Key Points
Question
What are the embolic outcomes associated with using direct oral anticoagulants for left ventricular thrombi, and how do they compare with outcomes associated with using warfarin for the same indication?
Findings
In this cohort study of 514 patients with echocardiographically diagnosed left ventricular thrombi, anticoagulation with direct oral anticoagulants was associated with a higher risk of ischemic stroke and systemic emboli compared with warfarin treatment.
Meaning
Off-label use of direct oral anticoagulants for left ventricular thrombi should be undertaken with caution until clinical trial data are available to compare their use with warfarin.
Abstract
Importance
Left ventricular (LV) thrombi can arise in patients with ischemic and nonischemic cardiomyopathies. Anticoagulation is thought to reduce the risk of stroke or systemic embolism (SSE), but there are no high-quality data on the effectiveness of direct oral anticoagulants (DOACs) for this indication.
Objective
To compare the outcomes associated with DOAC use and warfarin use for the treatment of LV thrombi.
Design, Setting, and Participants
A cohort study was performed at 3 tertiary care academic medical centers among 514 eligible patients with echocardiographically diagnosed LV thrombi between October 1, 2013, and March 31, 2019. Follow-up was performed through the end of the study period.
Exposures
Type and duration of anticoagulant use.
Main Outcomes and Measures
Clinically apparent SSE.
Results
A total of 514 patients (379 men; mean [SD] age, 58.4 [14.8] years) with LV thrombi were identified, including 300 who received warfarin and 185 who received a DOAC (64 patients switched treatment between these groups). The median follow-up across the patient cohort was 351 days (interquartile range, 51-866 days). On unadjusted analysis, DOAC treatment vs warfarin use (hazard ratio [HR], 2.71; 95% CI, 1.31-5.57; P = .01) and prior SSE (HR, 2.13; 95% CI, 1.22-3.72; P = .01) were associated with SSE. On multivariable analysis, anticoagulation with DOAC vs warfarin (HR, 2.64; 95% CI, 1.28-5.43; P = .01) and prior SSE (HR, 2.07; 95% CI, 1.17-3.66; P = .01) remained significantly associated with SSE.
Conclusions and Relevance
In this multicenter cohort study of anticoagulation strategies for LV thrombi, DOAC treatment was associated with a higher risk of SSE compared with warfarin use, even after adjustment for other factors. These results challenge the assumption of DOAC equivalence with warfarin for LV thrombi and highlight the need for prospective randomized clinical trials to determine the most effective treatment strategies for LV thrombi.
This cohort study compares the outcomes associated with direct oral anticoagulant use and warfarin use for the treatment of left ventricular thrombi.
Introduction
Left ventricular (LV) thrombi are associated with LV dysfunction.1 They can occur as a complication of acute myocardial infarction but may arise in nonischemic cardiomyopathies as well.2 Despite decades of study of LV thrombi, data of which we are aware regarding their treatment, particularly in the prevention of embolic events, are scarce. For example, the most rigorous study to date is a 1993 meta-analysis of 7 largely observational studies of warfarin conducted between 1979 and 1990.3 Although there are limited data on the use of low-molecular-weight heparin, there have been no prospective studies to date, to our knowledge, of alternative oral anticoagulants to prevent embolization of LV thrombi.4
In the interim, new oral anticoagulants have been brought to market. Oral factor Xa and direct thrombin inhibitors have been approved for use and are associated with a reduction in the risk of stroke or systemic embolism (SSE) in patients with nonvalvular atrial fibrillation (AF) and venous thromboembolism.5,6,7,8,9,10,11 Clinical use of these direct oral anticoagulants (DOACs) has been extended to off-label indications such as heparin-induced thrombocytopenia and antiphospholipid syndrome12,13,14; however, the assumption of generalized interchangeability between DOACs and warfarin is potentially problematic. For example, dabigatran etexilate used for thromboprophylaxis of mechanical heart valves demonstrated higher rates of both ischemic stroke and bleeding.15 In spite of this finding, there is growing enthusiasm about the use of DOACs by both patients and clinicians owing to their ease of administration, absence of a requirement for international normalized ratio monitoring, and freedom from dietary restrictions, among other factors that may improve patient quality of life.16,17 Perhaps because of these perceived advantages, there is a persistent clinical interest in the application of DOACs to conditions such as LV thrombi.18 Accordingly, we sought to assess the association between the use of DOACs for the treatment of LV thrombi and embolic outcomes, using warfarin as a reference, as part of the Retrospective Evaluation of DOACs and Vascular Endpoints of Left Ventricular Thrombi (RED VELVT) observational study.
