolism and serves as a cell death platform for apoptosis and necrosis, respectively. Since the heart is abundantly rich in mitochondria, perturbations that disrupt mitochondrial function profoundly influence cell viability. Indeed, earlier work from our laboratory demonstrated that Dox provoked severe mitochondrial injury that included impaired respiration, reactive oxygen species (ROS) production, increased mitochondrial calcium, and mitochondrial permeability transition pore (mPTP) opening.22,25–27 Notably, the mitochondrial injury induced by Dox was attributed to Bnip3-mediated mPTP opening.13,16,22 The mode by which Bnip3 provokes mPTP opening in cells treated with Dox is undetermined but it likely impinges on a putative mPTP regulator such as cyclophilin D (CypD).28–31
In this report, we provide new compelling evidence that NF-κB signalling pathway is impaired in cardiac myocytes treated with Dox. We further show that the loss of NF-κB activity promotes Bnip3 gene activation and mPTP opening and necrotic cell death through a mechanism contingent upon CypD. Importantly, restoring NF-κB signalling rapidly suppressed Dox-induced mPTP opening and necrotic cell death. Hence, our findings reveal a novel signalling axis that functionally couples Dox cardiomyopathy and NF-κB signalling pathway through a mechanism that is obligatorily linked to Bnip3 and CypD.
2. Methods
All animal experiments conducted in this study were approved from animal care committee of University of Manitoba and are in accordance with the guidelines laid for protection of animals used for scientific purposes by Canadian council for animal care, directive 2010/63/EU, and National Institutes of Health (NIH).
2.1 Cell culture and treatments
Cardiomyocytes were isolated from 1- to 2-day-old Sprague-Dawley rats, which were sacrificed by cervical dislocation. Hearts were excised and processed enzymatically to extract cardiomyocytes, which were then subjected to primary culture as previously reported.32 Briefly, cultured cells were transfected with expression plasmids using Effectene reagent (Qiagen, Inc.) or infected with adenovirus carrying the desired gene 24 h after plating under serum free DMEM conditions, as earlier reported.32 For the present study cells were treated with Dox (10 µM for 18 h, TEVA) or Cyclosporine A (CSA, 2 µM for 18 h) purchased from Sigma (Cat #239835).
2.2 Plasmids, siRNAs, and adenoviruses
Plasmids and adenovirus encoding wild-type IKKβ (IKKβ wt, inhibitor of nuclear kappa B kinase subunit β) which is kinase active form of IKKβ and IKKβK-M (kinase dead mutant of IKKβ) were employed for studying NF-κB signalling.6 Cells were infected for 24 h at a multiplicity of infection of 10–20 followed by treatment with vehicle or Dox, empty cytomegalovirus (CMV) adenovirus served as control for our studies. The small interfering RNA (siRNA) against Cyclophilin D (ppif) and NF-κB p65 were custom designed and synthesized from Invitrogen (see Supplementary material online, Tables S1–S4 for details). Briefly, cells were transfected with CypDsiRNA (40 nM), NF-κB p65 siRNA or scrambled siRNA (40 nM) for 7–8 h, followed by media change and treatments for 18 h.
2.3 Electron microscopy
Electron microscopy was performed in saline (0.9% physiological saline) and Dox (20 mg/kg body weight) treated mice (8–10 weeks) as previously reported.22 Mice were anaesthetized by intraperitoneal injection of a combination of ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight), euthanasia was performed by excision of the heart. Briefly, hearts were excised and washed in phosphate buffer saline, four random areas of left ventricle between mid-region and apex were cut into small cubes and fixed in 2% w/v glutaraldehyde. For studying ultrastructural details of the myocardium, tissues were osmicated, embedded in Epon, stained with uranyl acetate and lead citrate, as we previously reported.22 Experiments were approved by animal care committee, University of Manitoba and carried out according to guidelines provided.
2.4 Luciferase assay
Cells were transfected with a NF-κB luciferase reporter construct designated as (NF-κB luc) in the presence and absence of eukaryotic expression vectors encoding wild-type IKKβ and kinase dead mutant IKKβK-M as previously reported.8 Luciferase activity was recorded in the cells following 24 h of transfection, and the values were normalized to β-galactosidase activity to control for differences in transfection efficiency. Data are presented as average fold activation from control.
2.5 Cell viability assay
For viability assay, cardiomyocytes were incubated with the vital dyes calcein acetoxymethylester (Calcein-AM, 2 µM) to visualize green cells (live) and ethidium homodimer-1 (2 µM) to visualize red cells (dead) by epifluorescence microscopy using Olympus AX-70 research fluorescence microscope. Data are derived from at least three different experiments and are expressed as average ± SEM percent dead cells from control. High mobility group protein 1 (HMGB1) staining was used as necrotic marker and was assessed by immunostaining of cardiomyocytes with antibody directed against HMGB1 (Cell signalling#3935S, 1:100 dilution) and Alexa 488 goat anti-rabbit secondary antibody (Molecular Probes Inc., 1:200 dilution). Cells were counter stained with nuclear dye Hoechst and visualized with Zeiss fluorescence microscope. Images were captured with Carl Zeiss Axiovert microscope using the magnification 630× using Zen Software.
2.6 Mitochondrial health and functional assays
2.6.1. Mitochondrial morphology
Mitochondrial morphology was assessed on the cardiomyocytes fixed with 70% alcohol. Afterwards cells were immunostained with antibody against mitochondrial protein, Tom 20 (Santa Cruz-sc11415, dilution 1:100) and Alexa 546 goat anti-rabbit secondary antibody (Molecular Probes Inc., 1:200 dilution). Cells were counterstained with nuclear dye Hoechst and visualized under Zeiss fluorescence microscope as previously reported.33
2.6.2. Reactive oxygen species
For ROS assessment, cells were incubated with dihydroethidium (Molecular Probes, 2.5 μM) for 30 min and visualized by epifluorescence microscopy using Olympus AX-70 fluorescence microscope. Increase in red fluorescence indicates increase in superoxide species production.
2.6.3. Mitochondrial membrane potential (ΔΨM)
Mitochondrial membrane potential (ΔΨM) was assessed by incubating cells with tetra-methylrhodamine methyl ester perchlorate (TMRM, Molecular Probes, 50 nM) and visualized by epifluorescence microscopy. In case of TMRM, mitochondria with normal cells fluoresce bright red staining; however, red staining becomes diffuse or very light when mitochondrial membrane potential dissipates.
2.6.4. Mitochondrial permeability transition pore opening
mPTP opening was measured by incubating cells with Calcein-AM (Molecular probes, 5 μM) and Cobalt Chloride (5 mmol/L) for 30 min followed by visualizing under fluorescence research microscope. Loss of green fluorescence indicates mitochondrial pore opening.
2.6.5. Mitochondrial calcium uptake
For determining mitochondrial calcium, Rhod-2AM (Molecular probe) was reconstituted in DMSO following manufacturer’s instruction. Rhod-2AM was chemically reduced by addition of a little amount of sodium borohydride followed by mixing until solution became clear. Cells were incubated with media containing reduced Rhod-2AM (2.2 μM, 20 min) followed by a wash with serum free DMEM media (10 min). The dye binds to mitochondrial calcium and emits red fluorescence which was visualized either in live cells or after fixation with 4% paraformaldehyde. Rhod-2AM fluorescence was analysed by processing raw images using Image j software. To calculate Rhod-2AM fluorescence, background values were subtracted from the total fluorescence values and were plotted as histograms.
