Abstract
Hemophilia A (HemA) patients are currently treated with costly and inconvenient replacement therapy of short-lived factor VIII (FVIII) protein. Development of lipid nanoparticle (LNP)-encapsulated mRNA encoding FVIII can change this paradigm. LNP technology constitutes a biocompatible and scalable system to efficiently package and deliver mRNA to the target site. Mice intravenously infused with the luciferase mRNA LNPs showed luminescence signals predominantly in the liver 4 h after injection. Repeated injections of LNPs did not induce elevation of liver transaminases. We next injected LNPs carrying mRNAs encoding different variants of human FVIII (F8 LNPs) into HemA mice. A single injection of B domain-deleted F8 LNPs using different dosing regimens achieved a wide range of therapeutic activities rapidly, which can be beneficial for various usages in hemophilia treatment. The expression slowly declined yet remained above therapeutic levels up to 5–7 days post-injection. Furthermore, routine repeated injections of F8 LNPs in immunodeficient mice produced consistent expression of FVIII over time. In conclusion, F8 LNP treatment produced rapid and prolonged duration of FVIII expression that could be applied to prophylactic treatment and potentially various other treatment options. Our study showed potential for a safe and effective platform of new mRNA therapies for HemA.
Keywords: factor VIII, hemophilia A, lipid nanoparticle, nonviral, gene delivery, gene transfer, liver, mRNA, RNA therapy
Graphical Abstract

Messenger RNA (mRNA) encapsulated in lipid nanoparticles (LNPs) can be efficiently delivered into the liver. LNPs carrying FVIII mRNA (F8 LNPs) produced rapid and prolonged duration of FVIII expression in hemophilia A mice. This safe and effective platform of new mRNA therapies could be used for prophylactic treatment and potentially various other treatment options for hemophilia A patients.
Introduction
Coagulation factor VIII (FVIII) is an X-linked gene that encodes a large glycoprotein and participates in the intrinsic pathway of the coagulation cascade.1 FVIII assists formation of blood clots to prevent further blood loss after injury. The bleeding disorder resulting from insufficiency of FVIII protein level or deficiency of FVIII function is called hemophilia A (HemA). The occurrence of HemA is generally 1 in 5,000–10,000 males, and the mortality rates of patients with severe HemA increase when undergoing major surgery.2,3 Therapy for severe HemA patients requires not only on-demand treatment to rescue patients from excessive bleeding following trauma or surgery, but also prophylactic treatment to prevent bleeding without visible signs, including joints, soft tissues, or muscle hemorrhages. Frequent infusions (three to four times per week) of costly FVIII to prevent spontaneous bleeding episodes in these patients is required due to the short half-life (8–12 h) of FVIII protein.
An alternative for protein replacement therapy is to utilize gene therapy to introduce a functional FVIII gene into patients for longer-term FVIII expression, thus reducing the treatment frequency while also reducing risk of spontaneous bleeding events. However, the method of delivery needs careful consideration. For example, using viruses carrying genetic material increases the risk of oncogenic mutagenesis due to viral integration.4, 5, 6 In addition, FVIII transgene expression needs to be achieved and maintained at therapeutic levels, and sensitive genotoxicity detection assays remain yet to be developed for clinical gene therapy. Furthermore, immune responses to viral vectors and transgenes precluded its application to a significant portion of HemA patients. To avoid these problems encountered by DNA delivery using viral vectors, messenger RNA (mRNA)-based genetic materials can be used to rescue insufficient FVIII expression in HemA patients.
The advantages of mRNA therapy include no risk of oncogenic mutagenesis and rapid protein expression, as mRNAs do not translocate to the nucleus and are instead processed via translation in the cytoplasm. Recently, it was shown that functional protein was efficiently produced by using a 5-methoxy-U-modified codon-optimized mRNA successfully delivered into specific sites.7 For example, intradermal injections of modified mRNA encoding vascular endothelial growth factor A (VEGF-A) led to local functional VEGF-A protein expression and transient skin blood flow enhancement in men with type 2 diabetes mellitus (T2DM), indicating the therapeutic potential for regenerative angiogenesis.8 Furthermore, recent development of lipid nanoparticles (LNPs) enabled efficient packaging of mRNA and delivery to liver to produce a high level of protein expression.9,10 For example, delivery of LNP-encapsulated mRNA encoding human methylmalonyl-CoA mutase (MMUT) reduced circulating metabolites and dramatically improved survival and weight gain in a mouse model with isolated methylmalonic acidemia (MMA) syndrome.7 In addition, LNPs have been used to deliver factor IX mRNA into hemophilia B mice with good results.11,12
In this study, we used a non-viral carrier made of biodegradable lipids, LNPs, to encapsulate FVIII mRNAs (F8 LNPs), which were systemically delivered via intravenous injection to HemA mice. We showed that administration of F8 LNPs can rescue biological function and continually produce therapeutic levels of FVIII protein for 5 days. We also showed rapid and consistent expression of FVIII protein after repeated injections of F8 LNPs, indicating the translational potential of mRNA-based therapy for routine prophylactic treatment.
Results
LNPs Are Efficiently Delivered to the Liver
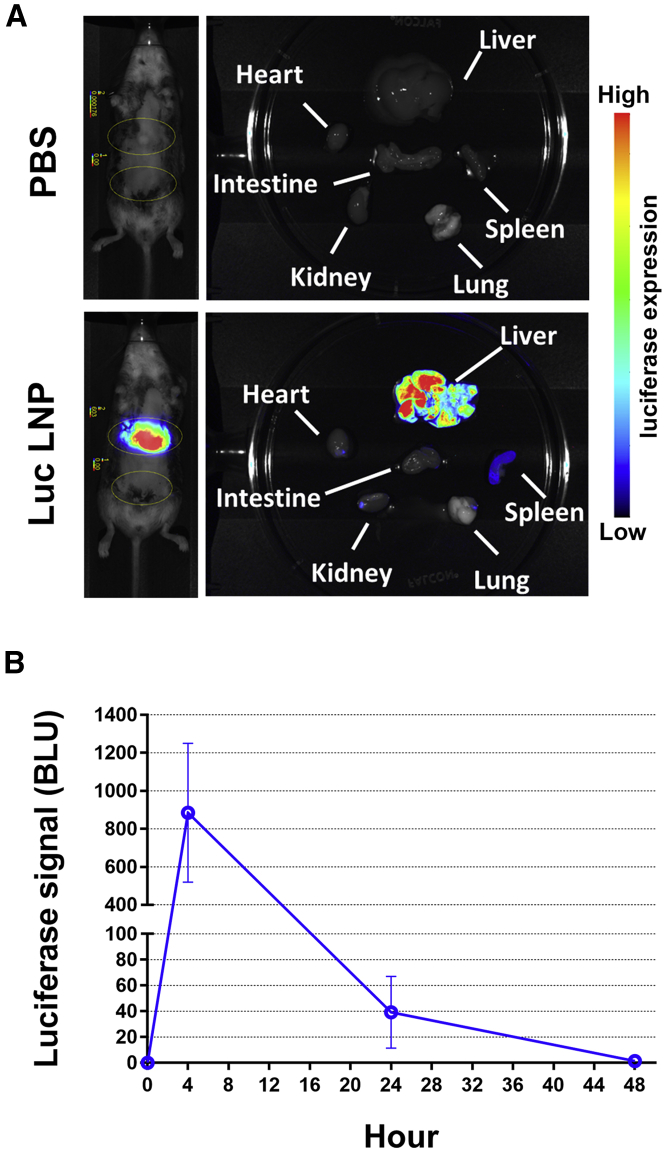
To test the delivery function of LNPs and therapeutic effect of mRNA-based treatment, we first examined the biodistribution and protein expression of luciferase mRNA carrying LNPs (Luc LNPs) in vivo. After injection of 2 mg/kg Luc LNPs into mice, luciferase signals were traced using a small animal live imaging method. Mice injected with Luc LNPs displayed high bioluminescence signals compared to control mice that were injected with PBS only. Images also showed that the bioluminescence signal was mainly detected in the liver, and weak or no signal was observed in spleen and other organs, suggesting that LNPs delivered mRNA to the liver efficiently (Figure 1A). The luciferase signal could be detected within 4 h, indicating highly efficient mRNA translation after Luc LNP administration (Figure 1B). Immunostaining of luciferase protein in liver of treated mice was also performed using endothelial markers and the nuclear markers. The results clearly demonstrated that Luc LNPs predominantly targeted hepatocyte cells rather than endothelial cells (Figure S1).
