These case-control studies assess whether mood homeostasis is a possible new therapeutic target by testing the hypothesis that people with low (vs high) mean mood and people with (vs without) a history of depression have impaired mood homeostasis.
Key Points
Question
Is impaired mood homeostasis (ie, failure to stabilize mood via mood-modifying activities) associated with low mood and a history of depression?
Findings
In 2 case-control studies including a total of 58 328 participants from low-, middle-, and high-income countries, mood homeostasis was lower in participants with low mood and those with a history of depression. Dynamic simulations showed that impaired mood homeostasis may lead to increased incidence and longer duration of depressive episodes.
Meaning
Mood homeostasis may be a new target for the development of novel treatments and the optimization of existing ones such as activity scheduling.
Abstract
Importance
Existing therapeutic options are insufficient to tackle the disease burden of depression, and new treatments are sorely needed. Defining new psychotherapeutic targets is challenging given the paucity of coherent mechanistic explanations for depression.
Objective
To assess whether mood homeostasis (ie, the stabilization of one’s mood by engaging in mood-modifying activities) is a possible new therapeutic target by testing the hypothesis that people with low (vs high) mean mood and people with (vs without) a history of depression have impaired mood homeostasis.
Design, Setting, and Participants
The quantitative association between mood and daily activities was computed in 2 large case-control studies based on the 58sec data set (collected from December 1, 2012, to May 31, 2014, and analyzed from April 1 to 30, 2019), and the World Health Organization Study on Global Aging and Adult Health (WHO SAGE) data set (collected from January 1, 2007, to December 31, 2010, and analyzed from June 1 to 30, 2019). The 58sec data set consists of self-enrolled participants from high-income countries. The WHO SAGE data set consists of nationally representative participants in low- and middle-income countries recruited via cluster sampling.
Main Outcomes and Measures
The main outcome (defined before data analysis) was the difference in mood homeostasis between people with high vs low mean mood (58sec data) and between people with vs without a history of depression (WHO SAGE data).
Results
A total of 28 212 participants from the 58sec data set (65.8% female; mean [SD] age, 28.1 [9.0] years) and 30 116 from the WHO SAGE data set (57.0% female; mean [SD] age, 57.8 [14.7] years) were included, for an overall study population of 58 328 participants. Mood homeostasis was significantly lower in people with low (vs high) mean mood (0.63 [95% CI, 0.45 to 0.79] vs 0.96 [95% CI, 0.96 to 0.98]; P < .001) and in people with (vs without) a history of depression (0.03 [95% CI, −0.26 to 0.24] vs 0.68 [95% CI, 0.55 to 0.75]; P < .001). In dynamic simulations, lower mood homeostasis led to more depressive episodes (11.8% vs 3.8% yearly risk; P < .001) that lasted longer (4.19 vs 2.90 weeks; P = .006).
Conclusions and Relevance
In this study, mood homeostasis appeared to have been impaired in people with low mood and in those with a history of depression. Mood homeostasis may therefore provide new insights to guide the development of treatments for depression.
Introduction
Major depressive disorder is the leading cause of disability worldwide.1 Existing pharmacological2 and nonpharmacological3,4 treatment options for depression achieve response rates of about 50%—a relatively modest contribution to the reduction of the disease burden.5 The development of treatments with novel or optimized mechanisms of action has therefore become a key strategic objective of research in psychiatry.6
We propose that a fundamental—yet unexplored—underlying mechanism of depression may lie in some people’s inability to stabilize mood through their choice of everyday activities. This idea was inspired by recent large-scale studies showing a robust pattern of associations between mood and choices of activities in the general population,7,8,9 termed the hedonic flexibility principle. According to this principle, people have a higher propensity to engage in mood-increasing activities when their mood is low and to sustain useful but mood-decreasing activities when their mood is high. We hypothesized that this principle reflects a homeostatic mechanism that helps stabilize mood in healthy people. Conversely, if weak or absent, the mechanism could increase the risk of a spiral down to depression.
