Abstract
Objective
Physical rehabilitation programs hold the potential to mitigate deterioration in health‐related quality of life (HRQoL) in patients with head and neck cancer. The objective was to assess development in relevant domains of HRQoL following a physical exercise and nutrition intervention administrated during or after treatment.
Methods
In a pilot study, 41 patients were randomized to resistance training and oral nutritional supplements during (EN‐DUR, n = 20) or after (EN‐AF, n = 21) radiotherapy. Global health status/QoL (GHS) and physical functioning (PF) were measured by the European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire at baseline, week 6, and week 14. Differences between the groups were assessed by analysis of covariance. A difference of ≥10 points in GHS and PF was interpreted as clinically relevant.
Results
No statistically significant differences were detected between the groups; however, clinically relevant changes and differences in GHS and PF were observed. From baseline to week 6, GHS decreased 9 points in the EN‐DUR group and 23 points in the EN‐AF group and PF decreased 13 points and 21 points, respectively. From week 6 to week 14, GHS increased 14 points in the EN‐DUR group and 26 points EN‐AF group and PF did not change (0 points) in the EN‐DUR group and increased 16 points in the EN‐AF group.
Conclusion
The findings from the present pilot study are promising and indicate that a physical rehabilitation program may have a positive impact on HRQoL during treatment and enhance recovery after treatment. A definitive randomized trial is warranted.
Level of Evidence
1b—Individual randomized controlled trial.
Keywords: head and neck cancer, health‐related quality of life, nutritional support, physical rehabilitation, resistance training
1. INTRODUCTION
Patients with head and neck cancer (HNC) are faced with specific challenges and needs due to the complex treatment involving changes to critical structures for speaking, eating, and breathing in addition to facial and neck disfigurement.1, 2 This may have a significant impact on function and body image that negatively affects health‐related quality of life (HRQoL) and survivorship.3, 4
Numerous observational studies have reported HRQoL throughout treatment and recovery in patients with HNC, demonstrating that symptoms such as pain, dry mouth, and sticky saliva increase steadily during the course of radiotherapy (+/− chemotherapy) while physical functioning (PF) and global health status/QoL decrease.5, 6, 7, 8, 9, 10, 11, 12, 13 The patients report maximum symptom burden and minimum functioning at the end of and immediately after radiotherapy.4, 7, 11 The post‐treatment period is normally characterized by gradual recovery and improvement; however, only global health status/QoL seems to reach pretreatment levels within 1 year after treatment completion.11, 13, 14 Thus, the following year(s) of HNC survivorship is characterized by persistent treatment‐related side effects accompanied by deteriorated functional status.15
Rehabilitation programs that include physical exercise and/or nutrition interventions hold the potential to mitigate some of the side effects and counteract the reduced functioning experienced by patients with HNC.16, 17 Although generally small in sample sizes and hampered by study design not tailored to study, several physical exercise intervention studies have indicated a beneficial impact of resistance training on PF, fatigue, and global health status/QoL during and immediately after tumor directed treatment.18, 19, 20, 21 The results from nutrition intervention studies are somewhat mixed, but two randomized controlled trials (RCTs) have demonstrated less deterioration in PF and global health status/QoL in patients receiving dietary counseling and/or oral nutritional supplements (ONS) during and after treatment.22, 23 However, to the best of our knowledge, no study has reported short‐ and long‐term HRQoL following an intervention combining physical exercise and nutritional support in patients with HNC.24
On this background, we conducted a randomized pilot study in 2015 to 2016 to evaluate the feasibility and compare the impact of a new rehabilitation program during radiotherapy (EN‐DUR) consisting of resistance training and ONS to a program after radiotherapy as part of an existing cancer rehabilitation program in the specialist health care in Norway. Previously we have reported data on feasibility and short‐term effects on lean‐body mass and body weight.25 Eighteen of 20 patients completed the EN‐DUR and 11 of 21 the EN‐AF intervention. The EN‐DUR intervention demonstrated high exercise‐adherence (81%) and moderate ONS‐adherence (57%), and a beneficial impact on lean body mass was indicated. The exercise and ONS adherence rates for the patients attending the EN‐AF intervention were even higher (94% and 76%, respectively). This raises several questions related to patient needs as well as timing and setting of rehabilitation services. Subgroup analyses of attenders and nonattenders may therefore provide valuable information regarding possible factors associated with needs and utilization of rehabilitation services in patients with HNC.
The objective of the present study was to assess short‐ and long‐term differences in HRQoL between the physical rehabilitation program administrated during vs after tumor directed treatment and describe within‐group changes in HRQoL during the first year after diagnosis of HNC. Due to the low attendance‐rate to the program after treatment, differences in HRQoL and sociodemographic and clinical characteristics between the attenders and nonattenders were explored.