Figure 1.
Luciferase mRNA Lipid Nanoparticles (Luc LNPs) Deliver mRNA Efficiently to Liver
(A and B) Localization and levels of luciferase expression in mice. 2 mg/kg Luc LNPs was intravenously administered to wild-type C57BL/6 mice (N = 6). Luc LNP-treated mice and control mice treated with PBS were anesthetized and infused with 10 μL/g luceferin at different time points. Luciferase expression was examined using a bioluminescent in vivo imaging system (IVIS). (A) Localization of luciferase expression in mice at 4 h post-treatment. Left panels: In vivo imaging was performed to examine the luciferase expression in whole animals. Right panels: The mice were sacrificed, and major organs were harvested for examination of luciferase expression. Luciferase expression in different organs is shown in color scales as indicated in the side bar, representing low to high levels of expression. (B) Luciferase expression levels in the treated mouse livers are presented as average luciferase signals ± standard deviations in bioluminescence light units (BLU).
Biocompatibility of mRNA LNPs
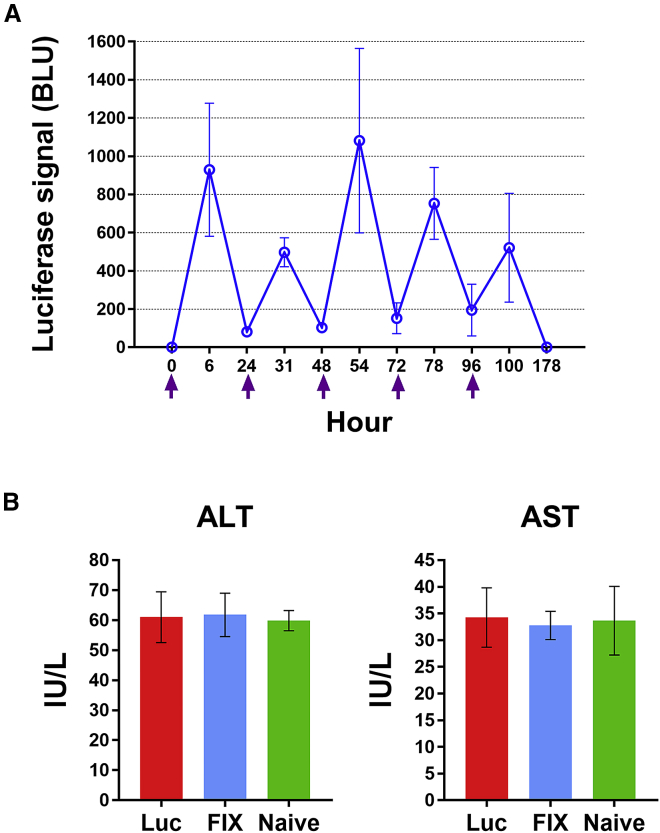
Routine prophylaxis is required to prevent frequent bleeding episodes in severe HemA patients. Examination of liver toxicity after multiple injections of therapeutic mRNA LNPs is necessary prior to clinical use. To evaluate biocompatibility of mRNA LNPs, mice were repeatedly injected once daily with 2 mg/kg Luc LNPs for 5 consecutive days. All mice showed consistent expression of luciferase (Figure 2A). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were monitored to assess the potential for liver inflammation after Luc LNP injection. Mice injected with nonfunctional factor IX (FIX) mRNA LNPs or PBS served as nonspecific and naive controls, respectively. No significant difference was observed in both ALT and AST values among all three groups of mice (Figure 2B). These results suggest that repeated infusions of mRNA LNPs did not induce any detectable liver injury, indicating that mRNA LNPs are suitable for therapeutic treatment.
Figure 2.
Biocompatibility of the LNPs Carrying mRNAs for In Vivo Delivery
2 mg/kg Luc LNPs was intravenously administrated to wild-type C57BL/6 mice (N = 4) for 5 consecutive days. (A) Luciferase expression in C57BL/6 mice following repeated injections of Luc LNPs examined by in vivo imaging. Arrows indicate injection time of Luc LNPs. Luciferase expression signals (BLU) were examined 4 h after each injection using an in vivo imaging system. (B) Liver enzyme tests following repeated injections of Luc LNPs in mice. ALT and AST levels in plasma were measured in treated mice. Mice injected with nonfunctional factor IX (FIX) mRNA LNPs (N = 4) or PBS (N = 2) served as the mRNA control and naive control, respectively. One-way ANOVA revealed no significant effect of LNPs on levels of ALT or AST (ALT, p = 0.9558; AST, p = 0.9040) between all groups. Data are presented as average values ± standard deviations.
To examine whether human F8 LNPs are suitable for treatment of HemA patients, we used HemA mice as the chosen hemophilic animal disease model. HemA mice were injected with 2 mg/kg F8 LNPs encapsulating each of several FVIII mRNA variants or control Luc LNPs (Figure 3A). The FVIII activities in treated mouse plasma were measured by an activated partial thromboplastin time (aPTT) clotting assay. HemA mice injected with F8 LNPs encapsulating full-length human FVIII mRNA (F8-FL LNPs) exhibited elevated clotting activity 6 h after injection and reached a peak activity level of 5.5% after 24 h (Figure 3B). As expected, mice treated with control Luc LNPs produced no FVIII activity.
Figure 3.
Injection of FVIII Variant mRNA LNPs (F8 LNPs) Restored Clotting Activity in HemA Mice
(A) Different furin cleavage site-mutated FVIII mRNAs were encapsulated into LNPs. Amino acids marked in red denote the residues contained in the furin cleavage recognition site. (B) FVIII activity of mice injected with F8-FL LNPs, F8-FL-F309S LNPs, and F8-N6Δ2-F309S LNPs were measured by an aPTT assay at different time points, respectively (N = 3/group). (C) FVIII activity of HemA mice injected with 2 mg/kg F8-N6-F309S, F8-N6Δ2-F309S, and F8-N6Δ4-F309S LNPs (N = 3–6/group), respectively. FVIII activity was measured in different time points by an aPTT assay. Data are presented as average FVIII activities ± standard deviations. (D and E) Western blot to detect plasma FVIII 1 day after LNP treatment in mice injected with 2 mg/kg F8-FL-F309S LNPs (FL) (D), and in mice injected with 2 mg/kg F8-N6-F309S (N6), F8-N6Δ2-F309S (N6Δ2), and F8-N6Δ4-F309S (N6Δ4) LNPs, respectively (E). Human normal pooled plasma (NPP) served as a positive control, and plasma from untreated HemA mice (HA) served as a negative control. Arrowheads in (E) indicate the single chain FVIII-N6 (N6-SC) and heavy chain of FVIII-N6 (N6-HC).