2.7 Western blot analysis and immunoprecipitation
For western blot (WB) analysis, cells were lysed in RIPA buffer and protein was extracted and measured by bicinchoninic acid (BCA). Lysate containing 20–30 μg of protein was resolved on denaturing SDS PAGE gels and were transferred to nitrocellulose membranes. Resultant membranes were probed with antibodies targeted against NF-κB p65 (Cell signalling#4764S), Cyclophilin F (Abcam#Ab110324), SOD2 (Cell signalling#13194), cIAPs 1/2(Santa Cruz#sc12410), and murine Bnip3 generated in house at 1:1000 dilution in TBS-T overnight at 4°C. Depending upon the source of primary antibody, membranes were incubated with secondary anti-murine or rabbit antibodies conjugated to HRP and were detected by enhanced ECL. For immunoprecipitation (IP), lysate derived from vehicle or Dox-treated cardiomyocytes (700 µg protein, lysed with RIPA buffer) was immunoprecipitated with murine Cyclophilin antibody (Abcam#Ab110324, 1 µg antibody/150 µg of protein) or IgG1 (Control for Cyclophilin antibody) using DynabeadsTM Protein G IP Kit and was probed with antibody against murine Bnip3 antibody.
2.8 Quantitative real-time PCR (qPCR)
RNA was extracted using GenElute mammalian total RNA kit from Sigma Aldrich and the reaction was amplified with NF-κB p65 and housekeeping gene L32 primers as explained previously.32
2.9 Statistical analysis
Multiple comparisons between groups were determined by one-way ANOVA and Bonferroni post hoc test. Unpaired two tailed Student’s t-test was used to compare mean differences between groups. Differences were considered to be statistically significant to a level of *P < 0.05. In all cases, the data were obtained from at least n = 3–6 independent myocyte isolations for each condition tested.
3. Results
3.1 Dox provokes morphological and functional defects to mitochondria
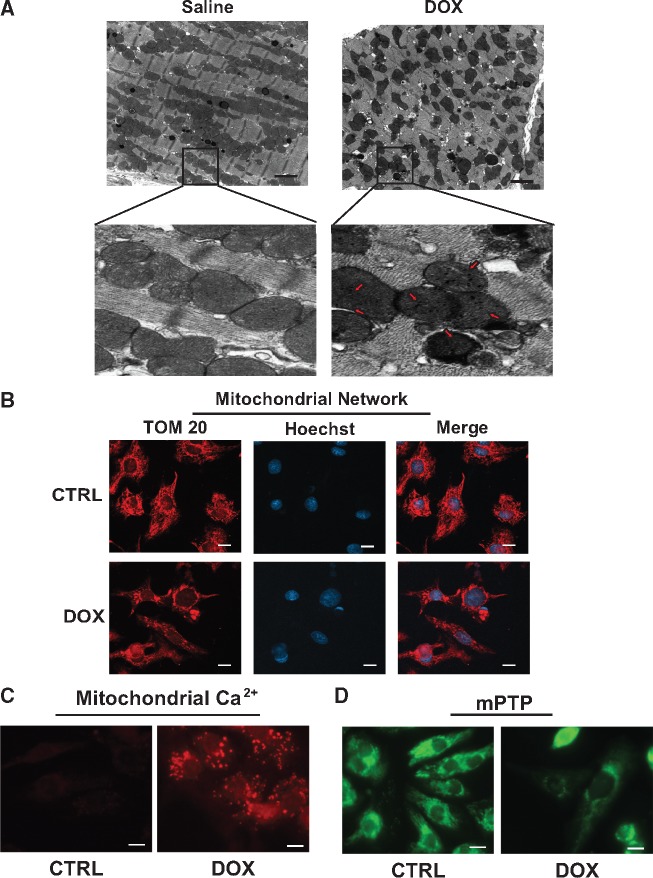
To begin to assess the mechanisms that underlie the molecular and cellular defects associated with Dox cardiomyopathy, we assessed the impact of Dox on cardiac ultrastructure in mice treated with Dox. For these studies, mice were treated with vehicle alone or with Dox and assessed for cardiac cell injury. As shown by electron microscopy (EM) in Figure 1A, in contrast to vehicle treated mice, mice treated with Dox exhibited severe ultrastructural defects including myofibril disorganization, cytoplasmic swelling, and mitochondria with disrupted cristae. Furthermore, evidence of mitochondrial localized electron dense granules resembling calcium deposits was also observed in hearts of mice treated with Dox. Together, these findings demonstrate that Dox is cytotoxic to cardiac myocytes. Since normal mitochondrial function is crucial for maintaining oxidative metabolism and ATP production, and based on electron micrographs indication of mitochondrial injury in Dox treated hearts, we tested the effect of Dox on mitochondrial morphology and function. For these studies, post-natal cardiac myocytes were treated with vehicle or Dox (18 h) and assessed for mitochondrial morphology and activity. As shown by fluorescence microscopy (Figure 1B), in contrast to control cells, mitochondrial reticular network in cardiac myocytes treated with Dox was disrupted and diffusely distributed, a finding consistent with the in vivo EM data. Notably, in electron micrographs, a marked increase in electron dense granules indicative of calcium accumulation were observed in virtually all mitochondria following Dox treatment. To verify that mitochondrial calcium was increased in cardiac mitochondria following Dox, we monitored mitochondrial calcium in the absence and presence of Dox in vitro. As shown in Figure 1C and Supplementary material online, Figure S1A, in contrast to vehicle treated control cells, mitochondria calcium was dramatically increased in cells treated with Dox—finding consistent with our EM data. Further, Dox-induced mitochondrial calcium uptake coincided with disrupted mitochondrial morphology, loss in mitochondrial membrane potential (TMRM), and increased ROS production compared to vehicle treated control cells (Supplementary material online, Figure S1B,C). Notably, Dox-induced mitochondrial calcium uptake resulted in mPTP opening (Figure 1D and Supplementary material online, Figure S1D).
Figure 1.
Dox provokes morphological and functional defects to mitochondria. Mitochondrial morphology in mice treated with saline (CTRL) or Dox. (A) Representative electron micrograph (5800× magnification) of heart muscle derived from saline (0.9%) and Dox (20 mg/kg) treated mice, with magnified insets below, scale bar 2 µm. (Left panel) Saline-treated mice exhibit normal mitochondrial morphology. (Right panel) Dox-treated mice displaying abnormal mitochondria with disrupted cristae and electron dense granules consistent with calcium deposits (red arrows). (B) Mitochondrial morphology assessed by epifluorescence microscopy using mitochondrial outer membrane protein Tom 20 (Red) and nuclear DNA (blue, Hoechst 33258), scale bar 10 µm. (C) Representative images of mitochondrial calcium uptake by Rhod-2AM (red fluorescence, see Section 2 for details), scale bar 10 µm. (D) Mitochondrial permeability transition pore (mPTP) opening assessed by using Calcein-AM in the presence of cobalt chloride, loss of green fluorescence indicates mPTP opening, scale bar 10 µm.
