Abstract
Previous studies have been reported that the fruit body of wild Phellinus baumii alleviates diabetes, and antioxidants are beneficial to diabetes by protecting the β-cell from damage due to oxidative stress. Large-scale cultivation of P. baumii fruit body has been successful in the past decade. This paper aimed to investigate whether the fruit body of the cultivated P. baumii has the same analogical effects as the wild. The cultivated P. baumii fruit body was extracted by 80% of ethanol extracts, and different fractions were obtained with the successive use of petroleum ether, ethyl acetate (EtOAc), n-butanol (n-BuOH), and water, which yielded 15.98 ± 1.56, 1.74 ± 0.34, 3.31 ± 0.41, 4.12 ± 0.37, and 1.38 ± 0.26% extract recovery, respectively. Results show that the EtOAc fraction exhibits the highest inhibitory effect on α-glucosidase activity (IC50 = 49.05 ± 3.14 μg mL–1), which is an order of magnitude higher than the positive control (acarbose, IC50 = 645.73 ± 7.86 μg mL–1). It was mainly composed of phenolic compounds with a purity of 79.45% and characterized by liquid chromatography-mass spectrometry as osmudacetone, hispidin, davallialactone, 2,5-bis(4,7-dihydroxy-8-methyl-2-oxo-2H-chromen-3-yl)cyclohexa-2,5-diene-1,4-dione, hypholomin B, and inoscavin A. Furthermore, the EtOAc fraction increased the glucose consumption of insulin-resistant HepG2 cells at a concentration range of 25–100 μg mL–1. The EtOAc fraction also demonstrated antioxidant activities by scavenging 1,1-diphenyl-2-picrylhydrazyl, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)diammonium salt, and hydroxyl radicals. In conclusion, the EtOAc fraction of the cultivated P. baumii fruit body exerted effective antidiabetic effects, possibly due to the high content of selective phenolic compounds. Hence, the cultivated fruit body of P. baumii can be a sustainable resource for treating diabetes, and our work also shed some light on its future utilization.
1. Introduction
Diabetes mellitus (DM), a chronic metabolic disorder characterized by hyperglycemia, can be classified into types 1 and 2 and gestational DM.1 Among them, type 2 DM accounts for about 90–95% of all cases of diabetes.2 With reference to the 2017 statistics taken from the International Diabetes Federation, about 425 million people suffer from diabetes worldwide, with the number growing at a surprising rate; it is estimated that it would reach 693 million by the year 2045. Hyperglycemia is the major cause of damage to various organs, including the eyes, kidneys, heart, and blood vessels.3 Inhibiting α-glucosidase to alleviate glucose formation and improving insulin resistance to promote glucose consumption are two frequently employed effective methods for treating diabetes.4,5 In addition, it is known that oxidative stress reduction can relieve tissue damage associated with glucose metabolism, thus facilitating diabetes management.6 In recent years, much attention has focused on various natural antidiabetic compounds derived from edible fungi in view of their low degree of side-effects.7
Phellinus baumii, well-known as “Sanghuang” in China, is a valued functional fungus that has been widely used over the centuries as a food source in several East Asian countries, including China, Korea, and Japan. P. baumii possesses a wide range of biological activities, such as decreasing blood lipid levels, antitumor, antiinfluenza, and antioxidation capacities, and regulating blood sugar levels.8−12 It consequently has the reputation of being called “forest gold.” The phenolics separated from the fruit body of wild Phellinus have been shown to exhibit a hypoglycemic effect; they consist of hispidin, chlorophellins C, gilvsins A, B, C, D, 7,8-dihydroxycoumarin, 3,4-dihydroxybenzalacetone, 7,3′-dihydroxy-5′-methoxyiso-flavone, and inoscavin C.13−16 However, wild Phellinus is a valuable and rare resource, which hinders its sustainable development and industrial utilization. Therefore, in order to meet increasing demands, great effort has been devoted during the past decade for its successful large-scale cultivation.
Previous studies have revealed that extracts and some phenolic compounds from the wild Phellinus fruit body demonstrate a hypoglycemic effect (Table 1). However, there are only a limited number of studies regarding the chemical compounds and biological activity of the fruit body derived from cultivated P. baumii, and their antidiabetic role has barely been investigated. Thus, the aim of this work is to investigate whether the fruit body of cultivated P. baumii has a hypoglycemic effect and whether it contains phenolics similar to those of its wild type. A sample from a cultivated P. baumii fruit body was extracted and fractioned using different polar solvents [petroleum ether (PET), ethyl acetate (EtOAc), and n-butanol (n-BuOH)] in succession. The in vitro hypoglycemic effects of different fractions were evaluated by α-glucosidase inhibition and glucose consumption assay in a IR-HepG2 cell model, and the phenolics in the active fraction were characterized by liquid chromatography–mass spectrometry (LC–MS). Furthermore, free-radical scavenging of 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)diammonium salt (ABTS), and hydroxyl were used to assess the antioxidative activities of the screened fraction.
Table 1. Antidiabetic Activities of Reported Extracts or Compounds from Genuses of P. baumii, Phellinus igniarius, and Phellinus linteus.
| antidiabetic approach | source | extracts/compounds | result | references |
|---|---|---|---|---|
| α-glucosidase inhibitory activity | P. baumii | crude exopolysaccharides | inhibition rate = 59.70% (5.0 mg/mL) | (21) |
| P. baumii | purification exopolysaccharides | inhibition rate = 53.73% (5.0 mg/mL) | (21) | |
| P. linteus | methanol extracts | IC50 = 477.33 ± 17.55 μg mL–1 | (22) | |
| reduction of oxidative stress | P. linteus | hispidin | oxygen species scavenging activity = 55% | (13) |
| enhancement of glucose uptake | P. igniarius | 7,8-dihydroxycoumarin, 3,4-dihydroxybenzalacetone, 7,3′-dihydroxy-5′-methoxyisoflavone, and inoscavin C | increased glucose uptake by 1.73-fold | (16) |
2. Results and Discussion
2.1. Fraction Yields and Their Total Polyphenolic Content
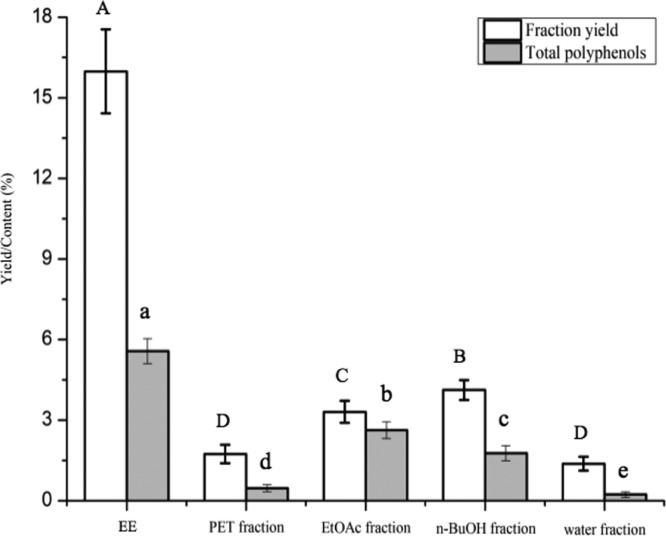
The compounds from the fruit body of cultivated P. baumii were fractionally extracted with different polar solvents. As shown in Figure 1, the yield of each fraction was 15.98 ± 1.56% [ethanol extracts (EE)], 1.74 ± 0.34% (PET fraction), 3.31 ± 0.41% (EtOAc fraction), and 4.12 ± 0.37% (n-BuOH fraction). In recent decades, phenolics have been excessively investigated as the main type of antioxidation compound and also in terms of other activities. Therefore, the contents of total polyphenols in different fractions of cultivated P. baumii were measured. The results revealed that the total phenolic content in the EE was 5.57 ± 0.46%; it was mainly present in the EtOAc fraction (2.63 ± 0.31%) and the n-BuOH fraction (1.77 ± 0.28%) but far less was present in the PET fraction (0.47 ± 0.13%) and the water fraction (0.23 ± 0.11%). The phenolic purity of cultivated P. baumii was of the order EtOAc fraction (79.45 ± 0.48%) > n-BuOH fraction (42.96 ± 2.94%) > EE (34.86 ± 0.52%) > PET fraction (27.01 ± 2.19%) > water fraction (16.67 ± 1.83%), calculated from the phenolic contents of each obtained fraction against its corresponding yield. The reason for this was possibly the polarity of phenol being much closer to those of ethyl acetate and n-butanol phase.
