Abstract
Background
Olfactory dysfunction is a prevalent problem with a significant impact on quality of life and increased mortality. Limited effective therapies exist. Platelet‐rich plasma (PRP) is an autologous biologic product with anti‐inflammatory and neuroprotective effects. This novel pilot study evaluated the role of PRP on olfactory neuroregeneration in patients with hyposmia.
Methods
Seven patients who had olfactory loss greater than 6 months in duration, no evidence of sinonasal inflammatory disease, and no improvement with olfactory training and budesonide topical rinses were enrolled in this preliminary study. Patients received a single intranasal injection of PRP into the mucosa of the olfactory cleft. The Sniffin' Sticks olfactory test consisting of threshold, discrimination, and identification measurements (TDI) was administered at the beginning of the study and at 1 and 3 months.
Results
All patients reported a subjective improvement of their smell shortly after injection but then stabilized. At 3‐month post‐treatment, two patients with functional anosmia (TDI < 16) did not improve significantly. Five patients with hyposmia (TDI > 16 but <30) showed an improvement with 60% achieving normosmia (TDI > 30) at 3‐month follow‐up. On average, patients with baseline TDI > 16 improved by 5.85 points with the most significant improvement in the threshold subcomponent. There were no adverse outcomes from intranasal PRP injections.
Conclusion
PRP appears safe for use in the treatment of olfactory loss, and preliminary data suggest possible efficacy, especially for those with moderate yet persistent loss. Further studies will help determine optimal frequency and duration of use.
Level of evidence: 2B
Keywords: hyposmia, olfaction, platelet‐rich plasma, postviral, smell loss
1. INTRODUCTION
Olfactory dysfunction is a prevalent disorder that affects up to 20% of the general population and has significant effects on a person's quality of life as well as increased morbidity and mortality.1, 2, 3 The etiology of olfactory dysfunction is quite varied, including postviral, post‐traumatic, and idiopathic loss of smell. Unfortunately, with these etiologies, the likelihood of spontaneous recovery is generally poor, with only approximately one‐third of people regaining function and the duration of loss negatively correlating with recovery rate.4, 5 Treatment for olfactory dysfunction is also limited. Best evidence studies recommend olfactory training and topical steroid nasal irrigations as potential therapeutics, yet both have limited efficacy.6, 7, 8, 9
Promisingly, the olfactory neuroepithelium and olfactory filae, peripheral nerve fibers that traverse the cribriform plate into the nasal cavity, have the ability to regenerate and thus may serve as potential therapeutic targets for patients with olfactory dysfunction. Platelet‐rich plasma (PRP) is an autologous biologic product derived from fresh whole blood containing a high concentration of platelets. PRP is known to have anti‐inflammatory and pro‐regenerative properties including upregulation of growth factors including transforming growth factor, vascular endothelial growth factor, epidermal growth factor, and insulin‐like growth factor.10 It has been used as a safe therapy, effective in treating inflammation, wound healing, and peripheral neuropathies in other clinical settings.11, 12, 13 In particular, PRP has been shown to promote axon regeneration and neuroregeneration.14, 15, 16, 17
The role of PRP on olfactory neuroregeneration and related inflammation is unknown, but a preliminary animal study showed potential functional benefits of topical PRP in an anosmia induced mouse model.18 One pilot study evaluated PRP for hyposmia in humans and reported subjective improvements in five patients following treatment.19 However, that study lacked quantitative measurements of olfaction pre‐ and post‐treatment and other standardized norms. Herein, we aimed to investigate the safety and role of PRP in patients with persistent olfactory loss as measured by validated olfactory testing. This represents a novel use of PRP to promote olfactory neuroregeneration and stimulate growth in an attempt to recover olfaction and taste.
2. METHODS
This was a single‐arm pilot study evaluating patients with smell loss for over 6 months but under 12 months. Informed consent and approval from the Stanford Institutional Review Board Committee were obtained.
2.1. Patient selection
Inclusion criteria included adult patients (>18 years of age) with quantitative olfactory dysfunction documented by a University of Pennsylvania's Smell Identification Test (UPSIT) score ≤33 during their initial visit to Stanford Sinus Center. Patients must have trialed both olfactory training and budesonide nasal irrigations for at least 3 months and had radiographic imaging that demonstrated normal paranasal anatomy and olfactory bulb. Exclusion criteria included a history of inflammatory sinonasal disease, prior sinonasal surgery, <6 months or >12 months of smell loss, or history of any bleeding disorders or use of blood thinner medications.
