Abstract
We present a case of a middle-age male who presented in emergency room with nonspecific abdominal pain. A contrast-enhanced computer tomography (ceCT) scan showed a reduced perfusion of both adrenal glands. The clinical examinations and the laboratory tests were negative for an adrenal pathological process. To reassess the adrenal ischemia, a second ceCT scan was performed 5 days later showing an acute bilateral adrenal hemorrhage. These findings demonstrated that the previous adrenal hypoperfusion represented the prodromal manifestation of a hemorrhagic intraglandular process. This case suggests that adrenal hypoperfusion detected on tomographic imaging dictates a prompt clinical management finalized to strictly monitor the potential evolution towards a more aggressive pathological condition and confirms the pivotal role of imaging in the diagnosis of such uncommon disorder.
Keywords: Adrenal, Hemorrhage, Tomographic imaging
Case report
A 53-years-old Caucasian male presented in emergency room referring asthenia, nausea, and slight nonspecific abdominal pain. Neither recent trauma nor previous surgical procedures were reported. He referred an acute cerebral hemorrhagic stroke 25 years before without any clinical sequelae and a story of essential hypertension managed with a calcium-antagonist drug (amlodipine). No further pharmacological treatments were reported.
Laboratory emergency investigations disclosed leukocytosis and increased inflammatory indexes: WBC 27.000/mm3 (normal range -n.r.- 4-10.000/mm3) with 90% neutrophils (n.r. 40%-75%) and C-Reactive Protein 28.6 mg/L (n.r. 0-10 mg/L). The other main laboratory results were unremarkable.
An abdominal ultrasound showed a centimetric biliary calculus in the gallbladder infundibulum, without any further notable findings. A ceCT was performed in clinical suspicion of an inflammatory abdominal process, confirming cholelithiasis in absence of acute cholecystitis signs. Moreover, a mild thickening of adrenal glands with reduced perfusion of right medial and left lateral limbs was observed (Fig. 1). No therapy was administered to the patient. Because of the persistence of abdominal complaint and deep asthenia, 5 days later a second ceCT scan was performed to reassess adrenal ischemia. Both adrenal glands presented an increased volume, rounded margins, spontaneous hyperdense appearance on basal images and no enhancement on postcontrast phases. These findings were suggestive of bilateral adrenal hemorrhage (Fig. 2).
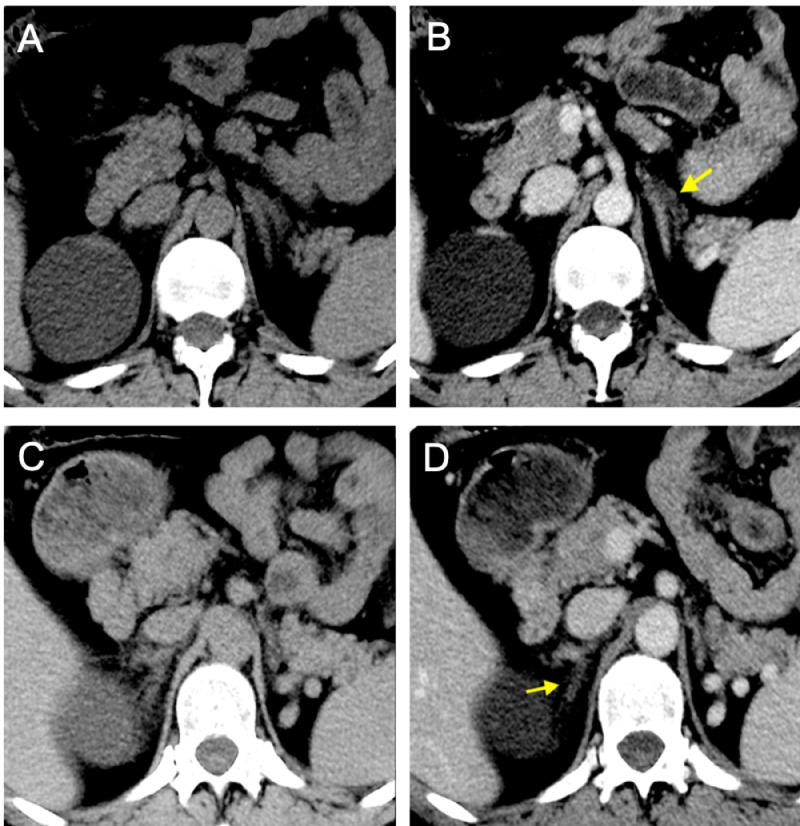
Fig. 1.
