Short abstract
Objective
This study aimed to evaluate the association between systolic blood pressure (SBP) and first ischemic stroke in older people with hypertension in the community.
Methods
This retrospective cohort study included 3315 residents who were hypertensive and older than 60 years in Guangdong, China.
Results
A total of 1475 men and 1840 women aged 71.41±7.20 years were included. All subjects had a median follow-up duration for 5.5 years and 206 subjects reached the endpoint. The prevalence of first ischemic stroke increased with a higher SBP. SBP expressed as a continuous variable (hazard ratio [HR], 1.01; 95% confidence interval [CI], 1.00–1.02) and categorical variable (HRs, 1.00, 1.06, 1.17, 1.39, and 1.60 for increasing blood pressure from < 120–≥150 mmHg), was significantly associated with a higher risk of first ischemic stroke. Moreover, a fully adjusted model indicated an obvious increased risk in the SBP ≥150 mmHg group (HR, 1.60; 95% CI, 1.15–2.71) and the SBP 140–149 mmHg group (HR, 1.39; 95% CI, 1.01–2.39).
Conclusions
High SBP was independently associated with the risk of first ischemic stroke in hypertensive residents in the community aged older than 60 years. SBP ≥140 mmHg increases the risk of first ischemic stroke.
Keywords: Systolic blood pressure, ischemic stroke, community, older population, hypertension, cardiovascular risk
Introduction
Arterial hypertension has a high prevalence in the older population. According to the 2017 American College of Cardiology and the American Heart Association (ACC/AHA), the prevalence of hypertension based on systolic blood pressure (SBP)/diastolic blood pressure (DBP) ≥140/90 mmHg or self-reported antihypertensive medication is 64% and 63% in 65- to 74-year-old men and women, respectively.1 The prevalence of hypertension is >70% in individuals aged 75 years or older.2,3 Current studies have shown that hypertension is an independent risk factor of ischemic stroke.4–7 Among older individuals, hypertension is a major risk factor for cardiovascular disease for 77% of hypertensive patients with incident stroke.8
According to the Guideline for the Primary Prevention of Stroke, aging and hypertension are risk factors of stroke.9 Increasing blood pressure is strongly, independently, predictively, and etiologically correlated with the risk of stroke.9 Blood pressure, especially SBP, rises as age increases in adults, and this may also progressively increase the risk of ischemic stroke.2,10 Nevertheless, the target for blood pressure control remains uncertain in the older population with hypertension.11 Current guidelines recommend different targets for controlling blood pressure for preventing stroke and other cardiovascular events.1,9,12,13
In the current study, we examined the clinical data of older patients in the community with hypertension. We aimed to estimate the correlation between SBP and first ischemic stroke, and to investigate an appropriate blood pressure target for older hypertensive patients to decrease the incidence of stroke.
Patients and methods
Study population and design
We performed a retrospective cohort study and recruited 3500 people from a rural residential population from 1 January 2010 to 31 December 2011 at Liaobu in Guangdong, China. Subjects were older hypertensive patients who met the following inclusion criteria: age ≥60 years, and SBP ≥140 mmHg and/or DBP ≥90 mmHg, or receiving antihypertensive medications within 2 weeks.14 We excluded patients with a previous stroke history (n = 132), lack of blood pressure (n = 37), and missing other physical examination data (n = 16). The study was undertaken on the basic principle of the Helsinki Declaration and was approved by the institutional medical ethical committee of Guangdong Provincial People’s Hospital, Guangzhou, China (No. 2012143H). All of the participants provided written informed consent.
Data collection
Blood pressure measurements were conducted according to the 2010 Chinese guidelines for management of hypertension.14 Measurements were taken by trained nurses or physicians. Participants were asked to avoid exercise, smoking, and caffeine for at least 30 minutes and have a rest for longer than 5 minutes before measurement. The measured arm was positioned at the level of the heart and circled with cuffs of an appropriate size. Blood pressure was measured simultaneously by an automated device (OMRONHBP1100u; Omron Corp., Tokyo, Japan). The arm with the highest blood pressure value was used for all subsequent measurements and data analysis.
