Abstract
Background:
Stroke is the leading cause of death and adult disability in Canada. Eighty percent of older adults (≥65 years) who have suffered a stroke will return to their homes, and 60% will require ongoing rehabilitation. The transition between hospital and home is often fragmented, leading to adverse health outcomes, hospital readmissions, and increased health-care costs. This study examined the feasibility of a 6-month integrated transitional care stroke intervention (TCSI), and explored its effects on health outcomes, patient and provider experience, and cost in 30 community-living older adults (≥55 years) with stroke and multimorbidity (≥2 chronic conditions) using outpatient stroke rehabilitation services.
Methods:
The TCSI is a 6-month intervention delivered by an interprofessional (IP) team (occupational therapist, physiotherapist, speech language pathologist, registered nurse, social worker). It involved care coordination, home visiting, and IP case conferences, supported by a web-based application. A qualitative descriptive approach was used to explore the feasibility of implementing the intervention. A prospective one-group pretest/posttest was used to evaluate the effects of the intervention on health outcomes and use and costs of health services, from baseline to 6 months.
Results:
Participants had an average of eight comorbid conditions. The intervention was feasible and acceptable to both older adults and providers. From baseline to 6 months, there was no statistically significant difference in health outcomes. However, there was a significant reduction in the total per person use and costs of health services.
Conclusions:
This study established the feasibility of conducting a larger randomized controlled trial of this intervention.
Keywords: Older adults, transitional care, integrated care, stroke rehabilitation, community living, mobile apps
Background
Stroke is the leading cause of death and adult disability in Canada. Eighty percent of older adults (≥65 years) who have suffered a stroke will return to their homes, and 60% will require ongoing rehabilitation in the community.1 Approximately 92% of older adults with stroke have at least two comorbid conditions and 75% have three or more.2 The transition from hospital to home is a vulnerable period in the continuum of care for this population. Research suggests that care transitions are frequent, complex, and risky for older adults with stroke and multimorbidity. This population is particularly vulnerable to transition-related risks, leading to hospital readmissions, increased health-care costs, reduced quality of life, reduced patient satisfaction and safety (e.g. medication errors, falls), and increased family burden.3,4 One-year all-cause hospital readmission rates are high, ranging from 31% to 49% according to various studies.5–7
These adverse outcomes have been attributed to factors such as (i) inadequate follow-up care following discharge from hospital8; (ii) lack of coordination of care between and among providers and across care settings, particularly between inpatient, outpatient, primary care, and community rehabilitation9; (iii) suboptimal use or delayed access to outpatient stroke, community rehabilitation, or other health and social services4; (iv) lack of knowledge about rehabilitation and recovery after stroke, and available health and social services4; and (v) lack of support for self-management and community reintegration following hospital discharge.4,8,10,11 The resulting fragmentation in care leads to many under-detected and unmet needs for older adults with stroke and multimorbidity and their caregivers.
Transitional care (TC) interventions have been recommended to address adverse outcomes in community-living older adults with complex needs transitioning from hospital to home.12,13 The aim of TC is to ensure the continuity and coordination of health care when patients transfer across care settings and supporting the older adult stroke patients’ reintegration back into the community. TC is considered to be part of integrated care, which occurs over a longer duration of care episodes.10,14 Integrated care aims to bring together services, providers, and organizations from across care settings and sectors to work together jointly so that together they are complementary to one another, are coordinated with each other, and represent a seamless unified system, with continuity for the client.15 Integrated care has the potential to improve patient, provider, and system outcomes by improving the quality of care and decreasing the cost of acute health-care service use.16 Older adults with stroke and multimorbidity particularly benefit from integrated care because their needs are complex and continuously changing, and they typically require a range of health and social services over a long time frame.17
Although TC interventions for older adults have been linked to several positive outcomes, including lower hospital readmissions, the effectiveness of these interventions for older adults with stroke and multimorbidity is uncertain.9,18 Current Canadian best practice guidelines for managing care transitions following stroke are largely built upon evidence from observational or qualitative studies, or expert consensus.13 The majority have focused on hospital-based, post-acute care (<3 months) initiatives, including early supported discharge interventions,19–21 while few have examined the role of outpatient or other community-based teams in supporting care transitions beyond the post-acute period. Recent meta-analyses22 and systematic reviews23 examining the effectiveness of TC interventions for stroke patients are inconsistent, some reporting benefits and others reporting negative effects, and most have focused predominantly on the management of stroke, with limited attention to the management of other comorbid conditions. Consequently, the effectiveness of these interventions for community-living older adults with stroke and multimorbidity is uncertain, and more information is needed on which intervention components are most effective, which populations are most likely to benefit, and which outcomes are important to evaluate.24 Moreover, this equivocal evidence base comes from studies that have paid little attention to (i) patient-relevant outcomes, (ii) broader social determinants of health, (iii) intervention implementation, and/or (iv) quality indicators (e.g. cost, safety, equity).8,10,25–27 Robust research studies using new, integrated TC interventions are needed to improve the experience and quality of transitioning from hospital to home for this vulnerable population.
Our team designed a new hospital-to-home TC intervention to provide an interdisciplinary and integrated strategy for older adults with stroke and multimorbidity. This intervention was designed to complement standard stroke care and improve Quadruple Aim outcomes28 (health outcomes, patient experience, provider experience, cost) by addressing gaps in TC for this complex and underserved population. Our intervention included elements of TC management and stroke rehabilitation that were evaluated in our previous randomized controlled trial (RCT) on interprofessional (IP) team approach to stroke rehabilitation in a home care setting. Results demonstrated improvements in physical and social functioning, from baseline to 6 months.25 The key ingredients of the IP team approach in this earlier study included up to six in-home visits by an IP team and monthly IP case conferences where providers discussed individual patients and developed a patient-centered plan of care for older adults with stroke receiving home care services. The current study differed from this earlier trial in that the target sample were community-living older adults with stroke and multimorbidity newly discharged from hospital and referred to outpatient stroke rehabilitation services; not home care services. A number of enhancements to the earlier study were included in the trial, including (i) adding a formal system navigator role, (ii) enhancing the focus on linkages to primary care and other health-care and community services, (iii) enhancing the focus on TC management following hospital discharge, (iv) enhancing the focus on patient self-management, and (v) use of a web-based app “My Stroke Team (MyST)” to support care coordination, communication, and information sharing within the team. We engaged a range of stakeholders—from patients to policy makers—in codesigning the intervention, with the goal of making the intervention patient-centered and feasible to implement, leading to greater uptake of results by the larger stroke rehabilitation system.
Objectives
The goal of this feasibility study was to investigate the feasibility of a larger RCT to examine the effectiveness of this integrated TC intervention for community-living older adults with stroke and multimorbidity newly discharged from hospital and referred to an outpatient stroke rehabilitation setting. Primary and secondary study objectives were designed to achieve this goal. The primary objective was to determine the feasibility of implementing the TC intervention. The secondary objectives were to (i) explore the preliminary effectiveness of the intervention based on changes in patient-reported health outcomes, and the costs of use of health and social services, from baseline to 6-month follow-up; (ii) determine the feasibility of the study methods; and (iii) determine the most appropriate primary outcome measure for a future RCT.
Methods
This study was conducted in accordance with the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans.29
Study design
A mixed-method design (QUANT + qual) was used to address the study objectives, and examine the interplay between the outpatient context, implementation of the occupational therapist (OT)-led strategy, and outcomes. A prospective one-group pretest/posttest pragmatic study design was used to evaluate the effectiveness of the TC intervention, implemented under real-world conditions, including reliance on existing health-care providers.30 Assessments were made at baseline (pretest) and immediately following the 6-month intervention period (posttest). Six months was selected for the intervention length for the feasibility study based on our previous research.25 A qualitative descriptive approach was utilized to explore feasibility of implementing the intervention.31,32 We drew on normalization process theory (NPT) to help identify, characterize, and explain key mechanisms that promote or inhibit implementation, embedding, and integration of the TC intervention. We focused on NPT constructs of most relevance to “normalizing” an intervention, specifically coherence, cognitive participation, collective action, and reflexive monitoring.33 A recent qualitative systematic review of 130 papers using NPT reported its effectiveness in supporting intervention design and implementation planning, as well as evaluating and understanding implementation processes.33 This includes describing the context of the study and supporting interpretation of the study results.34
Participants
This study was a collaborative project between researchers in the Aging, Community and Health Research Unit at McMaster University and the Regional Rehabilitation Outpatient Services Program at Hamilton Health Sciences; a large urban academic teaching hospital in Ontario, Canada. The center is in a large city (Hamilton population: 536,917) where older adults (≥65 years) represent 17.3% of the total population.35 The goal of the Aging, Community and Health Research Unit is to promote optimal aging at home for older adults with multimorbidity and to support their family/friend caregivers. To this end, the unit’s research program co-designs, implements, and evaluates innovative community-based interventions, and assesses the potential for scale-up of these interventions to improve Quadruple Aim outcomes.36,37 Inclusion criteria were older age (>55 years), hospitalization with a confirmed diagnosis of stroke (first ever or recurrent) within the past 12 months, two or more comorbid chronic conditions, newly referred to and receiving outpatient stroke rehabilitation services, living in the community (not in a long-term care home), mentally competent to give informed consent (or via a substitute decision maker), lived within the geographical boundaries of the outpatient stroke clinic (determined by postal code), and competent in English (or with an interpreter available).
Screening for eligibility and enrollment
A trained recruiter who was employed by the hospital-based outpatient services program identified potential participants based on the above inclusion criteria, then approached them either in person prior to their discharge from their acute hospital stay or by telephone following discharge from hospital to determine their eligibility for the study and obtain their verbal consent to be contacted by a research assistant (RA).
An RA conducted an interview either at home or at the outpatient clinic, obtaining written informed consent and completing baseline questionnaires. Older adults were deemed mentally competent and thus eligible to participate in the study if they had ≥5 correct answers on the Short Portable Mental Status Questionnaire (SPSMQ).38 Those who had <5 correct answers could be included if they had a substitute decision maker to provide consent and complete the questionnaires on their behalf. All eligible and consenting participants were assigned to the TC intervention.
