Abstract
To the best of our knowledge, a dose-response meta-analysis of the relationship between cardiovascular disease (CVD) and arsenic (As) exposure at drinking water As concentrations lower than the WHO provisional guideline value (10 µg/L) has not been published yet. We conducted a systematic review and meta-analyses to estimate the pooled association between the relative risk of each CVD endpoint and low-level As concentration in drinking water both linearly and non-linearly using a random effects dose-response model. In this study, a significant positive association was found between the risks of most CVD outcomes and drinking water As concentration for both linear and non-linear models (p-value for trend < 0.05). Using the preferred linear model, we found significant increased risks of coronary heart disease (CHD) mortality and CVD mortality as well as combined fatal and non-fatal CHD, CVD, carotid atherosclerosis disease and hypertension in those exposed to drinking water with an As concentration of 10 µg/L compared to the referent (drinking water As concentration of 1 µg/L) population. Notwithstanding limitations included, the observed significant increased risks of CVD endpoints arising from As concentrations in drinking water between 1 µg/L and the 10 µg/L suggests further lowering of this guideline value should be considered.
Keywords: arsenic, low level, cardiovascular disease, dose-response, systematic review, meta-analysis
1. Introduction
Originating from either geological or anthropogenic activities, arsenic (As) has been widely recognized since the 1950s as one of the most serious human carcinogens [1,2]. Ubiquitously present in the environment [3,4,5], exposure to As can take place via ingestion (oral), dermal contact, inhalation, and even parenteral routes [6], and can lead to a wide range of carcinogenic and non-carcinogenic end-points [7,8]. Long-term As exposure is known to result in lung, bladder, liver, skin and kidney cancers [9]. Concerning non-carcinogenic endpoints, while skin lesions are considered to be a primary marker of As toxicity [10], it has also been reported to be associated with different neurological problems [11], increased risk of immune problems in new-born [12], infant infections [13], some reproductive health problems in pregnant women [14] and, importantly, cardiovascular diseases (CVD). A prospective study conducted in Bangladesh found an increased risk of mortality from ischemic heart disease and cerebrovascular disease in populations exposed to high concentration of inorganic arsenic (iAs) in well water [15]. Similarly, in other studies, increased risk of hypertension [16], stroke [17] and changes in CVD biomarker levels [6,18] were observed associated with As exposure. Among all the adverse health risks from As exposure, CVD is regarded as the most serious non- carcinogenic detrimental health outcome [15,19].
The relationship between CVD and As exposure at As concentrations in drinking water greater than 100 µg/L is well established [17,18,20,21]. However, partly as a result of the joint effort by Environmental Protection Agency and National Academy of Sciences to define an As risk threshold, the need to determine the dose-response at lower concentrations is also well recognized [22]. Unfortunately, evidence for low concentration effects, especially below the WHO permissible limit (10 µg/L) has been controversial and inconsistent [23,24]. For example, a cross-sectional study conducted by Kunrath et al. [25] found an association between CVD risks and low-level As exposure, while other studies such as that of Jones, et al. [26] for hypertension and that of Monrad et al. [27] for myocardial infarction did not find similar associations. Such inconsistency might be related to limitations with sample size, exposure and outcome assessment as well as insufficient information on different CVD risk factors [26,28,29]. As the available scientific data do not allow characterization of a clear threshold below which CVD risk would be negligible [23,29] and the disagreements between individual studies are a clear problem for regulators who currently face mounting pressure to determine “better” regulatory limits for As in drinking water, the National Research Council has recommended the use of meta-analysis to quantify the dose-response relationship of different CVD endpoints to low-level As exposure [30]. Compared to individual studies where detecting the possible true effects is often prevented due to their relatively small number of participants, meta-analysis could lower the background noise, reduce error and bias and is a rigorous, transparent and systematic approach in evaluating aggregated evidence to answer a specific question [31,32].
Existing meta-analyses and systematic reviews that include the lower end of the As exposure range have not clearly and quantitatively specified any increased risk below 10 µg/L of water As concentration possibly because of a lack of epidemiological data. These reviews notably include a) a qualitative systematic review of the epidemiologic evidences on the association of CVD and low, moderate and high As exposure [33], b) a meta-regression of the association between risk of CVD and As exposure at concentrations lower than those recommended by previous studies [34], c) shaping the dose-response effects on incidence of CVD endpoints of chronic exposure to low-moderate (< 100 µg/L) and high levels (≥ 100 µg/L) of As [24], d) unveiling the epidemiologic evidence on the relationship between CVD and As exposure in studies that include the lower end of the exposure range [22], and e) qualitatively identifying an association between the prevalence of hypertension and both low and moderate-to-high As exposure [35].
Due to the recent publication of a number of key epidemiological studies [36] building upon our recent research in exposure science [37,38,39], we aimed to use meta-analysis to determine quantitatively the magnitude of increased CVD risks for the general population exposed at As concentrations lower than the WHO provisional guideline value for drinking water of 10 µg/L. We have also included some CVD biomarkers in the evaluation.
2. Materials and Methods
2.1. Data Sources
We searched ISI Web of Science for studies published before December 2019 on the association between CVD risks including overall CVD, coronary heart disease (CHD), stroke, carotid atherosclerosis disease, hypertension and associated clinic markers and As exposure and for the general population. The medical subject headings (MeSH) used were “arteriosclerosis”, “atherosclerosis", “carotid artery diseases”, “cardiovascular diseases”, “cerebrovascular disease”, “cerebrovascular disorders”, “coronary artery disease”, “heart diseases”, “hypertension”, “infarct”, “ischemia”, “ischemic heart disease”, “myocardial infarction”, “peripheral vascular diseases”, “peripheral arterial disease”, “stroke” and were combined with “arsenic”, “arsenic poisoning”, “arsenicals”, “arsenite” and “arsenate”. The terms were combined with the Boolean operators "OR" or "AND". The reference lists and cross-references of eligible studies were also searched as were the bibliographies of recent systematic reviews and meta-analyses reported by Navas-Acien et al. [33], Phung et al. [34], Moon et al. [24], Tsuji et al. [22], Abhyankar et al. [35] and Chowdhury et al. [40].
Studies not published in English, related to specific scenario like childhood exposure or exposure during pregnancy or occupational exposure, or based on a specific form of As like arsenic trioxide (which is not the common form of As exposure in the general population) were excluded. The review of literature was conducted in accordance with the techniques specified by Stroup et al. [41] for meta-analysis of observational studies in epidemiology (MOOSE) and reporting was done based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria [32].
Based on the abstract review, studies were firstly excluded if (a) they were case reports or case series; (b) no original data were presented; (c) had no human records; or (d) the outcome measures were not related with mortality or morbidity risk of relevant CVD types and/or clinical indicators considered in this study. The full text of studies which was regarded as potentially eligible was then assessed and further excluded if they were (a) not related with dietary As exposure or not for the general population; (b) no available relative risk (risk ratios, odds ratios) and measures of variability of different types of CVD were mentioned; or c) no categorical As exposure (at least three categories) was used (Figure S1). The methodological quality of the studies was also assessed and those with missing data on, or incomplete definitions of, the study design, population, exposure condition, or outcome variables were excluded [42,43,44,45,46,47,48,49]. Furthermore, as it is difficult to convert plasma As to water As, we excluded two studies which could only provide plasma As concentration to lower the bias of exposure assessment [50,51]. In addition, as there was only one study analysing each of soluble E-selectin, myeloperoxidase, plasminogen activator inhibitor-1 (PAI-1), soluble Intercellular Adhesion Molecule 1 (ICAM-1) and soluble Vascular cell adhesion protein 1 (VCAM-1) [18], carotid atherosclerosis indices (CAIs) [52], common carotid intima media thickness, plaque score and the presence of plaque in the common carotid [53], peripheral vascular disease [54,55] as well as some other CVD subtypes and clinic markers [21,27,56,57,58,59,60,61], respectively, these variables have not been included in our dose-response meta-analysis. Moreover, due to the fact that significant differences could be found regarding concentrations of matrix-metalloproteases (MMPs) using plasma vs. serum [62], we further excluded two studies which analysed the plasma MMPs and serum MMPs respectively to avoid bias in outcome ascertainment [18,21] (Figure S1). Two reviewers (LX and DM) independently reviewed the quality of the studies and differences were resolved by consensus and discussion.
2.2. Data Extraction
For each study that appeared to meet the inclusion criteria, we extracted data on the number of deaths or cases, person-years or total number of population, the measured risk values (e.g., rate ratio, hazard ratio, odds ratio) and their statistical uncertainty (95% confidence interval (CI)) for each As concentration category. In addition, we recorded the descriptive information including authors’ names, year of study publication, sampling method, sample size, study design, and outcome ascertainment as well as the adjustment for confounders.
Similar to the method used by Moon et al. [24], we combined all measures of association (e.g., rate ratio, hazard ratio, odds ratio) in different studies for each CVD endpoint together, as the relative risk. For the exposure assessment, we abstracted the mean, median values, and the range of As concentration in each concentration category. Though drinking water was the main metric of As exposure, for studies where drinking water data was not directly available, we estimated the drinking water As concentration from either toenail As [19] using the following formulae (see Equations (1) and (2)):
| (1) |
| (2) |
or from urinary As [63] using Equation (3):
| (3) |
where it was assumed that each participant has a urine creatinine concentration equal to the overall mean in the Strong Heart Study baseline visit (1.3 g/L, Moon KA, unpublished data). The credibility about the estimation of drinking water As concentration from toenail As and urinary As is indicated by their successful use in previous meta-analysis studies such as those of Moon et al. [24].
2.3. Statistical Analysis
A random-effects dose-response meta-analysis was conducted to estimate the pooled dose-response relationship between the log-transformed relative risk of each CVD outcome and water As concentrations from the summarized data of multiple studies using the package ‘dosresmeta’ in R statistical software [64,65]. This ‘dosresmeta’ meta-analysis consists of a two-stage procedure, whereby the study-specific trends are firstly estimated and then pooled across studies. In this analysis, we assumed both a constant log-linear (log-transformed water As concentration) and a flexible non-linear (restricted cubic splines with knots at the 10th, 50th and 90th percentiles of log-transformed water As concentration) associations. The median value was assigned as the concentration level for each concentration category and if the median value was not provided directly, the mean or the midpoint values were calculated instead [16,25,45,59,63,66,67,68,69,70,71,72,73,74,75]. For the calculation of the midpoint value for each concentration category, zero was used as the minimum if not available [16,25,66,68,69,70,71,72,73]. Furthermore, without available maxima values, the width of the highest concentration category was assumed to be equal to the next lowest category [16,25,66,68,69,70,71,72,73]. Pooled log-linear and non-linear relative risks for each CVD endpoint were estimated for water As concentration of 3 µg/L, 5 µg/L, 10 µg/L, 20 µg/L and 50 µg/L, using 1 µg/L of drinking water As as the reference exposure.
Dose-response relationships for individual studies with at least three concentration categories were plotted using the ‘ggplot’ function in the ‘ggplot2’ package in R and the pooled dose-response relationship for each CVD endpoint was plotted using the ‘matplot’ function in R to visualize the predicted relative risks at higher As level compared with the referent concentration (1 µg/L).
Forest plots were used to show the pooled relative risks (95% CI) for both individual studies and the overall one, comparing risk for chronic exposure to drinking water As of 10 µg/L with 1 µg/L using the ‘forestplot’ function of the ‘metafor’ package in R, with the sizes of the squares of individual study relative risks weighted by the inverse variance of the log-relative risk within each model. Specifically, both the individual and the overall pooled relative risks (95% CIs) in the forest plots were calculated via the dose-response meta-analysis through the ‘dosresmeta’ package in R. Model goodness-of-fit was assessed using deviance tests, coefficient of determination (R2 and adjusted R2) and Akaike’s information criterion (AIC) values via the ‘gof’ function in the ‘dosresmeta’ package in R. Furthermore, dose-response relationships for individual studies were overprinted by the pooled dose-response relationship for each CVD endpoint to visually test the model goodness-of-fit. Heterogeneity was assessed by P-heterogeneity (p < 0.05 being significant), I2-statistics (I2-statistic < 25% indicating low heterogeneity, 25%–50% moderate, and > 50% high) and Cochran’s Q-statistic [76].
In addition, to examine the potential publication bias and small study effects, funnel plots for all the CVD endpoints and Egger’s test of funnel plot asymmetry for those with greater than two studies were created using the ‘metafor’ package in R. To be specific, the effect estimated from each study (log-relative risk) was plotted against the standard error of log-relative risk, with the funnel centred at the overall model estimate.
Finally, sensitivity analyses were conducted. On one hand, to test the reliability and suitability of combining different exposure media, we conducted sensitivity analysis to estimate the relationships between CVD risks and drinking water As both linearly and non-linearly by excluding studies which did not provide water As concentrations directly. On the other hand, with the intention of analysing the effects of low-level As exposure, we also excluded studies with As concentration higher than 100 µg/L and conducted subgroup analysis only for low to moderate As concentrations (taken here to be < 100 µg/L), modelling its linear and non-linear associations with the CVD risks.