Methods
Patient Population
We evaluated the treatment patterns and outcomes of LV thrombi at 3 tertiary care academic medical centers. Patients with echocardiographically diagnosed LV thrombi were identified from October 1, 2013 (1 year after approval of the third DOAC, apixaban), through March 31, 2019. Baseline clinical, demographic, and echocardiographic information was recorded, as well as duration and timing of anticoagulation regimens. Echocardiographic data included the clinical interpretation regarding the morphologic characteristics and mobility of thrombi. The size of the thrombus was measured as the largest 2-dimensional area available on the index echocardiogram. Imaging characteristics were also evaluated from the subsequent echocardiogram (if performed) and from the most recent echocardiogram. Embolic events were cataloged as a composite outcome of clinically documented SSE. Patients who had not already experienced an SSE or censoring event (death, heart transplant, surgical LV assist device placement, or surgical thrombectomy) by medical record review were individually contacted by telephone for final ascertainment of events. In cases in which patients could not be contacted, information was extracted from the available medical record, with censoring at the time of their most recent hospital discharge or outpatient visit with a cardiologist or primary care clinician. The presenting embolism was defined as SSE occurring within 30 days prior to the index echocardiogram. Events that occurred during a period of bridging therapy with a parenteral anticoagulant to an oral agent were considered to be associated with the parenteral anticoagulant but not with the oral agent. The study protocol was approved by the University of Virginia, Virginia Commonwealth University, and the University of North Carolina Institutional Review Boards. Written consent was waived owing to minimal patient risk. Oral consent was obtained at the time of the telephone interview.
Statistical Analysis
The baseline characteristics between groups were compared using 1-way analysis of variance for continuous variables and the χ2 test for categorical variables. Pairwise testing was performed when the overall P < .05. Hazard ratios (HRs) were calculated with or without adjustment for covariates using Cox proportional hazards regression models. To better capture the detail of real-world anticoagulant use, including treatment switching and pauses, anticoagulation treatment during different time periods was treated as a time-dependent covariate. Nonparametric comparison of the risk of the composite end point (SSE) between anticoagulation strategies was evaluated using the method of Mantel and Byar.19 The association of anticoagulant treatment periods with SSE risk was illustrated visually with modified survival curves, using the method derived by Simon and Makuch.20 In this modification of standard Kaplan-Meier curves, the cohorts are continually updated such that a patient who takes both a DOAC and warfarin during different time intervals contributes to the DOAC cohort for only the period that a DOAC is taken and contributes to the warfarin cohort for only the period that warfarin is being taken. To determine factors associated with SSE, Cox proportional hazards regression analysis was performed. Clinical, demographic, and echocardiographic variables were evaluated via univariable Cox proportional hazards regression to determine whether they were factors significantly associated with SSE. Variables with P < .10 in a univariable model were then included in a stepwise multivariable Cox proportional hazards regression model, in which variables that produced P < .05 were considered statistically significant. Rather than using subdistribution hazards, we accounted for competing events (death) by evaluating the cause-specific hazard of each event type (primary end point and competing risk) as well as a composite end point without censoring.21 Statistical analyses were conducted using R Studio and R, version 3.5.1 (R Foundation for Statistical Computing).
Results
Patients
A total of 514 patients (379 men; mean [SD] age, 58.4 [14.8] years) who had echocardiographically diagnosed LV thrombi were identified. A more exhaustive review of the population studied is included the Supplement, including types of cardiomyopathy (eTable 1 in the Supplement), and timing of anticoagulation (eAppendix 1 in the Supplement). Three hundred patients were treated with warfarin, and 185 patients were treated with a DOAC. These groups included a mixed cohort of 64 patients (therapy-change group) who switched treatment, such that there were 236 patients treated exclusively with warfarin (warfarin-only group) and 121 patients treated exclusively with a DOAC (DOAC-only group). There was a total of 52 changes from warfarin to a DOAC and a total of 19 changes from a DOAC to warfarin. The most common cause of switching therapy from warfarin to a DOAC was convenience (10 switches [19.2%]), whereas the most common cause of switching therapy from a DOAC to warfarin was cost (6 switches [31.6%]). A comprehensive description of treatment switching is included in eAppendix 2 and eTable 3 in the Supplement. A total of 93 patients received no oral anticoagulation, including 43 patients who were not treated with any anticoagulants, oral or parenteral. Of the 185 patients treated with a DOAC, 141 (76.2%) were treated with apixaban, 46 (24.9%) were treated with rivaroxaban, and 9 (4.9%) were treated with dabigatran (Figure 1). Baseline demographic, clinical, and echocardiographic data in each treatment group are displayed in Table 1. Patients tended to be male, with a relatively balanced representation of races/ethnicities, and they were typically in their sixth decade of life.
Figure 1. Oral Anticoagulation Strategies.
Of the 514 patients with left ventricular thrombi, 421 were treated with an oral anticoagulant. Three hundred were treated with warfarin at any point during the follow-up period (any warfarin) and 185 were treated with a direct oral anticoagulant (DOAC; any DOAC). These groups included a mixed cohort of 64 patients (therapy change), who switched treatment, such that there were 236 patients treated exclusively with warfarin (warfarin only), and 121 patients treated exclusively with a DOAC (DOAC only). Among the patients treated with a DOAC, 141 were treated with apixaban, 46 with rivaroxaban, and 9 with dabigatran. No patients were treated with edoxaban.