Intervention
A detailed description of the transitional care stroke intervention (TCSI) is found elsewhere.39 The description of the intervention follows the Template for Intervention Description and Replication guidelines40 (Table 1A, Appendix 1). This feasibility study is pragmatic, which means that the intervention was implemented under real-world conditions, including reliance on existing staff from the existing outpatient services program to deliver the intervention, a non-prescriptive intervention protocol to enable it to be tailored to participant needs, and use of patient-relevant outcome measures.30 The intervention was offered in addition to usual stroke care and was provided by an IP team of five stroke rehabilitation providers stroke rehabilitation providers (registered nurse (RN), OT, physiotherapist (PT), speech language pathologist (SLP), social worker (SW)) from the outpatient services program. More than one-half (57%) of the providers had a master’s degree, about two-thirds (71%) had 16–20 years of experience working in their respective professions, and 57% reported working for 0–10 years in the outpatient services program. Only 28% had experience working in the community.
To support fidelity, the IP team were provided training prior to initiation of the intervention to convey key intervention activities, research study procedures, technology use, and underlying theories. A standardized training manual was developed that includes key content pertaining to all aspects of the intervention. Training was adapted to the needs of the providers including hands-on training on the use of the MyST app. Monthly outreach meetings were conducted to enable the investigators and the research coordinator to meet with the IP team to monitor intervention implementation and discuss any challenges.
Upon hospital discharge, outpatient services were available to patients by physician referral. Standard care included (i) routine outpatient clinic visits with an OT, PT, Occupational Therapist Assistant (OTA)/Physiotherapist Assistant (PTA), and/or SLP; (ii) a focus by these providers on functional goals for recovery; and (iii) provision of information and referral to community agencies over an average of 3 months. Providers delivering the intervention were also responsible for usual stroke care. Table 1 provides a summary of the key features of the TCSI intervention compared to usual care.
Table 1.
TCSI versus usual outpatient stroke care.
| Characteristics | TCSI | Usual outpatient stroke care |
|---|---|---|
| Outpatient stroke rehabilitation providers | Dedicated team of IP outpatient stroke rehabilitation providers (RN, OT, PT, SLP, SW) with specialized training in stroke rehabilitation, TC management, self-management, and the care of older adults with multimorbidity. | Outpatient stroke providers consisting of OT, PT, and/or SLP with specialized training in stroke rehabilitation working together without established mechanisms for coordinated care. |
| System navigation | Dedicated system navigator who is part of the IP outpatient stroke rehabilitation team. | No dedicated system navigator. |
| Access to outpatient stroke rehabilitation services | Structured and planned home visits and system navigation by all members of the IP team over a 6-month period and access to outpatient clinic services for an average of 3 months. | Outpatient clinic services by PT, OT, OTA/PTA, and/or SLP for an average of 3
months. No home visiting. |
| Mechanism for team communication and collaboration | Monthly IP team conferences and access to MyST to support care coordination, communication, and information sharing within the team. | No monthly IP case conferences. No electronic platform for documentation or sharing of information within the team. |
| Information systems | A single IP evidence-based plan of care that focuses on the patient’s preferences. | Provider-specific plan of care |
| Linkages between outpatient stroke rehabilitation services and community-based services | Development of relationships between IP outpatient stroke rehabilitation team and community-based providers and organizations. | Outpatient stroke providers may not be aware of all aspects of community-based services or have not yet developed and established relationships with these services and their providers. |
| Management of multimorbidity | Provision of training in care of older adults with multimorbidity and implementation of evidence-based guidelines for the provision of stroke rehabilitation that considers an individual’s MCC. | Outpatient stroke providers may require up-to-date training, resources, and tools on how to care for older adults with multimorbidity. |
| Approach to care | Focus on promoting and supporting self-management through focusing on improving patient’s problem-solving and self-efficacy, identifying patient goals and priorities. | Focus on functional goals for recovery with limited focus on promoting or supporting self-management. |
TC: transitional care; TCSI: transitional care stroke intervention; IP: interprofessional; OT: occupational therapist; PT: physiotherapist; SLP: speech language pathologist; RN: registered nurse; SW: social worker; MyST: My Stroke Team; MCC: multiple chronic conditions.
The TCSI was underpinned by Lorig and Holman’s self-management theory,41 and developed based on best practice guidelines and published research on TC, stroke,13,22,23 and multimorbidity,42 and qualitative interviews with health-care providers, older adults with stroke, and their caregivers. The TCSI was a 6-month intervention that supported patient self-management by improving patient/caregivers’ problem-solving and self-efficacy, identifying patient’s health goals, developing and implementing a patient-centered plan of care, engaging patients/caregivers as core members of the care team, improving patient/caregiver knowledge of and ability to access community resources, and facilitating care coordination across hospital, primary and other health-care or community care services to ensure care continuity.43
The TCSI was led by a system navigator from the participating outpatient services program, and consisted of four core components: (1) up to six home visits by a member of the IP team of stroke rehabilitation providers (RN, OT, PT, SLP, SW); (2) monthly IP case conferences where providers met in person and discussed individual patients and developed a patient-centered plan of care; (3) linkages to primary care and other health-care and community services; and (4) a web-based app “MyST” to support care coordination, communication, and information sharing within the team.
As part of the TCSI, the OT provided care coordination and system navigation. System navigation provided by individuals or teams is emerging as a strategy to reduce barriers to care for individuals with complex health and social support needs. While there is no commonly accepted definition of system navigation, we considered system navigation to refer to an individual or a team engaging in specific activities that include the following concepts: (i) facilitating access to health-related programs and social services for patients/families and caregivers; (ii) promoting and facilitating continuity of care; (iii) identifying and removing barriers to care; and (iv) effective and efficient use of the health-care system for both patients/families, caregivers, and practitioners.44 A recent scoping review involving 34 studies on system navigation reported that because of the diversity of navigation models in the literature, and the lack of suitable research designs, it is difficult to draw conclusions regarding the effectiveness of system navigation.44 However, one study was found that demonstrated the effectiveness of a Stroke Navigator role in increasing access to comprehensive, strengths-based assessment, planning, and referral facilitation.45
The navigator contacted participants by phone following hospital discharge, once they were referred to the outpatient program, to assess patient and caregiver needs and arrange timely admission to the outpatient program and access to other community services. Once the patient was admitted to the outpatient rehabilitation program, the navigator worked collaboratively with the patient and IP team to develop and implement a comprehensive stroke rehabilitation plan, in addition to receiving usual care. This included (1) conducting a needs assessment and engaging patients/caregivers in the TCSI; (2) identifying and addressing any risk factors for adverse events (e.g. hospital readmissions); (3) arranging community services such as home care and follow-up health-care appointments, including the primary care physician; (4) facilitating communication between the patient, their family caregiver, and the IP team; (5) supporting linkages and referrals to other relevant health and social service providers; and (6) initiating an individualized patient-centered plan of care.
Each participant was offered monthly in-home visits that were an average of 1 h in duration by a member of the IP team (i.e. OT, RN, PT, SLP, or SW) for 6 months as part of the TCSI, in addition to usual outpatient services. Usual outpatient services were only provided by the OT, PT, and SLP. The IP team’s main activities during the home visits included (i) conducting a holistic assessment of the health and social care needs of the older adult participant and their caregiver using standardized screening tools, (ii) medication review, (iii) providing self-management education and support using strengths-based practice,46–48 (iv) utilizing best practices for stroke care and other chronic conditions to inform the plan of care, (v) facilitating timely access to primary care and other health and social services (e.g. specialists, home care, community services such as recreation programs), and (vi) providing caregiver support. The frequency and timing of the home visits were flexible and based on individual needs and preferences.
Strengths-based practice was used because of its positive impact on self-efficacy, self-management, and quality of life.25 The intervention addressed the full range of stroke self-management activities within the context of multimorbidity but was inherently flexible so that it could be shaped by participants and tailored to their needs. Figure 1 displays the main intervention activities.
Figure 1.
TC intervention activities. TC: transitional care.
The IP team met once per month for 6 months to discuss patient-identified goals and develop a person-centered and evidence-based plan of care for each participant. Case conferences provided an opportunity to share observations about participants’ strengths and challenges, identify patient-centered goals related to stroke rehabilitation, and identify the need for other health professionals or community services. The plan included specific short-term and 6-month goals, a list of actions and referrals, a record of all recommendations, and the client’s progress toward their goals.
Our web-based app “MyST” was used by members of the IP team to support care coordination, communication, and information sharing within the team. MyST was codesigned with health-care providers,49 and included a patient profile and space that could be viewed by the IP team and patient/caregiver. However, only providers were able to add content or communicate through MyST in the outpatient setting as well as during the home visit. MyST was a technological tool intended to facilitate and enhance usual care and the intervention by providing a secure space for (i) detailed personal patient information; (ii) documenting and sharing visits, case conference records, standardized screening tool scores, client goals, and follow-up items; (iii) posting “alerts” for individuals or the team; and (iv) accessing resource links (e.g. stroke best practice guidelines, stroke educational materials, health and social services in the community).
Participant characteristics
Demographic characteristics were assessed using standard questions at baseline. Participants were asked about the number of months since their stroke and if they experienced known risk factors for stroke (e.g. hypertension, and other chronic conditions).
Variables and measures
Independent RAs assessed participants at baseline and again at 6 months immediately after intervention completion through a structured in-home interview lasting about 1 h. The RAs with previous experience working in community-based settings were trained in consent and data collection procedures; inter-rater reliability was good. Table 2A in Appendix 1 provides an overview of all outcome variables, measures, and methods of analyses.
Feasibility of implementing the intervention
Assessment of feasibility includes perceptions of the appropriateness and acceptability, as well as the benefits and convenience of implementing the intervention.50 Feasibility of intervention implementation was measured based on qualitative feedback from providers and participants regarding intervention acceptability and implementation barriers/facilitators. Qualitative feedback from providers was obtained during monthly outreach meetings with the researchers, and two focus groups held 3 and 9 months following initiation of the intervention. The qualitative feedback obtained during the monthly outreach meetings was recorded in the meeting minutes. Six-month exit qualitative interviews included a subset of older adult participants who completed the intervention regarding its perceived benefits, how it should be changed, and what they liked and did not like. Focus group sessions and qualitative interviews with participants were audiotaped and transcribed verbatim.
