Abstract
Objective
The term “labyrinthine concussion” has evolved to mean audiovestibular dysfunction in the absence of a temporal bone fracture (TBF). Despite a multitude of case descriptions of labyrinthine concussion, the precise pathophysiology remains poorly understood. Herein, we explore the historical otopathologic underpinnings of the diagnosis of labyrinthine concussion with a focus on the auditory pathway during the late 19th to the mid‐20th centuries and conclude with a discussion of its contemporary relevance.
Methods and Data Sources
A review of primary and secondary medical sources written in English, German, and French on otopathology labyrinthine concussion studies from the late‐19th to the mid‐20th centuries.
Results
Around the turn of the 20th century, otopathologists identified histologic changes in the temporal bones of individuals that sustained head injury without TBFs. Based on these otopathologic findings in humans, early experiments investigating the pathophysiology of labyrinthine concussion were performed in animals through either the delivery of blows to the head or direct introduction of a pressure wave into the labyrinthine fluid. Collectively, otopathologists hypothesized that predominant mechanisms for labyrinthine concussion included inner ear hemorrhage, cochleovestibular nerve traction injury, direct damage from a labyrinthine fluid pressure wave, or vasomotor dysfunction.
Conclusion
Historical study shows a variety of inner ear pathologies potentially responsible for auditory dysfunction following head injury. Understanding the history and otopathology of labyrinthine concussion may help clinicians focus on new pathways toward novel research and improved patient care.
Keywords: chronic traumatic encephalopathy, dementia pugilistica, head injury, hearing loss, history of otology, inner ear concussion, labyrinthine concussion
1. INTRODUCTION
“If we consider the anatomy of the labyrinth it can be understood that such a delicate structure is easily subjected to an indirect injury while it is well protected against a direct injury.”1
Auditory dysfunction, including hearing loss, tinnitus, and hyperacusis, has long been recognized as a potential consequence of head injury.2, 3 In cases of temporal bone fractures (TBF), auditory symptoms are commonly thought to be caused by direct anatomic disruption of the middle ear and/or inner ear sensory neuroepithelium.4, 5, 6 In the absence of a TBF, it can be difficult to predict whether a patient will sustain auditory pathology. The terms “labyrinthine concussion” and “inner ear concussion” have evolved to mean auditory and/or vestibular dysfunction of the inner ear following head trauma in the absence of TBF.7, 8
Despite the multitude of descriptions of labyrinthine concussion,9, 10, 11 the precise understanding and pathophysiology of labyrinthine concussion remain poorly understood.8 Descriptions of labyrinthine concussion date back centuries.9, 10, 11, 12, 13 Various types of terminology have been created to reflect the assumed pathophysiology of labyrinthine concussions. Prominent terms include “labyrintherschütterung” (labyrinthine concussion, or more literally, labyrinthine tremor, shock, or disruption),14, 15, 16, 17, 18 “commotio labyrinthi” (labyrinthine disturbance due to traumatic hemorrhage15, 19, 20, 21, 22 or a labyrinthine fluid pressure wave23), “otitis interna vasomotoria” (labyrinthine injury caused by circulatory disturbances or “vasomotory troubles”24), and “commotio cerebri” (cerebral disturbance).25 Many of these historic clinical studies, however, lacked rigorous methods of diagnosing TB fractures, such as high‐resolution imaging, and were not able to provide pathologic correlation.
Herein, we aim to explore the historical otopathological underpinnings of the diagnosis of labyrinthine concussion. The article will specifically focus on the otopathological findings in humans and in animal models of labyrinthine concussion from the late‐19th to mid‐20th centuries and then conclude by relating these findings and associated theories to the contemporary understanding of labyrinthine concussion. Understanding historical otopathology of labyrinthine concussion may help our current understanding of the diagnosis and shape future research pathways.
2. METHODOLOGY
A review of the literature was conducted to identify articles related to the phenomenon of labyrinthine concussion. Articles were identified by searching the MeSH terms “labyrinthine concussion,” “vestibular concussion,” “inner ear concussion,” “traumatic hearing loss,” and associated terms. A wide array of articles was identified, including basic and clinical studies, case series, and reviews. The references of these articles were then reviewed to identify primary sources and literature not captured by electronic search databases. In addition, historical book chapters on otology and manuscripts in English, German, and French were procured for additional review and analysis. Chapters and articles were translated by native German and French speakers. Attempts were made to identify original sources dating as far back as possible that would be amenable for review. Finally, auditory neuroscientists and experts in otopathology were contacted to ensure inclusion of the most relevant papers, data, and interpretations.
