Abstract
Objectives
There is a long‐standing interest in developing nicotinic acetylcholine receptor (nAChR) antagonists for concomitant use with nAChR agonists (e.g., nicotine replacement) as complementary smoking cessation aids. Previous studies demonstrate that daily nicotine treatment confers tolerance to some effects of nicotine, as well as cross‐tolerance to other nAChR agonists. The current study assessed the extent to which antagonism of nicotine varies as a function of daily nicotine treatment.
Methods
Schedule‐controlled responding and hypothermia were selected for study because they have been previously used to examine the pharmacology of nicotine, and both are sensitive to the development nicotine tolerance. The rate‐decreasing and hypothermic effects of nicotine, as well as antagonism of those effects, were examined in C57BL/6J mice before, during treatment with, and after discontinuation of three daily injections of 1.78 mg/kg nicotine. The nonselective nAChR antagonist mecamylamine and the β2 nAChR antagonist dihydro‐β‐erythroidine (DHβE) were studied in combination with nicotine.
Results
The ED50 values of nicotine to produce rate‐decreasing and hypothermic effects were, respectively, 0.44 and 0.82 mg/kg prior, 1.6 and 3.2 mg/kg during, and 0.74 and 1.1 mg/kg after discontinuation of daily nicotine treatment. Prior to daily nicotine treatment, mecamylamine decreased response rate and rectal temperature. However, during daily nicotine, mecamylamine (up to 5.6 mg/kg) only decreased rectal temperature. DHβE (up to 5.6 mg/kg) when studied prior to daily nicotine decreased rectal temperature, but that decrease was abolished during chronic nicotine treatment. Mecamylamine and DHβE antagonized the rate‐decreasing and hypothermic effects of nicotine before and after daily nicotine; however, during daily nicotine, mecamylamine and DHβE antagonized only the hypothermic effects of nicotine.
Conclusions
The differential antagonism of rate‐decreasing and hypothermic effects implicates differential involvement of nAChR subtypes. The decreased capacity of mecamylamine and DHβE to antagonize nicotine during chronic nicotine treatment may indicate that their effectiveness as smoking cessations might vary as a function of nicotine tolerance and dependence.
Keywords: dihydro‐β‐erythroidine, hypothermia, mecamylamine, nicotine, operant responding, tolerance
There is a loss of sensitivity to nicotinic antagonists in nicotine‐tolerant mice, which is unexpected based on receptor theory and the results of previous studies with other pharmacological classes. This finding has implications for the use of nicotinic antagonists as smoking cessation aids.
1. INTRODUCTION
Tobacco use is the leading cause of preventable death (World Health Organization, 2011). Current smoking cessation aids improve abstinence outcomes, although there is marked room for improvement inasmuch as 75% of individuals relapse to the use of tobacco products within 1 year of initiating pharmacotherapy (Hays, Ebbert, & Sood, 2008). There is a growing interest in new strategies to combat the tobacco epidemic, including the use of both drug and behavioral interventions (Brunzell, McIntosh, & Papke, 2014; Mooney et al., 2016; Vogeler, McClain, & Evoy, 2016; Windle et al., 2016). A potential strategy previously considered but not yet formally approved is the use of nicotinic acetylcholine receptor (nAChR) antagonists, which have been demonstrated to attenuate the reinforcing effects of nicotine as well as nicotine‐induced increases in mesolimbic dopamine (Corrigall, Franklin, Coen, & Clarke, 1992; Crooks, Bardo, & Dwoskin, 2014; Nisell, Nomikos, & Svensson, 1994). One such antagonist is the nonselective, noncompetitive nAChR ligand mecamylamine (Frishman, Mitta, Kupersmith, & Ky, 2006; Lancaster & Stead, 2000). The value of mecamylamine as a smoking cessation aid was underscored by reports that mecamylamine did not induce withdrawal in human tobacco users (Eissenberg, Griffiths, & Stitzer, 1996), which if it had would have created concerns regarding compliance. However, mecamylamine, and other antagonists, has not been widely used as smoking cessation aids. Studies have suggested that mecamylamine, when used alone, is only effective in approximately 15% of participants in clinical studies (Lancaster & Stead, 2000; Rose et al., 1994).
One issue to be considered when developing any pharmacotherapy for drug abuse is how chronic treatment with an abused drug alters the receptor systems mediating the effects of not only the abused drug, but also a pharmacotherapy that may act through the same receptor systems. In the same way, daily tobacco use confers tolerance to many of the effects of nicotine and other nAChR agonists (Cunningham & McMahon, 2011; de Moura & McMahon, 2016; Rodriguez et al., 2014; Rosecrans, Stimler, Hendry, & Meltzer, 1989), and the effects of nAChR antagonists might also be impacted. Repeated exposure to nicotine differentially impacts the expression levels of various nAChR subtypes within the CNS (Buisson & Bertrand, 2001; Fenster, Whitworth, Sheffield, Quick, & Lester, 1999; Nashmi et al., 2007; Olale, Gerzanich, Kuryatov, Wang, & Lindstrom, 1997). Whether neuroadaptations that occur as a result of nicotine exposure can impact the effects of nAChR antagonists, particularly their nicotine antagonist activity, has not been fully characterized.
The nonselective nAChR antagonist mecamylamine (Banerjee, Punzi, Kreilick, & Abood, 1990) and the selective β2 nAChR antagonist dihydro‐β‐erythroidine (DhβE; Papke et al., 2008) have been studied under conditions of chronic nicotine exposure to examine precipitated withdrawal (Damaj, Kao, & Martin, 2003; Vann, Balster, & Beardsley, 2006). However, to our knowledge, there is no published study that has examined the extent to which antagonism of nicotine varies as a function of chronic nicotine treatment. This study identified whether exposure to nicotine under conditions that produce tolerance but are unlikely to be sensitive to antagonist‐induced precipitated withdrawal (Damaj et al., 2003; de Moura & McMahon, 2016) disrupts the ability of the nAChR antagonists mecamylamine and DHβE to block the in vivo effects of nicotine. Mecamylamine was selected as a test drug because it is a nonselective nAChR antagonist which has been studied in human clinical studies as a potential smoking cessation aid, albeit with limited positive results (Crooks et al., 2014; Frishman et al., 2006; Lancaster & Stead, 2000). DHβE was selected as a test drug because the β2 subunit has been demonstrated to mediate the reinforcing effects of nicotine and nicotine‐induced dopamine release (Crooks et al., 2014; Picciotto et al., 1998; Salminen et al., 2007). Simple schedule‐controlled responding and hypothermia were selected for study because they have been previously used to examine the pharmacology of nicotine, and both are sensitive to the development of nicotine tolerance (Cunningham & McMahon, 2011; de Moura & McMahon, 2016; Rodriguez et al., 2014; Rosecrans et al., 1989).
2. MATERIALS AND METHODS
2.1. Subjects
Male C57BL/6J mice were purchased at 8 weeks of age (n = 8; Jackson Laboratories) and were housed individually in a temperature, controlled room (23°C), under a 14/10‐hr light/dark cycle. Mice were food restricted to 85% of their free‐feeding body weight, while water was available ad libitum. Food (Dustless Precision Pellets Grain‐Based Rodent Diet; Bio‐Serv) was available immediately following experimental sessions. Experimental conditions were in accordance with those set forth by the National Institute of Health's Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Research, 2011). The Institutional Animal Care and Use Committee of University of Texas Health San Antonio approved these experiments.