2.2. Procedure
Recruited patients partook in additional baseline olfactory testing using the validated Sniffin' Sticks to determine their odor threshold, discrimination, and identification scores (TDI) with each subscore ranging from 0 to 16 with a total 48 possible points.20 In our study, the threshold scores were scored from 0 to 16 rather than 1 to 16, with T = 0 representing those who fail to recognize the most intensely odorant pen (No. 1) and T = 1 representing those who only recognize pen No. 1. This scoring modification was recommended by prior studies and allowed us to distinguish between patients who initially had an inability to distinguish the most concentrated odorant pen and those who could recognize this concentration.21
Following olfactory testing, patients underwent a one‐time submucosal intranasal injection of 1 mL PRP in each olfactory cleft, under endoscopic visualization, first along the superior septum just posterior to the head of the middle turbinate and then again more posteriorly into the septum across from the leading edge of the superior turbinate, to cover the entire region of the olfactory epithelium along the septum as shown in Figure 1. The isolation of PRP was carried out as dictated by the GS30‐PURE II Protocol A (Emcyte, Ft Myers, Florida). This protocol isolates PRP products with high platelet, low granulocyte count, and minimal red blood cells. Briefly, 20 mL of patient's whole blood was drawn and added to 5 mL of sodium citrate (SC) anticoagulant. The blood was centrifuged at 4200 rpm for 1 minute upon which the platelet plasma suspension supernatant was aspirated and re‐centrifuged at 4200 rpm for 5 minutes. The subsequent supernatant or the platelet poor plasma was discarded until 2 mL of PRP remained. The PRP was drawn up into two separate 1‐mL syringes and injected intranasally with a 27‐g needle into the mucosa of the olfactory cleft that had been topically anesthetized with pledget application of 4% lidocaine and 0.1% phenylephrine. Patients were observed for 15 minutes postprocedure for any adverse effects and subsequently discharged. Patients returned to clinic for a subsequent Sniffin' Sticks olfaction testing at 1‐month and 3‐month intervals post‐treatment. Postprocedural nasal endoscopy was also performed to evaluate the health of the nasal mucosa.
Figure 1.
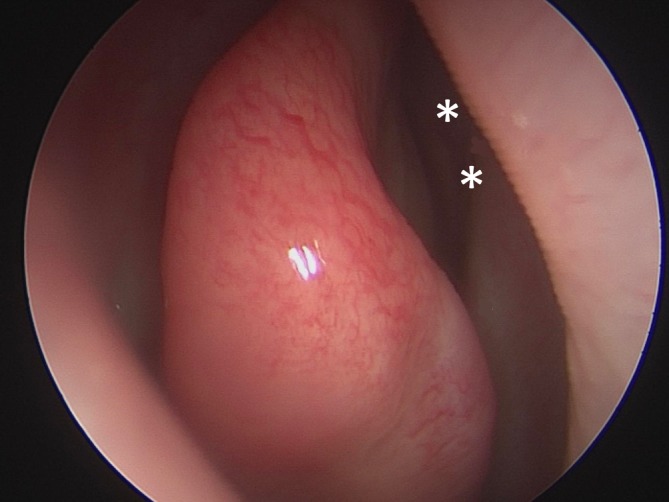
Endoscopic view of the right olfactory epithelium with the sites of PRP injection depicted by the two asterisks. A 1‐mL PRP was injected on each side of the nasal cavity and was split into two injections, first along the superior septum just posterior to the head of the middle turbinate and then again about 1 cm posteriorly into the septum across from the leading edge of the superior turbinate
2.3. Statistical analysis
A student's paired two‐tailed t test was utilized to compare overall TDI scores in our patients.
3. RESULTS
Seven female, nonsmoking patients with ages between 32 and 66 years were enrolled in this study (Table 1). Patients had smell loss duration from 6 to 11 months due to nonsinonasal disease’‐related etiologies. These included postviral, post‐traumatic, and postanesthetic exposure causes of olfactory loss. All patients had undergone multiple therapies including olfactory training and budesonide nasal irrigations for at least 3 months without improvement in their sense of smell.