2. MATERIALS AND METHODS
2.1. Patients and study design
Patients were recruited in the period between March 2015 and March 2016 from the Clinic of Ear‐Nose‐Throat, Eye and Maxillofacial Surgery (ENT‐clinic) at St. Olavs Hospital, Trondheim University Hospital in Norway. The patients were eligible if the following inclusion criteria were met: (a) a diagnosis of squamous cell carcinoma originated in the head and neck (naso, oro, or hypo pharynx, larynx and oral cavity, except from stage T1N0M0 laryngeal cancer), (b) referred for curative radiotherapy with or without chemotherapy, (c) 18 to 85 years of age, and (d) able to complete baseline assessments prior to start of radiotherapy.
The study was designed as a randomized pilot study and the patients were allocated to an exercise and nutrition intervention during radiotherapy (EN‐DUR) or after radiotherapy (EN‐AF). The EN‐DUR intervention was conducted from start to end of radiotherapy (6 weeks) at an outpatient training facility within the hospital area and consisted of 12 resistance training sessions (maximum 30 minutes per session). In addition, all patients received a booklet with nutritional advice specifically designed for patients with HNC and were provided with minimum one unit (200 mL) of ONS on weekdays (E+ by Tine SA, Norway, 350 kcal and 15 g protein per unit). On training days, the patients were asked to take one extra unit after the session. The EN‐AF intervention started 2 to 4 weeks after the end of radiotherapy and was conducted at a rehabilitation clinic as part of an established 3‐week cancer program. The program consisted of nine resistance training sessions (maximum 40 minutes per session), daily intake of ONS similar to the EN‐DUR intervention and dietary counseling once a week provided by a dietitian. A detailed description of the interventions has been published previously.25
2.2. Background variables
Sociodemographic data (age, sex, marital status, living situation, education, employment, and smoking status), nutritional status, and self‐reported physical activity were obtained by a questionnaire prior to start of radiotherapy (baseline), and clinical data (diagnosis date, type and stage, recurrence, type of treatment, and comorbidities) were obtained from the patients' medical journals. Karnofsky performance status (KPS) was scored by the involved physiotherapist (J.A.S.).26
Nutritional status was measured by the short form of the Patient‐Generated Subjective Global Assessment (PG‐SGA), and a total score was summarized ranging from 0 (no problem) to 36 (severe problems) based on the recommended use of the instrument.27, 28, 29, 30, 31 Self‐reported physical activity level was measured by the Nord‐Trøndelag Health Study Physical Activity Questionnaire (HUNT PA‐Q) with a total score calculated based on the product of frequency, duration, and intensity, ranging from 0 (no physical activity) to 15 (vigorous physical activity for more than 1 hour almost every day).32, 33, 34 Functional exercise capacity was measured by the field exercise test Modified Shuttle Walk Test (MSWT), and functional muscle strength was measured by the 30 seconds sit to stand test.35, 36, 37
2.3. Outcome variables
The patients completed HRQoL questionnaires at baseline, at the end of radiotherapy (week 6), at 2 months follow‐up (week 14), and 1 year later (1 year). HRQoL was measured by the European Organization for Research and Treatment of Cancer quality of life questionnaire (EORTC QLQ‐C30, version 3.0) and the HNC module EORTC QLQ‐H&N35.38, 39 The EORTC QLQ‐C30 consists of five functional scales, three symptom scales, a global health status/QoL scale, and six single items. Global health status and PF was considered relevant C30 scales in the present study. The EORTC QLQ‐H&N35 consists of seven multi‐item symptom scales that assess pain, swallowing, senses (taste and smell), speech, social eating, social contact and sexuality, and six single‐item symptom scales assessing side effects related to problems with teeth, opening mouth, dry mouth, sticky saliva and coughing, and the feeling of being ill. In addition, the questionnaire consists of five optional single‐item scales (ie, questions 31‐35) assessing the use of pain killers, nutritional supplements, and feeding tube and weight loss and gain. Pain, dry mouth and sticky saliva were considered relevant H&N35 scales. Scoring for the C30 and H&N35 questionnaires was conducted according to the EORTC QLQ‐C30 scoring manual recommendations and range from 0 to 100.40 A high score for global health status/QoL and PF represents high HRQoL/high functioning and a high score for a symptom scale represents a high level of symptoms/problems.
2.4. Statistical analyses
Descriptive statistics with confidence intervals (95% CI) was the focus of the analyses due to the pilot design of the study. Distributions of the included variables were checked for normality by inspection of histograms, Q‐Q‐plots and tests of normality, and presented as mean with SD if approximately normally distributed or median with interquartile range (IQR) if skewed. Differences between the groups were assessed by analysis of covariance (ANCOVA) at week 6, week 14, and 1 year with the respective baseline‐scores as covariate. Within‐group changes were assessed by paired sample t tests. A difference in HRQoL scores of 10 points or more was considered clinically relevant.41 All statistical analyses were performed using the IBM SPSS Statistics 22.0 software (IBM Corporation, Armonk, New York). P values < .05 were considered statistically significant.