3. HISTORIC OTOPATHOLOGIC MECHANISMS OF LABYRINTHINE CONCUSSION
Otopathologists in the late‐19th century and early‐20th century proposed several etiologies of labyrinthine concussion based on otopathology studies. In 1871, Samuel Moos (Germany) provided an early description of otopathology associated with this phenomenon—that of a Prussian soldier who developed hearing loss after receiving a glancing gunshot wound to his left mastoid.11 On postmortem investigation, the soldier's otic capsule was intact but there was “hemorrhage in the labyrinth [as] the consequence of concussion of the petrous bone by gunshot.” Since this time, additional etiologies were described, and the most commonly described mechanisms of labyrinthine concussion included (a) labyrinthine hemorrhage, (b) cochleovestibular nerve traction injury, or (c) direct damage from a traumatic labyrinthine fluid wave.
3.1. Labyrinthine Hemorrhage
One of the most predominant mechanistic explanations for inner ear concussion in the late‐19th and early‐20th centuries was inner ear hemorrhage. Otto Barnick (Austria) in 1897 was among the first to microscopically examine the temporal bone following head trauma. Barnick examined four patients who died a week following head trauma.10 The labyrinthine capsule was intact in two of these specimens and the prominent finding was the presence of hemorrhage within the labyrinth, including along the basal turn of the cochlea, which he believed explained the high frequency hearing loss typical of labyrinthine concussion.
Other early human otopathologic studies also identified the occurrence of hemorrhage in the inner ear without fracture.26, 27 Giuseppe Gradenigo (Italy) argued that minor blows to the head caused deafness by precipitating “commotion of the acoustic nerve” caused by extravasation of blood into the labyrinth.9 Gradenigo thought the presence of hemorrhage in the labyrinth could explain the worsening deficits over time which can follow exposure to an explosion.9 Adam Politzer (Hungary, Austria) assumed the importance of intralabyrinthine hemorrhage for labyrinthine concussion.14 Adolf Passow (Germany) also argued that labyrinthine bleeding could cause atrophy of the labyrinthine neuroepithelium and he used the phrase “commotio labyrinthi,” defining it as traumatic hemorrhage into the labyrinth.15
Additional proponents of labyrinthine hemorrhage, including Arthur Cheatle (United Kingdom),28 William Milligan (United Kingdom),28 and William Grove (United States),19, 29 supported their stance by citing the presence of labyrinthine hemorrhage in otopathologic10, 26, 27 as well as animal experiments.16, 30 Although Grove thought nerve traction injury, as described below, was occasionally responsible, he argued that symptoms of labyrinthine concussion were more often due to hemorrhage into the inner ear, damaging nerves from the pressure it exerts or by organizing and leading to atrophy of the finer nerve branches and degeneration of the neuroepithelium of both the cochlea and vestibule.19, 29
The theory of labyrinthine hemorrhage also had its share of detractors. Fifteen years following his own animal experiments which demonstrated labyrinthine hemorrhage, Hans Brunner (Austria, United States) in 1940 asserted that individuals attributed too much causal importance to inner ear hemorrhage.18, 24, 29, 31 He postulated that head trauma caused circulatory disturbances in the ear, “otitis interna vasomotoria,” which were responsible for the perivascular accumulation of lymphocytes and transudate which he saw in his experiments. Grove expanded on theories of brain concussion which postulated that midbrain trauma causes traumatic paralysis of vasoconstrictor nerves leading to impaired circulation in central vestibular areas.32, 33 He hypothesized that those changes led to impaired circulation in the labyrinth as well.29
Harold Schuknecht (United States) also did not believe that labyrinthine hemorrhage was the major etiology behind traumatic auditory injury. Schuknecht observed in many studies that “the location for blood had no correlation with the location of end‐organ damage…and that blood was found in the cochlea as long as four months after the head blow without causing reaction within the labyrinth.”34, 35 Schuknecht also thought that the etiology of delayed hearing loss was due to “secondary degenerative changes in the cochlea” and was not vasomotor in origin.34
3.2. Cochleovestibular Nerve Traction Injury
