Abstract
Major depressive disorder (MDD) represents a serious health problem estimated to affect 350 million people globally. Importantly, MDD has repeatedly emerged as an etiological or prognostic factor in cardiovascular disease (CVD) development, including vascular pathology. Several linking pathomechanisms between MDD and CVD involve abnormal autonomic regulation, inflammation, and endothelial dysfunction as an early preclinical stage of atherosclerosis. However, the cause of accelerated atherosclerosis in MDD patients remains unclear. Recently, the causal relationships between MDD and mediator (e.g., inflammation and/or endothelial dysfunction), as well as the causal pathways from the mediator to atherosclerosis, were discussed. Specifically, MDD is accompanied by immune dysregulation, resulting in increased production of proinflammatory cytokines (e.g., interleukin (IL)-6 and tumor necrosis factor (TNF)-α), which could lead to depression-linked abnormalities in brain function. Further, MDD has an adverse effect on endothelial function; for example, circulating markers of endothelial dysfunction (e.g., soluble adhesion molecules, von Willebrand factor) have been linked with depression. Additionally, MDD-linked autonomic dysregulation, which is characterized by disrupted sympathovagal balance associated with excessive circulating catecholamines, can contribute to CVD. Taken together, activated inflammatory response, endothelial dysfunction, and autonomic dysregulation could affect gradual atherosclerosis progression, resulting in a higher risk of developing CVD in MDD. This review focused on the pathomechanisms linking MDD and CVD with respect to neuroimmune regulation, and the description of promising biomarkers, which is important for the early diagnosis and personalized prevention of CVD in major depression.
Keywords: autonomic nervous system, atherosclerosis, biomarkers, endothelial dysfunction, inflammation, major depression
1. Introduction
Depression (major depressive disorder, MDD) is a common and serious medical illness that negatively affects emotional and cognitive functions, leading to feelings of sadness and/or a loss of interest in once-enjoyed activities. According to the World Health Organization (WHO) [1], depression is one of the most common health disorders, affecting more than 300 million people. Notably, MDD also represents a health problem in children and adolescents with increasing global prevalence; a recent review indicated the global prevalence of depressive disorder in children and adolescents to be 2.6% [2]. Furthermore, depressive disorder has been suggested as a risk factor for the development of cardiovascular disease (CVD). In this aspect, atherosclerosis and depression are listed in the most common medical conditions related to increased mortality and morbidity [1]. However, the cause of accelerated atherosclerosis in depressive patients remains unclear. One explanation is that depression is characterized by immune dysregulation, which links MDD and atherosclerosis. In particular, inflammation is closely associated with endothelial dysfunction as a preamble to atherosclerosis [3]. In this context, immune response is regulated by the autonomic nervous system via a sympathetic-cholinergic anti-inflammatory pathway that inhibits the release of proinflammatory cytokines [4,5]. The pathophysiological window into MDD–CVD complex relations remains the subject of extensive research to illuminate unresolved questions.
Therefore, the aim of this study was to summarize potential direct and associated pathophysiological mechanisms leading to depression-linked CVD with respect to immune, vascular, and autonomic dysfunction, as well as to emphasize potential promising biomarkers that could be used in the personalized prevention of CVD in major depression.
2. Role of Inflammation in Depressive Disorder
An immune response can affect brain function, contributing to neurodegenerative processes and cognitive decline [6]. In this aspect, evidence indicates that inflammatory cytokines contribute to the development of depression.
2.1. Cytokines in MDD Pathophysiology
Cytokines represent a broad group of secreted proteins that are important in cell signaling. These messenger molecules include chemokines, interferons (IFN), interleukins (IL), lymphokines, and tumor necrosis factors (TNFs). Cytokines are mainly released from immune cells such as monocytes, macrophages, and lymphocytes, and mast cells that are mobile within the body. Cytokines are characterized by diversity of the effects and functions. Cytokines are generally divided into proinflammatory cytokines, facilitating an inflammatory response (e.g., IL-1β, IL-6, and TNF-α), and anti-inflammatory cytokines, inhibiting an inflammatory response (e.g., IL-10) [7].
Cytokine production is not bound to a specific organ; endothelial cells, fibroblasts, and epithelial and stromal cells release cytokines on the body’s periphery, and microglia, neurons, and astrocytes produce central cytokines in brain regions such as the hypothalamus, hippocampus, cerebellum, forebrain, basal ganglia, or brain-stem nuclei [8,9]. Regarding peripheral cytokines, there are hypothesized complex pathways to enter the brain: a cytokine passage through leaky regions in the blood–brain barrier, such as the choroid plexus and the circumventricular organs (humoral pathway); binding to cytokine receptors associated with peripheral vagal-nerve afferents (neural pathway); and stimulation of microglia by proinflammatory cytokines to produce monocyte chemoattractant protein-1 (MCP-1) associated with the subsequent recruitment of monocytes to the meninges and brain parenchyma (cellular pathway) [8].
An inflammatory response in the brain could either be beneficial or detrimental. Specifically, the mild activation of microglia and astrocytes usually reveals neuroprotective effects, and ameliorates early symptoms of neurodegeneration by the modulation of synaptic plasticity and neuronal excitability. However, strong activation of glial cells, resulting in cytokine overexpression/dysregulation, accelerates neurodegeneration. Thus, the imbalance of inflammatory responses in the central nervous system (CNS) may represent an initial factor for many diseases [10].
In this context, the macrophage theory of depression first described a causal relationship between proinflammatory cytokines and depression [11]. Thereafter, the inflammatory and neurodegenerative hypothesis of depression [12] was based on evidence that depression is associated with inflammatory processes, as well as with neurodegeneration and reduced neurogenesis. Specifically, meta-analysis by Dowlati et al. [13] reported increased concentrations of proinflammatory cytokines TNF-α and IL-6 in MDD patients compared with controls, which strengthened the evidence of the inflammatory-response-system activation theory in depression. Further, recent meta-analysis characterized the MDD-linked inflammatory profile: IL-6, TNF-α, IL-10, IL-13, IL-18, and IL-12 levels were increased in MDD patients compared with those of controls; in contrast, peripheral levels of IL-1ß, IL-2, IL-4, IL-8, IL-5, IL-17, and transforming growth factor ß1 (TGF-ß1) did not differ significantly between depressive patients and controls [14]. Contrary to adults, the body of evidence on inflammatory alterations in children and adolescents is still limited. The first systematic review and meta-analysis [15] showed a trend for significantly higher levels of peripheral TNF-α without significant differences in any other cytokines (IFN-γ, IL-1ß, IL-4, IL-6, IL-8, IL-10) in depressive children and adolescents. It seems that altered pro- and anti-inflammatory cytokine profiles could be used as potential biomarkers for depressive disorder.