2.5. Ethics
The study was approved by the Regional Committees for Medical and Health Research Ethics (REK midt 2013/2098), and the study was registered at http://clinicaltrials.gov prior to study start (Identifier: NCT02439892). All patients provided written informed consent before entering the study.
3. RESULTS
The study sample consisted of 41 patients (25 male) with an average age of 63.2 years (SD = 9.3 years). Median time from diagnosis to baseline assessment was 14 days (IQR = 11 days) and 80% had a KPS score of ≥90 at baseline. Twenty patients were randomized to the EN‐DUR intervention and 21 patients to the EN‐AF intervention. Characteristics of the randomized groups and attendance, attrition, and adherence rates have been presented previously.25 At baseline, the mean global health status/QoL and PF scores were 61 and 83 points in the EN‐DUR group compared to 67 and 91 points in the EN‐AF group, and the mean symptom scores of pain, dry mouth, and sticky saliva were 29, 23, and 38 points in the EN‐DUR group compared to 23, 19, and 21 points in the EN‐AF group. The number of complete EORTC QLQ‐C30 and H&N35 forms in the EN‐DUR and EN‐AF groups is presented in Table 1. From baseline to 1‐year follow‐up, respectively, four patients died in the EN‐DUR group and two in the EN‐AF‐group.
Table 1.
The number of completed EORTC QLQ‐C30 and H&N35 forms at each assessment point
| Baseline | Week 6 | Week 14 | 1 year | |
|---|---|---|---|---|
| EN‐DUR group (n = 20) | 20 | 19 | 18 | 15 |
| EN‐AF group (n = 21) | 21 | 19 | 18 | 18 |
3.1. Changes in HRQoL
The ANCOVA‐analysis did not demonstrate any statistically significant differences between the EN‐DUR and EN‐AF groups in global health status/QoL, PF, or symptoms of pain, dry mouth and sticky saliva. Figure 1 presents the mean scores in global health status/QoL and PF in the two groups at baseline, week 6, week 14, and 1 year, based on the number of complete questionnaires as presented in Table 1.
Figure 1.
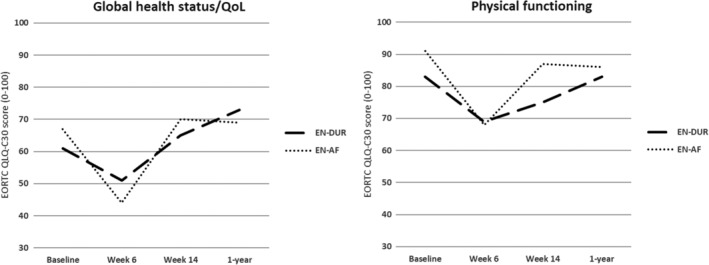
Mean scores in global health status/QoL and physical functioning in the EN‐DUR and EN‐AF groups at baseline, week 6, week 14, and 1 year. QoL, quality of life
However, clinically relevant changes in HRQoL from start to end of the study were observed within the EN‐DUR and EN‐AF groups as well as clinically relevant differences in change between the groups. From baseline to week 6, global health status/QoL decreased 9 points (95% CI: −20.6, −3.1) in the EN‐DUR group compared to 23 points (−34.0, −12.5) in the EN‐AF group, and PF decreased 13 points (−22.3, −3.0) compared to 21 points (−33.7, −9.0). From week 6 to week 14, global health status/QoL increased 14 points (0.9, 27.6) in the EN‐DUR group compared to 26 points (7.4, 43.6) in the EN‐AF group, while PF did not change in the EN‐DUR group (0 points, −6.8, 7.4) compared to an increase of 16 points (4.8, 26.6) in the EN‐AF group. Symptoms of pain, dry mouth, and sticky saliva increased in both groups from baseline to week 6 and decreased in both groups from week 6 to week 14 (see Table 2 for the respective scores).
Table 2.
Mean symptom scores at baseline and week 6 and at week 6 and week 14 for the EN‐DUR and EN‐AF groups, respectively
| EN‐DUR | EN‐AF | EN‐DUR | EN‐AF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 6 | Diff. (95% CI) | Baseline | Week 6 | Diff. (95% CI) | Week 6 | Week 14 | Diff. (95% CI) | Week 6 | Week 14 | Diff. (95% CI) | |
| Pain | 30 | 53 | 23 (11.6, 34.9) | 24 | 52 | 28 (17.1, 39.7) | 52 | 34 | −18 (−27.8, −7.5) | 53 | 22 | −31 (−40.6, −21.9) |
| Dry mouth | 24 | 77 | 53 (33.1, 72.2) | 19 | 77 | 58 (42.9, 72.9) | 76 | 74 | −2 (−13.2, 9.3) | 79 | 61 | −18 (−33.8, −1.5) |
| Sticky saliva | 39 | 84 | 45 (23.4, 67.8) | 21 | 84 | 63 (46.3, 80.0 | 82 | 61 | −21 (−41.6, −1.5) | 86 | 55 | −31 (−44.2, −18.6) |
Abbreviation: Diff., difference of mean scores.