We first tested this hypothesis by assessing the association between mood homeostasis and individuals’ mean mood among 28 212 people whose moods and activities were tracked in real time. We then sought to confirm the hypothesis by comparing mood homeostasis between people with and without a history of depression in an independent data set of 30 116 people across 6 countries obtained from the World Health Organization Study on Global Aging and Adult Health (WHO SAGE).10
Methods
Participants and Data
We used 2 independent and complementary case-control data sets. Demographic data for both studies are presented in the eTable in the Supplement. This study was approved by the Ethics Committee of ESADE (Escuela Superior de Administración y Dirección de Empresas) Business School, Barcelona, Spain. Participants provided written informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The first data set (referred to as the 58sec data set) was acquired in young adults (mean [SD] age: 28.1 [9.0] years) living in high-income francophone countries. Participants volunteered by downloading a mobile application for ecological momentary assessment of mood and behavior via short questionnaires presented at random times throughout the day. The 2 questions of interest were “How are you currently feeling?” (on a sliding scale from 0 [very unhappy] to 100 [very happy]) and “What are you currently doing?” (from a nonmutually exclusive list of 25 activities, eg, exercising, chatting, working) (eMethods 1 in the Supplement). We selected all participants who answered 2 consecutive questionnaires or more within 12 hours. This resulted in 28 212 participants and 216 794 pairs of observations. Details on the working of the app have been published previously8 (eMethods 2 in the Supplement). Data were collected from December 1, 2012, to May 31, 2014, and analyzed from April 1 to 30, 2019.
The second data set (WHO SAGE data set) forms part of the WHO SAGE study (wave 1),10 which consists of questionnaires administered to nationally representative samples in China, Ghana, India, Mexico, Russia, and South Africa. The participants were asked to name, in chronological order, the activities that they engaged in during the day11 (eg, religion, subsistence farming), their mood (eMethods 1 in the Supplement), and whether they were ever depressed; 30 116 participants recorded at least 2 activities (mean [SD], 4.4 [1.6]) and corresponding mood. Data were collected from January 1, 2007, to December 31, 2010, and analyzed from June 1 to 30, 2019.
Mood Homeostasis
We define mood homeostasis as the extent to which a person preferentially engages in mood-increasing activities such as exercising when their mood is low and saves the mood-decreasing activities such as housework for when their mood is higher. Thus, individuals who preferentially engage in mood-increasing activities when their mood is already high and unpleasant activities when their mood is already low would have a low mood homeostasis.
Mood homeostasis represents the extent to which people demonstrate hedonic flexibility.7 Mood homeostasis is an aspect of the broader concept of mood regulation,12 but it specifically refers to the moment-to-moment regulation of mood states via choices of activities. Details on mood homeostasis and its computation are presented in eMethods 3 to 5 and eFigures 1 and 2 in the Supplement.
Statistical Analysis
Mood homeostasis is positive if the probability of next engaging in an activity when current mood is low (estimated with a logistic regression) is positively correlated with the change in mood resulting from this activity (estimated with a linear regression). In the 58sec data set, we let the coefficients of interest (ie, the association between current mood and the probability of later engaging in a particular activity from the logistic regression model, and the resulting change in mood from the linear regression model) vary as a function of a participant’s mean mood. We evaluated mood homeostasis for mean moods ranging from 2 SDs below the population mean to 2 SDs above (ie, from 25 to 97 on a sliding scale of 0 to 100). We first tested the null hypothesis that mood homeostasis at any level of mean mood is equal to zero. We then tested the null hypothesis that mood homeostasis is identical between a mean mood equal to the mean in the top half of the population (75.2) and that in the bottom half of the population (46.9). Similarly, in the WHO SAGE data set, we calculated the coefficients of interest separately for people with and without a history of depression. We first tested the null hypotheses that mood homeostasis is equal to zero in each group (people with and people without a history of depression) and then the null hypothesis that there is no difference between the groups.
As in previous studies,7,8,9 the time of day and day of the week (only available for the 58sec data set) were included as potential confounders in the 2 regressions. The regression used to assess the association between current mood and the probability of later engaging in a particular activity also included a latency effect (ie, whether an individual was already engaged in that activity before), and the daily mean mood as potential covariates. Details on covariates are provided in eMethods 6 in the Supplement. Full details on the statistical analysis are presented in eMethods 7 and eFigure 3 in the Supplement, and robustness analysis is presented in eMethods 8 in the Supplement. Significance level was set at P < .05, and all statistical tests were 2-sided. Statistical tests were achieved using the nonparametric bootstrap method.
Dynamic Simulations
To further assess the association of low mood homeostasis with depressive episodes, we developed a generative model to simulate mood and activity timelines for a 5-year period for 200 simulated individuals: 100 with high and 100 with low mood homeostasis. This simulation is further described in eMethods 9 in the Supplement.