Table 1.
Patient demographics
| Subject | Gender | Age (years) | Etiology of loss | Duration of loss (months) | Baseline UPSIT | Smoker | Prior therapeutics |
|---|---|---|---|---|---|---|---|
| 1 | F | 66 | postviral | 11 | 17/40 anosmia | N | Topical steroid irrigations, olfactory training |
| 2 | F | 58 | postviral | 11 | 9/40 anosmia | N | Topical steroid irrigations, olfactory training, oral steroids, acupuncture |
| 3 | F | 57 | postviral | 6 | 27/40 moderate microsmia | N | Topical steroid irrigations, olfactory training, oral steroids |
| 4 | F | 56 | postviral | 9 | 28/40 moderate microsmia | N | Topical steroid irrigations, olfactory training, oral steroids |
| 5 | F | 44 | postviral | 10 | 18/40 anosmia | N | Topical steroid irrigations, olfactory training, oral steroids |
| 6 | F | 45 | post‐traumatic | 6 | 12/40 anosmia | N | Topical steroid irrigations, olfactory training |
| 7 | F | 32 | postanesthetic exposure | 6 | 9/40 anosmia | N | Topical steroid irrigations, olfactory training |
Abbreviation: UPSIT: University of Pennsylvania Smell Identification Test.
Following PRP injection, all patients reported a subjective improvement of their smell at their one‐month follow‐up visit (Table 2). Patients reported the ability to distinguish specific scents including rosemary, varnish, and soaps that were not previously detected. At 3‐month follow‐up, none of the patients reported a continued improvement in their sense of smell beyond the first month but felt improved compared to pretreatment baseline.
Table 2.
Change in olfaction based on TDI scores at 1 and 3 months following treatment with PRP
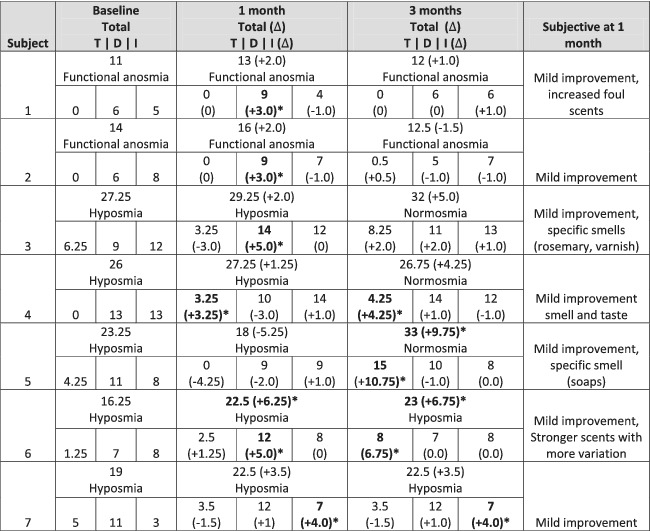
|
Abbreviations: ∆, change in score from baseline;T, threshold; D, discrimination; I, identification.
aAchieved minimally clinically important difference (∆5.5 total for TDI changes, subcategory: ∆2.5 for threshold, Δ3.0 for discrimination and identification).
For a majority of patients, there was continued improvement in their olfaction following PRP therapy up to 3 months post‐treatment. Overall, there was a significant improvement in average TDI values at the 3‐month follow‐up compared to baseline (23.6 vs 19.5, P = .026, Figure 2). Two patients had baseline functional anosmia (TDI score <16), suggestive of an inability to perceive any common or useful odors in daily life.22 For these patients (Patient 1 and 2), there was minimum to no improvement in olfaction scores at 3 months despite mild increases in their TDI at 1‐month and initial positive assessments. The remaining five other patients with baseline hyposmia (TDI > 16 but <30) but not anosmia showed continued improvement in olfaction at 3 months. Three patients achieved normosmia (TDI > 30) at 3‐month follow‐up. On average, the five hyposmic patients improved their overall TDI score by 5.85 points.
Figure 2.