2.2. Apparatus
Operant conditioning chambers (ENV‐307A‐CT; Med Associates) were kept in sound‐attenuating and ventilated boxes. Each chamber contained a light and a recessed 2.2‐cm‐diameter hole on one wall through which reinforcers could be presented. On the opposite wall were three identical holes arranged horizontally, spaced 5.5 cm apart. The centers of each hole were 1.6 cm from the floor of the chamber. During experimental sessions, when the middle hole was illuminated, a mouse could disrupt a photobeam by a nose poke, resulting in the presentation of a 0.01 ml of 50% v/v unsweetened condensed milk/water through the hole on the opposite wall. A computer was connected to the operant conditioning chambers through an interface (MED‐SYS‐8; Med Associates), and experimental sessions were controlled, and responses were recorded, using Med‐PC software (Med Associates). Rectal temperature was recorded using a rectal probe designed for mice attached to a digital thermometer (BAT7001H; Physitemp). The probe length was 2 cm. The probe diameter was 0.7112 mm, except for the tip, which was 1.651 mm (RET‐2‐ISO; Physitemp). The probe was inserted 2 cm into the rectum.
2.3. Experimental procedure
2.3.1. Phase 1: before daily treatment
Eight mice were trained to lever‐press for 50% condensed milk solution over 35–50 sessions as previously described (de Moura & McMahon, 2016). Mice were shaped to respond in the middle hole during 60‐min sessions by plugging the inactive holes as well as via hand‐shaping. Seven of these mice successfully acquired the operant response under a fixed ratio (FR20) and continued throughout the remainder of the study. These seven mice were submitted to a series of dose–response assessments, wherein the effects of nicotine alone, mecamylamine alone, DHβE alone, and nicotine + 3.2 mg/kg DHβE were assessed on rectal temperature and operant response rate.
Nicotine (0.56–5.6 mg/kg, s.c.), mecamylamine (1–5.6 mg/kg, i.p.), DHβE (1–5.6 mg/kg, s.c.), or a combination of drugs was administered per drug test, and all mice received drugs on the same day. Dose‐effect functions for rate‐decreasing and hypothermic effects were generated in the following order: nicotine, mecamylamine, DHβE, and nicotine in the presence of DHβE. Doses were administered in a non‐systematic order among the mice so that no more than 2 mice received the same dose or dose combination for that particular test. After drug tests, saline was administered during intervening sessions. Immediately before and after every experimental session, rectal temperature was measured. The dose‐effect function for nicotine was re‐determined twice: once before studies with mecamylamine and DHβE, and once immediately afterward. After the second nicotine dose‐effect determination, approximately day 50 after mice entered the acute testing paradigm, mice underwent the daily nicotine exposure paradigm.
2.3.2. Phase 2: daily nicotine treatment
The mice were then exposed to a daily treatment regimen of nicotine for 100 days. The timeline in Figure 1 shows the daily sequence (i.e., injections, temperature measurement, and operant session). Daily, mice received three injections of 1.78 mg/kg nicotine. For the first 10 days of daily nicotine treatment, these injections were separated by 90 min, and operant sessions were conducted following the third nicotine dose (Figure 1a). Operant sessions were conducted 60 min after the second injection. Rectal temperatures were recorded 30 min after each injection (as well as preceding the operant session). Starting on day 11, the 25‐min operant session was conducted 1 hr after the second nicotine dose, 30 min prior to the third nicotine dose (Figure 1b). Dose‐effect data were collected between days 16 and 100 of chronic nicotine exposure. On days 43–59, there was a lack of personnel to conduct the operant sessions, and instead, nicotine was administered, but operant sessions were not conducted.
Figure 1.
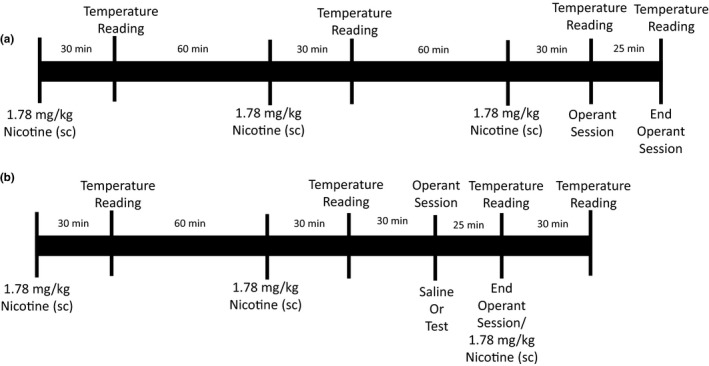
Schematic of experimental procedure during (a) days 1–10 and (b) days 11–100 of daily nicotine exposure. On days 1–10, 1.78 mg/kg nicotine was administered subcutaneously every 90 min, with a rectal temperature measurement 30 min after nicotine administration. Immediately following the third nicotine dose, the mice were placed in the operant chamber for a 25‐min experimental session; upon termination of the session, rectal temperature was measured again. On days 11–100, 1.78 mg/kg nicotine was administered every 90 min, and rectal temperature was measured 30 min after nicotine administration. However, operant sessions were conducted 60 min after the second nicotine dose and 30 min before the third nicotine dose. Operant sessions were conducted immediately following saline, or a dose of nicotine alone, or in combination with an antagonist. Only one test session was conducted per day. Rectal temperature was measured immediately upon termination of the operant session and was followed by the third injection of nicotine only if saline preceded the experimental session
2.3.3. Phase 3: after daily treatment
The mice were treated with saline instead of nicotine three times a day, but every 8th day received three injections of 1.0 mg/kg nicotine. This was used to assess recovery of response from daily nicotine exposure. Once sensitivity to the effects of 1 mg/kg nicotine was stable (i.e., not significantly different) for three consecutive tests, which occurred at approximately 30 days after daily nicotine discontinuation, dose‐effect functions for nicotine, nicotine in the presence of mecamylamine, and nicotine in the presence of DHβE were re‐determined in a similar fashion to Phase 1.
2.4. Drugs
The drugs were nicotine hydrogen tartrate salt (Sigma‐Aldrich), mecamylamine hydrochloride (Waterstone Technology, LLC), and dihydro‐β‐erythroidine hydrobromide (DHβE; Tocris Bioscience). Nicotine and DHβE were administered subcutaneously, while mecamylamine was administered intraperitoneally. DHβE and mecamylamine were administered 5 min prior to operant sessions. All drugs were administered in a volume of saline equal to 10 ml/kg; nicotine solutions were adjusted to pH of 7. Drug doses are expressed as the combined weight of base and salt, except for nicotine, which is expressed as the base weight.