Figure 1.
Extraction yield and total polyphenol contents of different fractions from the cultivated P. baumii fruit body. Results are means ± SD (n = 3). Different letters indicate a significant difference by Duncan’s multiple test at P < 0.05.
2.2. α-Glucosidase Inhibitory Effect of the Fractions
α-Glucosidase inhibition in the small intestine is a simple and effective way by which hyperglycemia treatment can manage type 2 DM.17 It was reported that different sources of phenolics play a role in inhibiting α-glucosidase; for instance, examples of such phenolics include anthocyanidin derivative from blueberry, blackcurrant, and blue honeysuckle fruits,18 catechins derivative derived from green tea, and rosmarinic acid derived from Perilla frutescens.19,20 Hence, it was of interest to explore whether the phenolics in the cultivated P. baumii fruit body had similar biological effects. The results of α-glucosidase inhibitory activities from P. baumii extracts are summarized in Table 2. The activities of α-glucosidase inhibition in these extracts were in the order of EtOAc fraction (IC50 = 49.05 ± 3.14 μg mL–1) > n-BuOH fraction (IC50 = 68.37 ± 3.83 μg mL–1) > PET fraction (IC50 = 95.47 ± 4.93 μg mL–1) > EE (IC50 = 179.81 ± 6.93 μg mL–1) > water fraction (IC50 = 378.97 ± 13.73 μg mL–1), where IC50 is the half-inhibitory concentration value. Results showed that all fractions displayed potent inhibitory activity as compared to the positive control of acarbose (IC50 = 645.73 ± 7.86 μg mL–1) and that the EtOAc fraction was the foremost. The inhibition tendency was almost consistent with that of the purity level. A previous study reported that the α-glucosidase inhibitory activity of bioactive exopolysaccharide from the cultured broth of P. baumii was lower than that of acarbose.21 Besides, the methanol extracts from the wild P. baumii fruit body inhibited α-glucosidase with an IC50 value of 477.33 ± 17.55 μg mL–1, which was better than the inhibition by acarbose22 but significantly inferior to our EtOAc fraction from cultivated P. baumii. The references indicated that small-molecule compounds might possess higher α-glucosidase inhibition activity than the macromolecular polysaccharides from P. baumii. Combining the experimental results, we inferred that the substances inhibiting α-glucosidase activity in the cultivated P. baumii fruit body were mainly derived from the extracts of ethyl acetate and probably belonged to phenolics according to our previous description. In short, the EtOAc fraction being rich in phenols from the cultivated P. baumii fruit body could dramatically inhibit α-glucosidase activities and had a potential effect of lowering the blood sugar levels.
Table 2. α-Glucosidase Inhibitory Activities of Samples from the Fruit Body of Cultivated P. baumiia.
| sample | IC50 (μg mL–1) |
|---|---|
| EE | 179.81 ± 6.93c |
| PET fraction | 95.47 ± 4.93d |
| EtOAc fraction | 49.05 ± 3.14f |
| n-BuOH fraction | 68.37 ± 3.83e |
| water fraction | 378.97 ± 13.73b |
| acarbose | 645.73 ± 7.86a |
Results are taken as means ± SD (n = 3). Different letters indicate a significant difference using Duncan’s multiple test at P < 0.05.
2.3. Identification of Ethyl Acetate Fraction Compounds
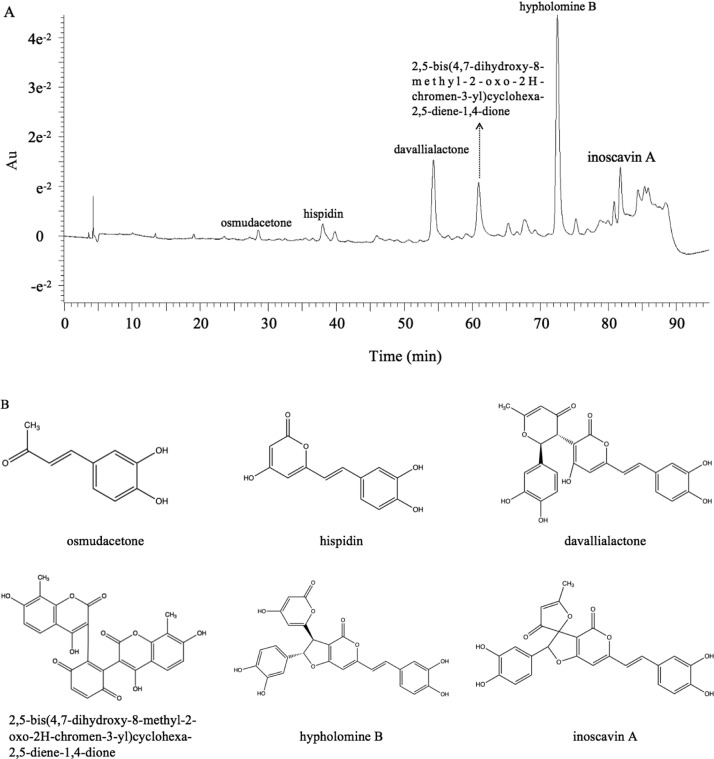
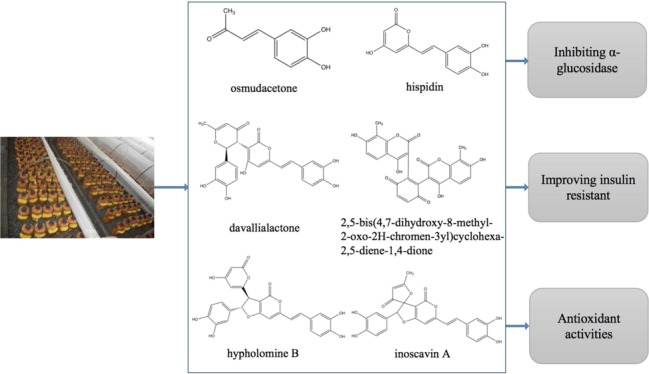
As presented in Table 3, six major polyphenolic compounds in the EtOAc fraction of cultivated P. baumii fruit bodies were tentatively identified by LC–MS. Figure 2A shows the high-performance liquid chromatography (HPLC) chromatograph at an ultraviolet wavelength of 254 nm; according to the data and literature, they were identified as osmudacetone, hispidin, davallialactone,2,5-bis(4,7-dihydroxy-8-methyl-2-oxo-2H-chromen-3-yl)cyclohexa-2,5-diene--1,4-dione, hypholomin B, and inoscavin A, and the relative area of each peak was 1.09, 2.96, 13.72, 10.70, 34.66, and 6.32%, respectively. Most of the compounds were previously identified in the wild Phellinus fruit body;23−25 nevertheless, 2,5-bis(4,7-dihydroxy-8-methyl-2-oxo-2H-chromen-3-yl)cyclohexa-2,5-diene-1,4-dione was rarely found in the Phellinus species. These compounds belong to the hydroxycoumarin derivatives. As seen from their structure (Figure 2B), most of them contained the major part of hispidin. According to research, 2,5-bis(4,7-dihydroxy-8-methyl-2-oxo-2H-chromen-3-yl)cyclohexa-2,5-diene-1,4-dione was a potent α-glucosidase inhibitor.26 Davallialactone and hypholomine B were found to inhibit the aldose reductase (AR) enzyme of rat (IC50 = 0.33 and 0.82 μM) and human (IC50 = 0.56 and 1.28 μM) lenses, respectively.27 Reduction of the polyol pathway flux by AR inhibitors was a potential therapeutic opening in the treatment and prevention of diabetic complications.28 Hispidin from the fruit body of wild Phellinus was shown to exhibit antidiabetic effects through preventing the damage of β-cells induced by reactive oxygen species.13,29,30 β-cell dysfunction is thought to be integral to the pathogenesis of type 2 DM.31 It was also found that davallialactone could prevent and treat liver damage.32 Nevertheless, the previous literature did not explicitly involve the use of these compounds in treating diabetes. Accumulated data showed that oxidation stress was one of the main mechanisms leading to type 2 diabetes,33 and the liver is an important organ for the metabolism of sugars in the body. Taken together, it was indicated that all these substances were associated with antidiabetic effects.