All our data analysis was performed using R statistical software (R Foundation for Statistical Computing, Vienna, Austria), version 3.4.3 (R Foundation for Statistical Computing) and R Studio (R Studio Desktop 1.1.423) [65,77,78].
3. Results
3.1. Study Characteristics
For our systematic review and meta-analysis, we identified 28 studies which satisfied the inclusion criteria (Table S1). There were considerable variations across studies in terms of the study design, study areas, exposure assessment, methods of outcome ascertainment and adjustment for confounders.
Among all these studies, there were 13 cohort studies [15,17,19,20,26,29,63,71,79,80,81,82], with one retrospective study [57], inferring causality between CVD endpoints and As exposure; six cross-sectional studies [59,66,67,68,70,75]; five case-control studies [16,69,72,73,83]; one ecological study [74]; and three case-cohort studies [20,36,84].
We have included studies from As-endemic areas, such as Bangladesh [15,17,20,67,70], China [66,68,79,83], Taiwan [69,71,72,81,82], Chile [16] and Mexico [59] where recorded As level in drinking water could be as high as 900 µg/L, and studies from what are generally considered as non-endemic areas such as parts of North America [19,63,84] and some European countries [29,74].
We observed substantial differences in the methods of exposure assessment across studies (Table S1): while most provided As concentrations directly in drinking water [17,20,67,68,69,72,73,74,79,80,81,82,84], some studies also reported exposure based on urine [26,29,36,63,66] or toenails [19]. In addition, although many studies analysed As exposure at the individual or household-level [59,73,80], some studies tested As exposure at the municipal- or village-level instead [74] and may have greater measurement error. Moreover, while several studies applied time-weighted average [17,29,70,80,84] or accumulative average [16,66,67,69,70,71,72], many studies still used As concentration analysed at one time point as a proxy for chronic exposure [15,20,68,73].
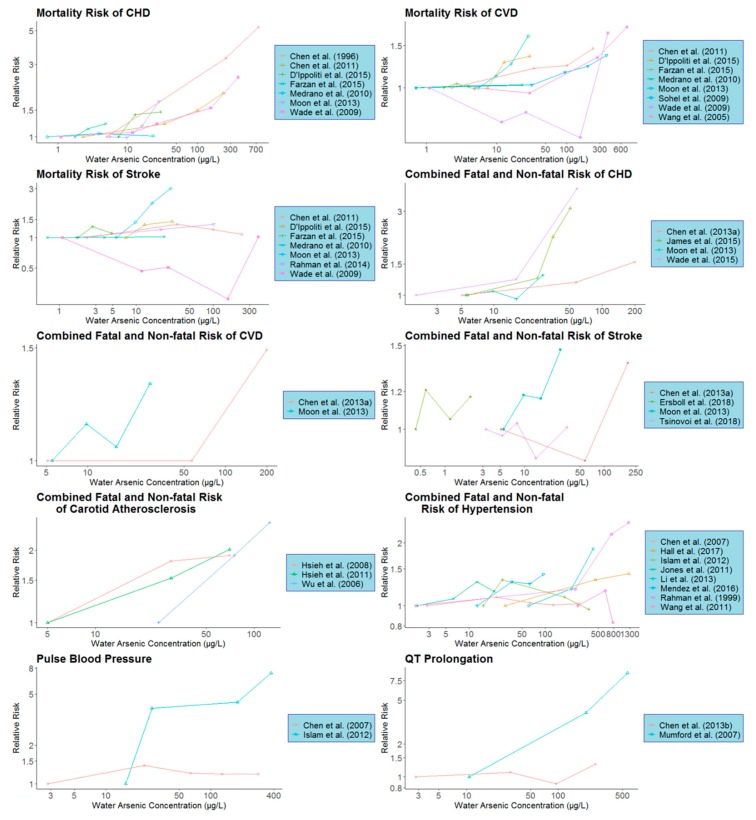
In this study, the different CVD outcomes considered were: carotid atherosclerosis [69,72,73], CHD [15,19,20,29,63,74,79,81,83,84], overall CVD [15,19,20,29,63,74,79,80,82], hypertension [16,26,59,66,67,70,71,75], stroke [15,17,19,20,29,36,57,63,74,79] and the CVD clinic markers considered were: QT prolongation [68,85] and pulse blood pressure [67,75]. There were considerable differences in the outcome ascertainment of these CVD endpoints with quality of those ascertainment methods varying across studies (Additional file 1: Table S1). On one hand, some researchers confirmed the outcomes through standard clinical practice or self-reporting information, for example, hypertension risk was identified via blood pressure tests by trained clinicians using a sphygmomanometer or self-reporting information from either a physician’s diagnosis or the record of anti-hypertensive medication usage [16,26,66,70]. On the other hand, some outcomes were identified by reviewing relevant database or records, ranging from the US National Death Index [19], National Institute for Statistics [74] to National Death Registry in Taiwan [81,82] and some other hospitalization and death records [63].
Association between CVD risks and As exposure could vary by age, gender, smoking status, alcohol consumption, physical activity, diabetes and obesity status, education level as well as some other socio-economic factors, all of which might be either powerful predictors of CVD risks or important factors in As toxicity [86,87,88,89,90,91,92,93,94]. However, varied adjustment for these well-identified potential confounders could be observed among different studies, leading to different magnitudes of associations estimated when combined in a meta-analysis. All the studies were adjusted for at least age and gender, while BMI [15,20,63,83,84], education level [15], and smoking status [19,20,63,81] were adjusted in some, and few studies had some other health and socio-economic indicators [29,63,74,81] adjusted for. Moon et al. [63], and Wade et al. [79] even adjusted for some more rarely used variables, notably estimated glomerular filtration rate, albuminuria and farm work.
3.2. Pooled Association between As Level and CVD Risk
Of the 28 selected studies, 22 studies included dose-response meta-analysis of mortality risk, of which there were seven for CHD, eight for CVD and seven for stroke. The 21 studies on combined fatal and non-fatal CVD risks included three studies on carotid atherosclerosis disease, four studies on CHD, two studies on CVD, eight studies on hypertension and four studies on stroke. Apart from those quantifying the risk of different CVD types, we also considered four studies for CVD clinic markers, including two studies for pulse blood pressure and two studies for QT prolongation (Table 1).
Table 1.
Characteristics of studies included for dose-response meta-analysis.
| Study (year) | Design | Cases | Person or Person-years | Exposure Media | Concentration Category | Median | RR (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|
| Mortality | |||||||||
| CHD | |||||||||
| Chen et al. [15] (2011) | ir | 14 | 20,064 | water (µg/L) | 0.1–12.0 | 2.3 | 1 (referent) | ||
| 16 | 19,109 | 12.1–62.0 | 34.0 | 1.22 | 0.56 | 2.65 | |||
| 15 | 18,699 | 62.1–148.0 | 101.0 | 1.49 | 0.70 | 3.19 | |||
| 26 | 19,380 | 148.1–864.0 | 237.0 | 1.94 | 0.99 | 3.84 | |||
| D’Ippoliti et al. [29] (2015) | ir | 684 | 771,860 | water (µg/L) | < 10 | 7.4 | 1 (referent) | ||
| 573 | 713,276 | 10–20 | 12.9 | 1.40 | 1.19 | 1.64 | |||
| 1014 | 904,129 | > 20 | 29.7 | 1.46 | 1.07 | 2.01 | |||
| Medrano et al. [74] (2010) | ci | 88,566 | 18,978,000 | water (µg/L) | < 1 | 0.7 | 1 (referent) | ||
| 19,709 | 4,803,000 | 1–10 | 3.9 | 1.05 | 1.01 | 1.10 | |||
| 4725 | 1,011,000 | > 10 | 23.3 | 1.02 | 0.96 | 1.08 | |||
| Moon et al. [63] (2013) | ir | 68 | 13,616 | urine (µg/g creatinine) | < 5.8 | 4.2 | 1 (referent) | ||
| 67 | 13,430 | 5.8–9.7 | 7.5 | 0.99 | 0.70 | 1.41 | |||
| 87 | 12,720 | 9.8–15.7 | 12.4 | 1.18 | 0.83 | 1.69 | |||
| 119 | 12,033 | > 15.7 | 21.8 | 1.71 | 1.19 | 2.44 | |||
| Chen et al. [81] (1996) | ir | 4 | 2748 | water (µg/L) | < 10 | 5 | 1 (referent) | ||
| 5 | 1417 | 10–500 | 255 | 3.30 | 0.80 | 13.69 | |||
| 16 | 4309 | ≥ 510 | 755 | 5.30 | 1.49 | 18.85 | |||
| Wade et al. [79] (2009) | ir | 44 | 14,636 | water (µg/L) | 0–5 | 1.1 | 1 (referent) | ||
| 26 | 9047 | 5.1–20 | 11.8 | 1.07 | 0.64 | 1.78 | |||
| 72 | 21,367 | 20.1–100 | 26.2 | 1.22 | 0.82 | 1.82 | |||
| 17 | 3313 | 100.1–300 | 156.1 | 1.55 | 0.88 | 2.73 | |||
| 2 | 249 | Over 300 | 387.9 | 2.47 | 0.50 | 12.18 | |||
| Farzan et al. [19] (2015) | ir | 57 | 898 | toenail (µg/g) | 0.01–0.07 | 0.05 | 1 (referent) | ||
| 51 | 852 | 0.07–0.11 | 0.09 | 1.13 | 0.77 | 1.67 | |||
| 46 | 754 | 0.11–3.26 | 0.23 | 1.22 | 0.82 | 1.82 | |||
| CVD | |||||||||
| Chen et al. [15] (2011) | ir | 43 | 20,064 | water (µg/L) | 0.1–12.0 | 2.3 | 1 (referent) | ||
| 51 | 19,109 | 12.1–62.0 | 34.0 | 1.21 | 0.80 | 1.84 | |||
| 41 | 18,699 | 62.1–148.0 | 101.0 | 1.24 | 0.80 | 1.93 | |||
| 63 | 19,380 | 148.1–864.0 | 237.0 | 1.46 | 0.96 | 2.20 | |||
| Sohel et al. [80] (2009) | ir | 147 | 114,068 | water (µg/L) | < 10 | 0.7 | 1 (referent) | ||
| 168 | 139,233 | 10–49 | 31.8 | 1.03 | 0.82 | 1.29 | |||
| 463 | 365,496 | 50–149 | 95.0 | 1.16 | 0.96 | 1.40 | |||
| 318 | 241,930 | 150–299 | 201.2 | 1.23 | 1.01 | 1.51 | |||
| 115 | 78,786 | > 300 | 371.5 | 1.37 | 1.07 | 1.77 | |||
| D’Ippoliti et al. [29] (2015) | ir | 2752 | 771,860 | water (µg/L) | < 10 | 7.4 | 1 (referent) | ||
| 2115 | 713,276 | 10–20 | 12.9 | 1.28 | 1.08 | 1.51 | |||
| 3514 | 904,129 | > 20 | 29.7 | 1.36 | 1.06 | 1.74 | |||
| Medrano et al. [74] (2010) | ci | 285,049 | 18,978,000 | water (µg/L) | < 1 | 0.7 | 1 (referent) | ||
| 62,739 | 4,803,000 | 1–10 | 3.9 | 1.02 | 0.99 | 1.06 | |||
| 13,962 | 1,011,000 | > 10 | 23.3 | 1.03 | 0.98 | 1.08 | |||
| Moon et al. [63] (2013) | ir | 86 | 13,616 | urine (µg/g creatinine) | < 5.8 | 4.2 | 1 (referent) | ||
| 95 | 13,430 | 5.8–9.7 | 7.5 | 1.12 | 0.83 | 1.52 | |||
| 115 | 12,720 | 9.8–15.7 | 12.4 | 1.26 | 0.92 | 1.73 | |||
| 143 | 12,033 | > 15.7 | 21.8 | 1.65 | 1.20 | 2.27 | |||
| Wade et al. [79] (2009) | ir | 97 | 14,636 | water (µg/L) | 0–5 | 1.1 | 1 (referent) | ||
| 42 | 9047 | 5.1–20 | 11.8 | 0.72 | 0.32 | 1.60 | |||
| 113 | 21,367 | 20.1–100 | 26.2 | 0.79 | 0.34 | 1.86 | |||
| 24 | 3313 | 100.1–300 | 156.1 | 0.62 | 0.10 | 3.70 | |||
| 3 | 249 | Over 300 | 387.9 | 1.70 | 0.51 | 5.72 | |||
| Farzan et al. [19] (2015) | ir | 125 | 1987 | toenail (µg/g) | 0.01–0.07 | 0.05 | 1 (referent) | ||
| 103 | 1691 | 0.07–0.11 | 0.09 | 1.04 | 0.80 | 1.35 | |||
| 84 | 1334 | 0.11–3.26 | 0.23 | 0.99 | 0.74 | 1.32 | |||