Another popular explanation for traumatic auditory injury was traction‐related injury to the cochleovestibular nerve. In early studies, Politzer interpreted the significant frequency of blood in the internal auditory canal of human temporal bones after head trauma as suggestive that the vestibulocochlear nerve may frequently be lacerated or its fibers torn in the porus acousticus.36 Later, Konrad Ulrich (Switzerland) and Walther Uffenorde (Germany), in their respective studies of human otopathology after trauma, concluded that stretching or tearing of the cochlear nerve was the most common and important histologic and pathophysiologic feature.37, 38, 39, 40 Ulrich and Uffenorde, along with F.R. Nager (Switzerland) and Arnold Klingenberg (Switzerland), doubted that direct damage to the labyrinth ever occurred following head injury in the absence of accompanying fracture of the otic capsule and instead concluded that nerve traction injury better accounted for auditory deficits.37, 38, 39, 40, 41, 42
Ulrich, referencing temporal bone studies, argued that if isolated labyrinthine injury in the absence of fracture were to exist, “one would find concussion of the inner ear on the healthy side more often in cases of fractures of the temporal bone.”24 Ulrich posited that the degree of nerve injury by stretching was inversely proportional to nerve length, explaining why after skull base fractures the cochleovestibular nerve is the most frequently injured nerve and the vagus nerve the least injured.39 In a later rebuttal, Brunner stated that nerve traction injury “hardly may occur when there is not any fracture of the base of the skull,”24 and Grove referenced the many cases of individuals in his study who had unilateral longitudinal TBFs with marked hearing loss in the contralateral ear.19
3.3. Traveling Pressure Wave
The mechanistic theory of a traveling labyrinthine fluid pressure wave causing direct inner ear damage became more popular as the inner ear hemorrhage theory fell out of favor. Karl Wittmaack (Germany) argued his experiments with rabbits, described in subsequent sections, suggested that a pressure wave could directly injure hair cells,23 and he along with Spira and Paul Stenger (Germany) argued a pressure wave could cause a “loosening of neurons or reversible biochemical change in nerve cell protoplasm.”34 Although Otto Voss (Germany) did not believe the “pushing wave” generated by Wittmaack's experiments was reflective of real life conditions, he did agree that a shock pulse on neuroepithelial cells in the labyrinth could cause degenerative changes.18
Schuknecht's experimentation using a feline head injury model, as detailed in later sections, supported the ability of a pressure wave to cause injury to the labyrinth and hearing deficits.43 By the mid‐20th century, however, the causal importance of the pressure wave became increasingly relegated to the acute phase of injury, with theories of vasomotor dysfunction,24, 30 secondary degenerative changes,34, 35 and progressive inflammatory states44 being favored for explaining the chronic deficits of labyrinthine concussion. The importance of a progressive inflammatory state was also alluded to by Politzer who stated that the development of inflammatory tissue along the labyrinth may cause ossification leading to deafness.36 Degenerative changes in the organ of Corti were observed in the temporal bones of patients with head injury without fracture.17, 23, 45 Labyrinthine scarring was also seen, with Politzer finding connective tissue in the labyrinth as early as the fifth week after “comparatively slight blows.”1 Other early otopathologists also commented on connective tissue and bone in the perilymphatic spaces of temporal bones from patients who suffered a head injury without fracture.41, 46, 47
4. HISTORIC ANIMAL OTOPATHOLOGY STUDIES
Early animal experiments aimed to establish causality between the histologic findings seen on human otopathology and the head traumas which were hypothesized to cause them. Paul Stenger (Germany) generated an early animal model of head injury in 1909 when he produced light blows with a hammer on skulls of live rats, which produced hemorrhages around the round window and basal turn of the cochlea.16 With heavier blows, Stenger noted more severe bleeding at the round window, extension of the cochlear hemorrhage to the helicotrema, blood in the ampullae, and petechial hemorrhages in the vestibular and cochlear nerves near the porus (Figure 1). Some animals also had degeneration of the organ of Corti and spiral ganglion, as well as intralabyrinthine connective tissue formation. As summarized by Fred Linthicum Sr. (United States), Stenger broadly concluded that the direct effect of labyrinthine concussion in the inner ear is 3‐fold: (a) degenerative changes of the nerve, ganglion cells, and nerve endings; (b) extravasation of blood; and (c) acute elevations in pressure in the perilymph and endolymph content of the labyrinth.48 Stenger's experimental design, pathological findings, and conclusions were repeated and reproduced by Alfred Linck (Germany) in 1921.49
Figure 1.