From baseline to 1‐year follow‐up, global health status/QoL increased 11 points (0.8, 21.4) in the EN‐DUR group compared to reaching baseline level in the EN‐AF group (0 points, −11.9, 11.9) and PF reached baseline values in the EN‐DUR group (0 points, −11.9, 11.9) compared to a decrease of 7 points (−13.9, −0.7) in the EN‐AF group. Symptoms of pain decreased 13 points (−27.7, 1.0) in the EN‐DUR group compared to an increase of 1 point (−12.7, 14.6) in the EN‐AF group. Dry mouth and sticky saliva increased 36 points (22.6, 48.5) and 9 points (−16.7, 34.5) in the EN‐DUR group compared to 46 points (26.5, 66.1) and 20 points (−1.0, 41.7) in the EN‐AF group.
3.2. Attenders vs nonattenders to the EN‐AF intervention
Only 11 of the 21 patients (52%) randomized to the EN‐AF intervention attended the program. Table 3 presents the background characteristics of the attenders and nonattenders to the EN‐AF intervention before the start of treatment (baseline). The median age of the attenders to the EN‐AF intervention was 61 years (IQR = 11 years) compared to 67.5 years (IQR = 15 years) among the nonattenders, and body mass index of the attenders was 25.7 kg/m2 (SD = 5.0) compared to 27.8 kg/m2 (SD = 9.0) among the nonattenders. More of the attenders were diagnosed with pharyngeal cancer compared to the nonattenders (n = 9 vs n = 1), and more of the attenders were scheduled for chemotherapy in addition to radiotherapy (n = 8 vs n = 2).
Table 3.
Baseline characteristics of the attenders and nonattenders to the EN‐AF intervention
| Attenders | Nonattenders | ||
|---|---|---|---|
| n = 11 | n = 10 | ||
| Age; years (median, IQR) | 61 (11) | 67.5 (15) | |
| Sex | |||
| Women | 5 | 6 | |
| BMI, kg/m 2 (mean, SD) | 25.7 (5.0) | 27.8 (9.0) | |
| Marital status | |||
| Married/cohabitant | 8 | 7 | |
| Single/widow | 3 | 3 | |
| Education | |||
| Primary or secondary school | 9 | 7 | |
| College/university | 2 | 3 | |
| Employment | |||
| Employed | 6 | 3 | |
| Retired | 3 | 7 | |
| Disability benefits | 2 | 0 | |
| Smoking status | |||
| Current smoker | 0 | 1 | |
| Past smoker | 7 | 6 | |
| Never smoked | 4 | 3 | |
| Karnofsky performance status | |||
| Score ≥ 90 | 9 | 9 | |
| Tumor site | |||
| Pharynx | 9 | 1 | |
| Larynx | 0 | 2 | |
| Oral cavity | 2 | 2 | |
| Salivary gland/nasal cavity | 0 | 5 | |
| Planned treatment | |||
| Concurrent chemoradiotherapy | 8 | 2 | |
| Radiotherapy | 3 | 8 | |
| Mean (SD) | Mean (SD) | Diff. a (95% CI) | |
| HRQoL | |||
| Global health status/QoL | 62.1 (22.8) | 72.5 (21.9) | 10.4 (−30.8, 10.1) |
| Physical functioning | 89.1 (20.7) | 92.5 (16.8) | 3.4 (−20.7, 13.9) |
| Pain | 29.5 (25.4) | 15.8 (24.7) | 13.7 (−9.2, 36.6) |
| Dry mouth | 18.2 (17.4) | 20.0 (23.3) | −1.8 (−20.5, 16.9) |
| Sticky saliva | 21.2 (34.2) | 20.0 (23.3) | 1.2 (−25.8, 28.2) |
| Nutritional status | 2.4 (3.4) | 3.5 (5.6) | 1.1 (−5.3, 3.1) |
| Physical activity level | 2.7 (2.7) | 1.8 (1.7) | 0.9 (−1.2, 3.0) |
| Functional capacity (m) | 683 (228) | 504 (154) | 179 (−5, 362) |
| Muscle strength (reps) | 15 (4) | 14 (3) | 1 (−2, 4) |
Abbreviations: BMI, body mass index; Diff., difference in mean scores; HRQoL, health‐related quality of life; IQR, interquartile range; reps, repetitions.
Difference in mean scores.
The attenders reported clinically relevant lower global health status/QoL compared to the nonattenders (62 vs 73 points) and more symptoms of pain (30 vs 16 points). Furthermore, the attenders reported a clinically relevant higher level of physical activity (2.7 vs 1.8 points) and had a higher functional capacity compared to the nonattenders (683 vs 504 m). No clinically relevant differences in PF, dry mouth, sticky saliva, nutritional status, or muscle strength were detected between the attenders and nonattenders.