Precontrast (A, C) and venous (B, D) phases of first CT scan showing reduced enhancement of left lateral adrenal gland limb (arrow in B) and right medial adrenal limb (arrow in D). Both limbs appear thickened.
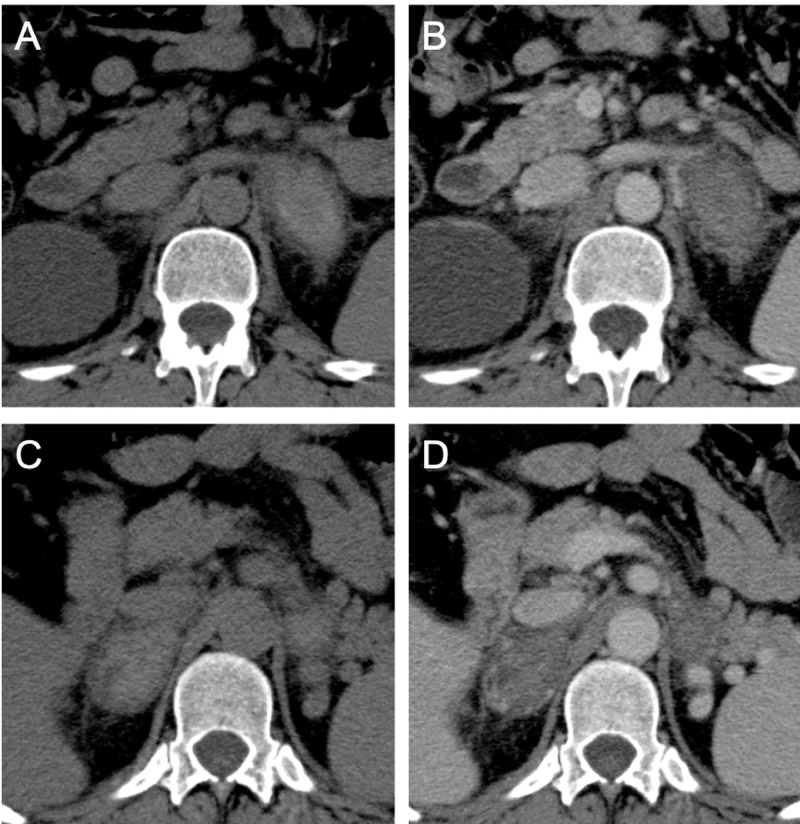
Fig. 2.
Precontrast (A, C) and venous (B, D) phases of CT scan showing the increased volume of left (A, B) and right (C, D) adrenal glands, which appear hyperattenuated on basal scans without enhancement on postcontrast images phase. Periadrenal fat stranding was evidenced. The presence of a bilateral adrenal hemorrhage was suggested.
Laboratory assessment of adrenal function was performed, showing the following values: adrenocorticotropic hormone (ACTH) 50 pg/mL (n.r 0-46 pg/mL), serum cortisol 80 nmol/L (n.r 171‐536 nmol/L), urinary cortisol 100 µg/24h (n.r 35-135 µg/24h), plasmatic renin 7.2 pg/mL (n.r 2.52-35.82 pg/mL), aldosterone 37 pg/mL (n.r 40-310 pg/mL), 17-hydroxyprogesterone 0.55 ng/mL (n.r 0.40-2.39 ng/mL). All necessary investigations were performed to define the cause of adrenal hemorrhage; however neither stressful events (e.g. congestive heart failure, pancreatitis, cholecystitis) nor blunt trauma, hemorrhagic diatheses (eg, anticoagulant use, thrombocytopenia and/or coagulation factor mutations), thrombophilia (eg, antiphospholipid antibody syndrome and/or presence of lupus-like antibodies), infective disease (QuantiFERON test, cultural exams to research bacteria as Neisseria meningitidis or Streptococcus pneumoniae), underlying tumors (eg, pheochromocytomas) and/or amyloidosis were demonstrated.