2.5. Data analyses
Data were plotted as mean ± standard error of the mean and analyzed as responses per s and change in °C, except for the analysis of DHβE in combination with nicotine during chronic nicotine treatment. For that analysis, data were expressed as a percentage of the individual saline‐control response rate and as a change in °C from the individual saline‐control rectal temperature. Saline control was defined as the running average of the 5 previous sessions during which saline was administered. Saline controls determined before, during, and after discontinuation of chronic nicotine treatment were compared with a one‐way repeated‐measures ANOVA followed by Dunnett's test (p < .05). Response rate following the daily nicotine dose (1.78 mg/kg) was examined with a one‐way repeated‐measures ANOVA, with consecutive days of treatment as the main factor, followed by Dunnett's test. A two‐way repeated‐measures ANOVA followed by Tukey's multiple comparison tests was used to analyze hypothermic effects, with daily nicotine dose (i.e., first, second, and third) as one factor and consecutive days of treatment was a second factor.
Straight lines were fitted to nicotine dose‐effect data using linear regression (GraphPad Prism version 6.0 for Windows, GraphPad Software). The linear portion of the function was determined per mouse. For response rate, the function included the largest dose producing no more than a 20% decrease up to and including the smallest dose producing greater than an 80% decrease. For rectal temperature, the function included the largest dose resulting in less than a 1°C change up to and including the smallest dose producing greater than a 5°C change. When the mean effect of a drug to produce a reduction in schedule‐controlled responding or a decrease in body temperature was significantly greater than 50%, the ED50 values and potency ratios were calculated from individual nicotine dose‐effect functions using a common best fitting slope (Tallarida, 2000). The ED50 values were significantly different when the 95% confidence limits calculated from the individual potency ratios did not include 1. Because mecamylamine and DHβE alone, up to the largest doses studied, were less effective than nicotine in decreasing response rate and rectal temperature, dose‐effect data were analyzed separately for each antagonist with one‐way repeated‐measures ANOVAs followed by Dunnett's tests.
One‐way repeated‐measures ANOVAs followed by Dunnett's test was used to compare the individual nicotine ED50 values determined before, during, and after discontinuation of treatment. Student's t test was used to compare potency ratios (i.e., magnitude of antagonism by DHβE) before and after discontinuation of chronic nicotine treatment. During chronic treatment, only the smallest doses of nicotine (0.56 and 1 mg/kg) appeared to be altered by mecamylamine or DHβE relative to the nicotine control; these dose‐effect data were analyzed separately per antagonist with two‐way repeated‐measures ANOVAs, with nicotine dose being one factor and saline versus antagonist being a second factor. Dunnett's tests were used to compare the effects of nicotine to saline or nicotine in combination with an antagonist to the effects of the antagonist alone. Sidak's tests were used to compare the effects of each dose of nicotine in the presence versus the absence of antagonist. Student's t test was used to compare the effects of saline to a dose of antagonist.
3. RESULTS
The effects of saline administered at the beginning of operant sessions were compared at each phase of the study including before, during, and after discontinuation of daily nicotine treatment. Once stable responding was achieved, the respective averages (±SEM) in responses per s were 0.68 ± 0.10, 0.51 ± 0.07, and 0.59 ± 0.11. These response rates were not significantly different from one another (F 2,12 = 3.4, p = .09). The respective rectal temperatures measured after these sessions were 37.1 ± 0.07, 36.7 ± 0.04, and 36.5 ± 0.11°C. These temperatures were significantly different from one another (F 2,12 = 30.7, p = .0001); rectal temperature before daily nicotine exposure was significantly higher than rectal temperature during and after daily nicotine exposure.
The nicotine dose‐effect functions, one determined before tests with DHβE and the second just before chronic nicotine treatment, were not significantly different from each other for response rate (F 2,42 = 2.8, p = .071) and hypothermia (F 2,42 = 1.98, p = .15). Therefore, the nicotine dose‐effect functions were averaged to produce a single control for further analyses. Nicotine dose dependently decreased response rate and rectal temperature (Figure 2); after saline pretreatment, 1.78 mg/kg nicotine decreased response rate to 0.0004 responses per s and decreased rectal temperature to 31.1°C (Figure 2, circles). The ED50 values of nicotine to decrease response rate and rectal temperature are shown in Table 1. DHβE (3.2 mg/kg) alone resulted in an average response rate and rectal temperature of 0.80 ± 0.16 responses per s and 37.2 ± 0.07°C, which were not significantly different from saline (t 7 = 1.2, p = .25 and t 7 = 0.08, p = .94, respectively; Figure 2, leftmost squares). DHβE shifted the nicotine dose‐effect functions for decreasing response rate and rectal temperature 2.9 (2.2–3.9)‐fold and 3.2 (2.7–3.8)‐fold to the right, respectively (Figure 2, squares, Table 1).
Figure 2.
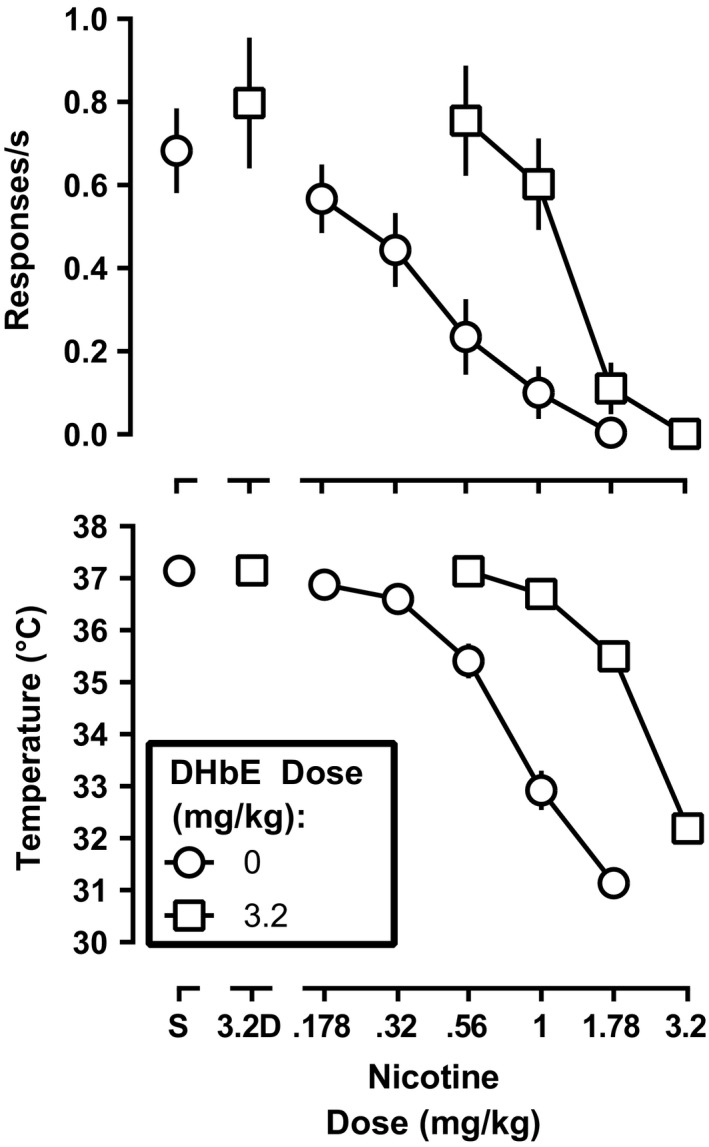
Rate of responding (top panel) and rectal temperature (bottom panel) following nicotine alone and in combination with 3.2 mg/kg DHβE (squares) prior to daily nicotine exposure. Ordinate: response rate expressed responses/s (top panel), and rectal temperature expressed as °C (bottom panel). Abscissa: nicotine dose in mg of the free base per kg body weight
Table 1.