4. DISCUSSION
The overall aim of the present pilot study was to assess differences and describe within‐group changes in HRQoL following two physical rehabilitation programs administered during or after radiotherapy (+/− chemotherapy). Due to the pilot design of the study (n = 41) with the inherent lack of power to detect statistically significant differences between groups, the main focus was to perform descriptive analyses to explore changes in HRQoL over time. Clinically relevant changes in global health status/QoL and PF were observed in both groups, with differences in change in favor of the active interventions both during and after treatment. Only half of the patients attended the intervention after treatment. Analysis of the attenders and nonattenders indicates that the two subgroups represent different populations. The attenders to the program administered after treatment reported clinically relevant lower global health status/QoL and more pain compared with the nonattenders. In addition, the attenders were younger, and more were diagnosed with pharyngeal cancer and scheduled for concurrent chemoradiotherapy than the nonattenders.
To our knowledge, this is the first intervention study that explores the development in HRQoL following a rehabilitation program administered during or after radiotherapy (+/− chemotherapy) consisting of a combination of resistance training and ONS in patients with HNC. Rogers et al conducted a randomized pilot study (n = 15) to assess the feasibility and preliminary effects of a 12‐week resistance training program initiated at start of radiotherapy in patients with HNC, and reported similar findings as in the present study with less decline in overall well‐being (FACT‐General) and HNC specific well‐being (FACT H&N) compared to standard care from baseline to week 6.19 However contrary to our findings, the intervention group reported less increase in overall and HNC specific well‐being compared to the control group from week 6 to week 12. Capozzi et al published an exploratory RCT (n = 60) in 2016 that evaluated the timing and effects of a 12‐week lifestyle intervention with resistance training and health education initiated during vs after radiotherapy.42 HRQoL was included as a secondary outcome, with a total symptom score and overall HRQoL measured before and after the interventions. In line with our results, no statistically significant differences were detected between the interventions in total symptom score or overall HRQoL, probably due to lack of power. However, contrary to our findings, no clinically relevant differences in mean scores were observed between the groups from start to end of the intervention initiated at start of treatment.42 Another single‐armed (n = 12) exercise trial by Lønkvist et al showed deterioration in most functional scales in EORTC QLQC‐30 during treatment, but only minor deterioration was reported in PF.43
The findings in the present study confirm earlier findings from numerous observational studies that global health status/QoL and PF decrease during radiotherapy (+/− chemotherapy) while symptoms of pain, dry mouth, and sticky saliva increase steadily, and that the post‐treatment period normally is characterized by gradual recovery and improvement in HRQoL and less reported symptoms.5, 6, 7, 8, 9, 10, 11, 12, 13 However, an interesting and novel finding in our study is that the patients receiving the active intervention during radiotherapy (EN‐DUR) experience clinically relevant less decline in global health status/QoL and PF from start to end of treatment compared with the patients receiving standard care. A similar trend was observed in the active intervention after treatment (EN‐AF), with clinically relevant larger improvements in global health status/QoL and PF from week 6 to week 14 compared to the “controls” (EN‐DUR). The results indicate that an intervention with resistance training and ONS either during or after radiotherapy may have positive impact on self‐reported health status/QoL and PF, and justifies the implementation of a full scale RCT to investigate whether exercise and nutrition interventions lead to statistically significant improvements. To optimize adherence and retention, we suggest starting the intervention already at the time of diagnosis and extending throughout the treatment period and into the acute post‐treatment phase compared to usual care. In addition, utilizing facilities close to where the patients live seems a sensible strategy to optimize retention especially in the post‐treatment phase. A next‐step study would profit from patient codesign on these issues.
The future RCT should probably include a combination of physical exercise and nutritional support initiated before start of tumor directed treatment with continuation during treatment and into the post‐treatment recovery phase compared to a usual care group. To our knowledge, the largest RCT that has investigated the effectiveness of a physical exercise program in patients with HNC undergoing chemoradiotherapy was recently published by Samuel et al, and conclude that physical exercise during treatment has the potential to enhance HRQoL.44 They studied the effect of an 11‐week program with aerobic and resistance training exercises on quality of life (using the generic Short Form‐36), functional capacity, and worsening of fatigue. Compared with the control group, there was a statistically significant difference in favor of the exercise group on all outcomes from start to end of the intervention. Both the mental and the physical quality of life score was maintained from baseline to immediately after treatment in the exercise group compared to a significant reduction in the control group.44 These are exciting results from a full‐scale trial supporting the findings in this article, although not directly comparable due to differences especially in content of interventions and measurement instruments.
