Abstract
The use of weekly chemotherapy for the treatment of patients with advanced-stage serous-type epithelial Tubo-ovarian cancer (ETOC), and primary peritoneal serous carcinoma (PPSC) is acceptable as the front-line postoperative chemotherapy after primary cytoreductive surgery (PCS). The main component of dose-dense chemotherapy is weekly paclitaxel (80 mg/m2), but it would be interesting to know what is the difference between combination of triweekly cisplatin (20 mg/m2) or triweekly carboplatin (carboplatin area under the curve 5-7 mg/mL per min [AUC 5-7]) in the dose-dense paclitaxel regimen. Therefore, we compared the outcomes of women with Gynecology and Obstetrics (FIGO) stage IIIC ETOC and PPSC treated with PCS and a subsequent combination of dose-dense weekly paclitaxel and triweekly cisplatin (paclitaxel–cisplatin) or triweekly carboplatin using AUC 5 (paclitaxel–carboplatin). Between January 2010 and December 2016, 40 women with International Federation of Gynecology and Obstetrics (FIGO) stage IIIC EOC, FTC, or PPSC were enrolled, including 18 treated with paclitaxel–cisplatin and the remaining 22 treated with paclitaxel–carboplatin. There were no statistically significant differences in disease characteristics of patients between two groups. Outcomes in paclitaxel–cisplatin group seemed to be little better than those in paclitaxel–carboplatin (median progression-free survival [PFS] 30 versus 25 months as well as median overall survival [OS] 58.5 versus 55.0 months); however, neither reached a statistically significant difference. In terms of adverse events (AEs), patients in paclitaxel–carboplatin group had more AEs, with a higher risk of neutropenia and grade 3/4 neutropenia, and the need for a longer period to complete the front-line chemotherapy, and the latter was associated with worse outcome for patients. We found that a period between the first-time chemotherapy to the last dose (6 cycles) of chemotherapy >21 weeks was associated with a worse prognosis in patients compared to that ≤21 weeks, with hazard ratio (HR) of 81.24 for PFS and 9.57 for OS. As predicted, suboptimal debulking surgery (>1 cm) also contributed to a worse outcome than optimal debulking surgery (≤1 cm) with HR of 14.38 for PFS and 11.83 for OS. Based on the aforementioned findings, both regimens were feasible and effective, but maximal efforts should be made to achieve optimal debulking surgery and following the on-schedule administration of dose-dense weekly paclitaxel plus triweekly platinum compounds. Randomized trials validating the findings are warranted.
Keywords: dose-dense weekly paclitaxel, epithelial tubo-ovarian cancer, FIGO stage IIIC, primary peritoneal serous carcinoma, triweekly carboplatin, triweekly cisplatin
1. Introduction
Epithelial tubo-ovarian cancer (ETOC) is the deadliest cancer among women, placing with fourth place of all the fetal diseases among women and ranking seventh of most common cancers in women’s cancer and women’s cancer-related deaths in Taiwan and globally in 2018 [1,2,3,4,5,6]. Serous-type is the most common subtype among EOCs in general; however, endometriosis-associated EOCs, such as clear cell type or endometrioid type are relatively common in certain populations, including Taiwan and Japan [7,8,9,10,11]. Clinically, the serous-type EOC and primary peritoneal serous carcinoma (PPSC) and primary Fallopian tube cancer (PFTC) are often considered the same group disease, based on the similar clinical behavior and possibly sharing the similar pathogenesis [12,13,14,15,16,17,18]. In 1996, McGuire and colleagues conducted a clinical trial to set up the standard therapy for patients with ETOC, including primary debulking surgery (PDS), also called primary cytoreductive surgery (PCS) plus adjuvant triweekly paclitaxel and cisplatin therapy [19]. The following studies have confirmed the superiority of survival benefits using paclitaxel into cisplatin-based regimens in place of cyclophosphamide for the treatment of patients with ETOC and PPSC [20,21]. Additionally, carboplatin in place of cisplatin in this combination has statistically significantly reduced the cisplatin-related potential toxicity of neural and renal systems and ameliorated the cisplatin-related high emetic effects [22,23,24,25].
1.1. Current Standard of Treatment
According to the National Comprehensive Cancer Network (NCCN) guideless version 1.2020 Ovarian Cancer/Fallopian Tube Cancer/Primary Perineal Cancer, principles of systemic therapy regimens for advanced-stage high-grade serous ETOC and PPSC included three parts [26]. Preferred regimens [26] include (1) the combination of intravenous (IV) paclitaxel 175 mg/m2 and IV carboplatin area under the curve ranging from 5 mg/mL per min (AUC 5) to 6 mg/mL per min (AUC 6) every three weeks (Q3W, also called triweekly) for 3-6 cycles [22,23,24,25]; (2) IV paclitaxel 175 mg/m2 day 1 followed by IV carboplatin AUC 6 Day 1 and bevacizumab 7.5 mg/kg Day 1 of cycle 1 Q3W for 5-6 cycles plus additional 12 cycles of bevacizumab Q3W for maintenance therapy; (3) IV paclitaxel 175 mg/m2 followed by IV carboplatin AUC 6 Day 1 Q3W for 6 cycles with 15 mg/kg bevacizumab added starting on Day 1 of cycle 2, Q3W for up to a total of 22 cycles [27,28,29,30,31].
Other recommended regimens [26] include the following four regimens, such as (1) IV paclitaxel 60 mg/m2 followed by IV carboplatin AUC 2 every week (weekly) [32,33], (2) IV docetaxel 60-75 mg/m2 and IV carboplatin AUC 5-6 Q3W [34,35], (3) IV carboplatin AUC 5 and IV pegylated liposomal doxorubicin 30 mg/m2 every four weeks (Q4W) [36,37,38], and (4) IV paclitaxel 80 mg/m2 weekly and IV carboplatin AUC 5 to AUC 7 triweekly [39,40,41,42].
The final part—which is especially useful in certain circumstances, such as in patients having optimally PCS stage II-IV diseases [26]—is the combination of IV paclitaxel 135 mg/m2 on Day 1, intraperitoneal (IP) cisplatin 75-100 mg/m2 on Day 2, and IV paclitaxel 60 mg/m2 on Day 8 Q3W for 6 cycles [43,44]. Although there are so many recommendations available as shown above, now, the most frequently acceptable standard chemotherapy for ETOC and PPSC is still the use of combination of triweekly carboplatin and paclitaxel [37,45,46,47,48,49,50,51].
However, the prognosis of patients with ETOC and PPSC is still disappointing, because of vague or free symptoms of patients, often misdiagnosed as less deadly gastro-enteral tract problems, and the absence of effective screening programs, and, in addition, its heterogeneous nature and different clinical development, accounting for late diagnosis in its advanced stages [52,53,54,55]. All result in a therapeutic challenge, contributing to low cure rate and high mortality rate, [1,2,3,4,5,19,20,21,22,23,24,25]. A median progression-free survival (PFS) is 16–21 months and an overall survival (OS) is 32–57 months [1,2,3,4,5,19,20,21,22,23,24,25]. Therefore, an improvement of therapeutic effect as well as a prolongation of PFS and/or OS is urgently needed.
Based on the relative worse prognosis of advanced-stage ETOC and PPSC, many new modalities and strategies have been recently developed [56,57,58,59]. Although some of them have been already recommended by updated NCCN guidelines for ETOC and PPSC treatment [4,26], they are not widely used in the routine clinical practice [60]. Among these, induction chemotherapy (neoadjuvant chemotherapy- NACT) using either standard form of paclitaxel and carboplatin or its modification form, including dose-dense or high-dose chemotherapy, and in additional adding bevacizumab, has become more and more popular, especially for those patients who are not candidates for immediate PCS [61,62,63,64,65,66,67,68,69].
In addition, the changed delivery route or warm-up of chemotherapy agents (IP administration or IP hyperthermia therapy) has been also accepted in the certain clinical situations [43,44,70,71,72,73,74,75]. Moreover, advancing drug development, including poly(adenosine diphosphate (ADP)-ribose) polymerase inhibitors (PARPi) as well as small molecules targeting various kinds of signaling pathway has shown the dramatic improvement in ETOC and PPSC treatment [76,77,78,79,80,81,82,83,84,85,86,87]. Finally, immune checkpoint inhibitors or immune system modulators has also provided a chance for ETOC and PPSC patients, although the therapeutic effect is debated [88,89]. However, there is no doubt that the high cost of the aforementioned new therapeutic approaches will limit the clinical use in routine [90], and in addition, the compliance may be compromised by the requirement of maintenance or continuous long-term therapy in some drugs.
1.2. Gap in Knowledge of Current Standard of Therapy
Based on the negative impact on the high cost of many targeted therapies and the poor compliance of long-term maintenance therapy in certain population, as well as no differences or the improvement of clinical outcomes in additional drugs added to platinum/taxane combination or the use of different platinum doublets [91], dose-dense platinum/taxane might be a good alternative, due to the similar duration of completing therapy and acceptable cost expense. In addition, some evidence also favored this regimen as a feasible front-line chemotherapy for patients after PCS, based on the possible PFS benefits and considerably robust cost-effectiveness [92,93].
In theory, the maximal tolerated doses of chemotherapy may yield the highest cytotoxicity to tumors, with subsequent higher cure rates [94]. However, such treatment may need a longer treatment-free period to wait normal host cells for recovery [94]. Among these, hematopoietic progenitors might be the best example [94]. Without this treatment-free period, some catastrophic and life-threatened conditions might occur. During the holiday of drug, cancer cells and cells in the tumor microenvironment may also re-grow, possibly resultant development of aggressive behavior and chemo-resistance clone of tumor cells. All decided a rule of thumb to design most chemotherapy combinations to be repeated every three or four weeks [95]. The triweekly carboplatin plus paclitaxel regimen is just followed by the aforementioned rule, and this protocol is still one of the best-known and golden-standard regimens in the management of EOC patients after PCS.
However, this “golden-standard” therapy only provides the relatively short PFS and unsatisfactory OS in these advanced-stage EOC patients, making the urgent needs of application of new strategies to improve therapeutic outcome and prolong the patients’ life. Since the use of 3-drug combinations and maintenance chemotherapy after the front-line chemotherapy is not recommended because of the absence of survival benefits but significantly increased adverse events (AEs) and possibly compromised quality of life [50,51,59], another strategy without the involving adding any new agents or targeted therapy is pursued [45]. Therefore, if single dose of treatment is decreased in their dosage, the holiday of drugs can be shortened. A dose-dense weekly prescription of drugs may fulfill the aforementioned requirement.
1.3. Rationale of Dose-Dense Therapy
Dose-dense weekly chemotherapy is based on the concept of shortening the timing of recycling by Goldie-Coldman’s hypothesis and the Norton–Simon model [96,97,98], providing the rationale, such as a reducing interval between treatment, an increasing duration of chemotherapy exposure, and an increasing dose-intensity or dose-density of cytotoxic agents, and offering a chance that tumor regrowth could be decreased by this approach [99,100,101,102]. In addition, dose-dense chemotherapy can not only preserve the immune system but also promote the treatment-mediated tumor-specific immunity, especially the antitumor kill cluster of differentiation 8+ (CD8+) T-cell response [103]. Therefore, hematological markers have been evaluated to try to establish their predictive value [104,105,106,107]. Dose intensity strategies include increasing the dose per cycle, decreasing the cycle interval (dose densification) and decreasing the interval plus increasing the dose [108]. However, the latter two strategies involved shortening the gap between chemotherapy, which has proven decisive in some neoplasms such as breast cancer [99], and, of most importance, this more frequent administration of cytotoxic agents within short intervals has also decreased the chemotherapy-related death associated with the use of massive doses, such as infection or sepsis caused by severe neutropenia or spontaneous hemorrhage caused by severe thrombocytopenia [49].
Dr. Muggia proposed a very interesting hypothesis to show the potential benefits of dose-dense sequential treatment designs for first line, such as an initial dose-dense cisplatin and following dose-dense paclitaxel based on unpublished data from GOG-132 and ICON3 (Gynecologic Cancer InterGroup (GCIG) International Collaboration on Ovarian Neoplasms 3) in 2003 [109]. In the next year (2004), the same author found that increasing the dose of cisplatin above a certain threshold is not recommended in EOC patients because the greater toxicity with higher dose of cisplatin was found [110], although some studies did not support a significant increase toxicity after weekly dose-dense cisplatin treatment (median dose intensity with 32 or 45 mg/m2/week) [108]. However, the therapeutic effect of dose-dense platinum is still controversial. Some are in favor [111,112], but many are against the resultant benefits of dose-dense platinum [108,113,114,115,116]. Biweekly dose-dense carboplatin (AUC5) combined with paclitaxel (175 mg/m2) is not feasible based on dose-limiting toxicity, even though GCSF was used for support [116]. Previous studies that the main determinant of dose-dense treatment response was not achieved by the relative dose intensity of platinum itself [108]. Indeed, one report showed that the administration of dose up to cisplatin of 25 mg/m2/week might reach the plateau of dose-response curve [113].