2.1.1. Tryptophan/Kynurenine Pathway
However, the underlying pathomechanisms with regard to coexistence of depression and inflammation are still unresolved. First, the immune system might contribute to depression through the tryptophan/kynurenine pathway linked to serotonergic transmission. Tryptophan represents a biochemical precursor for serotonin that is implicated in the pathogenesis of depressive disorder. Proinflammatory cytokines can induce tryptophan depletion by activating the indoleamine 2,3 dioxygenase (IDO) enzyme, which converts tryptophan to kynurenine (kynurenine pathway). Kynurenine is a precursor of the bioactive metabolites quinolinic acid and kynurenic acid. While quinolinic acid, as an N-methyl-D-aspartate (NMDA) receptor agonist, is potentially neurotoxic and thus potentially contributes to depression, kynurenic acid, as an NMDA receptor antagonist, is considered neuroprotective. According to the neurodegeneration hypothesis of depression [16], an inflammatory state can induce IDO activity, and increase the kynurenine/tryptophan ratio associated with a shift in the kynurenine pathway, resulting in an imbalance between neuroprotective (kynurenic acid) and neurodegenerative metabolites (3-hydroxyanthranilic acid, 3-hydroxy-kynurenine, and quinolinic acid). This could lead to neurodegenerative changes that may render the brain susceptible to depression [17].
2.1.2. Hypothalamus–Pituitary–Adrenal (HPA) Axis Pathway
Recently, studies regarding revisiting tryptophan-serotonin deficiency and the inflammatory hypotheses of major depression results argue for the validity of a biopsychosocial model of major depression [18]. In this aspect, major life stressors, such as interpersonal loss and social rejection, represent the strongest risk factors for depression [18]. Additionally, the social-signal-transduction theory of depression is based on the hypothesis that experiences of social threat and adversity increase proinflammatory cytokines, which, in turn, lead to behavioral changes (e.g., anhedonia, sad mood, fatigue, or social-behavioral withdrawal) [19]. Psychological stress can exert profound pathophysiological changes in the neuroimmune and endocrine regulatory systems associated with releasing stress hormones and proinflammatory markers [3,20]. Specifically, the activation of the HPA axis is associated with increased levels of glucocorticoids (cortisol), exerting anti-inflammatory actions, increasing anti-inflammatory cytokine levels, and decreasing proinflammatory cytokine levels. In chronic depression-linked stressful conditions, the HPA axis is activated to inhibit neuroinflammation; however, this mechanism fails owing to the desensitization of glucocorticoid receptors (GRs) and the impairment of control feedback regulatory processes [7]. In MDD, HPA axis hyperactivity and inflammation might be part of the same pathophysiological process: HPA axis hyperactivity is a marker of glucocorticoid resistance, leading to immune activation; inflammation could stimulate HPA axis activity via both direct cytokine action on the brain and by inducing glucocorticoid resistance [21]. In addition, diminished function and/or glucocorticoid-receptor expression is considered to be responsible for HPA axis hyperactivation in depressive disorder [22].
Taken together, an immune response resulting in the increased production of proinflammatory cytokines may induce symptoms of depression, predominantly via central neuroinflammation; a reduction in tryptophan availability, resulting in serotonin depletion along IDO activation; increased production of kynurenine neurotoxic metabolites; and HPA axis overdrive, including glucocorticoid-receptor downregulation.
3. Role of Endothelial Function in Depressive Disorder
With respect to MDD–CVD interaction, current research is focused on endothelial dysfunction as a critical intermediate phenotype in the complex relationship between depression, chronic inflammation, and vascular pathology, such as atherosclerosis [3].
The vascular endothelium, the monolayer of endothelial cells, covers the whole surface of the vascular system, providing the interface between circulating blood or lymph and the vessel wall [23]. This innermost layer of blood and lymphatic vessels represents a smart controller of blood flow with diverse biological roles in the micro- and macrovascular circulation, which is modulated through the release of paracrine, autocrine, and endocrine vasoactive substances in response to physical and chemical stimuli [24]. A healthy endothelium is characterized by a vasodilatory phenotype with high levels of nitric oxide (NO) and prostacyclin, and low levels of uric acid and reactive oxygen species (ROS). On the contrary, endothelial dysfunction is defined as a state of imbalance between the factors, with vasodilatory, antithrombogenic, and antimitogenic effects (mainly NO, prostacyclin, and endothelium-derived hyperpolarizing factor), and substances with vasoconstrictor, prothrombogenic, and proliferative effects (e.g., angiotensin II, endothelin 1, free oxygen radicals, and thromboxane) [25,26]. Thus, endothelial dysfunction predisposes the vessel wall to vasoconstriction, leukocyte adherence, platelet activation, mitogenesis, pro-oxidation, thrombosis, impaired coagulation, vascular inflammation, and atherosclerosis. Therefore, the noninvasive evaluation of endothelial dysfunction might be helpful to discriminate individuals at risk for atherosclerosis.
3.1. Evaluation of Endothelial Function
Endothelial function/dysfunction can be evaluated by examining endothelium-dependent brachial-artery flow-mediated dilation (FMD) and plethysmographic evaluation of the finger pulse-wave amplitude using peripheral-artery tonometry (PAT). In both methods, after recording the baseline resting parameters, the standard five-minute occlusion of blood flow through the evaluated arterial bed is applied, and the consequent reactive hyperemia (after release of the blood flow) is evaluated as a post-/pre-occlusion change in artery diameter (FMD), or pulse wave amplitude (PAT). In FMD, changes of the diameter of the brachial artery are evaluated from a single side recording, but in PAT, plethysmographic curves are simultaneously recorded at the index fingers of both hands (occluded vs. nonoccluded). This method allows to monitor contralateral changes in vasoconstriction, and to consequently correct the recorded parameters of reactive hyperemia, as well as diminish observer error [26,27]. In this aspect, FMD evaluates endothelial function predominantly dependent on NO bioavailability, and its predictive power for the estimation of cardiovascular risk is assumed to be greater in patients with an already developed process of atherosclerosis. In contrast, reactive hyperemia assessed by PAT seems to be mediated by NO release to approximately 60%, with the rest of the effect potentially attributable to regulation by the autonomic nervous system, and its application seems to be more significant in younger individuals with a lower degree of atherosclerotic changes [24,26,27].
Another approach to evaluate microvascular endothelial dysfunction is based on the measurement of plasma levels of endothelium-related markers. From the aspect of endothelium principal markers, Weibel–Palade bodies are specific endothelial organelles containing von Willebrand factor (VWF), P-selectin, and angiopoetin-2, which participate in platelet binding, leukocyte recruitment, and inflammation modulation, respectively [25]. With respect to vascular pathology, endothelial damage is characterized by increased plasma levels of soluble fractions of intercellular adhesion molecule-1 (ICAM-1), or vascular-cell adhesion molecule-1 (VCAM-1) [28]. A novel list of major and minor endothelial markers with their detailed characteristics is reported in a recent review by Goncharov et al. [25].
Furthermore, the high population of endothelial progenitor cells associated with low levels of endothelial microparticles and circulating endothelial cells indicates a normal function of the endothelium. Specifically, endothelial microparticles are circulating submicron-sized vesicles originated from damaged endothelial cells with various biological functions; as primary and secondary messengers of vascular inflammation, thrombosis, vasomotor response, angiogenesis, and endothelial survival [25]. Therefore, detachment of endothelial cells from the vascular wall represents another characteristic of endothelial damage, leading to a disrupted endothelium and facilitating platelets to interact with the exposed subendothelium. In other words, the presence of increased circulating endothelial cells and reduced endothelial progenitor cells was found in endothelial dysfunction-linked pathological states [29,30].
