Abstract
Effective and efficient delivery of healthcare services requires comprehensive collaboration and coordination between healthcare entities and their complex inter-reliant activities. This inter-relation and coordination lead to different networks among diverse healthcare stakeholders. It is important to understand the varied dynamics of these networks to measure the efficiency of healthcare delivery services. To date, however, a work that systematically reviews these networks outlined in different studies is missing. This article provides a comprehensive summary of studies that have focused on networks and administrative health data. By summarizing different aspects including research objectives, key research questions, adopted methods, strengths and weaknesses, this research provides insights into the inherently complex and interlinked networks present in healthcare services. The outcome of this research is important to healthcare management and may guide further research in this area.
Keywords: administrative health data, network study, network method
1. Introduction
Administrative health data are an important and the largest source of data collected from a large number of healthcare services provided by different healthcare stakeholders to patients [1,2]. These data are created when a healthcare customer connects with healthcare elements; for example, visiting a doctor, having medical diagnoses performed, being conceded into a medical clinic, or purchasing medicines from a pharmacy. The term ‘administrative health data’ also refers to “administrative data", "claim data", "electronic hospital record", “digital health data”, “digitized health data”, "electronic medical data" and "e-medical data". These data are used for patients’ care, diagnostic information, health treatments, or crosswise over various medicinal service offices (e.g., emergency clinics, aged care centers and nursing homes).
Recently, administrative health data have been used extensively in the healthcare community for research investigation and clinical decision making; for example, for disease risk prediction, analysis and restorative treatment [2]. Customarily, clinical choices have relied upon doctors’ estimation, capacity, experience and different clinical and diagnostic test outcomes. This practice culture, however, may prompt unsolicited favoritisms, faults, high admission cost and low service quality rendered to patients [2]. The combination of medical decision-making support with administrative claim data can significantly lessen medical errors and undesirable varieties of patient experiences, which could potentially increase patient safety [3]. Computer-supported information systems can play a significant role in this regard and have already seen increased application in healthcare data management [4]. A central aspect of such computerized healthcare information system is administrative health or claim data.
Administrative health data are used in a wider range of healthcare research as opposed to only for database record management; for example, medicinal services investigation [5], performance measurement of hospital care networks [6], identifying comparative cost of effective care [7], developing models for forecasting disease risk [8,9], observation of chronic illness [10] and monitoring sickness reappearance and medication results [11]. Additionally, these data are a significant resource for the examination and observation of chronic sicknesses [10].
Due to security and privacy issues, clinical datasets have very limited access permission; whereas, administrative datasets are available principally to multiple healthcare stakeholders. Access to these data occur through varied network architectures by varied healthcare entities. Further to patients’ health records, medicinal history and referral information [4], authoritative healthcare data captures these interactions including patient-doctor interactions, patient-nurse interactions, patient-pharmacist interactions, pharmacist-doctor interactions, and patient-medical center communications,
There is, however, a lack of a study that assesses these networks in an integrated fashion. This study is a wide-ranging systematic review of networks dependent on administrative health data. By assessing existing works, different types of networks have been identified in this study. The aims, methods and measures utilized in various articles are also noted and related objectives have been summarized. Moreover, the strengths and limitations of the different methods are examined. The outcomes of this study will assist different healthcare stakeholders (e.g., researchers, academics, patients, health care and insurance providers and Government) to quickly conceptualize developments that have occurred concerning networks using administrative health data and allow informed decision making. Further, since administrative datasets have wide application for research purposes, the outcomes will also guide future investigations.
2. Methodology
The search strategy of this study considered two keywords. They are: “administrative data” and “network”. Since the first keyword (i.e., “administrative data”) may appear in different forms in the present literature, we utilized different synonyms for this keyword. These synonyms are “administrative claim data”, “administrative health data”, “electronic health data” and “claim data”. For the second keyword (i.e., “network”), we did not consider any synonyms. This is because all synonyms that were used for this keyword in the existing literature (i.e., “network measure”, “network study”, “network review”, “network analysis” and “network comprehensive analysis”) contain the word “network”. This led to the development of the following search term for this study: ("administrative data" OR “administrative claim data” OR “administrative health data” OR “electronic health data” OR "claim data") AND “network".