By contrast, the toxicity of dose-dense taxane seemed to be well tolerated because of absence of relevant toxicity [94]. A pharmacokinetic study revealed that paclitaxel can be administered weekly at doses of 110 mg/m2 without interruption, while neutropenia precluded scheduled administration of doses ≥ 130 mg/m2 [94,117]. In theory, weekly dose-dense paclitaxel provided the better cytotoxicity to tumors, through more sustained exposure, limiting the emergence of tumors resistant to chemotherapy, enhancing the apoptotic and antiangiogenic effect, and improving the therapeutic index [94,118,119,120], although the single use of dose-dense paclitaxel for the first-line chemotherapy treatment of patients with ETOC seemed to be not approved by clinical trials [109,110].
Therefore, the application of a combination of taxane and platinum in ETOC has been conducted. In the management of all chemosensitive epithelial cancers, combination chemotherapy treatment has provided significant survival benefits compared to single agent chemotherapy when applied as initial therapy, and there is no doubt that the treatment of ETOC is similar in this regard [121]. Studies showed that combination of taxane and platinum compound appears both to attenuate the toxicity of the platinum compound and to facilitate the delivery of full dose on schedule and this full dose on schedule showed the strong correlation with patient outcomes [110]. Since both platinum and paclitaxel are the essential cytotoxic agents in ETOC patients after PCS, either any one or both can be administered as dose-dense protocol. Furthermore, dose-dense can be classified according to the types, including semiweekly dose-dense, in which paclitaxel was given weekly and carboplatin was given triweekly, and weekly dose dense, in which both paclitaxel and carboplatin were given weekly [47]. An accumulation dose of paclitaxel is 240–270 mg/m2 with separating into 80-90 mg/m2 per week compared to a single use of 175–180 mg/m2 every three weeks. By contrast, the dosage of carboplatin seems to be consistent with accumulation dose as AUC 5-7, regardless of administration triweekly (a single use of AUC 5-7) or weekly (AUC 2).
1.4. Previous Studies for Dose-Dense Therapy
Since the rational of dose-dense therapy supports the potential benefits for patients with advanced stage ETOC and PPSC, at least four large randomized clinical trials were conducted to test the hypothesis [32,33,39,40,41]. The Japanese Gynecologic Oncology Group (JGOG) 3016, named as JGOG 3016, provided a strong evidence to show the survival benefits in the combination regimen including dose-dense weekly paclitaxel (80 mg/m2) and triweekly carboplatin (AUC 6), which not only offered a longer PFS but also a significantly prolonged OS compared to those in a standard triweekly combination of paclitaxel and carboplatin treatment [32,33].
By contrast, this promising result was not reproducible in three other trials conducted in Western countries. The results of an NRG Oncology (the National Cancer Institute Cooperative Group Program plus the Radiation Therapy Oncology plus Gynecology Oncology Group)/GOG Study showed the similar PFS and OS in dose-dense regimen with adding 15 mg/kg bevacizumab treatment compared to the standard triweekly combination therapy [72]. MITO-7 (Multicentre Italian Trials in Ovarian cancer 7) showed that there was no statistically significant difference of median PFS between dose-dense weekly combination and standard triweekly combination therapy [32]. GOG-0262 showed no statistical difference of the median PFS between dose-dense weekly paclitaxel and triweekly carboplatin with/without adding 15 mg/kg bevacizumab treatment, respectively, and standard triweekly combination therapy [75]. The results from an ICON8 showed that the restricted mean PFS was not statistically significant different among the three groups (dose-dense or standard groups) [33].
A recent meta-analysis conducted by Marchetti and colleagues further updated these four randomized controlled trials containing 3698 patients, and found that dose-dense chemotherapy did not have a statistically significant benefit of PFS (HR 0.92, 95% CI 0.81-1.04) [47]. Additionally, the results were not changed with HR 1.01 (95% CI 0.93-1.10) and 0.82 (95% CI 0.63-1.08), respectively, even though the analysis was limited to both weekly and semi-weekly dose-dense data [47]. Therefore, the authors believed that conventional triweekly combination chemotherapy is still one of the golden-standard treatments of patients with advanced stage ETOC [47].
Why are the survival benefits only apparent in certain populations, such as Japanese or others? Although the real reasons associated with conflicted results are uncertain, genomic background of race, tumor heterogeneity, administration schedule, cisplatin or carboplatin, and the duration to complete postoperative adjuvant chemotherapy may all be attributable. Among these, we believe that platinum compound may play a certain role in the discrepancy.
In order to evaluate the effects and safety of dose-dense of paclitaxel combining with either cisplatin or carboplatin, the following retrospective study was conducted. Patients with FIGO IIIC serous-type ETOC and PPSC treated either with triweekly cisplatin (20 mg/m2) plus dose-dense weekly paclitaxel (80 mg/m2) or triweekly carboplatin (AUC 5) plus dose-dense weekly paclitaxel (80 mg/m2) as postoperative adjuvant chemotherapy after PCS were retrospectively reviewed. However, nearly all dose-dense paclitaxel-platinum combination chemotherapy regimens used as the front-line postoperative adjuvant therapy are focused on the dose-dense paclitaxel, without consideration of either the platinum compounds or the dose-dense agents.
2. Materials and Methods
2.1. Patient Population
After institutional review board approval (VGHIRB 2019-07-039BC), all patients between January 2010 and December 2016 who fulfilled the following inclusion (International Federation of Gynecology and Obstetrics (FIGO) stage IIIC histologically confirmed high-grade serous-type ETOC and PPSC, an initial PCS, a total of six cycles of weekly paclitaxel [80 mg/m2] plus either triweekly cisplatin [20 mg/m2] or triweekly carboplatin [AUC 5] regimen) and exclusion (NACT, other newly diagnosed cancer, previous chemotherapy, or radiotherapy in the past two years; incomplete chemotherapy or the delayed of the first course chemotherapy (>7 days after PCS), simultaneous use of other antineoplastic agents, antiangiogenic agents, or targeted therapy) criteria were identified from our gynecologic oncology registry. Since both cisplatin and carboplatin are available in our department, the selection of cisplatin or carboplatin was based on the patient’s will after shared decision making.
2.2. Treatment
All patients underwent PCS initially and then were treated with weekly paclitaxel (80 mg/m2) plus either triweekly cisplatin (20 mg/m2) or triweekly carboplatin (AUC 5). Paclitaxel was administered over 2 h intravenously on days 1, 8, and 15, and either cisplatin for 1 h or carboplatin for 1 h on day 1 was infused intravenously. Premedication including dexamethasone (20 mg), 2000 ml of normal saline, and palonosetron (250 ug) was prescribed intravenously during the chemotherapy therapy day. When grade 3/4 neutropenia occurred, the patients were treated with granulocyte colony-stimulating factor (GCSF) for three days before chemotherapy. However, treatment was delayed if febrile neutropenia or grade 3/4 neutropenia without correction was noted. If a three-fold increase in liver enzymes of patients was detected, paclitaxel (60 mg/m2) was used. The cisplatin dose was decreased to half dosage if the estimated glomerular filtration rate (eGFR) was decreased to 45–60 ml/min, which was calculated using the Cockcroft–Gault formula [122,123,124].
2.3. Assessments
The first course chemotherapy was prescribed within one week after PCS. The clinical symptoms or signs—including the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 5.0Adverse effects (AEs)—were used to evaluate the severity of adverse events before every cycle of treatment [125,126]. During the follow-up, the patients received both image evaluation, such as ultrasound, computed tomography, or magnetic resonance imaging and biochemical markers, such as CA-125 (the abbreviation of cancer antigen 125, carcinoma antigen 125, or carbohydrate antigen 125) examinations. The evaluation of image finding was based on Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 [127,128,129]. Criteria of progression or recurrence were based on findings of clinical symptoms or signs accompanied with image findings and tumor markers described elsewhere [41,130,131].
2.4. Statistical Analysis
The primary endpoint was PFS, defined as the time from the date patients first underwent PCS to the earliest date of disease progression, death from any cause, or the date of the last known follow-up. The secondary endpoint was OS, defined as the time from the date patients first underwent PCS to the date of death from any cause or the date of the last known follow-up. The Kaplan–Meier method was used to generate survival curves, and the log-rank test was used to detect the differences between survival curves. Prognostic factors for PFS or OS were evaluated using Cox proportional hazard methods. Multivariate analysis using Cox stepwise forward regression was conducted for the covariates selected in univariate analysis. A p value <0.05 was considered to be statistically significant. All statistical analyses were conducted with SAS version 9.3 (SAS Institute, Cary, NC) and Stata Statistical Software, version 12.0 (Stata Corporation, College Station, TX).
3. Results
3.1. Clinical Characteristics and Pathological Status
A total of 40 women with International Federation of Gynecology and Obstetrics (FIGO) stage IIIC ETOC or PPSC were analyzed, including 18 treated with paclitaxel–cisplatin and the remaining 22 treated with paclitaxel–carboplatin. Table 1 summarizes the characteristics of patients in each group. The mean age of the whole population was 59 years. Optimal PCS was achieved in 55% in overall, and 54.5% and 55.6% in the paclitaxel–carboplatin and paclitaxel–cisplatin groups, respectively. Patients in the paclitaxel–carboplatin group had a higher risk of a prolonged time to finish 6 cycles of the front-line chemotherapy after PCS than those in paclitaxel–cisplatin group (45.5% versus 11.1%, p = 0.018), which reached the statistically significant difference.
Table 1.
Characteristics of the patients with FIGO IIIC serous type epithelial tubo-ovarian cancer, or primary peritoneal carcinoma treated with weekly paclitaxel (80 mg/m2) plus either carboplatin (AUC 5) or cisplatin (20 mg/m2) combination chemotherapy triweekly.
| Carboplatin | Cisplatin | p | |
|---|---|---|---|
| Number of patients | 22 | 18 | |
| Age (years) | 58.5 ± 9.4 | 59.4 ± 9.4 | 0.768 |
| Size of residual tumors | 0.949 | ||
| ≤1cm | 12 (54.5%) | 10 (55.6%) | |
| >1cm | 10 (45.5%) | 8 (44.4%) | |
| Site of residual tumor | 0.676 | ||
| Localized | 12 (54.5%) | 11 (61.1%) | |
| Whole abdominal cavity | 10 (45.5%) | 7 (38.9%) | |
| Period to complete the front-line chemotherapy | 0.018 | ||
| ≤21 weeks | 12 (54.5%) | 16 (88.9%) | |
| >21 weeks | 10 (45.5%) | 2 (11.1%) | |
| ECOG | 0.884 | ||
| 0-1 | 21 (95.5%) | 17 (94.4%) | |
| 2-3 | 1 (4.5%) | 1 (5.6%) |
Carboplatin: carboplatin (AUC 5)-based dose dense chemotherapy; Cisplatin: cisplatin (20mg/m2)-based dose dense chemotherapy; ECOG: Eastern Cooperative Oncology Group Performance Status. Data are presented as a number (%) or the mean ± standard deviation.
3.2. Adverse Events (AEs)
Adverse events (AEs) are listed in Table 2. In the current study, no treatment-related death was found. The most common all-grade AEs in the entire cohort included anemia, nausea, neutropenia, and peripheral neuropathy. However, the occurrence of each AE was different in both groups. Patients who were treated with the paclitaxel–carboplatin regimen had a higher risk of development of neutropenia than those with the paclitaxel–cisplatin regimen. In addition, grade 3/4 neutropenia occurred more frequently in patients treated with the paclitaxel–carboplatin regimen compared to that in patients with the paclitaxel–cisplatin regimen. Both reached the statistically significant difference. In current study, cisplatin-related AEs, such as renal toxicity, neurotoxicity, nausea, or vomiting were mild or absent, as shown in Table 2.
Table 2.