To gain more insight into possible factors associated with the utilization and needs of rehabilitation within this population, background characteristics were compared between the attenders and nonattenders to the program administrated after treatment. A clear difference at baseline was that most of the attenders (8 of 11 patients) were scheduled for concurrent chemoradiotherapy compared with only two of the 10 nonattenders. Receiving chemotherapy concurrently with radiotherapy is associated with increased symptoms of mucositis, nausea, vomiting, and fatigue compared with only radiotherapy.45 Thus, an increase in symptoms could be expected among the attenders at the end of treatment. The younger age among the attenders may explain the higher level of self‐reported physical activity and the superior functional capacity compared to the nonattenders before treatment start. However, younger cancer patients often express an increased need for rehabilitation compared to older patients, and the utilization of cancer rehabilitation services has been found to be significantly higher in younger age groups.46, 47, 48 One of the reasons for this is suggested to be related to a greater self‐perceived loss of functioning among younger patients. The latter and the aspect of still being within working age may have influenced the choice to attend the post‐treatment program in the present study, possibly to regain physical and work‐related functioning. In addition, the setting of the program (ie, a rehabilitation clinic located 150 km from the hospital) may have affected the decision to decline participation, and the need for more local services needs to be addressed. As a further preparation for the definitive RCT, we will map out any existing outpatient and community‐based rehabilitation services within the current geographical area and, based on the findings, consider the need to design and implement new locally based interventions.
4.1. Strengths and limitations
The present data were obtained from a relatively small single‐center pilot study (n = 41) with no predefined clinically relevant difference or power and sample size calculations. Statistically significant differences between the interventions (EN‐DUR and EN‐AF) were neither the aim nor expected in this feasibility study; thus, we wanted to explore and compare absolute mean scores even in the absence of formal statistical significance. The two interventions administrated during and after tumor directed treatment respectively were not directly comparable due to differences in content, duration, and setting, and the reason for designing two different interventions was based on the current standards of post‐treatment rehabilitation programs in Norway and the need for testing new interventions during treatment. The utilized data in the analysis of the attenders and nonattenders were obtained from only one of the two intervention arms in the pilot trial (ie, the post‐treatment intervention), which implies a small number of patients (n = 21) split into smaller subgroups of attenders (n = 11) and nonattenders (n = 10).
5. CONCLUSION
No statistically significant differences in HRQoL between the physical rehabilitation programs were demonstrated, but interesting findings of importance for designing a full‐scale RCT were observed. The findings indicate that a physical rehabilitation program may have a positive impact on relevant HRQoL domains during treatment and enhance recovery after treatment in patients with HNC. In addition, the present findings also indicate an increased need for post‐treatment rehabilitation among patients receiving concurrent chemoradiotherapy.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception or design of the work: S.K., J.‐Å.L., and L.O.; Data collection: J.A.S., T.R.B., and A.B.; Data analysis and interpretation: J.A.S., E.S., and L.O.; Drafting the article: J.A.S., A.B., T.S.S., and L.O.; Critical revision of the article: A.B., T.S.S., T.R.B., L.T., E.S., S.K., J.‐Å.L., and L.O.; Final approval of the version to be published: J.A.S., A.B., T.S.S., T.R.B., L.T., E.S., S.K., J.‐Å.L., and L.O.
Sandmæl JA, Bye A, Solheim TS, et al. Physical rehabilitation in patients with head and neck cancer: Impact on health‐related quality of life and suitability of a post‐treatment program. Laryngoscope Investigative Otolaryngology. 2020;5:330–338. 10.1002/lio2.368
Funding information Norwegian Extra Foundation for Health and Rehabilitation
REFERENCES
- 1. Pfister DG, Ang KK, Brizel DM, et al. Head and neck cancers, version 2.2013. Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11(8):917‐923. [DOI] [PubMed] [Google Scholar]
- 2. Devins GM, Payne AY, Lebel S, et al. The burden of stress in head and neck cancer. Psychooncology. 2013;22(3):668‐676. [DOI] [PubMed] [Google Scholar]