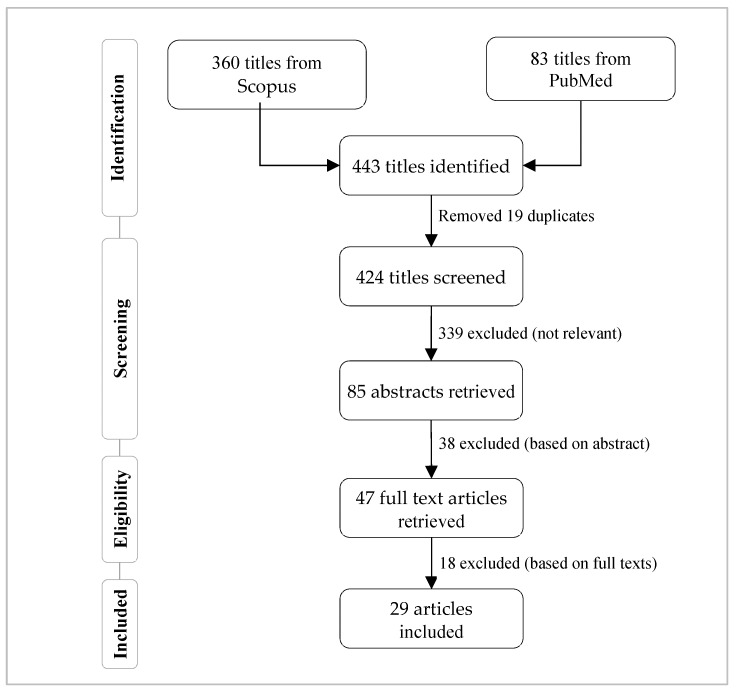
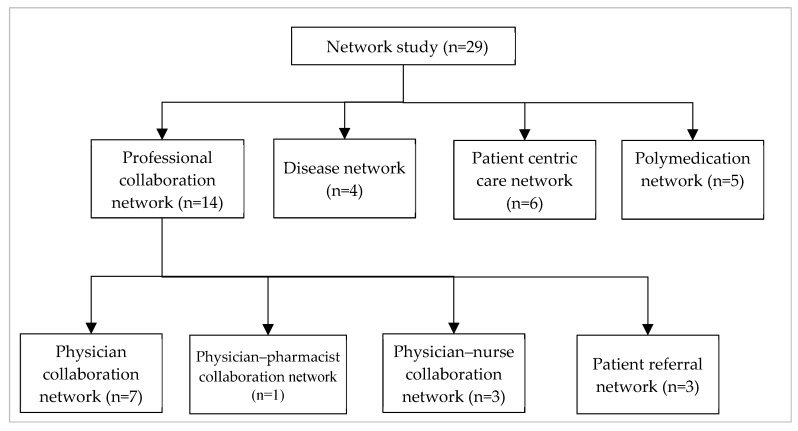
The above complex search term was scanned for articles in PubMed and Scopus databases. The metadata (i.e., title, abstract and keywords) of each scholarly article were considered during this search. We found 587 and 83 articles from PubMed and Scopus, respectively. We followed the steps described by DuGoff et al. [12] in selecting the articles that were considered to review in this study. After removing 19 duplicate titles, we screened the remaining 424 articles. We considered only those articles that are written in English and published in peer-reviewed journals or conferences. After this screening, 339 articles were excluded as they are not relevant to this study. In most cases, for conducting a network study, these 339 articles considered health data other than the administrative one. Subsequently, we manually reviewed the abstract first, and then the full text of the underlying articles to make the final selection of the 29 articles reviewed in this study. The complete flowchart of the study selection process followed in this study is depicted in Figure 1. These 29 articles were then split into different categories according to the type of healthcare stakeholders involved in the underlying studies. This categorization is presented in Figure 2.
Figure 1.
Flowchart for the selection of articles that were reviewed in this study.
Figure 2.
The list and number of various types of network studies (based on administrative health data) that were reviewed in this study.
3. Emergence of Network in Healthcare
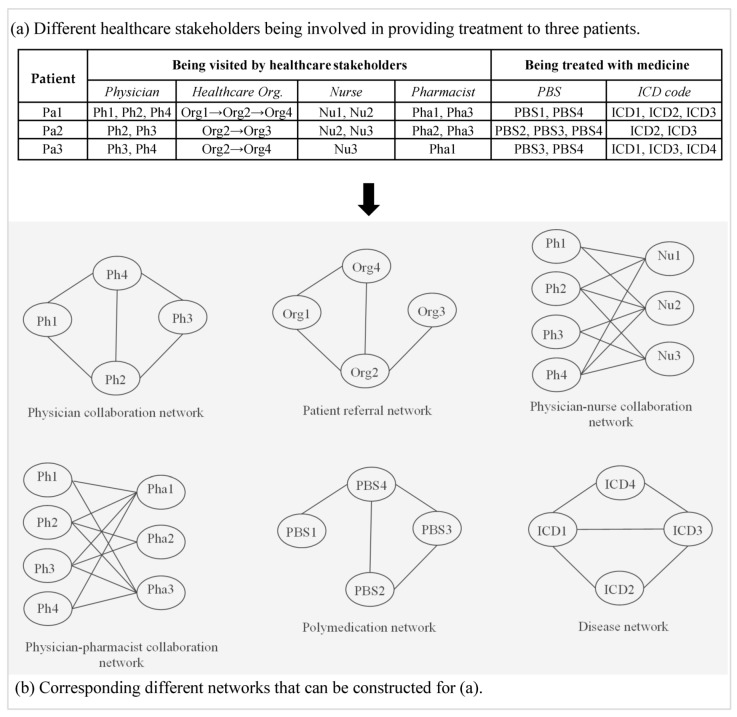
Networks can emerge in healthcare in different ways. A physician collaboration network (PCN), for example, can emerge among physicians when providing healthcare services to common patients. Such networks can also emerge when physicians visit common hospitalized patients [13]. In the current literature, such a network is also known as the patient-sharing network among physicians. In a similar way, other networks can emerge in healthcare among varied stakeholders involved. Based on an abstract dataset about the treatment information of three patients, Figure 3 provides an illustration of the construction of different networks emerged among various healthcare stakeholders while providing treatment to patients. As in Figure 3a, physicians Ph1, Ph2 and Ph4 visited patient Pa1, patient Pa2 is seen by physicians Ph2 and Ph3, patient Pa3 is visited by physicians Ph3 and Ph4. The top-left network shows the corresponding PCN. In this PCN, there are network connections between Ph1 and Ph2, Ph1 and Ph4, Ph2 and Ph3, Ph2 and Ph4, and Ph3 and Ph4 since they visit one or more common patients. Other networks constructed in Figure 3 follow a similar network construction logic for the respective healthcare setting. The following subsections provide details concerning these networks.