3.2 NF-κB signalling is impaired in cardiac myocytes treated with Dox
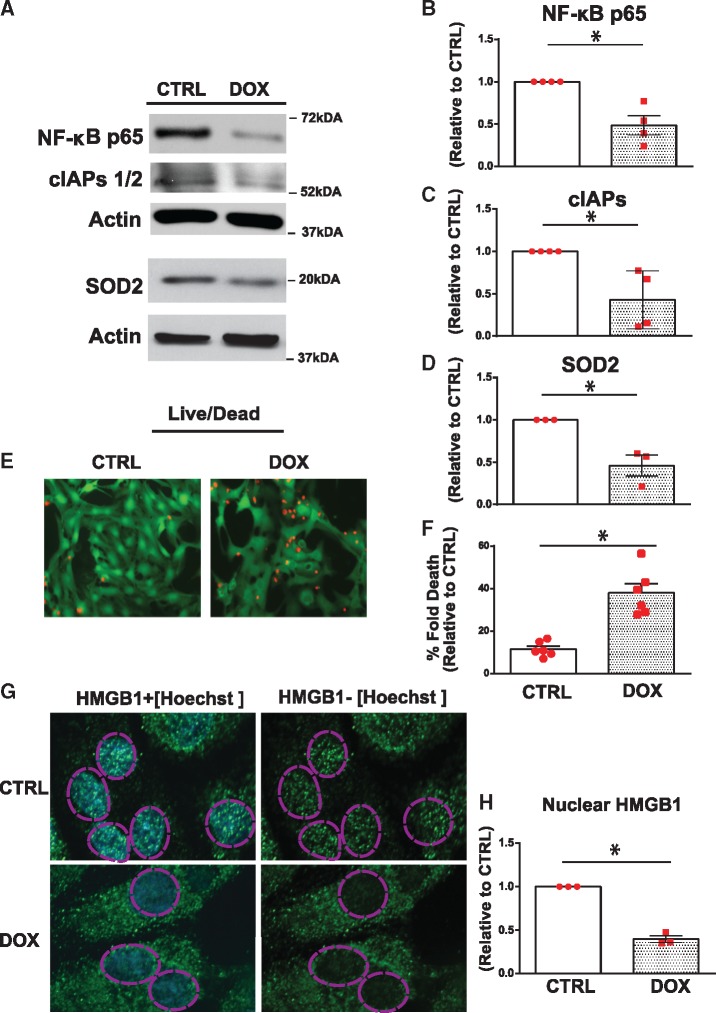
Earlier work by our laboratory established a critical survival role for NF-κB in cardiac myocytes; we reasoned that impaired NF-κB activity in cardiac myocytes treated with Dox may underlie the mitochondrial injury and cellular dysfunction associated with Dox treatment. To test this possibility, we assessed the expression levels of the p65 subunit of NF-κB in cardiac myocytes in the absence and presence of Dox. As shown by real-time qPCR (Supplementary material online, Figure S2A) and WB analysis (Figure 2A) in contrast to vehicle treated cells, a marked reduction in p65 NF-κB expression levels was observed in cells treated with Dox (18 h). This was consistent with downregulation of NF-κB transcriptional targets gene including inhibitor of apoptosis protein (IAP1/2) and superoxide dismutase 2 (SOD2; Figure 2A–D). Notably, the loss of NF-κB activity in Dox-treated cardiac myocytes coincided with a marked increase in cell death, Figure 2E,F, a finding concordant with the mitochondrial injury induced by Dox. Moreover, a loss of nuclear HMGB1 staining indicative of cell death is necrotic was observed in cardiac myocytes treated with Dox (Figure 2G,H). Taken together the data suggest that impaired NF-κB activation is associated with mitochondrial perturbations in cardiac myocytes treated with Dox. Moreover, a reduction in p65 NF-κB protein expression was observed in hearts of mice treated with Dox—a finding concordant with our in vitro findings (Supplementary material online, Figure S2B).
Figure 2.
Dox impairs NF-κB signalling and promotes necrotic cell death. NF-κB signalling and cell viability was determined in saline (CTRL) and Dox-treated cardiomyocytes. (A) Representative WBs of cell lysate derived from CTRL and Dox-treated cardiomyocytes. The filter was probed with antibodies directed against p65 subunit of NF-κB and its transcriptional targets, cIAPS1/2, and SOD2. Actin served as a protein loading control. (B–D) Quantitative analysis of proteins normalized to actin. Statistical significance determined using Student’s t-test for NF-κB, Dox vs. CTRL *P = 0.004; cIAPs, Dox vs. CTRL *P = 0.015 and SOD2, Dox vs. CTRL *P = 0.012. (E) Representative images of CTRL and Dox-treated cells assessed for cell viability with Calcein-AM and ethidium homodimer for detection of live (green) and dead (red) cells, respectively, scale bar 40µm. (F) Histogram depicts quantitative data for E. Data are expressed as mean ± SEM derived from at least n = 6 independent cardiomyocyte isolations. Unpaired two-tailed Student’s t-test was used to compare mean differences between groups. Statistically significant difference of Dox condition from control, *P = 0.002. (G) High mobility group box 1 immunostaining (HMGB1, green fluorescence) as an index of necrosis. Nuclear DNA stained with Hoechst 33528 (blue fluorescence). Dotted circle demarks nucleus, scale bar 10 µm. (H) Quantitative analysis of nuclear HMGB1 shown in G. Data are expressed as mean ± SEM derived from n = 3 independent cardiomyocyte isolations. Unpaired two tailed Student’s t-test was used to compare mean differences between groups. Statistically significant difference of Dox vs. CTRL *P = 0.0001.
3.3 Inhibition of cyclophilin D suppresses cell death in cardiac myocytes deficient for NF-κB signalling
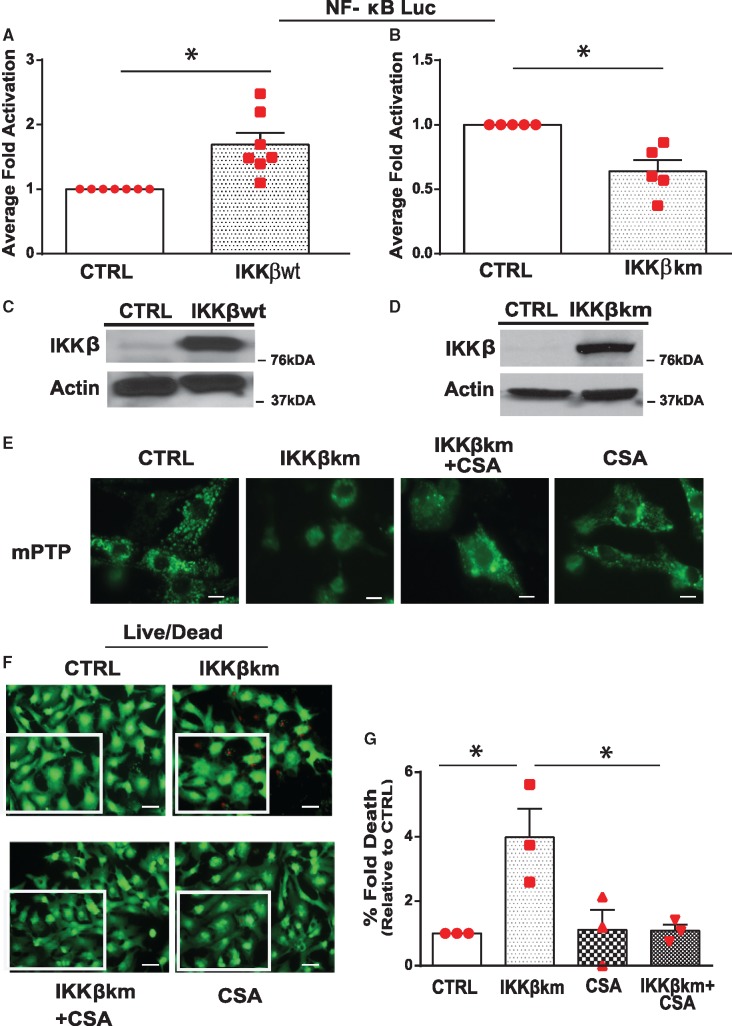
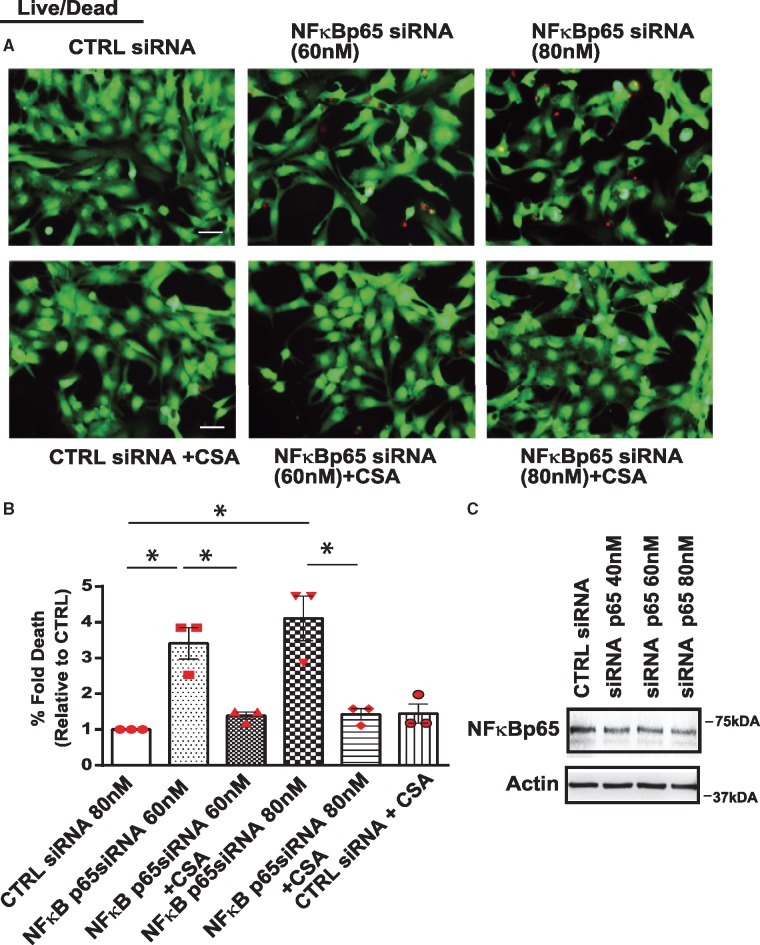