- 3. Vickery LE, Latchford G, Hewison J, Bellew M, Feber T. The impact of head and neck cancer and facial disfigurement on the quality of life of patients and their partners. Head Neck. 2003;25(4):289‐296. [DOI] [PubMed] [Google Scholar]
- 4. Sandstrom SK, Mazanec SR, Gittleman H, Barnholtz‐Sloan JS, Tamburro N, Daly BJ. A descriptive, longitudinal study of quality of life and perceived health needs in patients with head and neck cancer. J Adv Pract Oncol. 2016;7(6):640‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deleyiannis FW, Weymuller EA Jr, Coltrera MD. Quality of life of disease‐free survivors of advanced (stage III or IV) oropharyngeal cancer. Head Neck. 1997;19(6):466‐473. [DOI] [PubMed] [Google Scholar]
- 6. de Graeff A, de Leeuw RJ, Ros WJ, et al. A prospective study on quality of life of laryngeal cancer patients treated with radiotherapy. Head Neck. 1999;21(4):291‐296. [DOI] [PubMed] [Google Scholar]
- 7. List MA, Siston A, Haraf D, et al. Quality of life and performance in advanced head and neck cancer patients on concomitant chemoradiotherapy: a prospective examination. J Clin Oncol. 1999;17(3):1020‐1028. [DOI] [PubMed] [Google Scholar]
- 8. de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Long‐term quality of life of patients with head and neck cancer. Laryngoscope. 2000;110(1):98‐106. [DOI] [PubMed] [Google Scholar]
- 9. Weymuller EA, Yueh B, Deleyiannis FW, Kuntz AL, Alsarraf R, Coltrera MD. Quality of life in patients with head and neck cancer: lessons learned from 549 prospectively evaluated patients. Arch Otolaryngol Head Neck Surg. 2000;126(3):329‐335. [DOI] [PubMed] [Google Scholar]
- 10. Epstein JB, Robertson M, Emerton S, Phillips N, Stevenson‐Moore P. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck. 2001;23(5):389‐398. [DOI] [PubMed] [Google Scholar]
- 11. Bjordal K, Ahlner‐Elmqvist M, Hammerlid E, et al. A prospective study of quality of life in head and neck cancer patients. Part II: longitudinal data. Laryngoscope. 2001;111(8):1440‐1452. [DOI] [PubMed] [Google Scholar]
- 12. Vergeer MR, Doornaert PA, Rietveld DH, Leemans CR, Slotman BJ, Langendijk JA. Intensity‐modulated radiotherapy reduces radiation‐induced morbidity and improves health‐related quality of life: results of a nonrandomized prospective study using a standardized follow‐up program. Int J Radiat Oncol Biol Phys. 2009;74(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 13. Oskam IM, Verdonck‐de Leeuw IM, Aaronson NK, et al. Prospective evaluation of health‐related quality of life in long‐term oral and oropharyngeal cancer survivors and the perceived need for supportive care. Oral Oncol. 2013;49(5):443‐448. [DOI] [PubMed] [Google Scholar]
- 14. Klein J, Livergant J, Ringash J. Health related quality of life in head and neck cancer treated with radiation therapy with or without chemotherapy: a systematic review. Oral Oncol. 2014;50(4):254‐262. [DOI] [PubMed] [Google Scholar]
- 15. Verma N, Tan X, Knowles M, Bernard S, Chera B. Patient‐reported outcomes for dental health, shoulder‐neck dysfunction, and overall quality of life after treatment with radiation for head and neck cancer. Laryngoscope Investig Otolaryngol. 2019;4(3):300‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Capozzi LC, Nishimura KC, McNeely ML, Lau H, Culos‐Reed SN. The impact of physical activity on health‐related fitness and quality of life for patients with head and neck cancer: a systematic review. Br J Sports Med. 2016;50(6):325‐338. [DOI] [PubMed] [Google Scholar]
- 17. Langius JA, Zandbergen MC, Eerenstein SE, et al. Effect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo)radiotherapy: a systematic review. Clin Nutr. 2013;32(5):671‐678. [DOI] [PubMed] [Google Scholar]
- 18. Eades M, Murphy J, Carney S, et al. Effect of an interdisciplinary rehabilitation program on quality of life in patients with head and neck cancer: review of clinical experience. Head Neck. 2013;35(3):343‐349. [DOI] [PubMed] [Google Scholar]
- 19. Rogers LQ, Anton PM, Fogleman A, et al. Pilot, randomized trial of resistance exercise during radiation therapy for head and neck cancer. Head Neck. 2013;35(8):1178‐1188. [DOI] [PubMed] [Google Scholar]
- 20. Samuel SR, Maiya GA, Babu AS, Vidyasagar MS. Effect of exercise training on functional capacity & quality of life in head & neck cancer patients receiving chemoradiotherapy. Indian J Med Res. 2013;137(3):515‐520. [PMC free article] [PubMed] [Google Scholar]
- 21. Lonbro S, Dalgas U, Primdahl H, et al. Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy—results from the randomized DAHANCA 25B trial. Radiother Oncol. 2013;108(2):314‐319. [DOI] [PubMed] [Google Scholar]
- 22. Isenring EA, Capra S, Bauer JD. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br J Cancer. 2004;91(3):447‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ravasco P, Monteiro‐Grillo I, Marques Vidal P, Camilo ME. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck. 2005;27(8):659‐668. [DOI] [PubMed] [Google Scholar]