Demographic and medical data, including age, sex, and a history of smoking and alcoholism were obtained from interviews with patients or medical records. The past medical history, including cardiovascular diseases, cerebrovascular diseases, and type 2 diabetes mellitus, were collected from medical records and self-reports. The body mass index (BMI) was calculated as the ratio of weight in kilograms to the square of height in meters (kg/m2). The estimated glomerular filtration rate (eGFR) was calculated by the simplified Modification of Diet in Renal Disease equation.15 Antihypertensive medications were classified as angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), beta-blockers, and calcium channel blockers (CCBs).
Clinical outcome
The endpoints were obtained by reviewing medical records that included the last hospitalization and personal physical records. Based on previous studies, the primary endpoint was defined as first ischemic stroke, including cerebral infarction and transient ischemic attack. The diagnosis of ischemic stroke was based on a cranial computed tomography (CT) or contract vascular CT scan, magnetic resonance imaging of the brain, or cerebrovascular angiography. All stroke cases were ascertained from the local medical insurance system of the medical insurance bureau, and patients without medical records were followed up by phone call or face-to-face interview in the community. The duration of follow-up began at the time of the first visit and ended on 31 December 2016.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation, and categorical variables are presented as absolute values and percentages. SBP was divided into the following five groups: (1) < 120 mmHg, (2) 120 to 129 mmHg, (3) 130 to 139 mmHg, (4) 140 to 149 mmHg, and (5) ≥150 mmHg. The differences between groups were evaluated by ANOVA (normal distribution) or the Kruskal–Wallis H test (skewed distribution) for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. The multivariate Cox regression model was used to evaluate the hazard ratios (HRs) between SBP and ischemic stroke. Adjustments were made for age, sex, BMI, eGFR, antihypertensive medications, total cholesterol, triacylglycerol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, fasting blood glucose, type 2 diabetes mellitus, smoking, and drinking. SBP was also handled as a categorical variable according to SBP groups and the P for trend was estimated in each model. Subgroup analyses were performed by the multivariate Cox regression model. The interactions of subgroups for each variable were adjusted according to full adjustment. Survival analysis was performed using Kaplan–Meier curves, and the log-rank test was performed to examine between-group differences.
The collected data were double entered into EpiData software 3.1 (EpiData Associations, Odense, Denmark). Private identity information of all participants could not be ascertained by any approach in this study. All of the analyses were performed by SPSS version 22.0 (IBM Corp., Armonk, NY, USA) and R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria). The threshold of statistical significance was defined as P < 0.05 (two-sided).
Results
Demographic characteristics