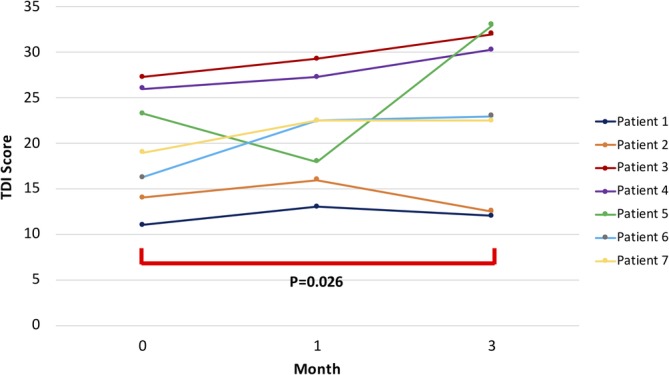
Total TDI scores at baseline, 1 and 3 months post‐PRP. Among the entire group, mean TDI scores increased from 19.5 at baseline to 23.6 points at 3 months, statistically significant at P = .026
A 5.5 point improvement in TDI score was previously determined to be the minimal clinically important difference (MCID) for olfactory improvement with a clinically notable improvement after a 2.5‐point increase in the threshold subscore and a 3.0‐point increase in the discrimination and identification subscores.23 Two patients achieved MCID in total TDI score at 3‐month follow‐up while two other patients achieved normosmia but had TDI improvements of 5 and 4.5 points (Table 2). At 1‐month follow‐up, most patients showed greatest improvement in their ability to discriminate odors with four of the seven patients achieving MCID in the discrimination subscore. However, by 3‐month postprocedure, most patients showed the greatest improvement in the threshold subcomponent.
In this study, there were no adverse outcomes from intranasal PRP injections. Follow‐up nasal endoscopy showed no evidence of intranasal synechiae, inflammation, or mucosal disturbances at the olfactory cleft bilaterally. No patient reported a significant decrease in their sense of smell during any period following PRP therapy.
4. DISCUSSION
This is a pilot study evaluating the role of PRP in neuro‐olfactory regeneration in the setting of hyposmia and is the first to demonstrate improvement in smell using validated olfactory measurements following PRP therapy. Importantly, there were no adverse outcomes following intranasal PRP injections. Patients reported no intranasal symptoms or decreases in sense of smell. Keeping in mind that there have been reports of visual loss from intranasal steroid injections, all linked to the large particles that are present within a steroid emulsion, we took care to ensure that the PRP was prepared and immediately used to decrease any opportunity for particle formation, even though the material itself is not in emulsion form at all and mixed with an anticoagulant, and thus risk is already exceptionally low for embolization. Standard injection precautions were taken to ensure no injection intravascularly and patients are awake during the procedure and would be able to report any visual changes immediately during the injection.
Thus far, there are few options for patients with olfactory loss particularly those unrelated to sinonasal inflammation. These patients had at least 6 months of olfactory loss as well as failed prior treatments with olfactory training and budesonide nasal irrigations, the only two validated treatments for nonsinonasal olfactory dysfunction with level 1 evidence. A failure to improve after 6 months as well as using these two therapies suggested that there was a lower likelihood of spontaneous recovery. After 12 months, we know peripheral nerve regeneration is unlikely.24 Thus, for the purposes of this initial study, we excluded patients with a greater 12‐month history of olfactory loss.
Following PRP therapy, three patients achieved normosmia at their 3‐month follow‐up and two patients achieved the MCID with a TDI increase of over six points. However, all patients subjectively continued to report smell loss. In light of recent findings suggesting spontaneous recovery is possible even after a year,4, 25 we should consider our results in that context. However, the direct temporal relationship of the improvement in our patients after injection causes us to consider this a finding worth investigating further.
In this study, interestingly, although five patients had a baseline UPSIT score that fell into the anosmia category (<19/40), only two patients were characterized as functional anosmics based on their Sniffin' sticks test. At baseline, these patients reported no or negligible sense of smell in everyday life. Both patients demonstrated little improvement in olfactory function compared to others in the study despite initial increases in scent discrimination at 1‐month postprocedure. This suggests that PRP therapy may be more beneficial in those with a mild to moderate loss and the potential for olfactory neuroregeneration may require a certain level of pre‐existing neural activity. It is important to also note that these two patients also were the two oldest patients in our sample size and their duration of loss was nearly 12 months, both factors known to be negative predictors in smell recovery. It is also interesting that in spite of lack of major quantitative improvement, these two patients had a similar subjective feeling of improvement following injection that all participants acknowledged. This may be due to the placebo effect of the treatment or suggest that an individual's everyday smell loss experience has nuances that cannot be completely captured by our olfactory test battery.