Table 1. Baseline Demographic, Clinical, and Echocardiographic Characteristics of Patients With LV Thrombia.
| Characteristic | Patients, No. (%) | P valueb | |||
|---|---|---|---|---|---|
| DOAC only (n = 121) | Warfarin only (n = 236) | Therapy change (n = 64) | Neither (n = 93) | ||
| Age, mean (SD), y | 58.1 (14.9) | 58.2 (15.1) | 55.5 (12.5) | 61.6 (14.9) | .06 |
| Male sex | 94 (77.7) | 170 (72.0) | 44 (68.8) | 71 (76.3) | .48 |
| White race/ethnicity | 73 (60.3) | 119 (50.4) | 32 (50.0) | 60 (64.5)c | .04 |
| Type 1 and 2 diabetes | 36 (29.8) | 92 (39.0) | 26 (40.6) | 41 (44.1) | .21 |
| Hypertension | 86 (71.1) | 177 (75.0) | 47 (73.4) | 66 (71.0) | .99 |
| Hyperlipidemia | 71 (58.7) | 126 (53.4) | 29 (45.3) | 47 (50.5) | .32 |
| Ischemic cardiomyopathy | 66 (54.5) | 148 (62.7) | 36 (56.3) | 60 (64.5) | .34 |
| Venous thromboembolism | 25 (20.7) | 38 (16.1) | 19 (29.7)c | 11 (11.8)d | .02 |
| Atrial fibrillation | 30 (24.8) | 45 (19.1) | 23 (35.9)c | 23 (24.7) | .04 |
| Prior SSE | 33 (27.3) | 51 (21.6) | 15 (23.4) | 12 (12.9) | .09 |
| Presenting embolism | 21 (17.4) | 34 (14.4) | 10 (15.6) | 8 (8.6) | .32 |
| BMI, mean (SD) | 28.2 (6.8) | 28.8 (7.4) | 30.5 (6.6) | 26.8 (5.1)d | .01 |
| Estimated GFR, mean (SD), mL/min/1.73 m2 | 80.5 (29.3) | 75.8 (29.8) | 79.4 (25.4) | 75.5 (32.1) | .45 |
| GFR, mL/min/1.73 m2 | |||||
| <30 | 5 (4.1) | 18 (7.6) | 1 (1.6) | 6 (6.5) | .24 |
| <15 | 1 (0.8) | 8 (3.4) | 0 | 0 | .07 |
| Echocardiographic contrast use | 46 (38.0) | 86 (36.4) | 25 (39.1) | 29 (31.2) | .70 |
| LV ejection fraction, mean (SD), % | 27.7 (13.8) | 28.2 (12.4) | 25.1 (11.7) | 26.6 (12.0) | .33 |
| Apical thrombus location | 115 (95.0) | 212 (89.8) | 56 (87.5) | 82 (88.2) | .23 |
| Mobile thrombus | 19 (15.7) | 39 (16.5) | 12 (18.8) | 19 (20.4) | .79 |
| Thrombus size, mean (SD), cm2 | 2.8 (2.1) | 2.8 (2.5) | 2.3 (1.5) | 2.9 (2.7) | .43 |
| Protruding or pedunculated thrombus morphologic characteristics | 12 (9.9) | 12 (5.1) | 3 (4.7) | 9 (9.7) | .22 |
| Antiplatelet therapy | 77 (63.6) | 164 (69.5) | 38 (59.4) | 61 (65.6) | .42 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DOAC, direct oral anticoagulant; GFR, glomerular filtration rate; LV, left ventricular; SSE, stroke or systemic embolism.
Additional data on types of antiplatelet regimens are available in eTable 2 in the Supplement.
Calculated from 1-way analysis of variance testing for continuous variables or χ2 test for categorical variables.
P < .05 compared with warfarin only group.
P < .05 compared with therapy change group.
Follow-up and Events
The median follow-up across the patient cohort was 351 days (interquartile range, 51-866 days). Additional data on follow-up, including follow-up period and treatment duration, are included in eAppendix 1 in the Supplement. There was a total of 54 SSE events, consisting of 36 ischemic strokes and 18 systemic emboli. The overall event rate was 0.065 SSE per patient-year of follow-up. Of the 54 SSE events, 17 occurred among patients taking a DOAC and 14 occurred among patients taking warfarin. There were also 115 deaths during the follow-up period. The total numbers of SSE events and deaths, organized by anticoagulant at time of event, are included in Table 2.
Table 2. Number of SSE Events, Deaths, and Bleeding Events Requiring Cessation in Anticoagulation in Patients With Left Ventricular Thrombi.
| Anticoagulant | Events, No. | ||
|---|---|---|---|
| SSE | Death | Bleeding event | |
| DOAC | 17 | 14 | 8 |
| Warfarin | 14 | 32 | 19 |
| Parenteral agent | 11 | 12 | 4 |
| None | 12 | 57 | NA |
| Total | 54 | 115 | 31 |
Abbreviations: DOAC, direct oral anticoagulant; NA, not applicable; SSE, stroke or systemic embolism.