Nascent translated FVIII proteins will translocate to the lumen of the endoplasmic reticulum (ER) and undergo post-translational modification before secretion from cells.13,14 However, retention of FVIII protein in the ER prevents Golgi transportation and FVIII secretion, leading to delayed elevation of plasma FVIII levels.2 To overcome delayed transportation of FVIII, a F309S mutation was introduced in the A1 domain of FVIII mRNA to decrease the retention time in the ER and increase secretion efficiency.15 Incorporating the F309S mutation showed a similar expression pattern, but higher clotting activity in HemA mice injected with F8 LNPs encapsulating F309S-mutated full-length FVIII mRNA (F8-FL-F309S LNPs). The mice administered F8-FL-F309S LNPs showed 20.5% FVIII clotting activity, which, however, dropped below 10% rapidly within 2 days (Figure 3B).
Owing to the biochemical characteristics of FVIII, full-length human FVIII mRNA is inefficiently translated compared to other comparably sized proteins. Biogenetic modification of FVIII protein is required to elevate the yield of FVIII protein.16 It has been reported that B domain-deleted human FVIII containing six N-linked glycosylation sites (BDD-hFVIII/N6) showed higher expression levels of FVIII compared to full-length FVIII in vitro and in vivo.17,18 To this end, BDD-FVIII/N6 mRNA was designed and encapsulated into LNPs (Figure 3A). In addition, previous studies suggest that the carboxyl terminus of the B domain contains a furin recognition motif (R-H-Q-R), which is the predominant cleavage site to separate FVIII into its heavy chain and light chain. Metal ion-dependent heterodimer FVIII protein is composed of these chains and is the major secreted form after post-translational modification.19 Compared to the heterodimer form of secreted human BDD-FVIII/N6, the single-chain form of BDD-canine FVIII exhibits higher activity and is more stable.20 Mutation of the furin cleavage site is reported to contribute to the enhanced secretion level that is associated with increased circulating FVIII.21 To further elevate the FVIII expression in HemA mice, we introduced a deletion in the furin recognition motif to partially disrupt the furin recognition motif (F8-N6Δ2-F309S) or fully delete the furin recognition motif (F8-N6Δ4-F309S) in BDD-FVIII/N6 mRNA. FVIII activity in HemA mice injected with either BDD-FVIII/N6 mRNA containing a wild-type (WT) (F8-N6-F309S) or mutated furin site (F8-N6Δ2-F309S or F8-N6Δ4-F309S) displayed significantly higher activity than did F8-FL-F309S LNPs. Among the F8 LNP variants, F8-N6Δ2-F309S LNPs produced a higher average FVIII activity than that of F8-N6-F309S and F8-N6Δ4-F309S variants (Figure 3C). After injection of 2 mg/kg LNPs carrying these three different BDD-FVIII variants, FVIII expression achieved peak expression levels higher than 1,000% FVIII activity and slowly declined during a 7-day period. In addition, we examined the kinetics of FVIII expression at multiple time points during the initial 24-h period after F8 LNP injection. The data showed that low levels of FVIII can already be detected at half an hour post-injection, and the levels continued to increase to peak levels at 24 h post-injection (Figure S2).
Immunoblotting was performed to verify FVIII proteins synthesized by F8 LNPs. The molecular size of FVIII detected from mice administrated with F8-FL-F309S LNPs was comparable to that in normal human plasma (Figure 3D). Immunoblotting analysis also showed diversity of molecular weight in furin mutant F8 LNP variants. FVIII proteins from F8-N6Δ2-F309S and F8-N6Δ4-F309S LNPs displayed high-molecular-weight single chains of BDD-FVIII/N6, whereas F8-N6-F309S LNPs without a furin site mutation showed predominantly the heavy chain form of BDD-FVIII/N6 and absence of the single chain form (Figure 3E).
Repeated Injections of Human F8 LNPs Induced FVIII Inhibitor Formation
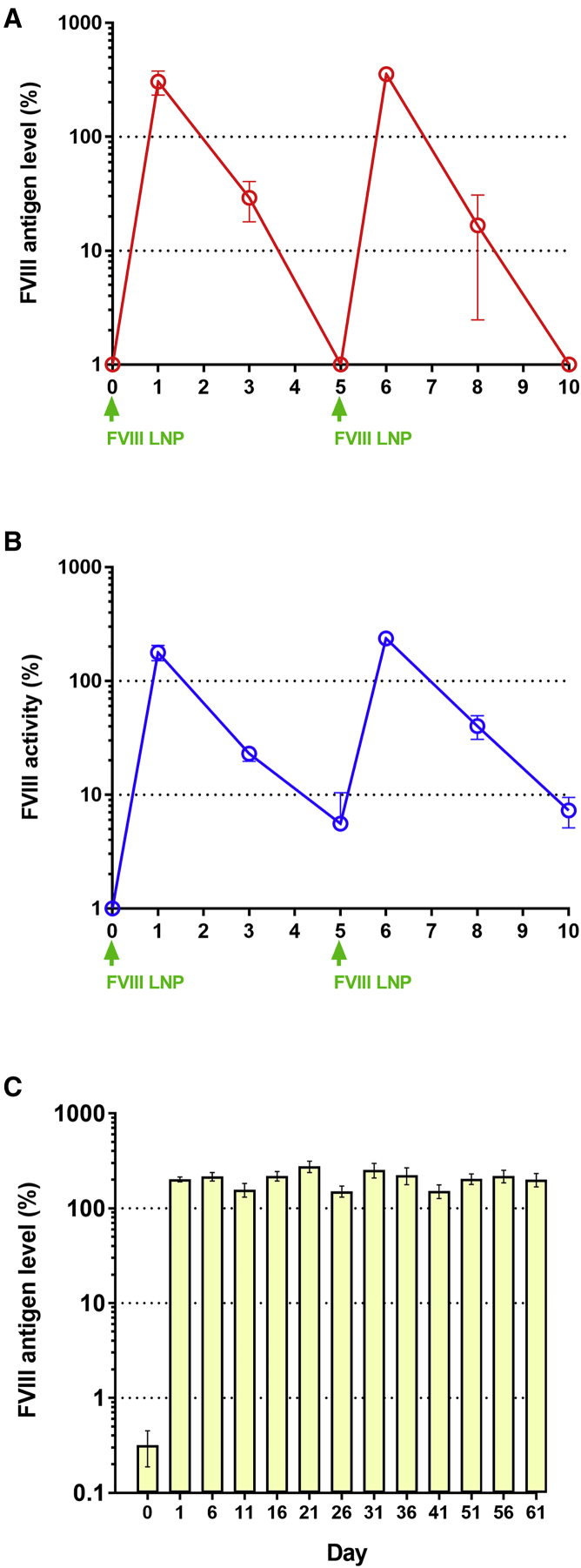
Since F8-N6Δ2-F309S LNPs showed higher activity than other F8 LNP variants on average, we used F8-N6Δ2-F309S LNPs in the following experiments. Mice injected with 2 mg/kg F8-N6Δ2-F309S LNPs can restore FVIII activity and maintain activity more than 7 days above a trough level of 1%; however, the association between elevated FVIII activity and increased thrombotic risk still needed to be evaluated. High plasma FVIII activity may result in a dose-dependent risk of venous thromboembolism.22 To evade thrombotic risk, different dosages of F8-N6Δ2-F309S were examined. 0.1, 0.2, and 0.4 mg/kg F8-N6Δ2-F309S LNPs were injected into HemA mice and antigen levels and FVIII activity were measured at days 1, 3, 5, and 7. The expression profile was similar in different mouse groups injected with different dosages of F8-N6Δ2-F309S LNPs. All mouse groups showed that the highest FVIII expression levels and activities were detected at day 1 and reached trough levels at day 5 or day 7 (Figures 4A and 4B). In order to verify whether blood clotting can be corrected in 0.2 mg/kg F8-N6Δ2-F309S LNP-treated mice at day 7, we examined the coagulation activity using whole blood isolated from treated mice by a rotational thromboelastometry (ROTEM) assay (Figure 4C). We found that at 7 days after F8 LNP delivery, the treated mice still had significant correction of coagulation parameters, including clotting time (CT) and clot formation time (CFT), compared with HemA mice (Figure 4D). The average half-life of FVIII:C expressed from F8-N6Δ2-F309S mRNA can reach about 22 h. In comparison, the FVIII levels from 0.1 mg/kg F8-N6Δ2-F309S LNP-treated mice were too low (undetectable) at day 5 for prophylactic treatment, whereas those from 0.4 mg/kg F8-N6Δ2-F309S LNP-treated mice were very high (867%) at day 1 with potentially increased thrombotic risk. Compared to treatment dosages of 0.1 and 0.4 mg/kg, mice treated with 0.2 mg/kg F8-N6Δ2-F309S LNPs showed optimal performance that exhibited ∼200% FVIII activity at day 1 and above 5% activity at day 7. Such levels are suitable for prophylactic treatment lasting 5–7 days.
