Abstract
Research on iron oxide-based magnetic nanoparticles and their clinical use has been, so far, mainly focused on the spherical shape. However, efforts have been made to develop synthetic routes that produce different anisotropic shapes not only in magnetite nanoparticles, but also in other ferrites, as their magnetic behavior and biological activity can be improved by controlling the shape. Ferrite nanoparticles show several properties that arise from finite-size and surface effects, like high magnetization and superparamagnetism, which make them interesting for use in nanomedicine. Herein, we show recent developments on the synthesis of anisotropic ferrite nanoparticles and the importance of shape-dependent properties for biomedical applications, such as magnetic drug delivery, magnetic hyperthermia and magnetic resonance imaging. A brief discussion on toxicity of iron oxide nanoparticles is also included.
Keywords: anisotropy, magnetic nanoparticles, hyperthermia, magnetic resonance imaging, drug delivery
1. Introduction
Many efforts are being made to overcome the flaws of cancer treatment and diagnosis. Nanomedicine is the field of nanotechnology responsible for the development of biomedical tools capable of a higher performance than conventional medicine [1,2]. Ferrite nanoparticles are magnetic nanoparticles that show several properties that result from finite-size and surface effects such as high magnetization, superparamagnetism and extra anisotropy contributions. The fact that these nanoparticles do not retain any magnetization upon removal of an applied magnetic field, along with the low toxicity, biocompatibility and strong magnetic properties, endow iron-based nanoparticles as suitable materials for use in bioimaging, cancer theranostics and drug delivery [3]. The dependence between magnetic behavior and size results from the structure of their magnetic domains. When size decreases to a threshold value (commonly 20 nm [4]), the ferrimagnetic material becomes a single domain which is characterized by a uniform magnetization [5,6]. Spinel ferrite nanoparticles have ferrimagnetic behavior, and this means that the material is composed of magnetic domains, each one composed of antiparallel magnetic moments with different magnitudes, resulting in a net spontaneous magnetic moment. When a magnetic field is applied, all domains have their magnetic moments aligned with the magnetic field, resulting in a large net magnetic moment [5,6,7,8].
Ferrite nanoparticles are composed of different atoms occupying two different lattice sites. Magnetite (Fe3O4) is a ferrite, but other ferrites can be formed when a Fe2+ ion is replaced by another cation (Mn2+, Zn2+, Co2+, Mg2+, Ni2+) [7,8]. The general structure of a normal spinel structure can be written as (Me2+)[Fe3+2]O4, where Me+2 refers to divalent cations, occupying tetrahedral positions, and Fe3+ serving as trivalent cations, occupying octahedral positions [7,8]. Given their unique structural and magnetic properties, ferrite nanoparticles have been widely used for magnetic drug delivery [9,10,11], magnetic hyperthermia [12,13,14] and magnetic resonance imaging [15,16,17]. Research on preparation methods and characterization of different anisotropic shapes of these nanoparticles is important and it is recently increasing, since desired physicochemical properties and applications can be achieved by simply change structural parameters [18,19,20,21,22]. The magnetic behavior of ferrite nanoparticles is highly influenced by shape anisotropy. For instance, magnetization of an elongated nanoparticles will be easier along their long axis than the short axis [23].
In this review, we aim to explore the recently developed synthetic routes of preparation of different anisotropic nanoparticles, namely elongated nanoparticles, nanofilms, sheets and plates, nanocubes and nanoflowers. Herein, we highlight the importance of utilization of anisotropic iron oxide nanoparticles as alternative materials for an enhanced performance in biomedical applications, such as magnetic drug delivery, magnetic hyperthermia and magnetic resonance imaging (MRI). Also, the influence of size, shape and administered dose on the toxicity of iron oxide nanoparticles is briefly discussed.
2. Synthesis of Shape Anisotropic Nanoparticles
Synthesis of nanoparticles with controlled shape has been a main challenge in the last decade [24], yet most of the described methods and strategies are confined to magnetite nanoparticles. A gap is thus kept between the shapes that have been already developed and the metal composition that would be of interest to afford improved magnetic and contrast properties, besides other technological applications.
High-quality nanoparticles are attained by different methods, which can be separated in three main classes: physical, chemical and biological [25]. Physical methods include pulsed laser ablation and pyrolysis, while chemical encompass a wide variety of methods, such as co precipitation, hydrothermal and solvothermal synthesis, thermal decomposition, sol-gel synthesis, sonochemical decomposition, microemulsion, microwave-assisted and electrochemical synthesis [24,25,26]. Biological routes include the bacterial and microorganism synthesis [24,25,26]. The advantages and disadvantages of the common methods are included in Table 1, besides examples of spherical and anisotropic shape nanoparticles obtained through each method.
Table 1.
Common methods used in the synthesis of iron oxide nanoparticles, advantages and disadvantages, and references. Examples of isotropic and anisotropic shape nanoparticles are included.
| Method | Advantages | Disadvantages | Ref. | Examples |
|---|---|---|---|---|
| Laser Ablation Synthesis in Solution (LASiS) | Green synthesis Different structures and composition |
Difficult control of particle size and clustering | [27,28] | [29,30] |
| Chemical vapor deposition (laser and spray pyrolysis) |
Easy to prepare Production of small particle size |
Expensive equipment Gaseous interferences |
[26,31,32,33] | [34,35,36,37] |
| Co-precipitation | Green Low-cost Scalable Facile Efficient |
Difficult to control size Polydispersity Lack of precise stoichiometric phase control |
[26,28,31,32,38] | [39,40,41] |
| Thermal decomposition | Small size particles Control of size and shape Monodisperse |
Requires multiple steps Toxic solvents Toxic and expensive precursors Laborious purification Requires surface treatment after synthesis |
[31,33] | [42,43] |
| Hydrothermal (solvothermal) | Green Versatile Control of morphology |
Need of autoclave Control of dispersity Slow reaction kinetics |
[28,33] | [44,45] |
| Sol-gel synthesis | Homogeneous Control of shape and length Low-cost High phase purity |
Requires post treatment By-products Safety Low efficiency |
[28,33] | [46,47] |
| Sonochemical decomposition | Mild experimental conditions Good crystallinity Versatile |
Mechanism not still understood | [26,32,43] | [48,49] |
| Microemulsion | Monodispersity Simple equipment High control of size and shape Room conditions Small sizes |
Removal of surfactants High solvent consumption Low-yield Difficult scale-up |
[26,28,31,33] | [50] |
| Electrochemical synthesis | Control of particle size Simple and fast |
Lack of reproducibility | [32] | [51,52] |
| Biosynthesis | High yield Reproducibility Scalability Low cost Room temperature |
Time-consuming Laborious |
[31,32] | [53,54] |
The crystal shape can be thermodynamically or kinetically controlled. A thermodynamic process is associated with the chemical potential of the reaction, that is related to parameters such as temperature and supersaturation of the solution, while a kinetic process happens when stable nucleation sites in supersaturated regions are present, which reduce the reaction energy barrier [55]. During the thermodynamically-driven growth, the reaction proceeds to reduce the particles surface free energy [23,55]. Here, the two mechanisms, reaction-limited and diffusion-limited, affect differently the rate of deposition of atoms in the nuclei surface. At high solution concentration, in the diffusion-limited regime, monomers are precipitated once on the surface of the NPs, which favours monodisperse nanoparticles, while at low concentration, in the reaction-limited regime, the surface reaction limits the growth and leads to different final shapes [23]. Therefore, parameters such as temperature, pH, solvent and concentration of the precursors affect the final shape [55,56]. Considering the relative surface energies of the crystal facets, different strategies have been studied to favour anisotropic shapes, including the use of shaping ligands, template-assisted synthesis and electrodeposition, and magnetic assembly of nanoparticles [23].
The use of shaping ligands is a common method to obtain anisotropic nanoparticles, which can be induced through: (I) steric inhibition of growth and modification of chemical reactivity of specific crystal planes by chelation of the ligand to specific planes; (II) coalescence of reactants or nuclei and consequent anisotropic growth in templated ligand assembly. Common ligands include surfactants such as oleylamine, dodecyldimethylammonium bromide, cetyltrimethylammonium bromide or chloride, octadecylamine and oleic acid [23,55,56]. Common polymers include polyethyleneimine (PEI), poly(ethylene glycol) (PEG), poly(vinylpyrrolidone) (PVP), poly(acrylic acid) (PAA) and poly(vinyl alcohol) (PVA) [23,55,57]. Small molecules containing halides, small polymers and solvents are also used for coordination of specific crystal planes [55,58]. Here, the functional groups have to be considered as they affect the size, shape and magnetic properties of the crystals [23,58]. Li and co-workers [58] demonstrated that the higher polarity of 3-aminopropanol compared to urea led to an increased confinement effect of the magnetite nanocrystal growth through hydrothermal synthesis, while urea was adequate to control nanoparticle shape owing for its surface plane preference σ{111} > σ{001} > σ{101}. Another example of the capping agent effect was studied by Fatima and co-workers in hydrothermal synthesis of magnetite [59], where cubes and octahedra of magnetite could be obtained using ferrous sulphate heptahydrate in ethylene glycol, and potassium hydroxide that was found to favour a faster growth rate of {111} plane than the {100} plane.
Furthermore, π-acceptor ligands (e.g. CO) are described to decrease saturation magnetization, Ms, values, while the σ-donors (e.g. NH2) do not affect the magnetic properties [23]. Also, post-synthesis surface-functionalization can affect phase distribution, as demonstrated by Daniel and co-workers [60]. The replacement of oleic acid by catechol surface ligands led to the separation into two distinctive magnetic entities, due to reduction of a nanoparticle surface fraction to magnetite, which reduced the anisotropy constant and, consequently, the effective magnetization [60].
2.1. Elongated Nanoparticles
Elongated magnetic nanoparticles, mainly nanorods, have been of interest in various industrial [61,62], and biomedical applications [63,64,65]. Classification is based on the external energy required for inversion of the magnetization [61]. Nanorods encompass structures with some nanometers of diameter and length up to 100 nm, while the length of nanowires is larger, and nanotubes are hollow nanorods [23]. The magnetic easy axis and magnetization directed along the long axis, which are determined by the shape anisotropy, lead to an anisotropic response to an external magnetic field: a S-like hysteresis loop is obtained when a magnetic field is applied parallel to the short axis, while along the long axis the hysteresis loop is square-like [23]. The reader is directed to articles [66,67,68,69] for more information on the magnetic behavior of elongated nanoparticles. Different strategies have been developed in recent years for the synthesis of elongated iron oxide nanoparticles of different chemical composition, which are summarized in Table 2.
Table 2.
Common methods used in the synthesis of elongated iron oxide-based nanoparticles. The size and saturation magnetization, Ms, values are also included.
| Shape | Structure | Method | Size (d × l, nm) |
Ms (emu/g) |
Ref. |
|---|---|---|---|---|---|
| Nanorods | Fe3O4 | Hydrothermal + shaping ligand (EDA) | 40–50 × 500–800 | 72.94 | [70] |
| 25 × 200 | 71.3 | [71] | |||
| Co-precipitation + shaping ligand (PVP) | 18.2 × 310.6 | 28 | [72] | ||
| Hydrothermal + sacrificial template (goethite) | ~70 × ~500 | 90 | [73] | ||
| Solvothermal + template-assisted (Fe3O4 microspheres) | 7–20 × 120–400 | 92.3 | [74] | ||
| MnFe2O4 | Hydrothermal + sacrificial template (MnO) | 25–40 × 300–400 | 72.45 | [75] | |
| CoFe2O4 | Hydrothermal | 19 × 400 | 73.36 | [76] | |
| Solvothermal (EG) | 100–200 l | 54.93 | [77] | ||
| Hydrothermal + shaping ligand (CTAB) | 25 × 120 | 66 | [78] | ||
| Co0.5Ni0.5Fe2O4 | Microemulsion | 30–200 l | 51.1 | [79] | |
| CuFe2O4 | Co-precipitation | 120–400 l | - | [80] | |
| Nanowires | Fe3O4 | Sol-gel + shaping ligand (EG and P123) | 10 d; >500 aspect ratio | 34.5 | [81] |
| MnFe2O4 | Hydrothermal | 100–300 d | 45.9 | [82] | |
| CoFe2O4 | Sol-gel + template-assisted | 40 d | - | [83] | |
| Template-assisted | 8–10 d | 51.81 | [84] | ||
| Nanotubes | Fe3O4 | Template-assisted | 30 d; 7 nm wall thickness | - | [85] |
| CoFe2O4 | Co-precipitation + Template-assisted | 217 d | 65 | [86] | |
| Sol-gel + template-assisted | 50 d × 1000 l; 5 nm wall thickness |
- | [87] |
Nanorods have been commonly obtained through hydrothermal reactions, which provides an economical and convenient route for the synthesis of crystals with compositional and morphological control, without requiring extremely high temperatures and sophisticated processing [75,78]. On the other hand, nanowires and nanotubes synthesis is commonly accomplished through template-assisted strategies. Opposite to other strategies, a reproducible method for the synthesis of homogeneous magnetite nanotubes (Figure 1A) was developed by Liu et al. [85], which consisted on the synthesis of MgO nanowires grown on Si/SiO2 substrates, pulsed laser deposition of Fe3O4 onto the nanowires and etching of the MgO inner cores with (NH4)2SO4. Yet, magnetic nanorods have been of higher interest as theranostic agents. Through the hydrothermal method, Wan et al. [71], using ethylenediamine as a shaping ligand, were able to synthesise uniform nanorods (Figure 1B), which were achieved later in a greener synthesis strategy by Ding et al. [70] without use of benzene. A tuneable strategy for the synthesis of magnetite nanorods (Figure 1C) has also been developed by Mohapatra et al. [88], which consists on the synthesis of PEI coated FeOOH nanorods and its reduction with oleylamine. The control of size between 25 nm and 70 nm is attained by varying PEI concentration, which adsorbs on the plane {200} of the nanorods [89,90]. The nanorods are then reduced to magnetite by oleylamine that acts as a reducing agent (electron donor) at 200 °C, besides acting as surfactant and solvent [88,91].