In this study, all the patients who were enrolled were coincidentally female. This is not surprising, as females tend to dominate the smell loss patient population. This is perhaps related to the greater subjective loss they may feel as a group, although population studies have shown that females tend to have higher baseline olfaction scores compared to male.22, 26, 27 Most patients suffered from a postviral smell loss etiology. However, one patient had a post‐traumatic loss, and one from a postanesthetic smell loss. Postanesthetic smell loss, although exceedingly rare, has been reported in the literature and can be permanent with a direct temporal relationship following general anesthesia.28 This etiology has been seen multiple times in our own smell loss center related to anesthesia for surgeries for areas far away from the head and neck region.
It is worth noting that our PRP protocol utilized a low concentration of SC as an anticoagulant for the purification of PRP. Compared to other anticoagulants, SC has been found to have high platelet recovery and mesenchymal stromal cell proliferation which makes it an optimal anticoagulant.29 However, there are studies that have suggested SC can improve olfactory dysfunction in postinfectious loss.30 In this particular study, a difference was seen when SC is used as a direct topical therapy but only for short term. In our protocol, SC is diluted to a final concentration of approximately 16%. Furthermore, studies by the PRP manufacturer have shown a 3‐ to 5‐fold increase in growth factor production with PRP + SC compared to SC alone (data unpublished, Emcyte, Ft Myers, Florida).
All patients subjectively noted an improvement in their smell immediately following PRP injections with enhanced detection of certain specific odors. However, this subjective improvement did not continue despite evidence of improved olfaction scores at their 3‐month follow‐up. In our pilot study, only a single PRP injection was performed to first assess the safety and feasibility of this therapy. Mavrogeni and colleagues described four PRP injections with 4‐week intervals in between without any safety concerns.19 Taking this together with our findings from this study, future studies may benefit from multiple PRP treatments.
Given its novelty and invasive experimental design, there is no standardized or optimal dosage or concentration recommended for PRP injections and commercial preparation techniques are varied. We injected 1 mL PRP in this pilot study as an appropriate amount that can be introduced into the olfactory cleft that can fill the entire region of the olfactory epithelium submucosal space without too much back pressure and leakage. Our technique relied on a double‐step centrifuge process to obtain enrichment of platelets that was up to five times baseline platelet concentration according to the manufacturer's brochure (Emcyte, Ft Myers, Florida). Prior studies using this same protocol as our study demonstrated an average yield with a 3.5‐fold increase in platelets concentration compared to whole blood.31 The concentration and volume of PRP injected may have a significant impact on olfaction recovery. A previous in vitro study showed that an intermediate PRP concentration was optimal in proliferation of Schwann cells for peripheral nerve regeneration.16 These studies suggest that PRP activity is modulated in a dose‐dependent mode and further investigation is needed to determine the optimal concentration of PRP in its therapy for olfactory outcomes.
As a single‐arm pilot study involving a small number of patients, a weakness is the lack of randomization and placebo arm, bringing the potential question of whether our results were simply spontaneous recovery. We recognize a control group would be optimal to assess true efficacy and rule out placebo effect. However, with a novel therapy and particularly one that has a procedural component involving a peripheral blood draw and intranasal injection, we felt strongly that a pilot single‐arm study with a small number of patients was more appropriate to establish safety, tolerability, and begin the investigation toward possible efficacy. Now that safety and tolerability have been established, we do plan on incorporating a placebo control in our upcoming randomized controlled trial.
Additionally, our exclusion criteria of patients who demonstrated persistent loss for 6 months and had not already trialed other proven therapies such as olfactory training do suggest that spontaneous recovery is not the main factor in our results. Additionally, our finding that the hyposmia group improved while the anosmia group did not follows the same paradigm that all neurologic injury follows with regard to severity and prognosis, with or without intervention.