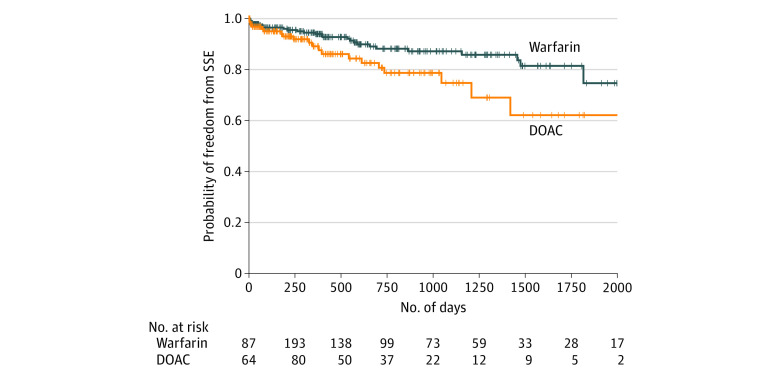
On univariable Cox proportional hazards regression analysis, a higher risk of SSE was significantly associated with DOAC treatment vs warfarin (HR, 2.71; 95% CI, 1.31-5.57; P = .01) (Table 3). Prior SSE was also significantly associated with SSE (HR, 2.13; 95% CI, 1.22-3.72; P = .01). Thrombus mobility, although not significantly associated with SSE, met the prespecified threshold for inclusion in the multivariable analysis (HR, 1.80; 95% CI, 0.96-3.38; P = .07). None of the other variables tested (including patient age, patient race/ethnicity, cardiomyopathy type, body mass index, estimated glomerular filtration rate, history of AF, history of venous thromboembolism, presenting SSE, use of bridging parenteral anticoagulation, antiplatelet therapy, LV ejection fraction, thrombus size, and thrombus morphologic characteristics) were significantly associated with the outcome of SSE in unadjusted Cox proportional hazards regression models. Additional analysis of the risk of SSE and history of AF is included in eAppendix 3 in the Supplement. On multivariable analysis, anticoagulation type (HR for DOAC vs warfarin, 2.64; 95% CI, 1.28-5.43; P = .01) and prior SSE (HR, 2.07; 95% CI, 1.17-3.66; P = .01) remained significantly associated with SSE. Thrombus mobility, however, was not associated with SSE (HR, 1.52; 95% CI, 0.80-2.87; P = .20). The probability of freedom from SSE by anticoagulation type is depicted in Figure 2.
Table 3. Results of Cox Proportional Hazards Regression Analysis.
| Variable | Univariable | Multivariablea | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| DOAC use (vs warfarin) | 2.71 (1.31-5.57) | .01 | 2.64 (1.28-5.43) | .01 |
| Prior SSE | 2.13 (1.22-3.72) | .01 | 2.07 (1.17-3.66) | .01 |
| Thrombus mobility | 1.80 (0.96-3.38) | .07 | 1.52 (0.80-2.87) | .20 |
| Patient age | 0.99 (0.97-1.01) | .19 | NA | NA |
| White ethnicity (vs other) | 1.57 (0.91-2.70) | .10 | NA | NA |
| Ischemic cardiomyopathy (vs nonischemic) | 0.89 (0.51-1.55) | .69 | NA | NA |
| Body mass index | 1.02 (0.99-1.06) | .16 | NA | NA |
| Estimated GFR | 1.00 (0.99-1.01) | .61 | NA | NA |
| History | ||||
| Atrial fibrillation | 0.94 (0.49-1.79) | .85 | NA | NA |
| Venous thromboembolism | 1.03 (0.52-2.06) | .93 | NA | NA |
| Antiplatelet therapy | 0.98 (0.70-1.36) | .90 | NA | NA |
| Bridging anticoagulation | 0.96 (0.45-2.00) | .90 | NA | NA |
| Presenting embolism | 1.46 (0.73-2.91) | .28 | NA | NA |
| Left ventricular ejection fraction | 1.00 (0.97-1.02) | .69 | NA | NA |
| Thrombus size | 1.05 (0.95-1.18) | .35 | NA | NA |
| Pedunculated or protruding thrombus morphologic characteristics | 1.00 (0.31-3.22) | .99 | NA | NA |
Abbreviations: DOAC, direct oral anticoagulant; GFR, glomerular filtration rate; HR, hazard ratio; NA, not applicable; SSE, stroke or systemic embolism.
In multivariable analysis, anticoagulation with DOAC (vs warfarin) and prior SSE were factors significantly associated with SSE. These were included in a multivariable Cox proportional hazards analysis, along with thrombus mobility, which was not a factor significantly associated with SSE in a univariable model but, with P < .10, met prespecified criteria for inclusion. In the multivariable model, prior SSE and anticoagulation with a DOAC were significantly associated with subsequent SSE.
Figure 2. Survival Curves for Freedom From Stroke and Systemic Embolism.
Survival curves are shown for freedom from stroke and systemic embolism (SSE) in patients with left ventricular thrombus after index echocardiogram, Mantel-Byar P < .001. DOAC indicates direct oral anticoagulant.