| Wang, et al. [82] (2005) | ir | 428 | 19,360 | water (µg/L) | <10 | 5.0 | 1 (referent) | ||
| 84 | 2130 | 10–49 | 29.5 | 0.95 | 0.74 | 1.21 | |||
| 116 | 2317 | 50–499 | 274.5 | 1.34 | 1.08 | 1.66 | |||
| 60 | 1165 | ≥500 | 724.5 | 1.80 | 1.36 | 2.38 | |||
| Stroke | |||||||||
| D’Ippoliti et al. [29] (2015) | ir | 660 | 771,860 | water (µg/L) | < 10 | 7.4 | 1 (referent) | ||
| 448 | 713,276 | 10–20 | 12.9 | 1.33 | 1.12 | 1.58 | |||
| 789 | 904,129 | > 20 | 29.7 | 1.44 | 1.16 | 1.78 | |||
| Farzan et al. [19] (2015) | ir | 15 | 233 | toenail (µg/g) | 0.01–0.07 | 0.05 | 1 (referent) | ||
| 16 | 243 | 0.07–0.11 | 0.09 | 1.28 | 0.64 | 2.61 | |||
| 12 | 161 | 0.11–3.26 | 0.23 | 1.10 | 0.50 | 2.40 | |||
| Rahman et al. [17] (2014) | ir | 62 | 38,198 | water (µg/L) | < 10 | 1.7 | 1 (referent) | ||
| 196 | 156,362 | 10–49 | 21.1 | 1.20 | 0.92 | 1.57 | |||
| 271 | 42,579 | > 50 | 102.2 | 1.35 | 1.04 | 1.75 | |||
| Moon et al. [63] (2013) | ir | 6 | 13,616 | urine (µg/g creatinine) | < 5.8 | 4.2 | 1 (referent) | ||
| 17 | 13,430 | 5.8–9.7 | 7.5 | 1.41 | 0.54 | 3.67 | |||
| 13 | 12,720 | 9.8–15.7 | 12.4 | 2.16 | 0.77 | 6.09 | |||
| 18 | 12,033 | > 15.7 | 21.8 | 3.03 | 1.08 | 8.50 | |||
| Chen et al. [15] (2011) | ir | 43 | 20,064 | water (µg/L) | 0.1–12.0 | 2.3 | 1 (referent) | ||
| 51 | 19,109 | 12.1–62.0 | 34.0 | 1.35 | 0.75 | 2.43 | |||
| 41 | 18,699 | 62.1–148.0 | 101.0 | 1.20 | 0.63 | 2.27 | |||
| 63 | 19,380 | 148.1–864.0 | 237.0 | 1.07 | 0.54 | 2.12 | |||
| Wade et al. [79] (2009) | ir | 53 | 14,636 | water (µg/L) | 0–5 | 1.1 | 1 (referent) | ||
| 16 | 9047 | 5.1–20 | 11.8 | 0.47 | 0.27 | 0.84 | |||
| 41 | 21,367 | 20.1–100 | 26.2 | 0.51 | 0.34 | 0.79 | |||
| 7 | 3313 | 100.1–300 | 156.1 | 0.25 | 1.10 | 2.95 | |||
| 1 | 249 | Over 300 | 387.9 | 1.02 | 0.16 | 6.71 | |||
| Medrano et al. [74] (2010) | ci | 81,368 | 18,978,000 | water (µg/L) | < 1 | 0.7 | 1 (referent) | ||
| 18,327 | 4,803,000 | 1–10 | 3.9 | 1.00 | 0.99 | 1.05 | |||
| 3895 | 1,011,000 | > 10 | 23.3 | 1.02 | 0.95 | 1.09 | |||
| Fatal and non-fatal | |||||||||
| Carotid atherosclerosis disease | |||||||||
| Wu et al. [69] (2006) | cc | 25 | 64 | water (µg/L) | ≤ 50.00 | 25 | 1 (referent) | ||
| 46 | 95 | 50.01–100.00 | 75 | 1.90 | 0.90 | 3.80 | |||
| 89 | 183 | ≥ 100.01 | 125 | 2.60 | 1.30 | 5.00 | |||
| Hsieh et al. [72] (2008) | cc | 17 | 48 | water (µg/L) | < 10 | 5 | 1 (referent) | ||
| 23 | 61 | 10.1–50 | 30 | 1.80 | 1.00 | 3.20 | |||
| 195 | 370 | > 50 | 70 | 1.90 | 1.10 | 3.10 | |||
| Hsieh et al. [73] (2011) | cc | 24 | 55 | water (µg/L) | < 10 | 5 | 1 (referent) | ||
| 31 | 81 | 10.1–50.0 | 30 | 1.53 | 0.67 | 3.50 | |||
| 325 | 720 | > 50.0 | 70 | 2.01 | 1.05 | 3.85 | |||
| CHD | |||||||||
| Wade et al. [83] (2015) | cc | 168 | 305 | water (µg/L) | < 10 | 1.9 | 1 (referent) | ||
| 105 | 236 | 10–39 | 16.0 | 1.23 | 0.78 | 1.93 | |||
| 11 | 26 | > 40 | 58.6 | 4.05 | 1.10 | 14.99 | |||
| Moon et al. [63] (2013) | ir | 202 | 12,146 | urine (µg/g creatinine) | < 5.8 | 4.2 | 1 (referent) | ||
| 206 | 11,701 | 5.8–9.7 | 7.5 | 1.05 | 0.86 | 1.28 | |||
| 197 | 11,305 | 9.8–15.7 | 12.4 | 0.95 | 0.77 | 1.19 | |||
| 241 | 10,586 | > 15.7 | 21.8 | 1.30 | 1.04 | 1.62 | |||
| James et al. [84] (2015) | ir | 58 | 4806 | water (µg/L) | 1–20 | 5.7 | 1 (referent) | ||
| 18 | 1335 | 20–30 | 25.3 | 1.25 | 0.70 | 2.31 | |||
| 16 | 534 | 30–45 | 35.1 | 2.14 | 1.22 | 3.98 | |||
| 4 | 98 | 45–88 | 50.5 | 3.12 | 1.12 | 9.02 | |||
| Chen et al. [20] (2013) | ir | 61 | 2823 | water (µg/L) | 0.1–25 | 5.1 | 1 (referent) | ||
| 72 | 2718 | 25.1–107 | 57.0 | 1.18 | 0.75 | 1.84 | |||
| 75 | 2770 | 108–864 | 198.5 | 1.54 | 1.02 | 2.31 | |||
| CVD | |||||||||
| Moon et al. [63] (2013) | ir | 265 | 12,146 | urine (µg/g creatinine) | < 5.8 | 4.2 | 1 (referent) | ||
| 297 | 11,701 | 5.8–9.7 | 7.5 | 1.14 | 0.95 | 1.35 | |||
| 291 | 11,305 | 9.8–15.7 | 12.4 | 1.05 | 0.87 | 1.26 | |||
| 331 | 10,586 | > 15.7 | 21.8 | 1.32 | 1.05 | 1.28 | |||
| Chen et al. [20] (2013) | ir | 114 | 2823 | water (µg/L) | 0.1–25 | 5.1 | 1 (referent) | ||
| 120 | 2718 | 25.1–107 | 57.0 | 1.00 | 0.67 | 1.50 | |||
| 132 | 2770 | 108–864 | 198.5 | 1.49 | 1.06 | 2.11 | |||
| Hypertension | |||||||||
| Wang et al. [71] (2011) | ir | 93 | 618 | water (µg/L) | < 538 | 269 | 1 (referent) | ||
| 103 | 721 | 538–700 | 619 | 1.18 | 0.60 | 2.34 | |||
| 83 | 634 | > 700 | 781 | 0.83 | 0.40 | 1.68 | |||
| Jones et al. [26] (2011) | ir | 418 | 952 | urine (µg/L) | < 4.2 | 2.1 | 1 (referent) | ||
| 451 | 1057 | 4.2 to 8.3 | 6.3 | 1.08 | 0.83 | 1.40 | |||
| 446 | 1090 | > 8.3 to 17.1 | 12.7 | 1.30 | 0.94 | 1.80 | |||
| 446 | 1068 | > 17.1 | 21.5 | 1.17 | 0.75 | 1.83 | |||
| Chen et al. [75] (2007) | cc | 289 | 2242 | water (µg/L) | 0.1–8.0 | 2.8 | 1 (referent) | ||
| 274 | 2116 | 8.1–40.8 | 23.2 | 1.10 | 0.90 | 1.33 | |||
| 273 | 2187 | 40.9–91.0 | 63.9 | 1.03 | 0.85 | 1.25 | |||
| 259 | 2181 | 91.1–176.0 | 128.1 | 1.01 | 0.83 | 1.22 | |||
| 265 | 2184 | 176.1–864.0 | 283.1 | 1.02 | 0.84 | 1.23 | |||
| Islam et al. [67] (2012) | cc | 22 | 291 | water (µg/L) | 10–22 | 15.5 | 1 (referent) | ||
| 19 | 208 | 23–32 | 27.5 | 1.33 | 0.67 | 2.62 | |||
| 13 | 252 | 33–261 | 180.0 | 1.10 | 0.49 | 2.44 | |||
| 12 | 243 | ≥ 262 | 376.0 | 0.96 | 0.42 | 2.23 | |||
| Li et al. [66] (2013) | cc | 29 | 120 | water (µg/L) | < 100 | 61.0 | 1 (referent) | ||
| 30 | 119 | 100 to 350 | 223.8 | 1.20 | 0.63 | 2.29 | |||
| 45 | 121 | > 350 | 427.7 | 1.87 | 1.02 | 3.42 | |||
| Mendez et al. [59] (2016) | cc | 106 | 260 | water (µg/L) | < 25.5 | 12.8 | 1 (referent) | ||
| 106 | 260 | 25.5–47.9 | 36.7 | 1.30 | 0.84 | 2.00 | |||
| 109 | 259 | 47.9–79.0 | 63.5 | 1.27 | 0.82 | 1.94 | |||
| 118 | 259 | ≥ 79.0 | 94.6 | 1.41 | 0.91 | 2.17 | |||
| Hall et al. [16] (2017) | cc | 140 | 323 | water (µg/L) | < 60 | 30.0 | 1 (referent) | ||
| 246 | 482 | 60–859 | 459.5 | 1.33 | 0.98 | 1.79 | |||
| 225 | 450 | > 859 | 1258.5 | 1.42 | 1.04 | 1.92 | |||
| Rahman et al. [70] (1999) | cc | 9 | 114 | water (µg/L) | < 0 | 2 | 1 (referent) | ||
| 50 | 623 | 0–500 | 250 | 1.20 | 0.60 | 2.30 | |||
| 93 | 576 | 500–1000 | 750 | 2.20 | 1.10 | 4.30 | |||
| 55 | 282 | > 1000 | 1250 | 2.50 | 1.20 | 4.90 | |||
| Stroke | |||||||||
| Tsinovoi et al. [36] (2018) | ir | 150 | 637 | water (µg/L) | 2.72–3.72 | 3.3 | 1 (referent) | ||
| 138 | 622 | 4.75–5.88 | 5.3 | 0.97 | 0.73 | 1.30 | |||
| 139 | 624 | 8.26–9.18 | 8.1 | 1.03 | 0.77 | 1.38 | |||
| 119 | 606 | 11.99–16.72 | 13.9 | 0.87 | 0.64 | 1.18 | |||
| 125 | 608 | 26.11–54.81 | 34.1 | 1.01 | 0.74 | 1.36 | |||
| Moon et al. [63] (2013) | ir | 55 | 12,146 | urine (µg/g creatinine) | < 5.8 | 4.2 | 1 (referent) | ||
| 75 | 11,701 | 5.8–9.7 | 7.5 | 1.18 | 0.82 | 1.69 | |||
| 62 | 11,305 | 9.8–15.7 | 12.4 | 1.16 | 0.77 | 1.72 | |||
| 72 | 10,586 | > 15.7 | 21.8 | 1.47 | 0.97 | 2.21 | |||
| Chen et al. [20] (2013) | ir | 50 | 2823 | water (µg/L) | 0.1–25 | 5.1 | 1 (referent) | ||
| 46 | 2718 | 25.1–107 | 57.0 | 0.86 | 0.49 | 1.51 | |||
| 52 | 2770 | 108–864 | 198.5 | 1.38 | 0.84 | 2.27 | |||
| Ersboll et al. [57] (2018) | ir | 486 | 172,202 | water (µg/L) | 0.049–0.573 | 0.435 | 1 (referent) | ||
| 657 | 180,891 | 0.573–0.760 | 0.584 | 1.21 | 1.07 | 1.36 | |||
| 475 | 169,470 | 0.760–1.933 | 1.174 | 1.05 | 0.92 | 1.19 | |||
| 577 | 173,856 | 1.933–25.34 | 2.109 | 1.17 | 1.04 | 1.32 | |||
| CVD markers | |||||||||
| Pulse blood pressure (SBP-DBP ≥ 55 mmHg)) | |||||||||
| Chen et al. [75] (2007) | cc | 205 | 2242 | water (µg/L) | 0.1–8.0 | 2.8 | 1 (referent) | ||
| 252 | 2116 | 8.1–40.8 | 23.2 | 1.39 | 1.14 | 1.71 | |||
| 232 | 2187 | 40.9–91.0 | 63.9 | 1.21 | 0.99 | 1.49 | |||
| 227 | 2181 | 91.1–176.0 | 128.1 | 1.19 | 0.97 | 1.45 | |||
| 233 | 2184 | 176.1–864.0 | 283.1 | 1.19 | 0.97 | 1.46 | |||
| Islam et al. [67] (2012) | cc | 5 | 291 | water (µg/L) | 10–22 | 15.5 | 1 (referent) | ||
| 10 | 208 | 23–32 | 27.5 | 3.87 | 1.22 | 12.2 | |||
| 10 | 252 | 33–261 | 180.0 | 4.32 | 1.23 | 15.11 | |||
| 16 | 243 | ≥ 262 | 376.0 | 7.32 | 2.18 | 24.60 | |||
| QT prolongation | |||||||||
| Chen et al. [85] (2013) | ir | 57 | 371 | water (µg/L) | 0.1–9 | 2.8 | 1 (referent) | ||
| 63 | 369 | 9.5–57 | 30.0 | 1.10 | 0.74 | 1.63 | |||
| 49 | 374 | 58–144 | 95.1 | 0.87 | 0.57 | 1.31 | |||
| 68 | 353 | 145–790 | 254.5 | 1.31 | 0.87 | 1.96 | |||
| Mumford et al. [68] (2007) | cc | 4 | 103 | water (µg/L) | < 21 | 10.7 | 1 (referent) | ||
| 12 | 108 | 100–350 | 199.9 | 3.83 | 1.13 | 12.99 | |||
| 21 | 102 | 430–690 | 568.3 | 8.85 | 2.72 | 28.75 | |||
CVD: cardiovascular disease; CHD: coronary heart disease. RR: Relative risk or approximation of the relative risk (rate ratio, risk ratio, odds ratio). ir: Risks estimated in the studies as rate ratio (incidence-rate data); ci: Risks estimated in the studies as risk ratio (cumulative incidence data); cc: Risks estimated in the studies as an odds ratio (see details reported by Orsini et al. [65]).