Results
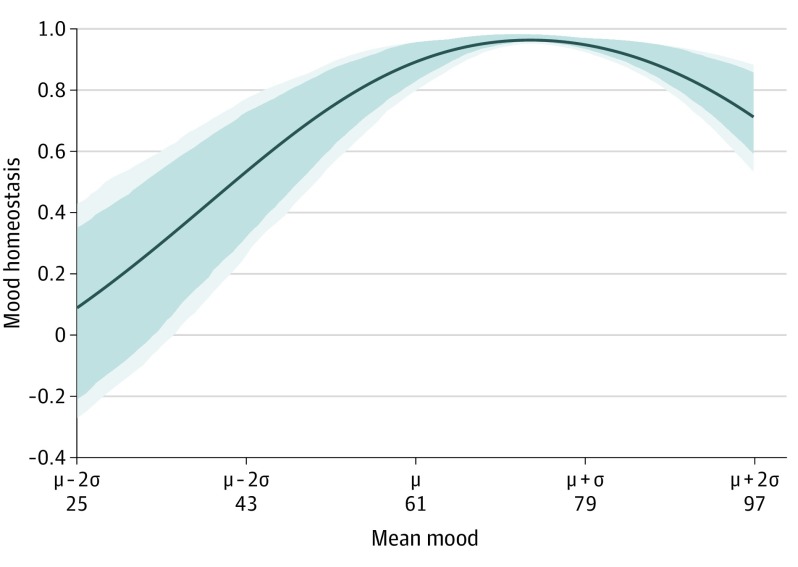
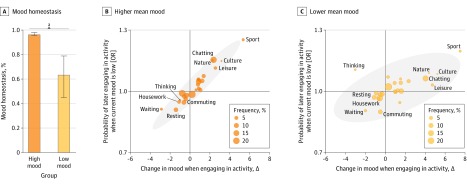
A total of 28 212 participants from the 58sec data set (18 504 [65.8%] female and 9621 [34.2%] male, among those who reported sex; mean [SD] age, 28.1 [9.0] years) and 30 116 from the WHO SAGE data set (17 175 [57.0%] female and 12 939 [43.0%] male, among those who reported sex; mean [SD] age, 57.8 [14.7] years) were included in the analysis, for a total study population of 58 328 participants. Mood homeostasis as a function of an individual’s mean mood in the 58sec data set is shown in Figure 1. For individuals’ mean moods below the population mean (μ = 61), mood homeostasis decreased monotonically. Its value fell to a range that included zero for mean moods below 33. Mood homeostasis for a high mean mood (taken as 75.2, which is the mean in the top half of the population) was significantly higher than mood homeostasis for a low mean mood (taken as 46.9, which is the mean in the bottom half of the population): 0.96 (95% CI, 0.96-0.98) vs 0.63 (95% CI, 0.45-0.79) (P < .001) (Figure 2A). Both values were significantly larger than zero (P < .001).
Figure 1. Mood Homeostasis as a Function of an Individual’s Mean Mood.
For mean moods below the population mean (μ), mood homeostasis decreases monotonically and eventually reaches values that are not significantly different from zero. Dark blue areas indicate 95% CI; light blue areas, 99% CI; and σ, population SD.
Figure 2. Group Differences in Mood Homeostasis in the 58sec Data Set.
A, People with low mean mood had significantly lower mood homeostasis than people with high mean mood. Error bars indicate 95% CI. B and C, Association in both groups between the change in mood that results from engaging in activities and the propensity to engage in them. Gray areas represent the 95% CIs. OR indicates odds ratio. See eFigure 6 in the Supplement for all the labels of the scatterplot.
aP < .001.
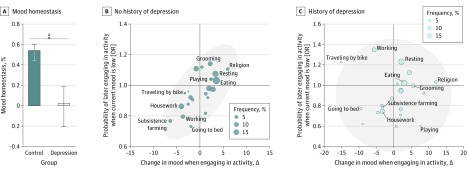
In the WHO SAGE data set, mood homeostasis among people without a history of depression was 0.68 (95% CI, 0.55-0.75) and significantly different from zero (P < .001) (Figure 3A). Among people with a history of depression, mood homeostasis was 0.03 (95% CI, −0.26 to 0.24) and not significantly different from zero (P = .68) (Figure 3A). The difference between the 2 was statistically significant (difference, 0.65; 95% CI, 0.46-0.93; P < .001), which implies that people with a history of depression had a disrupted mood homeostasis (which may even be effectively absent) compared with people without a history of depression. These findings were found to be robust when the data were randomly split in 2 independent subsets, when using parametric tests, when using multilevel regression models, and when adjusting for the country of origin (eFigure 4 in the Supplement).
Figure 3. Group Differences in Mood Homeostasis in the World Health Organization Study on Global Aging and Adult Health Data Set.