Imaging surveillance was variable, with 356 patients (69.3%) having at least 1 follow-up echocardiogram; the median time to first study was 81 days (interquartile range, 19-185 days). A second follow-up echocardiogram was taken for 320 patients (62.3%). The median time to the second study was 328 days (interquartile range, 105-890 days). A total of 231 patients had echocardiographically confirmed resolution of their thrombus, including 56 while being treated with a DOAC, 131 while being treated with warfarin, 21 during treatment with parenteral anticoagulation, and 23 without any anticoagulation. Of those with thrombus resolution, 20 patients (8.7%) experienced an SSE within 30 days of their echocardiogram documenting thrombus disappearance. In a Cox proportional hazards regression analysis of the patients who had follow-up imaging, oral anticoagulation use was not associated with thrombus resolution (HR of DOAC vs warfarin, 1.08; 95% CI, 0.78-1.50; P = .64).
Competing Risks
Given the high risk of death in a population with significant LV dysfunction, we accounted for this competing risk by evaluating the risk of death according to oral anticoagulation strategy. In unadjusted Cox proportional hazards regression models, with censoring at the time of SSE, anticoagulation type was not associated with death (HR for DOAC, 1.19; 95% CI, 0.72-1.96; P = .50). Finally, we examined the combined end point of death or SSE. In an unadjusted Cox proportional hazards regression model, treatment with a DOAC vs warfarin was significantly associated with death or SSE (HR, 1.55; 95% CI, 1.05-2.30; P = .03). Survival free of death or SSE is shown in the eFigure in the Supplement.
Landmark Analyses
To evaluate for the possibility of an uneven distribution of LV thrombi of such high risk that they would undergo rapid embolization regardless of anticoagulation strategy, we performed a landmark analysis with exclusion of events within the first 10 days after the index echocardiogram. This exclusion resulted in the removal of 14 events, leaving 40 total SSE events for analysis. After this adjustment, anticoagulation with DOAC vs warfarin remained significantly associated with SSE on univariable Cox proportional hazards regression (HR, 2.67; 95% CI, 1.25-5.70; P = .01).
Given the delayed divergence of survival curves and the potential for increased problems with confounding, we performed landmark analyses at 3 and 6 months, in accordance with the US and European guidelines.22,23 Within the first 3 months, there were 9 SSE events among patients being treated with oral anticoagulation, including 4 events among patients being treated with warfarin and 5 events among patients being treated with a DOAC. In this period, there was no statistical difference in the risk of SSE by oral anticoagulation (HR for DOAC vs warfarin, 2.33; 95% CI, 0.63-8.74; P = .21). In the interval from 3 months to the end of follow-up, there were 22 SSE events among patients being treated with oral anticoagulation, including 10 events among patients being treated with warfarin and 12 events among patients being treated with a DOAC. Anticoagulation with a DOAC vs warfarin during this period was associated with SSE on Cox proportional hazards regression (HR, 2.88; 95% CI, 1.22-6.80; P = .02). There were no events among patients being treated with oral anticoagulation between 3 and 6 months, such that the point estimates were unchanged using the European guideline–recommended 6-month time period as a landmark.
We performed an additional sensitivity analysis for patients who completed 90 days of anticoagulation therapy and then discontinued all oral anticoagulation. This analysis was motivated by the possibility that patients with uncomplicated, short periods of effective anticoagulation may result in underweighting the effectiveness of oral anticoagulation in a time-dependent analysis. In this analysis, there were 98 patients who discontinued therapy after completing at least 90 days of anticoagulation. Of these, 64 patients had follow-up associated with warfarin (total follow-up time, 45 043 days), and 34 patients had follow-up associated with a DOAC (total follow-up time, 11 715 days). Five events that had been considered as having occurred without oral anticoagulation were re-allocated to oral anticoagulants: 3 were re-allocated to warfarin use and 2 were re-allocated to DOAC use. After this adjustment and the use of univariable Cox proportional hazards regression, DOAC vs warfarin use remained associated with SSE (HR, 2.69; 95% CI, 1.37-5.26; P = .004).
We also performed an analysis censoring all events after 1 year. In the first year of follow-up, there were 15 SSE events among patients being treated with oral anticoagulation, including 6 events among patients being treated with warfarin and 9 events among patients being treated with a DOAC. On univariable Cox proportional hazards regression, SSE was associated with anticoagulation with a DOAC vs warfarin (HR, 3.10; 95% CI, 1.10-8.73; P = .03).
Chronic Thrombi
Given the more recent introduction of DOACs, it is possible that older, and thus more stable, thrombi may be disproportionately treated with warfarin. We attempted to account for this scenario by censoring all patients after the third quartile of follow-up time (866 days). In this analysis, there were 14 SSE events among patients being treated with a DOAC and 9 SSE events among patients being treated with warfarin. In univariable Cox proportional hazards regression with time-dependent covariates, anticoagulation with a DOAC vs warfarin was associated with SSE (HR, 3.35; 95% CI, 1.45-7.77; P = .005).