The day after the second CT scan, an MRI examination was performed to further characterize adrenal findings, depicting very low signal intensity of both glands on T2-weighted (-w) images, iso-hypointensity on T1-w images and hypointensity on diffusion-weighted images (DWI) with a peripheral hyperintensity showing restricted diffusion on apparent diffusion coefficient (ADC) maps; moreover, no enhancement was observed on T1-w postcontrast images. These findings were suggestive for an acute/very early subacute bleeding (Fig. 3). Two weeks after the event, the follow-up MRI documented the evolution of the blood products. Adrenal glands appeared patchy hyperintense on T2-w images, hypointense with subtle peripheral hyperintensity on T1-w images, hyperintense on DWI with restricted diffusion on ADC maps. An advanced delay subacute hemorrhage was suggested (Fig. 4). Adrenal hormone levels, electrolytes and overall hemodynamic stability were monitored for several weeks. The patient did not develop severe adrenal insufficiency; thus, no replacement treatment was necessary.
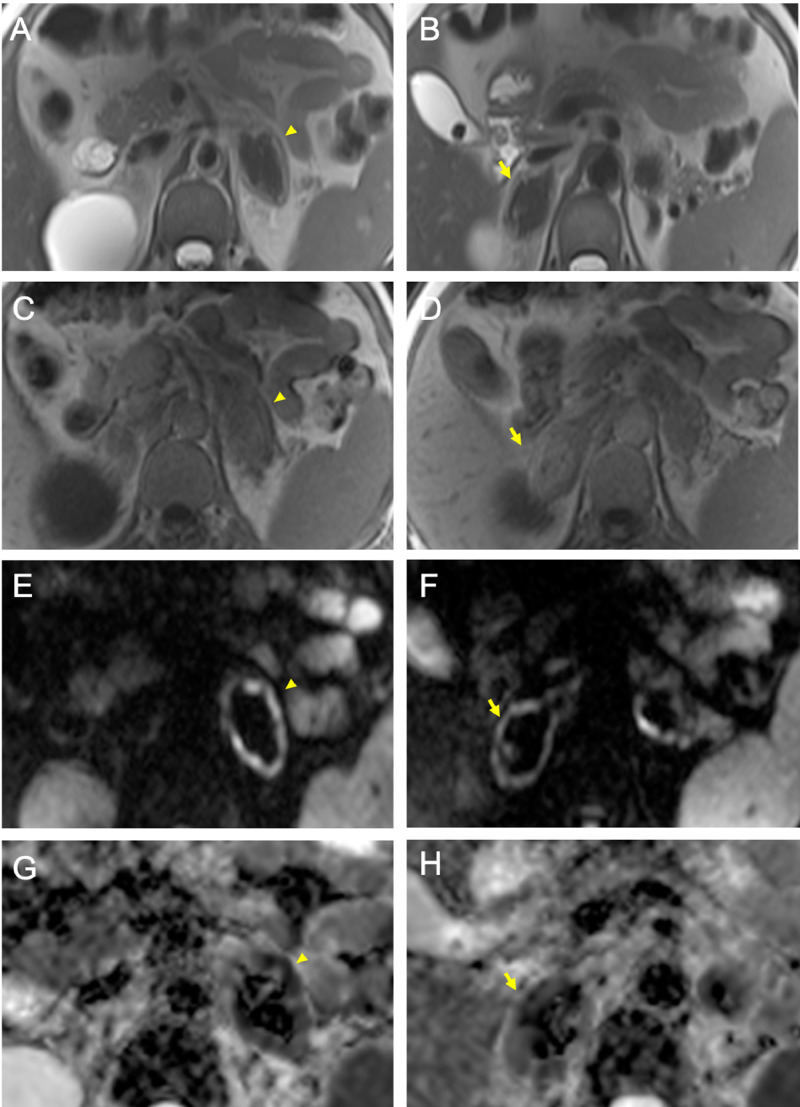
Fig. 3.