Figure 3.
Construction of different networks based on an abstract administrative health dataset for three patients: (a) treatment information by different stakeholders; and (b) corresponding different healthcare stakeholder networks. Here Pa stands for patient, Ph for physician, Org for organisation that provides healthcare to patients, Nu for nurse, Pha for pharmacist, PBS for pharmaceutical benefits scheme and ICD for international classification of diseases.
3.1. Professional Collaboration Network
Collaboration is a significant part of group healthcare [14] and is complex with numerous traits including the sharing of arrangements, decision making, tackling of issues, defining of objectives and acceptance of obligations [15,16]. Collaborative networks among people and groups are profoundly esteemed in associations in light of the fact that consolidating multi-dimensional endeavours and assorted expertise produce benefits more prominent than those accomplished through individual effort [17,18]. In a professional collaboration network, nodes represent healthcare service providers (e.g., physician, nurse, pharmacist and healthcare providing organisation), links between nodes indicates that the healthcare service providers have provided healthcare services to one or more common patients and the weights of those links represent the number of common patients. Different professional collaboration networks are found in the healthcare literature that used administrative claim data. They are briefly described in the following.
3.1.1. Physician Collaboration Network
It is a basic practice in the healthcare business that when doctors visit patients daily, they provide directions concerning treatments depending on the ailment and the past and recorded therapeutic history. Past advice from physicians is also taken into consideration during any shadow visits by other doctors to that patient. This comprehensive healthcare practice empowers academics to plot and prototype a physician collaboration network (PCN). There are many studies in the present healthcare literature that explore the impact of PCN structure on various healthcare outcomes [1,19,20]. For example, Uddin et al. [21] projected a classical model that utilizes specific engagement such as physician–physician relationships to acquire knowledge about actual collaboration and coordination in healthcare.
3.1.2. Physician–Pharmacist Collaboration Network
The collaboration between physicians and pharmacists has been explored to check whether such collaborations can make any difference to the concerned healthcare outcome(s). Chobanian et al. [22] conducted a study to assess whether, or not, a synergistic model between a doctor and a group of pharmacists in network-based workplaces could improve blood pressure (BP) control. In their study, clinical pharmacists made some medical treatments and drug suggestions to doctors, and medical attendants performed regular BP checking. They found that the synergistic model between a doctor and pharmacists in network-based workplaces can improve BP control.
3.1.3. Physician–Nurse Collaboration Network
A physician–nurse collaboration network evolves when a group of physicians and nurses provide healthcare services to one or more patients. A related research, undertaken by Knaus and his group, recognizes a notable connection between the level of physician–nurse joint effort and patient mortality in intensive care units [23].
3.1.4. Patient Referral Network
A patient referral network is the connections established among several healthcare service providers through referrals of patients by doctors. A patient referral network is generally a directed graph since ‘who is referring to whom’ is known. In a patient referral network, nodes are healthcare service providers and an edge (or link) between a pair of nodes is directed from the ‘visiting healthcare provider’ to the ‘referred healthcare provider’ [24,25].
3.2. Disease Network
Disease networks represent the development of different diseases, usually captured by the international classification of diseases (ICD) codes [26], within individual patients in a given period. In such networks, a node represents an individual disease or a comorbid condition, a link between a pair of nodes indicates that one or more patients developed the disease conditions represented by those nodes and the weight of a link represents the number of patients being treated for the diseases that are represented by the end nodes of that link. Khan et al. [27] developed a baseline disease network for type 2 diabetic patients by using their comorbid conditions captured by the Elixhauser index [28]. By applying graph theory and complex network methods, they used this baseline network for the predictive risk analysis of type 2 diabetes. A baseline disease network represents the succession of comorbidities sustained by patients derived from the restorative service histories.
3.3. Patient-Centric Care Collaboration Network
Different service providing units provide healthcare services to patients during their hospitalization periods. These include doctor–specialist units, pathology and diagnosis centers and health services units of the underlying healthcare service provider. In addition, triage doctors monitor patients depending on the emergency concerning their extreme urgencies [6]. A network between patient-centric care entities therefore emerges at the time of patients’ admissions and subsequent hospitalization periods [6]. For a given patient, a patient-centric care network and its different member nodes (actors) indicate the healthcare services she received during her hospitalization period from different hospital units. It further renders the level of engagement of these hospital units in providing healthcare services to that patient.
3.4. Polymedication Network
A polymedication network is produced from a regimen which comprises of a treatment plan and medical actions [29]. Such networks can be captured from the drug intake history of patients. Typically, different coding methods are followed to store patients’ drug intake records in different countries. The pharmaceutical benefits scheme (PBS), for example, is followed in Australia. The corresponding polymedication network of the abstract data (second last column) of Figure 3a is depicted in the lower middle figure of Figure 3b. A node of this network indicates the PBS code of the underlying medicine. A link between two nodes indicates that at least one patient was prescribed with the two medicines represented by those nodes. There is a potential relationship among patients’ age, medical and pharmaceutical expenses, and polymedication networks can assist pharmacovigilance and distinguishing adverse medication responses [29].