Treatment Switching
We performed an intention-to-treat–style analysis, categorizing patients according to the first oral anticoagulant used. Of 38 SSE events among patients treated with an oral anticoagulant at any point, 27 occurred among patients initially being treated with warfarin, and 11 occurred among patients initially being treated with a DOAC. In this analysis, 7 events that occurred among patients being treated with a DOAC were associated with warfarin, 6 events that occurred while patients were not receiving oral anticoagulation were associated with warfarin, and 1 event that occurred while patients were not receiving oral anticoagulation was associated with a DOAC (eTable 4 in the Supplement). On univariable Cox proportional hazards regression, the first oral anticoagulant was not associated with subsequent SSE (HR for DOAC vs warfarin, 1.42; 95% CI, 0.68-2.96; P = .35).
To account for the presence of unmeasured confounders associated with treatment switching, we compared the group-level risk of SSE events between the therapy-change, DOAC-only, and warfarin-only groups, without any time-dependent covariate analysis. On Cox proportional hazards regression analysis with the warfarin-only group as a reference, there was no significant increase in the risk of SSE for the DOAC-only group (HR, 1.99; 95% CI, 0.91-4.35; P = .08). Compared with the warfarin-only group, the therapy-change group demonstrated a similar risk of SSE (HR, 1.13; 95% CI, 0.48-2.70; P = .12). Additional analysis of events by type of cardiomyopathy and after treatment changes are in eAppendix 4 and eAppendix 5 in the Supplement.
Discussion
The present multicenter, retrospective study of patients with echocardiographically diagnosed LV thrombi is the largest of its kind to date, to our knowledge. Among the key findings was that DOAC treatment was associated with an increased risk of SSE events compared with warfarin use, even after adjustment for other factors. Prior SSE was also associated with SSE events. Of the 421 patients treated with an oral anticoagulant, 185 (43.9%) used an off-label DOAC for at least part of their treatment course.
The reason for the observed higher rate of vascular events with DOAC treatment is unclear, particularly given the comparability of DOACs and warfarin for the treatment of AF, but several factors can be considered. Anticoagulation for AF, the indication for which DOACs were developed, involves the prevention of thrombus development in addition to dissolution of existing thrombi. Only the latter is applicable to existing LV thrombi. In a prospective study of left atrial appendage thrombi, treatment with rivaroxaban was associated with a 41.5% rate of thrombus resolution.24 However, there was no direct comparison with warfarin. It is also possible that different classes of DOACs, and even individual agents, possess differing levels of effectiveness for treatment of LV thrombi. Among the DOACs in the present analysis, most were oral factor Xa inhibitors.
There are also intrinsic differences between thrombi in the LV and AF-related thrombi in the left atrium and its appendage that may complicate interchangeability. Whereas AF-related thrombi are thought to be primarily caused by stasis, LV thrombosis in settings such as acute myocardial infarction is associated with both stasis and endocardial changes.25 These differences in thrombogenesis may reasonably translate into differences in antithrombotic activity and thus anticoagulation responsiveness.
Also of interest is the late separation of event curves, largely after the initial anticoagulation window associated with LV thrombi. One potential explanation is that some late embolic events are associated with phenomena outside the LV, such as vascular atheroemboli or calcifications, leaflet thrombosis, or septic vegetations that, in turn, may be differentially suppressed by DOACs compared with warfarin. Finally, it may be that some LV thrombi are markers of longer-term thrombotic risk that persists after the initial period of anticoagulation and even despite thrombus resolution.
Other retrospective studies have addressed the issue of DOACs for LV thrombus, including by one of the centers in the present analysis.26,27,28,29 None has allowed for assessment of robust differences between DOACs and warfarin because they were limited to a single-center design, small numbers of patients (the largest consisting of 140 LV thrombi), shorter follow-up period, exclusive reliance on the medical record for event data and, most important, very few SSE events (none of the studies had more than 5 SSE events). By contrast, the present study is the largest and only multicenter study to date, to our knowledge, evaluating the use of DOACs to reduce embolic events in LV thrombi. In addition to a review of medical records, patients were contacted directly to obtain information about embolic events.
Other studies examining the use of DOACs for patients with LV thrombi have relied on resolution on imaging results as evidence of treatment effect; however, there was substantial variation in the presence and frequency of imaging follow-up in our retrospective study. More than one-third of patients with LV thrombus had no additional echocardiogram after initial diagnosis. Moreover, echocardiography has limited sensitivity for the diagnosis of LV thrombi.30 Finally, the absence of a visible thrombus could indicate one that embolized or one that has dissipated, further clouding the utility of this end point. For these reasons, a patient-centered end point focusing on clinically apparent embolic events was preferable.