Figure 4.
Dose Titration of F8 LNPs
To titrate the F8 LNP dosage best suited for treatment, HemA mice were injected with 0.1, 0.2, and 0.4 mg/kg F8-N6Δ2-F309S LNPs (N = 3/group). (A and B) FVIII antigen levels (A) and activities (B) were measured using ELISA and an aPTT assay, respectively. Coagulation activities of HemA mice were measured using a ROTEM assay 7 days after injection of 0.2 mg/kg F8-N6Δ2-F309S LNPs. Wild-type (WT) mice and HemA mice (HA) treated with Luc LNPs served as controls (N = 4–6/group). (C and D) Representative ROTEM graphs (C) and clotting times and clot formation time (D) of each group are shown. Data are presented as average experimental values ± standard deviation. Statistical comparisons were performed using one-way ANOVA. ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
To reduce the frequency of bleeding episodes in HemA patients, injection of FVIII protein ideally should be performed two to three times per week. To develop protocols for treating HemA patients, we monitored the FVIII expression performance in HemA mice after administration with 0.2 mg/kg F8-N6-F309S-Δ2 LNPs two to four times within 2 weeks. The results suggested that almost all treated mice can sustain FVIII activity higher than 1% within 2 weeks (Figure 5). However, FVIII expression levels decreased when the frequency of injections increased. Mice treated with two F8-N6Δ2-F309S LNP administrations within 2 weeks showed no difference in FVIII expression levels when compared between their first and second injections (Figures 5A and 5B), but mice that received three or four F8-N6Δ2-F309S LNP injections within 2 weeks displayed dramatic reduction of FVIII expression level (Figures 5C–5F). The plasma FVIII level decreased notably after the third injection and could not be detected after a fourth injection at day 11. The decreases of FVIII expression levels were found to be associated with the formation of inhibitors against FVIII, which were detected by a Bethesda assay at 2 weeks following multiple injections of F8-N6Δ2-F309S LNPs (Figure 5G). This anti-FVIII immune response is anticipated since this strain of HemA mice is prone to develop FVIII inhibitors following repeated FVIII treatment.23
Figure 5.
Repeated Injections of F8 LNPs into HemA Mice
(A–F) HemA mice were repeatedly injected with 0.2 mg/kg F8-N6Δ2-F309S LNPs (A and B) two times, (C and D) three times, and (E and F) four times within 2 weeks, respectively. FVIII antigen levels (A, C, and E) and FVIII activities (B, D, and F) were measured using ELISA and an aPTT assay. Arrows indicate the days of F8 LNP injections (N = 5/group). Each symbol represents data obtained from an individual mouse. (G) Anti-FVIII antibodies of F8 LNP repeatedly injected mice were measured by a Bethesda assay (N = 5/group). Data are presented as average experimental values ± standard deviation.
Routine Injections of Human F8 LNPs Induced FVIII Inhibitor Formation
Severe HemA patients have a high risk of spontaneous bleeding into muscles, soft tissue, skull, or joints, leading to serious complications. To improve the quality of life, routine injection of FVIII is required to prevent asymptomatic bleeding. According to clinical data, most HemA patients (∼70%) do not develop FVIII inhibitors.24, 25, 26 To evade human FVIII inhibitor development in the HemA mouse model, we evaluated the potential of F8-N6Δ2-F309S LNPs for prophylactic treatment using immunodeficient NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice. First, we used NSG HemA mice to examine the FVIII antigen and activity level after F8-N6Δ2-F309S LNP injections. Two injection cycles were carried out, and the results showed similar expression patterns of FVIII as those observed in immunocompetent HemA mice (Figures 6A and 6B). Then, NSG mice were routinely injected with F8-N6Δ2-F309S LNPs every 5 days for 2 months and the FVIII protein was detected by a human FVIII-specific ELISA. The treated mice consistently expressed FVIII with peak levels around 150%–220% after each injection, suggesting that systemic delivery of F8-N6Δ2-F309S LNPs can provide a potentially superior routine prophylaxis protocol for severe HemA patients (Figure 6C).
Figure 6.
NSG Mice Routinely Injected with F8 LNPs Can Consistently Express FVIII Protein
(A and B) NSG HemA mice were injected with 0.4 mg/kg F8-N6Δ2-F309S LNPs. FVIII antigen (A) and activity (B) levels were detected 1 day, 3 days, and 5 days after injection by ELISA and an aPTT assay, respectively. Two repeated injection cycles were examined (N = 3). (C) NSG mice were injected with 0.4 mg/kg F8-N6Δ2-F309S LNPs every 5 days for 2 months. FVIII antigen level was detected 1 day after injection by a human FVIII-specific ELISA (N = 7). The day 0 samples were collected before injection. Arrows indicate injection time of F8 LNPs. Data are presented as average values ± standard deviations.