This is the first study to use quantitative olfactory measures to demonstrate a potential improvement of smell with PRP intranasal injections. A small sample size was used as part of this pilot study and the findings should be interpreted with caution. There was a statistically significant improvement in the average TDI at P = .026. However, given the small number of patients in this study, statistical analysis is difficult to interpret, and this study was not powered to detect a difference.
5. CONCLUSION
PRP appears safe for use in the treatment of olfactory loss. In this study, we have preliminary data that suggests possible efficacy, especially for those with moderate yet persistent loss of smell. Further studies will help determine optimal frequency and duration of use.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
DISCLOSURES
Z.M.P.: Consultant for Medtronic Inc, Stryker, Intersect ENT, Optinose.
Yan CH, Mundy DC, Patel ZM. The use of platelet‐rich plasma in treatment of olfactory dysfunction: A pilot study. Laryngoscope Investigative Otolaryngology. 2020;5:187–193. 10.1002/lio2.357
This research was presented at the 2019 American Rhinologic Society Annual Meeting in New Orleans, LA, on September 14, 2019.
REFERENCES
- 1. Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life: an updated review. Chem Senses. 2014;39(3):185‐194. [DOI] [PubMed] [Google Scholar]
- 2. Nordin S, Brämerson A. Complaints of olfactory disorders: epidemiology, assessment and clinical implications. Curr Opin Allergy Clin Immunol. 2008;8(1):10‐15. [DOI] [PubMed] [Google Scholar]
- 3. Landis BN, Konnerth CG, Hummel T. A study on the frequency of olfactory dysfunction. Laryngoscope. 2004;114(10):1764‐1769. [DOI] [PubMed] [Google Scholar]
- 4. Cavazzana A, Larsson M, Münch M, Hähner A, Hummel T. Postinfectious olfactory loss: a retrospective study on 791 patients. Laryngoscope. 2018;128(1):10‐15. [DOI] [PubMed] [Google Scholar]
- 5. Kim DH, Kim SW, Hwang SH, et al. Prognosis of olfactory dysfunction according to etiology and timing of treatment. Otolaryngol—Head Neck Surg. 2017;156(2):371‐377. [DOI] [PubMed] [Google Scholar]
- 6. Yan CH, Overdevest JB, Patel ZM. Therapeutic use of steroids in non‐chronic rhinosinusitis olfactory dysfunction: a systematic evidence‐based review with recommendations. Int Forum Allergy Rhinol. 2019;9(2):165‐176. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen TP, Patel ZM. Budesonide irrigation with olfactory training improves outcomes compared with olfactory training alone in patients with olfactory loss. Int Forum Allergy Rhinol. 2018;8(9):977‐981. [DOI] [PubMed] [Google Scholar]
- 8. Damm M, Pikart LK, Reimann H, et al. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. Laryngoscope. 2014;124(4):826‐831. [DOI] [PubMed] [Google Scholar]
- 9. Pekala K, Chandra RK, Turner JH. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta‐analysis. Int Forum Allergy Rhinol. 2016;6(3):299‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet‐rich plasma. Am J Sports Med. 2011;39(10):2135‐2140. [DOI] [PubMed] [Google Scholar]
- 11. Anjayani S, Wirohadidjojo YW, Adam AM, Suwandi D, Seweng A, Amiruddin MD. Sensory improvement of leprosy peripheral neuropathy in patients treated with perineural injection of platelet‐rich plasma. Int J Dermatol. 2014;53(1):109‐113. [DOI] [PubMed] [Google Scholar]
- 12. Raeissadat SA, Karimzadeh A, Hashemi M, Bagherzadeh L. Safety and efficacy of platelet‐rich plasma in treatment of carpal tunnel syndrome; a randomized controlled trial. BMC Musculoskelet Disord. 2018;19(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sánchez M, Yoshioka T, Ortega M, Delgado D, Anitua E. Ultrasound‐guided platelet‐rich plasma injections for the treatment of common peroneal nerve palsy associated with multiple ligament injuries of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1084‐1089. [DOI] [PubMed] [Google Scholar]