A total of 3315 subjects (1475 men and 1840 women, mean age: 71.41±7.20 years) were included in this analysis. Demographic and clinical characteristics of the subjects, grouped by different SBP levels, are shown in Table 1. All of the participants were hypertensive. The comorbidities in this cohort included diabetes mellitus in 15.23% of the subjects and coronary atherosclerotic heart disease in 1.03%, 28.14% were smokers, and 10.20% had a history of drinking.
Table 1.
Demographic and clinical characteristics of the population at baseline.
| SBP groups | <120 (n = 645) | 120–129 (n = 825) | 130–139 (n = 891) | 140–149 (n = 452) | ≥150 (n = 502) | P value |
|---|---|---|---|---|---|---|
| SBP (mmHg) | 108.38±7.19 | 122.57±2.95 | 133.35±3.32 | 142.72±2.99 | 159.90±10.49 | |
| DBP (mmHg) | 69.89±7.14 | 75.84±6.30 | 79.32±6.84 | 82.61±8.99 | 87.74±10.37 | <0.001 |
| Age (years) | 70.63±6.22 | 71.24±6.99 | 71.56±7.11 | 72.12±7.84 | 71.81±8.13 | 0.006 |
| BMI (kg/m2) | 22.86±3.84 | 23.88±3.82 | 24.07±3.71 | 24.49±3.58 | 24.50±3.83 | <0.001 |
| HR (beats/min) | 68.77±9.63 | 71.26±10.64 | 71.72±10.26 | 71.10±10.06 | 73.35±10.74 | <0.001 |
| eGFR (mL/min/1.73 m2) | 100.84±40.07 | 102.13±52.28 | 105.17±42.21 | 95.67±43.82 | 104.42±47.50 | 0.438 |
| FBG (mmol/L) | 5.00±1.35 | 5.20±1.67 | 5.14± 1.52 | 5.19±1.18 | 5.35±1.53 | 0.002 |
| TC (mmol/L) | 11.10±2.45 | 11.32±2.58 | 11.46±2.65 | 11.20±2.42 | 11.21±2.45 | 0.073 |
| TG (mmol/L) | 6.98±4.49 | 8.35±6.77 | 8.49±6.63 | 8.62±5.66 | 9.50±7.90 | <0.001 |
| LDL-C (mmol/L) | 5.60±1.61 | 5.67±1.62 | 5.82±1.62 | 5.79±1.65 | 5.97±1.69 | 0.001 |
| HDL-C (mmol/L) | 2.91±0.79 | 2.78±0.70 | 2.80±0.87 | 2.79±0.73 | 2.83±0.76 | 0.028 |
| Sex (n, %) | 0.485 | |||||
| Male | 281 (43.57) | 379 (45.94) | 409 (45.90) | 197 (43.58) | 209 (41.63) | |
| Female | 364 (56.43) | 446 (54.06) | 482 (54.10) | 255 (56.42) | 293 (58.37) | |
| Diabetes (n, %) | 0.073 | |||||
| No | 557 (86.36) | 689 (83.52) | 749 (84.06) | 373 (82.52) | 442 (88.05) | |
| Yes | 88 (13.64) | 136 (16.48) | 142 (15.94) | 79 (17.48) | 60 (11.95) | |
| CAD (n, %) | 0.051 | |||||
| No | 642 (99.53) | 813 (98.55) | 881 (98.88) | 444 (98.23) | 501 (99.80) | |
| Yes | 3 (0.47) | 12 (1.45) | 10 (1.12) | 8 (1.77) | 1 (0.20) | |
| Smoking (n, %) | 0.004 | |||||
| No | 462 (71.63) | 558 (67.64) | 639 (71.72) | 336 (74.34) | 387 (77.09) | |
| Yes | 183 (28.37) | 267 (32.36) | 252 (28.28) | 116 (25.66) | 115 (22.91) | |
| Drinking (n, %) | 0.003 | |||||
| No | 580 (89.92) | 714 (86.55) | 803 (90.12) | 414 (91.59) | 466 (92.83) | |
| Yes | 65 (10.08) | 111 (13.45) | 88 (9.88) | 38 (8.41) | 36 (7.17) | |
| CCB (n, %) | <0.001 | |||||
| No | 603 (93.49) | 672 (81.45) | 714 (80.13) | 324 (71.68) | 346 (68.92) | |
| Yes | 42 (6.51) | 153 (18.55) | 177 (19.87) | 128 (28.32) | 156 (31.08) | |
| ACEI (n, %) | <0.001 | |||||
| No | 630 (97.67) | 779 (94.42) | 841 (94.39) | 405 (89.60) | 448 (89.24) | |
| Yes | 15 (2.33) | 46 (5.58) | 50 (5.61) | 47 (10.40) | 54 (10.76) | |
| ARB (n, %) | <0.001 | |||||
| No | 582 (90.23) | 683 (82.79) | 714 (80.13) | 326 (72.12) | 364 (72.51) | |
| Yes | 63 (9.77) | 142 (17.21) | 177 (19.87) | 126 (27.88) | 138 (27.49) | |
| Statins (n, %) | 0.164 | |||||
| No | 591 (91.63) | 741 (89.82) | 809 (90.80) | 420 (92.92) | 468 (93.23) | |
| Yes | 54 (8.37) | 84 (10.18) | 82 (9.20) | 32 (7.08) | 34 (6.77) | |
| Ischemic stroke (n, %) | 26 (4.03) | 44 (5.33) | 54 (6.06) | 35 (7.74) | 47 (9.36) | 0.002 |