A, People with a history of depression had significantly lower mood homeostasis than people without a history of depression and their mood homeostasis is not significantly different from zero. Error bars indicate 95% CI. B and C, Association in both groups between the change in mood that results from engaging in activities and the propensity to engage in them. Gray areas represent the 95% CIs. OR indicates odds ratio. See eFigure 6 in the Supplement for all the labels of the scatterplot.
aP < .001.
Factors Associated With Group Differences in Mood Homeostasis
In the 58sec data set, thinking was associated with a reduction in mood homeostasis in participants with low mean mood (Figure 2B and C). These participants tended to think more when their mood was low, although this was associated with a further decrease in their mood—the opposite of mood homeostasis. However, the group difference in mood homeostasis in terms of all other activities was also statistically significant (difference, 0.15; 95% CI, 0.04-0.26; P < .001) (eFigure 5 in the Supplement), suggesting that it must also be associated with other factors. No specific activity was found to be associated with the group difference in mood homeostasis in the WHO SAGE data set (Figure 3B and C and eFigure 6 in the Supplement).
The group differences in mood homeostasis were more closely associated with when people engage in mood-modifying activities than with how activities modify mood. In the 58sec data set, the change in mood resulting from engaging in different activities was virtually identical for people with high and low mean mood (correlation between the horizontal spread of activities, in Figure 2B and C, within the group with high mean mood and the horizontal spread of activities within the group with low mean mood: 0.955). For example, exercising was the activity that was associated with the largest boost in participants’ mood regardless of mean mood levels. In contrast, the 2 groups were less similar in their propensity to engage in different activities as a function of their current mood (correlation between vertical spreads in Figure 2B and C: 0.787). The difference between these 2 correlations was statistically significant (difference, 0.17; 95% CI, 0.07-0.32; P < .001). Similarly, in the WHO SAGE data set, people with and without a history of depression were relatively similar in the change in mood associated with different activities (correlation between horizontal spreads in Figure 3B and C: 0.782) but less similar in their propensity to engage in different activities as a function of their current mood (correlation between vertical spreads in Figure 3B and C: 0.135). The difference between these 2 correlations was statistically significant (difference, 0.65; 95% CI, 0.41-1.03; P < .001).
When people experience low mood, mood homeostasis can be achieved in 2 ways: individuals can refrain from engaging in mood-decreasing activities (negative valence; eg, postponing housework), or they can engage in mood-increasing activities (positive valence; eg, exercising). In the 58sec data set, activities with negative valence contributed more to the group difference in mood homeostasis than activities with positive valence. Specifically, the group difference in mood homeostasis between people with high and low mean mood for negative activities was 1.27 (0.76 vs −0.51 [95% CI, 1.07-1.59]; P < .001), whereas the group difference in mood homeostasis for positive activities was 0.22 (0.94 vs 0.72 [95% CI, 0.08-0.47]; P < .001), and the difference between the 2 was statistically significant (1.05 [95% CI, 0.81-1.41]; P < .001). A similar trend was observed in the WHO SAGE data set. The group difference in mood homeostasis between people with and without a history of depression was 0.860 for negative activities (0.559 vs −0.301 [95% CI, 0.314-1.456]; P = .003) and 0.285 for positive activities (0.383 vs 0.098 [95% CI, −0.002 to 0.937]; P = .054), although the difference between the 2 did not reach statistical significance (0.575 [95% CI, −0.320 to 1.226]; P = .24).
Dynamic Simulations
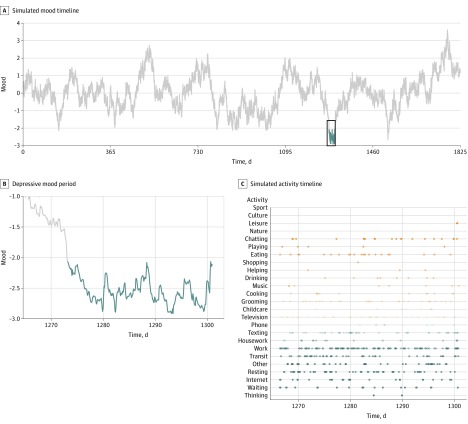
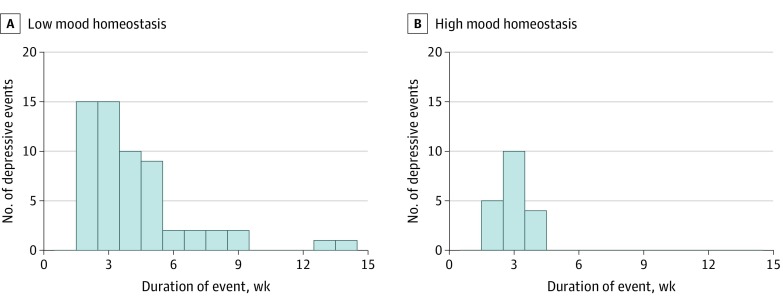
In simulations of mood and activity timelines (Figure 4), low mood homeostasis was associated with 3 times more depressive episodes than high mood homeostasis (11.8% vs 3.8% annual incidence of depressive episodes; P < .001) (Figure 5). When they occurred, these depressive episodes lasted significantly longer (4.19 vs 2.90 weeks; P = .006) (Figure 5). As illustrated in Figure 4C, when their mood was low, people with low-mood homeostasis may have been more likely to engage in mood-decreasing activities (blue dots) in higher proportion than mood-increasing activities (orange dots), which was further associated with a reduction in their mood.