Adverse events the patients with FIGO IIIC serous type epithelial tubo-ovarian cancer, or primary peritoneal carcinoma treated with weekly paclitaxel (80 mg/m2) plus either carboplatin (AUC 5) or cisplatin (20 mg/m2) combination chemotherapy triweekly.
| Events | Any grade, n (%) | Grade 3/4, n (%) | ||||
|---|---|---|---|---|---|---|
| CARBO | CIS | p | CARBO | CIS | p | |
| Neutropenia | 20 (90.9) | 7 (38.9) | < 0.0001 | 17 (77.3) | 5 (27.8) | 0.002 |
| Anemia | 21 (95.5) | 18 (100) | 0.360 | 5 (22.7) | 1 (5.6) | 0.130 |
| Thrombocytopenia | 5 (22.7) | 3 (16.7) | 0.634 | 3 (13.6) | 0 | 0.103 |
| Renal toxicity | 1 (4.5) | 3 (16.7) | 0.204 | 0 | 0 | |
| Proteinuria | 5 (22.7) | 1 (5.6) | 0.130 | 0 | 0 | |
| Peripheral neuropathy | 8 (36.4) | 7 (38.9) | 0.870 | 0 | 0 | |
| Nausea | 9 (40.9) | 9 (50.0) | 0.565 | 0 | 0 | |
CARBO: carboplatin (AUC 5)-based dose dense chemotherapy; CIS: cisplatin (20mg/m2)-based dose dense chemotherapy; n: number of patients; data are presented as numbers and percentages.
3.3. Outcomes
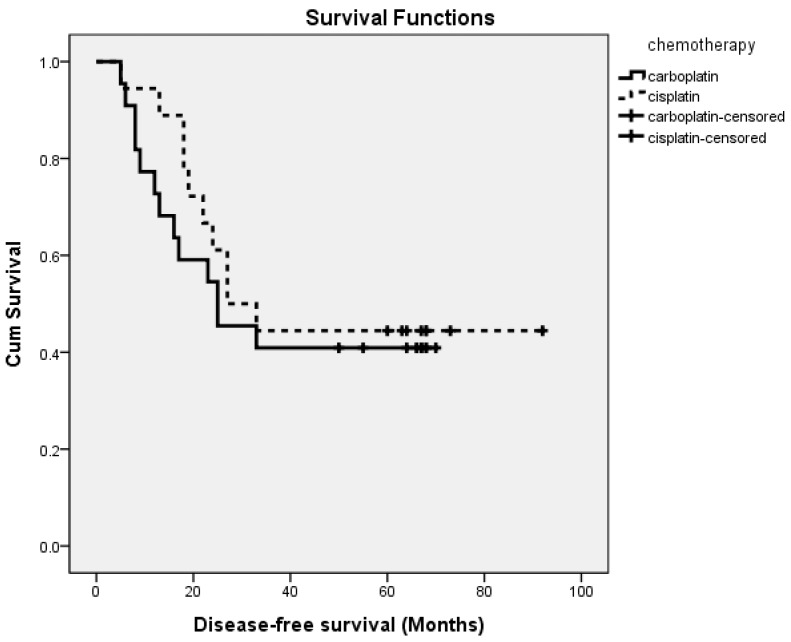
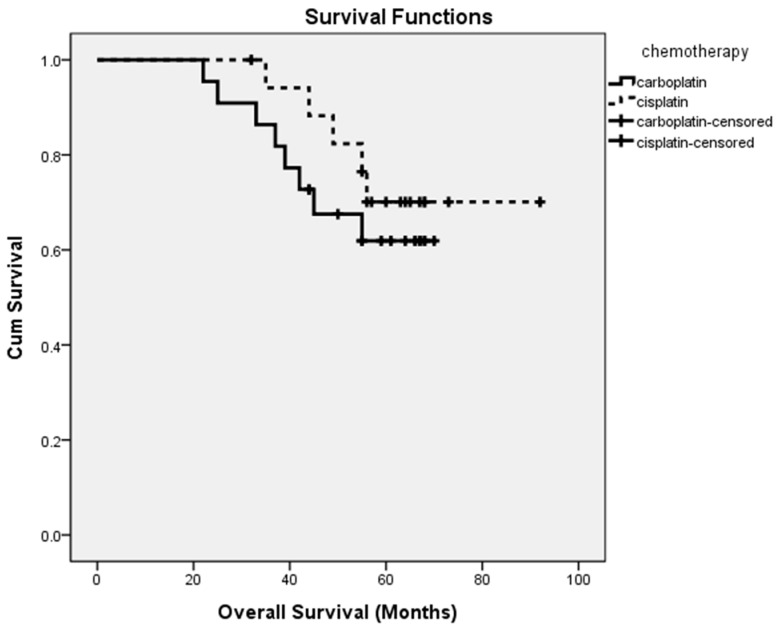
During the whole study period with a median follow-up time of 55 months (at the time of the data cutoff on 30 June 2019), disease progression and/or disease-related death occurred in 23 patients (57.5%). As was presented in Figure 1, the median PFS was 25.0 months in patients treated with the paclitaxel–carboplatin regimen, and 30.0 months in patients treated with the paclitaxel–cisplatin regimen, without a statistically significant difference. The median OS was 55.0 months and 58.5 months in patients treated with paclitaxel–carboplatin and paclitaxel–cisplatin regimens, respectively (Figure 2). There was also no statistically significant difference between the two groups.
Figure 1.
Progression-free survival curves for patients treated either with carboplatin-based dose-dense chemotherapy or with cisplatin-based dose-dense chemotherapy.
Figure 2.
Overall survival curves of patients for patients treated either with carboplatin-based dose-dense chemotherapy or with cisplatin-based dose-dense chemotherapy.
3.4. Prognostic Factors
To identify the prognostic factors for disease progression (PFS), a univariate analysis of clinic-pathologic factors showed that residual tumors more than 1 cm in size and the prolonged completion of postoperative adjuvant chemotherapy were associated with worse prognosis (Table 3). Both these factors were also independent risk factors when using the multivariate analysis model.
Table 3.
Association between baseline characteristics and progression-free survival.
| Parameters | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| n | HR (95% CI) | p | HR (95% CI) | p | |
| Size | |||||
| ≤1cm | 22 | 1(Ref) | 1(Ref) | ||
| >1cm | 18 | 8.68 (3.23–23.35) | <0.0001 | 14.38 (4.18–49.46) | <0.0001 |
| Site | |||||
| Localized | 23 | 1 (Ref) | 1 (Ref) | ||
| WAC | 17 | 1.38 (0.61–3.14) | 0.440 | 0.71 (0.28–1.82) | 0.470 |
| Period | |||||
| ≤21 weeks | 28 | 1 (Ref) | 1 (Ref) | ||
| >21 weeks | 12 | 28.49 (8.36–97.06) | <0.0001 | 81.24 (14.03–470.31) | <0.0001 |
n: number of patients; HR: hazard ratio; 95% CI: 95% confidence interval; Size: size of residual tumor; Site: site of residual tumor; Localized: a single anatomic site; WAC whole abdominal cavity similar to multiple anatomic sites; Period: period between the initial first time of chemotherapy and the end of final chemotherapy (six cycles of chemotherapy); Ref: reference.
Further evaluation was performed to analyze the prognostic factors associated with overall survival and the results showed that optimal debulking surgery was the most important independent prognostic factor to predict better outcomes in patients with FIGO IIIC ETOC and PPSC. However, the on-schedule administration of postoperative adjuvant chemotherapy regardless, in-time and on-time chemotherapy was also a very important predictor in those patients who had a better chance for prolonged OS (Table 4).
Table 4.
Association between baseline characteristics and overall survival.
| Parameters | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| n | HR (95% CI) | p | HR (95% CI) | p | |
| Size | |||||
| ≤1cm | 22 | 1(Ref) | 1(Ref) | ||
| >1cm | 18 | 22.37 (2.88–173.86) | 0.003 | 11.83 (1.48–94.72) | 0.020 |
| Site | |||||
| Localized | 23 | 1 (Ref) | 1 (Ref) | ||
| WAC | 17 | 2.41 (0.79–7.39) | 0.122 | 2.54 (0.76–8.53) | 0.131 |
| Period | |||||
| ≤21 weeks | 28 | 1 (Ref) | 1 (Ref) | ||
| >21 weeks | 12 | 15.31 (4.13–56.78) | <0.0001 | 9.57 (2.34–39.18) | 0.002 |
n: number of patients; HR: hazard ratio; 95% CI: 95% confidence interval; Size: size of residual tumor; Site: site of residual tumor; Localized: a single anatomic site; WAC whole abdominal cavity similar to multiple anatomic sites; Period: period between the initial first time of chemotherapy and the end of final chemotherapy (six cycles of chemotherapy); Ref: reference.
4. Discussion
4.1. Main Findings
The main findings of the current study showed that both regimens could be successfully used for the treatment of patients with FIGO IIIC ETOC or PPSC, offering 30 and 25 months of median PFS in the paclitaxel–cisplatin and paclitaxel–carboplatin groups, respectively. The median OS was 58.5 months in the paclitaxel–cisplatin group and 55.0 months in the paclitaxel–carboplatin group. Although neither PFS nor OS reached the statistically significant difference, it seemed to be little better in patients treated with the paclitaxel–cisplatin regimen compared to those treated with the paclitaxel–carboplatin regimen. However, to compare the frequency and severity of AEs, we found that patients in the paclitaxel–carboplatin group had more AEs than those in paclitaxel–cisplatin group did, including a higher risk of neutropenia and grade 3/4 neutropenia, and the need for a longer period to complete the front-line chemotherapy. The need for a longer period to finish the dose-dense therapy seemed to be associated with a worse outcome for patients. Further analysis showed that the period between the first-time chemotherapy to the last dose (6 cycles) of chemotherapy > 21 weeks was associated with worse prognosis in patients compared to that ≤21 weeks, with hazard ratio (HR) of 81.24 for PFS and 9.57 for OS. As predicted, suboptimal debulking surgery (>1 cm) also contributed to the worse outcome than optimal debulking surgery (≤1 cm) with a HR of 14.38 for PFS and 11.83 for OS (Table 3 and Table 4).
4.2. Summary of Studies Addressing Dose-Dense therapy: Survival Outcome (Table 5)
Table 5.
Summary of treatment efficacy and safety of dose-dense weekly paclitaxel plus platinum compounds in the management of patients with epithelial tubo-ovarian cancer and primary peritoneal serous carcinoma.
| Authors | Stage | n | Regimen | PFS | OS |
|---|---|---|---|---|---|
| Prospective randomized trials | |||||
| Katsumata [40] | II–IV | 312 | P 80 mg/m2 (D1,8,15), C 6 (D1) |
28.2 (M) | 100.5 (M) |
| Pignata [32] | IC-IV | 406 | P 60 mg/m2 (D1,8,15), C 2 (D1,8,15) |
18.3 (M) | |
| Chan [41] | II–IV | 340 | P 80 mg/m2 (D1,8,15), C 6 ±BEV (D1) |
14.7 (M) | - |
| 55 | P 80mg/m2 (D1,8,15), C 6 (D1) |
14.2 (M) | - | ||
| Clamp [33] | I-IV | P 80 mg/m2 (D1,8,15), C 5,6 ± BEV (D1) |
20.8 (M) | ||
| P 80 mg/m2 (D1,8,15), C 2 (D1,8,15) ± BEV (D1) |
21.0 (M) | ||||
| Walker [72] | II–IV | 521 | P 80 mg/m2 (D1,8,15), C 6 +BEV (D1) |
24.9 (M) | 75.5 (M) |
| Retrospective study, including phase II study | |||||
| Abaid [132] | III-IV | 88 | P 80 mg/m2 (D1,8,15), C 5 (D1), stop one week |
22.5 (M) | 31.5 (M) |
| Fleming [133] | III-IV | 33 | P 80 mg/m2 (D1,8,15), C 5 + BEV (D1) |
16.9-22.4 (M) | |
| Murphy [102] | III | 38 | P 80 mg/m2 (D1,8,15), C 5 (D1) |
31.3 (m) | 54.5 (m) |
| Boraska Jelavić [105] | I-IV | 43 | P 80 mg/m2 (D1,8,15), C 5 (D1) |
20-24 (M) | |
| Rettenmaier [78] | I-IV | 100 | P 80 mg/m2 (D1,8,15), C 5 (D1) |
27.6 (M) | |
| Cheng [101] | IIIC-IV | 32 | P 80 mg/m2 (D1,8,15), Cisplatin 20 mg/m2 (D1) |
27.0 (M) | 56 (m) |
| Current study | IIIC | 18 | P 80 mg/m2 (D1,8,15), Cisplatin 20 mg/m2 (D1) |
30.0 (M) | 58.5 (M) |
| 22 | P 80 mg/m2 (D1,8,15), C 5 (D1) |
25.0 (M) | 55.0 (M) | ||
Dose-dense chemotherapy is limited on the intravenous route. RCT: randomized control trial, containing co-administration of bevacizumab (BEV) 15 mg/kilograms at day 1 triweekly and patients treated with neoadjuvant chemotherapy; n: number of patients; PFS: median (M) or mean (m) progression-free survival calculated by months; OS: median or mean overall survival calculated by months; Stage: FIGO stage (International Federation of Gynecology and Obstetrics stage); P: paclitaxel; D: day; C 2, C 5 or C 6: carboplatin (AUC 2, AUC 5 or AUC 6: area under the curve 2, 5, or 6 mg/mL per minute).