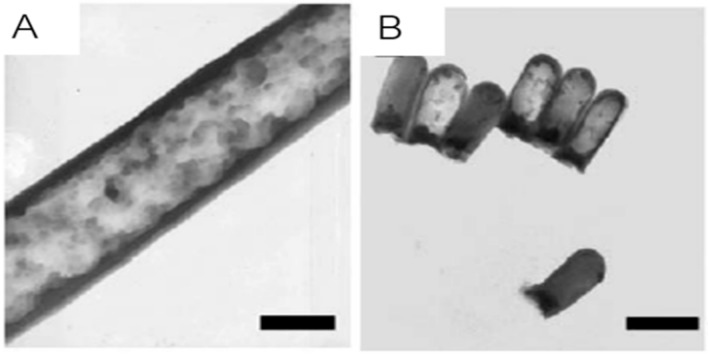
Figure 1.
(A) TEM image of a Fe3O4 nanotube. Upper inset: Selected area electron diffraction of the nanotube. Lower inset: HRTEM image taken on the tube wall. Reproduced from [85] with permission from American Chemical Society, 2020. (B) SEM image of magnetite nanorods described in [71]. Reproduced from [71] with permission from Elsevier, 2020. (C) Scheme of the two-step synthesis of magnetite nanorods. Adapted from [88] with permission from Royal Society of Chemistry, 2020.
Nanorods of CoFe2O4 are commonly obtained through a shaping ligand-assisted synthesis using oleic acid or CTAB as a surfactant [77,78,92]. Cobalt ferrite nanoparticles have remarkable properties, such as tuneable coercivity, large anisotropy and moderate saturation magnetization [77]. Yet, their application in the biomedical field is restricted owing to cobalt toxicity. Other ferrites, such as MnFe2O4, CaFe2O4 and MgFe2O4, have advantageous properties for biomedical applications; although, strategies for the synthesis of nanorods of these materials are lacking.
2.2. Nano- Films, Sheets and Plates
Two dimensional materials consist of plate-like magnetic nanoparticles, which magnetization in soft-magnetic materials lies parallel to the basal plane, requiring a strong magnetic field to align the magnetization out of the plane [23].
Thin films of magnetite have been commonly obtained through deposition techniques such as molecular beam epitaxy and pulsed-laser deposition [93]. Recently, Zukova et al. [93] were able to grow magnetite films using pulsed injection metallorganic chemical vapour deposition (PI MOCVD) on Al2O3 (0001) and MgO (001) substrates, which displayed a preferred (111) crystalline orientation. The authors denoted that the magnetic properties could be tuned through application of a 1T magnetic field during the film growth and also upon cooling. Another strategy to obtain magnetic nanofilms is the layer-by-layer method. For example, Grigoriev et al. [94,95] were able to synthesize polyelectrolyte/magnetite nanoparticle multilayer films by the layer-by-layer self-assembly technique and evaluated the dependence of the refractive index on the increase of layers.
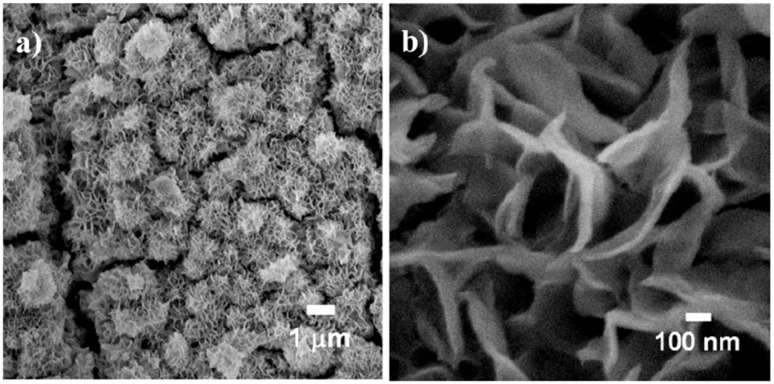
Other bidimensional shapes, such as nanosheets or nanoplates, have attracted more attention than nanofilms. Different strategies have already been used to obtain magnetite nanosheets, including hydrothermal synthesis [96], or soft-template assisted synthesis [97], affording nanosheets with good crystallinity and magnetic properties. A simple strategy was developed by Wang et al. [98], which consists of microwave-assisted coprecipitation of ferrous sulphate in a mixture of ethylene glycol and water, affording nanosheets with good crystallinity and saturation magnetization around 30 emu/g. Zhuang et al. [99] were able to synthesize magnetite nanosheets through a solvothermal method using diethylene glycol, which, through the inhibition of the crystal plane (111), favours the formation of magnetite sheet-like crystals. Furthermore, the authors demonstrated that the nanoparticles self-assemble into chain-like structures along the direction of the external magnetic field, known as photonic crystals, which display a magnetochromatic property. A scale-up of the synthesis of good quality and homogeneous nanosheets (Figure 2) has been developed by Chin et al. [100], which consisted in the immersion of Fe substrates into a 70 °C solution of HCl and KCl under an O2 rich environment. Strategies have also been developed for the synthesis of nanosheets based on other ferrites, mainly CoFe2O4 through template assisted [101,102,103], and thermal decomposition [104].
Figure 2.
SEM image at different magnifications of magnetite nanosheets. Adapted from [100] with permission from American Chemical Society, 2020.
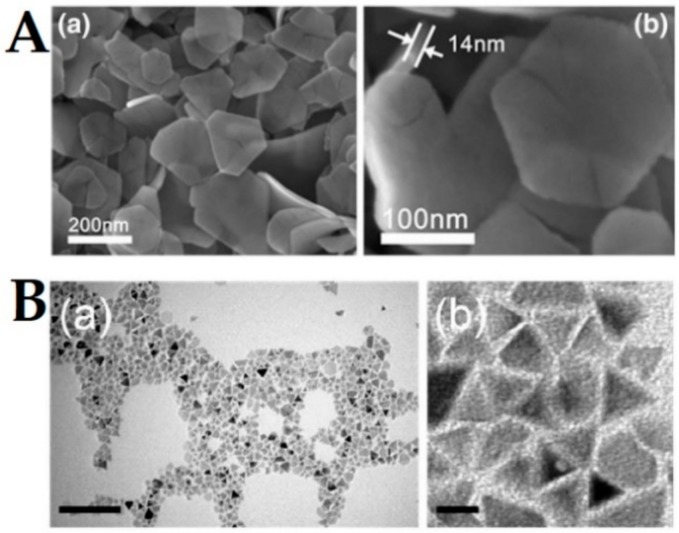
Magnetite nanoplates synthesis have also been reported through a hydrothermal route [105,106], with a saturation magnetization above 60 emu/g. An alternative to hydrothermal route has been proposed by Chen et al. [107], through the use of coprecipitation assisted with ultrasound irradiation, which does not require protection from oxygen and high temperatures, producing nanoplates with thickness ranging from 10 nm to 20 nm and lateral size between 50 nm and 90 nm. Hexagonal nanoplates have been obtained by Zhut et al. [108] through a hydrothermal synthesis using β-cyclodextrin and urea as, respectively, reducing agent and growth modifier. The nanoplates exhibit an average diameter of 400 nm and thickness of 40–80 nm, attaining a saturation magnetization close to 80 emu/g. Ma et al. [109] also synthesized hexagonal nanoplates with maximum magnetization above 70 emu/g through the Schikorr reaction in a hydrothermal route (Figure 3A), which is the formation of iron oxide and molecular hydrogen through oxidation of iron(II) hydroxide under anaerobic conditions. Recently, Xu et al. [110] were able to synthesize ultra-thin triangular magnetite nanoplates through a seed-mediated synthesis, where the seeds were obtained through the thermal decomposition of ferric oleate complex at 310 °C, and a growth-step (replenished with monomers) was carried out at a temperature (240 °C) lower than the required for nucleation (310 °C), but higher than the thermal decomposition of the complex (230 °C).
Figure 3.
(A) SEM images of hexagonal-shaped magnetite nanoplates at different magnifications. Reproduced from [109] published in Open Access by Springer. (B) TEM images of triangle-shaped magnetite ultra-thin nanoplates at different magnifications. Adapted from [110] with permission from Royal Society of Chemistry, 2020.
Recently, the synthesis of Co1-xCuxFe2O4 has been achieved through a two-step synthesis, starting with coprecipitation of the metal salts in the right ratio, which dried powder was subjected to thermal decomposition in air at 400 °C in a tubular furnace [111]. MnxFe1-xO nanoplates has also been recently reported, which are of biomedical interest owing to the resistance to oxidation in air compared to MnO and FeO, making them good candidates for magnetic contrast agents [112]. The synthesis was achieved through thermal decomposition of acetonate precursors under N2 atmosphere, affording nanoparticles that reach a saturation magnetization of 30 emu/g. The synthesis of Ni1-xMnxFe2O4 thin cubic nanoplates has also been obtained through coprecipitation of the metal salts in the right ratio and assisted with oleic acid as surfactant, to avert agglomeration during the reaction [113,114].
2.3. Nanocubes and Flowers
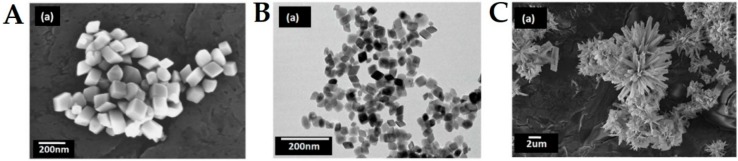
Despite the already described advances in the synthesis of colloidal iron oxide magnetic materials, efforts are still ongoing to attain facile and sustainable synthetic protocols for controlling the shape of nanoparticles. A protocol that allows control of nanoparticles was developed by Sayed and co-worker [115], in which through a microwave assisted method and by changing only the iron precursor, different shapes could be obtained such as distorted cubes, nanocubes and flowers (Figure 4).
Figure 4.
(A) SEM image of distorted cubes. (B) TEM image of nanocubes. (C) SEM image of self-oriented flowers. Reproduced from [115] with permission from Springer Nature, 2020.
A thermal decomposition method to synthesize magnetite flowers was developed by Guo et al. [116] using FeCl3.6H2O, urea and tetrabutylammonium bromide dissolved in ethylene glycol, which afforded particles with an average diameter of 1–3 µm and a saturation magnetization up to 80 emu/g. Later, Wang et al. [117] followed the reaction mechanism through electron microscopy, which proceeds as nucleation, aggregation and Ostwald ripening, and finally the oriented growth was also accompanied by Ostwald ripening. Flower-like particles have also been obtained through solvothermal routes. Li et al. [118] synthesized flower microspheres through solvothermal reaction with urea in ethylene glycol (200 °C, 4 h), which afforded particles that reach a magnetization in saturation up to 80 emu/g. A different particle architecture was developed by Wang et al. [119] through the solvothermal route, using CTAB and sodium acetate in ethylene glycol (200 °C, 24 h), that afforded hollow microspheres with a Ms of 80 emu/g. Other flower-like architecture is exhibited by the multicore particles, in which the interaction between the cores leads to a large magnetic moment and weak remanence magnetization [120]. Gavilán et al. [120] developed different methods to synthesize multi-core nanoparticles through the use of polyols as reducing agents, obtaining nanoflowers as small as 50 nm and Ms value of 100 emu/g. Through the use of succinic acid, urea and FeCl3.6H2O in a solvothermal route, Cheng et al. [121] were also able to synthesize flower-like multi-core nanoparticles with ca. 300 nm, while the use of 1,2-propylene glycol as solvent afforded nanoparticle clusters with ca. 50 nm. Recently, Shubitidze et al. [122] reported a coprecipitation method using dextran and NaNO3 that afforded nanoflowers with sizes of 20 nm to 40 nm.
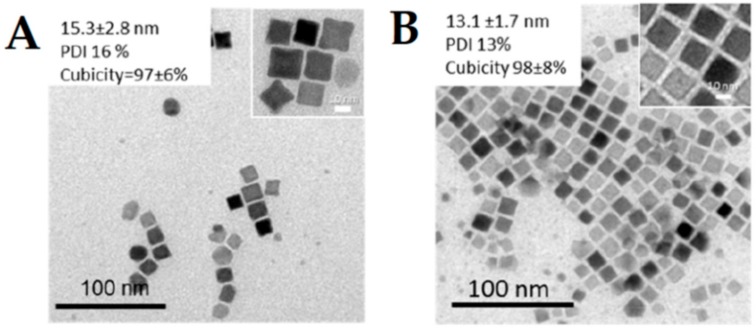
Anisotropic nanoparticles have been sought as a solution to improve T2 contrasting properties as the relaxivity can be enhanced by increasing the effective radius of the magnetic core, which is majorly morphologic dependent [123]. For example, Zhao et al. [100] synthesized octapods (Figure 5A) through thermal decomposition of iron oleate in the presence of NaCl. This strategy afforded nanoparticles with an average length of 30 nm, which displayed an ultrahigh transverse relaxivity value of 679.3 30 mM−1 s−1. Common methods for the synthesis of nanocubes include thermal decomposition of iron(II) oleate or iron(III) acetylacetonate complexes [124,125]. Recently, Muro-Cruces et al. [126] synthesized cubic MFe2O4 (M = Fe, Co, Mn) (Figure 5A,B) and Mn3O4 through the thermal decomposition of the right ratio of metal acetylacetonates in the presence of sodium oleate and oleic acid, in a mixture of 1-octadecene, dibenzyl ether and 1-tetradecene. Through this pathway, the authors were able to control the size variation in 1–2 nm and obtain monodisperse nanoparticles with high crystallinity, which is a critical control requirement to develop new materials for decreasing nuclear magnetic resonance relaxation time T2.
Figure 5.
TEM image of (A) CoFe2O4 and (B) MnFe2O4 nanocubes. Adapted from [126] with permission from American Chemical Society 2020.
3. Biomedical Applications of Anisotropic Iron Oxide Nanoparticles
The use of iron oxide nanoparticles (IONs) in cancer therapy and diagnosis (theranostics) has been of major interest regarding their unique physicochemical properties, namely, their superparamagnetic behavior, high magnetization and chemical stability [127,128,129]. The already reported influence of shape in the physicochemical properties and magnetic behavior of IONs [130,131,132,133] was a starting point to further research on the impact of shape anisotropy in biological behavior and imaging properties. Next sections are focused on recent developments in magnetic drug delivery, magnetic hyperthermia and magnetic resonance imaging (MRI).