Since our earlier studies revealed that NF-κB signalling was involved in mPTP regulation, we reasoned that loss of NF-κB signalling may underlie mPTP opening induced by Dox. To recapitulate the inhibitory effects of Dox on NF-κB signalling, we rendered cardiac myocytes defective for NF-κB activation with a kinase defective form of IKKβ (IKKβK-M), the principle IKK required for NF-κB activation in cardiac myocytes.6 As shown in Figure 3A, NF-κB gene activation was significantly increased by ectopic expression of IKKβwt but not by kinase defective form of IKKβ (IKKβK-M), Figure 3B. Figure 3C,D confirms the overexpression of IKKβwt and IKKβK-M. Moreover, in contrast to cardiac myocytes infected with control virus, a marked increase in mPTP opening and cell death was observed in cardiac myocytes deficient for NF-κB activation (Figure 3E–G, Supplementary material online, Figure S2C). Interestingly, mPTP opening and cell death in cells defective for NF-κB signalling was suppressed by inhibition of CypD with CSA. To further explore the direct involvement of p65NF-κB in the regulation of mPTP opening, we knocked-down p65 NF-κB subunit using siRNA as a complementary approach to NF-κB inhibition studies with IKKK-M. As shown in Figure 4A,B, a significant increase in cell death was observed following p65 NF-κB knock-down with siRNA (60–80 nM) which could be averted by CSA (Figure 4C). These findings substantiate that loss of NF-κB has a direct effect on mitochondrial mPTP opening through a mechanism that requires CypD.
Figure 3.
Inhibition of cyclophilin D suppresses cell death of cardiac myocytes expressing IKKβK-M. (A, B) NF-κB gene transcription was assessed by luciferase promoter reporter assay in 293 cells in the presence of IKKβwt (A) or kinase defective form of IKKβ (IKKβK-M) in B. Data were normalized to CTRL and presented as mean ± SEM derived from at least n = 3 experiments. Unpaired two-tailed Student’s t-test was used to compare mean differences between groups. Statistical significance between CTRL and IKKβwt, *P = 0.0026; between CTRL and IKKβK-M, *P = 0.003. (C, D) Expression of IKKβwt and IKKβK-M. (E–G) mPTP opening (C, scale bar 10 µm) and cell viability (scale bar 40 µm, D, E) of ventricular myocytes expressing IKKβK-M in the absence and presence of Cyclosporine A (CSA, 2μM, inhibitor of Cyclophilin D). mPTP opening and cell viability were assessed by epifluorescence microscopy (see Section 2 for details). Data are expressed as mean ± SEM (n = 3 experiments). Statistical significance between the groups was analysed by one-way ANOVA and Bonferroni post hoc test; IKKβK-M vs. CTRL,*P = 0.0145; IKKβK-M vs. IKKβK-M + CSA, *P = 0.0170; IKKβK-M vs. CSA, *P = 0.0177.
Figure 4.
Inhibition of cyclophilin D suppresses cell death in cardiac myocytes following NF-κB knock-down. Cardiomyocytes expressing scrambled siRNA or siRNA directed against NF-κB p65 (60 nM and 80 nM) assessed for cell viability assay in the absence and presence of CSA. (A) Representative images for cell viability, scale bar 40 µm. (B) Histogram shows quantitative data for A. Data are expressed as mean ± SEM derived from n = 3 independent experiments. Statistical significance between the groups marked in the histogram, analysed by one-way ANOVA and Bonferroni post hoc test, CTRL vs. NF-κB p65si 60 nM *P = 0.0015; CTRL vs. NF-κB p65si 80 nM *P = 0.0002; NF-κB p65si 60 nM vs. NF-κB p65si 60 nM+CSA *P = 0.006; NF-κB p65si 80 nM vs. NF-κB p65si 80 nM+CSA*P = 0.0006. (C) WB analysis of the cell lysate derived from cells expressing siRNA directed against NF-κB p65siRNA (40 nM, 60 nM, and 80 nM) or scrambled siRNA (80 nM). Actin served as a protein loading control.
3.4 Cyclophilin D mediates mPTP opening and cell death in cardiac myocytes treated with Dox
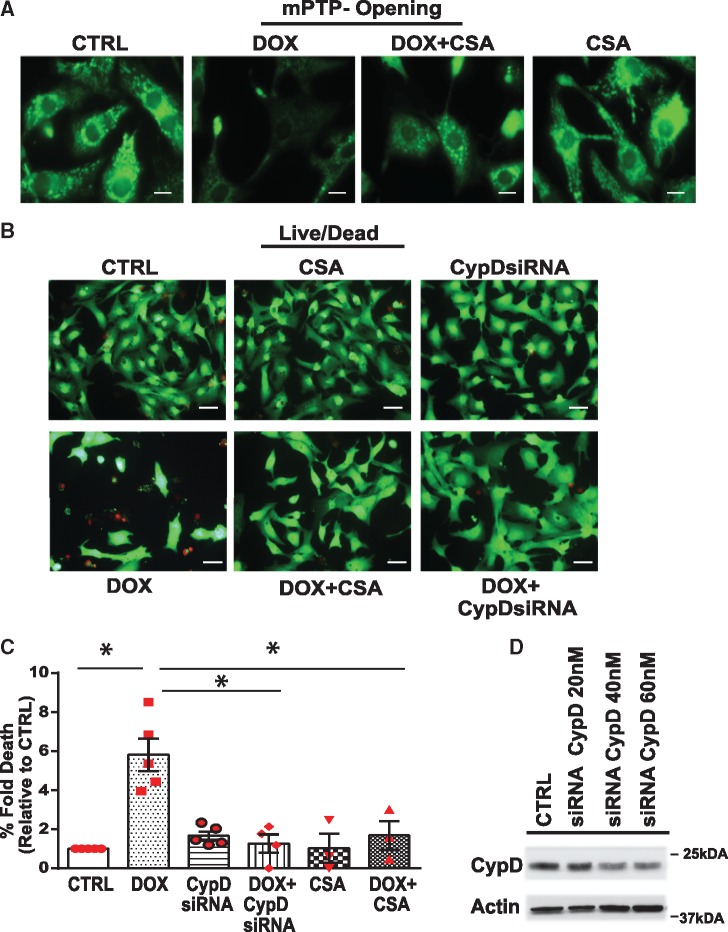
Based on these findings we next tested, whether CypD is involved in Dox-induced mPTP opening and cell death of cardiac myocytes. Interestingly, knock-down of CypD or treatment of cells with CSA dramatically suppressed mitochondrial mPTP opening and cell death induced by Dox (Figure 5A–C, Supplementary material online, Figure S2D). CypD knock-down was confirmed by WB analysis (Figure 5D). These data support the notion that Dox promotes cell death through a mechanism that involves CypD-mediated mPTP opening.