However, little is known about the effect of an acute mood state on endothelial function; for example, bipolar disorder, characterized by depressive/manic mood, was associated with endothelial dysfunction assessed by FMD [31]. Furthermore, a recent study showed that depression is associated with endothelial dysfunction indexed by reactive hyperemia index [32]. With respect to circulating biomarkers, depression-linked type-D personality, that is, the combination of negative affectivity and social inhibition, has already been associated with endothelial dysfunction assessed by soluble ICAM-1 and VCAM-1, E-selectin, and VWF [33]. In clinically manifested depression, circulating endothelial and progenitor cells, the plasma levels of soluble VWF, and VCAM-1 were altered in major depressive patients, and these biomarkers were normalized after antidepressant treatment, indicating the reversibility of endothelial dysfunction in major depression [29].
3.2. Endothelial Dysfunction—Role in MDD-Linked Vascular Pathology
Atherosclerosis is a chronic vascular inflammatory disease associated with endothelial dysfunction accelerated by a myriad of factors [34]. In this context, several local pathogenic proatherosclerotic mechanisms were considered: increased expression of adhesion molecules; oxidation of low-density lipoprotein (LDL); expression of scavenger receptors promoting increased uptake of oxidized LDL; influx of foam cells; resistance to apoptosis; altered vasomotor tone; and increased production of macrophage chemoattractant protein, growth factors, and matrix metalloproteinases [35,36]. Systemic proatherosclerotic mechanisms involve a higher release of cytokines stimulating the immune responses, activation, and proliferation of T-helper cells; elevated plasma levels of interferons (INF-α, INF-γ), TNF-α, IL-1β, IL-6, IL-17, and IL-18; decreased anti-inflammatory properties of high-density lipoprotein (HDL); ROS formation; and procoagulant activity [35,36].
Mechanisms linking depression and endothelium dysfunction are still unclear. First, several studies emphasized the crucial role of platelet reactivity and NO. A basal-level production of NO by endothelial cells contributes to regulating the vasomotor tone and preserving the nonthrombogenic behavior of the vascular lining [37]. Platelet hyperaggregability may contribute to alterations in the L-arginine–nitric oxide (NO)–cyclic guanosine monophosphate (cGMP) pathway and in platelet function, and consequently to the increased thrombotic risk in MDD [38]. Thus, both increased platelet reactivity and impaired NO production induced by reduced necessary cofactors for NO synthesis (e.g., lower endothelial NO synthase gene expression) could lead to depression-linked impaired endothelial function [9].
3.2.1. Endothelial Dysfunction and Oxidative Stress
Furthermore, several studies referred to the confluence of endothelial dysfunction and oxidative stress involved in the development of atherosclerosis [39]. Specifically, major ROS-producing systems in the vascular wall include a reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, xanthine oxidase, enzymes of the mitochondrial respiratory chain, and dysfunctional uncoupled endothelial NO synthase. While ROS at moderate concentrations have important signaling roles under physiological conditions, excessive ROS production leads to oxidative stress. Thus, key molecular mechanisms in vascular pathology, such as the oxidative modification of lipoproteins/phospholipids, endothelial-cell activation, and macrophage infiltration/activation, are facilitated by oxidative stress and inhibited by endothelial NO [40]. This assumption was confirmed by several studies. In experimental studies, oxidative-stress-induced reductions in NO bioavailability contributed to impaired endothelium dilation, indicating a causal role for increased oxidative stress in vascular pathology [41]. In depressive adults, increased oxidative-stress markers directly contribute to endothelial dysfunction via reductions in NO-dependent mechanisms. Acute superoxide scavenging or NADPH oxidase inhibition improves the NO-mediated component of endothelium-dependent dilation, suggesting that increased vascular oxidative stress, specifically increased superoxide, contributes to endothelial microvascular dysfunction associated with MDD [42]. Both oxidative stress and the reduction of endothelial NO could represent important pathomechanisms leading to endothelial dysfunction as a vascular phenotype predisposing to atherosclerosis development/progression in MDD [3,40]. As noted in a recent meta-analysis, depression is associated with high oxidative stress as a mediating factor in associations between depression and poor health outcomes [43].
3.2.2. Endothelial Dysfunction and Inflammation
Consequently, oxidative stress is closely related to the inflammatory pathway; proinflammatory cytokines are produced in reaction to oxidative stress, which, in turn, amplifies the inflammatory response [44]. Shihata et al. [45] reported that inflammation plays a key role in atherosclerosis progression. Several studies described the predictive value of the C-reactive protein (CRP), IL-6, and TNF-α on atherosclerosis development [46,47]. Specifically, the form of modified/monomeric CRP exerts potent proinflammatory actions on endothelial cells, endothelial progenitor cells, leukocytes, and platelets; thus, it may amplify inflammation [3]. Additionally, inflammation couples dyslipidemia to atherogenesis initiated by inflammatory processes in the endothelial cells of the vessel wall in response to LDL particles. On the other hand, endothelial dysfunction leads to the release of proinflammatory cytokines inducing inflammatory pathways to promote thrombus formation and vascular occlusion, leading to myocardial infarctions and strokes [48].
3.2.3. Endothelial Dysfunction and Glucocorticoids
Glucocorticoids exert a crucial effect on vascular smooth muscle and endothelial cells that is important in atherosclerosis progression. Specifically, glucocorticoids through GRs directly modulate endothelial function by regulating the expression of adhesion molecules (VCAM-1, ICAM-1, E-selectin), proinflammatory cytokines and chemokines (IL-6, IL-17F, IL-8, MCP-1), vasodilators (NO), and vasoconstrictors (angiotensin II and endothelin I) [49]. In this context, experimental studies emphasized that endothelial GRs are profoundly important for maintaining vascular homeostasis, and their loss, in combination with a second hit such as the inflammatory milieu, accelerates the disease [50,51]. Moreover, glucocorticoids may contribute to the process of atherosclerosis through their effect on lipid-metabolism regulation mediated by two isoforms of 11β-hydroxysteroid dehydrogenase (11β-HSD1 and 11β-HSD2), enzymes catalyzing the conversion of inactive cortisone to active cortisol, or vice versa, thus regulating the access of glucocorticoids to steroid receptors. Experimental studies revealed that inhibition of 11β-HSD1 slowed atherosclerosis [52,53], while 11β-HSD2 deficiency accelerated atherogenesis and caused proinflammatory changes in the endothelium [54]. Taken together, glucocorticoids certainly exert cardiovascular effects at the vasculature level, but despite the extensive clinical use of glucocorticoids, their detrimental effects on vascular functioning are still not fully characterized. From this perspective, further studies are needed to precisely elucidate this question, particularly from a clinical point of view.
A summary of the discussed biomarkers for depression-linked inflammatory activity and endothelial dysfunction is given in Table 1.
Table 1.