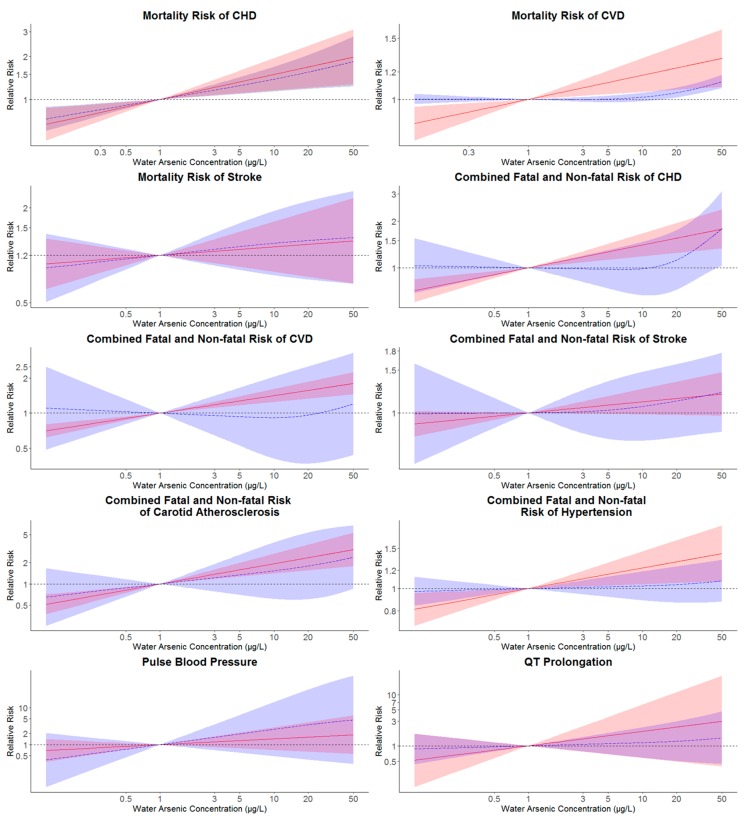
Individual dose-response information of all the studies included in our dose-response meta-analysis is presented in Table 1 and Figure 1. In the linear model, there was a significant overall trend for the mortality risk of CHD and CVD with an increase in As concentration (p < 0.05) (Table 2, Figure 2). Compared to 1 µg/L as the reference level, the relative mortality risk of CHD and CVD at 10 µg/L drinking water As concentration was 1.498 (95% CI: 1.153–1.948) and 1.174 (95% CI: 1.049–1.313), respectively (Table 2). Similarly, significantly overall trend (p < 0.05) was also obtained for the combined fatal and non-fatal risk of CHD, CVD, carotid atherosclerosis disease and hypertension with the relative risks of these four endpoints at 10 µg/L of water As concentration were 1.405 (95% CI: 1.183–1.667), 1.411 (95% CI: 1.242–1.603), 1.936 (95% CI: 1.403–2.671) and 1.231 (95% CI: 1.043–1.452), respectively, compared with 1 µg/L as the reference (Table 2 and Figure 2).
Figure 1.
Individual study dose-response characteristics for various CVD subtypes or biomarkers. Arsenic concentrations refer to the observed or estimated median arsenic concentrations for the given concentration category. Lines connect the dose-response data for each study and are for illustrative purposes only (CVD: cardiovascular disease; CHD: coronary heart disease).
Table 2.
Pooled relative risks (95% CIs) for different types of cardiovascular disease (CVD) and clinic markers in relation to water arsenic concentrations.
| Mortality Risk | Combined Fatal and Non-Fatal Risk | CVD Markers | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHD (7(25)) a | CVD (8(31)) a | Stroke (7(25)) a | CHD (4(14)) a | CVD (2(7)) a | Stroke (4(16)) a | Carotid Atherosclerosis Disease (3(9)) a | Hypertension (8(30)) a | Pulse Blood Pressure (2(9)) a | QT Prolongation (2(7)) a | |
| Log-linear dose-response association model | ||||||||||
| 1 µg/L b | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| 3 µg/L | 1.213 (1.070, 1.374) |
1.079 (1.023, 1.139) |
1.061 (0.891, 1.262) |
1.176 (1.083, 1.276) |
1.178 (1.108, 1.252) |
1.051 (0.992, 1.114) |
1.370 (1.175, 1.598) |
1.104 (1.020, 1.195) |
1.187 (0.848, 1.662) |
1.363 (0.770, 2.414) |
| 5 µg/L | 1.327 (1.105, 1.593) |
1.118 (1.034, 1.210) |
1.090 (0.844, 1.407) |
1.268 (1.125, 1.429) |
1.272 (1.163, 1.391) |
1.076 (0.989, 1.171) |
1.587 (1.267, 1.987) |
1.156 (1.030, 1.298) |
1.286 (0.785, 2.105) |
1.574 (0.682, 3.636) |
| 10 µg/L | 1.498 (1.153, 1.948) |
1.174 (1.049, 1.313) |
1.131 (0.784, 1.630) |
1.405 (1.183, 1.667) |
1.411 (1.242, 1.603) |
1.111 (0.984, 1.254) |
1.936 (1.403, 2.671) |
1.231 (1.043, 1.452) |
1.433 (0.707, 2.901) |
1.914 (0.578, 6.339) |
| 20 µg/L | 1.693 (1.203, 2.380) |
1.232 (1.064, 1.426) |
1.173 (0.729, 1.889) |
1.556 (1.245, 1.944) |
1.566 (1.325, 1.848) |
1.146 (0.979, 1.342) |
2.362 (1.553, 3.590) |
1.310 (1.057, 1.625) |
1.597 (0.637, 3.998) |
2.327 (0.490,11.052) |
| 50 µg/L | 1.988 (1.274, 3.103) |
1.313 (1.085, 1.589) |
1.233 (0.662, 2.295) |
1.781 (1.331, 2.383) |
1.796 (1.445, 2.230) |
1.195 (0.973 1.469) |
3.071 (1.777, 5.308) |
1.423 (1.074, 1.885) |
1.842 (0.555, 6.109) |
3.012 (0.394, 23.045) |
| coefficient | 0.1757 | 0.070 | 0.054 | 0.148 | 0.150 | 0.046 | 0.287 | 0.090 | 0.156 | 0.282 |
| p-value for trend c | 0.003 | 0.005 | 0.510 | < 0.001 | < 0.001 | 0.090 | < 0.001 | 0.014 | 0.320 | 0.290 |
| I2 d | 79.7% | 77.9% | 89.0% | 6.6% | 17.4% | 0.0% | 17.5% | 62.3% | 80.4% | 91.5% |
| Cochran’s Q-statistic | 29.54 | 31.70 | 54.78 | 3.21 | 1.21 | 2.88 | 2.43 | 18.56 | 5.10 | 11.7 |
| P-heterogeneity e | < 0.001 | < 0.001 | < 0.001 | 0.360 | 0.271 | 0.409 | 0.297 | 0.097 | 0.024 | 0.006 |
| Non-linear dose-response association model (restricted cubic splines) | ||||||||||
| 1 µg/L b | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| 3 µg/L | 1.163 (1.060, 1.276) |
0.999 (0.983, 1.014) |
1.092 (0.862, 1.382) |
0.985 (0.811, 1.197) |
0.954 (0.647, 1.406) |
1.011 (0.823, 1.241) |
1.225 (0.783, 1.917) |
1.012 (0.944, 1.085) |
1.578 (0.707, 3.523) |
1.070 (0.772, 1.483) |
| 5 µg/L | 1.250 (1.090, 1.433) |
1.001 (0.980, 1.023) |
1.136 (0.807, 1.596) |
0.978 (0.735, 1.302) |
0.933 (0.528, 1.648) |
1.026 (0.780, 1.349) |
1.347 (0.699, 2.594) |
1.018 (0.920, 1.128) |
1.951 (0.601, 6.326) |
1.105 (0.685, 1.781) |
| 10 µg/L | 1.387 (1.135, 1.695) |
1.015 (0.986, 1.043) |
1.192 (0.746, 1.902) |
0.986 (0.663, 1.468) |
0.915 (0.410, 2.040) |
1.063 (0.766, 1.474) |
1.537 (0.612, 3.863) |
1.027 (0.888, 1.187) |
2.601 (0.483, 14.001) |
1.155 (0.583, 2.288) |
| 20 µg/L | 1.557 (1.182, 2.052) |
1.045 (1.012, 1.080) |
1.241 (0.701, 2.195) |
1.124 (0.720, 1.754) |
0.963 (0.371, 2.499) |
1.120 (0.791, 1.586) |
1.800 (0.605, 5.353) |
1.041 (0.868, 1.249) |
3.449 (0.389, 30.605) |
1.229 (0.504, 2.996) |
| 50 µg/L | 1.846 (1.231, 2.769) |
1.125 (1.077, 1.176) |
1.295 (0.659, 2.542) |
1.795 (1.029, 3.131) |
1.199 (0.439, 3.273) |
1.214 (0.834, 1.769) |
2.394 (0.852, 6.728) |
1.082 (0.877, 1.334) |
4.642 (0.298, 72.343) |
1.433 (0.440, 4.667) |
| p-value for trend f | 0.006 | < 0.001 | 0.750 | 0.047 | 0.078 | 0.340 | < 0.001 | 0.200 | 0.150 | 0.270 |
| I2 d | 69.8% | 35.3% | 80.0% | 41.0% | 53.7% | 0.0% | 0.0% | 46.3% | 73.1% | 72.5% |
| Cochran’s Q-statistic | 39.75 | 21.65 | 60.02 | 10.16 | 4.32 | 5.59 | 2.58 | 26.07 | 7.43 | 7.27 |
| P-heterogeneity e | < 0.001 | 0.086 | < 0.001 | 0.117 | 0.115 | 0.470 | 0.629 | 0.025 | 0.024 | 0.026 |
CVD: cardiovascular disease; CHD: coronary heart disease. a: Sum of studies included; the total number of relative risks in each model. b: treat 1 µg/L water arsenic concentration as the referent. c: p-value for linear trend from a Wald test of the coefficient for drinking water arsenic concentrations. d: Proportion of total variance due to between-study heterogeneity. e: p-value for heterogeneity is chi-square p-value of the Q-statistic. f: Non-linear trend p-value for the non-linear spline coefficient in a model with arsenic concentrations entered as a restricted cubic spline with knots at 10th, 50th and 90th percentiles of water arsenic concentration.
Figure 2.