Four quantitative measures were also used to assess intervention feasibility: (1) percentage of participants (excluding deaths and transfers to long-term care) who completed the intervention, (2) percentage of completers who had at least one home visit over the 6-month intervention period (“engagement rate”), (3) mean number of home visits that each participant received over the 6-month intervention period (“dose”), and (4) percentage of participants who received the different components of the intervention (“fidelity to treatment”). Carroll et al.’s51 generic fidelity implementation model informed the development of a checklist that employed a simple, present/absent response format. One researcher reviewed source documents (e.g. visit and case conference records) to assess elements on the checklist (Table 3A, Appendix 1).
Feasibility of the study methods
Eligibility was defined as the percentage of clients screened that were eligible to participate in the study. Our target was ≥70%, based on the assumption that 92% of older adults with stroke have two or more other chronic conditions26; 75% would be deemed eligible. Recruitment was defined as the percentage of eligible clients that enrolled in the study. We set a target of ≥40% for this outcome based on a previous trial of older adults with stroke and multiple chronic conditions (MCC).25 Retention was defined as the percentage of enrolled clients that completed the 6-month program. We set a target of ≥80% for the retention rate, based on the notion that bias is a concern if attrition exceeds 20%.52 Representativeness was defined as the absence of substantial differences between completers and non-completers on a range of characteristics collected at baseline.
Questionnaires, administered by trained interviewers, were used to collect data at baseline and 6 months. Inter-rater reliability was established prior to data collection. At baseline, we also collected sociodemographic data and medical history. RAs provided feedback on interview length, clarity and acceptability of interview questions, applicability of questions to participants, and ease of collecting data. Researchers reviewed the data, explored possible reasons for missing or inconsistent responses, and reviewed results from the focus groups and interviews for indications of important issues relating to data collection or analysis.
Six-month change in outcome measures
Health-related quality of life (HRQoL) was measured using the Short Form-12 (SF-12), a patient-reported health outcome rating scale.53 The physical component summary score (PCS-12) and mental component summary score (MCS-12) were used to summarize the data. PCS-12 and MCS-12 scores range from 0 to 100 and higher scores indicate higher levels of HRQoL.54 Guidelines are available for judging clinical significance for the SF-12. SF-12 developers suggest a minimally important difference (MID) of 3 for interpreting group mean summary score differences (PCS, MCS).55 A recent systematic review of RCTs reporting non-significant results emphasized the importance of interpreting confidence intervals (CIs) in order to distinguish “negative” findings from “inconclusive” ones.56 This review suggests that p value alone does not allow readers to distinguish whether (1) the intervention does not have a clinically meaningful impact, and (2) the study is unable to rule out a clinically meaningful treatment effect, resulting in “inconclusive” findings. The authors recommend examining confidence limits in relation to the MID. We applied this recommendation to our feasibility study for the PCS and MCS of the SF-12 which has MIDs.
The use of all types of health services were measured using the Health and Social Services Utilization Inventory (HSSUI).57 The cost analysis applied unit costs to the service volumes reported in the HSSUI58 and assumed a societal perspective in order to inform the broad allocation of resources in the public interest. The HSSUI has been previously assessed for reliability and validity.59,60 Differences in median costs for each service for the 6 months prior to baseline (including the immediate post-stroke period), and the 6-month intervention period were calculated, and the change in each was compared to the hypothesized direction (increase in intervention costs, decrease in hospitalization and emergency room (ER) visit costs). Health service costs included the costs of delivering the intervention (e.g. home visits, case conferences). Costs for each service type were expressed as a median cost (Canadian dollar (CAD)).
Primary outcome for RCT
The candidate measures for the primary outcome for a future RCT included HRQoL, specifically, the MCS score and the PCS score from the SF-12. The criteria used to evaluate these measures included their applicability and relevance to the older adult study participants, face validity, and ease of data collection. RAs provided feedback on these criteria. Researchers also reviewed the data collected for each outcome measure, explored reasons for missing or inconsistent responses, and considered the responsiveness of each measure over the 6 months.
Sample size
The sample size for this study was based on feasibility considerations.52,61,62 A target sample size of between 20 and 40 participants was used to ensure ≥40% recruitment target and ≥80% retention target. The preliminary information on the estimates of change in outcomes from baseline to 6 months will be used to determine the effect size for estimating the sample size for a future RCT.
Statistical analysis
All analyses were performed using SAS version 9.4 for Windows. All statistical tests were performed using two-sided tests at the 0.05 level of significance. Descriptive analyses of participants’ characteristics at baseline were expressed as a mean (standard deviation (SD)) for continuous variables and count (percent) for categorical variables. Outcome data were treated as continuous variables and the change in effect from baseline to time 2 was expressed as a mean difference, with SDs and corresponding 95% CIs. Paired t-tests were performed to determine statistical significance of the change in outcomes from baseline to time 2. Health and social service use costs were determined by multiplying total volume over 6 months self-reported by patients (baseline, time 2) by the appropriate current unit cost. The costs of the intervention included the costs of home visits, which were conducted either in-person visits or by telephone. Visit costs were determined by multiplying the number of visits over 6 months by a blended provider rate (100 CAD/h, based on current hourly rates of PTs, OTs, SLPs, and RNs in Canada). Home visits (in person, telephone) were assumed to be an hour long. Due to the highly skewed nature of the cost data, the Wilcoxon signed-rank test was used to compare costs at baseline versus time 2. Median and quartiles (Q1, Q3) were used to describe costs by service type and total costs at baseline and time 2.
The qualitative data were transcribed verbatim and coded using NVivo Version 10 (QSR), including focus groups, interviews, and monthly outreach meeting minutes. A qualitative descriptive approach was used for analysis.31 An inductive and deductive approach63 was used to code the transcripts based on the main research questions and NPT constructs. Coding was completed line by line using an inductive approach, organizing the codes within NPT constructs deductively.33 Coding was conducted by the Research Coordinator (RC) (AB) and the co-principal investigator (RV) who have expertise in qualitative analysis. Analysis of the meeting minutes involved skimming (superficial examination), reading (through examination), and interpretation. This iterative process involved using the emerging themes in the focus group and interview data to organize the information into categories.
Results
Eligibility rate
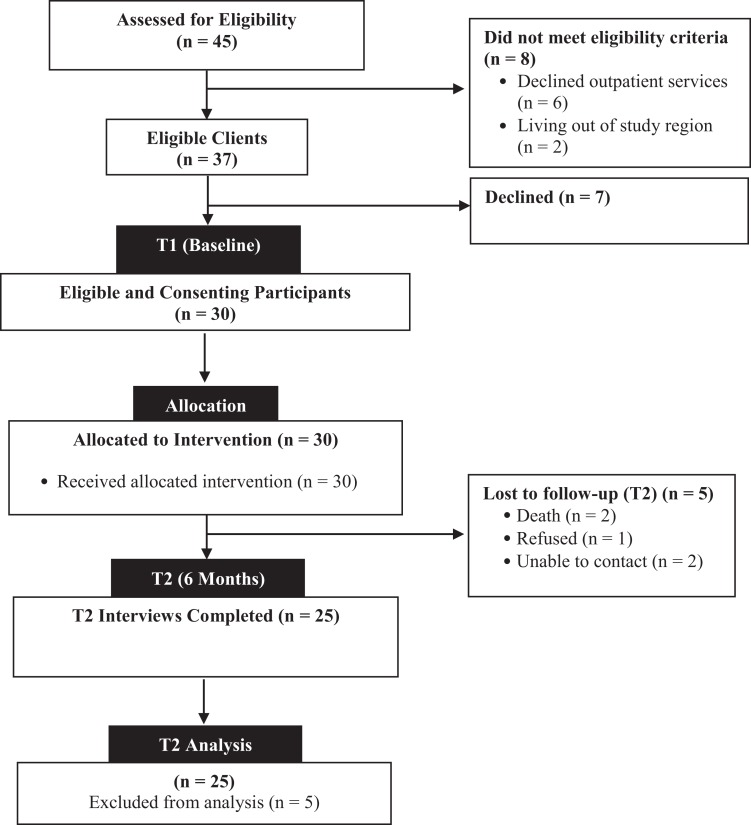
Recruitment was conducted over a 5-month period from May 2017 to October 2017. Figure 2 provides a summary of the flow through the study. A total of 45 consecutive older adults with stroke were screened for the study, and 82% (37/45) met all eligibility criteria (target of 70%). The most common reason for ineligibility (75%) was that potential participants declined outpatient rehabilitation services (6/8).
Figure 2.
Study flow of participants.
Enrollment rate
In total, 81% (30/37) of eligible older adults consented and entered the study (target of 40%). Reasons for refusal to enroll in the study were feeling overwhelmed (4/7, 57%) or not interested (3/7, 43%).
Attrition rate
Of the 30 enrolled participants, 25 successfully completed the 6-month follow-up, resulting in a retention rate of 83% (25/30) (target of 80%). Reasons for loss to follow-up are shown in Figure 2.
Comparison between dropouts and completers
No differences were observed between completers and non-completers on baseline demographic and stroke-related characteristics, SF-12 PCS and MCS scores, or Generalized Anxiety Disorder scale (GAD-7), Centre for Epidemiological Studies Depression scale (CES-D), and Self-Efficacy scores.
Baseline demographic profile and stroke-related characteristics
Baseline characteristics of the participants are displayed in Table 2. The majority (70%) had six or more comorbid chronic health conditions, with a mean of eight chronic conditions, and were taking an average of nine prescription medications. Almost all participants (93%) were within their first 6 months post-stroke, with an average of 2 months post-stroke. Participants displayed several risk factors for stroke, including cardiovascular disease (90%), hypertension (90%), diabetes (90%), and previous history of stroke (30%).
Table 2.