Some early phase studies have also been conducted to test the tolerability and efficacy of dose-dense weekly paclitaxel (80 mg/m2) and triweekly carboplatin (AUC 5) with other agents [132,133]. For example, one phase II study modified a dose-dense paclitaxel (80 mg/m2) and carboplatin (AUC 5) every four weeks for the treatment of advanced-stage ETOC patients, and found that the median PFS and OS were 22.5 and 31.5 months, respectively [132]. Another phase II study adding bevacizumab in dose-dense chemotherapy (weekly paclitaxel 80 mg/m2 and triweekly carboplatin AUC 5) showed that the median PFS was 16.9–22.4 months in advanced-stage ETOC patients [133].
Based on the impressive survival benefits of the earlier reports, the prospective, randomized trials may be the best method to test the efficacy and safety of dose-dense chemotherapy in the management of ETOC patients. The first large study, named as the JGOG 3016 has shown the survival benefits in dose-dense regimen containing weekly paclitaxel (80 mg/m2) and triweekly carboplatin (AUC 6), which not only offered a longer PFS (median PFS of 28.2 months, 95% CI 22.3-33.8 months) but also a significantly prolonged OS (median OS of 100.5 months, 95% CI 65.2-∞ months) than those in the standard triweekly paclitaxel–carboplatin regimen [32,33].
The results of an NRG Oncology/GOG Study showed a similar PFS (the median PFS of 24.9 months) and OS (the median OS of 75.5 months) in dose-dense weekly paclitaxel (80 mg/m2) and triweekly carboplatin (AUC 6) with 15 mg/kg bevacizumab treatment compared to the standard triweekly combination therapy [72].
MITO-7 has shown that there was no statistically significant difference in median PFS between dose-dense weekly combination (paclitaxel 60 mg/m2 plus carboplatin AUC 2) and standard triweekly combination therapy (18.3 months [95% CI 16.8-20.9 months] versus 17.3 months [95% CI 15.2-20.2 months]) with a HR of 0.96, 95% CI 0.80-1.16 [32].
GOG-0262 showed the median PFS of 14.9 and 14.2 months in dose-dense weekly paclitaxel (80 mg/m2) and triweekly carboplatin (AUC 6) with/without adding 15 mg/kg bevacizumab treatment, respectively, without a statistically significant difference compared to the standard triweekly combination therapy [41].
The results from an ICON8 have shown that the median PFS was 20.8 months (95% CI 11.9-59.0 months) and 21.0 months (95% CI 12.0-54.0 months) in dose-dense weekly paclitaxel (80 mg/m2) and triweekly carboplatin (AUC 5 or 6) and in dose-dense weekly paclitaxel (80 mg/m2) and weekly carboplatin (AUC 2) regimen, respectively, and none were statistically significant different from the 17.7-month median PFS in the standard-dose triweekly paclitaxel and triweekly carboplatin (AUC) treatment [33].
Although the evidence would be much more powerful if the results were obtained from prospective, randomized trials, the management of the individual patient who does not fulfill the criteria of a clinical trial or is enrolled into the clinical trial will be debated continuously. In addition, the strict criteria of a clinical trial, such as inclusive and exclusive criteria, may not reflect the real situations of patients. Therefore, clinical practices are not uniform, and retrospective evaluations may partly reveal real-world clinical practice. For example, the elderly population is often excluded in clinical trials. Dr. Bun and colleagues found dose-dense weekly paclitaxel plus triweekly carboplatin was feasible for elderly patients, although severe neuropathy might occur more frequently than in younger groups [134]. In fact, the elderly population might have a higher risk of receiving nonstandard chemotherapy [135].
4.3. Dose-Dense Therapy-Related Adverse Events: Prefer the Use of Low-Dose Cisplatin in Place of Carboplatin in Platinum-based Therapy
It is believed that the use of carboplatin in place of original cisplatin in the platinum compound-based paclitaxel doublet might provide a better quality of life (QOL) in the long-term, since this regimen—with carboplatin dosed using the Calvert formula—yielded convincing non inferior outcomes when compared with the prior, more toxic, cisplatin–paclitaxel regimen [136]. By contrast, bone marrow suppression, including the toxicity of hematological system might be more apparent in carboplatin administration, although Boyd and Muggia proposed that carboplatin–paclitaxel therapy is generally safe when the drug is properly dosed [136]. The occurrence of neutropenia is one of the most common causes necessitating postponing the therapy or modifying (decreasing) the dosage of therapeutic agent. One study from Japan in 2006 tried to determine the feasibility of docetaxel–cisplatin therapy Q3W compared with docetaxel–carboplatin therapy Q3W as a front-line for ETOC patients [136]. The results, as predicted, showed that the incidence of grade 4 neutropenia was much higher in the docetaxel–carboplatin group than that in the docetaxel–cisplatin group (74% versus 39%), suggesting the feasibility of docetaxel–cisplatin combination therapy as front-line therapy for ETOC patients [136]. In addition, the rationale of the use of “dose-dense” in place of “standard-dose” is the attempt to decrease the incidence of chemotherapy-related severe hematotoxicity, such as infection or sepsis caused by severe neutropenia or spontaneous hemorrhage caused by severe thrombocytopenia [49]. In Taiwan, GCSF is not routinely prescribed and insurance does not cover the cost if GCSF is used in a prophylactic role. Furthermore, the obstacle is still present when facing patients with chemotherapy-related thrombocytopenia [137,138,139].
Thrombocytopenia is a principal consideration, as well as the dose-limiting toxicity of carboplatin [140,141]. Although thrombocytopenia unlikely occurs in chemotherapy-naïve patients, this toxicity usually begins to appear after day 14 and is predictably cumulative, contributing to the consideration of a carboplatin dose reduction [140]. Due to aforementioned limitation, some gynecological oncologists favored the use of cisplatin as the drug in the platinum-based combination therapy, except for those patients with identified impaired renal function (eGFR < 60 ml/min). The current low-dose cisplatin plus dose-dense paclitaxel regimen was associated with lower rates of hematologic toxicities compared to the conventional dose-dense paclitaxel plus carboplatin regimen. The results from an ICON8 have also shown that patients treated with a weekly regimen had increased grade 3 or 4 toxic effects, although these high-grade toxicities were predominantly uncomplicated [33]. However, both regimens are carboplatin-based paclitaxel combinations.
Consistent with the increased toxicity of the use of carboplatin in previous studies [136], the incidence of grade 3/4 neutropenia was significantly lower in the cisplatin–paclitaxel arm than in that of the carboplatin–paclitaxel arm (27.8% versus 77.3%, p = 0.002) in our study. In addition, other parameters of hematological examination, including grade 3/4 anemia and grade 3/4 thrombocytopenia also favored the cisplatin–paclitaxel regimen with incidence of 5.6% and none, respectively, compared to 22.7% and 13.6% in the carboplatin–paclitaxel regimen in the current study. We believe that this carboplatin–paclitaxel combination therapy-related hematotoxicity might explain why more patients in the carboplatin–paclitaxel arm had a prolongation of the period between the initial first dose of chemotherapy and the final dose of chemotherapy (six cycles of chemotherapy) than those in the cisplatin–paclitaxel arm (45% versus 11.1%, p = 0.018). Further survival analysis showed that this prolongation of the therapeutic period might be associated with a worse outcome in FIGO IIIC ETOC and PPSC patients.
By contrast, the cisplatin-related renal, neural, and gastrointestinal system toxicity was lower in the current study. The main reason is the use of low-dose cisplatin (20 mg/m2) as a platinum compound in the platinum–paclitaxel combination therapy.
4.4. The Benefits of Maximal Cytoreductive Surgery and The Consideration of the Location of Residual Tumors
In agreement with well-known concepts [142,143,144,145,146], a rapid reduction in the tumor burden is the guarantee of successful treatment in cancer patients. Nearly all studies have confirmed that maximal cytoreduction surgery is the most critical step in the management of patients with advanced-stage ETOC or PPSC [142,143,144,145,146]. According to the definition of the Gynecologic Oncology group (GOG) for ’optimal’ as having residual tumor nodules which should be ≤ 1 cm in size, the Cochrane review revealed that the outcome of ETOC patients with residual disease ≤ 1 cm is better than of those with residual disease > 1 cm [143], although it is well known that PCS to no gross residual tumor might be associated with the longest PFS and OS [144,145]. One study showed an interesting finding that patients still had diverse outcomes despite undergoing optimal PCS (≤ 1 cm residual disease), in which it demonstrated that patients with a tumor limited in a single anatomic location had better median PFS and OS than patients with tumors widely distributed in multiple anatomic locations [146].
In our current study, we also tested the aforementioned findings. We found that patients with suboptimal PCS with a residual tumor > 1 cm indeed had a worse prognosis, with a hazard ratio (HR) of 14.38 (95% confidence interval [CI] 4.18-49.46) in PFS and a HR of 11.83 (95% CI 1.48-94.72) in OS, compared with those patients who could achieve optimal PCS with a residual tumor of a size less than 1 cm, supporting the rationale that small-volume tumors are more prone to clearance [61]. However, the widespread nature of tumors seemed to not be an independent risk factor for worse prognosis. We found that there was no statistically significant difference of outcome in patients whose residual tumors were in localized anatomical site or in multiple anatomical sites.
4.5. The Strengths and Limitations
The current study has the following strengths: All patients were serous-type ETOC and PPSC with FIGO stage IIIC. The follow-up period was long enough, of nearly 5 years (a mean follow-up period of 55 months). All patients were treated with by one senior doctor (P.-H.W). All the suggested study population was homogeneous.
There are some limitations in the current study. Retrospective data collection in nature may not be strong enough to show clear evidence. However, as shown above, this is a real-world clinical practice. In addition, only patients who underwent PCS with surgical confirmation of FIGO IIIC serous-type ETOC and PPSC and completed 6 cycles of dose-dense paclitaxel-platinum compounds were included, contributing to the risk of selection bias. Furthermore, the number of patients in the current study was small in each arm, contributing to wide confidence intervals. It demonstrated the higher risk of low precision in the estimates. Therefore, the findings of the current study should be used with caution. Finally, new therapeutic strategies—such as maintenance therapy, NACT, or intraperitoneal therapy—were not evaluated in the current report. However, we believe none of them impede the value of the current study.
5. Conclusions
Our study found that paclitaxel might be one of the most critical components for the successful administration of dose-dense adjuvant chemotherapy. In addition, the on-schedule delivery and shortening of the entire therapeutic period are a critical step for the survival benefits of patients who undergo a postoperative adjuvant dose-dense paclitaxel plus platinum compounds regimen. Of course, maximal efforts should be made to eradicate the tumor as much as possible. Although recent evidences highlighted the value of precise medicine, personalized and individual therapy, along with targeted therapy for cancer patients [147,148,149,150,151]—the net health benefits (NHBs), cost-effectiveness or quality of life may reflect the relative value of treatment options in EOC [100,152,153,154,155,156,157]. With the low risk of AEs, shortened therapeutic period, and, of most importance, working without compromising therapeutic effects—low-dose triweekly cisplatin plus dose-dense weekly paclitaxel might be associated with better NHBs, a better cost-effectiveness, and a better QOL compared to a carboplatin-based dose-dense paclitaxel regimen. More studies are encouraged to test our findings.