Table 3. Retention Time and Mass Spectral Information of Compounds 1–6 in the EtOAc Fraction.
| RT (min) | MS (m/z) | formula | fragment ions | peak area (%) | compound |
|---|---|---|---|---|---|
| 28.54 | 177.0569 | C10H10O3 | 177, 171, 121, 119 | 1.09 | osmudacetone |
| 38.07 | 245.0451 | C13H10O5 | 245, 144, 121 | 2.96 | hispidin |
| 54.31 | 463.1029 | C25H20O9 | 463, 405,121 | 13.72 | davallialactone |
| 60.94 | 487.0656 | C26H16O10 | 487, 277, 121 | 10.70 | 2,5-bis(4,7-dihydroxy-8-methyl-2-oxo-2H-chromen-3-yl)cyclohexa-2,5-diene-1,4-dione |
| 72.53 | 489.0816 | C26H18O10 | 489, 445, 335, 241 | 34.66 | hypholomine B |
| 81.98 | 461.0785 | C25H18O9 | 461, 459, 230 | 6.32 | inoscavin A |
Figure 2.
Major constituents of the EtOAc fraction from the fruit body of cultivated P. baumii (PB-EtOAc). (A) HPLC chromatogram of PB-EtOAc at 254 nm with peaks 1–6. (B) Chemical structures of compounds 1–6 identified from PB-EtOAc.
2.4. Establishment of an Insulin-Resistant HepG2 Cell Model
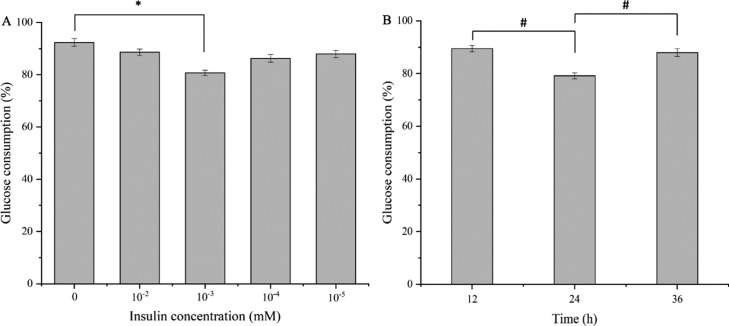
The establishment of the HepG2 cell insulin-resistant model depended on the insulin concentration and the culture time. Observed from Figure 3A, HepG2 cells were treated with different concentrations of insulin (10–2, 10–3, 10–4, and 10–5 mM) for 24 h, and a significant decrease (P < 0.05) in glucose consumption was found in the group at 10–3 mM with a value of 11.58 ± 0.35% as compared with the control group. HepG2 cells were then treated with an optimized concentration of 10–3 mM insulin and cultured for 12, 24, and 36 h. As shown in Figure 3B, the value of glucose consumption in the group at 24 h was significantly lower than those in the groups at 12 and 36 h. Therefore, HepG2 cells treated for 24 h with 10–3 mM insulin were at the optimized condition for cell modeling.
Figure 3.
Effects of different concentrations of insulin and cultured time on glucose consumption in HepG2 cells. Results are means ± SD (n = 3). (A) Glucose consumption in HepG2 cells under different concentrations of insulin. *P < 0.05, which is compared with the control. (B) Glucose consumption in HepG2 cells under different culture times. #P < 0.05, which is compared with 24 h group. Different letters indicate a significant difference by Tukey test.
2.5. Effects of Ethyl Acetate Fraction Concentration on Cell Viability
The concentration of the samples would have a strong impact on the HepG2 cells. As seen from the data in Figure 4, little toxic effects were induced on the cells when the concentration of ethyl acetate extracts was below 100 μg mL–1. At concentrations of 100–600 μg mL–1, the ethyl acetate extracts exerted certain inhibition on the cell viability. Hence, the concentrations of 25, 50, and 100 μg mL–1 were chosen in the subsequent experiments.
Figure 4.
MTT assay results of EtOAc fraction from the fruit body of cultivated P. baumii at different concentrations. Results are means ± SD (n = 3).
2.6. Effects of Ethyl Acetate Fraction on Insulin Resistance in HepG2 Cells
Insulin resistance is an important characteristic of type 2 DM. Therefore, it was further explored whether the EtOAc fraction from the fruit body of the cultivated P. baumii could alleviate the insulin resistance.