Figure 5.
Inhibition of cyclophilin D suppresses mPTP opening and cell death of cardiac myocytes treated with Dox. (A, B) mPTP opening (A, scale bar 10 µm) and cell viability (B, scale bar 40 µm) of cardiomyocytes treated with vehicle or Dox in the absence or presence of CypD inhibitor Cyclosporine A (CsA, μM) or siRNA directed against CypD (CypD siRNA, 40 nM). Representative fluorescent images derived for cell viability as described previously in Figure 2. (C) Histogram depicts percent fold increase in cell death in each condition shown in B, values were normalized to vehicle treated CTRL cells. Data are derived from at least n = 3 independent experiments. Statistical significance between the groups was analysed by one-way ANOVA and Bonferroni post hoc test, Dox vs. CTRL, *P < 0.0001; Dox vs. Dox +CSA, *P = 0.0002; Dox vs. Dox + CypDsi, *P = 0.0002. (D) WB analysis of the cell lysate derived from cells expressing siRNA directed against CypDsi (20 nM, 30 nM, and 40 nM) or scrambled siRNA (40 nM). Actin served as a protein loading control.
3.5 Bnip3 interacts with CypD and promotes mPTP opening and cell death in cardiac myocytes treated with Dox
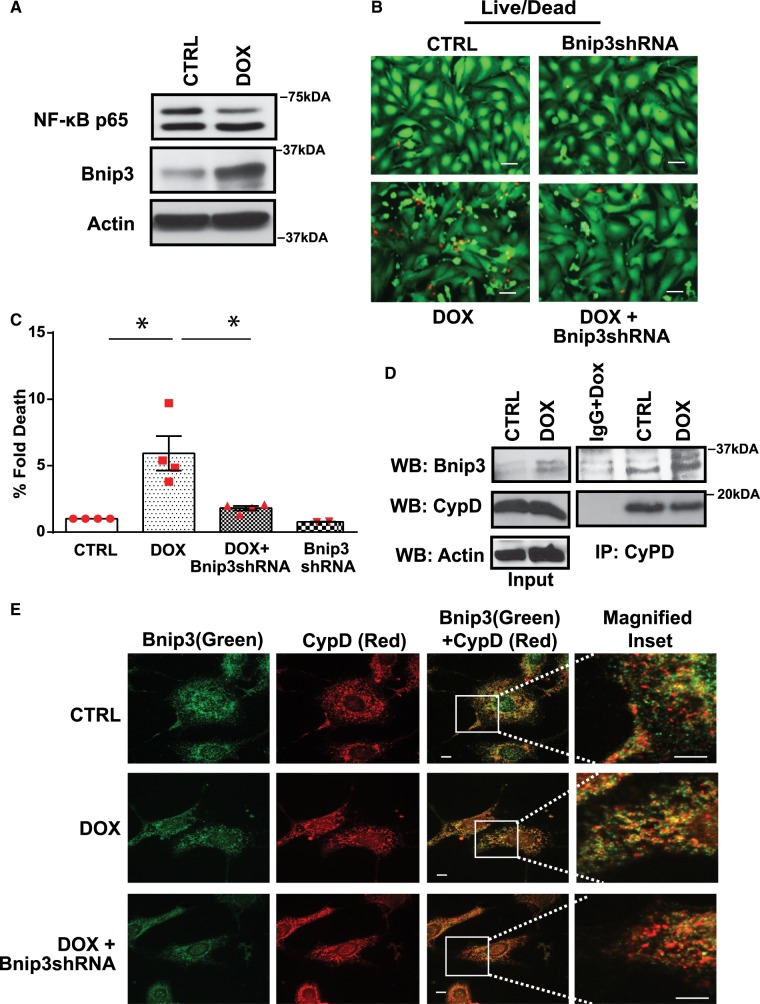
Bnip3 has been shown to provoke mitochondrial defects and cell death in ventricular myocytes treated with Dox. Since earlier studies from our laboratory demonstrated that Bnip3 promoter is transcriptionally silenced by NF-κB, we next ascertained whether loss of NF-κB signalling in cardiac myocytes treated with Dox would activate Bnip3. As shown by WB analysis in Figure 6A, in contrast to vehicle treated cells, Bnip3 expression was markedly increased in cardiac myocytes treated with Dox. Notably, Dox-induced cell death was suppressed by knock-down of Bnip3 using an adenovirus encoding Bnip3 shRNA (Figure 6B,C). These findings raise the intriguing possibility that Bnip3 may promote mPTP opening and necrotic cell death in Dox treated cardiac myocytes by a mechanism that impinges upon a key regulator of mPTP, such as CypD. To test this possibility, we performed co-IP assays on cell lysate derived from vehicle treated or cardiac myocytes treated with Dox (Figure 6D). As shown by WB analysis, in contrast to vehicle treated cells, Bnip3 readily formed protein complexes with CypD. Immunofluorescence studies verified an increase in co-localization of Bnip3 and CypD proteins following Dox treatment (Figure 6E).
Figure 6.
Bnip3 interacts with CypD and promotes cell death in cardiac myocytes deficient for NF-κB. (A) WB analysis of cardiac cell lysate derived from CTRL and Dox-treated cells to assess NF-κBp65 and Bnip3 protein expression. Actin served as a loading control. (B) Representative fluorescence images of cell viability for cardiac myocytes treated with vehicle or Dox in the absence and presence of Bnip3 short hairpin encoding (Bnip3shRNA) adenovirus, scale bar 40 µm. (C) Histogram represents quantitative data for B. Data are expressed as mean ± SEM derived from at least n = 3 independent cardiomyocyte isolations. Statistical significance between the groups marked in the histogram, analysed by one-way ANOVA and Bonferroni post hoc test, Dox vs. CTRL, *P = 0.002; Dox vs. Dox+Bnip3shRNA, *P = 0.0068. (D) IP assay was performed on CTRL and Dox treated ventricular myocytes, using Cyclophilin D antibody, the filter was blotted with Bnip3 antibody. (E) Immunofluorescence staining was performed for Bnip3 (green) and CypD (Red) on CTRL and Dox-treated ventricular myocytes in the absence and presence of Bnip3shRNA, scale bar 5µm.