Pooled log-linear and non-linear relative risks and 95% confidence intervals (CIs) of different CVD endpoints in relation to the estimated drinking water arsenic concentration. Pooled log-linear and non-linear relative risks of CVD endpoints were estimated for drinking water arsenic concentrations with reference to an arsenic concentration of 1 µg/L. Solid lines (red) correspond to pooled relative risks of linear models with their 95% CIs represented as shaded regions (red). Pooled relative risks of non-linear models were represented by long-dash lines (blue) and their 95% CIs were plotted as shaded areas (blue). Log-linear models were estimated with log-transformed estimated drinking water arsenic concentration and non-linear associations were estimated from models with restricted cubic splines of log-transformed water arsenic concentration with knots at the 10th, 50th and 90th percentiles of log-transformed water arsenic (CVD: cardiovascular disease; CHD: coronary heart disease).
In the non-linear analysis, though the overall trend was significant (p < 0.05) for CHD and CVD mortality risks as well as the combined fatal and non-fatal risk of CHD and carotid atherosclerosis disease, significant increases in pooled relative risks at 10 µg/L drinking water As compared with 1 µg/L as a reference were only found for the mortality risk of CHD (relative risk: 1.387; 95% CI: 1.135–1.695) (Table 2 and Figure 2).
3.3. Heterogeneity
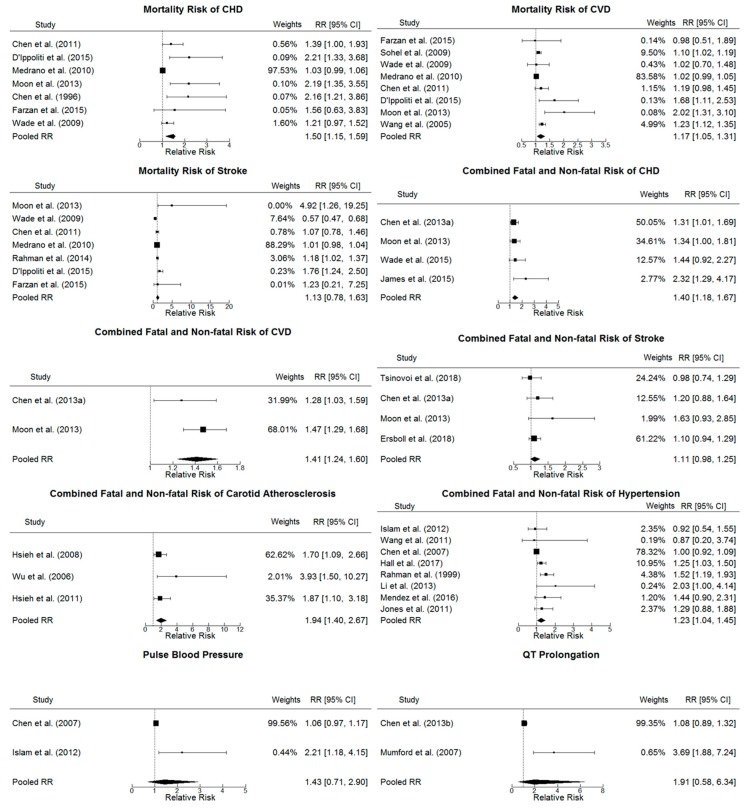
Combined with the estimated I2 statistic, Cochran’s Q-statistic and p-values for heterogeneity as presented in Table 2 and the individual and pooled relative risks of different CVD endpoints at 10 µg/L drinking water As in comparison with 1 µg/L shown in Figure 3, we found evidence of significantly high heterogeneity among the studies combined for the linear and non-linear analysis of the mortality risk of CHD, stroke and two CVD clinic markers (pulse blood pressure and QT prolongation) as well as the linear analysis of CVD mortality risk (heterogeneity p-values < 0.05 and I2 > 50%).
Figure 3.
Forest plot of individual study and pooled log-linear relative risks (95% confidence intervals (CIs)) of different CVD endpoints, comparing 10 µg/L with 1 µg/L drinking water arsenic concentration. The sizes of squares of the individual study relative risks were weighted by the inverse variance of the log-relative risk within each model (CVD: cardiovascular disease; CHD: coronary heart disease).
3.4. Model Goodness Assessment
Deviance, p-value, R2, adjusted R2 and AIC, as well as the difference between dose-response relationships for individual studies and the pooled dose-response relationship for each CVD endpoint, have been used for the goodness-of-fit assessment (Table 3 and Figure S2). According to the results, except for the linear analysis of pulse blood pressure (p-values lower than 0.05), deviance tests indicated no overall lack of fit for other CVD endpoints. In addition, there was only a slight increase in the R2 and the adjusted R2 for non-linear models compared with linear ones for most CVD endpoints, indicating that non-linear models explained more the variation of CVD risks. Linear models are considered a better fit for all the CVD endpoints due to their lower AIC values.
Table 3.
Goodness-of-fit assessment.
| Studies | Mortality Risk | Combined Fatal and Non-fatal Risk | CVD Markers | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHD | CVD | Stroke | CHD | CVD | Stroke | Carotid Atherosclerosis Disease | Hypertension | Pulse Blood Pressure | QT Prolongation | |
| Log-linear dose-response association model | ||||||||||
| Deviance a | 19.40 | 22.58 | 15.98 | 13.04 | 7.06 | 18.54 | 2.99 | 20.27 | 14.02 | 4.97 |
| Degrees of freedom b | 17 | 22 | 17 | 9 | 4 | 11 | 5 | 21 | 6 | 4 |
| p-value c | 0.306 | 0.426 | 0.526 | 0.161 | 0.133 | 0.070 | 0.702 | 0.504 | 0.029 | 0.291 |
| R2 | 0.320 | 0.258 | 0.027 | 0.537 | 0.798 | 0.134 | 0.844 | 0.230 | 0.066 | 0.185 |
| Adjusted R2 | 0.280 | 0.225 | −0.031 | 0.486 | 0.748 | 0.055 | 0.813 | 0.193 | −0.089 | −0.019 |
| AIC | 0.17 | −6.77 | 6.58 | −0.56 | 1.26 | −2.22 | 3.38 | −4.36 | 4.55 | 5.58 |
| Non-linear dose-response association model (restricted cubic splines) | ||||||||||
| Deviance a | 17.28 | 22.81 | 15.39 | 5.83 | 3.94 | 17.48 | 1.71 | 12.94 | 9.16 | 3.44 |
| Degrees of freedom b | 16 | 21 | 16 | 8 | 3 | 10 | 4 | 20 | 5 | 3 |
| p-value c | 0.367 | 0.354 | 0.496 | 0.666 | 0.267 | 0.064 | 0.789 | 0.880 | 0.103 | 0.328 |
| R2 | 0.373 | 0.620 | 0.035 | 0.512 | 0.564 | 0.110 | 0.892 | 0.199 | 0.292 | 0.435 |
| Adjusted R2 | 0.297 | 0.584 | −0.085 | 0.390 | 0.273 | −0.068 | 0.838 | 0.118 | 0.008 | 0.058 |
| AIC | 29.95 | 5.89 | 23.86 | 12.34 | 10.37 | 16.45 | 13.75 | 23.55 | 12.16 | 11.43 |
CVD: cardiovascular disease; CHD: coronary heart disease. a: Measure of the total absolute deviation between reported and predicted log-relative risk taking into account the covariance structure of the residuals. b: Degrees of freedom from the deviance statistic. c: p-value from test for model specification. AIC: Akaike’s information criterion.
3.5. Small-study Effects Evaluation
Funnel plots and Egger’s test of funnel plots asymmetry suggested potential bias for the mortality risk of CHD and CVD with their p-values for Egger’s Regression Test lower than 0.05 (Table S2 and Figure S3).
3.6. Sensitivity Analysis
Excluding studies which cannot provide drinking water As concentrations directly resulted in similar conclusions to the main test, with the relative risks for both pooled linear and non-linear models becoming lower but with heterogeneity remaining high (Table S3).
Excluding studies with drinking water As concentration > 100 µg/L, the estimated pooled relative risks were higher for both pooled linear and non-linear models but heterogeneity was not reduced (Table S4).
4. Discussion
To our knowledge, this is the first dose-response meta-analysis estimating CVD risks from low-level As exposure from drinking water with As concentrations lower than the WHO provisional guideline value of 10 µg/L, to which a large population is exposed globally [95]. In this study, we found a significant increase of 49.8 % and 17.4% of CHD and CVD mortality risks, as well as 40.5%, 41.1%, 93.6% and 23.1% increased risk for combined fatal and non-fatal CHD, CVD, carotid atherosclerosis disease and hypertension at a drinking water As concentration of 10 µg/L compared to 1 µg/L based on the linear model, which is preferred over the non-linear model. Considering the sizable population exposed to such low-level As concentrations [95] and the high morbidity and mortality of CVD worldwide [96], such a proportional increase in CVD risks due to low-level drinking water As may lead to hundreds of thousands of additional CVD cases or even premature deaths, causing a serious cardiovascular health issue for people and health services around the world.
Already, strong evidence of the relationships between As exposure and different CVD endpoints has been identified in several systematic reviews and meta-analysis. However, most reviews were either related with a higher level of As exposure (> 50 µg/L) [22,28,33,40,97] or only provided descriptive evaluations [23,28]. To our knowledge, only Moon et al. [24] dose-response meta-analysis was available on this subject, where 11 individual studies were integrated to evaluate linear and non-linear associations between CVD incident risks and low-moderate water As. Compared to Moon et al. [24], our present analysis included not only several CVD types, but also two CVD clinical markers (QT prolongation and pulse blood pressure), providing a more comprehensive view of the possible effects of low-level As exposure. In addition, Moon et al. [24] estimated CVD relative risk by comparing 20 µg/L with 10 µg/L water As concentration, while in our study, we determined the relative risk at 10 µg/L and below, using 1 µg/L as the referent, addressing the gap, at least partly, on the CVD risks from low-level As exposure especially for As concentrations lower than the WHO provisional guideline value, to which a large population is exposed worldwide [95].
To reduce model misspecification, we conducted both a constant linear and a smooth non-linear model (restricted cubic splines with knots at the 10th, 50th and 90th percentiles of log-transformed water As) via a two-stage ’dosresmeta’ function. Somewhat consistent with previous meta-analysis researches [22,24], differences in the linear and non-linear model outcomes could be observed; notably there was no significant increased risk at 10 µg/L As for any of the outcomes except for CHD mortality risk in the non-linear model. Such a phenomenon might be related to the small number of studies and relatively few exposure categories in each study, which may be underpowered to establish precise dose-response relationships between CVD risks and low As exposure. It was particularly the case for the combined fatal and non-fatal risk of CVD and two CVD markers, where only two studies were found for each of these outcomes.
Importantly, our study could provide guidelines for future modelling assessment in epidemiological studies. We found the linear model better using AIC as the selection criteria depicting the associations of low-level As concentration and CVD outcomes. Such a conclusion has already been confirmed by several epidemiological and biological studies [36,53,61]. However, it has been illustrated that several mechanisms of As toxicity, ranging from the generation of oxidative stress, inhibition of DNA repair to modulation of signal transduction pathways and perturbation of DNA methylation patterns, are likely to give rise to nonlinear dose-response relationships, especially at low concentrations [98,99]. Besides, one recent study also revealed a steeper increase at lower As concentrations for fatal CVD [29]. Considering our limited sample size which may prevent discerning statistical evidence of a departure from a constant linear dose-response association and the significantly non-linear trends detected for some CVD endpoints (p-value for non-linear trend lower than 0.05); it is, therefore, important to bear in mind that the non-linear analysis of As effects should not be neglected, calling for future individual-level studies with sufficient data on low level As exposure and adverse health effects.
Unfortunately, our study also has several limitations, the most important of which might be the unavoidable heterogeneity in our meta-analysis. We assumed that the above-mentioned substantial differences in underlying population characteristics, adjustment for confounding, methods for outcome ascertainment and exposure assessment might be responsible for such heterogeneity. It has been well-documented that assessing As exposure via a biomarker of internal dose is of great importance as it could provide an integrated measure of all sources of exposure, not only from drinking water, but also from diet, soil and air particles [100,101]. It is especially the case for areas with low to moderate drinking water As concentrations, where drinking water might not be the major exposure pathway [102,103]. Notwithstanding this, different biomarkers reflect different periods of As exposure. For example, as toenails normally grow about 0.75 mm per month [104], they could reflect a long-term exposure, even remaining constant for up to 6 years [105]. Similarly, As levels in hair could reflect exposure from the past several months [97]. In contrast, urine and blood are regarded as measuring recent As exposure for only several days or hours, respectively, being recommended as a more reliable method for examining short-term exposure patterns [6,106]. Besides, the relationships between As concentrations in internal biomarker (urine, toenail, hair or plasma) and drinking water still are not well understood, based not only on the magnitude, frequency and duration of environmental As exposure, but also on the individual variability associated to metabolism, body weight, urine dilution and toenail or hair characteristics [107,108,109]. Therefore, the pooled estimation with As concentrations from different exposure media in the present study may result in exposure misclassification, particularly at our low drinking water As concentrations at which both diet and water can contribute substantially to total As exposure. Nevertheless, some assumptions are necessary to pool studies with different exposure metrics and our sensitivity analysis excluding studies limited to biomarkers yielded similar conclusions.