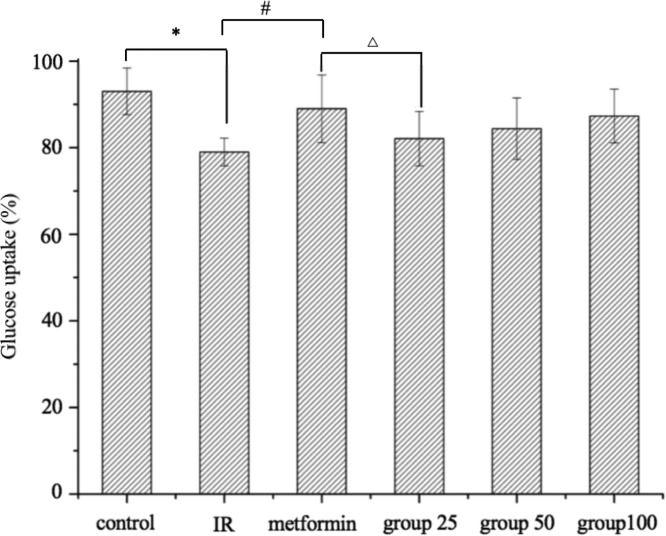
As shown in Figure 5, when the cells were in a state of insulin resistance (optimized cultured in 10–3 mM of insulin for 24 h), the glucose consumption of the cells was significantly reduced compared with the control group (P < 0.05). The number of insulin receptors on the cell surface was decreased at a relatively high concentration of insulin,34 which improved when treated with the EtOAc fraction of cultivated P. baumii. The results also showed that the alleviation effects of the EtOAc fraction on insulin resistance were in a dose-dependent manner at nontoxic concentrations. The positive control of metformin is a common medicine for the treatment of type 2 diabetes.35 Regulating hepatic glucose production and improving the insulin receptor sensitivity on the cell surface were attributed to the blood sugar lowering activities of metformin.36 At a concentration of 100 μg mL–1, the ability of the EtOAc fraction to improve HepG2 cell insulin resistance was approximately equivalent to that of metformin (100 μg mL–1). It was reported that EE rich in phenol from the wild P. igniarius fruit body could alleviate insulin resistance.16 Further test by Zheng also suggested that the extracts could enhance glucose uptake in L6 cells, and these phenolic compounds were identified as 7,8-dihydroxycoumarin, 3,4-dihydroxybenzalacetone, 7,3′-dihydroxy-5′-methoxyisoflavone, and inoscavin C. We report herein that the phenolic-rich extracts from the cultivated P. baumii fruit body also undertake the similar function in HepG2 cells, and the phenolic compounds were characterized as osmudacetone, hispidin, davallialactone, 2,5-bis(4,7-dihydroxy-8-methyl-2-oxo-2H-chromen-3-yl)cyclohexa-2,5-diene-1,4-dione, hypholomin B, and inoscavin A, which were totally different from the compounds found by Zheng, and it might be attributed to the difference in the type of raw material, the extraction method, and the consequent active substances. In conclusion, the cultivated P. baumii fruit body could be explored as novel therapeutic products on type 2 DM.
Figure 5.
Effects of the EtOAc fraction from the fruiting body of P. baumii on glucose consumption in insulin-resistant HepG2 cells. “IR” represents the insulin resistance group; “metformin” represents the positive control group of metformin. Results are means ± SD (n = 3). Different letters indicate a significant difference by Tukey test. *P < 0.05, which is compared with the control; #P < 0.05, which is compared with metformin; △P < 0.05, which is compared with IR.
2.7. In Vitro Antioxidant Activity
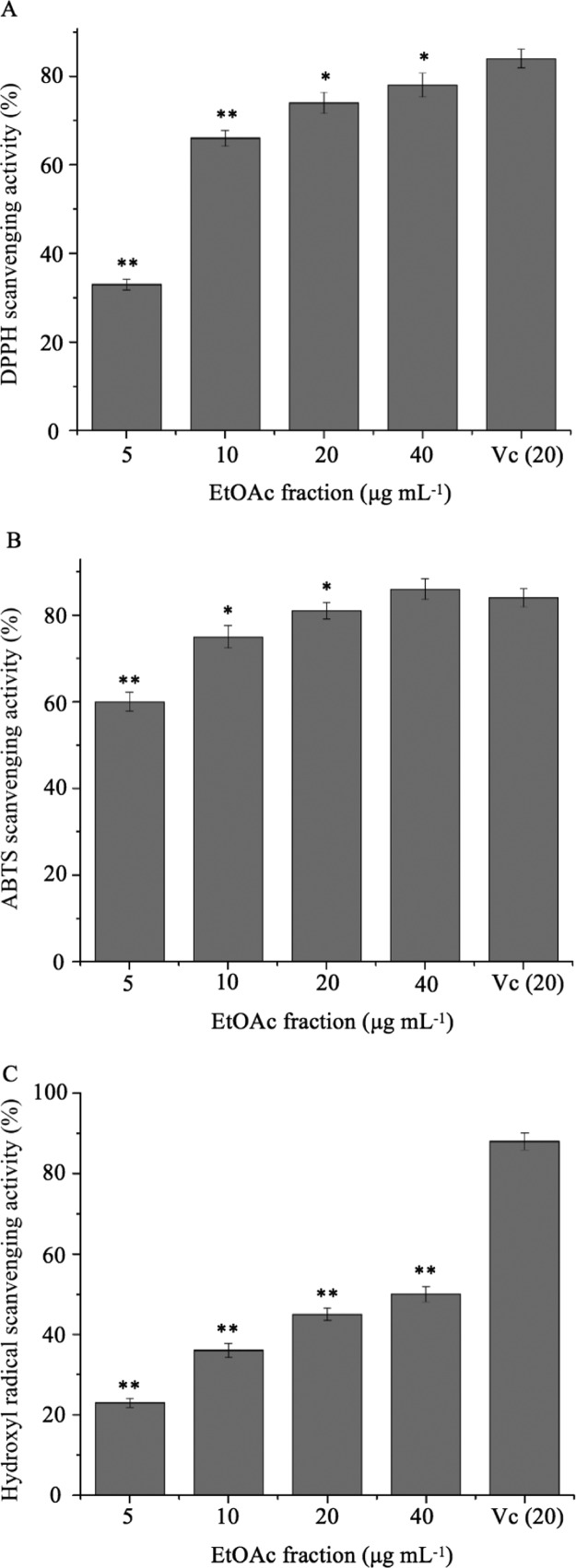
The results of the in vitro antioxidant activity of the EtOAc fraction are shown in Figure 6. The EtOAc fraction displayed dose-dependent antioxidant activity in the range of 5–40 μg mL–1.
Figure 6.
DPPH (A), ABTS (B), and hydroxyl radical (C) scavenging activities of the EtOAc fraction from the fruiting body of P. baumii. Data are expressed as means ± SD of at least three independent experiments. Different letters indicate a significant difference according to the Tukey test. *P < 0.05, **P < 0.01, which is compared with Vc.
DPPH has been used in various models to test the free radical quenching ability of natural products.37 As shown in Figure 6A, the DPPH radical scavenging ratio of the EtOAc fraction (40 μg mL–1) was 78.12 ± 2.73%.
In the ABTS analysis, ABTS•+ reacts with hydrogen donor molecules, and so there is a positive correlation between the radical scavenging activity of phenolic compounds and their hydroxyl number.38 Our results are consistent with the above theory. As shown in Figure 6B, the free-radical scavenging ability of the EtOAc fraction (40 μg mL–1) against ABTS•+ was 86.08 ± 2.47%, which was comparable to that of vitamin C (Vc) (20 μg mL–1, 84.03 ± 2.16%).
The hydroxyl radicals’ scavenging ability is a result of their combined action of reducing power, hydrogen supply, and scavenging for active oxygen molecules.39 As shown in Figure 6C, the hydroxyl radical scavenging ability of the EtOAc fraction is 50.12 ± 1.97% at a concentration of 40 μg mL–1.
The EtOAc fraction of cultivated P. baumii had equivalent free-radical scavenging effects at a concentration of 40 μg mL–1, as compared with that of the methanol extracts (500 μg mL–1) of wild Phellinus.40 The phenolic compounds had the ability to delay or inhibit the oxidative damage caused by free radicals.41 Oxidative stress is an important risk factor for the onset and development of type 2 DM.38 Oxidative stress might damage the tissue of β-cells, which are involved in glucose metabolism. Natural antioxidants might be used to treat diabetes by improving β-cell dysfunction and reducing these cells’ apoptosis.42 Hence, the EtOAc fraction’s antioxidant activity contributes to the potential antidiabetic effect.