3.6 Cyclophilin D is a downstream effector in Bnip3 induced mitochondrial defects and cell death of cardiac myocytes
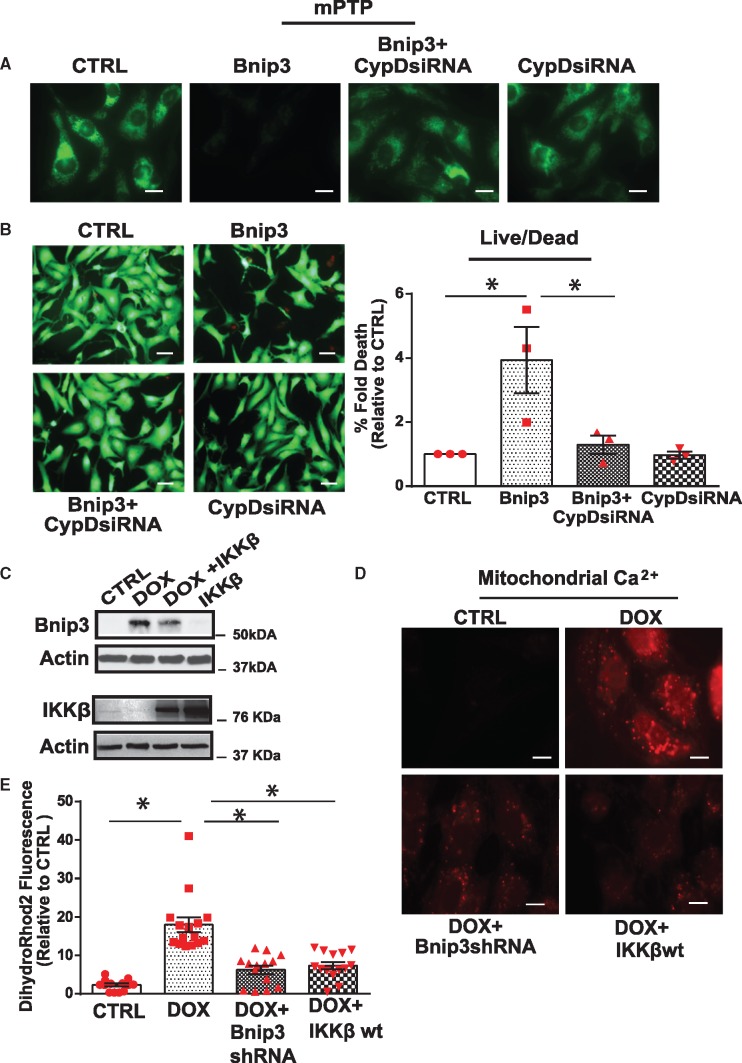
To determine whether CypD is involved in Bnip3-mediated mPTP opening and cell death of cardiac myocytes, we assessed whether Bnip3 would provoke mPTP opening and cell death in cardiac myocytes deficient for CypD. Interestingly, Bnip3-mediated mPTP opening, ROS production, and cell death were substantially reduced in cardiac myocytes following CypD knock-down (Figure 7A, B, Supplementary material online, Figure S3A,B)—supporting the notion that Bnip3-mediated mPTP involves CypD. This finding is in complete agreement with our data for the repression of Bnip3-mediated mPTP opening with CSA in cells deficient for NF-κB signalling. In aggregate, these findings demonstrate that Bnip3 promotes mPTP opening and cell death of cardiac myocytes through a mechanism that is contingent upon CypD.
Figure 7.
Cyclophilin D is required for mitochondrial perturbations and cell death of cardiac myocytes induced by Bnip3. Cardiomyocytes expressing Bnip3 in the presence of scrambled siRNA or siRNA directed against CypD (CypD siRNA 40 nM), assessed for mPTP opening and cell viability assay. (A) Representative fluorescent images for mPTP opening, scale bar 10 µm; (B) (Left panel) Representative images for cell viability, scale bar 40 µm. (right panel) Histogram shows quantitative data for B. Data are expressed as mean ± SEM derived from at least n = 3 independent experiments. Statistical significance between the groups marked in the histogram, analysed by one-way ANOVA and Bonferroni post hoc test, Bnip3 vs. CTRL *P = 0.0147; Bnip3 vs. CypDsiRNA *P = 0.0257. (C) WB analysis of the cardiac cell lysate derived from saline (CTRL) and Dox-treated cells in the absence and presence of IKKβwt. The filter was probed with murine antibodies directed against Bnip3, IKKβ, and actin. WB showing mitochondrial localized Bnip3 dimer (60 kDa) and actin. (D) Representative images of mitochondrial calcium by Rhod2-AM staining in cardiac myocytes treated with vehicle or Dox in the presence of adenovirus encoding Bnip3shRNA or IKKβwt, scale bar 10 µm. (E) Histogram represents quantitative data for E, data are normalized to CTRL. Fluorescence intensity was assessed using image j software data are expressed as mean ± SEM. Statistical significance between the groups marked in the histogram, analysed by one-way ANOVA and Bonferroni post hoc test, Dox vs. CTRL, *P < 0.0001; Dox vs. Dox+Bnip3shRNA, *P < 0.0001; Dox vs. Dox+IKKβ, *P < 0.0001.
3.7 Dox-induced mitochondrial calcium influx, mPTP opening, and necrotic cell death is suppressed by restoring NF-κB signalling
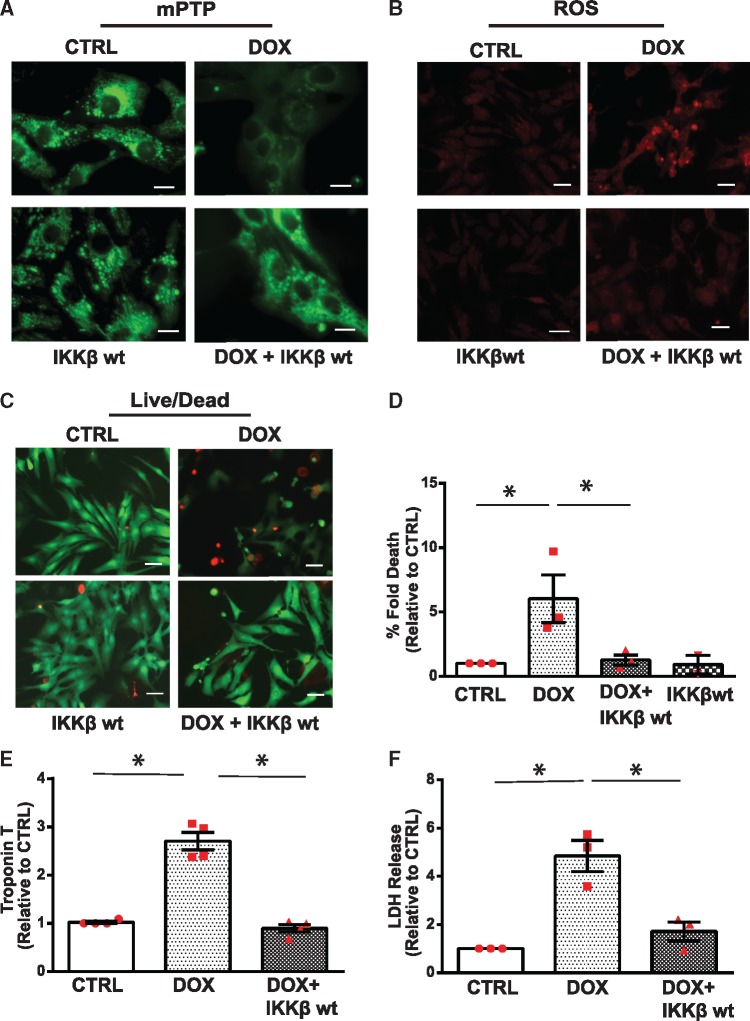
Based on the above findings, we next assessed whether restoring NF-κB signalling would suppress cardiac injury induced by Dox. For these studies, we tested whether restoring NF-κB signalling by IKKβwt overexpression would suppress Dox-induced mitochondrial perturbations and cell death of ventricular myocytes. As shown by WB analysis in Figure 7C and Supplementary material online, Figure S3C, in contrast to cardiac myocytes treated with Dox, cells treated with Dox and overexpressing IKKβ exhibited a reduction in Bnip3 expression. Notably, Dox-induced mitochondrial calcium overload was normalized by either inactivating Bnip3 with shRNA or restoring NF-κB signalling with IKKβ (Figure 7D,E). These findings support our contention that Bnip3 promotes mitochondrial perturbations and mitochondrial calcium overload. Interestingly, IKKβwt expression suppressed Dox-induced mPTP opening, ROS production and necrotic cell death indices lactate dehydrogenase (LDH) and TNT (Figure 8A–F; Supplementary material online, Figure S4A,B). To prove the protection conferred by IKKβwt against Dox-cardiotoxicity was related to Bnip3 inhibition, we tested whether forced expression of Bnip3 would overcome the protective effects of IKKβwt. As anticipated, cardioprotection conferred by IKKβ in cells treated with Dox was reduced upon Bnip3 overexpression (Supplementary material online, Figure S4C,D). Taken together these findings strongly suggest that impaired NF-κB signalling underlies the mitochondrial perturbations and necrotic cell death of cardiac myocytes treated with Dox.