Recent preview of peripheral biomarkers of inflammation activity and endothelial function in depression.
| Recent Studies | Measured Markers | Main Findings |
|---|---|---|
| Inflammation-Activity Biomarkers | ||
| Schmidt et al., 2014 [55] | IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, TNF-γ, TNF-α | Higher levels of IL-2, IL-5, IL-12, IL-13, INF-γ, and TNF-α were found in depressive patients compared with those in non-depressive subjects. |
| Haapakoski et al., 2015 [56] | IL-6, IL-1β, TNF-α | Meta-analysis confirmed higher serum IL-6 levels in depressive patients compared with those in non-depressive controls. |
| Al-Hakeim et al., 2015 [57] | IL-6, IL-18, TNF-α | Serum levels of IL-6, IL-18, and TNFα were significantly increased in depressive patients compared with those of the control group. |
| Muthuramalingam et al., 2016 [58] | IL-6, TNF-α, TGF-β | Depressive patients demonstrated significantly raised baseline levels of TNF-α and IL-6, but no difference in levels of TGF-β compared with healthy controls. |
| Goldsmith et al., 2016 [59] | IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α, INF-γ | IL-6, IL-10, IL-12, and TNF-α levels were significantly increased, and levels of IFN-γ and IL-4 were significantly decreased without significant differences in IL-1β and IL-2 levels in patients with MDD compared with controls. |
| Zou et al., 2018 [60] | IL-1β, IL-6, IL-8, IL-10, TNF-α, TGF-β1 | Increased levels of IL-1β, IL-10, and TNF-α, and decreased IL-8 levels, were found in MDD patients compared with healthy controls. |
| Ng et al., 2018 [61] | IL-1β, IL-6, TNF- α | Meta-analysis found elevated peripheral levels of IL-1β and IL-6 without TNF-α in depressive patients compared with controls. |
| Gariup et al., 2015 [62] | IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IFN-γ, and TNF-a | Depressive patients (aged 8–17 years) had significantly higher levels of IL-6, IL-8, and IL-1β compared with controls. |
| Pallavi et al., 2015 [63] | IL-1β, IL-2, IL-6, IL-10, IL-17, TNF-α, IFN-γ, and TGF-β | Male MDD adolescents had significantly higher levels of IL-2 compared with controls; female MDD adolescents had significantly elevated serum IL-2 and IL-6 compared with their healthy female counterparts. |
| Miklowitz et al., 2016 [64] | TNF-α, IL-1β, IL-6, IL-8, and IL-10 | No significant differences in measured cytokines between MDD and controls. |
| Perez-Sanchez et al., 2018 [65] | IL-2, IFN-γ, IL-1β, TNF-α, IL-6, IL-12, IL-4, IL-5, IL-13, and IL-10 | Adolescents with MDD at baseline showed significant increases in all mentioned cytokines, except for IL-10, compared with healthy subjects. |
| Endothelial-Function Biomarkers | ||
| Van Agtmaal et al., 2017 [66] | VCAM, ICAM, E-selectin, VWF | Meta-analysis revealed association between increased levels of all measured peripheral markers and depression. |
| Blum et al., 2017 [67] | VCAM-1, VEGF, EPCs | MDD patients had high levels of VCAM-1 and VEGF; significant inhibition of EPCs colonies. |
| Baghai et al., 2018 [68] | ICAM-1, P-selectin, E-Selectin | ICAM-1 was significantly elevated in MDD group compared with healthy controls. |
| Saleptsis et al., 2019 [69] | P-selectin | Depressive patients had higher levels of P-selectin compared with individuals free of depression. |
EPCs, endothelial progenitor cells; ICAM, intercellular adhesion molecule; IL, interleukin; IFN, interferon; MDD, major depressive disorder; TGF, transforming growth factor; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor; VWF, von Willebrand factor.
4. Role of Autonomic Nervous System in Depressive Disorder
The autonomic nervous system (ANS) is a central regulatory orchestrator in a mediating role of depression-inflammation-vascular dysfunction.
The ANS consists of two divisions: the sympathetic nervous system with principal neurotransmitter norepinephrine/epinephrine, and the parasympathetic nervous system with principal neurotransmitter acetylcholine. Both ANS divisions are highly coordinated in a dynamic sympathovagal balance aimed at maintaining physiological homeostasis. Disruption in ANS homeostasis, characterized by sympathetic overactivity and/or vagal underactivity, is an important contributor to adverse cardiac outcomes associated with depression [70,71]. Heart rate variability (HRV), that is, spontaneous oscillations of heart rate around its mean value, is determined by the dynamic interaction of the acceleratory sympathetic nervous system and deceleratory parasympathetic nervous system, indicating a healthy and adaptive organism. Despite the fact that deficient cardiac vagal control indexed by HRV is a common finding in major depression [72,73], a recent study concluded that parasympathetic predominance may precede the onset of depression, thus indicating a risk factor for future major depression [74]. Therefore, HRV analysis represents a promising biomarker—a noninvasive window into neurocardiac regulatory inputs in MDD.
Physiological functioning requires a close connection of the ANS and immune systems. For example, sympathetic output to lymphoid organs, including the spleen, induces anti-inflammatory responses via the β2 adrenergic receptors (adrenoceptors), expressed in multiple cells of the innate and adaptive immune systems. Vagal efferents affect immune responses in the gut via the enteric nervous system, and both vagal and dorsal root ganglion afferents trigger immunomodulatory responses via the antidromic release of neuropeptides and other signals at the target organs [5]. In addition, a recent study referred to the relationship between ANS function and chromosomal integrity in CD8+CD28- T lymphocytes [75]. With respect to regulation, the CNS controls the immune response through the ANS. Specifically, the presence of cytokines in the periphery is relayed via the vagus nerve to the CNS, particularly into the nucleus tractus solitarii, as the major area for neuroimmune communication. The efferent activity in the vagus nerve leads to parasympathetic neurotransmitter-acetylcholine release in the organs of the reticuloendothelial system, including the liver, heart, spleen, and gastrointestinal tract. Acetylcholine interacts with α7 subunits of containing acetylcholine receptors (α7nAChRs), expressed on macrophages that inhibit the release of cytokines—the cholinergic anti-inflammatory pathway [4]. However, this pathway model has recently been challenged, as there is no clear evidence of a synaptic connection from the vagus to splenic sympathetic nerves [76,77]. Currently, the review by Benarroch [5] has described the sympathetic-cholinergic anti-inflammatory pathway in detail; sympathetic output from the coeliac ganglia to the spleen promotes a potent anti-inflammatory response mediated by activation of β2-adrenoceptors in a population of T cells expressing choline acetyltransferase, important for acetylcholine synthesis. Subsequently, the activation of β2-adrenoceptors in these T cells evokes the release of acetylcholine that, via α7nAChRs on macrophages and other immune cells, suppresses the release of proinflammatory cytokines. Thus, the disruption of the sympathovagal dynamic balance could lead to activation of the proinflammatory pathway, resulting in a proinflammatory state. Recently, meta-analysis showed negative associations between HRV indices and inflammation markers [78].
A recent study summarized the associations of autonomic dysfunction, inflammation, and markers of atherosclerosis [79]. For example, intima media thickness, a marker of preclinical atherosclerosis, was associated with increased CRP and reduced HRV in patients suffering from depression [80]. Similarly, Ulleryd et al. [81] concluded that autonomic dysfunction is associated with atherosclerosis, and inflammation could play an important role in mediating this relationship. Moreover, ANS imbalance might alter not only inflammatory response, but also platelet aggregation, coagulation, or lipoprotein metabolism. Sympathetic activation provokes a simultaneous increase in both coagulation and fibrinolysis pathways, resulting in a hypercoagulability state. Thus, sympathetic chronic stimulation associated with concomitant hypercoagulable changes may contribute to gradual fibrin deposition at sites of atherosclerotic lesions and, in patients with endothelial dysfunction, an acute stress response could trigger the rupture of atherosclerotic plaque [82]. Therefore, the disruption of ANS homeostasis could play a fundamental role in the complex pathophysiological cascade linking major depression and CVD.