3. Conclusions
In this study, the fractions of PET, EtOAc, n-BuOH, and water were obtained after successive extractions of 80% EE from a cultivated P. baumii fruit body. In the α-glucosidase inhibition test, the EtOAc fraction with the highest content of phenolics (79.45 ± 0.48%) showed the optimal effect (IC50 = 49.05 ± 3.14 μg mL–1). Six phenolic compounds were characterized from the EtOAc fraction, namely, osmudacetone, hispidin, davallialactone, 2,5-bis(4,7-dihydroxy-8-methyl-2-oxo-2H-chromen-3-yl)cyclohexa-2,5-diene-1,4-dione, hypholomin B, and inoscavin A. The evaluation of the 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT) assay indicated that the EtOAc fraction had a nontoxic effect on the HepG2 cells of concentration less than 100 μg mL–1. The EtOAc fraction was able to increase glucose consumption in insulin-resistant HepG2 cells at a concentration range of 25–100 μg mL–1, and the values were equivalent to that of the positive control of metformin at a concentration of 100 μg mL–1. The EtOAc fraction displayed significant antioxidant activities in evaluations by DPPH, ABTS, and hydroxyl radical tests.
In summary, the results implied that the phenolic compounds in the cultivated P. baumii fruit body were able to inhibit glucose formation, improve glucose consumption, and reduce oxidative stress, which suggests that the fruit body is likely to be utilized as a sustainable medicinal or functional food resource for the regulation of type 2 DM. In general, this study fills in the gap of the antidiabetes effects of the phenolic compounds in cultivated P. baumii. Nevertheless, further in vitro and in vivo in-depth studies are required to explore the mechanisms involved in regulating DM.
4. Materials and Methods
4.1. Materials and Reagents
Cultivated P. baumii fruit body (Figure 7) harvested in May 2018 was promptly provided by the Sericultural Research Institute, Zhejiang Academy of Agricultural Sciences (Hangzhou, China), and the species was identified by Professor Lizhong Fu at the Zhejiang Chinese Medical University. α-Glucosidase from Saccharomyces cerevisiae (10 U mg–1), a glucose assay kit, bovine insulin, metformin, dimethyl sulfoxide (DMSO), antibiotic (100 U mL–1 penicillin and 100 μg mL–1 streptomycin), potassium persulfate, DPPH, and ABTS were all purchased from Sigma-Aldrich Co., Ltd. (St Louis, USA). High-glucose Dulbecco’s modified Eagle’s medium (DMEM) medium, phenol red-free, low-glucose DMEM medium, and fetal bovine serum (FBS) were all acquired from Hyclone (Logan, Utah, USA). All other reagents used were of analytical grade.
Figure 7.

Fruit bodies of P. baumii cultivated in a greenhouse.
4.2. Isolation and Determination of the Bioactive Compounds
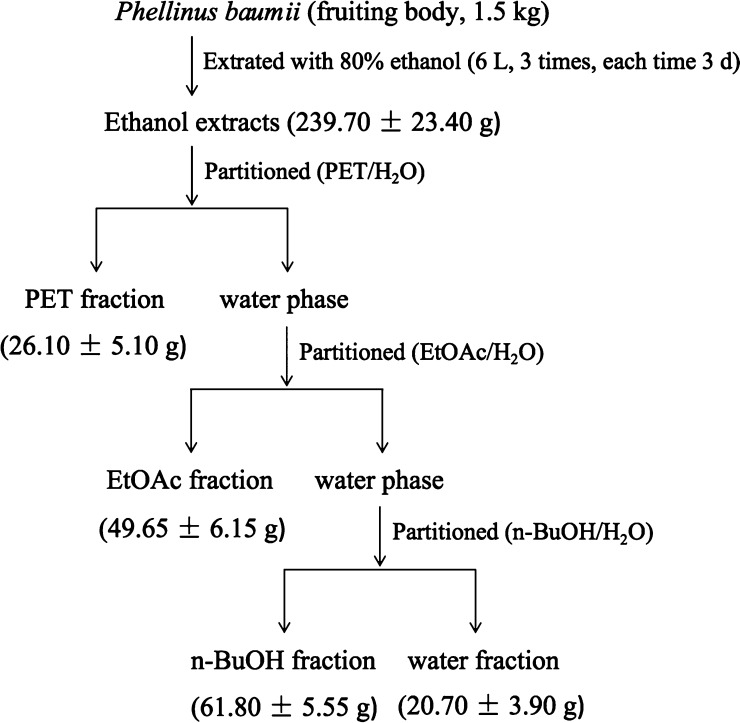
As shown in Figure 8, 1.5 kg of cultivated P. baumii fruit body was crushed and ground into powder and then dried by a forced-air oven (GZX-9070MBE, Boxun Medical Biological Instruments Co., Ltd, Shanghai, China) at 50 °C for 2 h. Afterward, it was extracted twice by 6.0 L of 80% ethanol at room temperature for 72 h. The EE were combined and filtered by a no. 1 Whatman filter paper, concentrated, and dried with a vacuum rotary evaporator (RV3 Flex, IKA Instrument and Equipment Co., Ltd, Guangzhou, China) at 45 °C. The dried matter was resuspended and homogenized in 2.0 L of purified water (1:10, v/v) in a 5.0 L separating funnel and then fractionally extracted with PET (4 × 2 L), ethyl acetate (4 × 2 L), and n-butanol (4 × 2 L), separately. Four fractions were subsequently obtained by vacuum drying and classified as PET, EtOAc, n-BuOH, and water fractions (the remains). The yield of each fraction was calculated by dividing its dry weight by that of the raw material.
Figure 8.
Flow chart of the fractions extracted from the fruit body of cultivated P. baumii.
4.3. Determination of Total Polyphenols
The total polyphenols in the fraction of the cultivated P. baumii were determined by the Folin-phenol method with a slight modification.43 In short, 10.0 mg of each fraction was accurately weighed and dissolved with methanol to a volume of 10 mL. Then, 0.5 mL of the sample solution was mixed in a test tube with 0.5 mL of 0.25 mol L–1 Folin–Ciocalteu reagent. After 3 min, 1.0 mL of 15% Na2CO3 solution was mixed and left for 30 min. The blue supernatant was obtained by centrifugation for 5 min at 3500 rpm. The absorbance was measured at 760 nm, with 15% Na2CO3 being used as the blank control. The total contents of the polyphenols in the sample were based on the standard pyrogallol curve.
4.4. α-Glucosidase Inhibitory Activity Assay
The α-glucosidase inhibitory activities of the fraction were assayed using a slight modification of the method.44 Briefly, 100 μL of α-glucosidase (0.5 U mL–1) in 100 mM pH 6.9 phosphate buffer and 50 μL of sample solution in 10% methanol were mixed in 96-well plates and incubated for 10 min at room temperature. After that, 50 μL of 5 mM p-NPG (4-nitrophenyl-β-d-glucopyranoside) in the phosphate buffer was added to initiate the reaction in each well and incubated for 10 min at room temperature, and then the optical density value was determined by a spectrophotometer at 405 nm. Acarbose was used as the positive control. Finally, the α-glucosidase inhibitory activity was calculated as follows:
where “Acontrol” represents the absorbance of the phosphate buffer and enzyme system; “Ablank control” represents the absorbance of the phosphate buffer; “Asample” represents the absorbance of the extracts, phosphate buffer, and enzyme system; and “Ablank sample” represents the absorbance of the extracts and phosphate buffer only. The inhibition activity was expressed as the half-inhibitory concentration (IC50) value.