The ED50 values calculated from dose–response data for nicotine alone and in combination with DHβE and mecamylamine before, during, and after daily nicotine, as shown in Figures 2, 5, and 6, respectively
| Treatment | ED50 value (95% confidence limits) in mg/kg | |
|---|---|---|
| Response rate | Body temperature | |
| Nicotine alone | ||
| Before | 0.44 (0.32–0.61) | 0.82 (0.72–0.94) |
| During | 1.60 (1.40–1.90) | 3.20 (2.70–3.80) |
| After | 0.74 (0.57–0.96) | 1.10 (0.93–1.20) |
| Pretreatment with DHβE | ||
| Before | 1.28 (0.70–2.38) | 2.62 (1.94–3.57) |
| During | 0.88 (0.58–1.33) | N.C. |
| After | 1.55 (0.86–2.69) | 2.86 (1.95–3.72) |
| Pretreatment with mecamylamine | ||
| Before | 2.41 (1.61–3.59)a | 4.1 (3.5–5.2)a |
| During | 0.84 (0.51–1.38) | N.C. |
| After | 1.41 (0.86–2.4) | 3.25 (2.54–4.16) |
On day 1 of daily nicotine treatment, response rate determined 10 min after the third dose (1.78 mg/kg) of nicotine was 0.44 responses per s, a decrease of 37% relative to the saline control determined before daily nicotine treatment (Figure 3, top). Responding after the last daily nicotine injection systematically decreased on subsequent days was lowest on day 4 and remained low (F 6,11 = 14, p = .0016) until the timing of sessions with nicotine injections was changed so that sessions were conducted 1 hr after the second daily dose of nicotine beginning on day 11 (Figure 1, compare top and bottom timelines). The first dose of nicotine on the first day of treatment decreased rectal temperature to 30°C. The second and third doses of nicotine also decreased rectal temperature (i.e., to 32 and 33°C, respectively), although the hypothermic effects of nicotine after the second and third nicotine daily doses were less than the first dose (Figure 3, bottom, day 1). Tolerance developed to the hypothermic effects produced by each dose of nicotine across days of repeated, daily nicotine treatment (F 21,126 = 54, p < .0001). Rate of responding was stable according to the established criterion on day 16 (Figure 3, top). During daily nicotine, the nicotine dose‐effect functions were significantly shifted to the right (Figure 4, black circles); the ED50 values for nicotine to produce rate‐decreasing and hypothermic effects are shown in Table 1. Daily nicotine exposure produced a 3.6 (2.6–5.0)‐ and 4.8 (3.9–5.9)‐fold rightward shift in the nicotine dose‐effect functions for rate‐decreasing and hypothermic effects, respectively.
Figure 3.
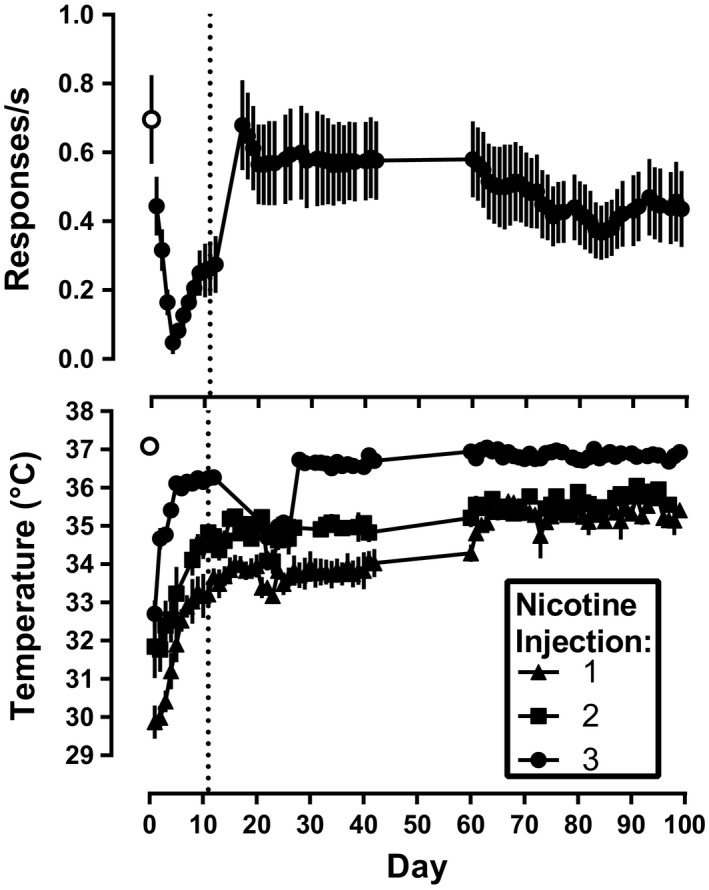
Response rate (top panel) and rectal temperature (bottom panel) during daily nicotine exposure with 3 doses of 1.78 mg/kg nicotine administered 90 min apart. For days 1–10, the third dose was administered at the beginning of operant conditioning sessions. From session 11 onward (vertical dashed line), operant conditioning sessions began 1 hr after the second nicotine dose. Temperature was measured 30 min after each dose throughout the experiment. Only the effects of 1.78 mg/kg nicotine are shown, that is, data from administration of other doses and drugs are omitted. Ordinate: response rate expressed responses/s (top panel), and rectal temperature expressed as °C (bottom panel). Abscissa: days of daily nicotine exposure
Figure 4.
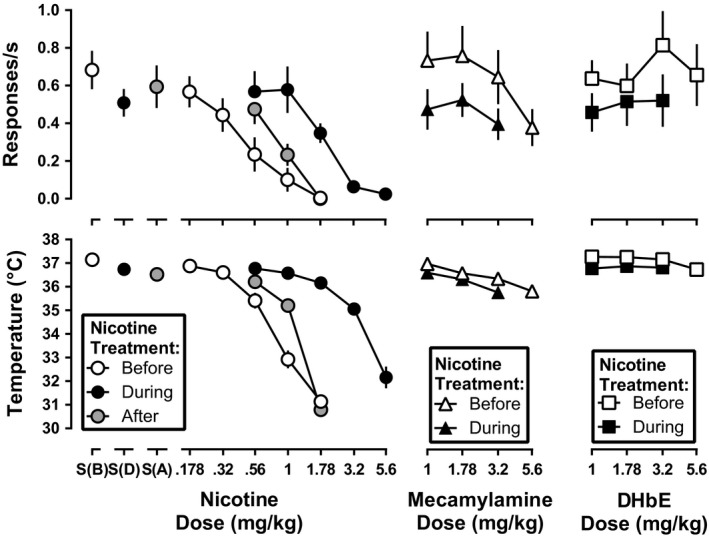
Rate of responding (top panels) and rectal temperature (bottom panels) following nicotine, mecamylamine, and DHβE before (open symbols) and during (filled symbols) daily nicotine exposure. The gray circles are the effects of nicotine after discontinuation of daily nicotine exposure. Ordinate: response rate expressed in responses/s (top panels), and rectal temperature expressed as °C (bottom panels). Abscissa: drug dose in mg/kg body weight
Mecamylamine, when studied alone and prior to daily nicotine exposure, significantly decreased response rate to 0.38 responses per s at a dose of 5.6 mg/kg (F 4,24 = 8.7, p = .0023; Figure 4, open triangles). Mecamylamine (3.2 and 5.6 mg/kg) significantly decreased rectal temperature as compared with saline control (Figure 4, open circle above S(B); F 4,24 = 25.5, p = .0002), with the largest decrease to 35.8°C. During daily nicotine exposure, mecamylamine no longer produced significant decreases in response rate relative to the corresponding saline control (F 3,18 = 0.27, p = .27; Figure 4, filled circle above S(D) and filled triangles), but did produce hypothermic effects at doses of 1.78 and 3.2 mg/kg as compared with the daily nicotine treatment saline control (Figure 4, filled circle above S(D); F 3,18 = 41.4, p < .0001). When normalized to their respective saline controls determined before and during nicotine treatment, the mecamylamine dose‐effect functions for decreasing response rate and rectal temperature were not significantly different (F 2,32 = 0.21, p = .21 and F 2,20 = 0.64, p = .54, respectively).