4. Results and Discussions
Since this study provides a literature review of network studies that used administrative health data, we first describe different network measures and methods that were used in our reviewed articles. This will ease to follow our findings in this study. Accordingly, Table 1 briefly outlines different network measures and methods under seven broad categories. These categories are node-level measure, network-level measure, edge-level measure, exponential random graph model, cohesive subgroup analysis, community analysis, and dyad and triad census analysis.
Table 1.
Explanation of major network methods and measures across different aspects.
| Aspects | Methods and Measures | Definition |
|---|---|---|
| Node level measure | Degree, closeness, betweenness, eigenvector and other similar measures | Degree centrality: It depicts the number of ties a node (or actor) has with other nodes in a network. It can be of two types (in-degree and out-degree) in a directed network [30]. Closeness centrality: For a node, it represents the extent it is close to the remaining nodes in a network [30]. Betweenness centrality: It represents the extent an actor is in a favoured position in terms of falling on the shortest paths between other actor pairs in the network [30]. Eigenvector: It measures the influence of a node in a network and can distinguish the degree centrality from cases where nodes having a wide range of degree values are connected [30]. |
| Network level measure | Network centralization, density, network diameter and other similar measures | Network centralization: The centralization of a network indicates how central its most central node is compared with how central other nodes within that network are [30]. Network density: It represents the ratio between the number of existing links in a network and the total number of possible links that can be presented among all network actors [30]. Network diameter: It represents the size of the largest path in a network [30]. |
| Edge level measure | Tie strength | Tie strength: It represents the strength of relation between a pair of actors in a network [30] and can be quantified from their duration of relation and the reciprocal services (that specify their tie) they have in common [31]. |
| Exponential random graph model | This model and its different variants | Exponential random graph model: It is a probabilistic model that can identify the building blocks of a given network with respect to different micro-level network substructures (e.g., dyad, triangle and 3-star) [32]. |
| Cohesive subgroup analysis | Clique, clan, n-clique, n-clan and other similar measures | Clique: A clique is a group of actors or nodes in a network that are directly connected with each other [30]. n-clique: An n-clique is also a clique where all member nodes are reachable to each other through at most (n-1) intermediate member nodes [30]. n-clan: An n-clan is also a clique where all member nodes are reachable to each other through at most (n-1) intermediate nodes [30]. The intermediate nodes may or may not be a member of the clique. |
| Community analysis | Community detection | Community detection: It helps to identify a group of nodes in a network that are densely connected among themselves but sparsely connected with other nodes of that network [30]. |
| Dyad and triad census analysis | Dyad and triad census | A dyad is a subgraph comprising two nodes or actors, while a triad is a subgraph consisting of three actors. Both dyads and triads can be formed with or without any links between their member actors [30]. Various dyadic and triadic structures (known as dyad and triad census) are used to explore the building block of networks. |
As illustrated in Figure 2, only one article based on the physician–pharmacist collaboration network met the search criteria of this study. However, there are many studies in the literature that analysed physician–pharmacist collaboration networks to address various research questions. Studies exploring such networks mostly used randomized control trials or survey methods to collect the corresponding research data [52,53]. Overall, for conducting this literature review, we did not find many articles that met our search criteria. Administrative health data were merely available to researchers globally even a couple of years before due to privacy reasons. This unavailability of administrative health data for research purposes may be a possible reason for this shortage of such articles. Many administrative data are now increasingly becoming available to researchers. The findings from this study implies that there is a scope for further research in this field, especially for less explored networks like the physician–pharmacist network.
As notable from Table 2, most studies have focused on the network structure of different professional collaborations and their impact on various performance measures (e.g., cost, length of stay and quality of care). Table 3 shows the key findings of each article reviewed in this study. It also illustrates the network measures or methods employed in each reviewed article. In the majority of the cases, the structure of the underlying network is correlated with the corresponding study goals. In few cases, socio-demographic attributes (e.g., patient age, patient sex and hospital geography) have been found to impact the development of the underlying healthcare professional networks. Based on the categorization of major network methods and measures as in Table 1, Figure 4 illustrates the frequency of different network measures and methods that were used by the 29 articles considered in this study. Finally, Table 4 outlines the strength and weaknesses of seven different type of networks identified in this study.
Table 2.