- 14. Ikumi A, Hara Y, Yoshioka T, Kanamori A, Yamazaki M. Effect of local administration of platelet‐rich plasma (PRP) on peripheral nerve regeneration: An experimental study in the rabbit model. Microsurgery. 2018;38(3):300‐309. [DOI] [PubMed] [Google Scholar]
- 15. Sariguney Y, Yavuzer R, Elmas C, Yenicesu I, Bolay H, Atabay K. Effect of platelet‐rich plasma on peripheral nerve regeneration. J Reconstr Microsurg. 2008;24(3):159‐167. [DOI] [PubMed] [Google Scholar]
- 16. Zheng C, Zhu Q, Liu X, et al. Effect of platelet‐rich plasma (PRP) concentration on proliferation, neurotrophic function and migration of Schwann cells in vitro. J Tissue Eng Regen Med. 2016;10(5):428‐436. [DOI] [PubMed] [Google Scholar]
- 17. Farrag TY, Lehar M, Verhaegen P, Carson KA, Byrne PJ. Effect of platelet rich plasma and fibrin sealant on facial nerve regeneration in a rat model. Laryngoscope. 2007;117(1):157‐165. [DOI] [PubMed] [Google Scholar]
- 18. Yasak AG, Yigit O, Araz Server E, Durna Dastan S, Gul M. The effectiveness of platelet‐rich plasma in an anosmia‐induced mice model. Laryngoscope. 2018;128(5):E157‐E162. [DOI] [PubMed] [Google Scholar]
- 19. Mavrogeni P, Kanakopoulos A, Maihoub S, Krasznai M, Szirmai A. Anosmia treatment by platelet rich plasma injection. Int Tinnitus J. 2017;20(2):102‐105. [DOI] [PubMed] [Google Scholar]
- 20. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. Sniffin' sticks: olfactory performance assessed by the combined testing of odour identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22(1):39‐52. [DOI] [PubMed] [Google Scholar]
- 21. Rumeau C, Nguyen DT, Jankowski R. How to assess olfactory performance with the Sniffin' sticks test(®). Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133(3):203‐206. [DOI] [PubMed] [Google Scholar]
- 22. Oleszkiewicz A, Schriever VA, Croy I, Hähner A, Hummel T. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol. 2019;276(3):719‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gudziol V, Lötsch J, Hähner A, Zahnert T, Hummel T. Clinical significance of results from olfactory testing. Laryngoscope. 2006;116(10):1858‐1863. [DOI] [PubMed] [Google Scholar]
- 24. Menorca RMG, Fussell TS, Elfar JC. Nerve physiology: mechanisms of injury and recovery. Hand Clin. 2013;29(3):317‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee DY, Lee WH, Wee JH, Kim J‐W. Prognosis of postviral olfactory loss: follow‐up study for longer than one year. Am J Rhinol Allergy. 2014;28(5):419‐422. [DOI] [PubMed] [Google Scholar]
- 26. Temmel AFP, Quint C, Schickinger‐Fischer B, Klimek L, Stoller E, Hummel T. Characteristics of olfactory disorders in relation to major causes of olfactory loss. Arch Otolaryngol Neck Surg. 2002;128(6):635‐641. [DOI] [PubMed] [Google Scholar]
- 27. Noel J, Habib A‐RR, Thamboo A, Patel ZM. Variables associated with olfactory disorders in adults: a U.S. population‐based analysis. World J Otorhinolaryngol—Head Neck Surg. 2017;3(1):9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elterman KG, Mallampati SR, Kaye AD, Urman RD. Postoperative alterations in taste and smell. Anesthesiol Pain Med. 2014;4(4):e18527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. do Amaral RJFC, da Silva NP, Haddad NF, et al. Platelet‐rich plasma obtained with different anticoagulants and their effect on platelet numbers and mesenchymal stromal cells behavior in vitro. Stem Cells Int. 2016;2016:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whitcroft KL, Ezzat M, Cuevas M, Andrews P, Hummel T. The effect of intranasal sodium citrate on olfaction in post‐infectious loss: results from a prospective, placebo‐controlled trial in 49 patients. Clin Otolaryngol. 2017;42(3):557‐563. [DOI] [PubMed] [Google Scholar]
- 31. Braun HJ, Kim HJ, Chu CR, Dragoo JL. The effect of platelet‐rich plasma formulations and blood products on human synoviocytes: implications for intra‐articular injury and therapy. Am J Sports Med. 2014;42(5):1204‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]