Stroke-related and clinical baseline characteristics.
| Characteristics | n = 30 | % |
|---|---|---|
| History of stroke | ||
| No | 21 | 70.0 |
| Yes | 9 | 30.0 |
| Time since stroke (months)a | ||
| <1 | 9 | 30.0 |
| 1–2 | 11 | 36.6 |
| >2 | 10 | 33.3 |
| Cognitive status | ||
| No impairment (score ≥5) | 29 | 96.7 |
| Impairment | ||
| Number of comorbid chronic conditions | ||
| <6 | 9 | 30.0 |
| ≥6 | 21 | 70.0 |
| Common conditions (sample prevalence ≥25%)b | ||
| Cardiovascular + hypertension | 27 | 90.0 |
| Diabetes + hyperlipidemia | 27 | 90.0 |
| Osteoarthritis + other arthritis | 13 | 43.3 |
| Stomach problems | 10 | 33.3 |
| Depression/anxiety | 9 | 30.0 |
| Chronic urinary problems | 9 | 30.0 |
| Vision and hearing | 9 | 30.0 |
| Respiratory | 8 | 26.7 |
| Fall within last 12 months | ||
| No | 13 | 43.0 |
| Yes | 17 | 56.7 |
| Number of prescription medications | ||
| <8 medications | 18 | 60.0 |
| ≥8 medications | 12 | 40.0 |
a Time since stroke calculated as baseline interview date minus date of index stroke.
b Cardiovascular defined as hypertension, atrial fibrillation, coronary artery disease, congenital malformed valve, or heart failure; osteoarthritis/arthritis/osteoporosis or rheumatoid arthritis, other inflammatory/systemic connective tissue disorders; diabetes including hyperlipidemia; respiratory as asthma, COPD, pulmonary fibrosis, or other lung condition; chronic urinary problems as bladder problems, bladder incontinence, fecal incontinence, constipation.
The majority (53.3%) of participants were men, living with a spouse or other family member (83.3%), with an average age of 71.6 years. A similar proportion were married (56.6%). Most (62%) had annual incomes of less than CAD$40,000. All participants reported receiving some form of support from a family member or friend. Almost all (96.7%) participants scored ≥5 on the SPSMQ, indicating that they were mentally competent. Older adult participants reported SF-12 PCS and MCS scores at baseline that were significantly lower than published norms for the Canadian population, indicating poor HRQoL.64 About one-third of participants (32%) screened positive for depressive symptoms (>10 on CES-D-10), and 60% screened positive for mild to moderate anxiety (≥5 on GAD-7).
Primary objective: Feasibility of implementing the intervention
We used NPT to identify, characterize, and explain key mechanisms that promote or inhibit implementation, embedding, and integration of the TCSI with a focus on coherence, cognitive participation, collective action, and reflexive monitoring.33
Coherence refers to what providers individually and collectively understand about the intervention. Providers understood that the intervention was designed to improve intersectoral and IP collaboration by enhancing communication about patient goals and the patient’s progress toward achieving their goals using case conferences and MyST. Providers understood that home visits were important for gaining an understanding of the patient and/or caregiver in the context of their day-to-day lives. Providers also understood that the intervention helped to forge connections between patients, health-care providers, and hospital and community-based services to promote community reintegration. Providers also credited the intervention with increasing their knowledge and use of best practices for stroke rehabilitation and community reintegration.
Cognitive participation refers to work done by the actors to get them to buy into the new intervention, how they can contribute to it, and how they reorganize themselves to get the work done. Providers worked to clarify and communicate the roles of each discipline on the IP team. Over time, the team learned about each other’s roles and scope of practice in relation to stroke rehabilitation. This helped the system navigator to coordinate care and refer more appropriately to the right provider to address individual patient and family needs. Providers acknowledged the importance of an IP team-based approach, particularly in allowing the providers to work to their full scope of practice. Each discipline used tools to identify and monitor functional and reintegration goals that were relevant for their discipline and the patient’s goals. Providers indicated that they needed to enhance their knowledge and skills related to the management of MCC, the use of standardized screening tools, and the use of a strengths-based approach to care. They also acknowledged a need to improve their knowledge of community-based services and supports in order to assist patients in navigating the health-care system.
Collective action refers to the operational work that people do to enact the intervention, such as interactional work, knowledge work, and the allocation of work. Although the providers were given release time from their regular positions to deliver the intervention, they felt that this time was insufficient to deliver the intervention. They also felt that the initial 1.5-day training session was not enough time to prepare them to deliver the intervention. Other challenges raised by the providers included the time spent on documentation. Because MyST was not considered the legal patient record, providers needed to document their activities in both the regular chart and the MyST app. Nevertheless, providers felt that the monthly case conference and MyST were an excellent forum for regular communication among the team. Providers felt that having dedicated resources, such as time and space for the case conferences, tablets to access MyST, and regular monthly meetings with the research team, were key factors supporting the successful implementation of the intervention. Another key factor supporting implementation of the intervention was having the navigator establish processes to coordinate and communicate with the team and manage the care of the patients around their schedules.
Reflexive monitoring refers to the work done by people to assess how a new intervention impacts them and those around them. The feedback from the providers, managers, and older adult participants was positive. Both providers and older adult participants felt that the home visits were very beneficial. Older adult participants credited the intervention plus usual care with several benefits, such as increasing their level of social support and ability to self-manage their care post-stroke, as well as reducing their anxiety. Participants noted that the providers were competent, knowledgeable, and empathetic. Providers felt that the intervention resulted in improvements in the quality of the care they provided to participants. Providers credited this improvement to the fact that the intervention helped to strengthen existing connections and forge new connections with patients, providers, and other community-based services. These connections were initiated by the system navigator following hospital discharge, immediately after referral to outpatient services, and during the 6-month intervention period. Participants and providers felt that the intervention assisted patients in navigating the health-care system and improving their timely access to community services and supports. The system navigator felt that the intervention could be improved by expanding their role across the care continuum so that they were working with inpatient providers and patients in the hospital setting to develop a comprehensive discharge plan. Implementation of the intervention was beneficial in other ways including the establishment of a permanent system navigator position in the outpatient rehabilitation program upon completion of the study.
Further evidence of the acceptability of the intervention to participants is provided by data on the uptake of the intervention. All the participants received at least one home visit by a member of the IP team during the 6-month intervention period. Five participants (17%) discontinued the intervention early. Two participants died during the 6-month follow-up, leaving 28 participants in the study. Twenty-five participants completed the 6-month interviews. This translates into an acceptability rate of 89% (25/28).
Intervention fidelity
Training protocols, manuals, and workshops were delivered to all providers, recruiters, and RAs. The intervention was tailored so participants had the option of declining some or the entire intervention; however, all the participants received at least one home visit by one of the IP team providers during the 6-month intervention period. Participants received an average of 4.73 home visits (1–3 visits, 13.3%; 4–5 visits, 63.3%; 6 visits, 26.7%) by one of the IP team providers over the 6-month study period. More than one-half (56%) of these visits were provided by the OT who also functioned as the system navigator. The remainder of visits were provided by the SW (17.6%), PT (14%), SLP (7.7%), and nurse (4.2%). All members of the IP team met on a monthly basis over the study period to discuss the study participants. The intervention was delivered as intended and was well received by both groups. Variation in the number of home visits received by participants was appropriate for a tailored, patient-driven intervention.
Secondary objective: 6-Month change in outcome measures
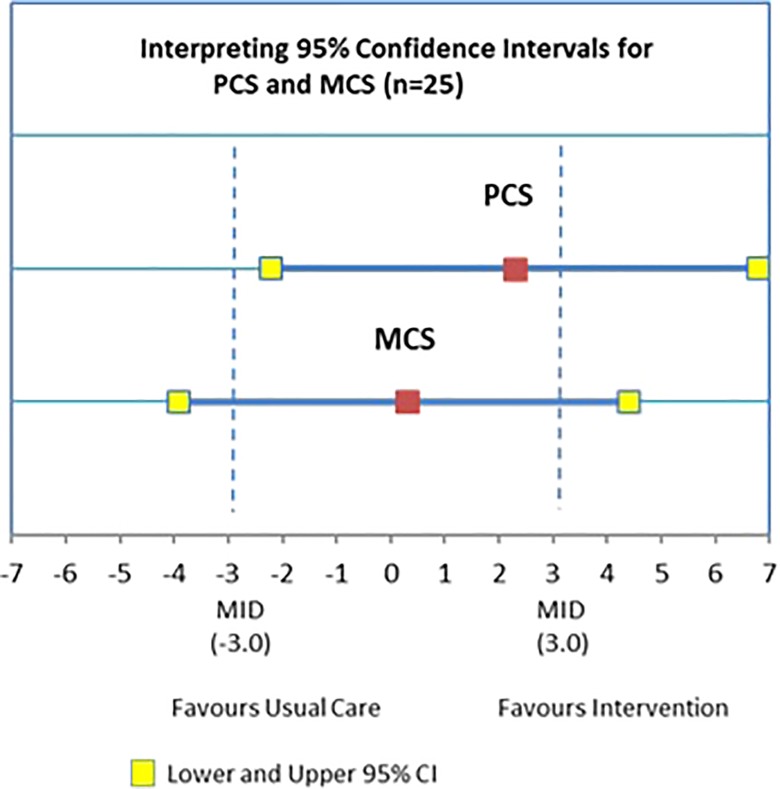
Table 3 shows values for the outcome measures at baseline and 6 months. At baseline, the mean score (SD) for the SF-12 PCS score was 32.76 (10.47), which is below the mean for general Canadian population.65 The results of the paired t-test showed no significant difference in the SF-12 PCS score from baseline to 6 months (mean difference: 2.32, 95% CI: −2.20, 6.83) for the 25 participants who completed the study.53 At baseline, the mean score (SD) for the SF-12 MCS score was 48.24 (11.03), which is also below mean for the general Canadian population.61 The results of the paired t-test showed no significant difference in the SF-12 MCS score from baseline to 6 months (mean difference: 0.25, 95% CI: −3.92, 4.43). Figure 3 provides a graphic interpretation of the MCS and the PCS findings. For PCS, the findings are inconclusive regarding superiority of the intervention but rule out the superiority of usual care, since the CI crosses 0 with the upper CI (favors the intervention) exceeds the MID and the lower CI (favors usual care) does not reach the MID. For MCS, the findings are inconclusive with either usual care or the intervention being potentially superior. There were no significant differences from baseline to 6 months for the Self-Efficacy score (mean difference: 0.72, 95% CI: −0.24, 1.68), the CES-D score (mean difference: −0.60, 95% CI: −1.70, 1.11), or the GAD-7 score (mean difference: 0.48, 95% CI: −1.17, 2.13) (Table 3).