3.1. Magnetic Drug Delivery
IONs can accumulate in tumor sites by passive or active targeting. Passive targeting occurs when nanoparticles can extravasate from the bloodstream and enter in tumor cells through the enhanced permeability and retention (EPR) effect [134,135]. On the other hand, active targeting with an applied magnetic field takes advantage on the responsiveness of magnetic nanoparticles towards a magnetic field [136,137]. IONs can also be coated with synthetic and natural polymers [130,138,139], surfactants and fatty acids [31,140] and functionalized with targeting ligands [130,141], which allows the use of these nanoparticles as drug delivery systems with improved selectivity and pharmacokinetics [142,143].
The superparamagnetic properties demand that nanoparticles have small sizes, preferably below ~20 nm [4]. However, at this size, magnetic moments of nanoparticles are small, so magnetic response can be compromised. Self-assembly of individual nanoparticles into nanoclusters is one possible strategy to overcome this problem. Kralj et al. [144] developed nanochains and nanobundles from nanoclusters of maghemite (γ-Fe2O3) with preserved superparamagnetism, zero coercivity and good colloidal stability. Other elongated structures, like nanotubes or nanorods, are also being investigated for drug delivery. Iron oxide nanorods have attracted attention due to their superparamagnetic behavior [145] and capacity of intracellular delivery with controlled-release profile and biocompatibility [146]. Nanotubes have the advantage of enabling the loading of large amounts of bioactive compounds in their inner voids, while the outer surface can be coated or functionalized with targeting ligands (Figure 6A,B) [147].
Figure 6.
TEM images of 200 nm (A) and 60 nm (B) nanotubes with magnetite. Scale bars, 200 nm. Adapted from [147] with permission from Elsevier, 2020.
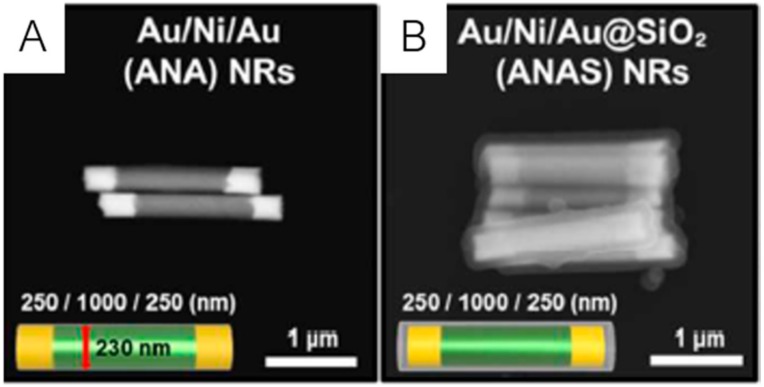
Magnetic nanotubes combine the magnetic and tubular properties allowing a differential functionalization of the inner and outer surfaces, which shows the potential of these nanostructures for magnetically assisted drug delivery and bioimaging [148]. A study regarding the use of iron oxide nanotubes as carriers for insoluble anticancer drugs showed that hematite nanorods were in a higher extent and more quickly internalized than nanospheres in human carcinoma cells [149]. After achieving this conclusion, the researchers developed PEGylated maghemite nanorods loaded with paclitaxel (PTX-PMNTs) and demonstrated that these nanocarriers have important properties for a successful magnetic drug delivery such as higher drug loading capacity, increased carcinoma uptake in the presence of a magnetic field and a pH-activated release profile. In 2014, Yu et al. [150] synthesized iron oxide porous nanorods functionalized with folic acid for targeted delivery of another low water-soluble anticancer drug, doxorubicin. In this study, the researchers demonstrated that the presence of folic acid on the surface of nanorods increased cellular uptake of nanorods and cytotoxicity of doxorubicin in HeLa cells, because of the specific binding between folic acid and folate receptors, thus showing the potential of these nanocarriers for targeted drug delivery in tumor cells. More recently, an innovative nanosystem was developed by Kwak et al. [65], being composed of Au blocks (plasmonically-active domain) and Ni blocks (magnetically-active domain), with a silica-coated surface for drug loading (Figure 7A,B).
Figure 7.
FE-SEM images of Au/Ni/Au nanorods (A) and silica-coated Au/Ni/Au nanorods (B) and respective schematic representations. Adapted from [65] with permission from Royal Society of Chemistry, 2020.
A rotating magnetic field will induce fluctuations of magnetic-responsive parts and consequently produce periodic fluctuations in optical measurements that can be converted by Fourier transform in a dominant frequency peak. The release rate of loaded drug can be controlled by changing the speed of the applied magnetic field, which in turn, is monitored by frequency peak shifting. In this study, doxorubicin was loaded in these nanorods and its release rate was higher when the speed of the rotating magnetic field increased, confirming the controlled release using magnetic modulation. Besides that, a linear decrease in cell viability was shown in HeLa cells as a function of magnetic field speed and release time. In 2013, Xiong et al. [151] produced Rubik-like magnetic nanoassemblies (MNAs) composed of four oleic acid-capped iron oxide nanocubes (Fe3O4@OA NCs) with a shell of dioleate-modified polyethylene glycol (OA2-PEG) (Figure 8A,B). Here, the researchers investigated the antitumor effect of paclitaxel-loaded MNAs in vitro using B16F10 culture cells and in vivo in B16F10 melanoma-bearing mice and demonstrated higher antitumor activity of these nanosystems, compared with the same amount of free paclitaxel (also known as taxol), as well as an enhanced intracellular delivery in the presence of a magnetic field. The pharmacokinetics study allowed concluding that the incorporation of paclitaxel in MNAs increased the amount and retention time of the drug in plasma, also an important parameter to be considered in drug delivery.
Figure 8.
TEM image (A) and schematic representation (B) of Rubik-like paclitaxel-loaded magnetic nanoassemblies. Adapted from [151] with permission from Elsevier, 2020.
3.2. Magnetic Hyperthermia Therapy
Cancer cells can be killed when exposed to high temperatures between 41 and 47 °C in a process known as hyperthermia [152]. Magnetic hyperthermia can be induced when magnetic nanoparticles are exposed to an alternating magnetic field (AMF) and produce heat due to Néel and Brownian relaxations of the rotating magnetic moments [152,153]. Metallic nanoparticles (Fe, Ag, Ni, Gd, TiO2) have the highest saturation magnetization, but their inherent toxicity and chemical instability make them not suitable for biomedical applications. Thus, the most used nanoparticles for hyperthermia applications are iron oxide nanoparticles that show low toxicity, facile fabrication and advantageous physicochemical properties [153,154]. This treatment can be combined with specific delivery of therapeutic drugs into the tumor cells, in order to increase the therapeutic effect and reduce the needed dosage and side effects [152,153,154].
The heating power of magnetic nanoparticles strongly depends on extrinsic and intrinsic properties, namely the external magnetic field amplitude, the saturation magnetization and the anisotropy energy, which in turn, depends on shape of nanoparticles, so a suitable design of these nanoparticles is an important step for enhanced hyperthermia efficiency [155]. The increase of heating efficiency allows the use of a lower dose of nanoparticles, preventing this way undesirable accumulation and cytotoxicity.
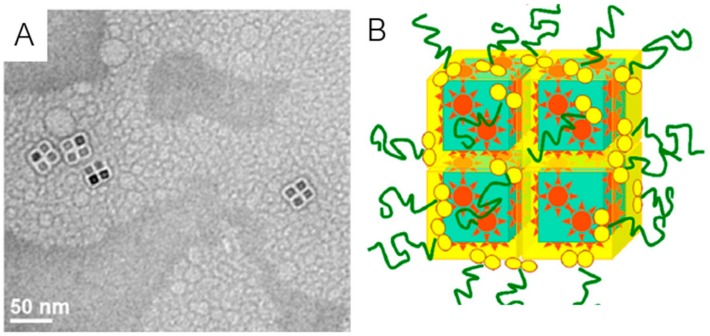
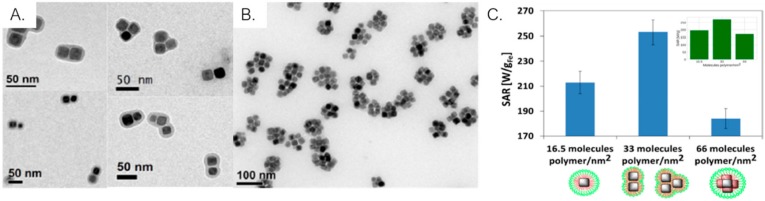
Nanocubes have been extensively studied for hyperthermia applications. Guardia et al. [156] reported significant SAR values of nanocubes with 19 nm of edge length, at frequencies and magnetic field amplitudes considered safe for clinical use (a SAR value of 875 W/g at 320 kHz and 10 kA.m−1), as well as high hyperthermia efficiency, promoting 50% of cancer cell mortality in a temperature range of 40 to 45 °C, at 110 kHz and 20 kA.m−1. Thermo-sensitive coated nanocubes have the ability of combining hyperthermia with chemotherapy by creating a synergistic therapeutic effect through heat triggered drug release, while maintaining their good magnetic properties and high SAR values [157]. The coating of magnetic nanoclusters seems to be a suitable strategy to create colloidally-stable solutions of nanoparticles aggregates taking advantage of magnetic and heating properties of different geometries. Niculaes et al. [158] prepared and compared oleic acid-coated iron oxide nanocubes with different geometries – single nanocubes, dimer and trimer assemblies, and centrosymmetric structures (with more than 4 nanocubes) – in terms of their magnetic properties and SAR values (Figure 9A–C). The SAR value of dimer and trimer structures was higher (253 W/g) than the one obtained for individual nanocubes (213 W/g) and a significantly decrease was observed for centrosymmetric assemblies (184 W/g). Dimer and trimer nanoclusters also had the highest saturation magnetization (MS) values. However, it was demonstrated that not Ms but magnetic dipolar effect is the major factor for the enhancement of SAR values (and not the MS values).
Figure 9.
TEM images of dimer and trimer (A) and centrosymmetric (with more than 4 nanocubes) (B) iron oxide nanoclusters; (C) SAR values of the different nanoassemblies shows that dimer and trimer structures have the highest SAR values. Adapted from [158] with permission from American Chemical Society 2020.
Geng et al. [159] demonstrated that iron oxide nanorods show enhanced specific absorption rates (SAR) (1072 W/g at 33 kA.m−1) comparing with their spherical counterparts (262 W/g). Other comparative study regarding the magnetic properties and heating efficiency of magnetite nanorods and spherical nanoparticles allowed demonstrating that the samples of nanorods possess higher Ms values than the ones with spherical nanoparticles of the same volume [160]. Then, AC (alternating current) hyperthermia experiments showed that the increase of the aspect ratio (for a limited concentration of nanoparticles, 3 mg/mL) and alignment of nanorods in the direction of the AC field are key factors for an enhanced heating efficiency. Additionally, the researchers obtained higher SAR values for magnetite nanorods (862 W/g at 800 Oe) when compared to nanocubes and nanospheres (314 W/g and 140 W/g, respectively).
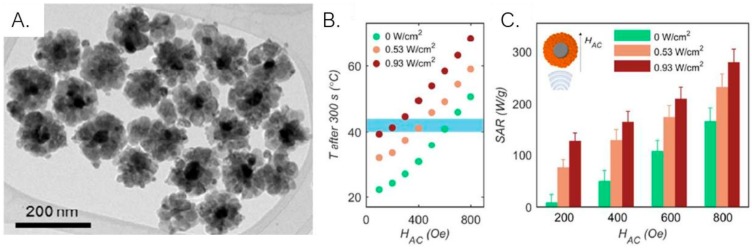
Not only nanorods and nanocubes are promising enhancers of heating efficiency. Magnetite nanodiscs were also investigated and, again, higher SAR values (2 times higher) for nanodiscs were obtained in comparison with their spherical counterparts [161]. More important, it was demonstrated that the high SAR value for nanodiscs in an aqueous solution (4.66 kW/g) is due to their parallel orientation to the applied AC field and can be maintained in a range of orientations of 60° to 90°. Ag/Fe3O4 core-shell nanoflowers were used in combined magnetic hyperthermia and photothermal therapy, taking advantage of both plasmonic and magnetic properties and allowing an increase of more than one order of magnitude of SAR values when a laser is simultaneously applied (Figure 10A–C) [162].
Figure 10.
(A) TEM image of Ag/Fe3O4 nanoflowers; (B) Temperature reached after 300 s of heating for different ac field intensities. The therapeutic temperature window (blue zone) is reached for lower magnetic field intensities when a laser is applied; (C) SAR values of Ag/Fe3O4 nanoflowers as a function of AC field intensity (HAC) for different laser power densities. SAR values are the highest when a laser is simultaneously applied. Adapted from [162] with permission from American Chemical Society, 2020.
As mentioned before, nanotubes have high MS and increased surface area that allows higher drug content due to their hollow morphology. The possibility of enhancing the therapeutic efficiency of a drug is now of major importance, justifying the development of multifunctional nanoparticles capable of a synergistic effect between efficient heating and drug delivery. Following this, Das et al. [163] prepared highly crystalline Fe3O4 nanotubes and measured the SAR values, in water and in 2% agar, for randomly dispersed and magnetically aligned nanotubes. A SAR value of 360 W/g for an AC magnetic field of 800 Oe was obtained for randomly dispersed nanotubes in water. For applied fields of 600 and 800 Oe, nanotubes aligned to the magnetic field had improvements in SAR of 65% and 80%, respectively, comparing to randomly dispersed nanotubes. This, again, confirms the importance of the alignment of anisotropic nanoparticles to the applied magnetic field in order to enhance the heating efficiency.
3.3. Magnetic Resonance Imaging (MRI)
Iron oxide nanoparticles have been extensively exploited as enhanced contrast agents in magnetic resonance imaging (MRI). The main advantages of MRI are high spatial resolution, soft tissue contrast and, most important, the possibility to early detect the presence of tumors, increasing therapy success [164]. Thus, the conception of new strategies that can optimize this technique is of major relevance. Superparamagnetic iron oxide nanoparticles (SPIONs) are strong candidates to be used as contrast agents due to their biocompatibility and ability to increase contrast enhancement [165]. A contrast in MRI image depends on nuclear relaxation times of the tissue protons, which can be longitudinal (T1) or transversal (T2) [166,167,168]. Positive contrast agents (T1) decrease longitudinal relaxation time, resulting in a brighter image, while negative contrast agents (T2) decrease transversal longitudinal relaxation time, causing a darkening in MRI image [166,167,168]. The behavior of a contrast agent essentially depends on longitudinal and transversal relaxivity, r1 and r2, respectively, indicating if it is more susceptible to be positive (T1) or negative (T2) [166,167,168]. Commonly, SPIONs are used for darkening T2-weighted images, but it was already reported that these nanoparticles are also capable to provide positive contrast enhancement, overpassing the toxicity of the usual gadolinium chelates contrast agents [169,170,171].