- 24. Pekmezi DW, Demark‐Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011;50(2):167‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sandmael JA, Bye A, Solheim TS, et al. Feasibility and preliminary effects of resistance training and nutritional supplements during versus after radiotherapy in patients with head and neck cancer: a pilot randomized trial. Cancer. 2017;123(22):4440‐4448. [DOI] [PubMed] [Google Scholar]
- 26. Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer. 1980;45(8):2220‐2224. [DOI] [PubMed] [Google Scholar]
- 27. Detsky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11(1):8‐13. [DOI] [PubMed] [Google Scholar]
- 28. Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition. 1996;12(1 suppl):S15‐S19. [DOI] [PubMed] [Google Scholar]
- 29. Gabrielson DK, Scaffidi D, Leung E, et al. Use of an abridged scored Patient‐Generated Subjective Global Assessment (abPG‐SGA) as a nutritional screening tool for cancer patients in an outpatient setting. Nutr Cancer. 2013;65(2):234‐239. [DOI] [PubMed] [Google Scholar]
- 30. Bauer J, Capra S, Ferguson M. Use of the scored Patient‐Generated Subjective Global Assessment (PG‐SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr. 2002;56(8):779‐785. [DOI] [PubMed] [Google Scholar]
- 31. Vigano AL, di Tomasso J, Kilgour RD, et al. The abridged patient‐generated subjective global assessment is a useful tool for early detection and characterization of cancer cachexia. J Acad Nutr Diet. 2014;114(7):1088‐1098. [DOI] [PubMed] [Google Scholar]
- 32. Bertheussen GF, Oldervoll L, Kaasa S, Sandmael JA, Helbostad JL. Measurement of physical activity in cancer survivors—a comparison of the HUNT 1 Physical Activity Questionnaire (HUNT 1 PA‐Q) with the International Physical Activity Questionnaire (IPAQ) and aerobic capacity. Support Care Cancer. 2013;21(2):449‐458. [DOI] [PubMed] [Google Scholar]
- 33. Bertheussen GF, Kaasa S, Hokstad A, et al. Feasibility and changes in symptoms and functioning following inpatient cancer rehabilitation. Acta Oncol. 2012;51(8):1070‐1080. [DOI] [PubMed] [Google Scholar]
- 34. Kurtze N, Rangul V, Hustvedt BE, Flanders WD. Reliability and validity of self‐reported physical activity in the Nord‐Trondelag Health Study: HUNT 1. Scand J Public Health. 2008;36(1):52‐61. [DOI] [PubMed] [Google Scholar]
- 35. Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax. 1992;47(12):1019‐1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bradley J, Howard J, Wallace E, Elborn S. Reliability, repeatability, and sensitivity of the modified shuttle test in adult cystic fibrosis. Chest. 2000;117(6):1666‐1671. [DOI] [PubMed] [Google Scholar]
- 37. Jones CJ, Rikli RE, Beam WC. A 30‐s chair‐stand test as a measure of lower body strength in community‐residing older adults. Res Q Exerc Sport. 1999;70(2):113‐119. [DOI] [PubMed] [Google Scholar]
- 38. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365‐376. [DOI] [PubMed] [Google Scholar]
- 39. Bjordal K, Hammerlid E, Ahlner‐Elmqvist M, et al. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire‐H&N35. J Clin Oncol. 1999;17(3):1008‐1019. [DOI] [PubMed] [Google Scholar]
- 40. Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. The EORTC QLQ‐C30 Scoring Manual. 3rd ed. Brussels, Belgium: European Organization for Research and Treatment of Cancer; 2001. [Google Scholar]
- 41. King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ‐C30. Qual Life Res. 1996;5(6):555‐567. [DOI] [PubMed] [Google Scholar]
- 42. Capozzi LC, McNeely ML, Lau HY, et al. Patient‐reported outcomes, body composition, and nutrition status in patients with head and neck cancer: results from an exploratory randomized controlled exercise trial. Cancer. 2016;122(8):1185‐1200. [DOI] [PubMed] [Google Scholar]
- 43. Lonkvist CK, Vinther A, Zerahn B, et al. Progressive resistance training in head and neck cancer patients undergoing concomitant chemoradiotherapy. Laryngoscope Investig Otolaryngol. 2017;2(5):295‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Samuel SR, Maiya AG, Fernandes DJ, et al. Effectiveness of exercise‐based rehabilitation on functional capacity and quality of life in head and neck cancer patients receiving chemo‐radiotherapy. Support Care Cancer. 2019;27:3913‐3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371(9625):1695‐1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gray RE, Goel V, Fitch MI, et al. Utilization of professional supportive care services by women with breast cancer. Breast Cancer Res Treat. 2000;64(3):253‐258. [DOI] [PubMed] [Google Scholar]
- 47. Plass A, Koch U. Participation of oncological outpatients in psychosocial support. Psychooncology. 2001;10(6):511‐520. [DOI] [PubMed] [Google Scholar]
- 48. Holm LV, Hansen DG, Johansen C, et al. Participation in cancer rehabilitation and unmet needs: a population‐based cohort study. Support Care Cancer. 2012;20(11):2913‐2924. [DOI] [PMC free article] [PubMed] [Google Scholar]