Table 3.
Changes in HRQoL, depression, anxiety, and self-efficacy over the study period (n = 25).a
| Time 1 | Time 2 | Difference in mean scores [T2 − T1] (95% CI) | p Value | |||
|---|---|---|---|---|---|---|
| Scale/subscale | Mean | SD | Mean | SD | ||
| HRQoL–Physical composite summary score (SF-12) | 32.8 | 10.5 | 35.1 | 10.5 | 2.3 (−2.2, 6.8) | 0.3 |
| HRQoL–Mental composite summary score (SF-12) | 48.2 | 11.0 | 48.5 | 8.9 | 0.3 (−3.9, 4.4) | 0.9 |
| Self-efficacy for managing chronic disease scale (SE-MCD) | 7.4 | 1.9 | 8.1 | 1.7 | 0.7 (−0.2, 1.7) | 0.1 |
| Centre for Epidemiological Studies Depression scale (CES-D-10) | 9.2 | 5.3 | 8.6 | 4.9 | −0.6 (−1.7, 2.9) | 0.6 |
| Generalized Anxiety Disorder scale (GAD-7) | 4.7 | 3.9 | 5.2 | 4.7 | 0.5 (-1.2, 2.1) | 0.6 |
HRQoL: health-related quality of life; SD: standard deviation; CI: confidence interval.
a Complete case analysis.
Figure 3.
Interpreting 95% CIs for PCS and MCS (n = 25). CI: confidence interval; PCS: physical component summary score; MCS: mental component summary score.
Health service use costs
The median cost of the TCSI intervention was CAD 616.00 (interquartile range CAD 492.80–716.00) per study participant (Table 4). The results of the Wilcoxon signed-rank test based on the 25 participants who completed the study showed a statistically significant reduction in the total median per person costs of use of all types of health services (including the intervention costs) from baseline to 6 months (difference: −CAD$28,550.06, p < 0.001) (Table 4). The direction of the changes in median costs from baseline to 6 months confirmed to our hypotheses: median intervention costs increased, including the cost of outpatient services (difference: CAD$4815.00, p < 0.001) and other community services (difference: CAD$184.00, p < 0.001); and non-intervention costs of use of acute care hospitalization (difference: −CAD$35,427.00, p < 0.001), ER visits (difference: −CAD$239.31, p < 0.001), ambulance, and 911 services (difference: −CAD$24.80, p < 0.001) decreased. The difference in acute care hospitalization costs from baseline to 6 months was due to a 92% reduction in the number of participants with one or more hospital admissions over the study period (from 100% at baseline to 8% at 6 months). There was also a significant increase in the use of physician specialists (difference: CAD$61.30, p < 0.001). There were no significant differences in the costs of use of other types of health services.
Table 4.
Costs (per patient) of use of health-care services at baseline versus 6 months (n = 25, CAD).
| Service | Time 1 | Time 2 | Difference in median costs [T2 − T1] (Q1, Q3) | p Value | ||
|---|---|---|---|---|---|---|
| Median | Q1, Q3 | Median | Q1, Q3 | |||
| Family physician | 191.42 | 77.20, 308.80 | 231.60 | 154.40, 308.80 | 77.20 (−37.01, 154.40) | 0.3077 |
| Physician specialist | 0.00 | 0.00, 61.30 | 122.60 | 61.30, 367.82 | 61.30 (0.00, 367.82) | <0.0001 |
| Home care | 0.00 | 0.00, 0.00 | 0.00 | 0.00, 0.00 | 0.00 (0.00, 0.00) | 1.00 |
| Ambulance services and 911 | 24.80 | 0.00, 264.80 | 0.00 | 0.00, 0.00 | −24.80 (−264.80, 0.00) | 0.0002 |
| ER visits | 239.31 | 239.31, 478.62 | 0.00 | 0.00, 0.00 | −239.31 (−239.31, −239.31) | 0.0002 |
| Acute care hospital | 35,427 | 11,809, 104,594 | 0.00 | 0.00, 0.00 | −35,427.00 (−104,594.00, −10,122.00) | <0.0001 |
| Outpatient rehabilitation | 0.00 | 0.00, 0.00 | 4815 | 1480.80, 7184.88 | 4815.00 (1480.08, 7184.88) | <0.0001 |
| TCSIa | 0.00 | 0.00, 0.00 | 616.00 | 492.80, 716.00 | 616.00 (492.80, 716.00) | <0.0001 |
| Prescription medications | 507.44 | 217.35, 834.92 | 571.75 | 251.78, 1044.27 | 11.42 (−179.52, 178.24) | 0.7639 |
| Equipment | 40.00 | 0.00, 245.00 | 50.00 | 0.00, 572.00 | 0.00 (−25.00, 438.00) | 0.1324 |
| Other community servicesb | 0.00 | 0.00, 61.23 | 250.00 | 81.23, 340.00 | 184.00 (20.00, 306.00) | <0.0001 |
| Total costs | 35,855.59 | 14,168.17, 106,370.75 | 8521.06 | (3697.90, 15,404.46) | −28,550.06 (−84,077.18, −8202.95) | <0.0001 |
ER: emergency room; TCSI: transitional care stroke intervention; Q1: lower quartile; Q3: upper quartile; CAD: Canadian dollar; T1: baseline; T2: 6 months.
a Transitional care intervention costs: home visits (includes transportation costs).
b Other: homemaker, optometrist, social and recreation services, community support, transportation.
Secondary objective: Feasibility of the study methods
The measures used to evaluate the feasibility of the study methods include the eligibility, recruitment, and retention rates reported previously. The results show that our targets were met for eligibility, recruitment, and retention rates.
Secondary objective: Primary outcome for RCT
Finally, we examined feedback from the RAs and researchers on the data collection and analysis procedures for the two outcomes that were viewed as potential candidates for the primary outcome for the RCT: MCS and/or PCS. The MCS and PCS are summary scores generated from the SF-12 questions and represent well-validated measures of HRQoL. We found that both measures were applicable and relevant to the older adult study population. However, the PCS, which captured physical functional ability, appeared to be a more important outcome for older adults with stroke and more responsive to the intervention at 6 months. RAs indicated that the length of time for administration of both the PCS and the MCS was 5–10 min. The completion rate of both measures was high, with minimal missing data.
Discussion
The overarching goal of this study was to determine the feasibility of conducting a large-scale RCT to evaluate a new integrated TC intervention for older adults with stroke and multimorbidity using outpatient stroke rehabilitation services. Our study is innovative given that it involved (1) testing a TC intervention that focuses on older adults with stroke and multimorbidity, (2) studying intervention implementation instead of focusing solely on effectiveness, (3) evaluating effectiveness of the intervention on patient-relevant outcomes within a multimorbidity context (i.e. HRQoL, depressive symptoms, anxiety, service use), (4) an intervention delivered over 6 months (evidence suggests that community reintegration takes up to 1 year post-stroke and almost all participants were within their first 6 months post-stroke, a time when individuals with stroke have the potential to make the most significant gains),66 and (5) implementing a knowledge translation plan that includes health system decision makers integrated in the proposal and engaged as study collaborators to maximize use of study results. The setting of this study is important because of the increasing emphasis on community-based stroke prevention and rehabilitation to improve patient outcomes and reduce health system costs.19
Feasibility of the intervention and study methods
Evidence of the feasibility of implementing the intervention was indicated by the acceptability of the intervention to providers and older adult participants. There was evidence of “buy-in” for the intervention from both groups, with providers envisioning ways to implement the intervention in practice. Their overall impression of the TCSI was very positive. Providers showed great enthusiasm for and a sense of purpose regarding their role related to the intervention. There was recognition by providers that the intervention resulted in improvements in the quality of care they provided to the participants. Providers credited this improvement to the fact that the intervention provided an improved understanding of the patient’s health status and context due to the combined effect of working in an IP team, and unique insights gained from the home visits. There was also recognition that the intervention helped to close the gap between hospital and home by strengthening existing connections and forging new connections with patients, providers, and community-based services. Further evidence of the acceptability of the intervention to participants was provided by the high level of engagement for the home visits. All study participants accepted at least one home visit by the IP team, and an average of five out of a possible six home visits over the 6-month intervention period.
Our study indicated that older adult participant’s interest in the intervention was high by exceeding our eligibility, enrollment, and retention targets. Results also showed that the study methods were feasible and effective in reaching our target population (e.g. the sample had an average of eight chronic conditions, and almost all (93%) were less than 6 months post-stroke). Overall, our findings support feasibility to deliver the intervention as intended.
Two outcomes were viewed as potential candidates for the primary outcome for the RCT: MCS and/or PCS. The MCS and PCS are summary scores generated from the SF-12 questions and represent well-validated measures of HRQoL. The PCS appeared more promising in that the PCS captured physical functional ability, which appears to be an important outcome for older adults with stroke,25 and is responsive to the intervention. A recent systematic review found that the PCS from the SF-12 was frequently used as a primary outcome in evaluation of interventions like ours for adults with chronic or long-term conditions.67 An argument could also be made for including hospital readmission as the primary outcome since this outcome was also responsive to the intervention and is frequently used in studies evaluating TC interventions. However, the PCS is more patient-relevant outcome than hospital readmissions.
Changes in patient-reported health outcomes and costs at 6 months
There were no statistically significant differences from baseline to 6 months in patient-reported health outcomes (HRQoL, depressive symptoms, anxiety, self-efficacy). However, for the PCS score, the findings are inconclusive regarding superiority of the intervention but rule out the superiority of usual care. For the MCS score, the findings are inconclusive with either usual care or the intervention being potentially superior, suggesting that further testing of the intervention is warranted.
The intervention identified and addressed numerous care gaps and forged new connections between the hospital-based outpatient rehabilitation team and community-based service providers. Future studies are warranted that include a longer follow-up period to determine if the benefits of addressing these care gaps would be seen.