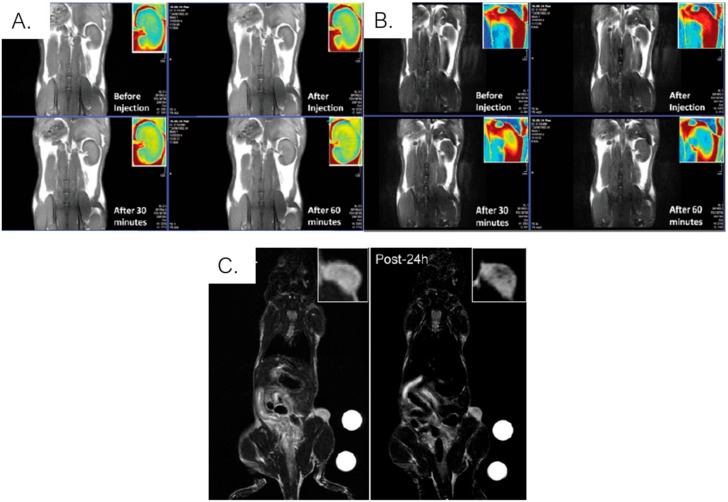
The beforementioned studies regarding the use of iron oxide nanoparticles in drug delivery and hyperthermia treatment evidenced that anisotropy of nanoparticles is a key factor for improving its properties. This way, researchers started to investigate the morphology dependence of nanoparticles in the enhancement of magnetic relaxivity in MRI [172,173]. Lee et al. [174] demonstrated that 22 nm-sized nanocubes have a higher r2 value (761 s−1 mM−1) compared with nanocubes with larger sizes and clear attenuation of the MRI signal in vivo, which opens the possibility of using nanoparticles with smaller sizes and high colloidal stability as T2 contrast agents. Sharma et al. [124] synthesized even smaller magnetite nanocubes (9.7 nm) with potential of simultaneous contrast enhancement of T1 and T2-weighted MRI, acting as dual contrast agents (Figure 11A,B). Later on, Cho et al. [175] prepared BSA-coated assembled iron oxide nanocubes with high r2 relaxivity (~500 s−1 mM−1). These nanoparticles, with an average size of 100 nm, were injected into mice bearing U87-MG tumor cells and a clear darkening of tumor mass was detected, confirming their accumulation (Figure 11C). Moreover, the tumor growth was successfully reduced with magnetic hyperthermia, which demonstrates the potential of these nanoparticles in combined diagnostics and therapy. Zhao et al. [123] synthesized iron oxide octapods with an edge length of 30 nm that exhibited a stronger contrast enhancement of T2-weighted images and a much higher r2 value (679 s−1 mM−1) than spherical iron oxide nanoparticles with similar geometric volume. Furthermore, these nanoparticles produced higher contrast in T2-weighted MR imaging of a hepatic carcinoma than the spherical ones, indicating that iron oxide octapods are efficient as T2 contrast agents. These results demonstrate that anisotropic shapes promote the increase of the effective radii of nanoparticles enhancing their performance in T2-weighted MRI. While the r2 values are influenced by not only the effective radii but also the saturation magnetization, r1 values depend mostly on metal exposure at the surface of nanoparticles that promotes proton coordination and chemical exchange [176,177]. This was emphasized by Zhou et al. [178] with the preparation of several anisotropic iron oxide nanostructures, namely Fe3O4{111} facet exposed plates, truncated octahedrons and tetrahedrons, and Fe3O4{100} facet exposed cubes, concaves, multibranches and assembled structures. In this study, the r1 and r2 values were obtained and compared for all nanostructures and it was demonstrated that Fe3O4{111} exposed cubes, octahedrons, tetrahedrons and Fe3O4{100} exposed cubes (with sizes of both 15 and 21 nm) have a greater T1 relaxation shortening effect than spherical nanoparticles, because of the metal-rich surfaces. The concaves and multibranches had lower r1 values similar to the one for spherical nanoparticles. The highest r1 and r2 values were obtained for 21 nm sized cubes, possibly due to spin-canting effect, in the case of T1 relaxation, and higher Ms and relatively large effective radii, in the case of T2 relaxation, when comparing with the remaining nanoparticles.
Figure 11.
T1- (A) and T2-weighted (B) in vivo MR images before injection, immediately after and after 30 and 60 minutes of silica coated nanocubes. The contrast enhancement is clearly observed in the coronal images of the kidney. Adapted from [124] with permission from Royal Society of Chemistry 2020. (C) T2-weighted in vivo MR images before (left) and 24 h after (right) injection of BSA-coated nanocubes into mice bearing U87-MG tumor cells. The darkening of the tumor mass is clearly visible after the injection of nanoparticles. Adapted from [175] with permission from MDPI 2020.
Among the elongated nanoparticles, nanorods were mentioned before as efficient systems for hyperthermia treatment because of their high MS values. Knowing that higher magnetization leads to a shortening effect of T2 relaxation time, it can be expected that nanorods are good T2 contrast agents. In fact, Mohapatra et al. [88] reported a high r2 value (608 s−1 mM−1) for magnetite nanorods of length 70 nm coated with polyethyleneimine (PEI). These nanorods were able to induce a stronger local magnetic field over a larger volume comparing with spherical nanoparticles with equivalent volume, due to their larger surface area, resulting in a faster dephasing of nearby protons and consequently a higher r2 value is obtained. Later, the same group [179] prepared silica-coated magnetite nanorods (Fe3O4@SiO2) with a length of ~520 nm and an r2 value of 192 s−1 mM−1. The nanorods promoted an r2 darkening effect, exhibited high loading efficiency (65%) of doxorubicin and a pH-stimulated release of the drug. These results indicate the potential of nanorods for combined therapy and diagnosis. The treatment and imaging of colon cancer in mice was investigated by Dehvari et al. [180], who developed pluronic-conjugated superparamagnetic nanorods loaded with paclitaxel. Here, tumor growth was suppressed, and in vivo localization and visualization of the tumor was possible due to the darkening effect promoted by the nanorods in T2-weighted MR images. More recently, elongated magnetite nanoparticles were synthesized by an environmentally friendly hydrothermal method, and it was demonstrated that the presence of larger pores resulted in the highest MS value (97 emu g−1) and transversal relaxivity (r2 = 343 s−1 mM−1) [63]. It is worth to note that the abovementioned iron oxide nanoparticles have higher transversal relaxivity values than the ones obtained for clinically approved T2 contrast agents, such as Ferumoxide (Endorem® or Feridex®) and Ferrixan (Resovist®) with r2 values of ~98 s−1 mM−1 [181] and ~150 s−1 mM−1 [182], respectively. More efficiency is now being achieved in anisotropic nanoparticles for MRI applications and further research on their effects is needed, namely in vitro and in vivo studies, in order to be clinically approved.
4. Cytotoxicity of Iron Oxide Nanoparticles
Besides the development of new strategies of synthesis and production of nanoparticles with different structural characteristics, the interaction of nanoparticles with biological systems is an important factor to be considered for long-term clinical use. The uptake and accumulation of iron oxide nanoparticles in tumor cells will depend on their size, shape, surface charge and coating. It is desirable that a great number of nanoparticles reaches the tumor environment without causing any harm to healthy cells when circulating inside the body. This way, controlled size and shape of iron oxide nanoparticles are fundamental parameters to be considered in their design for biomedical applications. The range of sizes from 10 nm to 100 nm has been reported as the ideal. Nanoparticles with sizes below 10 nm are easily cleared by kidneys, while nanoparticles with sizes larger than 100 nm are phagocytized by macrophages and accumulate in the liver and spleen [183]. Cytotoxicity promoted by iron oxide nanoparticles can result from different mechanisms, such as reactive oxygen species (ROS) production, oxidative stress, membrane damage, genotoxicity and inflammatory responses [184,185]. ROS production is mainly due to the large surface area of iron oxide nanoparticles for generation of free radicals and leaching of iron molecules from the surface by enzymatic degradation. This will lead to oxidative stress and possibly causing cell death by apoptosis or necrosis. The most common way of overcoming this problem and achieve colloidal stability is the surface coating of nanoparticles with different polymers such as dextran, polyethylene glycol (PEG) and liposomes [186].
However, some coatings can cause appreciable cytotoxicity. For example, Feng et al. [187] demonstrated in vivo dose-dependent lethal toxicity of 10 nm poly(ethylenimine)-coated iron oxide nanoparticles. Also, Ying et al. [188] concluded that, in a 24 h assay, iron oxide nanoparticles coated with carboxyl groups have higher toxicity than those with amine groups in A3 human T lymphocytes. Jarockyte et al. [189] prepared magnetite nanoparticles with a hydrodynamic size of about 50 nm and studied the accumulation and toxicity of these nanoparticles in mouse embryonic fibroblasts. After 48 h, nanoparticles were mainly accumulated in the perinuclear region. Then, cell viability was tested for 3 h, 24 h and 48 h, with incubation of 32.5 ng/mL and 65 ng/mL of nanoparticles. After 3 h and 24 h, no cytotoxicity was observed for both concentrations and, after 48 h, a dose-dependent decrease in cell viability is observed.
Not only size but also the morphology of nanoparticles can influence cytotoxicity [190,191]. In vitro, shape will mostly influence the nanoparticle-cell membrane interaction, which in turn will influence cellular uptake. Rod-shaped nanoparticles seem to have a more favorable cellular uptake when compared with spherical nanoparticles, due to the larger area of contact between the cell and the nanoparticle, so it is expectable that nanoparticles with this shape will accumulate in higher extent in tumor cells. On the other hand, a higher accumulation of these nanoparticles can lead to cell necrosis of non-tumorigenic cells, as demonstrated by Lee et al. [192]. In this study, rod-shaped iron oxide nanoparticles were found to be more cytotoxic to mouse macrophage cells than the spherical counterparts. On the other hand, Hu et al. [193] showed that iron oxide nanocubes produced low cytotoxicity up to 0.5 mg Fe mL−1 in a mice monocyte macrophage cell line.
In vitro and in vivo studies show that cytotoxicity is size-, shape- and dose-dependent, but these parameters differ according to the type of cell or tissue in which nanoparticles are being investigated [194,195,196,197]. So, further investigation on iron oxide nanotoxicity in different types of tissues is needed to achieve a more consistent knowledge on which range of sizes is the most suitable for a certain organ and which concentration is safe to be applied.
5. Conclusions and Future Perspectives
Over the past years, many different strategies have been developed for the reproducible synthesis of high-quality monodisperse anisotropic iron oxide-based magnetic nanoparticles, either through physical, chemical or biological methods. Although most of the developed strategies are focused solely in magnetite, methods for the synthesis of cobalt, manganese or nickel-doped ferrites have started to be a target of research. Remarkably, the number of reported syntheses of anisotropic shape nanoparticles has been continuously increasing, which is expected to improve further owing to the progress of scientific theories ruling the shape control. Yet, scaling remains a problem to be addressed and an evaluation of the therapeutic gains over the cost of production needs to be evaluated in future works, so to facilitate shape anisotropic synthesis at industrial scale with the desired properties.
Considering the nanoparticles shapes discussed in this article, the synthesis of elongate-shaped nanoparticles has been of major interest for drug delivery applications, owing to the superparamagnetic behaviour, the control over drug-release profiles and biocompatibility. Furthermore, elongated nanoparticles are able to induce a stronger local magnetic field over a larger volume than spherical nanoparticles with equivalent volume, making them highly important as T2 contrast agents. Particularly, nanotubes enable the possibility of differential functionalization of the inner and outer surfaces. Thus, the inner voids can be optimized for the loading of bioactive compounds, while the surface might be coated or functionalized with targeting ligands.
The high saturation magnetization of shape anisotropic nanoparticles allows the use of a lower dose of nanoparticles, averting undesirable adverse and side effects. Particularly, nanorods and nanocubes have shown promising hyperthermia results for cancer therapy. However, challenges still remain at the chemical composition level, as magnetite is prone to the generation of reactive oxygen species. Such can be overcome through investigation of other chemical compositions, such as ferrites doped with calcium or magnesium, or surface passivation with an inert material.
Hereby, future works should be focused on the facile fabrication of low toxicity anisotropic nanoparticles with other ferrite chemical composition, so that the physicochemical properties can be optimized and reduce the needed dosage of nanoparticles but also of the therapeutic drugs, and their associated side effects. Also, the development of nanoparticles that combine a plasmonically-active domain are of high interest, due to the possibility of taking advantage of both plasmonic and magnetic properties, such as the attainment of higher SAR values when a laser is simultaneously applied without requiring high magnetic hyperthermia conditions.