Acute/very early subacute phase of adrenal hemorrhage (arrowheads in A-C-E-G: left gland; arrows in B-D-F-H: right gland) detected on MRI resulting in low-signal intensity on T2-w images (A, B), intermediate to low-signal intensity on T1-w images (C, D), hypointensity with peripheral rim of hyperintensity on DWI (E, F) which showed restricted diffusion on ADC maps (G, H).
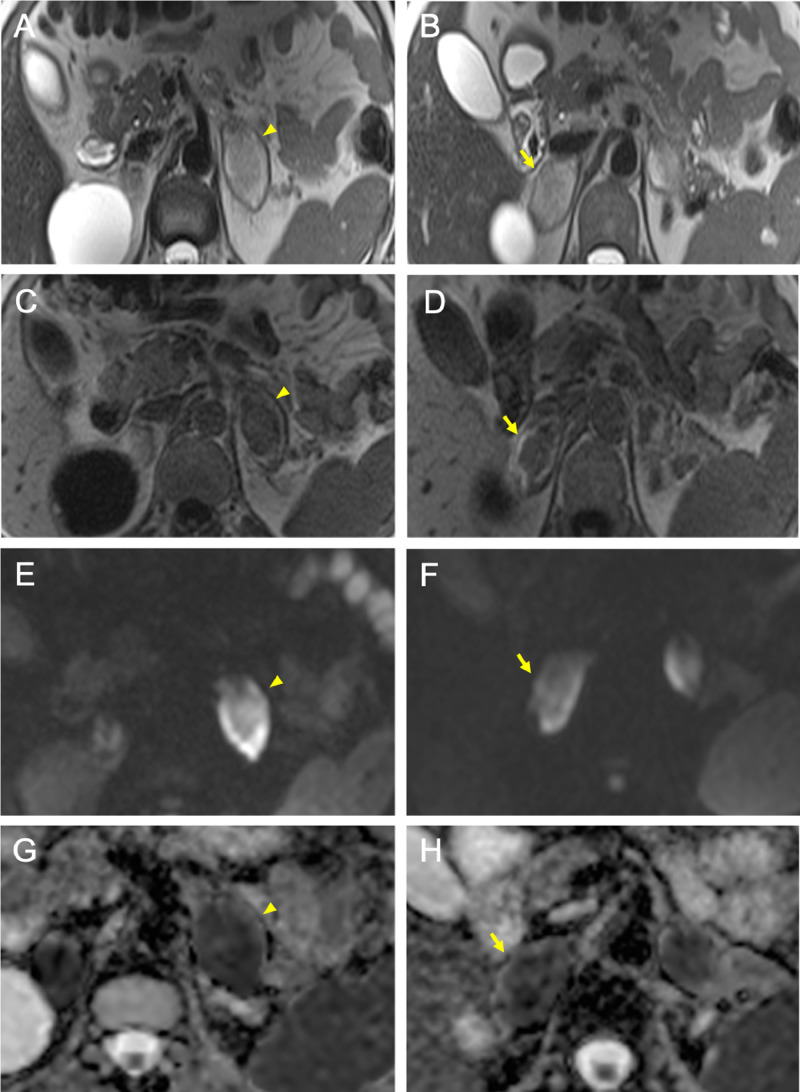
Fig. 4.
Advanced delay subacute phase of adrenal hemorrhage on follow-up MRI (arrowheads in A-C-E-G: left gland; arrows in B-D-F-H: right gland), resulting in increased signal intensity on T2-w images (A, B), in hypointensity with subtle peripheral hyperintensity on T1-w images (C, D), in hyperintesity on DWI (E, F) with restricted diffusion on ADC maps (G, H).