List of studies that focus on networks using administrative health data. This study considers only those study that used administrative health data to conduct a network study in a healthcare context. Network studies based on other health data (e.g., survey data) have been excluded.
| Network Type | Research Question(s) | Reference |
|---|---|---|
| Physician collaboration network (PCN) |
|
Uddin et al. [33] |
|
Uddin et al. [19] | |
|
Barnett et al. [34] | |
|
Uddin et al. [1] | |
|
Uddin et al. [20] | |
|
Landon et al. [13] | |
|
Pollack et al. [35] | |
| Patient-centric care coordination network |
|
Uddin et al. [36] |
|
Uddin [37] | |
|
Uddin and Hossain [38] | |
|
Uddin and Hossain [6] | |
|
Abbasi et al. [39] | |
|
Uddin et al. [21] | |
| Physician –nurse collaboration network |
|
Caricati et al. [40] |
|
Tschannen and Kalisch [41] | |
|
Yao et al. [42] | |
| Physician-pharmacist collaboration network |
|
DeMik et al. [43] |
| Patient referral network |
|
An et al. [24] |
|
Vukmir et al. [25] | |
|
Donker et al. [44] | |
| Disease network |
|
Khan et al. [45] |
|
Khan et al. [46] | |
|
Khan et al. [27] | |
|
Hossain and Uddin [47] | |
| Polymedication network |
|
Khan et al. [29] |
|
Zamora et al. [48] | |
|
Liu et al. [49] | |
|
Medhekar et al. [50] | |
|
Franchini et al. [51] |
Table 3.
Characteristics (i.e., network methods followed and key findings) of the included network studies.
| Network Type | Reference | Network Methods/Measures Used | Key Findings |
|---|---|---|---|
| Physician collaboration network (PCN) | Uddin et al. [33] | Exponential random graph model |
|
| Uddin et al. [19] | Network centralization |
|
|
| Barnett et al. [34] | Community detection |
|
|
| Uddin et al. [1] | Exponential random graph model |
|
|
| Uddin et al. [20] | Triad census, Clique and Clan |
|
|
| Landon et al. [13] | Network centrality |
|
|
| Patient-centric care coordination network | Uddin et al. [36] | Closeness centrality |
|
| Uddin [37] | Community detection |
|
|
| Uddin and Hossain [38] | Dyad and Network centrality |
|
|
| Uddin and Hossain [6] | Connectedness, Degree centrality and Tie strength |
|
|
| Abbasi et al. [39] | Network centrality |
|
|
| Uddin et al. [21] | Network centrality and Exponential random graph model |
|
|
| Physician–nurse collaboration network | Caricati et al. [40] | Community detection |
|
| Tschannen and Kalisch [41] | Network centrality |
|
|
| Yao et al. [42] | Network centrality |
|
|
| Physician–pharmacist collaboration network | DeMik et al. [43] | Community detection |
|
| Patient referral network | An et al. [24] | Network centrality and Triad census |
|
| Vukmir et al. [25] | Community detection |
|
|
| Donker et al. [44] | Degree centrality and Community detection |
|
|
| Disease network | Khan et al. [45] | Network centrality |
|
| Khan et al. [46] | Network centrality |
|
|
| Khan et al. [27] | Network centrality |
|
|
| Hossain and Uddin [47] | Network centrality |
|
|
| Polymedication network | Khan et al. [29] | Network centrality |
|
| Zamora et al. [48] | Betweenness centrality and Community detection |
|
|
| Liu et al. [49] | Network centrality |
|
|
| Medhekar et al. [50] | Community detection |
|
|
| Franchini et al. [51] | Network density |
|
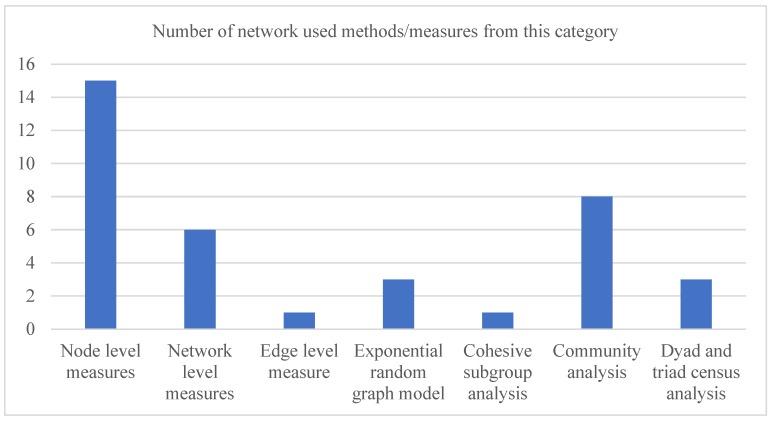
Figure 4.
Frequency of different network measures and methods that were used by the 29 articles considered in this study. Some of these articles employed network measures and methods from more than one category.
Table 4.
The strength and weakness of different types of network.
| Network Type | Strength | Weakness |
|---|---|---|
| Physician collaboration network |
|
|
| Patient-centric care coordination network |
|
|
| Physician–nurse collaboration network |
|
|
| Physician–pharmacist collaboration network |
|
|
| Patient referral network |
|
|
| Disease network |
|
|
| Polymedication network |
|
|
Except the articles that belong to the last two categories of Table 3 (i.e., Disease network and Polymedication network), all articles reviewed in this study analyzed different networks among various healthcare stakeholders (individuals or organizations) to understand how network structure affects the perceived level of different treatment outcomes. For example, all articles reviewed under the PCN category analyzed physician collaborations using diverse network measures. The ultimate goal of these studies was to figure out the PCN structure that is more conducive to better treatment outcomes. On the other side, the articles belonging to the ‘Disease network’ category attempted to map the co-appearance of different diagnostic codes in order to get a better understanding of the progression of a single disease or comorbidity of multiple diseases. All articles under the ‘Polymedication network’ category explored the complex relationships among various drugs.