Abbreviation
| Adverse events (AEs) |
| American Society of Clinical Oncology (ASCO) |
| Area under the curve (AUC) |
| Confidence interval (CI) |
| Epithelial tubo-ovarian cancer (ETOC) |
| Estimated glomerular filtration rate (eGFR) |
| Every four weeks (Q4W) |
| Every three weeks (Q3W) |
| Granulocyte colony-stimulating factor (GCSF) |
| Gynecologic Cancer InterGroup (GCIG) |
| Gynecologic Oncology Group (GOG) |
| Hazard ratio (HR) |
| International Collaboration on Ovarian Neoplasms (ICON) |
| International Federation of Gynecology and Obstetrics (FIGO) |
| Interval cytoreductive surgery (ISC) |
| Intravenous (IV) |
| Intraperitoneal (IP) |
| Japanese Gynecologic Oncology Group (JGOG) |
| National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE) |
| National comprehensive Cancer Network (NCCN) |
| Neoadjuvant chemotherapy (NACT) |
| Net health benefits (NHBs) |
| Magnetic resonance image (MRI) |
| Multicentre Italian Trials in Ovarian cancer (MITO) |
| Overall survival (OS) |
| Poly(ADP-ribose) polymerase (PARP) inhibitors (PARP inhibitors) |
| Primary peritoneal serous carcinoma (PPSC) |
| Primary cytoreductive surgery (PSC) |
| Primary debulking surgery (PDS) |
| Progression-free survival (PFS) |
| Progressive disease (PD) |
| Quality of life (QOL) |
| Response Evaluation Criteria in Solid Tumors (RECIST) |
Author Contributions
Conceptualization, C.-Y.H., M.C., W.-H.C., and P.-H.W.; formal analysis, H.-Y.H., W.-H.C., and P.-H.W.; investigation, C.-Y.H., W.-L.L., and P.-H.W.; resources, C.-Y.H., M.C., N.-R.L., and P.-H.W.; data curation, M.C., W.-H.C., and P.-H.W.; methodology, W.-L.L.,W.-H.C., and P.-H.W.; writing—original draft, C.-Y.H., W.-L.L., and P.-H.W.; writing—review and editing, C.-Y.H., and P.-H.W.; supervision, W.-H.C., and P.-H.W.; project administration, W.-H.C., and P.-H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the Taipei Veterans General Hospital (V108C-085, V109C-108, and V109A-022) and from the Ministry of Science and Technology, Executive Yuan (MOST: 106-2314-B-075-061-MY3), Taipei, Taiwan. The authors appreciate the financial support from the Female Cancer Foundation, Taipei, Taiwan.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Berek J.S., Kehoe S.T., Kumar L., Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2018;143(Suppl. 2):59–78. doi: 10.1002/ijgo.12614. [DOI] [PubMed] [Google Scholar]
- 2.Torre L.A., Trabert B., DeSantis C.E., Miller K.D., Samimi G., Runowicz C.D., Gaudet M.M., Jemal A., Siegel R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenzel A.E., Buas M.F., Moysich K.B. Survival disparities among racial/ethnic groups of women with ovarian cancer: An update on data from the Surveillance, Epidemiology and End Results (SEER) registry. Cancer. Epidemiol. 2019;62:101580. doi: 10.1016/j.canep.2019.101580. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong D.K., Alvarez R.D., Bakkum-Gamez J.N., Barroilhet L., Behbakht K., Berchuck A., Berek J.S., Chen L.M., Cristea M., DeRosa M., et al. NCCN guidelines insights: Ovarian cancer, version 1.2019. J. Natl. Compr. Cancer Netw. 2019;17:896–909. doi: 10.6004/jnccn.2019.0039. [DOI] [PubMed] [Google Scholar]
- 5.Hanchette C., Zhang C.H., Schwartz G.G. Ovarian cancer incidence in the U.S. and toxic emissions from pulp and paper plants: A geospatial analysis. Int. J. Environ. Res. Public Health. 2018;15:1619. doi: 10.3390/ijerph15081619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S.N., Chang R., Lin L.T., Chern C.U., Tsai H.W., Wen Z.H., Li Y.H., Li C.J., Tsui K.H. MicroRNA in ovarian cancer: Biology, pathogenesis, and therapeutic opportunities. Int. J. Environ. Res. Public Health. 2019;16:1510. doi: 10.3390/ijerph16091510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu H.C., Tseng K.Y., Wang H.C., Sung F.C., Ma W.F. Risk of endometriosis and subsequent ovary and breast cancers in nurses: A population-based cohort study in Taiwan. Int. J. Environ. Res. Public Health. 2019;16:3469. doi: 10.3390/ijerph16183469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oda K., Hamanishi J., Matsuo K., Hasegawa K. Genomics to immunotherapy of ovarian clear cell carcinoma: Unique opportunities for management. Gynecol. Oncol. 2018;151:381–389. doi: 10.1016/j.ygyno.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murakami K., Kotani Y., Shiro R., Takaya H., Nakai H., Matsumura N. Endometriosis-associated ovarian cancer occurs early during follow-up of endometrial cysts. Int. J. Clin. Oncol. 2020;25:51–58. doi: 10.1007/s10147-019-01536-5. [DOI] [PubMed] [Google Scholar]
- 10.Haraguchi H., Koga K., Takamura M., Makabe T., Sue F., Miyashita M., Urata Y., Izumi G., Harada M., Hirata T., et al. Development of ovarian cancer after excision of endometrioma. Fertil. Steril. 2016;106:1432–1437. doi: 10.1016/j.fertnstert.2016.07.1077. [DOI] [PubMed] [Google Scholar]
- 11.Su K.M., Wang P.H., Yu M.H., Chang C.M., Chang C.C. The recent progress and therapy in endometriosis-associated ovarian cancer. J. Chin. Med. Assoc. 2020;83:227–232. doi: 10.1097/JCMA.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 12.Kim J., Park E.Y., Kim O., Schilder J.M., Coffey D.M., Cho C.H., Bast R.C., Jr. Cell origins of high-grade serous ovarian cancer. Cancers. 2018;10:433. doi: 10.3390/cancers10110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lisio M.A., Fu L., Goyeneche A., Gao Z.H., Telleria C. High-grade serous ovarian cancer: Basic sciences, clinical and therapeutic standpoints. Int. J. Mol. Sci. 2019;20:952. doi: 10.3390/ijms20040952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura M., Obata T., Daikoku T., Fujiwara H. The association and significance of p53 in gynecologic cancers: The potential of targeted therapy. Int. J. Mol. Sci. 2019;20:5482. doi: 10.3390/ijms20215482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun M., Bao L., Shen H., Ji M., Yao L., Yi X., Jiang W. Unexpected primary fallopian tube carcinoma during gynecological operations: Clinicopathological and prognostic factors analyses of 67 cases. Taiwan J. Obstet. Gynecol. 2019;58:626–632. doi: 10.1016/j.tjog.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Coan M., Rampioni Vinciguerra G.L., Cesaratto L., Gardenal E., Bianchet R., Dassi E., Vecchione A., Baldassarre G., Spizzo R., Nicoloso M.S. Exploring the role of Fallopian ciliated cells in the pathogenesis of high-grade serous ovarian cancer. Int. J. Mol. Sci. 2018;19:2512. doi: 10.3390/ijms19092512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang C.C., Su K.M., Lu K.H., Lin C.K., Wang P.H., Li H.Y., Wang M.L., Lin C.K., Yu M.H., Chang C.M. Key immunological functions involved in the progression of epithelial ovarian serous carcinoma discovered by the gene ontology-based immunofunctionome analysis. Int. J. Mol. Sci. 2018;19:3311. doi: 10.3390/ijms19113311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sung P.L., Wen K.C., Chen Y.J., Chao T.C., Tsai Y.F., Tseng L.M., Qiu J.T., Chao K.C., Wu H.H., Chuang C.M., et al. The frequency of cancer predisposition gene mutations in hereditary breast and ovarian cancer patients in Taiwan: From BRCA1/2 to multi-gene panels. PLoS ONE. 2017;12:e0185615. doi: 10.1371/journal.pone.0185615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGuire W.P., Hoskins W.J., Brady M.F., Kucera P.R., Partridge E.E., Look K.Y., Clarke-Pearson D.L., Davidson M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N. Engl. J. Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 20.Piccart M.J., Bertelsen K., Stuart G., Cassidy J., Mangioni C., Simonsen E., James K., Kaye S., Vergote I., Blom R., et al. Long-term follow-up confirms a survival advantage of the paclitaxel–cisplatin regimen over the cyclophosphamide-cisplatin combination in advanced ovarian cancer. Int. J. Gynecol. Cancer. 2003;13:144–148. doi: 10.1111/j.1525-1438.2003.13357.x. [DOI] [PubMed] [Google Scholar]
- 21.Piccart M.J., Bertelsen K., James K., Cassidy J., Mangioni C., Simonsen E., Stuart G., Kaye S., Vergote I., Blom R., et al. Randomized intergroup trial of cisplatin–paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: Three-year results. J. Natl. Cancer Inst. 2000;92:699–708. doi: 10.1093/jnci/92.9.699. [DOI] [PubMed] [Google Scholar]
- 22.Alberts D.S. Carboplatin versus cisplatin in ovarian cancer. Semin. Oncol. 1995;22:88–90. [PubMed] [Google Scholar]
- 23.Neijt J.P., Engelholm S.A., Tuxen M.K., Sorensen P.G., Hansen M., Sessa C., de Swart C.A., Hirsch F.R., Lund B., van Houwelingen H.C. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J. Clin. Oncol. 2000;18:3084–3092. doi: 10.1200/JCO.2000.18.17.3084. [DOI] [PubMed] [Google Scholar]
- 24.du Bois A., Lück H.J., Meier W., Adams H.P., Mobus V., Costa S., Bauknecht T., Richter B., Warm M., Schröder W., et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J. Natl. Cancer Inst. 2003;95:1320–1329. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 25.Ozols R.F., Bundy B.N., Greer B.E., Fowler J.M., Clarke-Pearson D., Burger R.A., Mannel R.S., DeGeest K., Hartenbach E.M., Baerge R., et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J. Clin. Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 26.NCCN Clinical Practice Guideline in Oncology, Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer. Version 1.2020—March 11, 2020. [(accessed on 18 March 2020)]; Available online: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf.