Continuous variables are expressed as mean ± standard deviation, and categorical variables are shown as absolute values and percentages. Differences between groups were evaluated by the ANOVA or Kruskal–Wallis H test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. SBP: systolic blood pressure, DBP: diastolic blood pressure, BMI: body mass index, HR: heart rate, eGFR: estimated glomerular filtration rate, FBG: fasting blood glucose, TC: total cholesterol, TG: triacylglycerol, LDL-C: low-density lipoprotein cholesterol, HDL-C: high-density lipoprotein cholesterol, CAD: coronary atherosclerotic heart disease, CCB: calcium channel blocker, ACEI: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker
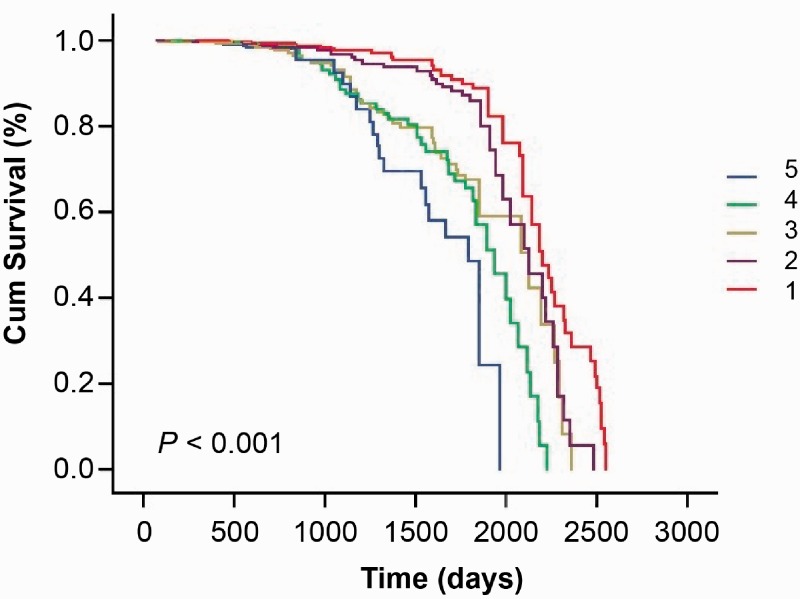
All patients in the cohort were followed for 5 to 7 years (median follow-up duration: 5.5 years). During the follow-up, 206 (6.21%) subjects reached the endpoint of first ischemic stroke. A total of 26 (4.03%), 44 (5.33%), 54 (6.06%), 35 (7.74%), and 47 (9.36%) patients had new-onset ischemic stroke in the < 120, 120 to 129, 130 to 139, 140 to 149, and ≥150 mmHg groups, respectively (Table 1). The morbidity of first ischemic stroke tended to be higher in the higher SBP groups. Kaplan–Meier curves showed that participants with a higher SBP were associated with a higher chance of first ischemic stroke among the SBP groups (log rank, P < 0.001) (Figure 1).
Figure 1.
Kaplan–Meier curves of systolic blood pressure and first ischemic stroke among the blood pressure groups. 1: SBP <120 mmHg, 2: SBP of 120–129 mmHg, 3: SBP of 130–139 mmHg, 4: SBP of 140–149 mmHg, and 5: SBP ≥150 mmHg. SBP: systolic blood pressure. The P value was derived from the log-rank test.
Relationship between SBP and first ischemic stroke
The association of SBP and first ischemic stroke was analyzed by the multivariate Cox regression model (Table 2). We found that a high SBP, expressed as a categorical variable and a continuous variable, was significantly associated with first ischemic stroke. When SBP was expressed as a continuous variable, high SBP was slightly associated with a higher risk of first ischemic stroke after adjustment for covariates (HR, 1.01; 95% confidence interval [CI], 1.00–1.02; P < 0.0135). Moreover, the effect size obviously changed when SBP was divided into different categories. In the fully adjusted model, the effect size sequentially increased in the higher SBP groups (HRs, 1.00, 1.06, 1.17, 1.39, 1.60; P for trend = 0.0381) (model 3). Therefore, with a higher SBP category, the trend of a higher risk of first ischemic stroke significantly increased. Similar results were found in non-adjusted (HRs, 1.00, 1.34, 1.54, 2.00, 2.46; P for trend < 0.0001) and minimally adjusted models (HRs, 1.00, 1.24, 1.40, 1.78, 2.20; P for trend < 0.0001) (models 1 and 2). Additionally, the risk of first ischemic stroke was significantly increased in non-adjusted (HR, 2.46; 95% CI, 1.50–4.03; P = 0.0004), minimally adjusted (HR, 2.20; 95% CI, 1.34–3.62; P = 0.002), and fully adjusted (HR, 1.60; 95% CI, 1.15–2.71; P = 0.0062) models in the SBP ≥150 mmHg group. Similarly, in the SBP 140–149 mmHg group, the risk of first ischemic stroke was also increased compared with the reference group (HR, 1.39; 95% CI, 1.01–2.39) in the fully adjusted model.
Table 2.
Relationship between systolic blood pressure and ischemic stroke in different models.