Discussion
Clinically, patients with hemophilia receive on-demand therapy with coagulation factor concentrates to prevent excessive bleeding in trauma, injury, and surgery. Patients with severe HemA require routine prophylactic infusion of FVIII to prevent spontaneous bleeding into joints or muscle. Conventional FVIII concentrates have a 12-h half-life on average. Severe HemA patients require a prophylaxis treatment regimen with three to four times per week repeated injections.27 Recent recombinant FVIII products modified with polyethylene glycol, Fc fragment, or albumin could extend the half-life of FVIII, thereby reducing the frequency of prophylaxis treatment to two to three times per week injections.28,29 However, individual dosing of half-life-extended recombinant FVIII differs between HemA patients to maintain levels higher than 1%. Given the process involved to produce safe and effective FVIII concentrates, current prophylactic treatment is a heavy financial burden for patients.30
Compared to protein replacement therapy, gene therapy can offer a change in the paradigm of hemophilia care. Gene transfer using adeno-associated virus (AAV) viral vectors carrying BDD-FVIII could achieve long-term correction of the phenotype. Clinical data suggest that HemA patients receiving gene therapy could reduce bleeding events compared to routine FVIII prophylaxis.31,32 However, FVIII expression in treated patients did not show consistent levels across patients. FVIII expression starts to decline in some patients within 2 years, as reported in a long-term clinical trial.33 Furthermore, the presence of anti-AAV vector antibodies reduces or abolishes the transduction efficiency of AAV and prevents repeated treatment in HemA patients.4,34 To date, there is no standard protocol to monitor the genotoxicity resulting from potential insertional mutations caused by AAV vectors in a clinical setting. More studies are needed to access the safety and efficacy of viral gene therapy.4,35, 36, 37
mRNA-based therapy has recently emerged as an important alternative since mRNA has no risk of integrational mutations as opposed to viral vector-based therapies. The mRNAs delivered into cells would not translocate to the nucleus and instead undergo translation in the cytoplasm with natural post-translational modification of encoded protein. Furthermore, modifications including replacement of uridine 5′-triphosphates (UTPs) by N1-methylpseudo-UTP and addition of cap-1 structure yielded mRNAs with increased stability and translational activity and reduced immunogenicity.38, 39, 40 Codon optimization of mRNA further enhanced protein expression levels.41,42 Moreover, the clinical-grade good manufacturing practice (GMP) production of clinical in vitro-transcribed (IVT) mRNA costs 5- to 10-fold less than that of production of recombinant protein.43
To improve safety, efficiency, and tissue-targeting of mRNA delivery, we developed optimized LNPs carrying hFVIII mRNA for efficient delivery into liver cells. The opsonized LNPs bound to apolipoprotein E (ApoE) and low-density lipoprotein (LDL) receptors and facilitated specific uptake of LNPs by hepatocytes.44,45 In addition, the formulation of F8 LNPs incorporated a “diffusible” polyethylene glycol (PEG) lipid that can shed from the LNP particle to significantly improve transfection.46 Of note, the total amount of PEG used in F8 LNP treatment was much lower than the acceptable daily intake of PEG in humans, and the use of diffusible PEG lipids reduced any potential immune responses to LNP-associated PEG.47 Following LNP delivery, mRNA was translated, and functional FVIII protein can be detected within half an hour. The average half-life of FVIII generated by hF8 LNPs (F8-N6Δ2-F309S LNPs) is about 22 h, which is much longer than that for commercial FVIII products.27 According to our in vivo data, one to two per week injections of 0.2 mg/kg hF8 LNPs are sufficient to maintain a trough level higher than 1% in HemA mice 7 days after F8 LNP delivery. Our F8 LNPs can provide a considerably higher flexible range of FVIII levels, compared to published formulated FVIII mRNA.48 In our protocol, we have used novel FVIII mutant molecules and achieved more potent treatment efficacy with longer half-life of FVIII expression and reproducible pharmacology in multidose studies. Furthermore, it was known that the half-life of infused hFVIII is significantly shorter in mice than in humans.49 Therefore, the frequency of F8 LNP dosing can be further reduced in clinical treatment. Compared to FVIII concentrates and recombinant FVIII protein, which require prophylaxis regimen of three to four times and two to three times weekly injections, respectively, we expect that once every 7 days or longer intervals of F8 LNP treatment will be sufficient for prophylactic treatment. Importantly, note that mRNA LNPs are biocompatible and lipids are rapidly degraded and eliminated after delivery and therefore can be safely administered with repeated injections.7
We observed FVIII gene expression, albeit at low levels, with full-length FVIII (F8-FL), indicating that FVIII mRNA can be correctly processed and expressed in liver cells following delivery of F8 LNPs. To elevate the efficiency of FVIII secretion, we introduced a F309S mutation and furin cleavage site deletion in FVIII mRNA. In accordance with those mutations, mice injected by LNPs with mutant FVIII mRNA showed higher FVIII level and clotting activity compared to WT FVIII. Most interestingly, FVIII expression levels were significantly increased up to 100- to 200-fold when mRNAs encoding B domain-deleted FVIII variants were encapsulated in LNPs. It is unclear at this point why such significant enhancement was obtained. It is potentially due to differences in delivery, translation, and secretion efficiency. As shown in the results, with higher dosages of F8-N6Δ2-F309S LNPs (0.2–2 mg/kg), 200%–1,000% activities can be achieved with a single treatment, providing a wide spectrum and flexibility for prophylactic treatment and other treatment options for hemophilia patients. As shown in our results, FVIII expression starts rapidly after F8LNP delivery. Even at half an hour after delivery, we already detected 2%–5% FVIII levels in mouse plasma, and FVIII levels continue to increase to peak levels at 24 h post-injection. With appropriate dosages, correction of blood clotting can be maintained to at least 7 days post-injection. With different dosing regimens, F8 LNPs can be used in various treatment scenarios. For example, F8 LNPs can be used for patients who are scheduled for major surgeries or had suffered from spontaneous bleeding in joints or muscle. Recently developed bispecific factor IXa and factor X antibody, Hemlibra (emicizumab-kxwh) was shown to successfully reduce the frequency of bleeding episodes in HemA patients with inhibitor. However, there are several limitations for treating hemophilia patients, and long-term toxicity and safety still need to be carefully assessed. HemA patients need to receive Hemlibra combined with other bypassing agents when undergoing major surgery;50 however, serious thrombotic side effects have occurred in some patients.51,52 There were also several instances when treated patients developed neutralizing antibody against the drug.53
Immunodeficient NSG mice were used to evaluate the routine treatment regimen of repeated injections of F8 LNPs for long-term therapeutic effect. First, we used NSG HemA mice to demonstrate their expression pattern: both antigen and activity levels were similar to those observed in 129/SV × C57BL/6 HemA mice, suggesting that different genetic background did not affect FVIII protein expression kinetics. Subsequently, we performed a long-term treatment of F8 LNPs in NSG mice. F8 LNP treatment was carried out at 5-day intervals for 2 months. NSG mice persistently express FVIII protein, suggesting that this protocol is suitable for routine prophylaxis treatment to achieve therapeutic efficacy. According to clinical data published on inhibitor formation incidence in severe HemA patients undergoing treatment of FVIII concentrates, more than 70% of patients do not generate FVIII inhibitors and are suitable for repeated F8 LNP treatment.24, 25, 26
Although inhibitor development is dependent on genetics and environmental factors, several studies indicated that recombinant FVIII products showed higher immunogenicity than did plasma-derived FVIII products. One of the possible reasons is that posttranslational modifications of recombinant FVIII such as glycosylation from non-human mammalian cell lines contribute to the increase of FVIII immunogenicity.54,55 In contrast to recombinant FVIII products, FVIII derived from F8 LNPs undergoes intrinsic post-translation modification, which potentially eliminates the increased risk of antibody formation induced against FVIII synthesized from different species. In addition, further genetic modification of FVIII mRNA can address the immunogenic issue of FVIII. For example, reducing FVIII immunogenicity may be achieved by either mutation of the FVIII sequence or fusion with specific proteins or peptides.56, 57, 58, 59
In conclusion, F8 LNP treatment offers several significant advantages over current protein replacement therapy and alternative therapies. Delivery of F8 LNPs produced rapid initial expression and a wide range of FVIII levels up to 1000% by administering different dosing regimens. These unique properties can lead to prophylactic application and various other treatment scenarios. Furthermore, the prolonged intervals between repeated treatment, flexibility of construct modification, native post-transcriptional modification of FVIII protein, high efficiency of F8 LNP delivery, and relative ease and reduced cost of scaling up to human use can potentially make F8 LNP treatment a much safer and superior protocol to substitute protein replacement therapy in the clinic. In addition, a major advantage of FVIII mRNA LNP therapy is that it is easy to improve FVIII function by modification of mRNA sequence encoding newly developed FVIII protein with higher activity or longer half-life. Future investigation in formulating F8 LNPs for subcutaneous injections is also currently ongoing, which will further increase the ease of administering the therapy and improve the quality of patient life.