Results are consistent with our previous RCT which also studied an IP team approach (delivered in a home care setting) to community-based stroke rehabilitation, and that found improvements in physical functioning after 1 year.25 The participants in this study are like those in the current study in that most had MCC and the majority (70%) were less than 6 months post-stroke. Results are also consistent with another Ontario-based study of a community-based stroke rehabilitation team that found improvements in the physical domain of the Stroke Impact Scale.68 While the findings from this study are inconclusive regarding the effect of the intervention on physical functioning, the components of the TCSI are well aligned with best practice guidelines for community-based stroke rehabilitation19 and the management of multimorbidity,69 evidence-based guidelines for personal care planning for older adults who are managing MCC,67 and guidelines for TC for complex patients.70 The intervention is well aligned with health-care reform in Ontario and Canada,70 which is focused on exploring new health-care models that integrate care around the patient and across providers in a way that makes sense for each community and improves outcomes.70
The impact of the intervention was shown by use of cost analysis. There was a statistically significant reduction in the total per person costs of use of all types of health services (including intervention costs) from baseline to 6 months. This was due primarily to a reduction in the use of hospitalization and ER visits (for any cause). There was a 92% reduction in the number of participants with one or more hospital admissions over the study period (from 100% at baseline to 8% at 6 months). While it is expected that older adults with stroke would use fewer acute care hospital services after discharge from hospital, the 8% readmission rate observed in this study is well below the 49%5–7 readmission rate reported for stroke survivors in the literature. The direction of the changes in median costs from baseline to 6 months confirmed to our hypotheses: median intervention costs increased; and non-intervention costs of acute care hospitalization, ER visits, ambulance, and 911 services decreased after hospital discharge, supporting the need for a larger trial. The potential impact on Quadruple Aim outcomes28 (health outcomes, patient experience, provider experience, cost) suggests that further research using an RCT design with a larger sample is needed to demonstrate intervention effectiveness alongside cost reductions.
The older adult participants in the present study are comparable to the general population of community-living adults with stroke described in the literature as reflected by their mean age (71 years) and the proportion of males (53%).64,71–73 However, the average number of self-reported chronic conditions (eight) was higher than the average of five chronic conditions reported in the literature.6,74–76 Caution should be exercised in interpreting this result, however, given the wide variation in the type of chronic conditions measured across the different studies.
While the prevalence of depressive symptoms (32%) is close to the 31% reported in the literature,77 our rate for anxiety (60%) was higher than the 24% reported in the literature.78 The higher rate of chronic conditions and anxiety may be due to the nature of our sample. That is, existing studies on stroke rehabilitation often excluded older adults with multimorbidity26 or a previous stroke, whereas, our study included them and consequently may have captured a group of patients more typical of the actual stroke population seen in practice. Further, our study population included patients who had recently had a stroke, and thus captured a more vulnerable subset of the stroke population.
Study strengths and limitations
Our study has several strengths. A key strength of this study was its focus on an intervention well-grounded in theory. Theory is an important consideration in the development of complex interventions, as it enables us to identify several hypotheses that can be tested in the RCT. The use of a pragmatic design optimizes applicability of the intervention to real-world practice by recruiting participants representative of the population presenting in clinical practice, flexible delivery of the intervention by providers, use of existing staff in practice, and the use of intention-to-treat analysis. It contributes to a limited body of research that explicitly targets older adults with stroke and comorbidities. The study also considered the costs of use of health and social services, which many intervention studies either omit entirely or approach less rigorously and comprehensively. Another strength of the study was the high rates of enrollment (81%), engagement with the intervention (100%), and follow-up (<20% dropout). The study evaluated the feasibility of implementing the intervention from multiple perspectives. Intervention fidelity was enhanced by multiple approaches. Notably, the interventionists were provided with training, a standardized training manual, and regular meetings were conducted with the research team throughout the study period.
Several limitations to this study should be noted. First, our study took place within one hospital-based outpatient program, which may limit the generalizability to other settings. Sites may differ on characteristics that can affect implementation of the intervention thereby influencing intervention effectiveness. For example, staffing is known to vary across outpatient stroke rehabilitation clinics in Ontario, with some having an OT, PT, SLP, RN, and SW (as at the study site) and others only having an OT, PT, and SLP.10 The future RCT should involve multiple sites, in order to explore how the intervention performs across a broader range of settings and contexts. Second, there was no comparison group in the one-group pretest/posttest design therefore the degree to which usual care accounted for the results was difficult to address. A future RCT on TCSI is needed to clarify our results. Third, the sample size was intentionally small to allow us to focus on the primary objective, the feasibility of the intervention. This small sample size limits the power of the statistical analyses and means that results offer only preliminary evidence of effectiveness of the intervention. Fourth, due to the complex nature of the intervention, effects cannot be attributed to specific intervention components.79 Finally, due to the small sample size, we were unable to explore whether the intervention was effective for subgroups, defined by differences in sex stroke severity, number of comorbid chronic conditions, or level of social support.
Conclusions
This pragmatic feasibility study of an integrated TCSI for older adults with stroke and multimorbidity conducted in an outpatient program in Ontario demonstrated (1) that the intervention was feasible to implement in real-world practice, (2) inconclusive in terms of the effectiveness for physical functioning (PCS of the SF-12), and (3) a significant reduction in the total costs of use of health services, from baseline to 6 months (primarily related to a reduction in the use of hospitalization and ER visits for any cause). Collectively, these findings have established the feasibility of conducting a large-scale study of this TC intervention for older adults with stroke and multimorbidity.
Funding has been obtained from the Canadian Institutes for Health Research for a large pragmatic RCT of the intervention. This RCT will consider factors that we have learned from this feasibility study, including (1) allocating additional time for training the IP stroke rehabilitation team and ensuring that training is standardized across sites, (2) increasing emphasis in the training program and the case conferences on self-management strategies appropriate for older adults with stroke within the context of multimorbidity, (3) allocating additional resources to all members of the IP team to deliver the intervention, (4) expanding the role of the navigator across the care continuum (working with inpatient providers and patients in the hospital to develop a comprehensive discharge plan), (5) allocating additional time for the navigator role to enable the expansion of their role across inpatient and outpatient settings, and (6) integrating MyST into the legal patient record to avoid duplication.
In Ontario, the jurisdiction where the TCSI was evaluated, a recent expert advisory panel on health-care reform identified several strategic policy recommendations to end hallway health care and build a sustainable health system. The TCSI has the potential to address many of these recommendations, such as putting patients at the center of their health care, improving patients’ and providers’ ability to navigate the health-care system, improving coordination and communication between services, and improving coordination at point of care by strengthening partnerships between health and social services.70 The alignment of the project with government policy, the ongoing relationships between researchers and decision makers, and the use of integrated knowledge translation strategies, will help to support the implementation and uptake of this novel intervention.
Acknowledgments
The authors thank the older adults who participated in this study, as well as the outpatient stroke rehabilitation clinic staff from the Regional Rehabilitation Outpatient Services Program at Hamilton Health Sciences who provided the intervention. The authors also thank the interviewers and recruiters, who gave their full cooperation so that this challenging study could be carried out.
Appendix 1
Table 1A.
The Template for Intervention Description and Replication checklist.
| Item # | Item | Where located |
|---|---|---|
| Primary paper (page or appendix number) | ||
| BRIEF NAME | ||
| 1. | Provide the name or a phrase that describes the intervention. | p. 11 |
| WHY | ||
| 2. | Describe any rationale, theory, or goal of the elements essential to the intervention. | p. 12 |
| WHAT | ||
| 3. | Materials: Describe any physical or informational materials used in the intervention, including those provided to participants or used in intervention delivery or in training of intervention providers. Provide information on where the materials can be accessed (e.g. online appendix, URL). | pp. 11 and 15 |
| 4. | Procedures: Describe each of the procedures, activities, and/or processes used in the intervention, including any enabling or support activities. | p. 11 |
| WHO PROVIDED | ||
| 5. | For each category of intervention provider (e.g. psychologist, nursing assistant), describe their expertise, background, and any specific training given. | p. 11 |
| HOW | ||
| 6. | Describe the modes of delivery (e.g. face-to-face or by some other mechanism, such as Internet or telephone) of the intervention and whether it was provided individually or in a group. | p. 12 |
| WHERE | ||
| 7. | Describe the type(s) of location(s) where the intervention occurred, including any necessary infrastructure or relevant features. | pp. 11–12 |
| WHEN and HOW MUCH | ||
| 8. | Describe the number of times the intervention was delivered and over what period including the number of sessions, their schedule, and their duration, intensity, or dose. | pp. 24–25 |
| TAILORING | ||
| 9. | If the intervention was planned to be personalized, titrated, or adapted, then describe what, why, when, and how. | pp. 14 and 25 |
| MODIFICATIONS | ||
| 10. | If the intervention was modified during the course of the study, describe the changes (what, why, when, and how). | N/A |
| HOW WELL | ||
| 11. | Planned: If intervention adherence or fidelity was assessed, describe how and by whom, and if any strategies were used to maintain or improve fidelity, describe them. | p. 11 |
| 12. | Actual: If intervention adherence or fidelity was assessed, describe the extent to which the intervention was delivered as planned. | pp. 24–25 |
Table 2A.
Variables and measures.
| Objective | Outcomes | Measures/approaches | Methods of analysis |
|---|---|---|---|
| Feasibility of study methods | Eligibility |
|
|
| Recruitment |
|
|
|
| Retention |
|
|
|
| Adequacy of data and data collection |
|
|
|
| Feasibility of the intervention | Fidelity to the intervention |
|
|
| Engagement |
|
|
|
Providers’ and Managers’ feedback on:
|
|
|
|
Older adult participant feedback on:
|
|
|
|
| Demographic and stroke-related characteristics | Age, gender, education, household income, marital status, ethnicity, accommodation, living arrangement, employment, informal support, technology use and comfort, comorbid health conditions, stroke history, falls history, medications use |
|
|
| HRQoL |
|
|
|
| Depressive symptoms |
|
|
|
| Anxiety |
|
|
|
| Self-efficacy |
|
|
|
| Costs of use of health services, from a societal perspective | Change in costs for use of health and social services:
|
|
|
IP: interprofessional; SD: standard deviation; MCS: mental health component summary score; PCS: physical health component summary score; CI: confidence interval; HRQoL: health-related quality of life; CCAC: Community Care Access Centre; NPT: normalization process theory; MyST: My Stroke Team; T1: baseline; T2: 6 months after baseline measures.