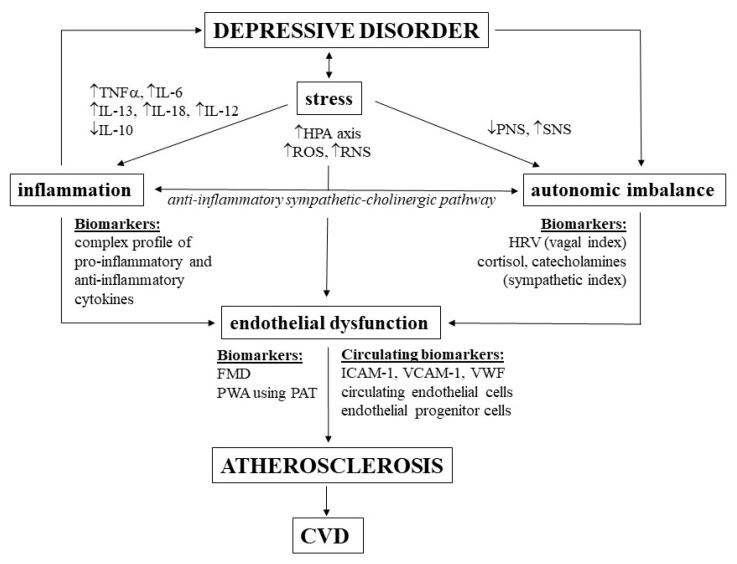
A summary of the discussed depression-linked pathomechanisms leading to atherosclerosis and biomarkers is given in Figure 1.
Figure 1.
Pathomechanisms and biomarkers related to depression-linked increased risk of cardiovascular diseases (CVDs). TNFα, tumor necrosis factor α; IL, interleukin; HPA, hypothalamic–pituitary–adrenal; ROS, reactive oxygen species; RNS, reactive nitrogen species; PNS, parasympathetic nervous system; SNS, sympathetic nervous system; HRV, heart rate variability; FMD, flow-mediated dilation; PWA, pulse-wave amplitude; PAT, peripheral arterial tonometry; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; VWF, von Willebrand factor.
5. Concluding Remarks and Perspectives
Major depression represents an independent factor for adverse cardiovascular outcomes. In this context, complex pathophysiological mechanisms connecting inflammation-vascular-autonomic dysregulation could represent pathways linking MDD and vascular pathology. Specifically, the depression-linked proinflammatory status is closely associated with endothelial dysfunction, and promotes atherosclerosis, resulting in an increased risk to develop CVD in major depression [83]. Further, ANS imbalance—sympathetic overactivity and vagal underactivity—stimulates neurophysiological pathways, leading to acceleration of atherosclerotic processes in MDD [79].
Future studies should clarify this issue from several aspects. First, it is important to illuminate pathomechanisms linking depression and vascular pathology in terms of developmental trajectory with a focus on the vulnerable and critical adolescent-age period. It is crucial for the early diagnosis and prevention of later cardiovascular complications in adults. Further clinical research is essential to clearly identify biomarkers for the assessment of inflammation-vascular-autonomic dysfunction, which could be used for personalized prevention and treatment of CVD in depressive patients. Currently, increasing attention is focused on the efficacy of anti-inflammatory treatment in major depressive disorder [84,85]; thus, the development of novel therapeutic interventions targeting the immune, vascular, and autonomic nervous systems in MDD is of particular importance.
Abbreviations
| MDD | Major depressive disorder |
| CVD | Cardiovascular disease |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| WHO | World Health Organization |
| IFN | Interferon |
| TGF | Transforming growth factor |
| CNS | Central nervous system |
| MCP | Monocyte chemoattractant protein |
| IDO | Indoleamine 2 |
| NMDA | N-methyl-D-aspartate |
| HPA | Hypothalamic-pituitary-adrenal |
| GRs | Glucocorticoid receptors |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| NO | Nitric oxide |
| FMD | Flow-mediated dilation |
| PAT | Peripheral-artery tonometry |
| VWF | von Willebrand factor |
| ICAM-1 | Intercellular adhesion molecule-1 |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
| cGMP | Cyclic guanosine monophosphate |
| NADPH | Reduced form of nicotinamide adenine dinucleotide phosphate |
| CRP | C-reactive protein |
| EPCs | Endothelial progenitor cells |
| VEGF | Vascular endothelial growth factor |
| ANS | Autonomic nervous system |
| SNS | Sympathetic nervous system |
| PNS | Parasympathetic nervous system |
| HRV | Heart rate variability |
Author Contributions
I.T., professor of normal and pathological physiology, and head of research team, wrote and contributed to the preparation of all parts of the original and revised manuscript, final version, supervision, and approval of the manuscript. L.B.O., N.S., and Z.V., clinicians and young researchers in the field of normal and pathological physiology. The contribution of all named authors was searching and selection of the scientific studies according to the topic of the manuscript and final editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Slovak Scientific Grant Agency under grants VEGA 1/0044/18 and VEGA 1/0190/20, and the project implementation Biomedical Center Martin, ITMS code: 26220220187, supported by the Operational Program Research and Innovation funded by the European Regional Development Fund (ERDF).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Depression. [(accessed on 13 January 2020)]; Available online: https://www.who.int/en/news-room/fact-sheets/detail/depression.