Materials and Methods
Synthesis of mRNA and LNPs
Formulation of LNPs was according to previous methods.7 Briefly, different variants of codon-optimized hFVIII mRNAs were synthesized from linearized DNA plasmids using in vitro transcription by T7 RNA polymerase, and ribonucleotide UTPs were replaced by 1-methylpseudo-UTP. A cap-1 structure and poly(A) tail were added to 5′ and 3′ UTRs of mRNA, respectively, to enhance mRNA translation efficiency. Lipid components (ionizable lipid:distearoylphosphatidylcholine (DSPC):cholesterol:PEG lipid, 50:10:38.5:1.5) were mixed with synthesized mRNAs at a volume ratio of 1:3 and assembled by NanoAssemblr system (Precision NanoSystems, San Francisco, CA, USA) to synthesize mRNA LNPs. All LNPs were tested for particle size, RNA encapsulation, and endotoxin to be <100 nm, >80%, and <10 endotoxin units (EU)/mL, respectively.
Animals
HemA mice generated by targeted disruption of the FVIII gene in exon 16 were used in mixed genetic background of 129/SV × C57BL/6 mice at the age of 8–12 weeks. NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (NSG mice, stock no. 0005557) were purchased from Jackson Laboratory (Sacramento, CA, USA). NSG HemA mice were purchased from the Gene Knockout Mouse Core Laboratory of the National Taiwan University Center of Genomic Medicine. All experimental mice were housed in a specific pathogen-free (SPF) facility in Seattle Children’s Research Institute according to the animal care guidelines of the National Institutes of Health and the Seattle Children’s Research Institute. The experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee of the Seattle Children’s Research Institute.
LNP Injection and Sample Collection
Mice were intravenously injected with mRNA LNPs through the retro-orbital plexus at different doses according to the experimental design. Blood samples from experimental mice were collected from the retro-orbital plexus at indicated time points and centrifuged immediately at 500 × g for 5 min to obtain sera. Samples were aliquoted and stored at −80°C for further experiments.
Live Imaging of Experimental Mice Using an Intravital Imaging System
Prior to the experiments, mice were anesthetized using isoflurane and hair was removed by hair removal cream (Nair, Ewing, NJ, USA). Mice were subcutaneously injected with 10 μL/g d-luciferin (PI88293, Fisher Scientific) in PBS 10 min before imaging. All images were monitored by Image Studio software for the Pearl Trilogy imaging system.
aPTT Assay and Bethesda Assay
Plasma hFVIII was examined using a modified clotting assay utilizing aPTT reagent and FVIII-deficient plasma.60,61 hFVIII activity was evaluated according to a standard curve obtained from serially diluted normal human pooled plasma. FVIII inhibitor levels were quantitated using Bethesda assays.
FVIII ELISA
hFVIII antigen levels introduced by FVIII-LNPs in HemA mice and NSG mice were examined by ELISA using murine anti-FVIII antibody (GMA-8020, Green Mountain Antibody, Burlington, VT, USA) and biotin-labeled murine anti-FVIII antibody (GMA-8015, Green Mountain Antibody). This pair of anti-FVIII antibodies does not cross-react with endogenous murine FVIII in NSG mice. Serially diluted normal human plasma was used as standards to evaluate human FVIII antigen level.
AST/ALT Assays
Liver cell injury of experimental mice was evaluated using an ALT reagent set (Teco Diagnostics, Anaheim, CA, USA) and an AST commercial enzyme kit (Randox, London, UK), respectively.
Western Blot
Plasma samples were electrophoresed in 4%–15% SDS-PAGE (Bio-Rad) and electrotransferred to polyvinylidene fluoride (PVDF) membranes using an iBlot gel transfer device (Thermo Fisher Scientific, Waltham, MA, USA). Membranes were blotted with non-fat skimmed milk subsequently. Human FVIII was detected by sheep-anti-human FVIII antibody (Affinity Biologicals, Ancaster, ON, Canada) following horseradish peroxidase-conjugated anti-sheep immunoglobulin G (IgG) (Thermo Fisher Scientific). ECL signals were developed with the enhanced chemiluminescence (ECL) reagent (Thermo Fisher Scientific).
Thromboelastography
Coagulation function of mice treated with F8 LNPs was examined 7 days after treatment. WT mice and HA (HemA) mice treated with Luc LNPs (HA) served as controls. Whole blood was collected by submental bleeding, and 3.8% citric acid was used as an anticoagulant. Clotting time and clot formation time were directly measured using an INTEM (activated intrinsic pathway) kit assay by rotational thromboelastometry (ROTEM delta, Instrumentation Laboratory, Bedford, MA, USA).
Statistical Analyses
All statistical analyses were carried out utilizing GraphPad Prism 7 software. The data were compared using a two-tailed unpaired Student’s t test and one-way or two-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc multiple comparison tests. A p value <0.05 was considered statistically significant.
Author Contributions
C.-Y.C. developed the study design, performed experiments, analyzed data, and wrote the manuscript. D.M.T. performed experiments and reviewed the manuscript. A.C., X.C., R.R., and M.J.L. performed experiments. P.G.V.M. reviewed the manuscript. C.H.M. developed the study concept, analyzed results, and wrote and revised the manuscript.
Conflicts of Interest
C.H.M. received funding of a Sponsored Research Administration (SRA) grant from Moderna, Inc. R.R. is a former Moderna employee. A.C., and P.G.V.M are employees of, and receive salary and stock options from, Moderna, Inc. C.H.M. and P.G.V.M are inventors of a patent on F8 LNP technology. The remaining authors declare no competing interests.
Acknowledgments
The work is partly supported by a Sponsored Research Administration (SRA) grant from Moderna Therapeutics.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtn.2020.03.015.
Supplemental Information
References
- 1.Davie E.W., Fujikawa K., Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30:10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 2.Escobar M.A., Brewer A., Caviglia H., Forsyth A., Jimenez-Yuste V., Laudenbach L., Lobet S., McLaughlin P., Oyesiku J.O.O., Rodriguez-Merchan E.C. Recommendations on multidisciplinary management of elective surgery in people with haemophilia. Haemophilia. 2018;24:693–702. doi: 10.1111/hae.13549. [DOI] [PubMed] [Google Scholar]
- 3.Tong K.M., Wang J.D., Chang S.T., Cheng Y.Y., Wang S.S. Outcome of perioperative hemostatic management in patients with hemophilia without inhibitors undergoing 161 invasive or surgical procedures. J. Chin. Med. Assoc. 2018;81:926–929. doi: 10.1016/j.jcma.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Colella P., Ronzitti G., Mingozzi F. Emerging Issues in AAV-mediated in vivo gene therapy. Mol. Ther. Methods Clin. Dev. 2017;8:87–104. doi: 10.1016/j.omtm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biasco L., Rothe M., Büning H., Schambach A. Analyzing the genotoxicity of retroviral vectors in hematopoietic cell gene therapy. Mol. Ther. Methods Clin. Dev. 2017;8:21–30. doi: 10.1016/j.omtm.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kao C.Y., Yang S.J., Tao M.H., Jeng Y.M., Yu I.S., Lin S.W. Incorporation of the factor IX Padua mutation into FIX-Triple improves clotting activity in vitro and in vivo. Thromb. Haemost. 2013;110:244–256. doi: 10.1160/TH13-02-0154. [DOI] [PubMed] [Google Scholar]