4.5. Cell Culture and MTT Assay
The cell culture protocol was followed as described previously.45 In brief, HepG2 cells were cultured in a complete medium (high-glucose DMEM + 10% FBS + 1% penicillin and streptomycin) and placed in culture flasks. The medium was renewed every second day, and the subsequent serial passage of the cells was performed by trypsin digestion.
The evaluation of cell viability was according to the MTT method.46 In short, the HepG2 cells were seeded on 96 multiwell plates at 4 × 103 cells per well and cultured for 48 h. Then, DMEM was added at different sample concentrations (25, 50, and 100 μg mL–1) and allowed to continue culturing for a further 24 h. After that, 20 μL of MTT solution (5.0 mg mL–1 in phosphate-buffered saline, pH 7.4) was added to each well and further incubated at 37 °C for 4 h, and then 100 μL of DMSO was added into each well and mixed on a gyratory shaker for 30 min. Finally, the plates were scanned at 570 nm.
4.6. IR-HepG2 Cell Model
An insulin-resistant cell model was induced according to the previous method with some modification.47 The cells were seeded in 96-well plates (104 cells per well) and then incubated in the complete medium for 24 h. In order to construct a model of cellular insulin resistance, the optional concentration of insulin was first investigated at different concentrations of 10–2, 10–3, 10–4, and 10–5 mM for 24 h, and then the optimized concentration of 10–3 mM insulin was cultured for 12, 24, and 36 h, respectively. After the cells were washed with DMEM, the glucose consumption was measured with a glucose kit.
The cells were divided into four groups: (1) the control group, with a culture of normal HepG2 cells in DMEM medium; (2) the insulin resistance group, with a culture of IR-HepG2 cells in DMEM medium; (3) the experimental group, with a culture of IR-HepG2 cells in DMEM medium with extracts (25, 50, and 100 μg mL–1); and (4) the metformin group, with a culture of IR-HepG2 cells in medium with 100 μg mL–1 of metformin. All cells were cultured in an incubator for 24 h at 37 °C. Each group had six parallel wells. The glucose consumption was detected by the glucose oxidase/peroxidase assay kit following the manufacturer’s instructions.
4.7. Chemical Characterization of the Ethyl Acetate Fraction
Chemical characterization of the ethyl acetate fraction was carried out on an LC–MS system (Waters UPLC Synapt G2-Si HDMS, Milford, MA, USA) with a Waters C18 column (5 μm, 4.6 × 150 mm). Purified water (A) and acetonitrile (B) containing 0.1% formic acid were used as mobile phases. The gradient program was used as follows: 0–20 min, 5–20% B; 20–60 min, 20–50% B; and 60–95 min, 50–100% B. The column was equilibrated for 5 min between each injection. The flow rate was 1.0 mL min–1, and the injection volume was 10.0 μL. Electrospray ionization-mass spectra were recorded in the negative ion mode. The capillary voltage was 2500 V, the cone hole voltage was 40 V, and the sample extraction voltage was 5.0 V. The ion source temperature was 120 °C, and the desolvent gas temperature was 350 °C. The spray gas was high purity nitrogen (N2), and the collision gas was high purity argon (Ar). The reverse gas flow rate was 80 L h–1, and the solvent removal gas flow rate was 800 L h–1. The MS scanning range was 100–1200 amu, and the scanning time was 0.3 s. Finally, the compounds were tentatively identified through comparisons with the literature data.
4.8. Hydroxyl Radical Assay
4.8.1. DPPH Assay
The DPPH assay was carried out according to the previous report with a slight modification.37 Briefly, 2.0 mL of the sample solution was added to 2.0 mL of the DPPH solution (0.1 mM); the reaction solution was vortexed and then left in the dark at room temperature for 30 min. The absorbance was measured at 517 nm, and Vc was used as the positive control. The activity was expressed as a percentage of DPPH scavenging using the following equation
where A0 is the absorbance of the control and A1 is the absorbance of the test sample.
4.8.2. ABTS Assay
The ABTS scavenging activity was assayed according to the previous report with a slight modification.48 Briefly, the ABTS•+ reagent was produced by reacting 10 mL of 7 mM ABTS and 179 mL of 140 mM potassium persulphate and incubated in the dark for 12 h at room temperature before use. The ABTS•+ solution was then diluted 20-fold with water prior to analysis. Different concentrations of samples (0.1 mL) was added to 3.0 mL of the ABTS•+ cation solution and then mixed thoroughly. The reaction mixture was kept at room temperature in the dark for 10 min, and the absorbance was recorded at 734 nm; Vc was used as the positive control. The antioxidant activity was expressed as the percentage of ABTS•+ scavenging using the following equation
where A0 is the absorbance of the control and A1 is the absorbance of the test samples.
4.8.3. Hydroxyl Radical Assay
The •OH scavenging activity assay was carried out according to the previous report with a slight modification.39 Briefly, different concentrations of samples were added to a reaction solution containing 1.0 mL of 6 mM FeSO4 and 1.0 mL of 6 mM H2O2 and left to stand for 10 min. After adding 1.0 mL of 6 mM salicylic acid, the reaction mixture was incubated at 37 °C for 30 min and centrifuged at 3000 rpm for 10 min. The absorbance was measured at 510 nm; Vc was used as the positive control. The percentage of the hydroxyl radical scavenging ability was subsequently calculated
where A0 is the absorbance of the control and A1 is the absorbance of the test samples.
4.9. Data Analysis
All data are presented as the mean ± standard deviation (SD) from at least triplicate analysis of each sample. The statistical analysis was performed by analyzing the one-way analysis of variance followed by Tukey’s or Duncan’s test. A probability (P) value of less than 0.05 was considered statistically significant, and less than 0.01 was considered extremely significant. Data were analyzed using version 20.0 SPSS Statistics (SPSS Inc., Chicago, IL).
Acknowledgments
This research was funded by the key research and development projects of Zhejiang Province (no. 2018C02003 and no. 2019C02040).
The authors declare no competing financial interest.