Dihydro‐β‐erythroidine, when studied prior to daily nicotine treatment, did not significantly decrease response rates (F 4,24 = 1.2, p = .33), but did produce a small yet significant decrease in rectal temperature at 5.6 mg/kg (F 4,24 = 5.7, p = .025; Figure 4, open squares). During daily nicotine exposure, DHβE (up to 3.2 mg/kg) did not significantly alter response rates (F 3,18 = 0.30, p = .69) or rectal temperature (F 3,18 = 0.65, p = .49; Figure 4, filled squares). When normalized to the respective saline controls, the dose‐effect functions of DHβE to decrease rectal temperature before versus during daily nicotine exposure were not significantly different from each other (F 2,24 = 1.8, p = .19).
During daily nicotine exposure, the dose‐effect function for nicotine to decrease response rate in the presence of 3.2 mg/kg mecamylamine was significantly different from the nicotine control dose‐effect function (main effect of nicotine dose, [F 5,34 = 26.5, p < .0001]; main effect of mecamylamine [F 1,6 = 13.6, p = .01]; effect of interaction, [F 5,34 = 2.9, p = .03]; Figure 5, top left). A Sidak's post hoc test demonstrated that response rate at only the 1 mg/kg dose of nicotine differed significantly in the presence versus the absence of 3.2 mg/kg mecamylamine (p < .05). Dunnett's multiple comparison test revealed that response rate after 1 mg/kg nicotine in combination with 3.2 mg/kg mecamylamine did not significantly differ from that after mecamylamine alone (p > .05). In contrast to response rate, 3.2 mg/kg mecamylamine significantly antagonized the hypothermic effects of nicotine (main effect of nicotine dose, [F 5,34 = 18.31, p < .0001]; main effect of mecamylamine [F 1,6 = 9.1, p = .003]; effect of interaction, [F 5,34 = 9.7, p < .001]). The dose‐effect functions for nicotine alone and nicotine in combination with 3.2 mg/kg DHβE were significantly different from each other for rate‐decreasing (main effect of nicotine dose, [F 5,30 = 23.7, p < .0001]; main effect of DHβE [F 1,6 = 15.6, p = .008]; effect of interaction [F 5,30 = 2.7, p = .04]) and hypothermic effects (main effect of nicotine dose, [F 5,30 = 26.82, p < .0001]; main effect of DHβE [F 1,6 = 23.7, p < .0001]; effect of interaction [F 5,30 = 5.4, p = .003]; Figure 5, right). Sidak's post hoc test indicated that the rate‐decreasing effects of 0.56 and 1 mg/kg nicotine were significantly different following pretreatment with 3.2 mg/kg DHβE as compared to pretreatment with saline (p < .05). However, Dunnett's post hoc test demonstrated that the rate‐decreasing effects of 0.56 and 1 mg/kg nicotine in the presence of 3.2 mg/kg DHβE were not significantly different from response rate after DHβE alone (p > .05).
Figure 5.
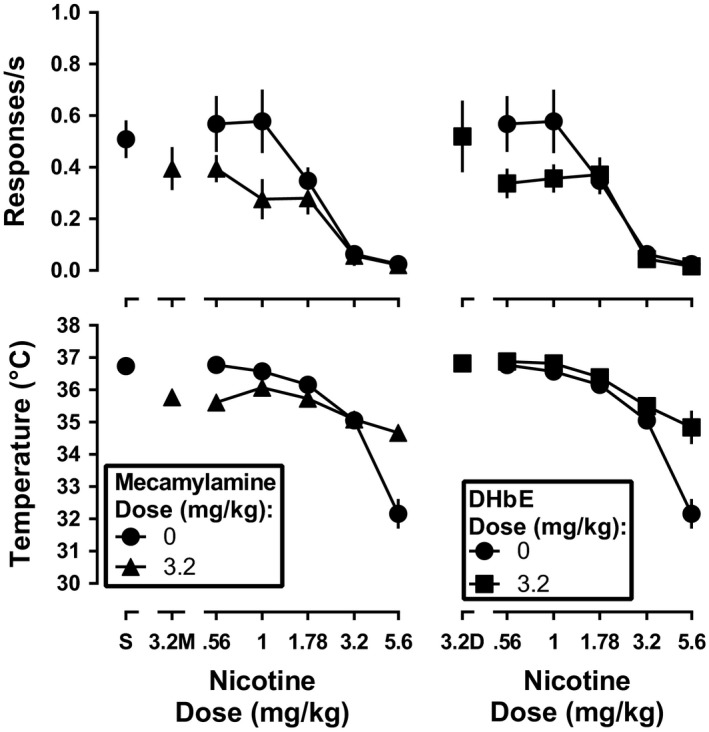
Response rate (top panels) and rectal temperature (bottom panels) following nicotine alone (filled circles) or in combination with 3.2 mg/kg mecamylamine (left panels; filled triangles) or DHβE (right panels; filled squares) during daily nicotine exposure. Ordinate: response rate expressed in responses/s (top panel), and rectal temperature expressed as °C (bottom panel). Abscissa: drug dose in mg/kg body weight
Following discontinuation of daily nicotine treatment, the effects of 1 mg/kg nicotine were determined every 8 days. The effects of 1 mg/kg nicotine on day 32 were not significantly different from the effects of nicotine on days 16 and 24 for both rate‐decreasing (F 2,12 = 0.72, p = .45) and hypothermic effects (F 2,12 = 0.59, p = .48). The nicotine dose‐effect function was re‐determined beginning on day 33 after discontinuation from daily nicotine treatment. The dose‐effect function for nicotine to produce rate‐decreasing (F 2,12 = 58.7, p < .0001) and hypothermic effects (F 2,12 = 39.5, p = .0007) significantly differed among the three conditions (i.e., before, during, and after discontinuation of nicotine treatment). The ED50 values for nicotine to produce rate‐decreasing and hypothermic effects following discontinuation of daily nicotine treatment are shown in Table 1 and depicted in Figure 4 (left panels, circles). Nicotine was 2.4 (1.6–3.4)‐ and 3.0 (2.4–3.6)‐fold more potent to produce rate‐decreasing and hypothermic effects, respectively, after discontinuation of daily nicotine, than during daily nicotine exposure. However, nicotine was 1.7 (1.2–2.4)‐ and 1.6 (1.3–1.8)‐fold more potent to produce rate‐deceasing and hypothermic effects, respectively, prior to daily nicotine exposure than after daily nicotine exposure.