- 27.Tewari K.S., Burger R.A., Enserro D., Norquist B.M., Swisher E.M., Brady M.F., Bookman M.A., Fleming G.F., Huang H., Homesley H.D., et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J. Clin. Oncol. 2019;37:2317–2328. doi: 10.1200/JCO.19.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chelariu-Raicu A., Coleman R.L., Sood A.K. Anti-angiogenesis therapy in ovarian cancer: Which patient is it most likely to benefit? Oncology. 2019;33:629378. [PubMed] [Google Scholar]
- 29.Oza A.M., Cook A.D., Pfisterer J., Embleton A., Ledermann J.A., Pujade-Lauraine E., Kristensen G., Carey M.S., Beale P., Cervantes A., et al. ICON7 trial investigators. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16:928–936. doi: 10.1016/S1470-2045(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perren T.J., Swart A.M., Pfisterer J., Ledermann J.A., Pujade-Lauraine E., Kristensen G., Carey M.S., Beale P., Cervantes A., Kurzeder C., et al. ICON7 Investigators. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 31.Burger R.A., Brady M.F., Bookman M.A., Fleming G.F., Monk B.J., Huang H., Mannel R.S., Homesley H.D., Fowler J., Greer B.E., et al. Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 32.Pignata S., Scambia G., Katsaros D., Gallo C., Pujade-Lauraine E., De Placido S., Bologna A., Weber B., Raspagliesi F., Panici P.B., et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014;15:396–405. doi: 10.1016/S1470-2045(14)70049-X. [DOI] [PubMed] [Google Scholar]
- 33.Clamp A.R., James E.C., McNeish I.A., Dean A., Kim J.W., O’Donnell D.M., Hook J., Coyle C., Blagden S., Brenton J.D., et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): Primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet. 2019;394:2084–2095. doi: 10.1016/S0140-6736(19)32259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasey P.A., Jayson G.C., Gordon A., Gabra H., Coleman R., Atkinson R., Parkin D., Paul J., Hay A., Kaye S.B., et al. Phase III randomized trial of docetaxel–carboplatin versus paclitaxel–carboplatin as first-line chemotherapy for ovarian carcinoma. J. Natl. Cancer Inst. 2004;96:1682–1691. doi: 10.1093/jnci/djh323. [DOI] [PubMed] [Google Scholar]
- 35.Mori T., Hosokawa K., Kinoshita Y., Watanabe A., Yamaguchi T., Kuroboshi H., Kato Y., Yasuda J., Fujita H., Nakata Y., et al. A pilot study of docetaxel–carboplatin versus paclitaxel–carboplatin in Japanese patients with epithelial ovarian cancer. Int. J. Clin. Oncol. 2007;12:205–211. doi: 10.1007/s10147-007-0656-z. [DOI] [PubMed] [Google Scholar]
- 36.Pignata S., Scambia G., Ferrandina G., Savarese A., Sorio R., Breda E., Gebbia V., Musso P., Frigerio L., Del Medico P., et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first-line treatment for patients with ovarian cancer: The MITO-2 randomized phase III trial. J. Clin. Oncol. 2011;29:3628–3635. doi: 10.1200/JCO.2010.33.8566. [DOI] [PubMed] [Google Scholar]
- 37.Bookman M.A., Brady M.F., McGuire W.P., Harper P.G., Alberts D.S., Friedlander M., Colombo N., Fowler J.M., Argenta P.A., De Geest K., et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: A Phase III Trial of the Gynecologic Cancer Intergroup. J. Clin. Oncol. 2009;27:1419–1425. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staropoli N., Ciliberto D., Botta C., Fiorillo L., Grimaldi A., Lama S., Caraglia M., Salvino A., Tassone P., Tagliaferri P. Pegylated liposomal doxorubicin in the management of ovarian cancer: A systematic review and metaanalysis of randomized trials. Cancer. Biol. Ther. 2014;15:707–720. doi: 10.4161/cbt.28557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katsumata N., Yasuda M., Takahashi F., Isonishi S., Jobo T., Aoki D., Tsuda H., Sugiyama T., Kodama S., Kimura E., et al. Japanese Gynecologic Oncology Group. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: A phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–1338. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 40.Katsumata N., Yasuda M., Isonishi S., Takahashi F., Michimae H., Kimura E., Aoki D., Jobo T., Kodama S., Terauchi F., et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol. 2013;14:1020–1026. doi: 10.1016/S1470-2045(13)70363-2. [DOI] [PubMed] [Google Scholar]
- 41.Chan J.K., Brady M.F., Penson R.T., Huang H., Birrer M.J., Walker J.L., DiSilvestro P.A., Rubin S.C., Martin L.P., Davidson S.A., et al. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N. Engl. J. Med. 2016;374:738–748. doi: 10.1056/NEJMoa1505067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujiwara K., Hasegawa K., Nagao S. Landscape of systemic therapy for ovarian cancer in 2019: Primary therapy. Cancer. 2019;125(Suppl. 24):4582–4586. doi: 10.1002/cncr.32475. [DOI] [PubMed] [Google Scholar]
- 43.Armstrong D.K., Bundy B., Wenzel L., Huang H.Q., Baergen R., Lele S., Copeland L.J., Walker J.L., Burger R.A., Gynecologic Oncology Group Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 44.Markman M., Bundy B.N., Alberts D.S., Fowler J.M., Clark-Pearson D.L., Carson L.F., Wadler S., Sickel J. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J. Clin. Oncol. 2001;19:1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 45.Chandra A., Pius C., Nabeel M., Nair M., Vishwanatha J.K., Ahmad S., Basha R. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019;8:7018–7031. doi: 10.1002/cam4.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lheureux S., Gourley C., Vergote I., Oza A.M. Epithelial ovarian cancer. Lancet. 2019;393:1240–1253. doi: 10.1016/S0140-6736(18)32552-2. [DOI] [PubMed] [Google Scholar]
- 47.Marchetti C., De Felice F., Di Pinto A., D’Oria O., Aleksa N., Musella A. Dose-dense weekly chemotherapy in advanced ovarian cancer: An updated meta-analysis of randomized controlled trials. Crit. Rev. Oncol. Hematol. 2018;125:30–34. doi: 10.1016/j.critrevonc.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Marth C., Reimer D., Zeimet A.G. Front-line therapy of advanced epithelial ovarian cancer: Standard treatment. Ann. Oncol. 2017;28:viii36–viii39. doi: 10.1093/annonc/mdx450. [DOI] [PubMed] [Google Scholar]
- 49.Kemp Z., Ledermann J. Update on first-line treatment of advanced ovarian carcinoma. Int. J. Womens Health. 2013;5:45–51. doi: 10.2147/IJWH.S30231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bookman M.A. First-line chemotherapy in epithelial ovarian cancer. Clin. Obstet. Gynecol. 2012;55:96–113. doi: 10.1097/GRF.0b013e31824b45da. [DOI] [PubMed] [Google Scholar]
- 51.Su M.H., Chen G.Y., Lin J.H., Lee H.H., Chung K.C., Wang P.H. Paclitaxel-related dermatological problems: Not only alopecia occurs. Taiwan. J. Obstet. Gynecol. 2019;58:877–879. doi: 10.1016/j.tjog.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Liu C.H., Horng H.C., Wang P.H. A case of ovarian cancer present with acute respiratory distress: Spontaneous rupture of diaphragm. Taiwan J. Obstet. Gynecol. 2019;58:712–714. doi: 10.1016/j.tjog.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 53.Su M.H., Cho S.W., Kung Y.S., Lin J.H., Lee W.L., Wang P.H. Update on the differential diagnosis of gynecologic organ-related diseases in women presenting with ascites. Taiwan J. Obstet. Gynecol. 2019;58:587–591. doi: 10.1016/j.tjog.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Hsieh S.F., Lau H.Y., Wu H.H., Hsu H.C., Twu N.F., Cheng W.F. Prognostic factors of early stage epithelial ovarian carcinoma. Int. J. Environ. Res. Public Health. 2019;16:637. doi: 10.3390/ijerph16040637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang H.T., Chiu M.L., Wang T.Y., Chen T.C., Chang C.L., Su T.H., Wang K.G., Wang K.L., Yang Y.C., Chen J.R. Effect of chemotherapy, laparoscopy, and cytology on stage IC ovarian clear cell carcinoma: A long-term, single-center study. Int. J. Environ. Res. Public Health. 2020;17:491. doi: 10.3390/ijerph17020491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee J.M., Minasian L., Kohn E.C. New strategies in ovarian cancer treatment. Cancer. 2019;125(Suppl. 24):4623–4629. doi: 10.1002/cncr.32544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsibulak I., Zeimet A.G., Marth C. Hopes and failures in front-line ovarian cancer therapy. Crit. Rev. Oncol. Hematol. 2019;143:14–19. doi: 10.1016/j.critrevonc.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Wendel Naumann R., Coleman R.L., Brown J., Moore K.N. Phase III trials in ovarian cancer: The evolving landscape of front line therapy. Gynecol. Oncol. 2019;153:436–444. doi: 10.1016/j.ygyno.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 59.Liu J., Matulonis U.A. New advances in ovarian cancer. Oncology. (Williston Park) 2010;24:721–728. [PubMed] [Google Scholar]
- 60.Markman M. Chemotherapy: Limited use of the intraperitoneal route for ovarian cancer-why? Nat. Rev. Clin. Oncol. 2015;12:628–630. doi: 10.1038/nrclinonc.2015.177. [DOI] [PubMed] [Google Scholar]
- 61.van der Burg M.E., van Lent M., Buyse M., Kobierska A., Colombo N., Favalli G., Lacave A.J., Nardi M., Renard J., Pecorelli S. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N. Engl. J. Med. 1995;332:629–634. doi: 10.1056/NEJM199503093321002. [DOI] [PubMed] [Google Scholar]
- 62.Vergote I., Trope C.G., Amant F., Kristensen G.B., Ehlen T., Johnson N., Verheijen R.H., van der Burg M.E., Lacave A.J., Panici P.B., et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 63.Vergote I., Tropé C.G., Amant F., Ehlen T., Reed N.S., Casado A. Neoadjuvant chemotherapy is the better treatment option in some patients with stage IIIc to IV ovarian cancer. J. Clin. Oncol. 2011;29:4076–4078. doi: 10.1200/JCO.2011.36.9785. [DOI] [PubMed] [Google Scholar]
- 64.Kusunoki S., Terao Y., Hirayama T., Fujino K., Ujihira T., Ota T., Takeda S. Safety and efficacy of neoadjuvant chemotherapy with bevacizumab in advanced-stage peritoneal/ovarian cancer patients. Taiwan J. Obstet. Gynecol. 2018;57:650–653. doi: 10.1016/j.tjog.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 65.Wang P.H. Neoadjuvant chemotherapy before definite operative approach for women with advanced-stage epithelial ovarian cancer. Taiwan J. Obstet. Gynecol. 2018;57:623–624. doi: 10.1016/j.tjog.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Bartels H.C., Rogers A.C., McSharry V., McVey R., Walsh T., O’Brien D., Boyd W.D., Brennan D.J. A meta-analysis of morbidity and mortality in primary cytoreductive surgery compared to neoadjuvant chemotherapy in advanced ovarian malignancy. Gynecol. Oncol. 2019;154:622–630. doi: 10.1016/j.ygyno.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 67.Shibutani T., Nagao S., Suzuki K., Kaneda M., Yamamoto K., Jimi T., Yano H., Kitai M., Shiozaki T., Matsuoka K., et al. Dose-dense paclitaxel and carboplatin vs. conventional paclitaxel and carboplatin as neoadjuvant chemotherapy for advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer: A retrospective study. Int. J. Clin. Oncol. 2019 doi: 10.1007/s10147-019-01567-y. [DOI] [PubMed] [Google Scholar]
- 68.Kim Y.N., Lee Y.J., Lee J.Y., Nam E.J., Kim S.W., Kim S., Kim Y.T. Comparison between weekly versus 3-weekly paclitaxel in combination with carboplatin as neoadjuvant chemotherapy in advanced ovarian cancer. J. Gynecol. Oncol. 2020;31:e23. doi: 10.3802/jgo.2020.31.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coleridge S.L., Bryant A., Lyons T.J., Goodall R.J., Kehoe S., Morrison J. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane. Database. Syst. Rev. 2019;10:CD005343. doi: 10.1002/14651858.CD005343.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hasegawa K., Shimada M., Takeuchi S., Fujiwara H., Imai Y., Iwasa N., Wada S., Eguchi H., Oishi T., Sugiyama T., et al. A phase 2 study of intraperitoneal carboplatin plus intravenous dose-dense paclitaxel in front-line treatment of suboptimal residual ovarian cancer. Br. J. Cancer. 2020 doi: 10.1038/s41416-020-0734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi T., Jiang R., Pu H., Yang H., Tu D., Dai Z., Cai Y., Zhang Y., Cheng X., Jia H., et al. Survival benefits of dose-dense early postoperative intraperitoneal chemotherapy in front-line therapy for advanced ovarian cancer: A randomised controlled study. Br. J. Cancer. 2019;121:425–428. doi: 10.1038/s41416-019-0543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walker J.L., Brady M.F., Wenzel L., Fleming G.F., Huang H.Q., DiSilvestro P.A., Fujiwara K., Alberts D.S., Zheng W., Tewari K.S., et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: An NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2019;37:1380–1390. doi: 10.1200/JCO.18.01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marchetti C., De Felice F., Perniola G., Palaia I., Musella A., Di Donato V., Cascialli G., Cascialli G., Muzii L., Tombolini V., et al. Role of intraperitoneal chemotherapy in ovarian cancer in the platimum-taxane-based era: A meta-analysis. Crit. Rev. Oncol. Hematol. 2019;136:64–69. doi: 10.1016/j.critrevonc.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 74.van Driel W.J., Koole S.N., Sikorska K., Schagen van Leeuwen J.H., Schreuder H.W.R., Hermans R.H.M., Hermans R.H.M., de Hingh I.H.J.T., van der Velden J., Arts H.J., et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N. Engl. J. Med. 2018;378:230–240. doi: 10.1056/NEJMoa1708618. [DOI] [PubMed] [Google Scholar]