- 2.Polanczyk G.V., Salum G.A., Sugaya L.S., Caye A., Rohde L.A. Annual Research Review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child Psychol. Psychiatry. 2015;56:345–365. doi: 10.1111/jcpp.12381. [DOI] [PubMed] [Google Scholar]
- 3.Halaris A. Inflammation-associated co-morbidity between depression and cardiovascular disease. Curr. Top. Behav. Neurosci. 2017;31:45–70. doi: 10.1007/7854_2016_28. [DOI] [PubMed] [Google Scholar]
- 4.Tracey K.J. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 5.Benarroch E.E. Autonomic nervous system and neuroimmune interactions: New insights and clinical implications. Neurology. 2019;92:377–385. doi: 10.1212/WNL.0000000000006942. [DOI] [PubMed] [Google Scholar]
- 6.Femenia T., Giménez-Cassina A., Codeluppi S., Fernández-Zafra T., Katsu-Jiménez Y., Terrando N., Eriksson L.I., Gómez-Galán M. Disrupted neuroglial metabolic coupling after peripheral surgery. J. Neurosci. 2018;38:452–464. doi: 10.1523/JNEUROSCI.1797-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim Y.K., Na K.S., Myint A.M., Leonard B.E. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:277–284. doi: 10.1016/j.pnpbp.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Himmerich H., Patsalos O., Lichtblau N., Ibrahim M.A., Dalton B. Cytokine research in depression: Principles, challenges, and open questions. Front. Psychiatry. 2019;10:1–16. doi: 10.3389/fpsyt.2019.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon S.W., Kim Y.K. Neuroinflammation and cytokine abnormality in major depression: Cause or consequence in that illness? World J. Psychiatry. 2016;6:283–293. doi: 10.5498/wjp.v6.i3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sochocka M., Diniz B.S., Leszek J. Inflammatory response in the CNS: Friend or foe? Mol. Neurobiol. 2017;54:8071–8089. doi: 10.1007/s12035-016-0297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith R.S. [(accessed on 13 January 2020)];Cytokines and Depression: How Your Immune System Causes Depression. Available online: http://www.cytokines-and-depression.com/
- 12.Maes M., Yirmyia R., Noraberg J., Brene S., Hibbeln J., Perini G., Kubera M., Bob P., Lerer B., Maj M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: Leads for future research and new drug developments in depression. Metab. Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 13.Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctôt K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 14.Köhler C.A., Freitas T.H., Maes M., de Andrade N.Q., Liu C.S., Fernandes B.S., Stubbs B., Solmi M., Veronese N., Herrmann N., et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017;135:373–387. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- 15.D’Acunto G., Nageye F., Zhang J., Masi G., Cortese S. Inflammatory cytokines in children and adolescents with depressive disorders: A systematic review and meta-analysis. J. Child Adolesc. Psychopharmacol. 2019;29:362–369. doi: 10.1089/cap.2019.0015. [DOI] [PubMed] [Google Scholar]
- 16.Myint A.M., Kim Y.K. Cytokine-serotonin interaction through IDO: A neurodegeneration hypothesis of depression. Med. Hypotheses. 2003;61:519–525. doi: 10.1016/S0306-9877(03)00207-X. [DOI] [PubMed] [Google Scholar]
- 17.Won E., Kim Y.K. Stress, the autonomic nervous system, and the immune-kynurenine pathway in the etiology of depression. Curr. Neuropharmacol. 2016;14:665–673. doi: 10.2174/1570159X14666151208113006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baranyi A., Amouzadeh-Ghadikolai O., von Lewinski D., Breitenecker R.J., Rothenhäusler H.B., Robier C., Baranyi M., Theokas S., Meinitzer A. Revisiting the tryptophan-serotonin deficiency and the inflammatory hypotheses of major depression in a biopsychosocial approach. PeerJ. 2017;5:1–22. doi: 10.7717/peerj.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slavich G.M., Irwin M.R. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol. Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson J.D., Barnard D.F., Kulp A.C., Mehta D.M. Neuroendocrine regulation of brain cytokines after psychological stress. J. Endocr. Soc. 2019;3:1302–1320. doi: 10.1210/js.2019-00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raison C.L., Capuron L., Miller A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shishkina G.T., Dygalo N.N. The glucocorticoid hypothesis of depression: History and prospects. Russ. J. Genet. Appl. Res. 2017;7:128–133. doi: 10.1134/S2079059717010142. [DOI] [Google Scholar]
- 23.Moncada S. The Vascular Endothelium. In: Luz P.L., Libby P., Chagas A.C., Laurindo F.R., editors. Endothelium and Cardiovascular Diseases. 1st ed. Academic Press; Cambridge, MA, USA: 2018. pp. 5–10. [Google Scholar]
- 24.Barthelmes J., Nägele M.P., Ludovici V., Ruschitzka F., Sudano I., Flammer A.J. Endothelial dysfunction in cardiovascular disease and Flammer syndrome-similarities and differences. EPMA J. 2017;8:99–109. doi: 10.1007/s13167-017-0099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goncharov N.V., Nadeev A.D., Jenkins R.O., Avdonin P.V. Markers and biomarkers of endothelium: When something is rotten in the state. Oxid. Med. Cell. Longev. 2017;2017:1–27. doi: 10.1155/2017/9759735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flammer A.J., Anderson T., Celermajer D.S., Creager M.A., Deanfield J., Ganz P., Hamburg N.M., Lüscher T.F., Shechter M., Taddei S., et al. The assessment of endothelial function: From research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonhajzerova I., Bona Olexova L., Jurko A., Spronck B., Jurko T., Sekaninova N., Visnovcova Z., Mestanikova A., Kudela E., Mestanik M. Novel biomarkers of early atherosclerotic changes for personalised prevention of cardiovascular disease in cervical cancer and human papillomavirus infection. Int. J. Mol. Sci. 2019;20:720. doi: 10.3390/ijms20153720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang Y., Li C., Shao R., Yu H., Zhang Q. The role of biomarkers of endothelial activation in predicting morbidity and mortality in patients with severe sepsis and septic shock in intensive care: A prospective observational study. Thromb. Res. 2018;171:149–154. doi: 10.1016/j.thromres.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Vilchez I., Diaz-Ricart M., Navarro V., Torramade S., Zamorano-Leon J., Lopez-Farre A., Galan A.M., Gasto C., Escolar G. Endothelial damage in major depression patients is modulated by SSRI treatment, as demonstrated by circulating biomarkers and an in vitro cell model. Transl. Psychiatry. 2016;6:1–8. doi: 10.1038/tp.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Regueiro A., Cuadrado-Godia E., Bueno-Betí C., Diaz-Ricart M., Oliveras A., Novella S., Gené G.G., Jung C., Subirana I., Ortiz-Pérez J.T., et al. Mobilization of endothelial progenitor cells in acute cardiovascular events in the PROCELL study: Time-course after acute myocardial infarction and stroke. J. Mol. Cell. Cardiol. 2015;80:146–155. doi: 10.1016/j.yjmcc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz S., Abosi O., Persons J., Sinkey C., Fiedorowicz J. Impact of mood on endothelial function and arterial stiffness in bipolar disorder. Hear. Mind. 2018;2:78–84. doi: 10.4103/hm.hm_20_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi H., Feng G., Wang Z., Zhou C., Zhong G., Hu Y., Wang G. Relationships between depressive symptoms and endothelial function among outpatients of a general hospital in China. Med. Sci. Monit. 2015;21:1812–1819. doi: 10.12659/MSM.893531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Dooren F.E., Verhey F.R., Pouwer F., Schalkwijk C.G., Sep S.J., Stehouwer C.D., Henry R.M., Dagnelie P.C., Schaper N.C., van der Kallen C.J., et al. Association of Type D personality with increased vulnerability to depression: Is there a role for inflammation or endothelial dysfunction?—The Maastricht Study. J. Affect. Disord. 2016;189:118–125. doi: 10.1016/j.jad.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 34.Marchio P., Guerra-Ojeda S., Vila J.M., Aldasoro M., Victor V.M., Mauricio M.D. Targeting early atherosclerosis: A focus on oxidative stress and inflammation. Oxid. Med. Cell. Longev. 2019;2019:1–32. doi: 10.1155/2019/8563845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pothineni N.V., Subramany S., Kuriakose K., Shirazi L.F., Romeo F., Shah P.K., Mehta J.L. Infections, atherosclerosis, and coronary heart disease. Eur. Heart J. 2017;38:3195–3201. doi: 10.1093/eurheartj/ehx362. [DOI] [PubMed] [Google Scholar]