After discontinuation of daily nicotine exposure, mecamylamine (1 mg/kg) significantly antagonized the rate‐decreasing (F 2,32 = 8.0, p = .0015) and hypothermic effects (F 2,34 = 54.3, p < .0001) of nicotine (Figure 6, left). Mecamylamine shifted the nicotine dose‐effect function for rate‐decreasing effects 1.9 (1.5–2.5)‐fold to the right (Table 1). Because of a significant difference in the slopes of the dose‐effect functions for producing hypothermia (F 1,34 = 19.3, p = .0001), a potency ratio for nicotine alone versus nicotine in combination with 1 mg/kg mecamylamine was not determined. DHβE antagonized the rate‐decreasing (F 2,34 = 7.4, p = .0021) and hypothermic effects of nicotine (F 2,32 = 32.4, p < .0001) following discontinuation of daily nicotine exposure (Figure 6, right). DHβE shifted the nicotine dose‐effect functions 2.1 (1.5–2.8)‐ and 2.6 (2.1–3.1)‐fold to the right for rate‐decreasing and hypothermic effects, respectively (Table 1). DHβE significantly differed in its antagonism of nicotine before daily nicotine exposure as compared with after discontinuation of daily nicotine exposure for both rate‐decreasing (F 2,12 = 20.1, p = .001) and hypothermic effects (F 2,12 = 46.8, p < .0001), with post hoc analysis indicating that antagonism of nicotine by DHβE was significantly less after discontinuation of daily nicotine exposure than before daily nicotine exposure.
Figure 6.
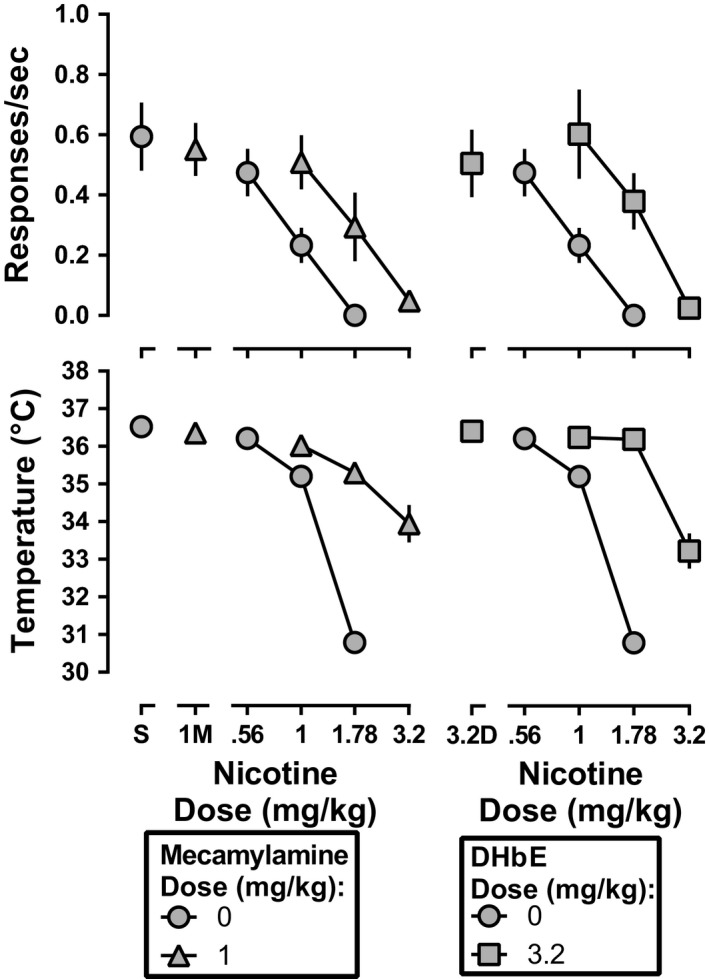
Response rate (top panels) and rectal temperature (bottom panels) following nicotine alone (gray circles) or in combination with 3.2 mg/kg mecamylamine (left panels; gray triangles) or DHβE (right panels; gray squares) after discontinuation of daily nicotine exposure. Ordinate: response rate expressed in responses/s (top panel), and rectal temperature expressed as °C (bottom panel). Abscissa: drug dose in mg/kg body weight. The control nicotine dose‐effect functions shown in the left and right panels are the same
4. DISCUSSION
Nicotine dose dependently decreased responding under a fixed ratio schedule of liquid food delivery and decreased rectal temperature before, during, and after daily nicotine exposure. Significant tolerance developed to these effects of nicotine during daily nicotine exposure as evidenced by a rightward shift in the nicotine dose‐effect functions. Before daily nicotine exposure, the effects of nicotine were antagonized by DHβE. The capacity of mecamylamine to antagonize nicotine prior to daily nicotine exposure was not studied because mecamylamine has been repeatedly demonstrated to antagonize the rate‐decreasing and hypothermic effects of nicotine under conditions identical to or similar with those here (Cunningham & McMahon, 2011; de Moura & McMahon, 2016; Rodriguez et al., 2014). During daily nicotine exposure, mecamylamine and DHβE no longer antagonized the rate‐decreasing effects of nicotine; however, the hypothermic effects of nicotine were still antagonized. Following discontinuation of daily nicotine exposure, both the rate‐decreasing and hypothermic effects of nicotine were antagonized by DHβE and mecamylamine. However, the degree to which DHβE shifted the nicotine dose‐effect functions rightward was significantly greater before daily nicotine exposure than after daily nicotine exposure. These results imply the following: The rate‐decreasing and hypothermic effects of nicotine are mediated by a different receptor types or mechanisms, the rate‐decreasing effects of nicotine in nicotine‐tolerant animals may be mediated by non‐nAChRs, and the antagonist actions of nAChR drugs are compromised in nicotine‐tolerant and perhaps nicotine‐dependent individuals (i.e., cigarette smokers).
As with many other drug classes (e.g., opioids), disruption of operant behavior has been used to examine precipitated withdrawal in dependent animals (Schulteis, Markou, Gold, Stinus, & Koob, 1994; Vann et al., 2006). Based on previous research demonstrating that 6 mg kg−1 day−1 of nicotine would not produce observable signs of withdrawal in C57BL/6J mice (Damaj et al., 2003), we predicted that our protocol would not increase sensitivity to the rate‐decreasing effects of mecamylamine and DHβE. As expected, there was no increased sensitivity to the rate‐decreasing and hypothermic effects of DHβE and mecamylamine, suggesting that the current regimen does not result in robust physical dependence.