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| SBP | 1.02 (1.01, 1.02), <0.0001 | 1.02 (1.01, 1.02), 0.0002 | 1.01 (1.00, 1.02), 0.0135 |
| SBP groups | |||
| <120 | 1.0 | 1.0 | 1.0 |
| 120–129 | 1.34 (0.82, 2.20), 0.2460 | 1.24 (0.75, 2.04), 0.3955 | 1.06 (0.64, 1.77), 0.8088 |
| 130–139 | 1.54 (0.95, 2.48), 0.0792 | 1.40 (0.87, 2.27), 0.1690 | 1.17 (0.71, 1.91), 0.5387 |
| 140–149 | 2.00 (1.19, 3.37), 0.0094 | 1.78 (1.05, 3.02), 0.0318 | 1.39 (1.01, 2.39), 0.0352 |
| ≥150 | 2.46 (1.50, 4.03), 0.0004 | 2.20 (1.34, 3.62), 0.0020 | 1.60 (1.15, 2.71), 0.0062 |
| P for trend | <0.001 | <0.001 | 0.0381 |
The results are expressed as the hazard ratio (95% confidence interval), followed by the P value. The SBP <120 mmHg group was regarded as the reference group. SBP: systolic blood pressure.
Model 1: non-adjusted model.
Model 2: minimally-adjusted model. Adjusted for age, sex, and body mass index.
Model 3: fully-adjusted model. Adjusted for age, sex, body mass index, estimated glomerular filtration rate, total cholesterol, triacylglycerol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, diabetes, smoking, drinking, fasting blood glucose, calcium channel blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers.
Effect size of SBP on first ischemic stroke in subgroups
The increased risk of first ischemic stroke in the higher SBP categories was still significant in men (HR, 1.02; 95% CI, 1.01–1.04; P < 0.0001) and in women (HR, 1.01; 95% CI, 1.00–1.02; P = 0.0288) when SBP was analyzed as a continuous variable in the Cox regression model (Table 3). The HRs showed a slight risk of ischemic stroke in most subgroups, but the sample size was limited. There were no significant differences between prespecified and exploratory subgroups of all variables according to the P for interaction.
Table 3.
Effect size of systolic blood pressure on ischemic stroke in prespecified and exploratory subgroups.
| n | HR (95% CI), P value | P for interaction | |
|---|---|---|---|
| Sex | 0.1324 | ||
| Male | 1475 | 1.02 (1.01, 1.04), <0.0001 | |
| Female | 1840 | 1.01 (1.00, 1.02), 0.0288 | |
| Diabetes | 0.1161 | ||
| No | 2810 | 1.02 (1.01, 1.03), <0.0001 | |
| Yes | 505 | 1.00 (0.98, 1.02), 0.7349 | |
| CAD | 0.5542 | ||
| No | 3281 | 1.02 (1.01, 1.03), <0.0001 | |
| Yes | 34 | 0.98 (0.86, 1.11), 0.7590 | |
| Smoking | 0.5139 | ||
| No | 2382 | 1.02 (1.01, 1.02), 0.0008 | |
| Yes | 933 | 1.02 (1.01, 1.04), 0.0061 | |
| Drinking | 0.5513 | ||
| No | 2977 | 1.02 (1.01, 1.02), 0.0001 | |
| Yes | 338 | 1.02 (1.00, 1.05), 0.0609 | |
| BMI | 0.1335 | ||
| <25 | 2127 | 1.01 (1.00, 1.02), 0.0368 | |
| ≥25 | 1188 | 1.02 (1.01, 1.04), 0.0001 | |
| FBG | 0.9237 | ||
| <6.1 | 2911 | 1.02 (1.01, 1.03), 0.0002 | |
| ≥6.1 | 404 | 1.02 (1.00, 1.04), 0.0630 | |
| TC | 0.1808 | ||
| <200 | 1576 | 1.01 (1.00, 1.02), 0.0348 | |
| ≥200 | 1673 | 1.02 (1.01, 1.03), <0.0001 | |
| TG | 0.7402 | ||
| <150 | 2157 | 1.02 (1.01, 1.03), 0.0015 | |
| ≥150 | 1092 | 1.01 (1.00, 1.03), 0.0247 | |
| LDL-C | 0.1160 | ||
| <130 | 2694 | 1.01 (1.00, 1.02), 0.0139 | |
| ≥130 | 553 | 1.04 (1.02, 1.05), <0.0001 | |
| HDL-C | 0.7150 | ||
| <40 | 557 | 1.01 (1.00, 1.03), 0.1038 | |
| ≥40 | 2690 | 1.02 (1.01, 1.03), 0.0001 | |
| Age | 0.4111 | ||
| <70 | 1645 | 1.02 (1.01, 1.03), 0.0032 | |
| ≥70, <80 | 1204 | 1.02 (1.01, 1.03), 0.0010 | |
| ≥80 | 466 | 1.01 (0.99, 1.03), 0.6075 | |