- 36.Sessa R., Di Pietro M., Filardo S., Turriziani O. Infectious burden and atherosclerosis: A clinical issue. World J. Clin. Cases. 2014;2:240–249. doi: 10.12998/wjcc.v2.i7.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gimbrone M.A., García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ormonde do Carmo M.B., Mendes-Ribeiro A.C., Matsuura C., Pinto V.L., Mury W.V., Pinto N.O., Moss M.B., Ferraz M.R., Brunini T.M. Major depression induces oxidative stress and platelet hyperaggregability. J. Psychiatr. Res. 2015;61:19–24. doi: 10.1016/j.jpsychires.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein B.I., Carnethon M.R., Matthews K.A., McIntyre R.S., Miller G.E., Raghuveer G., Stoney C.M., Wasiak H., McCrindle B.W. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 2015;132:965–986. doi: 10.1161/CIR.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 40.Förstermann U., Xia N., Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017;120:713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 41.Brooks S., Brnayan K.W., DeVallance E., Skinner R., Lemaster K., Sheets J.W., Pitzer C.R., Asano S., Bryner R.W., Olfert I.M., et al. Psychological stress-induced cerebrovascular dysfunction: The role of metabolic syndrome and exercise. Exp. Physiol. 2018;103:761–776. doi: 10.1113/EP086892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greaney J.L., Saunders E.F., Santhanam L., Alexander L.M. Oxidative stress contributes to microvascular endothelial dysfunction in men and women with major depressive disorder. Circ. Res. 2019;124:564–574. doi: 10.1161/CIRCRESAHA.118.313764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palta P., Samuel L.J., Miller E.R., Szanton S.L. Depression and oxidative stress: Results from a meta-analysis of observational studies. Psychosom. Med. 2014;76:12–19. doi: 10.1097/PSY.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Black C.N., Bot M., Scheffer P.G., Cuijpers P., Penninx B.W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164–175. doi: 10.1016/j.psyneuen.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 45.Shihata W.A., Michell D.L., Andrews K.L., Chin-Dusting J.P. Caveolae: A role in endothelial inflammation and mechanotransduction? Front. Physiol. 2016;7:628. doi: 10.3389/fphys.2016.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danesh J., Collins R., Peto R. Chronic infections and coronary heart disease: Is there a link? Lancet. 1997;350:430–436. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 47.Kop W.J., Gottdiener J.S. The role of immune system parameters in the relationship between depression and coronary artery disease. Psychosom. Med. 2005;67:37–41. doi: 10.1097/01.psy.0000162256.18710.4a. [DOI] [PubMed] [Google Scholar]
- 48.Tarantino G., Costantini S., Finelli C., Capone F., Guerriero E., La Sala N., Gioia S., Castello G. Carotid intima-media thickness is predicted by combined eotaxin levels and severity of hepatic steatosis at ultrasonography in obese patients with nonalcoholic fatty liver disease. PLoS ONE. 2014;9:1–8. doi: 10.1371/journal.pone.0105610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burford N.G., Webster N.A., Cruz-Topete D. Hypothalamic-pituitary-adrenal axis modulation of glucocorticoids in the cardiovascular system. Int. J. Mol. Sci. 2017;18:2150. doi: 10.3390/ijms18102150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodwin J.E., Feng Y., Velazquez H., Sessa W.C. Endothelial glucocorticoid receptor is required for protection against sepsis. Proc. Natl. Acad. Sci. USA. 2013;110:306–311. doi: 10.1073/pnas.1210200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodwin J.E., Zhang X., Rotllan N., Feng Y., Zhou H., Fernández-Hernando C., Yu J., Sessa W.C. Endothelial glucocorticoid receptor suppresses atherogenesis--brief report. Arterioscler. Thromb. Vasc. Biol. 2015;35:779–782. doi: 10.1161/ATVBAHA.114.304525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo M.J., Thieringer R., Springer M.S., Wright S.D., Hermanowski-Vosatka A., Plump A., Balkovec J.M., Cheng K., Ding G.J., Kawka D.W., et al. 11β-HSD1 inhibition reduces atherosclerosis in mice by altering proinflammatory gene expression in the vasculature. Physiol. Genomics. 2013;45:47–57. doi: 10.1152/physiolgenomics.00109.2012. [DOI] [PubMed] [Google Scholar]
- 53.Kipari T., Hadoke P.W., Iqbal J., Man T.Y., Miller E., Coutinho A.E., Zhang Z., Sullivan K.M., Mitic T., Livingstone D.E., et al. 11β-hydroxysteroid dehydrogenase type 1 deficiency in bone marrow-derived cells reduces atherosclerosis. FASEB J. 2013;27:1519–1531. doi: 10.1096/fj.12-219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deuchar G.A., McLean D., Hadoke P., Brownstein D.G., Webb D.J., Mullins J.J., Chapman K., Seckl J.R., Kotelevtsev Y.V. 11β-hydroxysteroid dehydrogenase type 2 deficiency accelerates atherogenesis and causes proinflammatory changes in the endothelium in apoe-/- mice. Endocrinology. 2011;152:236–246. doi: 10.1210/en.2010-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidt F.M., Lichtblau N., Minkwitz J., Chittka T., Thormann J., Kirkby K.C., Sander C., Mergl R., Faßhauer M., Stumvoll M., et al. Cytokine levels in depressed and non-depressed subjects, and masking effects of obesity. J. Psychiatr. Res. 2014;55:29–34. doi: 10.1016/j.jpsychires.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 56.Haapakoski R., Mathieu J., Ebmeier K.P., Alenius H., Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain. Behav. Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al-Hakeim H.K., Al-Rammahi D.A., Al-Dujaili A.H. IL-6, IL-18, sIL-2R, and TNFα proinflammatory markers in depression and schizophrenia patients who are free of overt inflammation. J. Affect. Disord. 2015;182:106–114. doi: 10.1016/j.jad.2015.04.044. [DOI] [PubMed] [Google Scholar]