Figure 4. Output of the Dynamic Simulation of Mood and Activity Timelines for 1 Subject.
A, Example of a mood timeline from a simulated individual with low mood homeostasis, with the depressive episode occurring in the fourth year highlighted in blue. B and C, Zoom on the squared portion of the curve in part A, showing the mood and activity timelines. In the latter, a dot is present if the simulated individual is engaged in that activity at that particular moment. The color of the dots corresponds to their effects on mood (blue dots have a negative effect on mood whereas orange dots have a positive effect), and darker dots indicate more influence of activity on mood. The preponderance of blue dots shows that the simulated individual is mostly engaged in activities that tend to decrease their mood despite their already depressed mood.
Figure 5. Distribution of the Number and Duration of Depressive Episodes in Dynamic Simulations.
Distribution in the 2 groups showing that the group with low mood homeostasis tends to develop more depressive episodes and that these episodes last longer.
Discussion
Across 2 large data sets including more than 58 000 people around the globe, we found that individuals with low mean mood had impaired mood homeostasis and that, at the group level, people with a history of depression showed no evidence of mood homeostasis at all. In dynamic simulations, low mood homeostasis predicted an increase in the incidence and duration of depressive episodes.
In this study, although the changes in mood associated with engaging in a specific activity at a specific time may appear small, the changes associated with engaging in multiple activities were additive. Thus, in the group with low mean mood, an individual who was both thinking and waiting would decrease their mood by a mean of 5 points (ie, 3 points for thinking and 2 points for waiting). The changes over time also appeared to be cumulative. For instance, an individual who is commuting and then starts thinking while commuting and continues to think while waiting for the elevator and then gets to work would see their mood decrease by a mean of 9.6 points compared with their mood before their commute. The decrease in mood from this (arguably not uncommon) sequence of activities corresponds to 29% of the median range of mood reports (see eResults 1 in the Supplement for details). In addition, the dynamic simulations enabled us to translate differences in mood homeostasis into differences in incidence and duration of depressive episodes that are more clinically interpretable. The finding that the simulated difference in mood homeostasis was associated with a 3-fold increase in the incidence of depressive episodes and a 44% increase in their duration thus also supports clinically significant associations between low mood homeostasis and depression.
The 2 data sets used in this study differed on many important aspects, including age, socioeconomic background, and data acquisition methods. The convergence of the findings from these data sets as well as in sensitivity analyses testify to their robustness. In addition, the consistency of the findings for high-income and low- and middle-income countries contributes to filling the gap between the burden of psychiatric disorders in low- and middle-income countries1 and the scarcity of research performed in them.13,14
Although findings converge in the 2 data sets, the strength of the association with mood homeostasis was substantially lower in people without a history of depression (WHO SAGE data set) than in people with high mean mood (58sec data set). This gap might be entirely accounted for by differences in sampling duration and mean mood level between the data sets (as shown in eResults 2 and eFigure 7 in the Supplement). Differences in age and income between data sets might also have been a factor: older adults and people with lower income might have daily activities that are less driven by their mood and more driven by external factors such as the time of day, day of the week, or immediate needs of the person or family. However, in eResults 2 and eFigure 8 in the Supplement, we show these were unlikely to have played significant roles. Finally, this gap in mood homeostasis may result from underreporting of depression by participants in the WHO SAGE data set (some of the control individuals would then have a history of depression, effectively lowering mood homeostasis in that group). This underreporting would explain why the prevalence of depression in that data set was lower than in the corresponding countries.15 Alternatively, the survey might have failed to reach a fully representative sample. Reliably establishing cross-cultural differences in mood homeostasis remains for future work.