Example questions based on NPT include the following: “What did you understand were your tasks and/or responsibilities in relation to the intervention?” and “What did you understand were your tasks and/or responsibilities in relation to using MyST?”; “How have you reorganized your routine and/or that of others on the team to contribute to and be involved in using the intervention?” and “How have you reorganized your routine and/or that of others on the team to contribute to and be involved in using MyST?”; and “What kinds of resources have been allocated to support you to deliver the intervention?” and “What kinds of resources have been allocated to support you to use MyST?”; and “Were these resources sufficient?”33
Table 3A.
Fidelity scale.
| Intervention components | Data source |
|---|---|
| Staffing and supervision | |
| IP team members (OT, PT, RN, SLP, SW) received standardized training | Attendance record |
| IP team members meet with investigators monthly | Attendance record Meeting minutes |
| Delivery of key components of intervention | |
| Monthly in-home visits by at least one member of the IP team for 6 months | MyST home visit record Home visit tracking record kept by care coordinator |
| Monthly IP case conferences over the study intervention period | MyST team meeting record |
| Activities during and between the home visits and telephone calls | |
| Use of standardized screening tools: | Standardized assessment forms in MyST |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Medication review and reconciliation | |
| Self-management education and support using strengths-based practice | |
| Caregiver engagement and support | |
| Use of evidence-based guidelines to prevent and manage stroke and other comorbidities | Number of links to evidence-based guidelines in MyST |
| Identification of patient-centered goals | Number of goals created and completed in MyST record |
| Single, patient-centered IP care plan | Individual goals assigned to IP team members in MyST |
| Referral to health and social service organizations | Number of links to community-based services in MyST Monthly research meeting minutes Focus group data |
IP: interprofessional; OT: occupational therapist; PT: physiotherapist; RN: registered nurse; SLP: speech language pathologist; SW: social worker; MyST; My Stroke Team; CES-D: Centre for Epidemiological Studies Depression.
Footnotes
Author contributions: All authors contributed to the design of this study. MMR, RV, RF, and AB contributed to the design and implementation of the intervention. MMR wrote the first draft of this manuscript, and all authors contributed to the discussion and editing. All authors read and approved the final manuscript. KF supervised the statistical analyses, and all authors had full access to the data.
Availability of data and materials: All data generated or analyzed during this study are included in this article
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval and consent to participate: Ethical approval for the study was obtained from the Hamilton Integrated Research Ethics Board (#14-612) and was renewed yearly, as required. All participants provided written informed consent.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the Canadian Institutes of Health Research Catalyst Grant: e-Health Innovations (http://www.cihr-irsc.gc.ca/; grant no. 134162) and the Max Bell Foundation (http://www.maxbell.org/). This study was also part of a program of research (Aging, Community and Health Research Unit) supported by the Canadian Institutes of Health Research Signature Initiative in Community-Based Primary Healthcare (http://www.cihr-irsc.gc.ca/e/43626.html) (funding reference no. TTF 128261) and the Ontario Ministry of Health and Long-Term Care Health System Research Fund Program (grant no. 06669). This research was also undertaken, in part, thanks to funding from Dr Markle-Reid’s Canada Research Chairs program.
ORCID iD: Maureen Markle-Reid  https://orcid.org/0000-0002-4019-7077
https://orcid.org/0000-0002-4019-7077
Jenny Ploeg  https://orcid.org/0000-0001-8168-8449
https://orcid.org/0000-0001-8168-8449
Jennifer Salerno  https://orcid.org/0000-0001-7282-5579
https://orcid.org/0000-0001-7282-5579
References
- 1. White JH, Alston MK, Marquez JL, et al. Community-dwelling stroke survivors: function is not the whole story with quality of life. Arch Phys Med Rehabil 2007; 88: 1140–1146. [DOI] [PubMed] [Google Scholar]
- 2. Gruneir A, Griffith LE, Fisher K, et al. Increasing comorbidity and health services utilization in older adults with prior stroke. Neurology 2016; 87: 2091–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cameron JI, Naglie G, Silver FL, et al. Stroke family caregivers’ support needs change across the care continuum: a qualitative study using the timing it right framework. Disabil Rehabil 2013; 35: 315–324. [DOI] [PubMed] [Google Scholar]
- 4. Cameron JI, Tsoi C, Marsella A. Optimizing stroke systems of care by enhancing transitions across care environments. Stroke 2008; 39: 2637–2643. [DOI] [PubMed] [Google Scholar]
- 5. Lainay C, Benzenine E, Durier J, et al. Hospitalization within the first year after stroke: the Dijon Stroke Registry. Stroke 2015; 46: 190–196. [DOI] [PubMed] [Google Scholar]
- 6. Johansen HL, Wielgosz AT, Nguyen K, et al. Incidence, comorbidity, case fatality and readmission of hospitalized stroke patients in Canada. Can J Cardiol 2006; 22: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. Br Med J 2001; 323: 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cott CA, Wiles R, Devitt R. Continuity, transition and participation: preparing clients for life in the community post-stroke. Disabil Rehabil 2007; 29: 1566–1574. [DOI] [PubMed] [Google Scholar]
- 9. Janzen S, Mirkowski M, McIntyre A, et al. Referral patterns of stroke rehabilitation inpatients to a model system of outpatient services in Ontario, Canada: a 7-year retrospective analysis. BMC Health Serv Res 2019; 19: 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allen J, Hutchinson AM, Brown R, et al. Quality care outcomes following transitional care interventions for older people from hospital to home: a systematic review. BMC Health Serv Res 2014; 14: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parke HL, Epiphaniou E, Pearce G, et al. Self-management support interventions for stroke survivors: a systematic meta-review. PLoS One 2015; 10: e0131448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coleman EA, Boult C. Improving the quality of transitional care for persons with complex care needs: position statement of the American Geriatrics Society Health Care Systems Committee. J Am Geriatr Soc 2003; 51: 556–557. [DOI] [PubMed] [Google Scholar]
- 13. Cameron JI, O’Connell C, Foley N, et al. Canadian stroke best practice recommendations: managing transitions of care following stroke, guidelines update 2016. Int J Stroke 2016; 11: 807–822. [DOI] [PubMed] [Google Scholar]
- 14. Threapleton DE, Chung RY, Wong SYS, et al. Integrated care for older populations and its implementation facilitators and barriers: a rapid scoping review. Int J Qual Health Care 2017; 29: 327–334. [DOI] [PubMed] [Google Scholar]
- 15. Canadian Council on Health Services Accreditation. CCHSA glossary. 6th ed Ottawa, Ontario, Canada: Canadian Council on Health Services Accreditation (CCHSA), 2007. [Google Scholar]
- 16. Oelke ND, Suter E, da Silva Lima MA, et al. Indicators and measurement tools for health system integration: a knowledge synthesis protocol. Syst Rev 2015; 4: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Young J, Murray J, Forster A. Review of longer-term problems after disabling stroke. Rev Clin Gerontol 2003; 13: 55–65. [Google Scholar]
- 18. Reeves MJ, Hughes AK, Woodward AT, et al. Improving transitions in acute stroke patients discharged to home: the Michigan Stroke Transitions Trial (MISTT) protocol. BMC Neurol 2017; 17: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hebert D, Lindsay MP, McIntyre A, et al. Canadian stroke best practice recommendations: stroke rehabilitation practice guidelines, update 2015. Int J Stroke 2016; 11: 459–484. [DOI] [PubMed] [Google Scholar]
- 20. Duncan PW, Bushnell CD, Rosamond WD, et al. The Comprehensive Post-Acute Stroke Services (COMPASS) study: design and methods for a cluster-randomized pragmatic trial. BMC Neurol 2017; 17: 133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McIntyre A, Richardson M, Janzen S, et al. The evolution of stroke rehabilitation randomized controlled trials. Int J Stroke 2014; 9: 789–792. [DOI] [PubMed] [Google Scholar]
- 22. Wang Y, Yang F, Shi H, et al. What type of transitional care effectively reduced mortality and improved ADL of stroke patients? A meta-analysis. Int J Environ Res Public Health 2017; 14: 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bettger JP, Alexander KP, Dolor RJ, et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med 2012; 157: 407–416. [DOI] [PubMed] [Google Scholar]
- 24. Puhr MI, Thompson HJ. The use of transitional care models in patients with stroke. J Neurosci Nurs 2015; 47: 223–234. [DOI] [PubMed] [Google Scholar]
- 25. Markle-Reid M, Orridge C, Weir R, et al. Interprofessional stroke rehabilitation for stroke survivors using home care. Can J Neurol Sci 2011; 38: 317–334. [DOI] [PubMed] [Google Scholar]
- 26. Nelson MLA, McKellar KA, Yi J, et al. Stroke rehabilitation evidence and comorbidity: a systematic scoping review of randomized controlled trials. Top Stroke Rehabil 2017; 24: 374–380. [DOI] [PubMed] [Google Scholar]
- 27. Nelson MLA, Kelloway L, Dawson D, et al. Stroke rehabilitation and patients with multimorbidity: a scoping review protocol. J Comorb 2015; 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bodenheimer T, Sinsky C. From Triple to Quadruple Aim: care of the patient requires care of the provider. Ann Fam Med 2014; 12: 573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada and Social Sciences and Humanities Research Council of Canada Tri-council policy statement: Ethical conduct for research involving humans; 2014. Government of Canada. [Google Scholar]
- 30. Loudon K, Treweek S, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015; 350: h2147. [DOI] [PubMed] [Google Scholar]
- 31. Sandelowski M. Focus on research methods: whatever happened to qualitative description? Res Nurs Health 2000; 23: 334–340. [DOI] [PubMed] [Google Scholar]
- 32. Sandelowski M. What’s in a name? Qualitative description revisited. Res Nurs Health 2010; 33: 77–84. [DOI] [PubMed] [Google Scholar]
- 33. May CR, Cummings A, Girling M, et al. Using Normalization Process Theory in feasibility studies and process evaluations of complex healthcare interventions: a systematic review. Implement Sci 2018; 13: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brooks H, Sanders C, Lovell K, et al. Re-inventing care planning in mental health: stakeholder accounts of the imagined implementation of a user/carer involved intervention. BMC Health Serv Res 2015; 15: 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Statistics Canada. Hamilton, City (CSD) - Ontario, https://www12.statcan.gc.ca/census-recensement/2016/as-sa/fogs-spg/Facts-CSD-Eng.cfm?TOPIC=1&&GK=CSD&GC=3525005 (2017, accessed 9 October 2018).