Limitations
The present study has several limitations by virtue of its retrospective nature, particularly the possibility of unmeasured confounders accounting for the difference in embolic events between DOACs and warfarin. However, there was no significant difference between most of the clinical variables that were measured. Even so, after adjustment for known factors associated with embolic risk from prior literature, including thrombus morphologic characterstics and mobility, as well as those identified here (history of SSE), the difference between DOAC treatment and warfarin treatment persisted. There was no centralized review of echocardiographic images; instead, we relied on the clinical report of each echocardiogram and the local measurement of the 2-dimensional thrombus area. Standardized expert assessment of thrombus morphologic characteristics and mobility by a core laboratory may have allowed for finer adjudication of these characteristics and thus better discrimination of embolic risk, which might have been unevenly distributed between patients taking DOACs and patients taking warfarin. We did not track bleeding events as a primary end point, ceding this question to the prior clinical trials for each DOAC.31,32,33,34 The absence of information on dosing, including use of regimens outside US Food and Drug Administration labeling for DOACs is another limitation. Finally, we did not obtain data on adherence to DOACs or the time in therapeutic range for warfarin treatment. Although we found no evidence that the observed differences in SSE events were caused by low adherence to DOACs, the present analysis cannot exclude this possibility.
Conclusions
In this multicenter, retrospective study of more than 500 patients with LV thrombi with 54 SSE events, 43.9% of patients treated with oral anticoagulation used an off-label DOAC for part of their course. Treatment with a DOAC was associated with a higher risk of SSE events compared with warfarin use, even after adjustment for other factors. These findings are limited by the lack of randomization and by the retrospective nature of this analysis. However, the findings argue against the assumption of equivalence of DOACs and warfarin for LV thrombi before outcomes can be compared in a prospective trial. In the interim, off-label use of DOACs for LV thrombi should be undertaken with caution. Randomized clinical trials are needed to determine the most effective treatment strategies for patients with LV thrombi.
eTable 1. Types of Cardiomyopathy
eTable 2. Antiplatelet Therapy
eTable 3. Timing and Reasons for Anticoagulation Changes
eTable 4. Comparison of Oral Anticoagulant at the Time of SSE With the Initial Agent
eAppendix 1. Patient Follow-Up and Anticoagulation Duration
eAppendix 2. Treatment Switching
eAppendix 3. Atrial Fibrillation
eAppendix 4. Cardiomyopathy Type
eAppendix 5. Events After Treatment Changes
eFigure. Survival Curves for Survival Free of Stroke and Systemic Embolism
References
- 1.Cabin HS, Roberts WC. Left ventricular aneurysm, intraaneurysmal thrombus and systemic embolus in coronary heart disease. Chest. 1980;77(5):586-590. doi: 10.1378/chest.77.5.586 [DOI] [PubMed] [Google Scholar]
- 2.Robinson AA, Jain A, Gentry M, McNamara RL. Left ventricular thrombi after STEMI in the primary PCI era: a systematic review and meta-analysis. Int J Cardiol. 2016;221:554-559. doi: 10.1016/j.ijcard.2016.07.069 [DOI] [PubMed] [Google Scholar]
- 3.Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol. 1993;22(4):1004-1009. doi: 10.1016/0735-1097(93)90409-T [DOI] [PubMed] [Google Scholar]
- 4.Meurin P, Tabet JY, Renaud N, et al. Treatment of left ventricular thrombi with a low molecular weight heparin. Int J Cardiol. 2005;98(2):319-323. doi: 10.1016/j.ijcard.2004.02.014 [DOI] [PubMed] [Google Scholar]
- 5.Hughes B. First oral warfarin alternative approved in the US. Nat Rev Drug Discov. 2010;9(12):903-906. doi: 10.1038/nrd3322 [DOI] [PubMed] [Google Scholar]
- 6.Mullard A. 2011 FDA drug approvals. Nat Rev Drug Discov. 2012;11(2):91-94. doi: 10.1038/nrd3657 [DOI] [PubMed] [Google Scholar]
- 7.Mullard A. 2012 FDA drug approvals. Nat Rev Drug Discov. 2013;12(2):87-90. doi: 10.1038/nrd3946 [DOI] [PubMed] [Google Scholar]
- 8.Minor C, Tellor KB, Armbruster AL. Edoxaban, a novel oral factor Xa inhibitor. Ann Pharmacother. 2015;49(7):843-850. doi: 10.1177/1060028015579426 [DOI] [PubMed] [Google Scholar]
- 9.Pradaxa (dabigatran). Package insert. Boehringer Ingelheim Pharmaceuticals Inc; 2010.
- 10.Xarelto (rivaroxaban). Package insert. Janssen Pharmaceuticals Inc; 2011.
- 11.Eliquis (apixaban). Package insert. Bristol Myers Squibb; 2013.