Discussion
Adrenal hemorrhage is an uncommon process, especially when involving both glands. The disease represents a possible cause of systemic shock that must be recognized and treated as early as possible [1,2]. The causes of adrenal hemorrhage can be classified as nontraumatic or traumatic. The following nontraumatic etiologies have been reported: (a) stress, (b) hemorrhagic diathesis or coagulopathy, (c) neonatal stress, (d) underlying adrenal tumors, and (e) idiopathic disease [3]. A well-defined etiology has not been disclosed in our case; thus, an idiopathic form has been suggested.
The underlying mechanism of bleeding propensity of adrenal glands has not been fully elucidated, particularly it has not been defined whether hemorrhage results from infarction or precedes it. The “adrenal dam,” a term used to describe the peculiar vascular anatomy of these glands, has been called into question. The rich arterial supply associated to a single central draining vein determines an abrupt flow changes into medullary sinusoids [4]. The abrupt flow changes in the capillary plexus creates hemodynamic turbulence leading to platelets aggregation and as a consequence to microvascular thrombosis, especially in situations of procoagulable states or during episodes of hypotension or high stress-induced levels of catecholamines and ACTH [5]. It is likely that venous thrombosis is antecedent to hemorrhage, that occurs at the time of reperfusion because of necrotic vessels bleeding [6]. The pathological process may be worsened by reduced capillary resistance associated to aging [7].
In our case, the first CT scan documented a mild thickening of adrenal glands associated to reduced perfusion on postcontrast images, which was more evident on venous phase supporting the hypothesis of venous thrombosis as the event preceding the bleeding. The 5 days later CT scan demonstrated a further adrenal dimensional increase with spontaneous hyperdensity on precontrast images and absence of enhancement on postcontrast images, suggesting an infarction with conspicuous amount of hemorrhage.
The MRI documented signal changes associated to the degradation of blood products. The first set of images disclosed the acute/very early subacute (3 days-1 week) phase of adrenal hemorrhage. The strongly paramagnetic effect of intracellular deoxyhemoglobin resulted in substantial signal loss on T2-w images, in a little signal change on T1-w images and hypointensity with peripheral hyperintensity on DWI. The 2 weeks later MRI documented the advanced delay subacute (range between 1 week and 1 month) phase of bleeding. After red cells break down, extracellular methemoglobin determined an increased signal intensity on T2-w and DWI and determined a subtle peripheral hyperintensity on T1-w images.
Acute adrenal infarction on CT images has well been described as an enlarged low-attenuation unilateral or bilateral adrenal gland with no significant enhancement after intravenous contrast material injection, often surrounded by a thin high-attenuation rim, known as the capsular sign [8]. The presence of hemorrhage is depicted depending on the basal density on CT or intensity on MRI; the different phases of such process in terms of attenuation modifications on CT scan or blood signal intensity changes on MRI have been well described [1,9,10]. Regarding the early stage of these processes, the evidence of diffuse slight thickening of glands with periadrenal fat stranding is reported as a sign of a precious phase of adrenal injury resulting from the vascular congestion within the glands [11]. These findings were similar to those depicted in our first CT scan; in addition, we focalized the reduced perfusion of the adrenal limbs as a sign of the early ischemia preceding the bleeding in absence of a known trigger. In a clinical context of a life-threatening condition such as adrenal hemorrhage, the radiologist has to be confident with the precocious ischemic adrenal changes associated to this pathological process, allowing an early diagnosis. This point is mandatory because the initial symptoms may be poor and nonspecific, as well as a massive (>90%) bilateral adrenal involvement is needed to promote specific clinical manifestations. Moreover, during the initial phase of the pathological process, the basal steroid levels can be normal, while the adrenal reserve may decrease making the cortisol secretion suboptimal only in response to stress situations [2]. In our case, laboratory tests showed a minimal initial adrenal function impairment, suggesting the absence of a massive necrotic involvement of both glands. Nevertheless, monitoring vital functions of patient was crucial to evaluate the potential aggressive evolution of the pathological process. In conclusion, tomographic imaging is confirmed to play a fundamental role in the initial phase of adrenal hemorrhage allowing physicians to perform a prompt and correct treatment management before any potential clinical and laboratory manifestations appear.