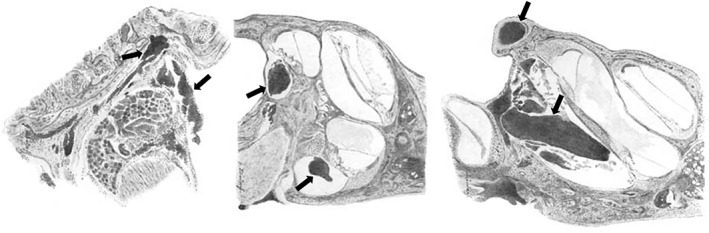
Stenger's drawings of the rat inner ear after head injury. Figures demonstrate accumulation of blood in the labyrinth (arrows) after the delivery of blows to the skull with a hammer in Stenger's 1909 experiment. Modified and translated from German16
Brunner conducted a similar experiment to Stenger and Linck using guinea pigs in 1925.30 In postmortem analysis, Brunner observed perivascular infiltrates in the region of the spiral vein, and hemorrhage in the scala tympani and cochlear aqueduct (Figure 2). To account for these findings, Brunner surmised that the force of head trauma is also exerted against the inner walls of the skull, including the temporal bone, thereby producing a wavelike motion of perilymph and endolymph by exerting force upon the endolymphatic sac and the internal auditory canal. He hypothesized that this force was eventually reflected to, and predominantly experienced by, the round window thereby accounting for the observed surrounding hemorrhages, as well as the bulging or potential rupture of the round window membrane into the middle ear at the time of injury. In addition to the acute damage to the internal ear at the time of injury, Brunner suggested that the “vasomotory troubles,” or alterations in tone and permeability, affected the labyrinthine artery resulting in the observed accumulation of lymphocytes and transudate within the internal ear; and, thus explaining the delayed hearing loss observed to occur over 2 years or more after the initial insult.24 The vascular findings inspired him to alternatively refer to labyrinthine concussion as “otitis interna vasomotoria.”
Figure 2.
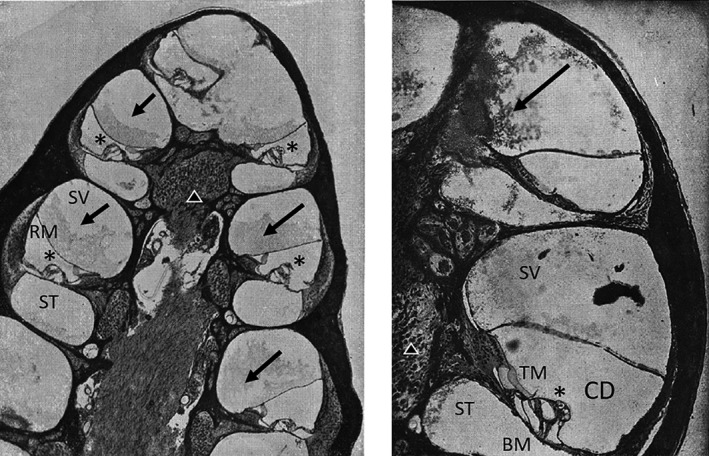
Otopathology from Brunner's head injury experiments in guinea pigs. Postmortem hematoxylin and eosin (H&E) stained cochlea of guinea pigs after blows to the skull with a hammer. (Left) Low power magnification H&E stained slide of several turns of the guinea pig cochlea. Exudate (arrows) can be seen in the scala vestibula (SV) of all turns. (Right) Higher power magnification of mid and apical portions of the guinea pig cochlea. Fibrin clot in the helicotrema and scala vestibuli (arrow). Tectorial membrane (TM), cochlear duct (CD), basement membrane (BM), organ of Corti (*), cochlear neurons (triangle). Modified and translated from German30
In 1932, Wittmaack sought to investigate the causal role of a labyrinthine fluid pressure wave in generating inner ear pathology.23 Wittmaack employed a rabbit model and displaced the stapes into the vestibule before connecting a water‐filled cannula to create a fluid pressure wave via the oval window (Figure 3). Findings included destruction of the organ of Corti and the macula sacculi, with lesser effect on the macula utriculi (Figure 4). The spiral ganglion cells lost their Nissl granules, became vacuolated, and degenerated over the course of 2 weeks. These investigations, however, were heavily scrutinized by Voss and Brunner for being “rough” experiments that did not accurately model the forces generated in human head injury.18, 24 Additionally, Brunner criticized Wittmaack for placing too much emphasis on the fluid wave theory as the predominate source of damage in labyrinthine concussion, citing the delayed hearing loss seen in patients as an overwhelming source of invalidation.24
Figure 3.