Antagonism of nicotine by DHβE before and after chronic nicotine is consistent with the results of previous studies (de Moura & McMahon, 2016) and suggests that the rate‐decreasing and hypothermic effects of nicotine are mediated by β2‐containing nAChRs. Failure of DHβE and mecamylamine to antagonize the rate‐decreasing effects of nicotine during daily nicotine exposure suggests that in nicotine‐tolerant animals, the rate‐decreasing effects of nicotine are produced by actions at non‐nAChRs. In contrast, the hypothermic effects of nicotine continue to be mediated by β2 nAChRs as evidenced by antagonism of nicotine by both mecamylamine and DHβE. Because daily nicotine exposure decreases functional nAChRs (Giniatullin, Nistri, & Yakel, 2005; Quick & Lester, 2002), lack of antagonism of the rate‐decreasing effects of nicotine by mecamylamine and DHβE suggests that nicotine may act at other receptor types (e.g., non‐nAChR) to disrupt behavior. Differential antagonism of the rate‐decreasing and hypothermic effects of nicotine suggests that different receptors mediate these effects.
The current results are consistent with previous research, demonstrating that multiple nAChRs mediate the in vivo effects of nicotine (de Moura & McMahon, 2016; Stolerman, Chandler, Garcha, & Newton, 1997). For instance, the nAChR agonist cytisine will only partially substitute for nicotine in rats trained to discriminate 0.32 mg/kg nicotine; however, when the training dose of nicotine is increased to 1.78 mg/kg, cytisine will fully substitute for nicotine (Jutkiewicz, Brooks, Kynaston, Rice, & Woods, 2011). This result suggests that nAChR subtypes differentially mediate the discriminative stimulus effects of nicotine as a function of training dose, where low doses of nicotine may activate only a subset of the nAChR subtypes that are activated at higher doses. Furthermore, Stolerman et al. (1997) demonstrated that DHβE differentially antagonizes various behavioral effects of nicotine. Of particular note, DHβE antagonized the discriminative stimulus effects of nicotine, but not its rate‐decreasing effects (Stolerman et al., 1997). From these results, the authors concluded that nAChR subtypes differentially mediate the behavioral effects of nicotine. The failure of DHβE to antagonize the rate‐decreasing effects of nicotine in Stolerman et al. (1997) might suggest that tolerance had developed to the rate‐decreasing effects of nicotine over the course of discrimination training and testing, thereby decreasing the ability of DHβE to antagonize rate‐decreasing effects.
The finding that nicotine was more potent to decrease response rate and rectal temperature before as compared with approximately 5 weeks after discontinuation of daily nicotine treatment is consistent with previously published reports (Rosecrans et al., 1989). The loss of potency to the effects of nicotine on schedule‐controlled responding even after several weeks of discontinued daily nicotine treatment could reflect behavioral tolerance; however, because tolerance to nicotine under these treatment conditions is greater than cross‐tolerance to cytisine and cocaine, as reported previously (de Moura & McMahon, 2016), pharmacodynamic changes at the level of nAChRs (i.e., desensitization) appear to play an important role. Moreover, the persistent loss of potency to a physiological effect of nicotine (i.e., hypothermia) suggests that behavioral tolerance is insufficient to explain the results obtained with schedule‐controlled responding. The persistent loss of nicotine's potency as a consequence of daily nicotine treatment, coupled with the decreased effectiveness of DHβE as an antagonist, suggests that neuroadaptations in nAChR signaling persist long after chronic treatment. This result is consistent with studies that demonstrated changes in receptor expression levels following various amounts of exposure to nicotine (Buisson & Bertrand, 2001; Fenster et al., 1999; Nashmi et al., 2007; Olale et al., 1997). However, it is also possible that the development of tolerance to the response‐suppressing and hypothermic effects of nicotine via thrice daily injection in the present study could be attributed to an altered stress response, rather than a specific nicotine‐induced adaptation (Matta et al., 2007).
While there is interest in using nAChR antagonists to treat tobacco use in a manner similar to that underlying the use of the µ‐opioid antagonist naltrexone as a treatment for opioid use disorder, these results suggest that antagonists may be limited in their capacity to function as smoking cessation aids. It is unclear why mecamylamine and DHβE are no longer able to antagonize the rate‐decreasing effects of nicotine after daily nicotine exposure. It is possible that these effects of nicotine are mediated by non‐nAChRs in tolerant mice or that repeated nicotine exposure changes how mecamylamine and DHβE interact with the nAChR. However, these results suggest that multiple nAChRs mediate the various in vivo effects of nicotine, which is consistent with previously published reports (de Moura & McMahon, 2016; Stolerman et al., 1997). Future research should attempt to identify which receptors mediate the effects of nicotine during repeated nicotine exposure. Identification of these receptors may provide a blueprint in the development of more effective tobacco cessation pharmacotherapies.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTION
de Moura and McMahon participated in research design. de Moura conducted experiments. de Moura, Wilkerson, and McMahon performed data analysis. de Moura, Wilkerson, and McMahon wrote or contributed to the writing of the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Morgan Cocke and Julia Threadgill for their technical assistance and Dr. Brett Ginsburg for suggestions for data analyses. This work was supported by U.S. Public Health Service, National Institutes of Health, National Institute on Drug Abuse (R01‐DA25267) and (UG3‐DA48353).
de Moura FB, Wilkerson JL, McMahon LR. Unexpected loss of sensitivity to the nicotinic acetylcholine receptor antagonist activity of mecamylamine and dihydro‐β‐erythroidine in nicotine‐tolerant mice. Brain Behav. 2020;10:e01581 10.1002/brb3.1581
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.1581
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available at Open Science Framework at https://osf.io [https://doi.org/10.17605/OSF.IO/TNWMQ].