It is also important to understand that even though several perspective studies, such as the ones conducted by D’Ippoliti et al. [29] and Ersboll et al. [57] examined As exposure for a period of more than 20 years, the follow-up periods was still too short for the analysis of such low-level exposures (< 10 µg/L). Thus, more studies with longer periods of study period are warranted to confirm this association. Our meta-analysis might also be underpowered because of the small number of studies and concentration categories included in each CVD endpoint, producing a challenge for distinguishing the sources of heterogeneity. Furthermore, there was a lack-of-fit for the linear analysis of pulse blood pressure in this study, requiring further model improvement. Overall, given the high prevalence of low As exposure from different sources worldwide, long-term prospective studies with sufficient and high quality outcomes and exposure assessment at the individual level are needed [28].
These findings shed light, at least in part, on low-level As exposure induced health risks, suggesting that a consideration of downward revision of the WHO provisional guide value may be indicated to protect better human health and to reduce the economic burden arising from CVD related outcomes, even in countries such as the UK, where relatively few individuals consume drinking water with more than 10 µg/L As [39], but over 20 million people consume drinking water with between 1 µg/L As and 10 µg/L As [38]. Future carefully designed larger-scale perspective studies with sufficient individual data of CVD risk and low level environmental As exposure from different sources are necessary to evaluate the association between As exposure and increases in CVD risk.
5. Conclusions
Our meta-analysis indicates an overall positive association between CVD risks and low level (1 µg/L to 10 µg/L) As concentration in drinking water. For the general population, those with chronic exposure to As in drinking water at the WHO provisional guideline value limit (10 µg/L) compared to a reference population with 1 µg/L As are modelled to have significant excess CHD (50% (95% CI: 15%–95%)) and CVD (17% (95% CI: 5%–31%)) mortality risks, as well as significant excess combined fata land non-fatal risks of CHD (41 % (95% CI: 18%–67%)), CVD (41% (95% CI: 24%–60%)), carotid atherosclerosis (94% (95% CI: 40%–167%)) and hypertension (23% (95% CI: 4%–45%)). Given the high mortality and morbidity of CVD risks in the world [96], such an increase in the relative risks related to low-level As exposure would give rise to a large absolute number of CVD patients and avoidable premature deaths, as well as a substantive increase in the financial burden on health service systems. Uncertainties and limitations of the available data and models indicate that further studies particularly those aiming to provide a deeper mechanistic understanding of the link between CVD and exposure to As are warranted, as well as a consideration of tightening the existing WHO provisional guideline value for arsenic in drinking water.
Acknowledgments
LX acknowledges receipt of a University of Manchester President’s Doctoral Award.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/17/7/2536/s1, Table S1: Epidemiological studies of arsenic (As) exposure and cardiovascular disease (CVD) included in the systematic review, Table S2: Egger’s regression test of funnel plot asymmetry, Table S3: Pooled relative risks (95% confidence intervals) for different CVD types and clinical markers in relation to drinking water arsenic concentrations with the exclusion of studies which do provide drinking water As concentrations directly, Table S4: Pooled relative risks (95% confidence intervals) for different CVD types and CVD markers in relation to drinking water arsenic concentrations lower than 100 ppb, Figure S1: Flow diagram of study selection procedure, Figure S2: Association of CVD endpoints with drinking water arsenic concentrations, Figure S3: Funnel Plots for the analysis of publication bias.
Author Contributions
Conceptualization, L.X. and D.M.; methodology, L.X.; software, L.X.; validation, L.X., D.A.P. and D.M.; formal analysis, L.X.; writing—original draft preparation, L.X.; writing—review and editing, D.A.P. and D.M.; visualization, L.X.; supervision, D.A.P. and D.M. All authors have read and agreed to the published version of the manuscript.
Funding
LX was funded by the University of Manchester.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Currie A. The role of arsenic in carcinogenesis. Br. Med. Bull. 1947;4:402–405. doi: 10.1093/oxfordjournals.bmb.a072834. [DOI] [PubMed] [Google Scholar]
- 2.Pinto S.S., Bennett B. Effect of arsenic trioxide exposure on mortality. Arch. Environ. Health. 1963;7:583–591. doi: 10.1080/00039896.1963.10663587. [DOI] [PubMed] [Google Scholar]
- 3.Culioli J.L., Fouquoire A., Calendini S., Mori C., Orsini A. Trophic transfer of arsenic and antimony in a freshwater ecosystem: A field study. Aquat. Toxicol. 2009;94:286–293. doi: 10.1016/j.aquatox.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Bundschuh J., Nath B., Bhattacharya P., Liu C.W., Armienta M.A., López M.V.M., Lopez D.L., Jean J.S., Cornejo L., Macedo L.F.L. Arsenic in the human food chain: The Latin American perspective. Sci. Total Environ. 2012;429:92–106. doi: 10.1016/j.scitotenv.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 5.Huda N., Hossain S., Rahman M., Karim M.R., Islam K., Al Mamun A., Hossain M.I., Mohanto N.C., Alam S., Aktar S., et al. Elevated levels of plasma uric acid and its relation to hypertension in arsenic-endemic human individuals in Bangladesh. Toxicol. Appl. Pharmacol. 2014;281:11–18. doi: 10.1016/j.taap.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Arsenic. Department of Health and Human Services, Public Health Service; Atlanta, GA, USA: 2007. [PubMed] [Google Scholar]
- 7.Nigra A.E., Sanchez T.R., Nachman K.E., Harvey D.E., Chillrud S.N., Graziano J.H., Navas-Acien A. The effect of the Environmental Protection Agency maximum contaminant level on arsenic exposure in the USA from 2003 to 2014: An analysis of the National Health and Nutrition Examination Survey (NHANES) Lancet Public Health. 2017;2:E513–E521. doi: 10.1016/S2468-2667(17)30195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tchounwou P.B., Patlolla A.K., Centeno J.A. Carcinogenic and systemic health effects associated with arsenic exposure--a critical review. Toxicol. Pathol. 2003;31:575–588. doi: 10.1080/714044691. [DOI] [PubMed] [Google Scholar]
- 9.Ferreccio C., Smith A.H., Duran V., Barlaro T., Benitez H., Valdes R., Aguirre J.J., Moore L.E., Acevedo J., Vasquez M.I., et al. Case-Control Study of Arsenic in Drinking Water and Kidney Cancer in Uniquely Exposed Northern Chile. Am. J. Epidemiol. 2013;178:813–818. doi: 10.1093/aje/kwt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jomova K., Jenisova Z., Feszterova M., Baros S., Liska J., Hudecova D., Rhodes C.J., Valko M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011;31:95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- 11.Nahar M.N., Inaoka T., Fujimura M., Watanabe C., Shimizu H., Tasnim S., Sultana N. Arsenic contamination in groundwater and its effects on adolescent intelligence and social competence in Bangladesh with special reference to daily drinking/cooking water intake. Environ. Health Prev. Med. 2014;19:151–158. doi: 10.1007/s12199-013-0369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadeau K.C., Li Z., Farzan S., Koestler D., Robbins D., Fei D.L., Malipatlolla M., Maecker H., Enelow R., Korrick S., et al. In utero arsenic exposure and fetal immune repertoire in a US pregnancy cohort. Clin. Immunol. 2014;155:188–197. doi: 10.1016/j.clim.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farzan S.F., Li Z., Korrick S.A., Spiegelman D., Enelow R., Nadeau K., Baker E., Karagas M.R. Infant Infections and Respiratory Symptoms in Relation to in Utero Arsenic Exposure in a US Cohort. Environ. Health Perspect. 2016;124:840–847. doi: 10.1289/ehp.1409282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butts C.D., Bloom M.S., Neamtiu I.A., Surdu S., Pop C., Anastasiu D., Fitzgerald E.F., Gurzau E.S. A pilot study of low-moderate drinking water arsenic contamination and chronic diseases among reproductive age women in Timis County, Romania. Environ. Toxicol. Pharmacol. 2015;40:1001–1004. doi: 10.1016/j.etap.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y., Graziano J.H., Parvez F., Liu M., Slavkovich V., Kalra T., Argos M., Islam T., Ahmed A., Rakibuzzaman M. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: Prospective cohort study. BMJ Br. Med. J. 2011;342:d2431. doi: 10.1136/bmj.d2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall E.M., Acevedo J., Lopez F.G., Cortes S., Ferreccio C., Smith A.H., Steinmaus C.M. Hypertension among adults exposed to drinking water arsenic in Northern Chile. Environ. Res. 2017;153:99–105. doi: 10.1016/j.envres.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahman M., Sohel N., Yunus M., Chowdhury M.E., Hore S.K., Zaman K., Bhuiya A., Streatfield P.K. A prospective cohort study of stroke mortality and arsenic in drinking water in Bangladeshi adults. BMC Public Health. 2014;14:1–8. doi: 10.1186/1471-2458-14-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu F., Jasmine F., Kibriya M.G., Liu M., Wójcik O., Parvez F., Rahaman R., Roy S., Paulbrutus R., Segers S. Association Between Arsenic Exposure From Drinking Water and Plasma Levels of Cardiovascular Markers. Am. J. Epidemiol. 2012;175:1252–1261. doi: 10.1093/aje/kwr464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farzan S.F., Chen Y., Rees J.R., Zens M.S., Karagas M.R. Risk of death from cardiovascular disease associated with low-level arsenic exposure among long-term smokers in a US population-based study. Toxicol. Appl. Pharmacol. 2015;287:93–97. doi: 10.1016/j.taap.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y., Wu F., Liu M.L., Parvez F., Slavkovich V., Eunus M., Ahmed A., Argos M., Islam T., Rakibuz-Zaman M., et al. A Prospective Study of Arsenic Exposure, Arsenic Methylation Capacity, and Risk of Cardiovascular Disease in Bangladesh. Environ. Health Perspect. 2013;121:832–838. doi: 10.1289/ehp.1205797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Islam M.S., Mohanto N.C., Karim M.R., Aktar S., Hoque M.M., Rahman A., Jahan M., Khatun R., Aziz A., Salam K.A., et al. Elevated concentrations of serum matrix metalloproteinase-2 and-9 and their associations with circulating markers of cardiovascular diseases in chronic arsenic-exposed individuals. Environ. Health. 2015;14:1–12. doi: 10.1186/s12940-015-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuji J.S., Perez V., Garry M.R., Alexander D.D. Association of low-level arsenic exposure in drinking water with cardiovascular disease: A systematic review and risk assessment. Toxicology. 2014;323:78–94. doi: 10.1016/j.tox.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Navas-Acien A., Sanchez T.R., Mann K., Jones M.R. Arsenic Exposure and Cardiovascular Disease: Evidence Needed to Inform the Dose-Response at Low Levels. Curr. Epidemiol. Rep. 2019;6:81–92. doi: 10.1007/s40471-019-00186-5. [DOI] [Google Scholar]