The association between mood homeostasis and depression may provide important insights into the treatment of depression. Most treatments are developed in pragmatic ways poorly informed by mechanistic understandings of mood regulation, hampering their refinement and optimization.5 For example, activity scheduling—a therapeutic technique in which patients elaborate activity charts, predict their resulting pleasure, and actively evaluate with the therapist whether they have had the anticipated effect16—is part of a wide range of psychotherapies for depression, such as cognitive-behavioral therapy17 and behavioral activation.18,19 However, its mechanism of action remains largely unknown.20 Activity scheduling avoids situations in which many unpleasant activities are scheduled consecutively, which is critical for achieving mood homeostasis. This may be an important determinant of its therapeutic benefit, in which case focusing explicitly on mood homeostasis might further increase its efficacy. Moreover, measuring mood homeostasis at baseline might help to predict which patients will most benefit from such treatments or from antidepressants. Similar to the early effect of antidepressants on emotional processing,21 a gain of mood homeostasis could also be an early biomarker of drug action.
A question for future investigation is the extent to which behavior is driven primarily by conscious recognition of mood states and active choice of subsequent activities, or whether mood homeostasis occurs largely outside of awareness. Either way, our findings could also be important in designing new psychotherapeutic interventions. By monitoring mood in real time,22 intelligent systems might be able to make activity recommendations to increase mood homeostasis. Such an intervention could be delivered remotely,23,24 improving access to treatment for patients for whom face-to-face care is unavailable, including in low- and middle-income countries.25,26 Importantly, some associations between activities and mood were highly culture specific. For instance, exercise led to the highest increase in mood in high-income countries, whereas religion did so in low- and middle-income countries. Therefore, it seems that interventions aimed at increasing mood homeostasis will need to be culture specific—or even individual specific—and account for people’s constraints and preferences. If a gap in mood homeostasis is found to be driven by a few specific activities, then interventions could directly target them. For instance, the effect of thinking on mood homeostasis in the 58sec data set might reflect the tendency for some people to ruminate when feeling depressed.27 In those people, an intervention that targets rumination28 could restore mood homeostasis. Using the concept of mood homeostasis might therefore unify different therapeutic approaches by expressing their outcome as a quantifiable measurement of mood stability.
Limitations
Although our hypothesis is that weak or absent mood homeostasis may lead to depression, it is possible that low mood itself drives this association because this study was cross-sectional. Using simulations, we have explored the possibility of a direct link between the two. However, additional studies are needed to establish whether impaired mood homeostasis may indeed cause depression. The large number of participants guards against extreme unrepresentativeness, but we know relatively little about their medical and social histories. Hence, it is difficult to exclude various forms of selection bias resulting, for example, from access to mobile technologies (58sec cohort) or recruitment in different countries (WHO SAGE study). Finally, measuring mood with a sliding scale in the 58sec data set was motivated by the need for simplicity (to be used multiple times a day within a mobile application) while preserving a degree of granularity. However, clinically validated mood scales29 could be used in future studies, or the sliding scale could be validated against them.
Conclusions
This study found that lower mood homeostasis was associated with low mood and a history of depression, and dynamic simulations showed a plausible causal association linking the two. These mechanistically informed findings may prompt the development of new treatments for depression or the optimization of existing ones, such as activity scheduling. As a quantitative approach, measuring mood homeostasis may play a role in personalized psychiatry by identifying the patients most likely to benefit from a variety of treatments. Additional studies are needed to demonstrate a causal link between mood homeostasis and depression. We believe our findings thus open the door to new research avenues that may ultimately help reduce the disease burden of depression.
eTable. Demographic Information for the Complete and Analyzed Data Sets
eMethods 1. List of Activities and Emotions
eMethods 2. Description of the 58sec Project
eMethods 3. Formal Definition of Mood Homeostasis
eMethods 4. Illustration of the Practical Calculation of Mood Homeostasis
eMethods 5. Mathematical Demonstration of the Invariance of Mood Homeostasis
eMethods 6. Covariates
eMethods 7. Details of Statistical Analysis
eMethods 8. Robustness Analysis
eMethods 9. Dynamic Simulation Process
eResults 1. Interpretation of the Effect Sizes
eResults 2. Differences in Mood Homeostasis Between the 2 Data Sets
eFigure 1. Procedure Followed to Calculate Mood Homeostasis From the Input Data and From the 2 Regression Models
eFigure 2. Mood Homeostasis is Invariant to Difference in Mood Level, Difference in Fluctuation Amplitude, and Combinations Thereof
eFigure 3. Different Factors May Drive Group Differences in Mood Homeostasis
eFigure 4. Effect of Sampling Mood and Activities for Only 1 Day on the Value of Mood Homeostasis and Compounded Effect of Sampling Duration and Different Average Mood on Mood Homeostasis
eFigure 5. Mood Homeostasis Does Not Significantly Differ Between Age Group (A) and Between Income Levels (B)
eFigure 6. Results of the Robustness Analyses Carried Out in Both Data Sets
eFigure 7. Group Difference in Mood Homeostasis Between High and Low Average Mood in the 58sec Dataset After Excluding Thinking Which Was Found to be Partially Driving the Results
eFigure 8. Same Figure as eFigure 2C-D but With All Activity Labels Included
eReferences.