Author Contributions
Conceptualization, R.G.D.A., S.R.S.V. and E.M.S.C.; Writing—Original Draft Preparation, R.G.D.A. and S.R.S.V.; Writing—Review and Editing, E.M.S.C.; Supervision, E.M.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Portuguese Foundation for Science and Technology (FCT) in the framework of the Strategic Funding of CF-UM-UP (UID/FIS/04650/2019) and through the research project PTDC/QUI–QFI/28020/2017 (POCI-01–0145-FEDER-028020), financed by European Fund of Regional Development (FEDER), COMPETE2020 and Portugal2020. The APC was also funded by FCT. S.R.S. Veloso acknowledges FCT for a PhD grant (SFRH/BD/144017/2019).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chen G., Roy I., Yang C., Prasad P. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016;116:2826–2885. doi: 10.1021/acs.chemrev.5b00148. [DOI] [PubMed] [Google Scholar]
- 2.Shi J., Kantoff P., Wooster R., Farokhzad O. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer. 2016;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akbarzadeh A., Samiei M., Davaran S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012;7:1–13. doi: 10.1186/1556-276X-7-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim B., Rutka J., Chan W. Nanomedicine. N. Engl. J. Med. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 5.Kolhatkar A., Jamison A., Litvinov D., Willson R., Lee T. Tuning the magnetic properties of nanoparticles. Int. J. Mol. Sci. 2013;14:15977–16009. doi: 10.3390/ijms140815977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang C., Hou Y., Gao S. Nanomagnetism: Principles, nanostructures, and biomedical applications. Chin. Phys. B. 2014;23:1–8. doi: 10.1088/1674-1056/23/5/057505. [DOI] [Google Scholar]
- 7.Issa B., Obaidat I., Albiss B., Haik Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013;14:21266–21305. doi: 10.3390/ijms141121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathew D., Juang R. An overview of the structure and magnetism of spinel ferrite nanoparticles and their synthesis in microemulsions. Chem. Eng. J. 2007;129:51–65. doi: 10.1016/j.cej.2006.11.001. [DOI] [Google Scholar]
- 9.Rana S., Gallo A., Srivastava R., Misra R. On the suitability of nanocrystalline ferrites as a magnetic carrier for drug delivery: Functionalization, conjugation and drug release kinetics. Acta Biomater. 2007;3:233–242. doi: 10.1016/j.actbio.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Sulaiman N., Ghazali M., Majlis B., Yunas J., Razali M. Superparamagnetic calcium ferrite nanoparticles synthesized using a simple sol-gel method for targeted drug delivery. Biomed. Mater. Eng. 2015;26:103–110. doi: 10.3233/BME-151295. [DOI] [PubMed] [Google Scholar]
- 11.Dey C., Baishya K., Ghosh A., Goswami M., Ghosh A., Mandal K. Improvement of drug delivery by hyperthermia treatment using magnetic cubic cobalt ferrite nanoparticles. J. Magn. Magn. Mater. 2017;427:168–174. doi: 10.1016/j.jmmm.2016.11.024. [DOI] [Google Scholar]
- 12.Lee S., Bae S., Takemura Y., Shim I., Kim T., Kim J., Lee H., Zurn S., Kim C. Self-heating characteristics of cobalt ferrite nanoparticles for hyperthermia application. J. Magn. Magn. Mater. 2007;310:2868–2870. doi: 10.1016/j.jmmm.2006.11.080. [DOI] [Google Scholar]
- 13.Doaga A., Cojocariu A., Amin W., Heib F., Bender P., Hempelmann R., Caltun O. Synthesis and characterizations of manganese ferrites for hyperthermia applications. Mater. Chem. Phys. 2013;143:305–310. doi: 10.1016/j.matchemphys.2013.08.066. [DOI] [Google Scholar]
- 14.Sharifi I., Shokrollahi H., Amiri S. Ferrite-based magnetic nanofluids used in hyperthermia applications. J. Magn. Magn. Mater. 2012;324:903–915. doi: 10.1016/j.jmmm.2011.10.017. [DOI] [Google Scholar]
- 15.Shultz M., Calvin S., Fatouros P., Morrison S., Carpenter E. Enhanced ferrite nanoparticles as MRI contrast agents. J. Magn. Magn. Mater. 2007;311:464–468. doi: 10.1016/j.jmmm.2006.10.1188. [DOI] [Google Scholar]
- 16.Tromsdorf U., Bigall N., Kaul M., Bruns O., Nikolic M., Mollwitz B., Sperling R., Reimer R., Hohenberg H., Parak W., et al. Size and surface effects on the MRI relaxivity of manganese ferrite nanoparticle contrast agents. Nano Lett. 2007;7:2422–2427. doi: 10.1021/nl071099b. [DOI] [PubMed] [Google Scholar]
- 17.Bárcena C., Sra A., Chaubey G., Khemtong C., Liu J., Gao J. Zinc ferrite nanoparticles as MRI contrast agents. Chem. Comm. 2008;19:2224–2226. doi: 10.1039/b801041b. [DOI] [PubMed] [Google Scholar]
- 18.Yang L., Zhou Z., Song J., Chen X. Anisotropic nanomaterials for shape-dependent physicochemical and biomedical applications. Chem. Soc. Rev. 2019;48:5140–5176. doi: 10.1039/C9CS00011A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islam M., Masud M., Nguyen N., Gopalan V., Alamri H., Alothman Z., Hossain M., Yamauchi Y., Lamd A., Shiddiky M. Gold-loaded nanoporous ferric oxide nanocubes for electrocatalytic detection of microRNA at attomolar level. Biosens. Bioelectron. 2018;101:275–281. doi: 10.1016/j.bios.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Orza A., Wu H., Xu Y., Lu Q., Mao H. One-step facile synthesis of highly magnetic and surface functionalized iron oxide nanorods for biomarker-targeted applications. ACS Appl. Mater. Interfaces. 2017;9:20719–20727. doi: 10.1021/acsami.7b02575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling T., Yan D., Jiao Y., Wang H., Zheng Y., Zheng X., Mao J., Du X., Hu Z., Jaroniec M., et al. Engineering surface atomic structure of single-crystal cobalt (II) oxide nanorods for superior electrocatalysis. Nat. Commun. 2016;7:12876. doi: 10.1038/ncomms12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azevedo J., Fernández-García M., Magén C., Mendes A., Araújo J., Sousa C. Double-walled iron oxide nanotubes via selective chemical etching and Kirkendall process. Sci. Rep. 2019;9:11994. doi: 10.1038/s41598-019-47704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lisjak D., Mertelj A. Anisotropic magnetic nanoparticles: A review of their properties, syntheses and potential applications. Prog. Mater. Sci. 2018;95:286–328. doi: 10.1016/j.pmatsci.2018.03.003. [DOI] [Google Scholar]
- 24.Xie W., Guo Z., Gao F., Gao Q., Wang D., Liaw B., Cai Q., Sun X., Wang X., Zhao L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics. 2018;8:3284–3307. doi: 10.7150/thno.25220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruschi M., de Toledo L. Pharmaceutical applications of iron-oxide magnetic nanoparticles. Magnetochemistry. 2019;5:50. doi: 10.3390/magnetochemistry5030050. [DOI] [Google Scholar]
- 26.Hosu O., Tertis M., Cristea C. Implication of magnetic nanoparticles in cancer detection, screening and treatment. Magnetochemistry. 2019;5:55. doi: 10.3390/magnetochemistry5040055. [DOI] [Google Scholar]
- 27.Amendola V., Meneghetti M. What controls the composition and the structure of nanomaterials generated by laser ablation in liquid solution? Phys. Chem. Chem. Phys. 2013;15:3027–3046. doi: 10.1039/C2CP42895D. [DOI] [PubMed] [Google Scholar]
- 28.Arias L., Pessan J., Vieira A., Lima T., Delbem A., Monteiro D. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics. 2018;7:46. doi: 10.3390/antibiotics7020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amendola V., Meneghetti M., Granozzi G., Agnoli S., Polizzi S., Riello P., Boscaini A., Anselmi C., Fracasso G., Colombatti M., et al. Top-down synthesis of multifunctional iron oxide nanoparticles for macrophage labelling and manipulation. J. Mater. Chem. 2011;21:3803. doi: 10.1039/c0jm03863f. [DOI] [Google Scholar]
- 30.Amendola V., Riello P., Meneghetti M. Magnetic nanoparticles of iron carbide, iron oxide, iron@iron oxide, and metal iron synthesized by laser ablation in organic solvents. J. Phys. Chem. C. 2010;115:5140–5146. doi: 10.1021/jp109371m. [DOI] [Google Scholar]
- 31.Xu J., Zhang F., Sun J., Sheng J., Wang F., Sun M. Bio and nanomaterials based on Fe3O4. Molecules. 2014;19:21506–21528. doi: 10.3390/molecules191221506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali A., Zafar H., Zia M., ul Haq I., Phull A., Ali J., Hussain A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016;9:49–67. doi: 10.2147/NSA.S99986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majidi S., Zeinali Sehrig F., Farkhani S., Soleymani Goloujeh M., Akbarzadeh A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2014;44:722–734. doi: 10.3109/21691401.2014.982802. [DOI] [PubMed] [Google Scholar]
- 34.Zhang D., Liu Z., Han S., Li C., Lei B., Stewart M., Tour J., Zhou C. Magnetite (Fe3O4) core–shell nanowires: Synthesis and magnetoresistance. Nano Lett. 2004;4:2151–2155. doi: 10.1021/nl048758u. [DOI] [Google Scholar]
- 35.Liu F., Cao P., Zhang H., Tian J., Xiao C., Shen C., Li J., Gao H. Novel nanopyramid arrays of magnetite. Adv. Mater. 2005;17:1893–1897. doi: 10.1002/adma.200500367. [DOI] [Google Scholar]
- 36.Mathur S., Barth S., Werner U., Hernandez-Ramirez F., Romano-Rodriguez A. Chemical vapor growth of one-dimensional magnetite nanostructures. Adv. Mater. 2008;20:1550–1554. doi: 10.1002/adma.200701448. [DOI] [Google Scholar]
- 37.Ding Y., Morber J., Snyder R., Wang Z. Nanowire structural evolution from Fe3O4 to ϵ-Fe2O3. Adv. Funct. Mater. 2007;17:1172–1178. doi: 10.1002/adfm.200601024. [DOI] [Google Scholar]
- 38.Movlaee K., Ganjali M., Norouzi P., Neri G. Iron-based nanomaterials/graphene composites for advanced electrochemical sensors. Nanomaterials. 2017;7:406. doi: 10.3390/nano7120406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahn T., Kim J., Yang H., Lee J., Kim J. Formation pathways of magnetite nanoparticles by coprecipitation method. J. Phys. Chem. C. 2012;116:6069–6076. doi: 10.1021/jp211843g. [DOI] [Google Scholar]
- 40.Zhang W., Jia S., Wu Q., Ran J., Wu S., Liu Y. Convenient synthesis of anisotropic Fe3O4 nanorods by reverse co-precipitation method with magnetic field-assisted. Mater. Lett. 2011;65:1973–1975. doi: 10.1016/j.matlet.2011.03.101. [DOI] [Google Scholar]
- 41.Shen L., Qiao Y., Guo Y., Meng S., Yang G., Wu M., Zhao J. Facile co-precipitation synthesis of shape-controlled magnetite nanoparticles. Ceram. Int. 2014;40:1519–1524. doi: 10.1016/j.ceramint.2013.07.037. [DOI] [Google Scholar]
- 42.Unni M., Uhl A., Savliwala S., Savitzky B., Dhavalikar R., Garraud N., Arnold D., Kourkoutis L., Andrew J., Rinaldi C. Thermal decomposition synthesis of iron oxide nanoparticles with diminished magnetic dead layer by controlled addition of oxygen. ACS Nano. 2017;11:2284–2303. doi: 10.1021/acsnano.7b00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lian S., Wang E., Kang Z., Bai Y., Gao L., Jiang M., Hu C., Xu L. Synthesis of magnetite nanorods and porous hematite nanorods. Solid State Commun. 2004;129:485–490. doi: 10.1016/j.ssc.2003.11.043. [DOI] [Google Scholar]
- 44.Li W., Yao X., Guo Z., Liu J., Huang X. Fe3O4 with novel nanoplate-stacked structure: Surfactant-free hydrothermal synthesis and application in detection of heavy metal ions. J. Electroanal. Chem. 2015;749:75–82. doi: 10.1016/j.jelechem.2015.04.038. [DOI] [Google Scholar]
- 45.Zhu T., Chen J., Lou X. Glucose-assisted one-pot synthesis of FeOOH nanorods and their transformation to Fe3O4@carbon nanorods for application in lithium ion batteries. J. Phys. Chem. C. 2011;115:9814–9820. doi: 10.1021/jp2013754. [DOI] [Google Scholar]
- 46.Sundar S., Venkatachalam G., Kwon S. Sol-gel mediated greener synthesis of γ-Fe2O3 nanostructures for the selective and sensitive determination of uric acid and dopamine. Catalysts. 2018;8:512. doi: 10.3390/catal8110512. [DOI] [Google Scholar]
- 47.Woo K., Lee H., Ahn J., Park Y. Sol-gel mediated synthesis of Fe2O3 nanorods. Adv. Mater. 2003;15:1761–1764. doi: 10.1002/adma.200305561. [DOI] [Google Scholar]
- 48.Kumar R., Koltypin Y., Xu X., Yeshurun Y., Gedanken A., Felner I. Fabrication of magnetite nanorods by ultrasound irradiation. J. Appl. Phys. 2001;89:6324–6328. doi: 10.1063/1.1369408. [DOI] [Google Scholar]
- 49.Abbas M., Takahashi M., Kim C. Facile sonochemical synthesis of high-moment magnetite (Fe3O4) nanocube. J. Nanopart. Res. 2013;15:1354. doi: 10.1007/s11051-012-1354-y. [DOI] [Google Scholar]
- 50.Zhang D., Tong Z., Li S., Zhang X., Ying A. Fabrication and characterization of hollow Fe3O4 nanospheres in a microemulsion. Mater. Lett. 2008;62:4053–4055. doi: 10.1016/j.matlet.2008.05.023. [DOI] [Google Scholar]