- 24.Moon K.A., Oberoi S., Barchowsky A., Chen Y., Guallar E., Nachman K.E., Rahman M., Sohel N., D’Ippoliti D., Wade T.J., et al. A dose-response meta-analysis of chronic arsenic exposure and incident cardiovascular disease. Int. J. Epidemiol. 2017;46:1924–1939. doi: 10.1093/ije/dyx202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunrath J., Gurzau E., Gurzau A., Goessler W., Gelmann E.R., Thach T.T., Mccarty K.M., Yeckel C.W. Blood pressure hyperreactivity: An early cardiovascular risk in normotensive men exposed to low-to-moderate inorganic arsenic in drinking water. J. Hypertens. 2013;31:361–369. doi: 10.1097/HJH.0b013e32835c175f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones M.R., Tellezplaza M., Sharrett A.R., Guallar E., Navasacien A. Urine Arsenic and Hypertension in U.S. Adults: The 2003–2008 NHANES. Epidemiology. 2011;22:153–161. doi: 10.1097/EDE.0b013e318207fdf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monrad M., Ersboll A.K., Sorensen M., Baastrup R., Hansen B., Gammelmark A., Tjonneland A., Overvad K., Raaschou-Nielsen O. Low-level arsenic in drinking water and risk of incident myocardial infarction: A cohort study. Environ. Res. 2017;154:318–324. doi: 10.1016/j.envres.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Moon K., Guallar E., Navas-Acien A. Arsenic Exposure and Cardiovascular Disease: An Updated Systematic Review. Curr. Atheroscler. Rep. 2012;14:542–555. doi: 10.1007/s11883-012-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Ippoliti D., Santelli E., De Sario M., Scortichini M., Davoli M., Michelozzi P. Arsenic in Drinking Water and Mortality for Cancer and Chronic Diseases in Central Italy, 1990-2010. PLoS ONE. 2015;10:e0138182. doi: 10.1371/journal.pone.0138182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Research Council . Critical Aspects of EPA’s IRIS Assessment of Inorganic Arsenic: Interim Report. The National Academies Press; Washington, DC, USA: 2013. p. 127. [Google Scholar]
- 31.Wang W., Cheng S., Zhang D. Association of inorganic arsenic exposure with liver cancer mortality: A meta-analysis. Environ. Res. 2014;135:120–125. doi: 10.1016/j.envres.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 32.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 33.Navas-Acien A., Sharrett A.R., Silbergeld E.K., Schwartz B.S., Nachman K.E., Burke T.A., Guallar E. Arsenic exposure and cardiovascular disease: A systematic review of the epidemiologic evidence. Am. J. Epidemiol. 2005;162:1037–1049. doi: 10.1093/aje/kwi330. [DOI] [PubMed] [Google Scholar]
- 34.Phung D., Connell D., Rutherford S., Chu C. Cardiovascular risk from water arsenic exposure in Vietnam: Application of systematic review and meta-regression analysis in chemical health risk assessment. Chemosphere. 2017;177:167–175. doi: 10.1016/j.chemosphere.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Abhyankar L.N., Jones M.R., Guallar E., Navas-Acien A. Arsenic Exposure and Hypertension: A Systematic Review. Environ. Health Perspect. 2012;120:494–500. doi: 10.1289/ehp.1103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsinovoi C.L., Xun P.C., McClure L.A., Carioni V.M.O., Brockman J.D., Cai J.W., Guallar E., Cushman M., Unverzagt F.W., Howard V.J., et al. Arsenic Exposure in Relation to Ischemic Stroke The Reasons for Geographic and Racial Differences in Stroke Study. Stroke. 2018;49:19–26. doi: 10.1161/STROKEAHA.117.018891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerjee M., Banerjee N., Bhattacharjee P., Mondal D., Lythgoe P.R., Martínez M., Pan J., Polya D.A., Giri A.K. High arsenic in rice is associated with elevated genotoxic effects in humans. Sci. Rep. 2013;3:2195. doi: 10.1038/srep02195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polya D.A., Xu L., Launder J., Gooddy D., Ascott M. Arsenic hazard in public water supplies in the United Kingdom: Implications for exposure, health and regulation; Proceedings of the 7th International Congress and Exhibition on Arsenic in the Environment (As 2018); Beijing, China. 1–6 July 2018; pp. 22–25. [Google Scholar]
- 39.Middleton D.R.S., Watts M.J., Hamilton E.M., Ander E.L., Close R.M., Exley K.S., Crabbe H., Leonardi G.S., Fletcher T., Polya D.A. Urinary arsenic profiles reveal exposures to inorganic arsenic from private drinking water supplies in Cornwall, UK. Sci. Rep. 2016;6:25656. doi: 10.1038/srep25656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chowdhury R., Ramond A., O’Keeffe L.M., Shahzad S., Kunutsor S.K., Muka T., Gregson J., Willeit P., Warnakula S., Khan H., et al. Environmental toxic metal contaminants and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ. 2018;362:k3310. doi: 10.1136/bmj.k3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B., et al. Meta-analysis of observational studies in epidemiology—A proposal for reporting. JAMA-J. Am. Med. Assoc. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 42.Li W.F., Sun C.W., Cheng T.J., Chang K.H., Chen C.J., Wang S.L. Risk of carotid atherosclerosis is associated with low serum paraoxonase (PON1) activity among arsenic exposed residents in Southwestern Taiwan. Toxicol. Appl. Pharmacol. 2009;236:246–253. doi: 10.1016/j.taap.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 43.Nong Q., Zhang Y., Guallar E., Zhong Q. Arsenic Exposure and Predicted 10-Year Atherosclerotic Cardiovascular Risk Using the Pooled Cohort Equations in U.S. Hypertensive Adults. Int. J. Environ. Res. Public Health. 2016;13:1093. doi: 10.3390/ijerph13111093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tseng C.H., Chong C.K., Tseng C.P., Hsueh Y.M., Chiou H.Y., Tseng C.C., Chen C.J. Long-term arsenic exposure and ischemic heart disease in arseniasis-hyperendemic villages in Taiwan. Toxicol. Lett. 2003;137:15–21. doi: 10.1016/S0378-4274(02)00377-6. [DOI] [PubMed] [Google Scholar]
- 45.Li Y.F., Wang D., Li X., Zheng Q.M., Sun G.F. A Potential Synergy between Incomplete Arsenic Methylation Capacity and Demographic Characteristics on the Risk of Hypertension: Findings from a Cross-Sectional Study in an Arsenic-Endemic Area of Inner Mongolia, China. Int. J. Environ. Res. Public Health. 2015;12:3615–3632. doi: 10.3390/ijerph120403615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsueh Y.M., Lin P.P., Chen H.W., Shiue H.S., Chung C.J., Tsai C.T., Huang Y.K., Chiou H.Y., Chen C.J. Genetic polymorphisms of oxidative and antioxidant enzymes and arsenic-related hypertension. J. Toxicol. Environ. Health-Part A-Curr. Issues. 2005;68:1471–1484. doi: 10.1080/15287390590967414. [DOI] [PubMed] [Google Scholar]
- 47.Mazumder D.G., Purkayastha I., Ghose A., Mistry G., Saha C., Nandy A.K., Das A., Majumdar K.K. Hypertension in chronic arsenic exposure: A case control study in West Bengal. J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2012;47:1514–1520. doi: 10.1080/10934529.2012.680329. [DOI] [PubMed] [Google Scholar]
- 48.Hsueh Y.M., Wu W.L., Huang Y.L., Chiou H.Y., Tseng C.H., Chen C.J. Low serum carotene level and increased risk of ischemic heart disease related to long-term arsenic exposure. Atherosclerosis. 1998;141:249–257. doi: 10.1016/S0021-9150(98)00178-6. [DOI] [PubMed] [Google Scholar]
- 49.Lisabeth L.D., Ahn H.J., Chen J.J., Sealy-Jefferson S., Burke J.F., Meliker J.R. Arsenic in Drinking Water and Stroke Hospitalizations in Michigan. Stroke. 2010;41:2499–2504. doi: 10.1161/STROKEAHA.110.585281. [DOI] [PubMed] [Google Scholar]
- 50.Wen Y., Huang S.L., Zhang Y.W., Zhang H.M., Zhou L., Li D., Xie C.H., Lv Z.Q., Guo Y.S., Ke Y.B., et al. Associations of multiple plasma metals with the risk of ischemic stroke: A case-control study. Environ. Int. 2019;125:125–134. doi: 10.1016/j.envint.2018.12.037. [DOI] [PubMed] [Google Scholar]
- 51.Yuan Y., Xiao Y., Feng W., Liu Y.Y., Yu Y.Q., Zhou L., Qiu G.K., Wang H., Liu B., Liu K., et al. Plasma Metal Concentrations and Incident Coronary Heart Disease in Chinese Adults: The Dongfeng-Tongji Cohort. Environ. Health Perspect. 2017;125:107007–107016. doi: 10.1289/EHP1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang C.H., Jeng J.S., Yip P.K., Chen C.L., Hsu L.I., Hsueh Y.M., Chiou H.Y., Wu M.M., Chen C.J. Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation. 2002;105:1804–1809. doi: 10.1161/01.CIR.0000015862.64816.B2. [DOI] [PubMed] [Google Scholar]
- 53.Mateen F.J., Grau-Perez M., Pollak J.S., Moon K.A., Howard B.V., Umans J.G., Best L.G., Francesconi K.A., Goessler W., Crainiceanu C., et al. Chronic arsenic exposure and risk of carotid artery disease: The Strong Heart Study. Environ. Res. 2017;157:127–134. doi: 10.1016/j.envres.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newman J.D., Navas-Acien A., Kuo C.C., Guallar E., Howard B.V., Fabsitz R.R., Devereux R.B., Umans J.G., Francesconi K.A., Goessler W., et al. Peripheral Arterial Disease and Its Association With Arsenic Exposure and Metabolism in the Strong Heart Study. Am. J. Epidemiol. 2016;184:806–817. doi: 10.1093/aje/kww002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tseng C.H., Chong C.K., Chen C.J., Tai T.Y. Dose-response relationship between peripheral vascular disease and ingested inorganic arsenic among residents in blackfoot disease endemic villages in Taiwan. Atherosclerosis. 1996;120:125–133. doi: 10.1016/0021-9150(95)05693-9. [DOI] [PubMed] [Google Scholar]
- 56.Moon K.A., Zhang Y., Guallar E., Francesconi K.A., Goessler W., Umans J.G., Best L.G., Howard B.V., Devereux R.B., Okin P.M., et al. Association of low-moderate urine arsenic and QT interval: Cross-sectional and longitudinal evidence from the Strong Heart Study. Environ. Pollut. 2018;240:894–902. doi: 10.1016/j.envpol.2018.04.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ersboll A.K., Monrad M., Sorensen M., Baastrup R., Hansen B., Bach F.W., Tjonneland A., Overvad K., Raaschou-Nielsen O. Low-level exposure to arsenic in drinking water and incidence rate of stroke: A cohort study in Denmark. Environ. Int. 2018;120:72–80. doi: 10.1016/j.envint.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 58.Liao Y.T., Chen C.J., Li W.F., Hsu L.I., Tsai L.Y., Huang Y.L., Sun C.W., Chen W.J., Wang S.L. Elevated lactate dehydrogenase activity and increased cardiovascular mortality in the arsenic-endemic areas of southwestern Taiwan. Toxicol. Appl. Pharmacol. 2012;262:232–237. doi: 10.1016/j.taap.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 59.Mendez M.A., Gonzálezhorta C., Sánchezramírez B., Ballinascasarrubias L., Cerón R.H., Morales D.V., Terrazas F.A.B., Ishida M.C., Gutiérreztorres D.S., Saunders R.J. Chronic Exposure to Arsenic and Markers of Cardiometabolic Risk: A Cross-Sectional Study in Chihuahua, Mexico. Environ. Health Perspect. 2016;124:104–111. doi: 10.1289/ehp.1408742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang J.Y., Liu M.L., Parvez F., Wang B.H., Wu F., Eunus M., Bangalore S., Newman J.D., Ahmed A., Islam T., et al. Association between Arsenic Exposure from Drinking Water and Longitudinal Change in Blood Pressure among HEALS Cohort Participants. Environ. Health Perspect. 2015;123:806–812. doi: 10.1289/ehp.1409004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moon K.A., Navas-Acien A., Grau-Perez M., Francesconi K.A., Goessler W., Guallar E., Umans J.G., Best L.G., Newman J.D. Low-moderate urine arsenic and biomarkers of thrombosis and inflammation in the Strong Heart Study. PLoS ONE. 2017;12:e0182435. doi: 10.1371/journal.pone.0182435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jonsson A., Hjalmarsson C., Falk P., Ivarsson M.L. Levels of matrix metalloproteinases differ in plasma and serum—Aspects regarding analysis of biological markers in cancer. Br. J. Cancer. 2016;115:703–706. doi: 10.1038/bjc.2016.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moon K.A., Guallar E., Umans J.G., Devereux R.B., Best L.G., Francesconi K.A., Goessler W., Pollak J., Silbergeld E.K., Howard B.V., et al. Association Between Exposure to Low to Moderate Arsenic Levels and Incident Cardiovascular Disease. Ann. Intern. Med. 2013;159:649–659. doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Q., Cook N.R., Bergstrom A., Hsieh C.C. A two-stage hierarchical regression model for meta-analysis of epidemiologic nonlinear dose-response data. Comput. Stat. Data Anal. 2009;53:4157–4167. doi: 10.1016/j.csda.2009.05.001. [DOI] [Google Scholar]