Figure 8.
Restoration of NF-κB signalling by IKKβ suppresses Dox-induced mitochondrial perturbations and necrotic cell death of ventricular myocytes. (A) Cardiomyocytes were treated with vehicle or Dox in the absence or presence of an adenovirus encoding IKKβwt and assessed for mPTP opening, scale bar 10 µm. (B) ROS production, scale bar 40 µm; (C) Cell viability, scale bar 40 µm; (D) Histogram presents quantitative data for C. Statistical significance between the groups shown in the histogram, analysed by one-way ANOVA and Bonferroni post hoc test, Dox vs. CTRL, *P = 0.033; Dox vs. Dox+IKKβ, *P = 0.0423; (E, F) Cardiac troponin T (cTNT) release and lactate dehydrogenase assessment from the supernatants derived from saline and Dox-treated cardiac myocytes in the absence and presence of IKKβwt. Data are expressed as mean ± SEM derived from at least n = 3 cardiomyocyte isolations. Statistical significance between the groups marked in the histogram, analysed by one-way ANOVA and Bonferroni post hoc test. For TNT, difference between Dox and CTRL, *P < 0.0001; Dox vs. Dox+IKKβ, *P < 0.0001. For LDH, statistically significant difference between Dox and CTRL, *P = 0.0024; Dox vs. Dox+IKKβ, *P = 0.007.
4. Discussion
Herein, we provide a novel evidence that NF-κB signalling pathway was severely impaired in Dox cardiomyopathy. This was attributed to a loss in NF-κB activation from the inhibition of critical components of the signalling pathway required for NF-κB activation in cardiac myocytes. Indeed, IKKβ expression, the principle kinase required for activating NF-κB in the heart was inhibited in cardiac myocytes cells following Dox treatment in vivo and in vitro supports the notion that IKKβ-mediated activation of NF-κB is crucial for cell survival of ventricular myocytes. The fact that restoring IKKβ-mediated NF-κB signalling in cardiac myocytes suppressed the cytotoxic effects induced by Dox supports our contention that NF-κB plays a critical survival role in cardiac myocytes. This view is substantiated by the embryonic lethality of mice germ-line deleted for p65 NF-κB and increased sensitivity of cardiac myocytes to tumour necrosis factor alpha (TNFα)-mediated necrosis in cardiac myocytes deficient for NF-κB activation.10,12
While the mode by which NF-κB averts cell death remains to be fully understood, its survival properties have been linked to its well-established role as a transcriptional activator.14,15 However, a less appreciated feature of NF-κB is its ability to transcriptionally repress activation of certain cell death promoting genes such as Bnip3.17 Earlier work by our laboratory established that NF-κB suppressed cell death of cardiac myocytes by transcriptionally silencing basal and inducible expression of Bnip3 in cardiac myocytes.16,17 Since de-regulated Bnip3 activation would otherwise be lethal to cells and provoke cell death, its suppression by NF-κB represents an important underappreciated survival mechanism for averting cell death in cardiac myocytes. As such, the loss of NF-κB signalling such as seen here, would result in de-regulated Bnip3 expression and widespread cell death. The functional impairment of NF-κB activity from loss of IKKβ in cardiac myocytes treated with Dox, coincident with increased Bnip3 expression and cell death is consistent with this view. Hence, the findings of the present study highlight a novel survival pathway that functionally links IKKβ–NF-κB mediated suppression of Bnip3 to cell survival during Dox cardiomyopathy. This view is further supported by the finding that inhibition of Bnip3 suppressed Dox-induced mitochondrial perturbations such calcium influx, ROS production, and mPTP opening. This implies that Bnip3 is necessary for provoking mitochondrial injury and cell death of cardiac myocytes.
Another salient feature of our study was the finding that inhibition of CypD suppressed Bnip3-mediated mPTP opening and cell death in cardiac myocytes deficient for NF-κB signalling or treated with Dox. Even more profound was our finding that Bnip3 readily formed protein complexes with CypD in cardiac myocytes treated with Dox. These findings support the notion that Bnip3 promotes mPTP opening and necrosis in cardiac cells treated with Dox through a mechanism that involves CypD. Bnip3 had been previously shown to disrupt mitochondrial oxidative metabolism by disrupting protein complexes between uncoupling protein 3 and cytochrome c oxidase subunit I of complex IV of electron transport chain.22 However, the relationship to CypD to form mPTP opening had not been explored.
The finding that pharmacological inhibition of CypD with CSA or genetic knock-down by siRNA each suppressed Bnip3-mediated mPTP opening in cells treated with Dox, strongly suggests that CypD is essential for Bnip3-mediated mPTP opening in cardiac myocytes. At present the exact relationship between Bnip3, CypD and mPTP opening is unknown. It is equally undermined whether the association of Bnip3 with CypD is direct, indirect or requires additional factors. It is also undetermined whether Bnip3 influences other putative regulators of the mPTP, such as the F0/F1 ATPase, this is an active area of investigation in our laboratory.26,34 Nevertheless, the fact that Dox-induced mitochondrial calcium uptake which is requisite event for mPTP opening was suppressed by Bnip3 inhibition or by restoring IKKβ–NF-κB signalling, supports a model in which de-regulated Bnip3 activation serves as a primary trigger for mitochondrial calcium influx and CypD-mediated mPTP opening. This notion is in agreement with Dox-induced mitochondrial defects and cell death of cardiac myocytes following Bnip3 activation. However, although the Bnip3–CypD appears to be important for Dox-induced cardiotoxicity, the functional importance of this complex will require further studies to examine the impact of disrupting Bnip3-CypD interaction on mPTP under pathophysiological conditions.
Herein, we provide the first evidence that NF-κB activation is severely impaired in cardiac myocytes treated with Dox. We further show that functional loss of NF-κB signalling predisposes cardiac myocytes to Dox-induced mitochondrial injury and CypD-mediated mPTP opening through activation of Bnip3. Hence, interventions that preserve NF-κB signalling in cardiac myocytes may prove beneficial in suppressing the cardiac dysfunction in cancer patients undergoing Dox treatment by preventing Bnip3–CypD interaction.
Supplementary Material
Acknowledgements
We thank Ms Floribeth Aguilar for technical assistance.
Conflict of interest: none declared.
Funding
This work was supported by a Foundation grant to L.A.K. from the Canadian Institute for Health Research (CIHR) and Heart and Stroke Foundation of Canada; I.R-N. is supported by Post-doctoral fellowship from CIHR. L.A.K. holds a Canada Research Chair in Molecular Cardiology.
Time for primary review: 22 days
Translational perspective
Doxorubicin (Dox) is commonly used for treating variety of human cancers; however, it is highly toxic to cardiac myocytes and promotes heart failure. NF-κB regulates vital cellular processes including survival and is impaired in cardiac myocytes treated with Dox. The death gene Bnip3 is activated by Dox and provokes mitochondrial perturbations and necrotic cell death contingent upon Cyclophilin D. Re-establishing NF-κB signalling, suppressed Dox-induced Bnip3-mediated mitochondrial injury and Dox cardiomyopathy. Hence, interventions that preserve NF-κB signalling may prove beneficial in mitigating cardiac dysfunction and heart failure in cancer patients treated with Dox.