References
- 1.Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197-2223. [published correction appears in Lancet. 2013;381(9867):628] doi: 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 2.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746-758. doi: 10.1016/S0140-6736(09)60046-5 [DOI] [PubMed] [Google Scholar]
- 3.Leichsenring F. Comparative effects of short-term psychodynamic psychotherapy and cognitive-behavioral therapy in depression: a meta-analytic approach. Clin Psychol Rev. 2001;21(3):401-419. doi: 10.1016/S0272-7358(99)00057-4 [DOI] [PubMed] [Google Scholar]
- 4.Holmes EA, Craske MG, Graybiel AM. Psychological treatments: a call for mental-health science. Nature. 2014;511(7509):287-289. doi: 10.1038/511287a [DOI] [PubMed] [Google Scholar]
- 5.Holmes EA, Ghaderi A, Harmer CJ, et al. The Lancet Psychiatry Commission on psychological treatments research in tomorrow’s science. Lancet Psychiatry. 2018;5(3):237-286. doi: 10.1016/S2215-0366(17)30513-8 [DOI] [PubMed] [Google Scholar]
- 6.National Institute of Mental Health. Strategic Plan for Research. NIH Publication Number 15-6368. Published 2015. Accessed March 19, 2020. https://www.nimh.nih.gov/about/strategic-planning-reports/nimh_strategicplanforresearch_508compliant_corrected_final_149979.pdf
- 7.Taquet M, Quoidbach J, de Montjoye Y-A, Desseilles M, Gross JJ. Hedonism and the choice of everyday activities. Proc Natl Acad Sci U S A. 2016;113(35):9769-9773. doi: 10.1073/pnas.1519998113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quoidbach J, Taquet M, Desseilles M, de Montjoye Y-A, Gross JJ. Happiness and social behavior. Psychol Sci. 2019;30(8):1111-1122. doi: 10.1177/0956797619849666 [DOI] [PubMed] [Google Scholar]
- 9.Quoidbach J, Sugitani Y, Gross JJ, Taquet M, Akutsu S. From affect to action: how pleasure shapes everyday decisions in Japan and the US. Motiv Emot. Published online July 23, 2019. doi: 10.1007/s11031-019-09785-7 [DOI] [Google Scholar]
- 10.Kowal P, Chatterji S, Naidoo N, et al. ; SAGE Collaborators . Data resource profile: the World Health Organization Study on Global Ageing and Adult Health (SAGE). Int J Epidemiol. 2012;41(6):1639-1649. doi: 10.1093/ije/dys210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahneman D, Krueger AB, Schkade DA, Schwarz N, Stone AA. A survey method for characterizing daily life experience: the day reconstruction method. Science. 2004;306(5702):1776-1780. doi: 10.1126/science.1103572 [DOI] [PubMed] [Google Scholar]
- 12.Gross JJ. Emotion regulation: current status and future prospects. Psychol Inq. 2015;26(1):1-26. doi: 10.1080/1047840X.2014.940781 [DOI] [Google Scholar]
- 13.Tomlinson M, Rudan I, Saxena S, Swartz L, Tsai AC, Patel V. Setting priorities for global mental health research. Bull World Health Organ. 2009;87(6):438-446. doi: 10.2471/BLT.08.054353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chisholm D, Flisher AJ, Lund C, et al. ; Lancet Global Mental Health Group . Scale up services for mental disorders: a call for action. Lancet. 2007;370(9594):1241-1252. doi: 10.1016/S0140-6736(07)61242-2 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Depression and other common mental disorders: global health estimates. Published 2017. Accessed March 19, 2020. https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf
- 16.Beck JS. Cognitive Therapy: Basics and Beyond. Guilford Press; 1997:71. [Google Scholar]
- 17.Jacobson NS, Dobson KS, Truax PA, et al. A component analysis of cognitive-behavioral treatment for depression. J Consult Clin Psychol. 1996;64(2):295-304. doi: 10.1037/0022-006X.64.2.295 [DOI] [PubMed] [Google Scholar]