- 65.Orsini N., Li R.F., Wolk A., Khudyakov P., Spiegelman D. Meta-Analysis for Linear and Nonlinear Dose-Response Relations: Examples, an Evaluation of Approximations, and Software. Am. J. Epidemiol. 2012;175:66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X., Li B., Xi S.H., Zheng Q.M., Wang D., Sun G.F. Association of urinary monomethylated arsenic concentration and risk of hypertension: A cross-sectional study from arsenic contaminated areas in northwestern China. Environ. Health. 2013;12:37–46. doi: 10.1186/1476-069X-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Islam M.R., Khan I., Attia J., Hassan S.M.N., McEvoy M., D’Este C., Azim S., Akhter A., Akter S., Shahidullah S.M., et al. Association between Hypertension and Chronic Arsenic Exposure in Drinking Water: A Cross-Sectional Study in Bangladesh. Int. J. Environ. Res. Public Health. 2012;9:4522–4536. doi: 10.3390/ijerph9124522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mumford J.L., Wu K.G., Xia Y.J., Kwok R., Wang Z.H., Foster J., Sanders W.E. Chronic arsenic exposure and cardiac repolarization abnormalities with QT interval prolongation in a population-based study. Environ. Health Perspect. 2007;115:690–694. doi: 10.1289/ehp.9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu M.M., Chiou H.Y., Hsueh Y.M., Hong C.T., Su C.L., Chang S.F., Huang W.L., Wang H.T., Wang Y.H., Hsieh Y.C., et al. Effect of plasma homocysteine level and urinary monomethylarsonic acid on the risk of arsenic-associated carotid atherosclerosis. Toxicol. Appl. Pharmacol. 2006;216:168–175. doi: 10.1016/j.taap.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 70.Rahman M., Tondel M., Ahmad S.A., Chowdhury I.A., Faruquee M.H., Axelson O. Hypertension and arsenic exposure in Bangladesh. Hypertension. 1999;33:74–78. doi: 10.1161/01.HYP.33.1.74. [DOI] [PubMed] [Google Scholar]
- 71.Wang S.L., Li W.F., Chen C.J., Huang Y.L., Chen J.W., Chang K.H., Tsai L.Y., Chou K.M. Hypertension incidence after tap-water implementation: A 13-year follow-up study in the arseniasis-endemic area of southwestern Taiwan. Sci. Total Environ. 2011;409:4528–4535. doi: 10.1016/j.scitotenv.2011.07.058. [DOI] [PubMed] [Google Scholar]
- 72.Hsieh Y.C., Hsieh F.I., Lien L.M., Chou Y.L., Chiou H.Y., Chen C.J. Risk of carotid atherosclerosis associated with genetic polymorphisms of apoliploprotein E and inflammatory genes among arsenic exposed residents in Taiwan. Toxicol. Appl. Pharmacol. 2008;227:1–7. doi: 10.1016/j.taap.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 73.Hsieh Y.C., Lien L.M., Chung W.T., Hsieh F.I., Hsieh P.F., Wu M.M., Tseng H.P., Chiou H.Y., Chen C.J. Significantly increased risk of carotid atherosclerosis with arsenic exposure and polymorphisms in arsenic metabolism genes. Environ. Res. 2011;111:804–810. doi: 10.1016/j.envres.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 74.Medrano M.J., Boix R., Pastor-Barriuso R., Palau M., Damian J., Ramis R., del Barrio J.L., Navas-Acien A. Arsenic in public water supplies and cardiovascular mortality in Spain. Environ. Res. 2010;110:448–454. doi: 10.1016/j.envres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y., Factor-Litvak P., Howe G.R., Graziano J.H., Brandt-Rauf P., Parvez F., van Geen A., Ahsan H. Arsenic exposure from drinking water, dietary intakes of B vitamins and folate, and risk of high blood pressure in Bangladesh: A population-based, cross-sectional study. Am. J. Epidemiol. 2007;165:541–552. doi: 10.1093/aje/kwk037. [DOI] [PubMed] [Google Scholar]
- 76.Quansah R., Armah F.A., Essumang D.K., Luginaah I., Clarke E., Marfoh K., Cobbina S.J., Nketiah-Amponsah E., Namujju P.B., Obiri S., et al. Association of Arsenic with Adverse Pregnancy Outcomes/Infant Mortality: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2015;123:412–421. doi: 10.1289/ehp.1307894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ginestet C. ggplot2: Elegant graphics for data analysis. J. R. Stat. Soc. Ser. A. 2011;174:245–246. doi: 10.1111/j.1467-985X.2010.00676_9.x. [DOI] [Google Scholar]
- 78.R Core Team . A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2017. [(accessed on 20 October 2018)]. Available online: https://www.R-project.org/ [Google Scholar]
- 79.Wade T.J., Xia Y., Wu K., Li Y., Ning Z., Le X.C., Lu X., Feng Y., He X., Mumford J.L. Increased mortality associated with well-water arsenic exposure in Inner Mongolia, China. Int. J. Environ. Res Public Health. 2009;6:1107–1123. doi: 10.3390/ijerph6031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sohel N., Persson L.A., Rahman M., Streatfield P.K., Yunus M., Ekstrom E.C., Vahter M. Arsenic in Drinking Water and Adult Mortality A Population-based Cohort Study in Rural Bangladesh. Epidemiology. 2009;20:824–830. doi: 10.1097/EDE.0b013e3181bb56ec. [DOI] [PubMed] [Google Scholar]
- 81.Chen C.J., Chiou H.Y., Chiang M.H., Lin L.J., Tai T.Y. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler. Thromb. Vasc. Biol. 1996;16:504–510. doi: 10.1161/01.ATV.16.4.504. [DOI] [PubMed] [Google Scholar]
- 82.Wang C.H., Chen C.L., Hsu L.I., Chiou H.Y., Hsueh Y.M., Chen S.Y., Wu M.M., Hsiao C.K. Chronic Arsenic Exposure Increases Mortality from Ischemic Heart Disease and Stroke: A Follow-Up Study on 26,851 Residents in Taiwan. National Taiwan University; Taipei, Taiwan: 2005. [Google Scholar]
- 83.Wade T.J., Xia Y.J., Mumford J., Wu K.G., Le X.C., Sams E., Sanders W.E. Cardiovascular disease and arsenic exposure in Inner Mongolia, China: A case control study. Environ. Health. 2015;14:35–44. doi: 10.1186/s12940-015-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.James K.A., Byers T., Hokanson J.E., Meliker J.R., Zerbe G.O., Marshall J.A. Association between Lifetime Exposure to Inorganic Arsenic in Drinking Water and Coronary Heart Disease in Colorado Residents. Environ. Health Perspect. 2015;123:128–134. doi: 10.1289/ehp.1307839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Y., Wu F., Parvez F., Ahmed A., Eunus M., McClintock T.R., Patwary T.I., Islam T., Ghosal A.K., Islam S. Arsenic exposure from drinking water and QT-interval prolongation: Results from the Health Effects of Arsenic Longitudinal Study. Environ. Health Perspect. 2013;121:427–432. doi: 10.1289/ehp.1205197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaplan G.A., Keil J.E. Socioeconomic factors and cardiovascular disease: A review of the literature. Circulation. 1993;88:1973–1998. doi: 10.1161/01.CIR.88.4.1973. [DOI] [PubMed] [Google Scholar]
- 87.Lear S.A., Hu W.H., Rangarajan S., Gasevic D., Leong D., Iqbal R., Casanova A., Swaminathan S., Anjana R.M., Kumar R., et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: The PURE study. Lancet. 2017;390:2643–2654. doi: 10.1016/S0140-6736(17)31634-3. [DOI] [PubMed] [Google Scholar]
- 88.Notara V., Panagiotakos D., Kogias Y., Stravopodis P., Antonoulas A., Zombolos S., Mantas Y., Pitsavos C. The impact of education status on the 10-year (2004-2014) cardiovascular disease incidence and all cause mortality, among Acute Coronary Syndrome patients: The GREECS longitudinal study. J. Prev. Med. Public Health. 2016;4:220–229. doi: 10.3961/jpmph.16.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Owolabi M.O., Sarfo F., Akinyemi R., Gebregziabher M., Akpa O., Akpalu A., Wahab K., Obiako R., Owolabi L., Ovbiagele B., et al. Dominant modifiable risk factors for stroke in Ghana and Nigeria (SIREN): A case-control study. Lancet Glob. Health. 2018;6:E436–E446. doi: 10.1016/S2214-109X(18)30002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shah A.D., Langenberg C., Rapsomaniki E., Denaxas S., Pujades-Rodriguez M., Gale C.P., Deanfield J., Smeeth L., Timmis A., Hemingway H. Type 2 diabetes and incidence of cardiovascular diseases: A cohort study in 1-9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neaton J.D., Wentworth D. Serum Cholesterol, Blood Pressure, Cigarette Smoking, and Death From Coronary Heart Disease Overall Findings and Differences by Age for 316099 White Men. Arch. Intern. Med. 1992;152:56–64. doi: 10.1001/archinte.1992.00400130082009. [DOI] [PubMed] [Google Scholar]
- 92.Wood A.M., Kaptoge S., Butterworth A.S., Willeit P., Warnakula S., Bolton T., Paige E., Paul D.S., Sweeting M., Burgess S., et al. Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet. 2018;391:1513–1523. doi: 10.1016/S0140-6736(18)30134-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Donnell M.J., Chin S.L., Rangarajan S., Xavier D., Liu L.S., Zhang H.Y., Rao-Melacini P., Zhang X.H., Pais P., Agapay S., et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): A case-control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 94.Banack H.R., Kaufman J.S. The obesity paradox: Understanding the effect of obesity on mortality among individuals with cardiovascular disease. Prev. Med. 2014;62:96–102. doi: 10.1016/j.ypmed.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 95.Smedley P.L., Kinniburgh D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002;17:517–568. doi: 10.1016/S0883-2927(02)00018-5. [DOI] [Google Scholar]
- 96.Wang H., Naghavi M., Allen C., Barber R.M., Bhutta Z.A., Carter A., Casey D.C., Charlson F.J., Chen A.Z., Coates M.M. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang C.H., Hsiao C.K., Chen C.L., Hsu L.I., Chiou H.Y., Chen S.Y., Hsueh Y.M., Wu M.M., Chen C.J. A review of the epidemiologic literature on the role of environmental arsenic exposure and cardiovascular diseases. Toxicol. Appl. Pharmacol. 2007;222:315–326. doi: 10.1016/j.taap.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 98.Chavan H., Christudoss P., Mickey K., Tessman R., Ni H.-m., Swerdlow R., Krishnamurthy P. Arsenite effects on mitochondrial bioenergetics in human and mouse primary hepatocytes follow a nonlinear dose response. Oxidative Med. Cell. Longev. 2017;2017:1–12. doi: 10.1155/2017/9251303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schoen A., Beck B., Sharma R., Dubé E. Arsenic toxicity at low doses: Epidemiological and mode of action considerations. Toxicol. Appl. Pharmacol. 2004;198:253–267. doi: 10.1016/j.taap.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 100.Lin P.D., Bromage S., Mostofa M.G., Allen J., Oken E., Kile M.L., Christiani D.C. Associations between Diet and Toenail Arsenic Concentration among Pregnant Women in Bangladesh: A Prospective Study. Nutrients. 2017;9:420. doi: 10.3390/nu9040420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hughes M.F. Biomarkers of exposure: A case study with inorganic arsenic. Environ. Health Perspect. 2006;114:1790–1796. doi: 10.1289/ehp.9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davis M.A., Mackenzie T.A., Cottingham K.L., Gilbert-Diamond D., Punshon T., Karagas M.R. Rice Consumption and Urinary Arsenic Concentrations in U.S. Children. Environ. Health Perspect. 2012;120:1418–1424. doi: 10.1289/ehp.1205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leese E., Morton J., Tan E., Gardiner P.H.E., Carolan V.A. LCICP-MS Determinations of Unexposed UK Urinary Arsenic Speciation Reference Values. J. Anal. Toxicol. 2014;38:24–30. doi: 10.1093/jat/bkt090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hopps H.C. The biologic bases for using hair and nail for analyses of trace elements. Sci. Total Environ. 1977;7:71–89. doi: 10.1016/0048-9697(77)90018-3. [DOI] [PubMed] [Google Scholar]
- 105.Garland M., Morris J.S., Rosner B.A., Stampfer M.J., Spate V.L., Baskett C.J., Willett W.C., Hunter D.J. Toenail trace element levels as biomarkers: Reproducibility over a 6-year period. Cancer Epidemiol. Prev. Biomark. 1993;2:493–497. [PubMed] [Google Scholar]
- 106.Rahbar M.H., Samms-Vaughan M., Ma J., Bressler J., Loveland K.A., Ardjomand-Hessabi M., Dickerson A.S., Grove M.L., Shakespeare-Pellington S., Beecher C., et al. Role of Metabolic Genes in Blood Arsenic Concentrations of Jamaican Children with and without Autism Spectrum Disorder. Int. J. Environ. Res. Public Health. 2014;11:7874–7895. doi: 10.3390/ijerph110807874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Middleton D.R.S., Watts M.J., Lark R.M., Milne C.J., Polya D.A. Assessing urinary flow rate, creatinine, osmolality and other hydration adjustment methods for urinary biomonitoring using NHANES arsenic, iodine, lead and cadmium data. Environ. Health. 2016;15:68. doi: 10.1186/s12940-016-0152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pomroy C., Charbonneau S., McCullough R., Tam G. Human retention studies with 74As. Toxicol. Appl. Pharmacol. 1980;53:550–556. doi: 10.1016/0041-008X(80)90368-3. [DOI] [PubMed] [Google Scholar]
- 109.Marchiset-Ferlay N., Savanovitch C., Sauvant-Rochat M.-P. What is the best biomarker to assess arsenic exposure via drinking water? Environ. Int. 2012;39:150–171. doi: 10.1016/j.envint.2011.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.