| eGFR | 0.2369 | ||
| <60 | 362 | 1.00 (0.98, 1.02), 0.9790 | |
| ≥60, <90 | 1084 | 1.02 (1.00, 1.03), 0.0064 | |
| ≥90 | 1797 | 1.02 (1.01, 1.03), 0.0010 | |
| CCB | 0.1184 | ||
| No | 2659 | 1.02 (1.01, 1.03), 0.0003 | |
| Yes | 656 | 1.00 (0.99, 1.02), 0.6129 | |
| ACEI | 0.0519 | ||
| No | 3103 | 1.02 (1.01, 1.03), <0.0001 | |
| Yes | 212 | 0.99 (0.97, 1.02), 0.5406 | |
| ARB | 0.1385 | ||
| No | 2669 | 1.02 (1.01, 1.03), <0.0001 | |
| Yes | 646 | 1.01 (0.99, 1.02), 0.4921 | |
| Statins | 0.1480 | ||
| No | 3029 | 1.02 (1.01, 1.03), <0.0001 | |
| Yes | 286 | 0.98 (0.95, 1.01), 0.2253 |
HR: hazard ratio, CI: confidence interval, CAD: coronary atherosclerotic heart disease, BMI: body mass index, FBG: fasting blood glucose, TC: total cholesterol, TG: triacylglycerol, LDL-C: low-density lipoprotein cholesterol, HDL-C: high-density lipoprotein cholesterol, eGFR: estimated glomerular filtration rate, CCB: calcium channel blocker, ACEI: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker.
Discussion
This study showed that SBP was significantly associated with the risk of first ischemic stroke in the older population with hypertension in the community, regardless of conventional cardiovascular risk factors. SBP ≥140 mmHg was a significant risk factor for first ischemic stroke. Therefore, we suggest controlling blood pressure to < 140 mmHg to prevent ischemic stroke in older hypertensive patients.
Hypertension remains a vital risk factor of ischemic stroke in the older population, and antihypertensive treatment is still the first strategy to prevent stroke.2,16–18 The Guideline for the Primary Prevention of Stroke regards aging as a non-modifiable risk factor of ischemic stroke and intracerebral hemorrhage for increasing cardiovascular risk in older individuals.9,19 The seventh report of the Joint National Committee suggested that SBP is a more important cardiovascular risk factor than DBP in those aged older than 50 years.20 A meta-analysis that pooled 23 randomized trails estimated that 32% risk of ischemic stroke was decreased in any antihypertensive drug group compared with the no treatment group (risk ratio, 0.68; 95% CI, 0.61–0.76; P = 0.004).21
Isolated systolic hypertension (SBP ≥140 mmHg and DBP < 90 mmHg) should be accounted for when controlling blood pressure.22–24 The Systolic Hypertension in the Elderly Program (SHEP) included 4736 older patients aged ≥60 years with isolated systolic hypertension (SBP, 160–219 mmHg; DBP < 90 mmHg). The 5-year incidence of total stroke was 5.2 versus 8.2 per 100 participants in active treatment versus placebo (risk ratio, 0.64).25 Eighty-five participants suffered from ischemic stroke in the active treatment group during follow-up and 132 suffered from ischemic stroke in the placebo group.26 The Systolic Hypertension in China (Syst-China) study focused on Chinese people older than 60 years with isolated systolic hypertension.22 This previous study compared the incidence of stroke and other cardiovascular complications between active and placebo treatment. This incidence in the active treatment group was reduced by 38% compared with the placebo group (13.0 versus 20.8, P = 0.01).22 Our study examined the association between SBP and the risk of ischemic stroke, which may be predictive and significant in older patients with isolated systolic hypertension in future subgroup analysis.