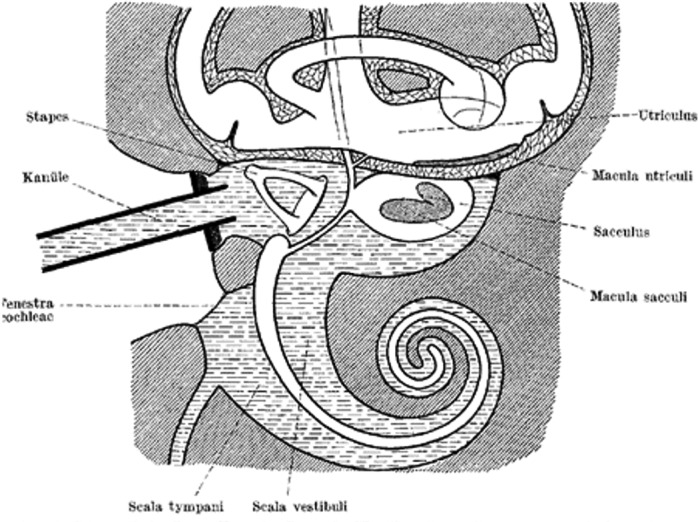
Illustration of Wittmaack's experimental setup in rabbits. The stapes were dislocated into the vestibule and a plunger (kanüle) was used to produce a labyrinthine fluid wave. Translated from German23
Figure 4.
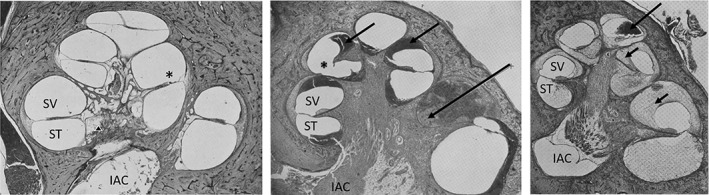
Otopathology from Wittmaack's experiment in rabbits. (Left) Low power H&E stained slide of the cat cochlea following head injury. Reissner's membrane is missing in the lower turn and collapsed in the middle turn. There is missing Reissner's and tectorial membrane (*). (Middle) Basilar membrane and a portion of the osseous spiral lamina are missing (*).The scala vestibuli (SV) contains blood (arrows). (Right) Low power photomicrograph of cochlea 15 days after injury noting tissue and blood in the cochlea (arrows). There is also atrophy of the cochlear neurons (not shown). Spiral vestibuli (SV), scala tympani (ST), internal auditory canal (IAC), cochlear neurons (triangle). Modified and translated from German23
Nearly 30 years later, Schuknecht in 1951 returned to studying traumatic hearing loss. Classic studies by Schuknecht in a feline head injury model are the best evidence to date of potential etiologies of labyrinthine concussion. Schuknecht behaviorally conditioned cats to respond to sounds at various frequencies. He then subjected the cats to head blows using an iron rod.43 Schuknecht found that most of the head blows delivered over the temporal and parietal bones near the experimental ear resulted in hearing loss. In contrast, blows to the contralateral side of the skull failed to produce deafness in the studied ear in several experiments. Schuknecht found a 15‐40 dB recovery of hearing acuity over the first 2 weeks postinjury (Figure 5). Hearing losses were greatest for high frequencies, particularly for the range from 3 to 8 KHz. Schuknecht posited that this loss in pure tones was similar to noise and blast‐induced hearing loss.
Figure 5.
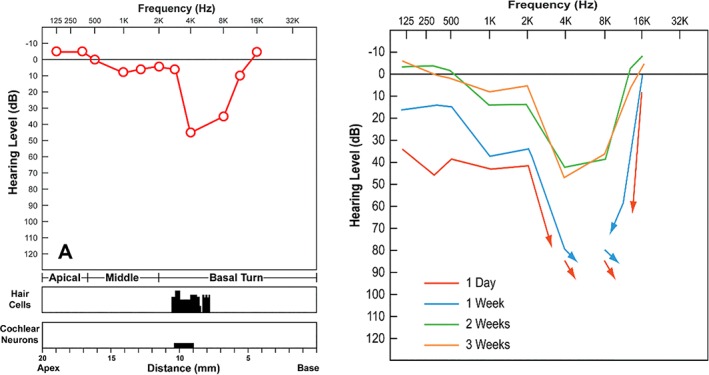
Post head injury behavioral audiogram from Schuknecht's experiment in feline head injury model. (Left) A behavioral audiogram and cytocochleogram from a cat which was sacrificed on the 22nd day after experimental head blows showing 2000 to 8000 Hz hearing loss, which was pathologically correlated with loss of outer hair cells along the upper basal coil of the cochlea.7 (Right) Serial behavioral audiograms from the same cat showing improvement in hearing thresholds during a 2‐week period following head injury7
Temporal bone histopathological analysis demonstrated that 4 out of the 10 animals had skull fractures, one involving the temporal bone (Figures 6 and 7). Otopathological findings on a cellular level were also variable, ranging from slight anatomical degeneration of the outer hair cells and supporting cells to extensive loss of hair cells to complete degeneration of the organ of Corti. Blood in the scala tympani and vestibuli was found in several of the cats, with no evidence of local tissue reaction. Leukocytes were also identified only in a small number of cats euthanized 2 days after injury. Additionally, only animals euthanized 3 weeks or longer after injury presented with nerve degeneration, although changes were less severe than what was found in the organ of Corti. Histological slides dating back to 1949 were recently rediscovered at the Massachusetts Eye and Ear Infirmary, and they likely served as the foundation for a series of classic papers on the topic43, 50, 51 (Figures 8 and 9).