REFERENCES
- Banerjee, S. , Punzi, J. S. , Kreilick, K. , & Abood, L. G. (1990). [3H]mecamylamine binding to rate brain membranes. Studies with mecamylamine and nicotine analogues. Biochemical Pharmacology, 40, 2105–2110. [DOI] [PubMed] [Google Scholar]
- Brunzell, D. H. , McIntosh, J. M. , & Papke, R. L. (2014). Diverse strategies targeting α7 homomeric and α6β2* heteromeric nicotinic acetylcholine receptors for smoking cessation. Annals of the New York Academy of Sciences, 1327, 27–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson, B. , & Bertrand, D. (2001). Chronic exposure to nicotine upregulates the human α4β2 nicotinic acetylcholine receptor function. Journal of Neuroscience, 21, 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall, W. A. , Franklin, K. B. , Coen, K. M. , & Clarke, P. B. (1992). The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology, 107, 285–289. [DOI] [PubMed] [Google Scholar]
- Crooks, P. A. , Bardo, M. T. , & Dwoskin, L. P. (2014). Nicotinic receptor antagonists as treatments for nicotine abuse. Advances in Pharmacology, 69, 513–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, C. S. , & McMahon, L. R. (2011). The effects of nicotine, varenicline, and cytisine on schedule‐controlled responding in mice: Differences in α4β2 nicotinic receptor activation. European Journal of Pharmacology, 654, 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj, M. I. , Kao, W. , & Martin, B. R. (2003). Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. Journal of Pharmacology and Experimental Therapeutics, 307, 526–534. [DOI] [PubMed] [Google Scholar]
- de Moura, F. B. , & McMahon, L. R. (2016). Differential antagonism and tolerance/cross‐tolerance among nicotinic acetylcholine receptor agonists: Scheduled‐controlled responding and hypothermia in C57BL/6J mice. Behavioural Pharmacology, 27, 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg, T. , Griffiths, R. R. , & Stitzer, M. L. (1996). Mecamylamine does not precipitate withdrawal in cigarette smokers. Psychopharmacology, 127, 328–336. [DOI] [PubMed] [Google Scholar]
- Fenster, C. P. , Whitworth, T. L. , Sheffield, E. B. , Quick, M. W. , & Lester, R. A. J. (1999). Upregulation of α4β2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. Journal of Neuroscience, 19, 4804–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frishman, W. H. , Mitta, W. , Kupersmith, A. , & Ky, T. (2006). Nicotine and non‐nicotine smoking cessation pharmacotherapies. Cardiology in Review, 14, 57–73. [DOI] [PubMed] [Google Scholar]
- Giniatullin, R. , Nistri, R. , & Yakel, J. L. (2005). Desensitization of nicotinic Ach receptors: Shaping cholinergic signaling. Trends in Neurosciences, 28, 371–378. [DOI] [PubMed] [Google Scholar]
- Hays, J. T. , Ebbert, J. O. , & Sood, A. (2008). Efficacy and safety of varenicline for smoking cessation. American Journal of Medicine, 121, S32–S42. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Research (2011). Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press. [Google Scholar]
- Jutkiewicz, E. M. , Brooks, E. A. , Kynaston, A. D. , Rice, K. C. , & Woods, J. H. (2011). Patterns of nicotinic receptor antagonism: Nicotine discrimination studies. Journal of Pharmacology and Experimental Therapeutics, 339, 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster, T. , & Stead, L. F. (2000). Mecamylamine (a nicotine antagonist) for smoking cessation. Cochrane Database Systematic Review, 2, CD001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta, S. G. , Balfour, D. J. , Benowitz, N. L. , Boyd, R. T. , Buccafusco, J. J. , Caggiula, A. R. , … Zirger, J. M. (2007). Guidelines on nicotine dose selection for in vivo research. Psychopharmacology, 190, 269–319. 10.1007/s00213-006-0441-0 [DOI] [PubMed] [Google Scholar]
- Mooney, M. E. , Schmitz, J. M. , Allen, S. , Grabowski, J. , Pentel, P. , Oliver, A. , & Hatsukami, D. K. (2016). Bupropion and naltrexone for smoking cessation: A double‐blind randomized placebo‐controlled clinical trial. Clinical Pharmacology and Therapeutics, 100, 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi, R. , Xiao, C. , Deshpande, P. , McKinney, S. , Grady, S. R. , Whiteaker, P. , … Lester, H. A. (2007). Chronic nicotine cell specifically upregulates functional α4* nicotinic receptors: Basis for both tolerance in midbrain and enhanced long‐term potentiation in perforant path. Journal of Neuroscience, 27, 8202–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisell, M. , Nomikos, G. G. , & Svensson, T. H. (1994). Systemic nicotine‐induced dopamine release in the rate nucleus accumbens in regulated by nicotinic receptors in the ventral tegmental area. Synapse, 16, 36–44. [DOI] [PubMed] [Google Scholar]
- Olale, F. , Gerzanich, V. , Kuryatov, A. , Wang, F. , & Lindstrom, J. (1997). Chronic nicotine exposure differentially affects the function of human α3, α4, and α7 neuronal nicotinic receptor subtypes. Journal of Pharmacology and Experimental Therapeutics, 283, 675–683. [PubMed] [Google Scholar]
- Papke, R. L. , Dwoskin, L. P. , Crooks, P. A. , Zheng, G. , Zhang, Z. , McIntosh, J. M. , & Stokes, C. (2008). Extending the analysis of nicotinic receptor antagonists with the study of alpha6 nicotinic receptor subunit chimeras. Neuropharmacology, 54, 1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto, M. R. , Zoli, M. , Rimondini, R. , Léna, C. , Marubio, L. M. , Pich, E. M. , … Changeux, J. P. (1998). Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature, 391, 173–177. [DOI] [PubMed] [Google Scholar]
- Quick, M. W. , & Lester, R. A. J. (2002). Desensitization of neuronal nicotinic receptors. Developmental Neurobiology, 53, 457–478. [DOI] [PubMed] [Google Scholar]
- Rodriguez, J. S. , Cunningham, C. S. , Moura, F. B. , Ondachi, P. , Carroll, F. I. , & McMahon, L. R. (2014). Discriminative stimulus and hypothermic effects of some derivatives of the nAChR agonist epibatidine in mice. Psychopharmcology (Berl), 231, 4455–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, J. E. , Behm, F. M. , Westman, E. C. , Levin, E. D. , Stein, R. M. , & Ripka, G. V. (1994). Mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone. Clinical Pharmacology and Therapeutics, 56, 86–99. [DOI] [PubMed] [Google Scholar]
- Rosecrans, J. A. , Stimler, C. A. , Hendry, J. S. , & Meltzer, L. E. T. (1989). Nicotine‐induced tolerance and dependence in rats and mice: Studies involving schedule‐controlled behavior. Brain Research, 79, 239–248. [DOI] [PubMed] [Google Scholar]
- Salminen, O. , Drapeau, J. A. , McIntosh, J. M. , Collins, A. C. , Marks, M. J. , & Grady, S. R. (2007). Pharmacology of alpha-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol, 71, 1563–1571. [DOI] [PubMed] [Google Scholar]
- Schulteis, G. , Markou, A. , Gold, L. H. , Stinus, L. , & Koob, G. F. (1994). Relative sensitivity to naloxone of multiple indices of opiate withdrawal: A quantitative dose‐response analysis. Journal of Pharmacology and Experimental Therapeutics, 271, 1391–1398. [PubMed] [Google Scholar]
- Stolerman, I. P. , Chandler, C. J. , Garcha, H. S. , & Newton, J. M. (1997). Selective antagonism of behavioural effects of nicotine by dihydro‐β‐erythroidine in rats. Psychopharmacology, 129, 390–397. [DOI] [PubMed] [Google Scholar]
- Tallarida, R. J. (2000). Drug synergism and dose‐effect data analysis. Boca Raton, FL: Chapman Hall/CRC Press. [Google Scholar]
- Vann, R. E. , Balster, R. L. , & Beardsley, P. M. (2006). Dose, duration, and pattern of nicotine administration as determinants of behavioral dependence in rats. Psychopharmacology, 184, 482–493. [DOI] [PubMed] [Google Scholar]
- Vogeler, T. , McClain, C. , & Evoy, K. E. (2016). Combination bupropion SR and varenicline for smoking cessation: A systematic review. American Journal of Drug and Alcohol Abuse, 42, 129–139. [DOI] [PubMed] [Google Scholar]
- Windle, S. B. , Filion, K. B. , Mancini, J. G. , Adye‐White, L. , Joseph, L. , Gore, G. C. , … Eisenberg, M. J. (2016). Combination therapies for smoking cessation: A hierarchical Bayesian meta‐analysis. American Journal of Preventive Medicine, 51, 1060–1071. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2011). WHO report on the global tobacco epidemic, 2011: Warning about the dangers of tobacco. Geneva, Switzerland: World Health Organization. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available at Open Science Framework at https://osf.io [https://doi.org/10.17605/OSF.IO/TNWMQ].