- 75.Auer R.C., Sivajohanathan D., Biagi J., Conner J., Kennedy E., May T. Indications for hyperthermic intraperitoneal chemotherapy with cytoreductive surgery: A systematic review. Eur. J. Cancer. 2020;127:76–95. doi: 10.1016/j.ejca.2019.10.034. [DOI] [PubMed] [Google Scholar]
- 76.du Bois A., Floquet A., Kim J.W., Rau J., del Campo J.M., Friedlander M., Pignata S., Fujiwara K., Vergote I., Colombo N., et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J. Clin. Oncol. 2014;32:3374–3382. doi: 10.1200/JCO.2014.55.7348. [DOI] [PubMed] [Google Scholar]
- 77.du Bois A., Kristensen G., Ray-Coquard I., Reuss A., Pignata S., Colombo N., Denison U., Vergote I., Del Campo J.M., Ottevanger P., et al. AGO Study Group led Gynecologic Cancer Intergroup/European Network of Gynaecologic Oncology Trials Groups Intergroup Consortium. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2016;17:78–89. doi: 10.1016/S1470-2045(15)00366-6. [DOI] [PubMed] [Google Scholar]
- 78.Coleman R.L., Oza A.M., Lorusso D., Aghajanian C., Oaknin A., Dean A., Colombo N., Weberpals J.I., Clamp A., Scambia G., et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–1961. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu C.H., Chang Y., Wang P.H. Poly(ADP-ribose) polymerase (PARP) inhibitors and ovarian cancer. Taiwan J. Obstet. Gynecol. 2017;56:713–714. doi: 10.1016/j.tjog.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 80.Moore K., Colombo N., Scambia G., Kim B.G., Oaknin A., Friedlander M., Lisyanskaya A., Floquet A., Leary A., Sonke G.S., et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 81.Vergote I., du Bois A., Floquet A., Rau J., Kim J.W., Del Campo J.M., Friedlander M., Pignata S., Fujiwara K., Colombo N., et al. Overall survival results of AGO-OVAR16: A phase 3 study of maintenance pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced ovarian cancer. Gynecol. Oncol. 2019;155:186–191. doi: 10.1016/j.ygyno.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 82.Boussios S., Karihtala P., Moschetta M., Karathanasi A., Sadauskaite A., Rassy E., Pavlidis N. Combined strategies with poly (ADP-Ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer: A literature review. Diagnostics. 2019;9:87. doi: 10.3390/diagnostics9030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feng Y., Huang H., Wan T., Zhang C., Tong C., Liu J. Comparison of PARPis with angiogenesis inhibitors and chemotherapy for maintenance in ovarian cancer: A network meta-analysis. Adv. Ther. 2019;36:3368–3380. doi: 10.1007/s12325-019-01106-1. [DOI] [PubMed] [Google Scholar]
- 84.Vergote I., Scambia G., O’Malley D.M., Van Calster B., Park S.Y., Del Campo J.M., Meier W., Bamias A., Colombo N., Wenham R.M., et al. Trebananib or placebo plus carboplatin and paclitaxel as first-line treatment for advanced ovarian cancer (TRINOVA-3/ENGOT-ov2/GOG-3001): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20:862–876. doi: 10.1016/S1470-2045(19)30178-0. [DOI] [PubMed] [Google Scholar]
- 85.Coleman R.L., Fleming G.F., Brady M.F., Swisher E.M., Steffensen K.D., Friedlander M., Okamoto A., Moore K.N., Efrat Ben-Baruch N., Werner T.L., et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N. Engl. J. Med. 2019;381:2403–2415. doi: 10.1056/NEJMoa1909707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beaver J.A., Coleman R.L., Arend R.C., Armstrong D.K., Bala S., Mills G.B., Sood A.K., Herzog T.J. Advancing drug development in gynecologic malignancies. Clin. Cancer Res. 2019;25:4874–4880. doi: 10.1158/1078-0432.CCR-19-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee W.L., Wang P.H. Aberrant sialylation in ovarian cancers. J. Chin. Med. Assoc. 2020;83:337–344. doi: 10.1097/JCMA.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 88.Matanes E., Gotlieb W.H. Immunotherapy of gynecological cancers. Best Pract. Res. Clin. Obstet. Gynaecol. 2019;60:97–110. doi: 10.1016/j.bpobgyn.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 89.Lee W.L., Wang P.H. Immunology and ovarian cancers. J. Chin. Med. Assoc. 2020;83:425–432. doi: 10.1097/JCMA.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 90.Suidan R.S., He W., Sun C.C., Zhao H., Rauh-Hain J.A., Fleming N.D., Lu K.H., Giordano S.H., Meyer L.A. Total and out-of-pocket costs of different primary management strategies in ovarian cancer. Am. J. Obstet. Gynecol. 2019;221:136.e1–136.e9. doi: 10.1016/j.ajog.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Markman M., Liu P.Y., Wilczynski S., Monk B., Copeland L.J., Alvarez R.D., Jiang C., Alberts D., Southwest Oncology Group. Gynecologic Oncology Group Southwest Oncology, Oncology G. Gynecologic, Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: A Southwest Oncology Group and Gynecologic Oncology Group trial. J. Clin. Oncol. 2003;21:2460–2465. doi: 10.1200/JCO.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 92.de Jongh F.E., de Wit R., Verweij J., Sparreboom A., van den Bent M.J., Stoter G., van der Burg M.E. Dose-dense cisplatin/paclitaxel. a well-tolerated and highly effective chemotherapeutic regimen in patients with advanced ovarian cancer. Eur. J. Cancer. 2002;38:2005–2013. doi: 10.1016/S0959-8049(02)00242-3. [DOI] [PubMed] [Google Scholar]
- 93.Viret F., Bertucci F., Genre D., Gravis G., Chabannon C., Conte M., Houvenaeghel G., Maraninchi D., Viens P. Intensive sequential dose dense chemotherapy with stem cell support as first-line treatment in advanced ovarian carcinoma: A phase II study. Bone, Marrow, Transplant. 2002;30:879–884. doi: 10.1038/sj.bmt.1703762. [DOI] [PubMed] [Google Scholar]
- 94.Marchetti P., Urien S., Cappellini G.A., Ronzino G., Ficorella C. Weekly administration of paclitaxel: Theoretical and clinical basis. Crit. Rev. Oncol. Hematol. 2002;44:S3–S13. doi: 10.1016/S1040-8428(02)00109-9. [DOI] [PubMed] [Google Scholar]
- 95.Fizazi K., Zelek L. Is one cycle every three or four week’s obsolete? A critical review of dose-dense chemotherapy in solid neoplasms. Ann. Oncol. 2000;11:133–149. doi: 10.1023/A:1008344014518. [DOI] [PubMed] [Google Scholar]
- 96.Goldie J.H., Coldman A.J. The genetic origin of drug resistance in neoplasms: Implication for systemic therapy. Cancer. Res. 1984;44:3643–3653. [PubMed] [Google Scholar]
- 97.Norton L., Simon R. The Norton-Simon hypothesis revisited. Cancer. Treat. Rep. 1986;70:163–169. [PubMed] [Google Scholar]
- 98.Simon R., Norton L. The Norton-Simon hypothesis: Designing more effective and less toxic chemotherapeutic regimens. Nat. Clin. Pract. Oncol. 2006;3:406–407. doi: 10.1038/ncponc0560. [DOI] [PubMed] [Google Scholar]
- 99.Sparano J.A., Wang M., Martino S., Jones V., Perez E.A., Saphner T., Wolff A.C., Sledge G.W., Jr., Wood W.C., Davidson N.E. Weekly paclitaxel in the adjuvant treatment of breast cancer. N. Engl. J. Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Milani A., Kristeleit R., McCormack M., Raja F., Luvero D., Widschwendter M., MacDonald N., Mould T., Olatain A., Hackshaw A., et al. Switching from standard to dose-dense chemotherapy in front-line treatment of advanced ovarian cancer: A retrospective study of feasibility and efficacy. ESMO Open. 2017;1:e000117. doi: 10.1136/esmoopen-2016-000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rettenmaier M.A., Micha J.P., Bohart R., Goldstein B.H. A retrospective study comparing the efficacy of dose-dense chemotherapy, intraperitoneal chemotherapy and dose-dense chemotherapy with hyperthermic intraperitoneal chemotherapy in the treatment of advanced stage ovarian carcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;44:101–105. doi: 10.1016/j.ejogrb.2019.10.047. [DOI] [PubMed] [Google Scholar]
- 102.Murphy M., Martin G., Mahmoudjafari Z., Bivona C., Grauer D., Henry D. Intraperitoneal paclitaxel and cisplatin compared with dose-dense paclitaxel and carboplatin for patients with stage III ovarian cancer. J. Oncol. Pharm. Pract. 2020:1078155219899460. doi: 10.1177/1078155219899460. [DOI] [PubMed] [Google Scholar]
- 103.Chang C.L., Hsu Y.T., Wu C.C., Lai Y.Z., Wang C., Yang Y.C., Wu T.C., Hung C.F. Dose-dense chemotherapy improves mechanisms of antitumor immune response. Cancer. Res. 2013;73:119–127. doi: 10.1158/0008-5472.CAN-12-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stasenko M., Reynolds R.K., Johnston C., Brackman M., McLean K., Uppal S. Adherence to hematologic hold parameters in carboplatin and dose-dense paclitaxel chemotherapy for ovarian malignancies: A survey of NCCN member institutions. J. Natl. Compr. Cancer Netw. 2016;14:849–853. doi: 10.6004/jnccn.2016.0089. [DOI] [PubMed] [Google Scholar]
- 105.Boraska Jelavić T., Boban T., Brčić L., Vrdoljak E. Is macrocytosis a potential biomarker of the efficacy of dose-dense paclitaxel–carboplatin combination therapy in patients with epithelial ovarian cancer? Anticancer Drugs. 2017;28:922–927. doi: 10.1097/CAD.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 106.Huang C.Y., Yang Y.C., Wang K.L., Chen T.C., Chen J.R., Weng C.S., Chien H.J., Chang C.L. Possible surrogate marker for an effective dose-dense chemotherapy in treating ovarian cancer. Taiwan J Obstet. Gynecol. 2016;55:405–409. doi: 10.1016/j.tjog.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 107.Lee W.L., Chan I.S., Wang P.H. Does a simple hematological examination predict the response and side effects in patients undergoing induction chemotherapy and/or neoadjuvant chemotherapy? J. Chin. Med. Assoc. 2020;83:107–108. doi: 10.1097/JCMA.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 108.Vasey P.A. “Dose dense” chemotherapy in ovarian cancer. Int. J. Gynecol. Cancer. 2005;15(Suppl. 3):226–232. doi: 10.1111/j.1525-1438.2005.00438.x. [DOI] [PubMed] [Google Scholar]
- 109.Muggia F.M. Sequential single agents as first-line chemotherapy for ovarian cancer: A strategy derived from the results of GOG-132. Int. J. Gynecol. Cancer. 2003;13(Suppl. 2):156–162. doi: 10.1136/ijgc-00009577-200311001-00005. [DOI] [PubMed] [Google Scholar]
- 110.Muggia F.M. Relevance of chemotherapy dose and schedule to outcomes in ovarian cancer. Semin. Oncol. 2004;31(Suppl. 15):19–24. doi: 10.1053/j.seminoncol.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 111.van der Burg M.E., van der Gaast A., Vergote I., Burger C.W., van Doorn H.C., de Wit R., Stoter G., Verweij J. What is the role of dose-dense therapy? Int. J. Gynecol. Cancer. 2005;15(Suppl. 3):233–240. doi: 10.1111/j.1525-1438.2005.00432.x. [DOI] [PubMed] [Google Scholar]
- 112.van den Bent M.J., van Putten W.L., Hilkens P.H., de Wit R., van der Burg M.E. Retreatment with dose-dense weekly cisplatin after previous cisplatin chemotherapy is not complicated by significant neuro-toxicity. Eur. J. Cancer. 2002;38:387–391. doi: 10.1016/S0959-8049(01)00381-1. [DOI] [PubMed] [Google Scholar]
- 113.Vasey P.A., Kaye S.B. Dose intensity in ovarian cancer. In: Gershenson D.M., McGuire W.P., editors. Ovarian Cancer Controversies in Management. Churchill Livingstone; New York, NY, USA: 1997. pp. 139–169. [Google Scholar]
- 114.Fruscio R., Garbi A., Parma G., Lissoni A.A., Garavaglia D., Bonazzi C.M., Dell’anna T., Mangioni C., Milani R., Colombo N. Randomized phase III clinical trial evaluating weekly cisplatin for advanced epithelial ovarian cancer. J. Natl. Cancer. Inst. 2011;103:347–351. doi: 10.1093/jnci/djq530. [DOI] [PubMed] [Google Scholar]
- 115.Boere I.A., van der Burg M.E. Review of dose-intense platinum and/or paclitaxel containing chemotherapy in advanced and recurrent epithelial ovarian cancer. Curr. Pharm. Des. 2012;18:3741–3753. doi: 10.2174/138161212802002634. [DOI] [PubMed] [Google Scholar]