Figure 6.
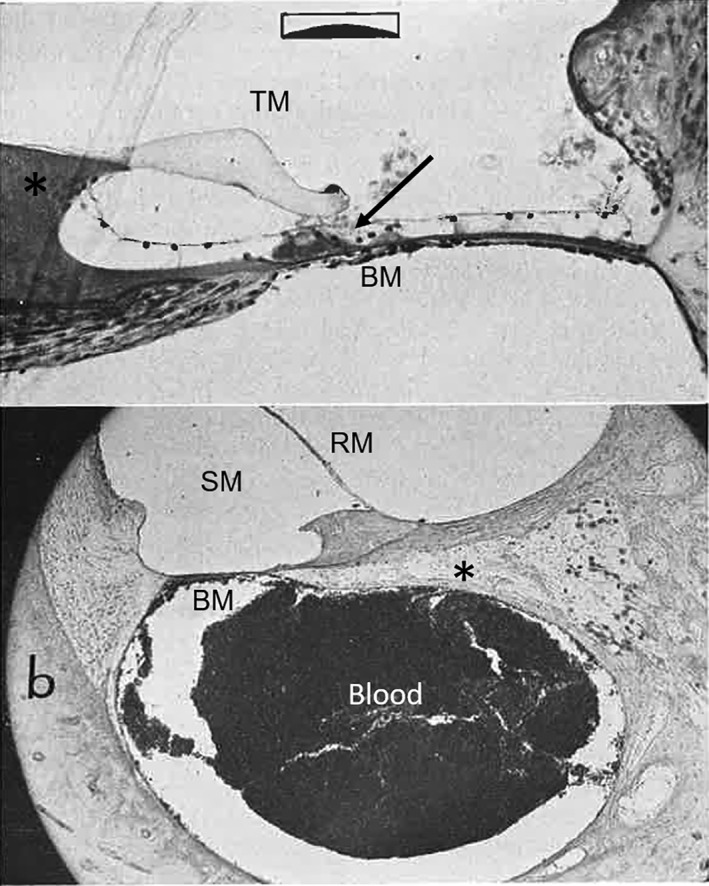
Otopathology from Schuknecht's experiment in feline head injury model. (Top panel) A view through the upper basal coil of the cochlea demonstrating degeneration (arrow) of the organ of Corti. Basilar membrane (BM). Tectorial membrane (TM), limbus (*). (Bottom panel) Blood in the scala tympani, absence of the tectorial membrane, and loss of ganglion cells (*). Scala media (SM). Reissner's membrane (RM)43
Figure 7.
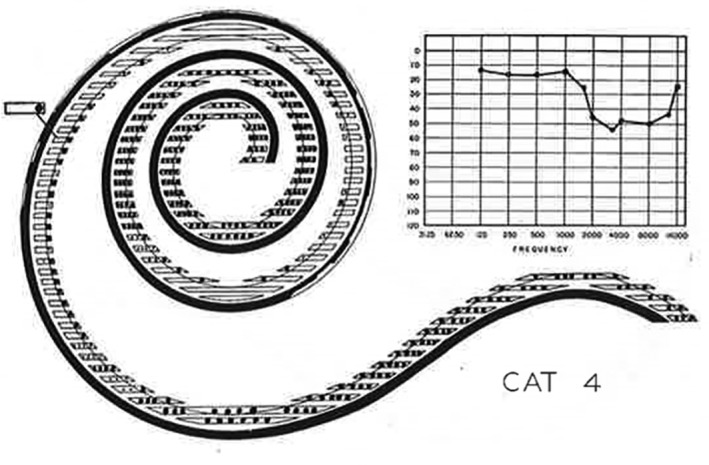
Behavioral audiogram and hair cell counts after head injury in a feline model. Representative illustration demonstrating both hair cell counts (left) and behavioral audiogram (right). Cat 4 was sacrificed 19 days after injury and had a 50 db hearing loss between 1500 and 16 000 Hz. On otopathologic examination, most of the outer hair cells were missing and many inner hair cells are injured or missing as well43
Figure 8.