Although all articles considered in this study conducted network analysis utilizing administrative health data and different network measures, a close examination of Table 3 shows a comprehensive relationship between the network utilized in the various investigations and their key findings. Community analysis and exponential random graph model were mostly utilized in investigations that intended to explore diverse coordination and joint efforts for different health conditions.
It is evident, from Figure 4, that the ‘node level measures’ has been used more often (15 times) in the literature. Commonly used measures of this category were degree centrality and betweenness centrality. The second most employed category of network measures and methods is the ‘community analysis’. There are eight reviewed articles that used this method. The ‘network level measures’ stands as the third most used category. The measures of this category have appeared in six reviewed articles. The common measures of this category included network density and network centralization. Notably, only limited focus has been made in literature on ‘edge level measure’ and ‘cohesive subgroup analysis’—again, an indication that there are opportunities to undertake further research in these areas.
5. Conclusions
This study provides a complete review of network studies based on only administrative claim data. We utilized the search technique explained in the method section to extract the 29 articles considered in this study. All reviewed articles of this study utilized administrative health data to conduct a network study. The findings of this study can be used by healthcare policymakers in developing future research strategies. They can also be used by prospective future researchers for a summarized comprehension of the current research on network studies that use administrative health data.
Similar to other review studies, this study may miss articles that utilized administrative health data for a network study. This could be a possible limitation of this study. However, this limitation will not block the essential point of this study in giving an image of administrative health data utilization for network studies. This review examined various network studies that used administrative claim data. A comprehensive understanding of different networks can provide insights that are important for improving healthcare systems. The capability of administrative health data in terms of their usefulness in conducting network study is also effectively demonstrated in this study, which could be a great extent for healthcare research.
Acknowledgments
The authors wish to acknowledge Arif Khan and Ekramul Hossain for sharing their valuable insights and reviewing the paper.
Author Contributions
Conceptualization, S.K. and S.U.; data analysis and investigation, S.K.; writing—original draft preparation, S.K.; writing—review and editing, S.U., T.I. and M.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Uddin S., Kelaher M., Srinivasan U. A framework for administrative claim data to explore healthcare coordination and collaboration. Aust. Health Rev. 2016;40:500–510. doi: 10.1071/AH15058. [DOI] [PubMed] [Google Scholar]
- 2.Palaniappan S., Awang R. Intelligent heart disease prediction system using data mining techniques; Proceedings of the 2008 IEEE/ACS International Conference on Computer Systems and Applications; Doha, Qatar. 31 March–4 April 2008; Piscataway, NJ, USA: IEEE; 2008. [Google Scholar]
- 3.Wu R., Peters W., Morgan M. The next generation of clinical decision support: Linking evidence to best practice. J. Healthc. Inf. Manag. JHIM. 2002;16:50–55. [PubMed] [Google Scholar]
- 4.Ohno-Machado L., Kim J., Gabriel R.A., Kuo G.M., Hogarth M.A. Genomics and electronic health record systems. Hum. Mol. Genet. 2018;27:R48–R55. doi: 10.1093/hmg/ddy104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culler S.D., Parchman M.L., Przybylski M. Factors related to potentially preventable hospitalizations among the elderly. Med. Care. 1998;36:804–817. doi: 10.1097/00005650-199806000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Uddin M.S., Hossain L. Social networks enabled coordination model for cost management of patient hospital admissions. J. Healthc. Qual. 2011;33:37–48. doi: 10.1111/j.1945-1474.2011.00118.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee P.P., Levin L.A., Walt J.G., Chiang T., Katz L.M., Dolgitser M., Doyle J.J., Stern L.S. Cost of patients with primary open-angle glaucoma: A retrospective study of commercial insurance claims data. Ophthalmology. 2007;114:1241–1247. doi: 10.1016/j.ophtha.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Davis D.A., Chawla N.V., Christakis N.A., Barabási A.-L. Time to CARE: A collaborative engine for practical disease prediction. Data Min. Knowl. Discov. 2010;20:388–415. doi: 10.1007/s10618-009-0156-z. [DOI] [Google Scholar]
- 9.McCormick T., Rudin C., Madigan D. A Hierarchical Model for Association Rule Mining of Sequential Events: An Approach to Automated Medical Symptom Prediction. Ann. Appl. Stat. 2011 doi: 10.2139/ssrn.1736062. [DOI] [Google Scholar]
- 10.Yiannakoulias N., Schopflocher D., Svenson L. Using administrative data to understand the geography of case ascertainment. Chronic Dis. Can. 2009;30:20–28. [PubMed] [Google Scholar]