- 7.An D., Schneller J.L., Frassetto A., Liang S., Zhu X., Park J.S., Theisen M., Hong S.J., Zhou J., Rajendran R. Systemic messenger RNA therapy as a treatment for methylmalonic acidemia. Cell Rep. 2017;21:3548–3558. doi: 10.1016/j.celrep.2017.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gan L.M., Lagerström-Fermér M., Carlsson L.G., Arfvidsson C., Egnell A.C., Rudvik A., Kjaer M., Collén A., Thompson J.D., Joyal J. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat. Commun. 2019;10:871. doi: 10.1038/s41467-019-08852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassett K.J., Benenato K.E., Jacquinet E., Lee A., Woods A., Yuzhakov O., Himansu S., Deterling J., Geilich B.M., Ketova T. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao J., An D., Galduroz M., Zhuo J., Liang S., Eybye M., Frassetto A., Kuroda E., Funahashi A., Santana J. mRNA therapy improves metabolic and behavioral abnormalities in a murine model of citrin deficiency. Mol. Ther. 2019;27:1242–1251. doi: 10.1016/j.ymthe.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeRosa F., Guild B., Karve S., Smith L., Love K., Dorkin J.R., Kauffman K.J., Zhang J., Yahalom B., Anderson D.G., Heartlein M.W. Therapeutic efficacy in a hemophilia B model using a biosynthetic mRNA liver depot system. Gene Ther. 2016;23:699–707. doi: 10.1038/gt.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramaswamy S., Tonnu N., Tachikawa K., Limphong P., Vega J.B., Karmali P.P., Chivukula P., Verma I.M. Systemic delivery of factor IX messenger RNA for protein replacement therapy. Proc. Natl. Acad. Sci. USA. 2017;114:E1941–E1950. doi: 10.1073/pnas.1619653114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorner A.J., Wasley L.C., Kaufman R.J. Increased synthesis of secreted proteins induces expression of glucose-regulated proteins in butyrate-treated Chinese hamster ovary cells. J. Biol. Chem. 1989;264:20602–20607. [PubMed] [Google Scholar]
- 14.Tagliavacca L., Wang Q., Kaufman R.J. ATP-dependent dissociation of non-disulfide-linked aggregates of coagulation factor VIII is a rate-limiting step for secretion. Biochemistry. 2000;39:1973–1981. doi: 10.1021/bi991896r. [DOI] [PubMed] [Google Scholar]
- 15.Fantacini D.M., Fontes A.M., de Abreu Neto M.S., Covas D.T., Picanço-Castro V. The F309S mutation increases factor VIII secretion in human cell line. Rev. Bras. Hematol. Hemoter. 2016;38:135–140. doi: 10.1016/j.bjhh.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch C.M., Israel D.I., Kaufman R.J., Miller A.D. Sequences in the coding region of clotting factor VIII act as dominant inhibitors of RNA accumulation and protein production. Hum. Gene Ther. 1993;4:259–272. doi: 10.1089/hum.1993.4.3-259. [DOI] [PubMed] [Google Scholar]
- 17.Miao H.Z., Sirachainan N., Palmer L., Kucab P., Cunningham M.A., Kaufman R.J., Pipe S.W. Bioengineering of coagulation factor VIII for improved secretion. Blood. 2004;103:3412–3419. doi: 10.1182/blood-2003-10-3591. [DOI] [PubMed] [Google Scholar]
- 18.Wang X., Shin S.C., Chiang A.F., Khan I., Pan D., Rawlings D.J., Miao C.H. Intraosseous delivery of lentiviral vectors targeting factor VIII expression in platelets corrects murine hemophilia A. Mol. Ther. 2015;23:617–626. doi: 10.1038/mt.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman R.J., Wasley L.C., Dorner A.J. Synthesis, processing, and secretion of recombinant human factor VIII expressed in mammalian cells. J. Biol. Chem. 1988;263:6352–6362. [PubMed] [Google Scholar]
- 20.Sabatino D.E., Freguia C.F., Toso R., Santos A., Merricks E.P., Kazazian H.H., Jr., Nichols T.C., Camire R.M., Arruda V.R. Recombinant canine B-domain-deleted FVIII exhibits high specific activity and is safe in the canine hemophilia A model. Blood. 2009;114:4562–4565. doi: 10.1182/blood-2009-05-220327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen G.N., George L.A., Siner J.I., Davidson R.J., Zander C.B., Zheng X.L., Arruda V.R., Camire R.M., Sabatino D.E. Novel factor VIII variants with a modified furin cleavage site improve the efficacy of gene therapy for hemophilia A. J. Thromb. Haemost. 2017;15:110–121. doi: 10.1111/jth.13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins P.V., Rawley O., Smith O.P., O’Donnell J.S. Elevated factor VIII levels and risk of venous thrombosis. Br. J. Haematol. 2012;157:653–663. doi: 10.1111/j.1365-2141.2012.09134.x. [DOI] [PubMed] [Google Scholar]
- 23.Ye P., Thompson A.R., Sarkar R., Shen Z., Lillicrap D.P., Kaufman R.J., Ochs H.D., Rawlings D.J., Miao C.H. Naked DNA transfer of factor VIII induced transgene-specific, species-independent immune response in hemophilia A mice. Mol. Ther. 2004;10:117–126. doi: 10.1016/j.ymthe.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Tuddenham E.G. Molecular biological aspects of inhibitor development. Vox Sang. 1999;77(Suppl 1):13–16. doi: 10.1159/000056707. [DOI] [PubMed] [Google Scholar]
- 25.Ehrenforth S., Kreuz W., Scharrer I., Linde R., Funk M., Güngör T., Krackhardt B., Kornhuber B. Incidence of development of factor VIII and factor IX inhibitors in haemophiliacs. Lancet. 1992;339:594–598. doi: 10.1016/0140-6736(92)90874-3. [DOI] [PubMed] [Google Scholar]
- 26.Kreuz W., Becker S., Lenz E., Martinez-Saguer I., Escuriola-Ettingshausen C., Funk M., Ehrenforth S., Auerswald G., Kornhuber B. Factor VIII inhibitors in patients with hemophilia A: epidemiology of inhibitor development and induction of immune tolerance for factor VIII. Semin. Thromb. Hemost. 1995;21:382–389. doi: 10.1055/s-2007-1000659. [DOI] [PubMed] [Google Scholar]
- 27.Cafuir L.A., Kempton C.L. Current and emerging factor VIII replacement products for hemophilia A. Ther. Adv. Hematol. 2017;8:303–313. doi: 10.1177/2040620717721458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mancuso M.E., Santagostino E. Outcome of clinical trials with new extended half-life FVIII/IX concentrates. J. Clin. Med. 2017;6:6. doi: 10.3390/jcm6040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lieuw K. Many factor VIII products available in the treatment of hemophilia A: an embarrassment of riches? J. Blood Med. 2017;8:67–73. doi: 10.2147/JBM.S103796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorat T., Neumann P.J., Chambers J.D. Hemophilia burden of disease: a systematic review of the cost-utility literature for hemophilia. J. Manag. Care Spec. Pharm. 2018;24:632–642. doi: 10.18553/jmcp.2018.24.7.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George L.A. Hemophilia gene therapy comes of age. Blood Adv. 2017;1:2591–2599. doi: 10.1182/bloodadvances.2017009878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rangarajan S., Walsh L., Lester W., Perry D., Madan B., Laffan M., Yu H., Vettermann C., Pierce G.F., Wong W.Y., Pasi K.J. AAV5-Factor VIII Gene Transfer in Severe Hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 33.BioMarin . 2019. BioMarin provides 3 years of clinical data from ongoing phase 1/2 study of valoctocogene roxaparvovec gene therapy for severe hemophilia A. PRNewswire, May 28, 2019.https://investors.biomarin.com/2019-05-28-BioMarin-Provides-3-Years-of-Clinical-Data-from-Ongoing-Phase-1-2-Study-of-Valoctocogene-Roxaparvovec-Gene-Therapy-for-Severe-Hemophilia-A [Google Scholar]