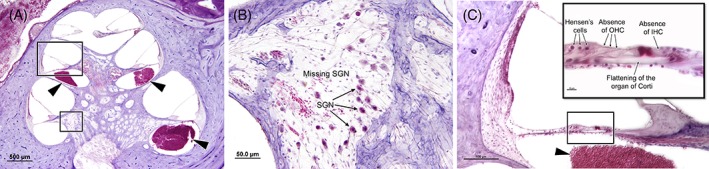
Low‐ and high‐power view of the cochlea following injury. A, Low‐power view of the cochlea, showing blood in the scala tympani of the basal and middle turns (arrowhead). B, High‐power view of the Rosenthal's canal presenting reduced population of cochlear ganglion neurons. C, High‐power view of the middle turn of the cochlea, showing flattening of the organ of Corti, with complete degeneration of the outer and inner hair cells. (Schuknecht et al. 1949, unpublished)
Figure 9.
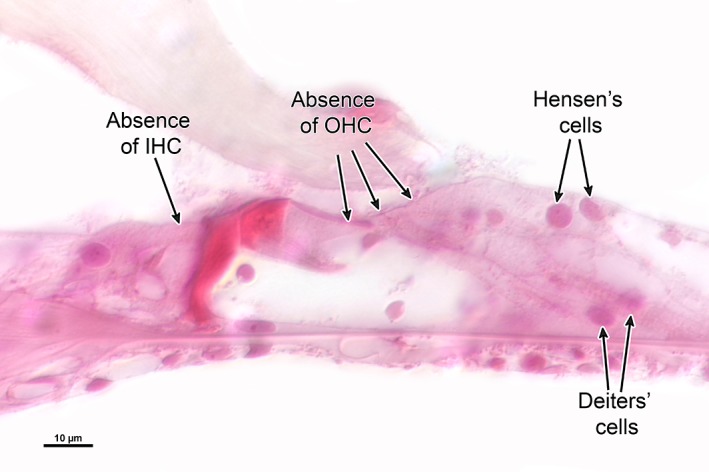
High‐power view of the cochlear upper basal turn. Photomicrograph shows severe disruption of the organ of Corti, with loss of inner and outer hair cells. (Schuknecht et al. 1949, unpublished)
By evaluation of the otopathology of the cats sacrificed at varying days after head injury, Schuknecht delineated successive stages of inner ear damage: (a) loss of external hair cells; (b) loss of external and internal hair cells; (c) flattening of the organ of Corti; and finally (d) complete disappearance of the organ of Corti. Schuknecht remarked that these changes were almost identical to previous otopathological studies from animal and human subjects exposed to intense airborne sound stimuli.52, 53, 54
Schuknecht built on Brunner's initial theory regarding labyrinthine fluid waves by theorizing that head injuries create pressure waves transmitted through bone to the cochlea, which in turn produces a “shock pulse” comparable to an airborne blast wave transmitted by a conductive mechanism to the inner ear. This pressure pulse leads to violent displacement of the basilar membrane, causing injury of the organ of Corti. Schuknecht was also puzzled with high frequencies being the most commonly involved in his experiment, in particular the range from 3 to 8 KHz. He considered some possible explanations for this finding, such as an inherent fragility in the upper region of the basal turn, or physical properties of the impulse.
Finally, cats euthanized 2 days after trauma had less profound otopathological changes than what would be expected given their severe hearing loss on audiometric evaluation. Schuknecht conjectured that this may be caused by an insufficient time for auditory threshold recovery, since he only observed recovery after 3 weeks in his other cats. From these observations, Schuknecht concluded that the severe audiometric changes were not reflected in histopathological specimens. He theorized that additional cellular changes caused by the injury were potentially too subtle to be detected by contemporary histopathological preparation of his era.