- 18.Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: a meta-analysis. Clin Psychol Rev. 2007;27(3):318-326. doi: 10.1016/j.cpr.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 19.Patel V, Weobong B, Weiss HA, et al. The Healthy Activity Program (HAP), a lay counsellor-delivered brief psychological treatment for severe depression, in primary care in India: a randomised controlled trial. Lancet. 2017;389(10065):176-185. doi: 10.1016/S0140-6736(16)31589-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagy GA, Cernasov P, Pisoni A, Walsh E, Dichter GS, Smoski MJ. Reward network modulation as a mechanism of change in behavioral activation. Behav Modif. 2020;44(2):186-213. doi: 10.1177/0145445518805682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? a cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry. 2009;195(2):102-108. doi: 10.1192/bjp.bp.108.051193 [DOI] [PubMed] [Google Scholar]
- 22.Spathis D, Servia-Rodriguez S, Farrahi K, Mascolo C, Rentfrow J Passive mobile sensing and psychological traits for large scale mood prediction. In: Proceedings of the 13th EAI International Conference on Pervasive Computing Technologies for Healthcare. ACM; 2019:272-281. [Google Scholar]
- 23.Leigh S, Flatt S. App-based psychological interventions: friend or foe? Evid Based Ment Health. 2015;18(4):97-99. doi: 10.1136/eb-2015-102203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazdin AE, Rabbitt SM. Novel models for delivering mental health services and reducing the burdens of mental illness. Clin Psychol Sci. 2013;1(2):170-191. doi: 10.1177/2167702612463566 [DOI] [Google Scholar]
- 25.Naslund JA, Aschbrenner KA, Araya R, et al. Digital technology for treating and preventing mental disorders in low-income and middle-income countries: a narrative review of the literature. Lancet Psychiatry. 2017;4(6):486-500. doi: 10.1016/S2215-0366(17)30096-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel V, Saxena S, Lund C, et al. The Lancet Commission on global mental health and sustainable development. Lancet. 2018;392(10157):1553-1598. doi: 10.1016/S0140-6736(18)31612-X [DOI] [PubMed] [Google Scholar]
- 27.Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. J Abnorm Psychol. 1991;100(4):569-582. doi: 10.1037/0021-843X.100.4.569 [DOI] [PubMed] [Google Scholar]
- 28.Watkins ER, Mullan E, Wingrove J, et al. Rumination-focused cognitive-behavioural therapy for residual depression: phase II randomised controlled trial. Br J Psychiatry. 2011;199(4):317-322. doi: 10.1192/bjp.bp.110.090282 [DOI] [PubMed] [Google Scholar]
- 29.McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18(4):1007-1019. doi: 10.1017/S0033291700009934 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Demographic Information for the Complete and Analyzed Data Sets
eMethods 1. List of Activities and Emotions
eMethods 2. Description of the 58sec Project
eMethods 3. Formal Definition of Mood Homeostasis
eMethods 4. Illustration of the Practical Calculation of Mood Homeostasis
eMethods 5. Mathematical Demonstration of the Invariance of Mood Homeostasis
eMethods 6. Covariates
eMethods 7. Details of Statistical Analysis
eMethods 8. Robustness Analysis
eMethods 9. Dynamic Simulation Process
eResults 1. Interpretation of the Effect Sizes
eResults 2. Differences in Mood Homeostasis Between the 2 Data Sets
eFigure 1. Procedure Followed to Calculate Mood Homeostasis From the Input Data and From the 2 Regression Models
eFigure 2. Mood Homeostasis is Invariant to Difference in Mood Level, Difference in Fluctuation Amplitude, and Combinations Thereof
eFigure 3. Different Factors May Drive Group Differences in Mood Homeostasis
eFigure 4. Effect of Sampling Mood and Activities for Only 1 Day on the Value of Mood Homeostasis and Compounded Effect of Sampling Duration and Different Average Mood on Mood Homeostasis
eFigure 5. Mood Homeostasis Does Not Significantly Differ Between Age Group (A) and Between Income Levels (B)
eFigure 6. Results of the Robustness Analyses Carried Out in Both Data Sets
eFigure 7. Group Difference in Mood Homeostasis Between High and Low Average Mood in the 58sec Dataset After Excluding Thinking Which Was Found to be Partially Driving the Results
eFigure 8. Same Figure as eFigure 2C-D but With All Activity Labels Included
eReferences.