References
- Cudworth A. G.; Woodrow J. C. Classification of diabetes. Lancet 1977, 309, 949–950. 10.1016/s0140-6736(77)92241-3. [DOI] [PubMed] [Google Scholar]
- Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care 2018, 41, S13–S27. 10.2337/dc18-s002. [DOI] [PubMed] [Google Scholar]
- Mirmiran P.; Bahadoran Z.; Azizi F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: a review. World J. Diabetes 2014, 5, 267–281. 10.4239/wjd.v5.i3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H.; Zhao D.; Xue Z. Exploring the interaction between Salvia miltiorrhiza and α-glucosidase: insights from computational analysis and experimental studies. RSC Adv. 2018, 8, 24701–24710. 10.1039/c8ra04772c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilcampos M.; Canete R.; Gil A. Adiponectin, the missing link in insulin resistance and obesity. Clin. Nutr. 2004, 23, 963–974. 10.1016/j.clnu.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Nowotny K.; Jung T.; Höhn A.; Weber D.; Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomole 2015, 5, 194–222. 10.3390/biom5010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wińska K.; Maczka W.; Gabryelska K.; Grabarczyk M. Mushrooms of the genus Ganoderma used to treat diabetes and insulin resistance. Molecules 2019, 24, 4075. 10.3390/molecules24224075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q.; Chen L.; Teng H.; Song H.; Wu X.; Xu M. Phenolic compounds ameliorate the glucose uptake in HepG2 cells’ insulin resistance via activating AMPK. J. Funct. Foods 2015, 19, 487–494. 10.1016/j.jff.2015.09.020. [DOI] [Google Scholar]
- Liu M.-M.; Zeng P.; Li X.-T.; Shi L.-G. Antitumor and immunomodulation activities of polysaccharide from Phellinus baumii. Int. J. Biol. Macromol. 2016, 91, 1199–1205. 10.1016/j.ijbiomac.2016.06.086. [DOI] [PubMed] [Google Scholar]
- Hwang B. S.; Lee I.-K.; Choi H. J.; Yun B.-S. Anti-influenza activities of polyphenols from the medicinal mushroom Phellinus baumii. Bioorg. Med. Chem. Lett. 2015, 25, 3256–3260. 10.1016/j.bmcl.2015.05.081. [DOI] [PubMed] [Google Scholar]
- Lee I.-K.; Han M.-S.; Lee M.-S.; Kim Y.-S.; Yun B.-S. Styrylpyrones from the medicinal fungus Phellinus baumii and their antioxidant properties. Bioorg. Med. Chem. Lett. 2010, 20, 5459–5461. 10.1016/j.bmcl.2010.07.093. [DOI] [PubMed] [Google Scholar]
- Luo J.; Liu J.; Ke C.; Qiao D.; Ye H.; Sun Y.; Zeng X. Optimization of medium composition for the production of exopolysaccharides from Phellinus baumii Pilát in submerged culture and the immuno-stimulating activity of exopolysaccharides. Carbohydr. Polym. 2009, 78, 409–415. 10.1016/j.carbpol.2009.04.038. [DOI] [Google Scholar]
- Jang J. S.; Lee J. S.; Lee J. H.; Kwon D. S.; Lee K. E.; Lee S. Y.; Hong E. K. Hispidin produced from Phellinus linteus protects pancreatic β-cells from damage by hydrogen peroxide. Arch Pharm. Res. 2010, 33, 853–861. 10.1007/s12272-010-0607-5. [DOI] [PubMed] [Google Scholar]
- Lee I.-K.; Lee J.-H.; Yun B.-S. Polychlorinated compounds with PPAR-γ agonistic effect from the medicinal fungus Phellinus ribis. Bioorg. Med. Chem. Lett. 2008, 18, 4566–4568. 10.1016/j.bmcl.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Liu H.-K.; Tsai T.-H.; Chang T.-T.; Chou C.-J.; Lin L.-C. Lanostane-triterpenoids from the fungus Phellinus gilvus. Phytochemistry 2009, 70, 558–563. 10.1016/j.phytochem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Zheng S.; Deng S.; Huang Y.; Huang M.; Zhao P.; Ma X.; Wen Y.; Wang Q.; Yang X. Anti-diabetic activity of a polyphenol-rich extract from Phellinus igniarius in KK-Ay mice with spontaneous type 2 diabetes mellitus. Food Funct. 2018, 9, 614–623. 10.1039/c7fo01460k. [DOI] [PubMed] [Google Scholar]
- Bagri P.; Chester K.; Khan W.; Ahmad S. Aspects of extraction and biological evaluation of naturally occurring sugar-mimicking sulfonium-ion and their synthetic analogues as potent α-glucosidase inhibitors from Salacia: a review. RSC Adv. 2017, 7, 28152–28185. 10.1039/c7ra02955a. [DOI] [Google Scholar]
- Zhang J.; Sun L.; Dong Y.; Fang Z.; Nisar T.; Zhao T.; Wang Z.-C.; Guo Y. Chemical compositions and α-glucosidase inhibitory effects of anthocyanidins from blueberry, blackcurrant and blue honeysuckle fruits. Food Chem. 2019, 299, 125102. 10.1016/j.foodchem.2019.125102. [DOI] [PubMed] [Google Scholar]
- Xu L.; Li W.; Chen Z.; Guo Q.; Wang C.; Santhanam R. K.; Chen H. Inhibitory effect of epigallocatechin-3-O-gallate on α-glucosidase and its hypoglycemic effect via targeting PI3K/AKT signaling pathway in L6 skeletal muscle cells. Int. J. Biol. Macromol. 2019, 125, 605–611. 10.1016/j.ijbiomac.2018.12.064. [DOI] [PubMed] [Google Scholar]
- Ha T. J.; Lee J. H.; Lee M.-H.; Lee B. W.; Kwon H. S.; Park C.-H.; Shim K.-B.; Kim H.-T.; Baek I.-Y.; Jang D. S. Isolation and identification of phenolic compounds from the seeds of Perilla frutescens (L.) and their inhibitory activities against α-glucosidase and aldose reductase. Food Chem. 2012, 135, 1397–1403. 10.1016/j.foodchem.2012.05.104. [DOI] [PubMed] [Google Scholar]
- Wang Y.-Y.; Ma H.; Ding Z.-C.; Yang Y.; Wang W.-H.; Zhang H.-N.; Yan J.-K. Three-phase partitioning for the direct extraction and separation of bioactive exopolysaccharides from the cultured broth of Phellinus baumii. Int. J. Biol. Macromol. 2019, 123, 201–209. 10.1016/j.ijbiomac.2018.11.065. [DOI] [PubMed] [Google Scholar]
- Stojkovic D.; Smiljkovic M.; Ciric A.; Glamoclija J.; Van Griensven L.; Ferreira I. C. F. R.; Sokovic M. An insight into antidiabetic properties of six medicinal and edible mushrooms: Inhibition of α-amylase and α-glucosidase linked to type-2 diabetes. South Afr. J. Bot. 2019, 120, 100–103. 10.1016/j.sajb.2018.01.007. [DOI] [Google Scholar]
- Jung J.-Y.; Lee I.-K.; Seok S.-J.; Lee H.-J.; Kim Y.-H.; Yun B.-S. Antioxidant polyphenols from the mycelial culture of the medicinal fungi Inonotus xeranticus and Phellinus linteus. J. Appl. Microbiol. 2008, 104, 1824–1832. 10.1111/j.1365-2672.2008.03737.x. [DOI] [PubMed] [Google Scholar]
- Lee N.-H.; Lee Y.-H.; Bhattari G.; Lee I.-K.; Yun B.-S.; Jeon J.-G.; Hwang P.-H.; Yi H.-K. Reactive oxygen species removal activity of davallialactone reduces lipopolysaccharide-induced pulpal inflammation through inhibition of the extracellular signal-regulated kinase 1/2 and nuclear factor kappa B pathway. J. Endod. 2011, 37, 491–495. 10.1016/j.joen.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Kojima K.; Ogihara Y.; Sakai Y.; Mizukami H.; Nagatsu A. HPLC profiling of Phellinus linteus. J. Nat. Med. 2008, 62, 441–446. 10.1007/s11418-008-0260-1. [DOI] [PubMed] [Google Scholar]