- 58.Muthuramalingam A., Menon V., Rajkumar R.P., Negi V. Is depression an inflammatory disease? Findings from a cross-sectional study at a tertiary care center. Indian J. Psychol. Med. 2016;38:114–119. doi: 10.4103/0253-7176.178772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldsmith D.R., Rapaport M.H., Miller B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry. 2016;21:1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zou W., Feng R., Yang Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS ONE. 2018;13:1–10. doi: 10.1371/journal.pone.0197267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ng A., Tam W.W., Zhang M.W., Ho C.S., Husain S.F., McIntyre R.S., Ho R.C. IL-1β, IL-6, TNF- α and CRP in elderly patients with depression or Alzheimer’s disease: Systematic review and meta-analysis. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-30487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gariup M., Gonzalez A., Lázaro L., Torres F., Serra-Pagès C., Morer A. IL-8 and the innate immunity as biomarkers in acute child and adolescent psychopathology. Psychoneuroendocrinology. 2015;62:233–242. doi: 10.1016/j.psyneuen.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 63.Pallavi P., Sagar R., Mehta M., Sharma S., Subramanium A., Shamshi F., Sengupta U., Pandey R.M., Mukhopadhyay A.K. Serum cytokines and anxiety in adolescent depression patients: Gender effect. Psychiatry Res. 2015;229:374–380. doi: 10.1016/j.psychres.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 64.Miklowitz D.J., Portnoff L.C., Armstrong C.C., Keenan-Miller D., Breen E.C., Muscatell K.A., Eisenberger N.I., Irwin M.R. Inflammatory cytokines and nuclear factor-kappa B activation in adolescents with bipolar and major depressive disorders. Psychiatry Res. 2016;241:315–322. doi: 10.1016/j.psychres.2016.04.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pérez-Sánchez G., Becerril-Villanueva E., Arreola R., Martínez-Levy G., Hernández-Gutiérrez M.E., Velasco-Velásquez M.A., Alvarez-Herrera S., Cruz-Fuentes C., Palacios L., De La Peña F., et al. Inflammatory profiles in depressed adolescents treated with fluoxetine: An 8-week follow-up open study. Mediators Inflamm. 2018;2018:1–12. doi: 10.1155/2018/4074051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Agtmaal M.J., Houben A.J., Pouwer F., Stehouwer C.D., Schram M.T. Association of microvascular dysfunction with late-life depression: A systematic review and meta-analysis. JAMA Psychiatry. 2017;74:729–739. doi: 10.1001/jamapsychiatry.2017.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blum A., Pastuck N., Zaroura I., Rotem J., Kamal F. Impaired ability to grow colonies of endothelial stem cells could be the mechanism explaining the high cardiovascular morbidity and mortality of patients with depression. QJM. 2017;110:501–506. doi: 10.1093/qjmed/hcx059. [DOI] [PubMed] [Google Scholar]
- 68.Baghai T.C., Varallo-Bedarida G., Born C., Häfner S., Schüle C., Eser D., Zill P., Manook A., Weigl J., Jooyandeh S., et al. Classical risk factors and inflammatory biomarkers: One of the missing biological links between cardiovascular disease and major depressive disorder. Int. J. Mol. Sci. 2018;19:1740. doi: 10.3390/ijms19061740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saleptsis V., Papaliaga M., Spanos K., Kouvelos G., Labropoulos N., Halaris A., Giannoukas A. Carotid intima-media thickness and circulation markers of inflammation in patients with depression. J. Diagnostic Med. Sonogr. 2019;36:111–119. doi: 10.1177/8756479319890579. [DOI] [Google Scholar]
- 70.Shi S., Liang J., Liu T., Yuan X., Ruan B., Sun L., Tang Y., Yang B., Hu D., Huang C. Depression increases sympathetic activity and exacerbates myocardial remodeling after myocardial infarction: Evidence from an animal experiment. PLoS ONE. 2014;9:1–12. doi: 10.1371/journal.pone.0101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ngampramuan S., Tungtong P., Mukda S., Jariyavilas A., Sakulisariyaporn C. Evaluation of autonomic nervous system, saliva cortisol levels, and cognitive function in major depressive disorder patients. Depress. Res. Treat. 2018;2018:1–8. doi: 10.1155/2018/7343592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mestanikova A., Mestanik M., Ondrejka I., Hrtanek I., Cesnekova D., Jurko A., Visnovcova Z., Sekaninova N., Tonhajzerova I. Complex cardiac vagal regulation to mental and physiological stress in adolescent major depression. J. Affect. Disord. 2019;249:234–241. doi: 10.1016/j.jad.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 73.Koch C., Wilhelm M., Salzmann S., Rief W., Euteneuer F. A meta-analysis of heart rate variability in major depression. Psychol. Med. 2019;49:1948–1957. doi: 10.1017/S0033291719001351. [DOI] [PubMed] [Google Scholar]
- 74.An H., Han J.W., Jeong H.G., Kim T.H., Lee J.J., Lee S.B., Park J.H., Kim K.W. Parasympathetic predominance is a risk factor for future depression: A prospective cohort study. J. Affect. Disord. 2020;260:232–237. doi: 10.1016/j.jad.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 75.Shahane A.D., LeRoy A.S., Denny B.T., Fagundes C.P. Connecting cognition, cardiology, and chromosomes: Cognitive reappraisal impacts the relationship between heart rate variability and telomere length in CD8+CD28– cells. Psychoneuroendocrinology. 2020;112:1–7. doi: 10.1016/j.psyneuen.2019.104517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bratton B.O., Martelli D., McKinley M.J., Trevaks D., Anderson C.R., McAllen R.M. Neural regulation of inflammation: No neural connection from the vagus to splenic sympathetic neurons. Exp. Physiol. 2012;97:1180–1185. doi: 10.1113/expphysiol.2011.061531. [DOI] [PubMed] [Google Scholar]
- 77.Martelli D., McKinley M.J., McAllen R.M. The cholinergic anti-inflammatory pathway: A critical review. Auton. Neurosci. Basic Clin. 2014;182:65–69. doi: 10.1016/j.autneu.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 78.Williams D.P., Koenig J., Carnevali L., Sgoifo A., Jarczok M.N., Sternberg E.M., Thayer J.F. Heart rate variability and inflammation: A meta-analysis of human studies. Brain. Behav. Immun. 2019;80:219–226. doi: 10.1016/j.bbi.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 79.Kadoya M., Koyama H. Sleep, autonomic nervous function and atherosclerosis. Int. J. Mol. Sci. 2019;20:794. doi: 10.3390/ijms20040794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pizzi C., Manzoli L., Mancini S., Bedetti G., Fontana F., Costa G.M. Autonomic nervous system, inflammation and preclinical carotid atherosclerosis in depressed subjects with coronary risk factors. Atherosclerosis. 2010;212:292–298. doi: 10.1016/j.atherosclerosis.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 81.Ulleryd M.A., Prahl U., Börsbo J., Schmidt C., Nilsson S., Bergström G., Johansson M.E. The association between autonomic dysfunction, inflammation and atherosclerosis in men under investigation for carotid plaques. PLoS ONE. 2017;12:e0174974. doi: 10.1371/journal.pone.0174974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pagano G., Talamanca A.A., Castello G., Cordero M.D., d’Ischia M., Gadaleta M.N., Pallardó F.V., Petrović S., Tiano L., Zatterale A. Oxidative stress and mitochondrial dysfunction across broad-ranging pathologies: Toward mitochondria-targeted clinical strategies. Oxid. Med. Cell. Longev. 2014;2014:1–27. doi: 10.1155/2014/541230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caruso G., Fresta C.G., Grasso M., Santangelo R., Lazzarino G., Lunte S.M., Caraci F. Inflammation as the common biological link between depression and cardiovascular diseases: Can carnosine exert a protective role? Curr. Med. Chem. 2019;26:1. doi: 10.2174/0929867326666190712091515. [DOI] [PubMed] [Google Scholar]
- 84.Köhler-Forsberg O., N. Lydholm C., Hjorthøj C., Nordentoft M., Mors O., Benros M.E. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: Meta-analysis of clinical trials. Acta Psychiatr. Scand. 2019;139:404–419. doi: 10.1111/acps.13016. [DOI] [PubMed] [Google Scholar]
- 85.Liu J.J., Wei Y.B., Strawbridge R., Bao Y., Chang S., Shi L., Que J., Gadad B.S., Trivedi M.H., Kelsoe J.R., et al. Peripheral cytokine levels and response to antidepressant treatment in depression: A systematic review and meta-analysis. Mol. Psychiatry. 2020;25:339–350. doi: 10.1038/s41380-019-0474-5. [DOI] [PubMed] [Google Scholar]