- 51.Cabrera L., Gutierrez S., Menendez N., Morales M., Herrasti P. Magnetite nanoparticles: Electrochemical synthesis and characterization. Electrochim. Acta. 2008;53:3436–3441. doi: 10.1016/j.electacta.2007.12.006. [DOI] [Google Scholar]
- 52.Karami H., Chidar E. Pulsed-electrochemical synthesis and characterizations of magnetite nanorods. Int. J. Electrochem. Sci. 2012;7:2077–2090. [Google Scholar]
- 53.Bharde A., Wani A., Shouche Y., Joy P., Prasad B., Sastry M. Bacterial aerobic synthesis of nanocrystalline magnetite. J. Am. Chem. Soc. 2005;127:9326–9327. doi: 10.1021/ja0508469. [DOI] [PubMed] [Google Scholar]
- 54.Tuo Y., Liu G., Dong B., Zhou J., Wang A., Wang J., Jin R., Lv H., Dou Z., Huang W. Microbial synthesis of Pd/Fe3O4, Au/Fe3O4 and PdAu/Fe3O4 nanocomposites for catalytic reduction of nitroaromatic compounds. Sci. Rep. 2015;5:13515. doi: 10.1038/srep13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu Z., Yang S., Wu W. Shape control of inorganic nanoparticles from solution. Nanoscale. 2016;8:1237–1259. doi: 10.1039/C5NR07681A. [DOI] [PubMed] [Google Scholar]
- 56.Qiao L., Fu Z., Li J., Ghosen J., Zeng M., Stebbins J., Prasad P., Swihart M. Standardizing size- and shape-controlled synthesis of monodisperse magnetite (Fe3O4) nanocrystals by identifying and exploiting effects of organic impurities. ACS Nano. 2017;11:6370–6381. doi: 10.1021/acsnano.7b02752. [DOI] [PubMed] [Google Scholar]
- 57.Kasparis G., Erdocio A., Tuffnell J., Thanh N. Synthesis of size-tuneable β-FeOOH nanoellipsoids and a study of their morphological and compositional changes by reduction. CrystEngComm. 2019;21:1293–1301. doi: 10.1039/C8CE01778F. [DOI] [Google Scholar]
- 58.Li S., Qin T.G., Pei W., Ren Y., Zhang Y., Esling C., Zuo L. Capping groups induced size and shape evolution of magnetite particles under hydrothermal condition and their magnetic properties. J. Am. Ceram. Soc. 2009;92:631–635. doi: 10.1111/j.1551-2916.2009.02928.x. [DOI] [Google Scholar]
- 59.Fatima H., Lee D., Yun H., Kim K. Shape-controlled synthesis of magnetic Fe3O4 nanoparticles with different iron precursors and capping agents. RSC Adv. 2018;8:22917–22923. doi: 10.1039/C8RA02909A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daniel P., Shylin S., Lu H., Tahir M., Panthöfer M., Weidner T., Möller A., Ksenofontov V., Tremel W. The surface chemistry of iron oxide nanocrystals: Surface reduction of γ-Fe2O3 to Fe3O4 by redox-active catechol surface ligands. J. Mater. Chem. C. 2018;6:326–333. doi: 10.1039/C7TC04795A. [DOI] [Google Scholar]
- 61.Ramzannezhad A., Gill P., Bahari A. Fabrication of magnetic nanorods and their applications in medicine. BioNanoMaterials. 2017;18:20170008. doi: 10.1515/bnm-2017-0008. [DOI] [Google Scholar]
- 62.Schrittwieser S., Reichinger D., Schotter J. Applications, surface modification and functionalization of nickel nanorods. Materials. 2017;11:45. doi: 10.3390/ma11010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Avolio M., Gavilán H., Mazario E., Brero F., Arosio P., Lascialfari A., Puerto Morales M. Elongated magnetic nanoparticles with high-aspect ratio: A nuclear relaxation and specific absorption rate investigation. Phys. Chem. Chem. Phys. 2019;21:18741–18752. doi: 10.1039/C9CP03441B. [DOI] [PubMed] [Google Scholar]
- 64.Mazuel F., Mathieu S., Di Corato R., Bacri J., Meylheuc T., Pellegrino T., Reffay M., Wilhelm C. Forced- and self-rotation of magnetic nanorods assembly at the cell membrane: A biomagnetic torsion pendulum. Small. 2017;13:1701274. doi: 10.1002/smll.201701274. [DOI] [PubMed] [Google Scholar]
- 65.Kwak M., Jung I., Kang Y., Lee D., Park S. Multi-block magnetic nanorods for controlled drug release modulated by Fourier transform surface plasmon resonance. Nanoscale. 2018;10:18690–18695. doi: 10.1039/C8NR05412F. [DOI] [PubMed] [Google Scholar]
- 66.Schlörb H., Haehnel V., Khatri M., Srivastav A., Kumar A., Schultz L., Fähler S. Magnetic nanowires by electrodeposition within templates. Phys. Status Solidi. 2010;247:2364–2379. doi: 10.1002/pssb.201046189. [DOI] [Google Scholar]
- 67.Pecko D., Arshad M., Sturm S., Kobe S., Rozman K. Magnetization-switching study of fcc Fe–Pd nanowire and nanowire arrays studied by in-field magnetic force microscopy. IEEE Trans. Magn. 2015;51:1–4. doi: 10.1109/TMAG.2015.2449773. [DOI] [Google Scholar]
- 68.Li C., Wu Q., Yue M., Xu H., Palaka S., Elkins K., Ping Liu J. Manipulation of morphology and magnetic properties in cobalt nanowires. AIP Adv. 2017;7:056229. doi: 10.1063/1.4977890. [DOI] [Google Scholar]
- 69.Huang W., Yang F., Zhu L., Qiao R., Zhao Y. Manipulation of magnetic nanorod clusters in liquid by non-uniform alternating magnetic fields. Soft Matter. 2017;13:3750–3759. doi: 10.1039/C7SM00488E. [DOI] [PubMed] [Google Scholar]
- 70.Ding Y., Liu F., Jiang Q., Du B., Sun H. 12-Hydrothermal synthesis and characterization of Fe3O4 nanorods. J. Inorg. Organomet. Polym. 2012;23:379–384. doi: 10.1007/s10904-012-9789-2. [DOI] [Google Scholar]
- 71.Wan J., Chen X., Wang Z., Yang X., Qian Y. A soft-template-assisted hydrothermal approach to single-crystal Fe3O4 nanorods. J. Cryst. Growth. 2005;276:571–576. doi: 10.1016/j.jcrysgro.2004.11.423. [DOI] [Google Scholar]
- 72.Feng L., Jiang L., Mai Z., Zhu D. Polymer-controlled synthesis of Fe3O4 single-crystal nanorods. J. Colloid Interface Sci. 2004;278:372–375. doi: 10.1016/j.jcis.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 73.Han C., Ma J., Wu H., Yao W., Hu K. A low-cost and high-yield production of magnetite nanorods with high saturation magnetization. J. Chil. Chem. Soc. 2015;60:2799–2802. doi: 10.4067/S0717-97072015000100005. [DOI] [Google Scholar]
- 74.Xi G., Wang C., Wang X. The Oriented self-assembly of magnetic Fe3O4 nanoparticles into monodisperse microspheres and their use as substrates in the formation of Fe3O4 nanorods. Eur. J. Inorg. Chem. 2008;2008:425–431. doi: 10.1002/ejic.200700863. [DOI] [Google Scholar]
- 75.Hou X., Feng J., Xu X., Zhang M. Synthesis and characterizations of spinel MnFe2O4 nanorod by seed-hydrothermal route. J. Alloy. Compd. 2010;491:258–263. doi: 10.1016/j.jallcom.2009.10.029. [DOI] [Google Scholar]
- 76.Sodaee T., Ghasemi A., Razavi R. Controlled growth of large-area arrays of gadolinium-substituted cobalt ferrite nanorods by hydrothermal processing without use of any template. Ceram. Int. 2016;42:17420–17428. doi: 10.1016/j.ceramint.2016.08.042. [DOI] [Google Scholar]
- 77.Jia Z., Ren D., Zhu R. Synthesis, characterization and magnetic properties of CoFe2O4 nanorods. Mater. Lett. 2012;66:128–131. doi: 10.1016/j.matlet.2011.08.056. [DOI] [Google Scholar]
- 78.Ji G., Tang S., Ren S., Zhang F., Gu B., Du Y. Simplified synthesis of single-crystalline magnetic CoFe2O4 nanorods by a surfactant-assisted hydrothermal process. J. Cryst. Growth. 2004;270:156–161. doi: 10.1016/j.jcrysgro.2004.06.025. [DOI] [Google Scholar]
- 79.Gao Y., Zhao Y., Jiao Q., Li H. Microemulsion-based synthesis of porous Co–Ni ferrite nanorods and their magnetic properties. J. Alloy. Compd. 2013;555:95–100. doi: 10.1016/j.jallcom.2012.12.057. [DOI] [Google Scholar]
- 80.Singh S., Yadav B., Prakash R., Bajaj B., Lee J. Synthesis of nanorods and mixed shaped copper ferrite and their applications as liquefied petroleum gas sensor. Appl. Surf. Sci. 2011;257:10763–10770. doi: 10.1016/j.apsusc.2011.07.094. [DOI] [Google Scholar]
- 81.Huang Z., Zhang Y., Tang F. Solution-phase synthesis of single-crystalline magnetic nanowires with high aspect ratio and uniformity. Chem. Commun. 2005:342–344. doi: 10.1039/b410463c. [DOI] [PubMed] [Google Scholar]
- 82.Cui H., Shi J., Yuan B., Fu M. Synthesis of porous magnetic ferrite nanowires containing Mn and their application in water treatment. J. Mater. Chem. A. 2013;1:5902. doi: 10.1039/c3ta01692g. [DOI] [Google Scholar]
- 83.Ji G., Tang S., Xu B., Gu B., Du Y. Synthesis of CoFe2O4 nanowire arrays by sol-gel template method. Chem. Phys. Lett. 2003;379:484–489. doi: 10.1016/j.cplett.2003.08.090. [DOI] [Google Scholar]
- 84.El-Sheikh S., Harraz F., Hessien M. Magnetic behavior of cobalt ferrite nanowires prepared by template-assisted technique. Mater. Chem. Phys. 2010;123:254–259. doi: 10.1016/j.matchemphys.2010.04.005. [DOI] [Google Scholar]
- 85.Liu Z., Zhang D., Han S., Li C., Lei B., Lu W., Fang J., Zhou C. Single crystalline magnetite nanotubes. ChemInform. 2005;127:6–7. doi: 10.1021/ja0445239. [DOI] [PubMed] [Google Scholar]
- 86.Menchaca-Nal S., Londoño-Calderón C., Cerrutti P., Foresti M., Pampillo L., Bilovol V., Candal R., Martínez-García R. Facile synthesis of cobalt ferrite nanotubes using bacterial nanocellulose as template. Carbohyd. Polym. 2016;137:726–731. doi: 10.1016/j.carbpol.2015.10.068. [DOI] [PubMed] [Google Scholar]
- 87.Ji G., Su H., Tang S., Du Y., Xu B. Simplified synthesis of cobalt ferrite nanotubes using sol–gel method. Chem. Lett. 2005;34:86–87. doi: 10.1246/cl.2005.86. [DOI] [Google Scholar]
- 88.Mohapatra J., Mitra A., Tyagi H., Bahadur D., Aslam M. Iron oxide nanorods as high-performance magnetic resonance imaging contrast agents. Nanoscale. 2015;7:9174–9184. doi: 10.1039/C5NR00055F. [DOI] [PubMed] [Google Scholar]
- 89.Moon J., Wei A. Uniform gold nanorod arrays from polyethylenimine-coated alumina templates. J. Phys. Chem. B. 2005;109:23336–23341. doi: 10.1021/jp054405n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen L., Yin Y., Chen C., Chiou J. Influence of polyethyleneimine and ammonium on the growth of zno nanowires by hydrothermal method. J. Phys. Chem. C. 2011;115:20913–20919. doi: 10.1021/jp2056199. [DOI] [Google Scholar]
- 91.Mourdikoudis S., Liz-Marzán L. Oleylamine in nanoparticle synthesis. Chem. Mater. 2013;25:1465–1476. doi: 10.1021/cm4000476. [DOI] [Google Scholar]
- 92.Lalwani S., Marichi R., Mishra M., Gupta G., Singh G., Sharma R. Edge enriched cobalt ferrite nanorods for symmetric/asymmetric supercapacitive charge storage. Electrochim. Acta. 2018;283:708–717. doi: 10.1016/j.electacta.2018.07.008. [DOI] [Google Scholar]
- 93.Zukova A., Teiserskis A., Rohava Y., Baranov A., van Dijken S., Gun’ko Y. Deposition of magnetite nanofilms by pulsed injection MOCVD in a magnetic field. Nanomaterials. 2018;8:1064. doi: 10.3390/nano8121064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grigoriev D., Gorin D., Sukhorukov G., Yashchenok A., Maltseva E., Möhwald H. Polyelectrolyte/magnetite nanoparticle multilayers: Preparation and structure characterization. Langmuir. 2007;23:12388–12396. doi: 10.1021/la700963h. [DOI] [PubMed] [Google Scholar]
- 95.Gorin D., Yashchenok A., Koksharov Y., Neveshkin A., Serdobintsev A., Grigoriev D., Khomutov G. Surface morphology and optical and magnetic properties of polyelectrolyte/magnetite nanoparticles nanofilms. Tech. Phys. 2009;54:1675–1680. doi: 10.1134/S1063784209110206. [DOI] [Google Scholar]
- 96.Kafshgari L., Ghorbani M., Azizi A. Synthesis and characterization of manganese ferrite nanostructure by co-precipitation, sol-gel, and hydrothermal methods. Particul. Sci. Technol. 2018;37:904–910. doi: 10.1080/02726351.2018.1461154. [DOI] [Google Scholar]
- 97.De-hui S., De-xin S., Hao Y. Controlled synthesis of Fe3O4 nanosheets via P123 micelle template. Mater. Sci. Forum. 2011;663–665:1125–1128. [Google Scholar]
- 98.Wang W., Zhu Y. Microwave-assisted synthesis of magnetite nanosheets in mixed solvents of ethylene glycol and water. Curr. Nanosci. 2007;3:171–176. doi: 10.2174/157341307780619233. [DOI] [Google Scholar]
- 99.Zhuang L., Zhang W., Zhao Y., Shen H., Lin H., Liang J. Preparation and characterization of Fe3O4 particles with novel nanosheets morphology and magnetochromatic property by a modified solvothermal method. Sci. Rep. 2015;5:9320. doi: 10.1038/srep09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chin K., Chong G., Poh C., Van L., Sow C., Lin J., Wee A. Large-scale synthesis of Fe3O4 nanosheets at low temperature. J. Phys. Chem. C. 2007;111:9136–9141. doi: 10.1021/jp070873g. [DOI] [Google Scholar]
- 101.Dong B., Li M., Xiao C., Ding D., Gao G., Ding S. Tunable growth of perpendicular cobalt ferrite nanosheets on reduced graphene oxide for energy storage. Nanotechnology. 2016;28:055401. doi: 10.1088/1361-6528/28/5/055401. [DOI] [PubMed] [Google Scholar]
- 102.Gao H., Xiang J., Cao Y. Hierarchically porous CoFe2O4 nanosheets supported on Ni foam with excellent electrochemical properties for asymmetric supercapacitors. Appl. Surf. Sci. 2017;413:351–359. doi: 10.1016/j.apsusc.2017.04.067. [DOI] [Google Scholar]