References
- 1.Alexandraki K.I., Grossman A. Adrenal insufficiency. In: Feingold K.R., Anawalt B., Boyce A., editors. Vol. 2000. MDText.com, Inc.; South DartmouthMA: 2018. https://www.ncbi.nlm.nih.gov/books/NBK279122/ (Endotext). Available from. [Google Scholar]
- 2.Rao R.H., Vagnucci A.H., Amico J.A. Bilateral massive adrenal hemorrhage: early recognition and treatment. AnnInternMed 1. 1989;110(3):227–235. doi: 10.7326/0003-4819-110-3-227. http://doi:10.7326/0003-4819-110-3-227 [DOI] [PubMed] [Google Scholar]
- 3.Kawashima A., Sandler C.M., Ernst R.D., Takahashi N., Roubidoux M.A., Goldman S.M. Imaging of nontraumatic hemorrhage of the adrenal gland. RadioGraphics. 1999;19(4):949–963. doi: 10.1148/radiographics.19.4.g99jl13949. https://doi:10.1148/radiographics.19.4.g99jl13949 [DOI] [PubMed] [Google Scholar]
- 4.Dhawan N., Bodukam V.K., Thakur K., Singh A., Jenkins D., Bahl J. Idiopathic bilateral adrenal hemorrhage in a 63-year-old male: a case report and review of the literature. Case Rep Urol. 2015:1–4. doi: 10.1155/2015/503638. http://doi:10.1155/2015/503638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riddell A.M., Khalili K. Sequential adrenal infarction without MRI-detectable hemorrhage in primary antiphospholipid-antibody syndrome. Am J Roentgenol. 2004;183(1):220–222. doi: 10.2214/ajr.183.1.1830220. [DOI] [PubMed] [Google Scholar]
- 6.Fox B. Venous infarction of the adrenal glands. J Pathol. 1976;119(2):65–89. doi: 10.1002/path.1711190202. http://doi:10.1002/path.1711190202 [DOI] [PubMed] [Google Scholar]
- 7.McDonald F.D., Myers A.R., Pardo R. Adrenal hemorrhage during anticoagulant therapy. JAMA. 1966;198(10):1052–1056. doi: 10.1001/jama.1966.03110230068014. [DOI] [PubMed] [Google Scholar]
- 8.Moschetta M., Telegrafo M., Pignatelli A., Stabile Ianora A.A., Angelelli G. Value of the CT capsular sign as a potential indicator of acute adrenal ischemia. Emerg Radiol. 2015;22(5):533–538. doi: 10.1007/s10140-015-1327-4. http://doi:10.1007/s10140-015-1327-4 [DOI] [PubMed] [Google Scholar]
- 9.Maurea S., Imbriaco M., Mollica C., Pace L., Salvatore M. Quantitative imaging characterization of hypersecreting or nonhypersecreting adrenal adenomas: comparison between iodine-131 norcholesterol uptake and magnetic resonance signal intensity ratios. Nucl Med Commun. 2011;32(6):535–541. doi: 10.1097/MNM.0b013e32834319e3. http://doi:10.1097/MNM.0b013e32834319e3 [DOI] [PubMed] [Google Scholar]
- 10.Sayit A.T., Sayit E., Gunbey H.P., Aslan K. Imaging of unilateral adrenal hemorrhages in patients after blunt abdominal trauma: report of two cases. Chin J Traumatol. 2017. 2017;20(1):52–55. doi: 10.1016/j.cjtee.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serafino M.D., Severino R., Coppola V., Gioioso M., Rocca R., Lisanti F. Nontraumatic adrenal hemorrhage: the adrenal stress. Radiol Case Rep. 2017;12(3):483–487. doi: 10.1016/j.radcr.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]