The Systolic Blood Pressure Intervention Trial (SPRINT) randomized 9361 hypertensive patients (aged ≥50 years, SBP: 130–180 mmHg) to an SBP target of < 120 mmHg (intensive treatment group) or < 140 mmHg (standard treatment group).27 This previous study especially excluded patients with previous stroke or diabetes mellitus. The annual stroke rate was 0.41% versus 0.47% in the intensive and standard treatment groups, respectively (HR, 0.89; 95% CI, 0.63–1.25).27 Subgroup analysis of the SPRINT study included 2636 participants aged ≥75 years. With a median follow-up of 3.14 years, the rate of stroke was 0.67% in the intensive group versus 0.85% in the standard treatment group (HR, 0.72; 95% CI, 0.34–1.21).28 This finding indicates the advantage of intensive blood pressure lowering on preventing stroke. The Hypertension in the Very Elderly Trial (HYVET) showed effectiveness of antihypertensive therapy for reducing the risk of cardiovascular and total mortality, regardless of the frailty status of older individuals aged ≥80 years.29 Similar to the SPRINT study, the HYVET showed no significant difference in estimation of stroke between the active drug and placebo groups. Evidence on blood pressure lowering to reduce the risk of stroke is still limited in older patients with hypertension. These results may be related to special age categories and limited follow-up periods. Intensive treatment of hypertension may decrease the risk of ischemic stroke in the general population, but not lead to significant results in older age groups.
Moreover, the current guidelines have different cut-off values of age for older individuals and controversial blood pressure targets for older hypertensive patients.16,30 The Guideline for the Primary Prevention of Stroke recommends that hypertensive patients should be treated with hypertensive drugs to a target blood pressure of < 140/90 mmHg.9 In 2017, the ACC/AHA recommended a target SBP < 130 mmHg for community-dwelling older patients with hypertension.1 Additionally, the European Society of Cardiology and European Society of Hypertension recommended that older hypertensive patients should reach a target of SBP < 140 mmHg.12 Although these guidelines recommend different blood pressure control targets, they are consistent in the view that the risk of stroke and adverse effects are decreased by progressively lowering blood pressure.1,8,9,12,13 In our study, the threshold of controlling blood pressure conformed with most current guidelines and studies, suggesting that SBP < 140 mmHg is an ideal target. Further studies are required to determine the appropriate control target of blood pressure in older hypertensive individuals.
This study showed a significant association between SBP and ischemic stroke in the older hypertensive population and provided an optimal blood pressure target, but did not discuss the relationship between DBP and stroke. However, we adjusted DBP in Cox regression model analysis to avoid confounding. Moreover, there were various antihypertensive therapies used in our study, which may have led to underestimation of the cardiovascular risk because of potential poor blood pressure control and hypotension. Additionally, this study did not examine different types of ischemic stroke separately. This issue should be evaluated in a further study. Finally, because we focused on hypertensive patients in this study, we could not estimate the baseline characteristics and clinical outcome in general subjects. Therefore, future studies are required to investigate the risk factors of ischemic stroke in the general population.
In conclusion, SBP is independently associated with the risk of first ischemic stroke in hypertensive patients older than 60 years in the Chinese community. SBP ≥140 mmHg obviously increases the risk of first ischemic stroke in the older population. Undoubtedly, lowering SBP to < 140 mmHg is an effective and moderate method of decreasing the risk of first ischemic stroke in older hypertensive patients.
Acknowledgement
We gratefully acknowledge all of the participants for their collaboration.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Science and Technology Program of Guangzhou (Grant Nos. 201604020143, 201604020018, 201604020186, and 201803040012), the National Key Research and Development Program of China (Grant Nos. 2017YFC1307603, 2016YFC1301305), and the Key Area R&D Program of Guangdong Province (Grant no. 2019B020227005) and Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention (No. 2017B030314041).