- 11.Fisher E.S., Malenka D.J., Wennberg J.E., Roos N.P. Technology assessment using insurance claims: Example of prostatectomy. Int. J. Technol. Assess. Health Care. 1990;6:194–202. doi: 10.1017/S0266462300000714. [DOI] [PubMed] [Google Scholar]
- 12.DuGoff E.H., Fernandes-Taylor S., Weissman G.E., Huntley J.H., Pollack C.E. A scoping review of patient-sharing network studies using administrative data. Transl. Behav. Med. 2018;8:598–625. doi: 10.1093/tbm/ibx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landon B.E., Keating N.L., Barnett M.L., Onnela J.-P., Paul S., O’Malley A.J., Keegan T., Christakis N.A. Variation in patient-sharing networks of physicians across the United States. JAMA. 2012;308:265–273. doi: 10.1001/jama.2012.7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham S.J., Dillon S.M. Authorship patterns in information systems. Scientometrics. 1997;39:19. doi: 10.1007/BF02457428. [DOI] [Google Scholar]
- 15.Beaver D., Rosen R. Studies in scientific collaboration: Part I. The professional origins of scientific co-authorship. Scientometrics. 1978;1:65–84. doi: 10.1007/BF02016840. [DOI] [Google Scholar]
- 16.Luukkonen T., Tijssen R., Persson O., Sivertsen G. The measurement of international scientific collaboration. Scientometrics. 1993;28:15–36. doi: 10.1007/BF02016282. [DOI] [Google Scholar]
- 17.Norman C., Axelsson R. Co-operation as a strategy for provision of welfare services—A study of a rehabilitation project in Sweden. Eur. J. Public Health. 2007;17:532–536. doi: 10.1093/eurpub/ckm001. [DOI] [PubMed] [Google Scholar]
- 18.Uddin S., Hossain L. Disaster coordination preparedness of soft-target organisations. Disasters. 2011;35:623–638. doi: 10.1111/j.1467-7717.2011.01229.x. [DOI] [PubMed] [Google Scholar]
- 19.Uddin S., Hossain L., Kelaher M. Effect of physician collaboration network on hospitalization cost and readmission rate. Eur. J. Public Health. 2011;22:629–633. doi: 10.1093/eurpub/ckr153. [DOI] [PubMed] [Google Scholar]
- 20.Uddin S., Hossain M.E., Khan A. Triad Census and Subgroup Analysis of Patient-Sharing Physician Collaborations. IEEE Access. 2018;6:72233–72240. doi: 10.1109/ACCESS.2018.2880514. [DOI] [Google Scholar]
- 21.Uddin S., Khan A., Piraveenan M. Administrative claim data to learn about effective healthcare collaboration and coordination through social network; Proceedings of the 2015 48th Hawaii International Conference on System Sciences; Kauai, HI, USA. 5–8 January 2015; Piscataway, NJ, USA: IEEE; 2015. [Google Scholar]
- 22.Chobanian A.V., Bakris G.L., Black H.R., Cushman W.C., Green L.A., Izzo Jr J.L., Jones D.W., Materson B.J., Oparil S., Wright J.T., Jr. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003;289:2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 23.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. An evaluation of outcome from intensive care in major medical centers. Ann. Intern. Med. 1986;104:410–418. doi: 10.7326/0003-4819-104-3-410. [DOI] [PubMed] [Google Scholar]
- 24.An C., O’Malley A.J., Rockmore D.N., Stock C.D. Analysis of the US patient referral network. Stat. Med. 2018;37:847–866. doi: 10.1002/sim.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vukmir R.B., Kremen R., Dehart D.A., Menegazzi J. Compliance with emergency department patient referral. Am. J. Emerg. Med. 1992;10:413–417. doi: 10.1016/0735-6757(92)90065-6. [DOI] [PubMed] [Google Scholar]
- 26.Roberts R.F., Innes K.C., Walker S.M. Introducing ICD-10-AM in Australian hospitals. Med J. Aust. 1998;169:S32–S35. doi: 10.5694/j.1326-5377.1998.tb123473.x. [DOI] [PubMed] [Google Scholar]
- 27.Khan A., Uddin S., Srinivasan U. Comorbidity network for chronic disease: A novel approach to understand type 2 diabetes progression. Int. J. Med. Inform. 2018;115:1–9. doi: 10.1016/j.ijmedinf.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med. Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Khan A., Srinivasan U., Uddin S. Development and exploration of polymedication network from pharmaceutical and medicare benefits scheme data; Proceedings of the Australasian Computer Science Week Multiconference; Sydney, NSW, Australia. 29–31 January 2019; New York, NY, USA: ACM; 2019. [Google Scholar]
- 30.Wasserman S., Faust K. Social Network Analysis: Methods and Applications. Cambridge University Press; Cambridge, UK: 2003. [Google Scholar]
- 31.Granovetter M. The strength of weak ties. Am. J. Sociol. 1973;78:1360–1380. doi: 10.1086/225469. [DOI] [Google Scholar]
- 32.Robins G., Pattison P., Kalish Y., Lusher D. An introduction to exponential random graph (p*) models for social networks. Soc. Netw. 2007;29:173–191. doi: 10.1016/j.socnet.2006.08.002. [DOI] [Google Scholar]
- 33.Uddin S., Hamra J., Hossain L. Mapping and modeling of physician collaboration network. Stat. Med. 2013;32:3539–3551. doi: 10.1002/sim.5770. [DOI] [PubMed] [Google Scholar]