- Shen Q.; Shao J.; Peng Q.; Zhang W.; Ma L.; Chan A. S. C.; Gu L. Hydroxycoumarin derivatives: novel and potent α-glucosidase inhibitors. J. Med. Chem. 2010, 53, 8252–8259. 10.1021/jm100757r. [DOI] [PubMed] [Google Scholar]
- Lee Y. S.; Kang Y.-H.; Jung J.-Y.; Kang I.-J.; Han S.-N.; Chung J.-S.; Shin H.-K.; Lim S. S. Inhibitory constituents of aldose reductase in the fruiting body of Phellinus linteus. Biol. Pharm. Bull. 2008, 31, 765–768. 10.1248/bpb.31.765. [DOI] [PubMed] [Google Scholar]
- Patel D. K.; Kumar R.; Sairam K.; Hemalatha S. Pharmacologically tested aldose reductase inhibitors isolated from plant sources - A concise report. Chin. J. Nat. Med. 2012, 10, 388–400. 10.1016/s1875-5364(12)60078-8. [DOI] [Google Scholar]
- Kim G.-Y.; Roh S.-I.; Park S.-K.; Ahn S.-C.; Oh Y.-H.; Lee J.-D.; Park Y.-M. Alleviation of experimental septic shock in mice by acidic polysaccharide isolated from the medicinal mushroom Phellinus linteus. Biol. Pharm. Bull. 2003, 26, 1418–1423. 10.1248/bpb.26.1418. [DOI] [PubMed] [Google Scholar]
- Yayeh T.; Lee W. M.; Ko D.; Park S.-C.; Cho J. Y.; Park H.-J.; Lee I.-K.; Kim S.-H.; Hong S.-B.; Kim S.; Yun B.-S.; Rhee M. H. Phellinus baumii ethyl acetate extract alleviated collagen type II induced arthritis in DBA/1 mice. J. Nat. Med. 2013, 67, 807–813. 10.1007/s11418-013-0752-5. [DOI] [PubMed] [Google Scholar]
- Sampathkumar K.; Riyajan S.; Tan C. K.; Demokritou P.; Chudapongse N.; Loo S. C. J. Small-intestine-specific delivery of antidiabetic extracts from Withania coagulans using polysaccharide-based enteric-coated nanoparticles. ACS Omega 2019, 4, 12049–12057. 10.1021/acsomega.9b00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh J.-R.; Kim Y.-H.; Hwang J. H.; Gang G.-T.; Kim K.-S.; Lee I.-K.; Yun B.-S.; Lee C.-H. Davallialactone protects against acetaminophen overdose-induced liver injuries in mice. Food Chem. Toxicol. 2013, 58, 14–21. 10.1016/j.fct.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Heidari F.; Rabizadeh S.; Mansournia M. A.; Mirmiranpoor H.; Salehi S. S.; Akhavan S.; Esteghamati A.; Nakhjavani M. Inflammatory, oxidative stress and anti-oxidative markers in patients with endometrial carcinoma and diabetes. Cytokine 2019, 120, 186–190. 10.1016/j.cyto.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Murakami K.; Yokoyama S.-i.; Murata N.; Ozawa Y.; Irie K.; Shirasawa T.; Shimizu T. Insulin receptor mutation results in insulin resistance and hyperinsulinemia but does not exacerbate Alzheimer’s-like phenotypes in mice. Biochem. Biophys. Res. Commun. 2011, 409, 34–39. 10.1016/j.bbrc.2011.04.101. [DOI] [PubMed] [Google Scholar]
- Feinglos M.; Dailey G.; Cefalu W.; Osei K.; Tayek J.; Canovatchel W.; Chaiken R.; Kourides I. Effect on glycemic control of the addition of 2.5 mg glipizide GITS to metformin in patients with T2DM. Diabetes Res. Clin. Pract. 2005, 68, 167–175. 10.1016/j.diabres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Zhou T.; Xu X.; Du M.; Zhao T.; Wang J. A preclinical overview of metformin for the treatment of type 2 diabetes. Biomed. Pharmacother. 2018, 106, 1227–1235. 10.1016/j.biopha.2018.07.085. [DOI] [PubMed] [Google Scholar]
- Da Porto C.; Calligaris S.; Celotti E.; Nicoli M. C. Antiradical properties of commercial cognacs assessed by the DPPH test. J. Agric. Food Chem. 2000, 48, 4241–4245. 10.1021/jf000167b. [DOI] [PubMed] [Google Scholar]
- Cao G.; Sofic E.; Prior R. L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radicals Biol. Med. 1997, 22, 749–760. 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- Smirnoff N.; Cumbes Q. J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. 10.1016/0031-9422(89)80182-7. [DOI] [Google Scholar]
- Shon M.; Kim T. H.; Sung N. J. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem. 2003, 82, 593–597. 10.1016/s0308-8146(03)00015-3. [DOI] [Google Scholar]
- Lee D.-S.; Woo J.-Y.; Ahn C.-B.; Je J.-Y. Chitosan-hydroxycinnamic acid conjugates: Preparation, antioxidant and antimicrobial activity. Food Chem. 2014, 148, 97–104. 10.1016/j.foodchem.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Karan S. K.; Mishra S. K.; Pal D.; Mondal A. Isolation of β-sitosterol and evaluation of antidiabetic activity of Aristolochia indica in alloxan-induced diabetic mice with a reference to in-vitro antioxidant activity. J. Med. Plants Res. 2012, 6, 1219–1223. 10.5897/jmpr11.973. [DOI] [Google Scholar]
- Li X.; Hu Q.; Jiang S.; Li F.; Lin J.; Han L.; Hong Y.; Lu W.; Gao Y.; Chen D. Flos Chrysanthemi indici protects against hydroxyl-induced damages to DNA and MSCs via antioxidant mechanism: a chemistry study. J. Saudi Chem. Soc. 2015, 19, 454–460. 10.1016/j.jscs.2014.06.004. [DOI] [Google Scholar]
- Pinto M. d. S.; Kwon Y. I.; Apostolidis E.; Lajolo F. M.; Genovese M. I.; Shetty K. Functionality of bioactive compounds in brazilian strawberry (Fragaria × ananassa Duch.) cultivars: evaluation of hyperglycemia and hypertension potential using in vitro models. J. Agric. Food Chem. 2008, 56, 4386–4392. 10.1021/jf0732758. [DOI] [PubMed] [Google Scholar]
- Wang C.; Liu X.; Lian C.; Ke J.; Liu J. Triterpenes and aromatic meroterpenoids with antioxidant activity and neuroprotective effects from Ganoderma lucidum. Molecules 2019, 24, 4353. 10.3390/molecules24234353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Wang Y.; Lei J.-C.; Hao Y.; Yang Y.; Yang C.-X.; Yu J.-Q. Sensitisation of ovarian cancer cells to cisplatin by flavonoids from Scutellaria Barbata. Nat. Prod. Res. 2014, 28, 683–689. 10.1080/14786419.2013.871547. [DOI] [PubMed] [Google Scholar]
- Huang Q.; Chen L.; Teng H.; Song H.; Wu X.; Xu M. Phenolic compounds ameliorate the glucose uptake in HepG2 cells’ insulin resistance via activating AMPK. J. Funct. Foods 2015, 19, 487–494. 10.1016/j.jff.2015.09.020. [DOI] [Google Scholar]
- Ozgen M.; Reese R. N.; Tulio A. Z.; Scheerens J. C.; Miller A. R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl(DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. 10.1021/jf051960d. [DOI] [PubMed] [Google Scholar]