- 103.Ravindran Madhura T., Viswanathan P., Gnana kumar G., Ramaraj R. Nanosheet-like manganese ferrite grown on reduced graphene oxide for non-enzymatic electrochemical sensing of hydrogen peroxide. J. Electroanal. Chem. 2017;792:15–22. doi: 10.1016/j.jelechem.2017.03.014. [DOI] [Google Scholar]
- 104.Yao X., Kong J., Tang X., Zhou D., Zhao C., Zhou R., Lu X. Facile synthesis of porous CoFe2O4 nanosheets for lithium-ion battery anodes with enhanced rate capability and cycling stability. RSC Adv. 2014;4:27488–27492. doi: 10.1039/C4RA02835J. [DOI] [Google Scholar]
- 105.Lu J., Jiao X., Chen D., Li W. Solvothermal synthesis and characterization of Fe3O4 and γ-Fe2O3 nanoplates. J. Phys. Chem. C. 2009;113:4012–4017. doi: 10.1021/jp810583e. [DOI] [Google Scholar]
- 106.Moradlou O., Dehghanpour Farashah S., Masumian F., Banazadeh A. Magnetite nanoplates decorated on anodized aluminum oxide nanofibers as a novel adsorbent for efficient removal of As(III) Int. J. Sci. Environ. Technol. 2016;13:1149–1158. doi: 10.1007/s13762-016-0941-3. [DOI] [Google Scholar]
- 107.Cheng J., Ma R., Shi D., Liu F., Zhang X. Rapid growth of magnetite nanoplates by ultrasonic irradiation at low temperature. Ultrason. Sonochem. 2011;18:1038–1042. doi: 10.1016/j.ultsonch.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 108.Zhu J., Chen M., Li D., Jiang D. Facile synthesis and characterisation of hexagonal magnetite nanoplates. Micro Nano Lett. 2013;8:383–385. doi: 10.1049/mnl.2013.0152. [DOI] [Google Scholar]
- 109.Ma M., Zhang Y., Guo Z., Gu N. Facile synthesis of ultrathin magnetic iron oxide nanoplates by Schikorr reaction. Nanoscale Res. Lett. 2013;8:16. doi: 10.1186/1556-276X-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu Z., Wei Z., He P., Duan X., Yang Z., Zhou Y., Jia D. Seed-mediated growth of ultra-thin triangular magnetite nanoplates. Chem. Commun. 2017;53:11052–11055. doi: 10.1039/C7CC05723G. [DOI] [PubMed] [Google Scholar]
- 111.Kamta Tedjieukeng H., Tsobnang P., Fomekong R., Etape E., Joy P., Delcorte A., Lambi J. Structural characterization and magnetic properties of undoped and copper-doped cobalt ferrite nanoparticles prepared by the octanoate coprecipitation route at very low dopant concentrations. RSC Adv. 2018;8:38621–38630. doi: 10.1039/C8RA08532C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Song H., Zink J., Khashab N. Selective Magnetic Evolution of MnxFe1−xO Nanoplates. J. Phys. Chem. C. 2015;119:10740–10748. doi: 10.1021/acs.jpcc.5b01938. [DOI] [Google Scholar]
- 113.Maaz K., Duan J., Karim S., Chen Y., Zhai P., Xu L., Yao H., Liu J. Fabrication and size dependent magnetic studies of NixMn1−xFe 2O4 (x = 0.2) cubic nanoplates. J. Alloy. Compd. 2016;684:656–662. doi: 10.1016/j.jallcom.2016.05.246. [DOI] [Google Scholar]
- 114.Khan M., Duan J., Chen Y., Yao H., Lyu S., Shou H., Heng K., Xu Q. Superparamagnetic nickel–substituted manganese ferrite (Mn0.8Ni0.2Fe2O4) nanoplates as anode materials for lithium-ion batteries. J. Alloy. Compd. 2017;701:147–152. doi: 10.1016/j.jallcom.2017.01.113. [DOI] [Google Scholar]
- 115.Sayed F., Polshettiwar V. Facile and sustainable synthesis of shaped iron oxide nanoparticles: Effect of iron precursor salts on the shapes of iron oxides. Sci. Rep. 2015;5:9733. doi: 10.1038/srep09733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guo C., Xia F., Wang Z., Zhang L., Xi L., Zuo Y. Flowerlike iron oxide nanostructures and their application in microwave absorption. J. Alloy. Compd. 2015;631:183–191. doi: 10.1016/j.jallcom.2015.01.087. [DOI] [Google Scholar]
- 117.Wang D., Yang P., Huang B. Three-dimensional flowerlike iron oxide nanostructures: Morphology, composition and metal ion removal capability. Mater. Res. Bull. 2016;73:56–64. doi: 10.1016/j.materresbull.2015.08.008. [DOI] [Google Scholar]
- 118.Li X., Tian L., Ali Z., Wang W., Zhang Q. Design of flexible dendrimer-grafted flower-like magnetic microcarriers for penicillin G acylase immobilization. J. Mater. Sci. 2017;53:937–947. doi: 10.1007/s10853-017-1581-9. [DOI] [Google Scholar]
- 119.Wang X., Huang H., Li G., Liu Y., Huang J., Yang D. Hydrothermal synthesis of 3D hollow porous Fe3O4 microspheres towards catalytic removal of organic pollutants. Nanoscale Res. Lett. 2014;9:648. doi: 10.1186/1556-276X-9-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gavilán H., Kowalski A., Heinke D., Sugunan A., Sommertune J., Varón M., Bogart L., Posth O., Zeng L., González-Alonso D., et al. Colloidal flower-shaped iron oxide nanoparticles: Synthesis strategies and coatings. Part. Part. Syst. Charact. 2017;34:1700094. doi: 10.1002/ppsc.201700094. [DOI] [Google Scholar]
- 121.Cheng C., Xu F., Gu H. Facile synthesis and morphology evolution of magnetic iron oxide nanoparticles in different polyol processes. New J. Chem. 2011;35:1072. doi: 10.1039/c0nj00986e. [DOI] [Google Scholar]
- 122.Shubitidze F., Kekalo K., Stigliano R., Baker I. Magnetic nanoparticles with high specific absorption rate of electromagnetic energy at low field strength for hyperthermia therapy. J. Appl. Phys. 2015;117:094302. doi: 10.1063/1.4907915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao Z., Zhou Z., Bao J., Wang Z., Hu J., Chi X., Ni K., Wang R., Chen X., Chen Z., et al. Octapod iron oxide nanoparticles as high-performance T2 contrast agents for magnetic resonance imaging. Nat. Commun. 2013;4:2266. doi: 10.1038/ncomms3266. [DOI] [PubMed] [Google Scholar]
- 124.Sharma V., Alipour A., Soran-Erdem Z., Aykut Z., Demir H. Highly monodisperse low-magnetization magnetite nanocubes as simultaneous T1–T2 MRI contrast agents. Nanoscale. 2015;7:10519–10526. doi: 10.1039/C5NR00752F. [DOI] [PubMed] [Google Scholar]
- 125.Kim D., Lee N., Park M., Kim B., An K., Hyeon T. Synthesis of uniform ferrimagnetic magnetite nanocubes. J. Am. Chem. Soc. 2009;131:454–455. doi: 10.1021/ja8086906. [DOI] [PubMed] [Google Scholar]
- 126.Muro-Cruces J., Roca A., López-Ortega A., Fantechi E., del-Pozo-Bueno D., Estradé S., Peiró F., Sepúlveda B., Pineider F., Sangregorio C., et al. Precise size control of the growth of Fe3O4 nanocubes over a wide size range using a rationally designed one-pot synthesis. ACS Nano. 2019;13:7716–7728. doi: 10.1021/acsnano.9b01281. [DOI] [PubMed] [Google Scholar]
- 127.Indira T., Lakshmi P. Magnetic nanoparticles—A Review. Int. J. Pharm. Sci. Nanotechnol. 2010;3:1035–1042. [Google Scholar]
- 128.Zhu K., Ju Y., Xu J., Yang Z., Gao S., Hou Y. Magnetic nanomaterials: Chemical design, synthesis, and potential applications. Acc. Chem. Res. 2018;51:404–413. doi: 10.1021/acs.accounts.7b00407. [DOI] [PubMed] [Google Scholar]
- 129.Gupta A., Naregalkar R., Vaidya V., Gupta M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine. 2007;2:23–39. doi: 10.2217/17435889.2.1.23. [DOI] [PubMed] [Google Scholar]
- 130.Veiseh O., Gunn J., Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010;62:284–304. doi: 10.1016/j.addr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Song Q., Zhang Z. Shape Control and Associated Magnetic Properties of Spinel Cobalt Ferrite Nanocrystals. J. Am. Chem. Soc. 2004;126:6164–6168. doi: 10.1021/ja049931r. [DOI] [PubMed] [Google Scholar]
- 132.Salazar-Alvarez G., Qin J., Šepelák V., Bergmann I., Vasilakaki M., Trohidou K., Ardisson J., Macedo W., Mikhaylova M., Muhammed M., et al. Cubic versus spherical magnetic nanoparticles: The role of surface anisotropy. J. Am. Chem. Soc. 2008;130:13234–13239. doi: 10.1021/ja0768744. [DOI] [PubMed] [Google Scholar]
- 133.Zhen G., Muir B., Moffat B., Harbour P., Murray K., Moubaraki B., Suzuki K., Madsen I., Agron-Olshina N., Waddington L., et al. Comparative study of the magnetic behavior of spherical and cubic superparamagnetic iron oxide nanoparticles. J. Phys. Chem. C. 2010;115:327–334. doi: 10.1021/jp104953z. [DOI] [Google Scholar]
- 134.Fang J. Enhanced permeability and retention effect based nanomedicine, a solution for cancer. World J. Pharmacol. 2015;4:168–171. doi: 10.5497/wjp.v4.i2.168. [DOI] [Google Scholar]
- 135.Greish K. Enhanced permeability and retention effect for selective targeting of anticancer nanomedicine: Are we there yet? Drug Discov. Today Technol. 2012;9:161–166. doi: 10.1016/j.ddtec.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 136.Singh A., Sahoo S. Magnetic nanoparticles: A novel platform for cancer theranostics. Drug Discov. Today. 2014;19:474–481. doi: 10.1016/j.drudis.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 137.Cole A., Yang V., David A. Cancer theranostics: The rise of targeted magnetic nanoparticles. Trends Biotechnol. 2011;29:323–332. doi: 10.1016/j.tibtech.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Castelló J., Gallardo M., Busquets M., Estelrich J. Chitosan (or alginate)-coated iron oxide nanoparticles: A comparative study. Colloids Surf. A Physicochem. Eng. Aspects. 2015;468:151–158. doi: 10.1016/j.colsurfa.2014.12.031. [DOI] [Google Scholar]
- 139.Berry C., Wells S., Charles S., Curtis A. Dextran and albumin derivatised iron oxide nanoparticles: Influence on fibroblasts in vitro. Biomaterials. 2003;24:4551–4557. doi: 10.1016/S0142-9612(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 140.Rodrigues A.R.O., Ramos J., Gomes I.T., Almeida B., Araújo J.P., Queiroz M.J.R.P., Coutinho P.J.G., Castanheira E.M.S. Magnetoliposomes based on manganese ferrite nanoparticles as nanocarriers for antitumor drugs. RSC Adv. 2016;6:17302–17313. doi: 10.1039/C5RA27058H. [DOI] [Google Scholar]
- 141.Couto D., Freitas M., Carvalho F., Fernandes E. Iron oxide nanoparticles: An insight into their biomedical applications. Curr. Med. Chem. 2015;22:1808–1828. doi: 10.2174/0929867322666150311151403. [DOI] [PubMed] [Google Scholar]
- 142.Tran N., Webster T. Magnetic nanoparticles: Biomedical applications and challenges. J. Mater. Chem. 2010;20:8760–8767. doi: 10.1039/c0jm00994f. [DOI] [Google Scholar]
- 143.Wu M., Huang S. Magnetic nanoparticles in cancer diagnosis, drug delivery and treatment (Review) Mol. Clin. Oncol. 2017;7:738–746. doi: 10.3892/mco.2017.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kralj S., Makovec D. Magnetic assembly of superparamagnetic iron oxide nanoparticle clusters into nanochains and nanobundles. ACS Nano. 2015;9:9700–9707. doi: 10.1021/acsnano.5b02328. [DOI] [PubMed] [Google Scholar]
- 145.Nath S., Kaittanis C., Ramachandran V., Dalal N., Perez J. Synthesis, magnetic characterization, and sensing applications of novel dextran-coated iron oxide Nanorods. Chem. Mater. 2009;21:1761–1767. doi: 10.1021/cm8031863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu P., Wang W., Huang Y., Sheu H., Lo Y., Tsai T., Shieh D., Yeh C. Porous iron oxide based nanorods developed as delivery nanocapsules. Chem. A Eur. J. 2007;13:3878–3885. doi: 10.1002/chem.200601372. [DOI] [PubMed] [Google Scholar]
- 147.Son S., Bai X., Nan A., Ghandehari H., Lee S. Template synthesis of multifunctional nanotubes for controlled release. J. Control. Release. 2006;114:143–152. doi: 10.1016/j.jconrel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 148.Son S., Reichel J., He B., Schuchman M., Lee S. Magnetic nanotubes for magnetic-field-assisted bioseparation, biointeraction, and drug delivery. J. Am. Chem. Soc. 2005;127:7316–7317. doi: 10.1021/ja0517365. [DOI] [PubMed] [Google Scholar]
- 149.Yue Z., Wei W., You Z., Yang Q., Yue H., Su Z., Ma G. Iron Oxide Nanotubes for magnetically guided delivery and pH-activated release of insoluble anticancer drugs. Adv. Funct. Mater. 2011;21:3446–3453. doi: 10.1002/adfm.201100510. [DOI] [Google Scholar]
- 150.Yu P., Xia X., Wu M., Cui C., Zhang Y., Liu L., Wu B., Wang C., Zhang L., Zhou X., et al. Folic acid-conjugated iron oxide porous nanorods loaded with doxorubicin for targeted drug delivery. Colloids Surf. B Biointerfaces. 2014;120:142–151. doi: 10.1016/j.colsurfb.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 151.Xiong F., Chen Y., Chen J., Yang B., Zhang Y., Gao H., Hua Z., Gu N. Rubik-like magnetic nanoassemblies as an efficient drug multifunctional carrier for cancer theranostics. J. Control. Release. 2013;172:993–1001. doi: 10.1016/j.jconrel.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 152.Fortin J., Gazeau F., Wilhelm C. Intracellular heating of living cells through Néel relaxation of magnetic nanoparticles. Eur. Biophys. J. 2007;37:223–228. doi: 10.1007/s00249-007-0197-4. [DOI] [PubMed] [Google Scholar]