- 34.Barnett M.L., Landon B.E., O’Malley A.J., Keating N.L., Christakis N.A. Mapping physician networks with self-reported and administrative data. Health Serv. Res. 2011;46:1592–1609. doi: 10.1111/j.1475-6773.2011.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollack C.E., Weissman G.E., Lemke K.W., Hussey P.S., Weiner J.P. Patient sharing among physicians and costs of care: A network analytic approach to care coordination using claims data. J. Gen. Intern. Med. 2013;28:459–465. doi: 10.1007/s11606-012-2104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uddin S., Khan A., Hossain L., Piraveenan M., Carlsson S. A topological framework to explore longitudinal social networks. Comput. Math. Organ. Theory. 2015;21:48–68. doi: 10.1007/s10588-014-9176-3. [DOI] [Google Scholar]
- 37.Uddin S. Exploring the impact of different multi-level measures of physician communities in patient-centric care networks on healthcare outcomes: A multi-level regression approach. Sci. Rep. 2016;6:20222. doi: 10.1038/srep20222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uddin S., Hossain L. Social networks in exploring healthcare coordination. Asia Pac. J. Health Manag. 2014;9:53–62. [Google Scholar]
- 39.Abbasi A., Uddin S., Hossain L. Socioeconomic analysis of patient-centric networks: Effects of patients and hospitals’ characteristics and network structure on hospitalization costs. Eur. J. Health Econ. 2012;13:267–276. doi: 10.1007/s10198-011-0303-5. [DOI] [PubMed] [Google Scholar]
- 40.Caricati L., Mancini T., Sollami A., Guidi C., Prandi C., Bianconcini M., Silvano R., Taffurelli C., Artioli G. Nurse-physician collaboration scale: A contribution to the italian validation. TPM-Test. Psychom. Methodol. Appl. Psychol. 2013;20:263–276. [Google Scholar]
- 41.Tschannen D., Kalisch B.J. The impact of nurse/physician collaboration on patient length of stay. J. Nurs. Manag. 2009;17:796–803. doi: 10.1111/j.1365-2834.2008.00926.x. [DOI] [PubMed] [Google Scholar]
- 42.Yao N., Zhu X., Dow A., Mishra V.K., Phillips A., Tu S.-P. An exploratory study of networks constructed using access data from an electronic health record. J. Interprof. Care. 2018;32:666–673. doi: 10.1080/13561820.2018.1496902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeMik D.E., Vander Weg M.W., Lundt E.S., Coffey C.S., Ardery G., Carter B.L. Using theory to predict implementation of a physician–pharmacist collaborative intervention within a practice-based research network. Res. Soc. Adm. Pharm. 2013;9:719–730. doi: 10.1016/j.sapharm.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donker T., Wallinga J., Grundmann H. Patient referral patterns and the spread of hospital-acquired infections through national health care networks. PLoS Comput. Biol. 2010;6:e1000715. doi: 10.1371/journal.pcbi.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan A., Uddin S., Srinivasan U. Understanding chronic disease comorbidities from baseline networks: Knowledge discovery utilising administrative healthcare data; Proceedings of the Australasian Computer Science Week Multiconference; Geelong, Australia. 31 January–3 February 2017; New York, NY, USA: ACM; 2017. [Google Scholar]
- 46.Khan A., Uddin S., Srinivasan U. Chronic disease prediction using administrative data and graph theory: The case of type 2 diabetes. Expert Syst. Appl. 2019;136:230–241. doi: 10.1016/j.eswa.2019.05.048. [DOI] [Google Scholar]
- 47.Hossain M.E., Uddin S. Understanding the comorbidity of multiple chronic diseases using a network approach; Proceedings of the Australasian Computer Science Week Multiconference; Sydney, NSW, Australia. 29–31 January 2019; New York, NY, USA: ACM; 2019. [Google Scholar]
- 48.Zamora M., Baradad M., Amado E., Cordomí S., Limón E., Ribera J., Arias M., Gavaldà R. Characterizing chronic disease and polymedication prescription patterns from electronic health records; Proceedings of the 2015 IEEE International Conference on Data Science and Advanced Analytics (DSAA); Paris, France. 19–21 October 2015; Piscataway, NJ, USA: IEEE; 2015. [Google Scholar]
- 49.Liu Q., Liu J., Wang P., Zhang Y., Li B., Yu Y., Dang H., Li H., Zhang X., Wang Z. Poly-dimensional network comparative analysis reveals the pure pharmacological mechanism of baicalin in the targeted network of mouse cerebral ischemia. Brain Res. 2017;1666:70–79. doi: 10.1016/j.brainres.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Medhekar R., Aparasu R., Bhatara V., Johnson M., Alonzo J., Schwarzwald H., Chen H. Risk factors of psychotropic polypharmacy in the treatment of children and adolescents with psychiatric disorders. Res. Soc. Adm. Pharm. 2019;15:395–403. doi: 10.1016/j.sapharm.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Franchini M., Pieroni S., Fortunato L., Molinaro S., Liebman M. Poly-pharmacy among the elderly: Analyzing the co-morbidity of hypertension and diabetes. Curr. Pharm. Des. 2015;21:791–805. doi: 10.2174/1381612820666141024150901. [DOI] [PubMed] [Google Scholar]
- 52.Carter B.L., Bergus G.R., Dawson J.D., Farris K.B., Doucette W.R., Chrischilles E.A., Hartz A.J. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J. Clin. Hypertens. 2008;10:260–271. doi: 10.1111/j.1751-7176.2008.07434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carter B.L., Ardery G., Dawson J.D., James P.A., Bergus G.R., Doucette W.R., Chrischilles E.A., Franciscus C.L., Xu Y. Physician and pharmacist collaboration to improve blood pressure control. Arch. Intern. Med. 2009;169:1996–2002. doi: 10.1001/archinternmed.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]