- 34.Miesbach W., O’Mahony B., Key N.S., Makris M. How to discuss gene therapy for haemophilia? A patient and physician perspective. Haemophilia. 2019;25:545–557. doi: 10.1111/hae.13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandler R.J., LaFave M.C., Varshney G.K., Trivedi N.S., Carrillo-Carrasco N., Senac J.S., Wu W., Hoffmann V., Elkahloun A.G., Burgess S.M., Venditti C.P. Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J. Clin. Invest. 2015;125:870–880. doi: 10.1172/JCI79213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donsante A., Miller D.G., Li Y., Vogler C., Brunt E.M., Russell D.W., Sands M.S. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- 37.Rosas L.E., Grieves J.L., Zaraspe K., La Perle K.M., Fu H., McCarty D.M. Patterns of scAAV vector insertion associated with oncogenic events in a mouse model for genotoxicity. Mol. Ther. 2012;20:2098–2110. doi: 10.1038/mt.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwon H., Kim M., Seo Y., Moon Y.S., Lee H.J., Lee K., Lee H. Emergence of synthetic mRNA: in vitro synthesis of mRNA and its applications in regenerative medicine. Biomaterials. 2018;156:172–193. doi: 10.1016/j.biomaterials.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 39.Karikó K., Muramatsu H., Welsh F.A., Ludwig J., Kato H., Akira S., Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaidyanathan S., Azizian K.T., Haque A.K.M.A., Henderson J.M., Hendel A., Shore S., Antony J.S., Hogrefe R.I., Kormann M.S.D., Porteus M.H., McCaffrey A.P. Uridine depletion and chemical modification increase Cas9 mRNA activity and reduce immunogenicity without HPLC purification. Mol. Ther. Nucleic Acids. 2018;12:530–542. doi: 10.1016/j.omtn.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward N.J., Buckley S.M., Waddington S.N., Vandendriessche T., Chuah M.K., Nathwani A.C., McIntosh J., Tuddenham E.G., Kinnon C., Thrasher A.J., McVey J.H. Codon optimization of human factor VIII cDNAs leads to high-level expression. Blood. 2011;117:798–807. doi: 10.1182/blood-2010-05-282707. [DOI] [PubMed] [Google Scholar]
- 42.Mauro V.P., Chappell S.A. A critical analysis of codon optimization in human therapeutics. Trends Mol. Med. 2014;20:604–613. doi: 10.1016/j.molmed.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 44.Sabnis S., Kumarasinghe E.S., Salerno T., Mihai C., Ketova T., Senn J.J., Lynn A., Bulychev A., McFadyen I., Chan J. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 2018;26:1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulkarni J.A., Cullis P.R., van der Meel R. Lipid nanoparticles enabling gene therapies: from concepts to clinical utility. Nucleic Acid Ther. 2018;28:146–157. doi: 10.1089/nat.2018.0721. [DOI] [PubMed] [Google Scholar]
- 46.Ambegia E., Ansell S., Cullis P., Heyes J., Palmer L., MacLachlan I. Stabilized plasmid-lipid particles containing PEG-diacylglycerols exhibit extended circulation lifetimes and tumor selective gene expression. Biochim. Biophys. Acta. 2005;1669:155–163. doi: 10.1016/j.bbamem.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Judge A., McClintock K., Phelps J.R., Maclachlan I. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol. Ther. 2006;13:328–337. doi: 10.1016/j.ymthe.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 48.Russick J., Delignat S., Milanov P., Christophe O., Boros G., Denis C.V., Lenting P.J., Kaveri S.V., Lacroix-Demazes S. Correction of bleeding in experimental severe hemophilia A by systemic delivery of factor VIII-encoding mRNA. Haematologica. 2020;105 doi: 10.3324/haematol.2018.210583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dwarki V.J., Belloni P., Nijjar T., Smith J., Couto L., Rabier M., Clift S., Berns A., Cohen L.K. Gene therapy for hemophilia A: production of therapeutic levels of human factor VIII in vivo in mice. Proc. Natl. Acad. Sci. USA. 1995;92:1023–1027. doi: 10.1073/pnas.92.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kizilocak H., Yukhtman C.L., Marquez-Casas E., Lee J., Donkin J., Young G. Management of perioperative hemostasis in a severe hemophilia A patient with inhibitors on emicizumab using global hemostasis assays. Ther. Adv. Hematol. 2019;10 doi: 10.1177/2040620719860025. 2040620719860025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langer A.L., Etra A., Aledort L. Evaluating the safety of emicizumab in patients with hemophilia A. Expert Opin. Drug Saf. 2018;17:1233–1237. doi: 10.1080/14740338.2019.1551356. [DOI] [PubMed] [Google Scholar]
- 52.Wada H., Matsumoto T., Katayama N. Emicizumab prophylaxis in hemophilia A with inhibitors. N. Engl. J. Med. 2017;377:2193–2194. doi: 10.1056/NEJMc1712683. [DOI] [PubMed] [Google Scholar]
- 53.Young G., Liesner R., Chang T., Sidonio R., Oldenburg J., Jiménez-Yuste V., Mahlangu J., Kruse-Jarres R., Wang M., Uguen M. A multicenter, open-label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. 2019;134:2127–2138. doi: 10.1182/blood.2019001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calvez T., Chambost H., d’Oiron R., Dalibard V., Demiguel V., Doncarli A., Gruel Y., Huguenin Y., Lutz P., Rothschild C., for FranceCoag Collaborators Analyses of the FranceCoag cohort support differences in immunogenicity among one plasma-derived and two recombinant factor VIII brands in boys with severe hemophilia A. Haematologica. 2018;103:179–189. doi: 10.3324/haematol.2017.174706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai J., Hough C., Tarrant J., Lillicrap D. Biological considerations of plasma-derived and recombinant factor VIII immunogenicity. Blood. 2017;129:3147–3154. doi: 10.1182/blood-2016-11-750885. [DOI] [PubMed] [Google Scholar]
- 56.Ettinger R.A., Liberman J.A., Gunasekera D., Puranik K., James E.A., Thompson A.R., Pratt K.P. FVIII proteins with a modified immunodominant T-cell epitope exhibit reduced immunogenicity and normal FVIII activity. Blood Adv. 2018;2:309–322. doi: 10.1182/bloodadvances.2017013482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muczynski V., Casari C., Moreau F., Aymé G., Kawecki C., Legendre P., Proulle V., Christophe O.D., Denis C.V., Lenting P.J. A factor VIII-nanobody fusion protein forming an ultrastable complex with VWF: effect on clearance and antibody formation. Blood. 2018;132:1193–1197. doi: 10.1182/blood-2018-01-829523. [DOI] [PubMed] [Google Scholar]
- 58.Powell J.S., Josephson N.C., Quon D., Ragni M.V., Cheng G., Li E., Jiang H., Li L., Dumont J.A., Goyal J. Safety and prolonged activity of recombinant factor VIII Fc fusion protein in hemophilia A patients. Blood. 2012;119:3031–3037. doi: 10.1182/blood-2011-09-382846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dumont J.A., Liu T., Low S.C., Zhang X., Kamphaus G., Sakorafas P., Fraley C., Drager D., Reidy T., McCue J. Prolonged activity of a recombinant factor VIII-Fc fusion protein in hemophilia A mice and dogs. Blood. 2012;119:3024–3030. doi: 10.1182/blood-2011-08-367813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu C.L., Ye P., Lin J., Djukovic D., Miao C.H. Long-term tolerance to factor VIII is achieved by administration of interleukin-2/interleukin-2 monoclonal antibody complexes and low dosages of factor VIII. J. Thromb. Haemost. 2014;12:921–931. doi: 10.1111/jth.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng B., Ye P., Blazar B.R., Freeman G.J., Rawlings D.J., Ochs H.D., Miao C.H. Transient blockade of the inducible costimulator pathway generates long-term tolerance to factor VIII after nonviral gene transfer into hemophilia A mice. Blood. 2008;112:1662–1672. doi: 10.1182/blood-2008-01-128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.