- 153.Makridis A., Topouridou K., Tziomaki M., Sakellari D., Simeonidis K., Angelakeris M., Yavropoulou M., Yovos J., Kalogirou O. In vitro application of Mn-ferrite nanoparticles as novel magnetic hyperthermia agents. J. Mater. Chem. B. 2014;2:8390–8398. doi: 10.1039/C4TB01017E. [DOI] [PubMed] [Google Scholar]
- 154.Hedayatnasab Z., Abnisa F., Daud W. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater. Des. 2017;123:174–196. doi: 10.1016/j.matdes.2017.03.036. [DOI] [Google Scholar]
- 155.Cotin G., Perton F., Blanco-Andujar C., Pichon B., Mertz D., Bégin-Colin; S. Design of anisotropic iron-oxide-based nanoparticles for magnetic hyperthermia. In: Fratila R.M., De la Fuente J.M., editors. Nanomaterials for Magnetic and Optical Hyperthermia Applications. Elsevier; Amsterdam, The Netherlands: 2019. pp. 41–60. (Micro and Nano Technologies). [Google Scholar]
- 156.Guardia P., Di Corato R., Lartigue L., Wilhelm C., Espinosa A., Garcia-Hernandez M., Gazeau F., Manna L., Pellegrino T. Water-soluble iron oxide Nanocubes with high values of specific absorption rate for cancer cell hyperthermia treatment. ACS Nano. 2012;6:3080–3091. doi: 10.1021/nn2048137. [DOI] [PubMed] [Google Scholar]
- 157.Kakwere H., Leal M., Materia M., Curcio A., Guardia P., Niculaes D., Marotta R., Falqui A., Pellegrino T. Functionalization of strongly interacting magnetic nanocubes with (Thermo)Responsive coating and their application in hyperthermia and heat-triggered drug delivery. ACS Appl. Mater. Interfaces. 2015;7:10132–10145. doi: 10.1021/am5088117. [DOI] [PubMed] [Google Scholar]
- 158.Niculaes D., Lak A., Anyfantis G., Marras S., Laslett O., Avugadda S., Cassani M., Serantes D., Hovorka O., Chantrell R., et al. Asymmetric assembling of iron oxide nanocubes for improving magnetic hyperthermia performance. ACS Nano. 2017;11:12121–12133. doi: 10.1021/acsnano.7b05182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Geng S., Yang H., Ren X., Liu Y., He S., Zhou J., Su N., Li Y., Xu C., Zhang X., et al. Anisotropic magnetite nanorods for enhanced magnetic hyperthermia. Chem. Asian J. 2016;11:2996–3000. doi: 10.1002/asia.201601042. [DOI] [PubMed] [Google Scholar]
- 160.Das R., Alonso J., Nemati Porshokouh Z., Kalappattil V., Torres D., Phan M., Garaio E., García J., Sanchez Llamazares J., Srikanth H. Tunable high aspect ratio iron oxide nanorods for enhanced hyperthermia. J. Phys. Chem. C. 2016;120:10086–10093. doi: 10.1021/acs.jpcc.6b02006. [DOI] [Google Scholar]
- 161.Yang Y., Liu X., Lv Y., Herng T., Xu X., Xia W., Zhang T., Fang J., Xiao W., Ding J. Orientation mediated enhancement on magnetic hyperthermia of Fe3O4 nanodisc. Adv. Funct. Mater. 2014;25:812–820. doi: 10.1002/adfm.201402764. [DOI] [Google Scholar]
- 162.Das R., Rinaldi-Montes N., Alonso J., Amghouz Z., Garaio E., García J., Gorria P., Blanco J., Phan M., Srikanth H. Boosted hyperthermia therapy by combined AC magnetic and photothermal exposures in Ag/Fe3O4 nanoflowers. ACS Appl. Mater. Interfaces. 2016;8:25162–25169. doi: 10.1021/acsami.6b09942. [DOI] [PubMed] [Google Scholar]
- 163.Das R., Cardarelli J., Phan M., Srikanth H. Magnetically tunable iron oxide nanotubes for multifunctional biomedical applications. J. Alloy. Compd. 2019;789:323–329. doi: 10.1016/j.jallcom.2019.03.024. [DOI] [Google Scholar]
- 164.Revia R., Zhang M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today. 2016;19:157–168. doi: 10.1016/j.mattod.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sahoo B., Devi K., Dutta S., Maiti T., Pramanik P., Dhara D. Biocompatible mesoporous silica-coated superparamagnetic manganese ferrite nanoparticles for targeted drug delivery and MR imaging applications. J. Colloid Interface Sci. 2014;431:31–41. doi: 10.1016/j.jcis.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 166.Yadollahpour A., Asl H., Rashidi S. Applications of nanoparticles in magnetic resonance imaging: A comprehensive review. Asian J. Pharm. 2017;11:1–7. [Google Scholar]
- 167.Vuong Q., Gillis P., Roch A., Gossuin Y. Magnetic resonance relaxation induced by superparamagnetic particles used as contrast agents in magnetic resonance imaging: A theoretical review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017;9:1–22. doi: 10.1002/wnan.1468. [DOI] [PubMed] [Google Scholar]
- 168.Bao Y., Sherwood J., Sun Z. Magnetic iron oxide nanoparticles as T1 contrast agents for magnetic resonance imaging. J. Mater. Chem. C. 2018;6:1280–1290. doi: 10.1039/C7TC05854C. [DOI] [Google Scholar]
- 169.Tromsdorf U., Bruns O., Salmen S., Beisiegel U., Weller H. A Highly Effective, nontoxic T1 MR Contrast agent based on Ultrasmall PEGylated iron oxide nanoparticles. Nano Lett. 2009;9:4434–4440. doi: 10.1021/nl902715v. [DOI] [PubMed] [Google Scholar]
- 170.Taboada E., Rodríguez E., Roig A., Oró J., Roch A., Muller R. Relaxometric and magnetic characterization of ultrasmall iron oxide nanoparticles with high magnetization. Evaluation as potential T1 magnetic resonance imaging contrast agents for molecular imaging. Langmuir. 2007;23:4583–4588. doi: 10.1021/la063415s. [DOI] [PubMed] [Google Scholar]
- 171.Huang G., Li H., Chen J., Zhao Z., Yang L., Chi X., Chen Z., Wang X., Gao J. Tunable T1 and T2 contrast abilities of manganese-engineered iron oxide nanoparticles through size control. Nanoscale. 2014;6:10404–10412. doi: 10.1039/C4NR02680B. [DOI] [PubMed] [Google Scholar]
- 172.Park J., von Maltzahn G., Zhang L., Schwartz M., Ruoslahti E., Bhatia S., Sailor M. Magnetic iron oxide nanoworms for tumor targeting and imaging. Adv. Mater. 2008;20:1630–1635. doi: 10.1002/adma.200800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Park M., Lee N., Choi S., An K., Yu S., Kim J., Kwon S., Kim D., Kim H., Baek S., et al. Large-Scale synthesis of Ultrathin manganese oxide nanoplates and their applications to T1 MRI contrast agents. Chem. Mater. 2011;23:3318–3324. doi: 10.1021/cm200414c. [DOI] [Google Scholar]
- 174.Lee N., Choi Y., Lee Y., Park M., Moon W., Choi S., Hyeon T. Water-dispersible ferrimagnetic iron oxide nanocubes with extremely high r2 relaxivity for highly sensitive in vivo MRI of tumors. Nano Lett. 2012;12:3127–3131. doi: 10.1021/nl3010308. [DOI] [PubMed] [Google Scholar]
- 175.Cho M., Cervadoro A., Ramirez M., Stigliano C., Brazdeikis A., Colvin V., Civera P., Key J., Decuzzi P. Assembly of Iron Oxide Nanocubes for Enhanced Cancer Hyperthermia and Magnetic Resonance Imaging. Nanomaterials. 2017;7:72. doi: 10.3390/nano7040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhou Z., Zhao Z., Zhang H., Wang Z., Chen X., Wang R., Chen Z., Gao J. Interplay between longitudinal and transverse contrasts in Fe3O4 Nanoplates with (111) exposed surfaces. ACS Nano. 2014;8:7976–7985. doi: 10.1021/nn5038652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Park J., Baek M., Choi E., Woo S., Kim J., Kim T., Jung J., Chae K., Chang Y., Lee G. Paramagnetic Ultrasmall gadolinium oxide nanoparticles as advanced T1 MRI contrast agent: Account for Large longitudinal relaxivity, optimal particle diameter, and in Vivo T1 MR Images. ACS Nano. 2009;3:3663–3669. doi: 10.1021/nn900761s. [DOI] [PubMed] [Google Scholar]
- 178.Zhou Z., Zhu X., Wu D., Chen Q., Huang D., Sun C., Xin J., Ni K., Gao J. Anisotropic shaped iron oxide nanostructures: Controlled synthesis and proton relaxation shortening effects. Chem. Mater. 2015;27:3505–3515. doi: 10.1021/acs.chemmater.5b00944. [DOI] [Google Scholar]
- 179.Beg M., Mohapatra J., Pradhan L., Patkar D., Bahadur D. Porous Fe3O4-SiO2 core-shell nanorods as high-performance MRI contrast agent and drug delivery vehicle. J. Magn. Magn. Mater. 2017;428:340–347. doi: 10.1016/j.jmmm.2016.12.079. [DOI] [Google Scholar]
- 180.Dehvari K., Chen Y., Tsai Y., Tseng S., Lin K. Superparamagnetic iron oxide nanorod carriers for paclitaxel delivery in the treatment and imaging of colon cancer in mice. J. Biomed. Nanotechnol. 2016;12:1734–1745. doi: 10.1166/jbn.2016.2283. [DOI] [PubMed] [Google Scholar]
- 181.Wang Y.X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011;1:35–40. doi: 10.3978/j.issn.2223-4292.2011.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang Y.X.J. Current status of superparamagnetic iron oxide contrast agents for liver magnetic resonance imaging. World J. Gastroenterol. 2015;21:13400–13402. doi: 10.3748/wjg.v21.i47.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Arami H., Khandhar A., Liggitt D., Krishnan K. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015;44:8576–8607. doi: 10.1039/C5CS00541H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vakili-Ghartavol R., Momtazi-Borojeni A., Vakili-Ghartavol Z., Aiyelabegan H., Jaafari M., Rezayat S., Arbabi Bidgoli S. Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artif. Cells Nanomed. Biotechnol. 2020;48:443–451. doi: 10.1080/21691401.2019.1709855. [DOI] [PubMed] [Google Scholar]
- 185.Mahmoudi M., Hofmann H., Rothen-Rutishauser B., Petri-Fink A. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem. Rev. 2011;112:2323–2338. doi: 10.1021/cr2002596. [DOI] [PubMed] [Google Scholar]
- 186.Yu M., Huang S., Yu K., Clyne A. Dextran and Polymer Polyethylene Glycol (PEG) coating reduce both 5 and 30 nm iron oxide nanoparticle cytotoxicity in 2D and 3D Cell culture. Int. J. Mol. Sci. 2012;13:5554–5570. doi: 10.3390/ijms13055554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Feng Q., Liu Y., Huang J., Chen K., Huang J., Xiao K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018;8:2082–2095. doi: 10.1038/s41598-018-19628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ying E., Hwang H. In vitro evaluation of the cytotoxicity of iron oxide nanoparticles with different coatings and different sizes in A3 human T lymphocytes. Sci. Total Environ. 2010;408:4475–4481. doi: 10.1016/j.scitotenv.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 189.Jarockyte G., Daugelaite E., Stasys M., Statkute U., Poderys V., Tseng T., Hsu S., Karabanovas V., Rotomskis R. Accumulation and toxicity of superparamagnetic iron oxide nanoparticles in cells and experimental animals. Int. J. Mol. Sci. 2016;17:1193. doi: 10.3390/ijms17081193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hamilton R., Wu N., Porter D., Buford M., Wolfarth M., Holian A. Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Part. Fibre Toxicol. 2009;6:35–46. doi: 10.1186/1743-8977-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Stoehr L., Gonzalez E., Stampfl A., Casals E., Duschl A., Puntes V., Oostingh G. Shape matters: Effects of silver nanospheres and wires on human alveolar epithelial cells. Part. Fibre Toxicol. 2011;8:36–51. doi: 10.1186/1743-8977-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lee J., Ju J., Kim B., Pak P., Choi E., Lee H., Chung N. Rod-shaped iron oxide nanoparticles are more toxic than sphere-shaped nanoparticles to murine macrophage cells. Environ. Toxicol. Chem. 2014;33:2759–2766. doi: 10.1002/etc.2735. [DOI] [PubMed] [Google Scholar]
- 193.Hu B., Zeng M., Chen J., Zhang Z., Zhang X., Fan Z., Zhang X. External magnetic field-induced targeted delivery of highly sensitive iron oxide nanocubes for MRI of myocardial infarction. Small. 2016;12:4707–4712. doi: 10.1002/smll.201600263. [DOI] [PubMed] [Google Scholar]
- 194.Szalay B., Tátrai E., Nyírő G., Vezér T., Dura G. Potential toxic effects of iron oxide nanoparticles in in vivo and in vitro experiments. J. Appl. Toxicol. 2011;32:446–453. doi: 10.1002/jat.1779. [DOI] [PubMed] [Google Scholar]
- 195.Li L., Jiang L., Zeng Y., Liu G. 2013. Toxicity of superparamagnetic iron oxide nanoparticles: Research strategies and implications for nanomedicine. Chin. Phys. B. 2013;22:127503–127514. doi: 10.1088/1674-1056/22/12/127503. [DOI] [Google Scholar]
- 196.Prodan A., Iconaru S., Ciobanu C., Chifiriuc M., Stoicea M., Predoi D. Iron oxide magnetic nanoparticles: Characterization and toxicity evaluation byin vitroandin vivoassays. J. Nanomater. 2013;2013:1–10. doi: 10.1155/2013/587021. [DOI] [Google Scholar]
- 197.Patil U., Adireddy S., Jaiswal A., Mandava S., Lee B., Chrisey D. In vitro/in vivo toxicity evaluation and quantification of iron oxide nanoparticles. Int. J. Mol. Sci. 2015;16:24417–24450. doi: 10.3390/ijms161024417. [DOI] [PMC free article] [PubMed] [Google Scholar]