References
- 1. Beg AA, Finco TS, Nantermet PV, Baldwin AS.. Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol 1993;13:3301–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ducut Sigala JL, Bottero V, Young DB, Shevchenko A, Mercurio F, Verma IM.. Activation of transcription factor NF-kappaB requires ELKS, an IkappaB kinase regulatory subunit. Science 2004;304:1963–1967. [DOI] [PubMed] [Google Scholar]
- 3. Baeuerle P, Baltimore D.. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science 1988;242:540–546. [DOI] [PubMed] [Google Scholar]
- 4. Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T.. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev 1995;9:1586–1597. [DOI] [PubMed] [Google Scholar]
- 5. Ghosh S, Karin M.. Missing pieces in the NF-kappaB puzzle. Cell 2002;109 Suppl:S81–S96. [DOI] [PubMed] [Google Scholar]
- 6. Regula KM, Ens K, Kirshenbaum LA.. IKKβ is required for Bcl-2-mediated NF-κB activation in ventricular myocytes. J Biol Chem 2002;277:38676–38682. [DOI] [PubMed] [Google Scholar]
- 7. Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting J-Y, Baldwin AS.. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev 2008;22:1490–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dhingra R, Gang H, Wang Y, Biala AK, Aviv Y, Margulets V, Tee A, Kirshenbaum LA.. Bidirectional regulation of nuclear factor-κb and mammalian target of rapamycin signaling functionally links bnip3 gene repression and cell survival of ventricular myocytes. Circ Hear Fail 2013;6:335–343. [DOI] [PubMed] [Google Scholar]
- 9. Tanaka M, Fuentes ME, Yamaguchi K, Durnin MH, Dalrymple SA, Hardy KL, Goeddel DV.. Embryonic lethality, liver degeneration, and impaired NF-kappa B activation in IKK-beta-deficient mice. Immunity 1999;10:421–429. [DOI] [PubMed] [Google Scholar]
- 10. Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D.. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature 1995;376:167–170. [DOI] [PubMed] [Google Scholar]
- 11. Mustapha S, Kirshner A, Moissac DD, Kirshenbaum LA.. A direct requirement of nuclear factor-kappa B for suppression of apoptosis in ventricular myocytes. Am J Physiol Heart Circ Physiol 2000;279:H939–H945. [DOI] [PubMed] [Google Scholar]
- 12. Gang H, Shaw J, Dhingra R, Davie JR, Kirshenbaum LA.. Epigenetic regulation of canonical TNFα pathway by HDAC1 determines survival of cardiac myocytes. Am J Physiol Circ Physiol 2013;304:H1662–H1669. [DOI] [PubMed] [Google Scholar]
- 13. Regula KM, Baetz D, Kirshenbaum LA.. Nuclear factor-κB represses hypoxia-induced mitochondrial defects and cell death of ventricular myocytes. Circulation 2004;110:3795–3802. [DOI] [PubMed] [Google Scholar]
- 14. Stehlik C, Martin R. D, Binder BR, Lipp J.. Cytokine induced expression of porcine inhibitor of apoptosis protein (iap) family member is regulated by NF-kappa. Biochem Biophys Res Commun 1998;243:827–832. [DOI] [PubMed] [Google Scholar]
- 15. Gordon JW, Shaw JA, Kirshenbaum LA.. Multiple facets of NF-κB in the heart. Circ Res 2011;108:1122–1132. [DOI] [PubMed] [Google Scholar]
- 16. Baetz D, Regula KM, Ens K, Shaw J, Kothari S, Yurkova N, Kirshenbaum LA.. Nuclear factor-κB-mediated cell survival involves transcriptional silencing of the mitochondrial death gene BNIP3 in ventricular myocytes. Circulation 2005;112:3777–3785. [DOI] [PubMed] [Google Scholar]
- 17. Shaw J, Zhang T, Rzeszutek M, Yurkova N, Baetz D, Davie JR, Kirshenbaum LA.. Transcriptional silencing of the death gene BNIP3 by cooperative action of NF-κB and histone deacetylase 1 in ventricular myocytes. Circ Res 2006;99:1347–1354. [DOI] [PubMed] [Google Scholar]
- 18. Yurkova N, Shaw J, Blackie K, Weidman D, Jayas R, Flynn B, Kirshenbaum LA.. The cell cycle factor E2F-1 activates Bnip3 and the intrinsic death pathway in ventricular myocytes. Circ Res 2008;102:472–479. [DOI] [PubMed] [Google Scholar]
- 19. Gang H, Dhingra R, Wang Y, Mughal W, Gordon JW, Kirshenbaum LA.. Epigenetic regulation of E2F-1-dependent Bnip3 transcription and cell death by nuclear factor-κB and histone deacetylase-1. Pediatr Cardiol 2011;32:263.. [DOI] [PubMed] [Google Scholar]
- 20. Lipshultz SE, Cochran TR, Franco VI, Miller TL.. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat Rev Clin Oncol 2013;10:697–710. [DOI] [PubMed] [Google Scholar]
- 21. Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL.. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 2012;52:1213–1225. [DOI] [PubMed] [Google Scholar]
- 22. Dhingra R, Margulets V, Chowdhury SR, Thliveris J, Jassal D, Fernyhough P, Dorn GW, Kirshenbaum LA.. Bnip3 mediates doxorubicin-induced cardiac myocyte necrosis and mortality through changes in mitochondrial signaling. Proc Natl Acad Sci USA 2014;111:E5537–E5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singal PK, Siveski-Iliskovic N, Hill M, Thomas TP, Li T.. Combination therapy with probucol prevents adriamycin-induced cardiomyopathy. J Mol Cell Cardiol 1995;27:1055–1063. [DOI] [PubMed] [Google Scholar]
- 24. Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, Mutharasan RK, Naik TJ, Ardehali H.. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest 2014;124:617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zaglia T, Ceriotti P, Campo A, Borile G, Armani A, Carullo P, Prando V, Coppini R, Vida V, Stølen TO, Ulrik W, Cerbai E, Stellin G, Faggian G, Stefani DD, Sandri M, Rizzuto R, Lisa F, Di Pozzan T, Catalucci D, Mongillo M.. Content of mitochondrial calcium uniporter (MCU) in cardiomyocytes is regulated by microRNA-1 in physiologic and pathologic hypertrophy. Proc Natl Acad Sci USA 2017;114:E9006–E9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Antoniel M, Jones K, Antonucci S, Spolaore B, Fogolari F, Petronilli V, Giorgio V, Carraro M, Lisa FD, Forte M, Szabó I, Lippe G, Bernardi P.. The unique histidine in OSCP subunit of F‐ATP synthase mediates inhibition of the permeability transition pore by acidic pH. EMBO Rep 2018;19:257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bernardi P, Lisa F.. Di Cyclosporine before PCI in Acute Myocardial Infarction. N Engl J Med 2016;374:88–90. [DOI] [PubMed] [Google Scholar]
- 28. Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD.. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol 2007;9:550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nguyen TT, Stevens MV, Kohr M, Steenbergen C, Sack MN, Murphy E.. Cysteine 203 of cyclophilin D is critical for cyclophilin D activation of the mitochondrial permeability transition pore. J Biol Chem 2011;286:40184–40192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, Karch J, Gabel S, Farber J, Force T, Heller Brown J, Murphy E, Molkentin JD.. Cyclophilin D controls mitochondrial pore-dependent Ca2+ exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest 2010;120:3680–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bernardi P, Rasola A, Forte M, Lippe G.. The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol Rev 2015;95:1111–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gang H, Hai Y, Dhingra R, Gordon JW, Yurkova N, Aviv Y, Li H, Aguilar F, Marshall A, Leygue E, Kirshenbaum LA.. A novel hypoxia-inducible spliced variant of mitochondrial death gene Bnip3 promotes survival of ventricular myocytes. Circ Res 2011;108:1084–1092. [DOI] [PubMed] [Google Scholar]
- 33. Gang H, Dhingra R, Lin J, Hai Y, Aviv Y, Margulets V, Hamedani M, Thanasupawat T, Leygue E, Klonisch T, Davie JR, Kirshenbaum LA.. PDK2-mediated alternative splicing switches Bnip3 from cell death to cell survival. J Cell Biol 2015;210:1101–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan S, Du F, Wu L, Zhang Z, Zhong C, Yu Q, Wang Y, Lue L-F, Walker DG, Douglas JT, Yan SS.. F1 F0 ATP synthase–cyclophilin D interaction contributes to diabetes-induced synaptic dysfunction and cognitive decline. Diabetes 2016;65:3482–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.