ORCID iD
Yuling Yu https://orcid.org/0000-0002-8255-3770
References
- 1.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71: e127–e248. DOI: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Asplund K, Karvanen J, Giampaoli S, et al. Relative risks for stroke by age, sex, and population based on follow-up of 18 European populations in the MORGAM Project. Stroke 2009; 40: 2319–2326. DOI: 10.1161/STROKEAHA.109.547869. [DOI] [PubMed] [Google Scholar]
- 3.Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension 1995; 25: 305–313. [DOI] [PubMed] [Google Scholar]
- 4.Thompson AM, Hu T, Eshelbrenner CL, et al. Antihypertensive treatment and secondary prevention of cardiovascular disease events among persons without hypertension: a meta-analysis. JAMA 2011; 305: 913–922. DOI: 10.1001/jama.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franklin SS. Cardiovascular risks related to increased diastolic, systolic and pulse pressure. An epidemiologist’s point of view. Pathol Biol (Paris) 1999; 47: 594–603. [PubMed] [Google Scholar]
- 6.Turnbull F. and Blood Pressure Lowering Treatment Trialists C . Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003; 362: 1527–1535. DOI: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 7.Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA 2002; 287: 1003–1010. DOI: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 8.Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Soc Hypertens 2011; 5: 259–352. DOI: 10.1016/j.jash.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 3754–3832. DOI: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913. DOI: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 11.Benetos A, Bulpitt CJ, Petrovic M, et al. An expert opinion from the European Society of Hypertension-European Union Geriatric Medicine Society Working Group on the management of hypertension in very old, frail subjects. Hypertension 2016; 67: 820–825. DOI: 10.1161/hypertensionaha.115.07020. [DOI] [PubMed] [Google Scholar]
- 12.Schunkert H. [ Management of arterial hypertension: ESC/ESH guidelines 2018]. Herz 2018; 43: 695–700. DOI: 10.1007/s00059-018-4758-3. [DOI] [PubMed] [Google Scholar]
- 13.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 2160–2236. DOI: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 14.Liu LS. and Writing Group of Chinese Guidelines for the Management of H. [ 2010 Chinese guidelines for the management of hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi 2011; 39: 579–615. [PubMed] [Google Scholar]
- 15.Ketteler M, Block GA, Evenepoel P, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney Int 2017; 92: 26–36. DOI: 10.1016/j.kint.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation 2016; 134: 904–905. DOI: 10.1161/circulationaha.116.022536. [DOI] [PubMed] [Google Scholar]
- 17.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358: 1887–1898. DOI: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 18.Sipahi I, Swaminathan A, Natesan V, et al. Effect of antihypertensive therapy on incident stroke in cohorts with prehypertensive blood pressure levels: a meta-analysis of randomized controlled trials. Stroke 2012; 43: 432–440. DOI: 10.1161/strokeaha.111.636829. [DOI] [PubMed] [Google Scholar]
- 19.Douros A, Tolle M, Ebert N, et al. Control of blood pressure and risk of mortality in a cohort of older adults: the Berlin Initiative Study. Eur Heart J 2019; 40: 2021–2028. DOI: 10.1093/eurheartj/ehz071. [DOI] [PubMed] [Google Scholar]
- 20.Chaturvedi S. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): is it really practical? Natl Med J India 2004; 17: 227. [PubMed] [Google Scholar]
- 21.Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA 2003; 289: 2534–2544. DOI: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Wang JG, Gong L, et al. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16: 1823–1829. [DOI] [PubMed] [Google Scholar]
- 23.Franklin SS, Jacobs MJ, Wong ND, et al. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 2001; 37: 869–874. [DOI] [PubMed] [Google Scholar]
- 24.Staessen J, Fagard R, Amery A. Isolated systolic hypertension in the elderly: implications of Systolic Hypertension in the Elderly Program (SHEP) for clinical practice and for the ongoing trials. J Hum Hypertens 1991; 5: 469–474. [PubMed] [Google Scholar]
- 25.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265: 3255–3264. [PubMed]
- 26.Perry HM, Jr, Davis BR, Price TR, et al. Effect of treating isolated systolic hypertension on the risk of developing various types and subtypes of stroke: the Systolic Hypertension in the Elderly Program (SHEP). JAMA 2000; 284: 465–471. DOI: 10.1001/jama.284.4.465. [DOI] [PubMed] [Google Scholar]
- 27.Group SR, Wright JT, Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373: 2103–2116. DOI: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged >/ = 75 years: a randomized clinical trial. JAMA 2016; 315: 2673–2682. DOI: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beckett N, Peters R, Tuomilehto J, et al. Immediate and late benefits of treating very elderly people with hypertension: results from active treatment extension to Hypertension in the Very Elderly randomised controlled trial. BMJ 2011; 344: d7541. DOI: 10.1136/bmj.d7541. [DOI] [PubMed] [Google Scholar]
- 30.Molander L, Lovheim H. Blood pressure change and antihypertensive treatment in old and very old people: evidence of age, sex and cohort effects. J Hum Hypertens 2013; 27: 197–203. DOI: 10.1038/jhh.2012.14. [DOI] [PubMed] [Google Scholar]