- 116.Tiersten A.D., Sill M.W., Knight D., Muggia F., Garcia A.A., Swensen R., Warshal D.P., Mannel R.S., Fracasso P.M. A phase I trial of dose-dense (biweekly) carboplatin combined with paclitaxel and pegfilgrastim: A feasibility study in patients with untreated Stage III and IV ovarian, tubal or primary peritoneal cancer: A Gynecologic Oncology Group study. Gynecol. Oncol. 2010;118:303–307. doi: 10.1016/j.ygyno.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Briasoulis E., Karavasilis V., Tzamakou E., Haidou C., Piperidou C., Pavlidis N. Pharmacodynamics of non-break weekly paclitaxel (Taxol) and pharmacokinetics of Cremophor-EL vehicle: Results of a dose-escalation study. Anticancer Drugs. 2002;13:481–489. doi: 10.1097/00001813-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 118.Thomas H., Rosenberg P. Role of weekly paclitaxel in the treatment of advanced ovarian cancer. Crit. Rev. Oncol. Hematol. 2002;44:S43–S51. doi: 10.1016/S1040-8428(02)00103-8. [DOI] [PubMed] [Google Scholar]
- 119.Mori T., Kinoshita Y., Watanabe A., Yamaguchi T., Hosokawa K., Honjo H. Retention of paclitaxel in cancer cells for 1 week in vivo and in vitro. Cancer. Chemother. Pharmacol. 2006;58:665–672. doi: 10.1007/s00280-006-0209-6. [DOI] [PubMed] [Google Scholar]
- 120.Kumar A., Hoskins P.J., Tinker A.V. Dose-dense paclitaxel in advanced ovarian cancer. Clin. Oncol. (R. Coll. Radiol) 2015;27:40–47. doi: 10.1016/j.clon.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 121.Kaye S.B. First-line chemotherapy for ovarian cancer—the controversy continues. Br. J. Cancer. 2002;87:813–814. doi: 10.1038/sj.bjc.6600568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fiseha T., Mengesha T., Girma R., Kebede E., Gebreweld A. Estimation of renal function in adult outpatients with normal serum creatinine. BMC Res. Notes. 2019;12:462. doi: 10.1186/s13104-019-4487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chang W.H., Horng H.C., Yeh C.C., Guo C.Y., Chou Y.J., Huang N., Lee W.L., Wang P.H. Risks of female genital tract related cancers (gynecological cancers) or breast cancer in women with and without chronic kidney disease: A population-based cohort study in Taiwan. Medicine. 2018;97:e0157. doi: 10.1097/MD.0000000000010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang B.S., Chang W.H., Wang K.C., Huang N., Guo C.Y., Chou Y.J., Lee W.L., Wang P.H. Endometriosis might be inversely associated with developing chronic kidney disease: A population-based cohort study in Taiwan. Int. J. Mol. Sci. 2016;17:1079. doi: 10.3390/ijms17071079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chung A.E., Shoenbill K., Mitchell S.A., Dueck A.C., Schrag D., Bruner D.W., Minasian L.M., St Germain D., O’Mara A.M., Baumgartner P., et al. Patient free text reporting of symptomatic adverse events in cancer clinical research using the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) J. Am. Med. Inform. Assoc. 2019;26:276–285. doi: 10.1093/jamia/ocy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schoen M.W., Basch E., Hudson L.L., Chung A.E., Mendoza T.R., Mitchell S.A., St Germain D., Baumgartner P., Sit L., Rogak L.J., et al. Software for administering the National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events: Usability Study. JMIR Hum. Factors. 2018;5:e10070. doi: 10.2196/10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Raimondi A., Randon G., Sepe P., Claps M., Verzoni E., de Braud F., Procopio G. The evaluation of response to immunotherapy in metastatic renal cell carcinoma: Open challenges in the clinical practice. Int. J. Mol. Sci. 2019;20:4263. doi: 10.3390/ijms20174263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seymour L., Bogaerts J., Perrone A., Ford R., Schwartz L.H., Mandrekar S., Lin N.U., Litière S., Dancey J., Chen A., et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet. Oncol. 2017;18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Beaumont H., Bertrand A.S., Klifa C., Patriti S., Cippolini S., Lovera C., Iannessi A. Radiology workflow for RECIST assessment in clinical trials: Can we reconcile time-efficiency and quality? Eur. J. Radiol. 2019;118:257–263. doi: 10.1016/j.ejrad.2019.07.030. [DOI] [PubMed] [Google Scholar]
- 130.Cheng M., Lee H.H., Chang W.H., Lee N.R., Huang H.Y., Chen Y.J., Horng H.C., Lee W.L., Wang P.H. Weekly dose-dense paclitaxel and triweekly low-dose cisplatin: A well-tolerated and effective chemotherapeutic regimen for first-line treatment of advanced ovarian, fallopian tube, and primary peritoneal cancer. Int. J. Environ. Res. Public Health. 2019;16:4794. doi: 10.3390/ijerph16234794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang Z.J., Zhao B.B., Li L. The significance of the change pattern of serum CA125 level for judging prognosis and diagnosing recurrences of epithelial ovarian cancer. J. Ovarian. Res. 2016;9:57. doi: 10.1186/s13048-016-0266-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 132.Abaid L.N., Micha J.P., Rettenmaier M.A., Brown J.V., Mendivil A.A., Lopez K.L., Goldstein B.H. A phase II study of modified dose-dense paclitaxel and every 4-week carboplatin for the treatment of advanced-stage primary epithelial ovarian, fallopian tube, or peritoneal carcinoma. Cancer. Chemother. Pharmacol. 2013;72:101–107. doi: 10.1007/s00280-013-2173-2. [DOI] [PubMed] [Google Scholar]
- 133.Fleming N.D., Coleman R.L., Tung C., Westin S.N., Hu W., Sun Y., Bhosale P., Munsell M.F., Sood A.K. Phase II trial of bevacizumab with dose-dense paclitaxel as first-line treatment in patients with advanced ovarian cancer. Gynecol. Oncol. 2017;147:41–46. doi: 10.1016/j.ygyno.2017.07.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bun S., Yunokawa M., Ebata T., Shimomura A., Shimoi T., Kodaira M., Yonemori K., Shimizu C., Fujiwara Y., Kato T., et al. Feasibility of dose-dense paclitaxel/carboplatin therapy in elderly patients with ovarian, fallopian tube, or peritoneal cancer. Cancer. Chemother. Pharmacol. 2016;78:745–752. doi: 10.1007/s00280-016-3100-0. [DOI] [PubMed] [Google Scholar]
- 135.Bun S., Yunokawa M., Ebata T., Kobayashi Kato M., Shimoi T., Kato T., Tamura K. Feasibility of initial treatment in elderly patients with ovarian cancer in Japan: A retrospective study. Int. J. Clin. Oncol. 2019;24:1111–1118. doi: 10.1007/s10147-019-01449-3. [DOI] [PubMed] [Google Scholar]
- 136.Minagawa Y., Kigawa J., Kanamori Y., Itamochi H., Terakawa N., Okada M., Kitada F. Feasibility study comparing docetaxel–cisplatin versus docetaxel–carboplatin as first-line chemotherapy for ovarian cancer. Gynecol. Oncol. 2006;101:495–498. doi: 10.1016/j.ygyno.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 137.Morton J.M., George J.N. Microangiopathic hemolytic anemia and thrombocytopenia in patients with cancer. J. Oncol. Pract. 2016;12:523–530. doi: 10.1200/JOP.2016.012096. [DOI] [PubMed] [Google Scholar]
- 138.Mones J.V., Soff G. Management of thrombocytopenia in cancer patients. Cancer. Treat. Res. 2019;179:139–150. doi: 10.1007/978-3-030-20315-3_9. [DOI] [PubMed] [Google Scholar]
- 139.Al-Samkari H., Connors J.M. Managing the competing risks of thrombosis, bleeding, and anticoagulation in patients with malignancy. Blood Adv. 2019;3:3770–3779. doi: 10.1182/bloodadvances.2019000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Boyd L.R., Muggia F.M. Carboplatin/paclitaxel induction in ovarian cancer: The finer points. Oncol. (Williston Park) 2018;32:418–420. [PubMed] [Google Scholar]
- 141.Egorin M.J., Van Echo D.A., Tipping S.J., Olman E.A., Whitacre M.Y., Thompson B.W., Aisner J. Pharmacokinetics and dosage reduction of cis-diammine(1,1-cyclobutanedicarboxylato)platinum in patients with impaired renal function. Cancer. Res. 1984;44:5432–5438. [PubMed] [Google Scholar]
- 142.Wang P.H., Yuan C.C., Shyong W.Y., Chiang S.C., Chao J.Y., Yen M.S., Ng H.T. Optimal debulking surgery is an independent prognostic factor in patients with FIGO IIIC primary epithelial ovarian carcinoma. Zhonghua Yi Xue Za Zhi (Taipei) 2000;63:220–225. [PubMed] [Google Scholar]
- 143.Elattar A., Bryant A., Winter-Roach B.A., Hatem M., Naik R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst. Rev. 2011:CD007565. doi: 10.1002/14651858.CD007565.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Javellana M., Hoppenot C., Lengyel E. The road to long-term survival: Surgical approach and longitudinal treatments of long-term survivors of advanced-stage serous ovarian cancer. Gynecol. Oncol. 2019;152:228–234. doi: 10.1016/j.ygyno.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 145.Manning-Geist B.L., Hicks-Courant K., Gockley A.A., Clark R.M., Del Carmen M.G., Growdon W.B., Horowitz N.S., Berkowitz R.S., Muto M.G., Worley M.J., Jr. Moving beyond “complete surgical resection” and “optimal”: Is low-volume residual disease another option for primary debulking surgery? Gynecol. Oncol. 2018;150:233–238. doi: 10.1016/j.ygyno.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 146.Sioulas V.D., Schiavone M.B., Kadouri D., Zivanovic O., Roche K.L., O’Cearbhaill R., Abu-Rustum N.R., Levine D.A., Sonoda Y., Gardner G.J., et al. Optimal primary management of bulky stage IIIC ovarian, fallopian tube and peritoneal carcinoma: Are the only options complete gross resection at primary debulking surgery or neoadjuvant chemotherapy? Gynecol. Oncol. 2017;145:15–20. doi: 10.1016/j.ygyno.2017.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lu J., Jiang Y., Qian M., Lv L., Ying X. The improved effects of a multidisciplinary team on the survival of breast cancer patients: Experiences from China. Int. J. Environ. Res. Public Health. 2020;17:277. doi: 10.3390/ijerph17010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Natarajan R., Aljaber D., Au D., Thai C., Sanchez A., Nunez A., Resto C., Chavez T., Jankowska M.M., Benmarhnia T., et al. Environmental exposures during puberty: Window of breast cancer risk and epigenetic damage. Int. J. Environ. Res. Public Health. 2020;17:493. doi: 10.3390/ijerph17020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Atlan M., Neman J. Targeted transdermal delivery of curcumin for breast cancer prevention. Int. J. Environ. Res. Public Health. 2019;16:4949. doi: 10.3390/ijerph16244949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Paulauskiene J., Stelemekas M., Ivanauskiene R., Petkeviciene J. The cost-effectiveness analysis of cervical cancer screening using a systematic invitation system in Lithuania. Int. J. Environ. Res. Public Health. 2019;16:5035. doi: 10.3390/ijerph16245035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lipscomb J., Escoffery C., Gillespie T.W., Henley S.J., Smith R.A., Chociemski T., Almon L., Jiang R., Sheng X., Goodman M., et al. Improving screening uptake among breast cancer survivors and their first-degree relatives at elevated risk to breast cancer: Results and implications of a randomized study in the state of Georgia. Int. J. Environ. Res. Public Health. 2020;17:977. doi: 10.3390/ijerph17030977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harano K., Terauchi F., Katsumata N., Takahashi F., Yasuda M., Takakura S., Takano M., Yamamoto Y., Sugiyama T. Quality-of-life outcomes from a randomized phase III trial of dose-dense weekly paclitaxel and carboplatin compared with conventional paclitaxel and carboplatin as a first-line treatment for stage II-IV ovarian cancer: Japanese Gynecologic Oncology Group Trial (JGOG3016) Ann. Oncol. 2014;25:251–257. doi: 10.1093/annonc/mdt527. [DOI] [PubMed] [Google Scholar]
- 153.Dalton H.J., Yu X., Hu L., Kapp D.S., Benjamin I., Monk B.J., Chan J.K. An economic analysis of dose dense weekly paclitaxel plus carboplatin versus every-3-week paclitaxel plus carboplatin in the treatment of advanced ovarian cancer. Gynecol. Oncol. 2012;124:199–204. doi: 10.1016/j.ygyno.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 154.Seagle B.L., Shahabi S. Cost-effectiveness analysis of dose-dense versus standard intravenous chemotherapy for ovarian cancer: An economic analysis of results from the Gynecologic Oncology Group protocol 262 randomized controlled trial. Gynecol. Oncol. 2017;145:9–14. doi: 10.1016/j.ygyno.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 155.Herzog T.J., Cohn D.E. Dose dense chemotherapy for front-line ovarian cancer treatment: The price is right? Gynecol. Oncol. 2017;145:1–2. doi: 10.1016/j.ygyno.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 156.Foote J., Secord A.A., Liang M., Cohn D.E., Jewell E., Havrilesky L.J. ASCO value framework highlights the relative value of treatment options in ovarian cancer. J. Oncol. Pract. 2017;13:e1030–e1039. doi: 10.1200/JOP.2017.025106. [DOI] [PubMed] [Google Scholar]
- 157.Wright J.D., Hou J.Y., Burke W.M., Tergas A.I., Chen L., Hu J.C., Ananth C.V., Neugut A.I., Hershman D.L. Utilization and toxicity of alternative delivery methods of adjuvant chemotherapy for ovarian cancer. Obstet. Gynecol. 2016;127:985–991. doi: 10.1097/AOG.0000000000001436. [DOI] [PMC free article] [PubMed] [Google Scholar]