- 36. Markle-Reid M, Ploeg J, Valaitis R, et al. Protocol for a program of research from the Aging, Community and Health Research Unit: promoting optimal aging at home for older adults with multimorbidity. J Comorb 2018; 8: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aging, Community and Health Research Unit. About ACHRU, https://achru.mcmaster.ca/ (2018, accessed 9 October 2018).
- 38. Pfeiffer E. A Short Portable Mental Status Questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 1975; 23: 433–441. [DOI] [PubMed] [Google Scholar]
- 39. Markle-Reid M, Valaitis R, Bartholomew A, et al. Feasibility and preliminary effects of an integrated hospital-to-home transitional care intervention for older adults with stroke and multimorbidity: a study protocol. J Comorb 2019; 9: 2235042X19828241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Network. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide, https://www.equator-network.org/reporting-guidelines/tidier/(2019). Accessed November 30, 2019. [DOI] [PubMed]
- 41. Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med 2003; 26: 1–7. [DOI] [PubMed] [Google Scholar]
- 42. Smith M, Wallace E, O’Dowd T, et al. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev 2016; 3: CD006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kennedy A, Rogers A, Bower P. Support for self care for patients with chronic disease. BMJ 2007; 335: 968–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carter N, Valaitis RK, Lam A, et al. Navigation delivery models and roles of navigators in primary care: a scoping literature review. BMC Health Serv Res 2018; 18: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anderson JE, Larke SC. The Sooke Navigator project: using community resources and research to improve local service for mental health and addictions. Ment Health Fam Med 2009; 6: 21–28. [PMC free article] [PubMed] [Google Scholar]
- 46. Lorig R, Sobel S, Ritter L, et al. Effects of a self-management program for patients with chronic disease. Eff Clin Pract 2001; 4: 256–262. [PubMed] [Google Scholar]
- 47. Saleebey D. The strengths perspective in social work practice: extensions and cautions. Soc Work 1996; 41: 296–305. [PubMed] [Google Scholar]
- 48. Rapp C, Saleebey D, Sullivan PW. The future of strengths-based social work practice. 4th ed Boston, USA: Pearson Education, 2008. [Google Scholar]
- 49. McDonald S, Edwards HM, Zhao T. Exploring think-alouds in usability testing: an international survey. IEEE Trans Prof Commun 2012; 55: 2–19. [Google Scholar]
- 50. Sidani S, Braden CJ. Design, evaluation, and translation of nursing interventions. Hoboken: John Wiley & Sons, 2011. [Google Scholar]
- 51. Carroll C, Patterson M, Wood S, et al. A conceptual framework for implementation fidelity. Implement Sci 2007; 2: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marcellus L. Are we missing anything? Pursuing research on attrition. Can J Nurs Res 2004; 36: 82–98. [PubMed] [Google Scholar]
- 53. Ware JE, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–233. [DOI] [PubMed] [Google Scholar]
- 54. Ware JE, Kosinski M, Keller SD. SF-12: How to score the SF-12 physical and mental health summary scales. Lincoln, RI: Quality Metric Inc, 1998. [Google Scholar]
- 55. Maruish ME. Users manual for the SF-12v2. 3rd ed Lincoln, RI: Quality Metric Inc, 2012. [Google Scholar]
- 56. Gewandter JS, McDermott MP, Kitt RA, et al. Interpretation of CIs in clinical trials with non-significant results: systematic review and recommendations. BMJ Open 2017; 7: e017288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Browne G, Gafni A, Roberts J. Approach to the measurement of resource use and costs. Working paper s06-01 Hamilton, Canada: McMaster University, System-Linked Research Unit on Health and Social Service Utilization, 2006. [Google Scholar]
- 58. Ministry of Health and Long-term Care. Health data branch web portal, https://hsimi.on.ca/hdbportal/ (2015, accessed 1 March 2015).
- 59. Browne G, Roberts J, Gafni A, et al. Economic evaluations of community-based care: lessons from twelve studies in Ontario. J Eval Clin Pract 1999; 5: 367–385. [DOI] [PubMed] [Google Scholar]
- 60. Browne G, Roberts J, Byrne C, et al. The costs and effects of addressing the needs of vulnerable populations: results of 10 years of research. Can J Nurs Res 2001; 33: 65–76. [PubMed] [Google Scholar]
- 61. Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016; 355: i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health 2008; 31: 180–191. [DOI] [PubMed] [Google Scholar]
- 63. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs 2008; 62: 107–115. [DOI] [PubMed] [Google Scholar]
- 64. Hopman WM, Verner J. Quality of life during and after inpatient stroke rehabilitation. Stroke 2003; 34: 801–805. [DOI] [PubMed] [Google Scholar]
- 65. Hopman WM, Towheed T, Anastassiades T, et al. Canadian normative data for the SF-36 health survey. CMAJ 2000; 163: 265–271. [PMC free article] [PubMed] [Google Scholar]
- 66. Horgan NF, O’Regan M, Cunningham CJ, et al. Recovery after stroke: a 1-year profile. Disabil Rehabil 2009; 31: 831–839. [DOI] [PubMed] [Google Scholar]
- 67. Coulter A, Entwistle VA, Eccles A, et al. Personalised care planning for adults with chronic or long-term health conditions. Cochrane Database Syst Rev 2015; 3: CD010523 doi: 10.1002/14651858.CD010523.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Allen L, Richardson M, McIntyre A, et al. Community stroke rehabilitation teams: providing home-based stroke rehabilitation in Ontario, Canada. Can J Neurol Sci 2014; 41: 697–703. [DOI] [PubMed] [Google Scholar]
- 69. Kernick D, Chew-Graham CA, O’Flynn N. Clinical assessment and management of multimorbidity: NICE guideline. Br J Gen Pract 2017; 67: 235–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. . Health Quality Ontario. Quality Standards. Transitions from Hospital to Home: Care for People of All Ages. April 2019, http://www.hqontario.ca/Portals/0/documents/qi/health-links/bp-improve-package-transitions-en.pdf. Accessed September 1, 2019.
- 71. Clarke LH, Bennett EV. Constructing the moral body: self-care among older adults with multiple chronic conditions. Health 2013; 17: 211–228. [DOI] [PubMed] [Google Scholar]
- 72. Mayo NE, Wood-Dauphinee S, Côté R, et al. Activity, participation, and quality of life 6 months poststroke. Arch Phys Med Rehabil 2002; 83: 1035–1042. [DOI] [PubMed] [Google Scholar]
- 73. Robinson-Smith G, Johnston MV, Allen J. Self-care self-efficacy, quality of life, and depression after stroke. Arch Phys Med Rehabil 2000; 81: 460–464. [DOI] [PubMed] [Google Scholar]
- 74. Fischer U, Arnold M, Nedeltchev K, et al. Impact of comorbidity on ischemic stroke outcome. Acta Neurol Scand 2006; 113: 108–113. [DOI] [PubMed] [Google Scholar]
- 75. Karatepe AG, Gunaydin R, Kaya T, et al. Comorbidity in patients after stroke: impact on functional outcome. J Rehabil Med 2008; 40: 831–835. [DOI] [PubMed] [Google Scholar]
- 76. Liu M, Tsuji T, Tsujiuchi K, et al. Comorbidities in stroke patients as assessed with a newly developed comorbidity scale. Am J Phys Med Rehabil 1999; 78: 416–424. [DOI] [PubMed] [Google Scholar]
- 77. Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psychiatry 2016; 173: 221–231. [DOI] [PubMed] [Google Scholar]
- 78. Wright F, Wu S, Chun HY, et al. Factors associated with poststroke anxiety: a systematic review and meta-analysis. Stroke Res Treat 2017; 2017: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Busetto L, Luijkx KG, Elissen AMJ, et al. Intervention types and outcomes of intagrated care for diabetes mellitus type 2: a systematic review. J Eval Clin Pract 2016; 22: 299–310. [DOI] [PubMed] [Google Scholar]
- 80. Hamilton Health Sciences and Aging Community Health Research Unit Stroke safety checklist, 2017. [Google Scholar]
- 81. Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003; 41: 1284–1294. [DOI] [PubMed] [Google Scholar]
- 82. Björgvinsson T, Kertz SJ, Bigda-Peyton JS, et al. Psychometric properties of the CES-D-10 in a psychiatric sample. Assessment 2013; 20: 429–436. [DOI] [PubMed] [Google Scholar]
- 83. Nasreddine Z. MoCA Montreal - Cognitive Assessment, https://www.mocatest.org/wp-content/uploads/2015/03/Montreal-cognitive-assessment-Basic-English-FINAL-VERSION-4-June-2014.pdf (2018, accessed 27 September 2018).
- 84. Confusion Assessment Method. © 1988, Hospital Elder Life Program. All rights reserved. Adapted from: Inouye SK et al. Ann Intern Med 1990; 113: 941–948. [DOI] [PubMed] [Google Scholar]
- 85. American Geriatrics Society and British Geriatrics Society. AGS/BGS clinical practice guideline: Prevention of falls in older persons. New York: American Geriatrics Society, 2010. [Google Scholar]
- 86. Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc 1986; 34: 119–126. [DOI] [PubMed] [Google Scholar]
- 87. Wood-Dauphinee SL, Opzoomer MA, Williams JI, et al. Assessment of global function: the Reintegration to Normal Living Index. Arch Phys Med Rehabil 1988; 69: 583–590. [PubMed] [Google Scholar]
- 88. Thornton M, Travis SS. Analysis of the reliability of the Modified Caregiver Strain Index. J Gerontol B Psychol Sci Soc Sci 2003; 58: S127–132. [DOI] [PubMed] [Google Scholar]