- 12.Vinter N, Linder M, Andersen M, et al. Classification and characteristics of on-label and off-label apixaban use in Denmark and Sweden. Pharmacoepidemiol Drug Saf. 2019;28(6):867-878. doi: 10.1002/pds.4778 [DOI] [PubMed] [Google Scholar]
- 13.Davis KA, Davis DO. Direct acting oral anticoagulants for the treatment of suspected heparin-induced thrombocytopenia. Eur J Haematol. 2017;99(4):332-335. doi: 10.1111/ejh.12921 [DOI] [PubMed] [Google Scholar]
- 14.Dufrost V, Risse J, Reshetnyak T, et al. Increased risk of thrombosis in antiphospholipid syndrome patients treated with direct oral anticoagulants: results from an international patient-level data meta-analysis. Autoimmun Rev. 2018;17(10):1011-1021. doi: 10.1016/j.autrev.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 15.Eikelboom JW, Connolly SJ, Brueckmann M, et al. ; RE-ALIGN Investigators . Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206-1214. doi: 10.1056/NEJMoa1300615 [DOI] [PubMed] [Google Scholar]
- 16.Boom MS, Berghuis EM, Nieuwkerk PT, Pinedo S, Büller HR. When do patients prefer a direct oral anticoagulant over a vitamin K antagonist? Neth J Med. 2015;73(8):368-372. [PubMed] [Google Scholar]
- 17.Keita I, Aubin-Auger I, Lalanne C, et al. Assessment of quality of life, satisfaction with anticoagulation therapy, and adherence to treatment in patients receiving long-course vitamin K antagonists or direct oral anticoagulants for venous thromboembolism. Patient Prefer Adherence. 2017;11:1625-1634. doi: 10.2147/PPA.S131157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smetana KS, Dunne J, Parrott K, et al. Oral factor Xa inhibitors for the treatment of left ventricular thrombus: a case series. J Thromb Thrombolysis. 2017;44(4):519-524. doi: 10.1007/s11239-017-1560-7 [DOI] [PubMed] [Google Scholar]
- 19.Mantel N, Byar DP. Evaluation of response-time data involving transient states: an illustration using heart-transplant data. J Am Stat Assoc. 1974;69(345):81-86. doi: 10.1080/01621459.1974.10480131 [DOI] [Google Scholar]
- 20.Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med. 1984;3(1):35-44. doi: 10.1002/sim.4780030106 [DOI] [PubMed] [Google Scholar]
- 21.Poguntke I, Schumacher M, Beyersmann J, Wolkewitz M. Simulation shows undesirable results for competing risks analysis with time-dependent covariates for clinical outcomes. BMC Med Res Methodol. 2018;18(1):79. doi: 10.1186/s12874-018-0535-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kernan WN, Ovbiagele B, Black HR, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236. doi: 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 23.Ibanez B, James S, Agewall S, et al. ; ESC Scientific Document Group . 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the Management of Acute Myocardial Infarction in Patients Presenting With ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119-177. doi: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 24.Lip GY, Hammerstingl C, Marin F, et al. ; X-TRA study and CLOT-AF registry investigators . Left atrial thrombus resolution in atrial fibrillation or flutter: results of a prospective study with rivaroxaban (X-TRA) and a retrospective observational registry providing baseline data (CLOT-AF). Am Heart J. 2016;178:126-134. doi: 10.1016/j.ahj.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 25.Delewi R, Zijlstra F, Piek JJ. Left ventricular thrombus formation after acute myocardial infarction. Heart. 2012;98(23):1743-1749. doi: 10.1136/heartjnl-2012-301962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson A, Ruth B, Dent J. Direct oral anticoagulants compared to warfarin for left ventricular thrombi: a single center experience. J Am Coll Cardiol. 2018;71(11)(suppl):A981. doi: 10.1016/S0735-1097(18)31522-5 [DOI] [Google Scholar]
- 27.Cochran JM, Jia X, Hamzeh I, Birnbaum Y. Direct oral anticoagulant use for left ventricular thrombus: a single center experience. Circulation. 2018;138(suppl_1):A16411. [Google Scholar]
- 28.McCarthy CP, Murphy S, Venkateswaran RV, et al. Left ventricular thrombus: contemporary etiologies, treatment strategies, and outcomes. J Am Coll Cardiol. 2019;73(15):2007-2009. doi: 10.1016/j.jacc.2019.01.031 [DOI] [PubMed] [Google Scholar]
- 29.Fleddermann AM, Hayes CH, Magalski A, Main ML. Efficacy of direct acting oral anticoagulants in treatment of left ventricular thrombus. Am J Cardiol. 2019;124(3):367-372. doi: 10.1016/j.amjcard.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 30.Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J. 2006;152(1):75-84. doi: 10.1016/j.ahj.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 31.Connolly SJ, Ezekowitz MD, Yusuf S, et al. ; RE-LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151. doi: 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 32.Patel MR, Mahaffey KW, Garg J, et al. ; ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891. doi: 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 33.Granger CB, Alexander JH, McMurray JJ, et al. ; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. doi: 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 34.Giugliano RP, Ruff CT, Braunwald E, et al. ; ENGAGE AF-TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104. doi: 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Types of Cardiomyopathy
eTable 2. Antiplatelet Therapy
eTable 3. Timing and Reasons for Anticoagulation Changes
eTable 4. Comparison of Oral Anticoagulant at the Time of SSE With the Initial Agent
eAppendix 1. Patient Follow-Up and Anticoagulation Duration
eAppendix 2. Treatment Switching
eAppendix 3. Atrial Fibrillation
eAppendix 4. Cardiomyopathy Type
eAppendix 5. Events After Treatment Changes
eFigure. Survival Curves for Survival Free of Stroke and Systemic Embolism