5. CONTEMPORARY RELEVANCE OF LABYRINTHINE CONCUSSION
The contemporary definition of labyrinthine concussion has a storied history based on human and animal otopathologic analysis. Although many definitions incorporated the hypothesized pathophysiology of labyrinthine concussion, those that followed and remain in use today are largely agnostic on pathophysiology.8 The current definition largely serves as a catchall phrase that incorporates all the theorized etiologies.
Although there has been additional experimentation since the mid‐20th century,55, 56, 57 the diagnosis of labyrinthine concussion remains “mysterious,” as described by Ulrich.37 The pathophysiology of labyrinthine concussion may remain elusive for a host of reasons: variable human and animal head injury models, rudimentary hematoxylin and eosin staining techniques, and application of outdated understanding of auditory physiology. Validated large and small animal models of head injury, such as trauamtic brain injury, are necessary to better understand the mechanisms of auditory dysfunction.58
Our understanding of inner ear pathologies has grown and now includes an enhanced understanding of several different pathologies, including synaptopathies. For example, the degeneration of synaptic connections has been demonstrated in numerous animal models and in postmortem human samples and is correlated with noise‐exposure that leads to tinnitus, hyperacusis, and varying levels of hearing loss. Interestingly, patients who have sustained mild head injuries also appear to present with similar complaints of tinnitus and hyperacusis. Although the concept of degeneration of synaptic connections and resultant tinnitus and hyperacusis has been shown to result from noise‐induced hearing loss, patients following head injury also appear to present with similar auditory complaints.59, 60 Our evolving understanding of cochlear synaptopathy may provide a new paradigm to frame mild auditory dysfunction and experimental protocols.61, 62
Additionally, the term “labyrinthine concussion” seems predominantly used in the otolaryngology and audiology literature, other medical specialties, such as neurology, neurosurgery, physical medicine, and rehabilitation, have also identified this phenomenon,63, 64, 65 and there may be central auditory pathway etiologies.66, 67, 68 Indeed, literature on TBI,69, 70, 71, 72 sports‐related concussion,59, 60, 73 as well as emerging literature on chronic traumatic encephalopathy (CTE)74 highlight potential auditory symptoms without fracture of the temporal bone. CTE was initially known as Punch Drunk Syndrome 75 and Dementia Pugilistica.76 These diagnoses described a range of neurologic symptoms commonly seen in boxers, such as tremors, slowed movements and speech, confusion, memory loss, and psychiatric issues.75, 77, 78 Auditory and vestibular symptoms have been associated with Punch Drunk Syndrome, Dementia Pugilistica, and CTE, since the earliest reports of these conditions by pathologists Martland and Millspaugh.75, 76 To date, however, discrete pathologic changes that may occur to the auditory pathway in individuals with CTE are unknown. There is no evidence of discourse among Martland, Millspaugh, and otopathologists of their era. Additionally, recent studies have linked hearing loss to decreased executive functioning and dementia.79, 80 Further research is needed to characterize the interplay between cognition and hearing loss.
Schuknecht thought that inner ear damage following head injury resembled the one caused by intense noise and/or blast impulse trauma. However, recent experimental studies of blast‐induced trauma in Chinchillas have demonstrated particular features of damage, including complete separation of outer hair cells, Deiter cells, and Hansen cells from the basal membrane,81 in addition to evidence of cochlear synaptopathy with focal damage in the midcochlear and basal regions of the cochlea,82 which differ somewhat from those findings reported in labyrinthine concussion studies. Even though blast injury and labyrinthine concussion may share some anatomical indicators of injury, it still does not fully explain all the histopathological changes.
Further, the high pressure wave caused by blast is mainly transmitted to the cochlea via air conduction,83 a blow to the head can potentially create a significant bone conducted pressure instead. Recent studies on vibratory stimuli transmission to the inner ear have shown a transcranial delay and attenuation when bone‐conducted sound crosses over the contralateral cochlea.84, 85 This same mechanism could potentially explain why blows to the contralateral side of the skull failed to produce deafness in Schuknecht's experiment, in which the bone conducted pressure may be attenuated. Cases of contralateral sensorineural hearing loss following head injury in the absence of TBF have been described.86, 87, 88, 89 It is thought to be secondary to contra‐coup labyrinthine concussion, in a very similar way that head injury can lead to cerebral concussion. Therefore, it should be taken in consideration that differences in mechanisms of injury could result in distinct patterns of inner ear damage as well.
Although our historical study focuses on the auditory pathway, we acknowledge vestibular dysfunction may also occur following head injury. Balance complaints are among the most commonly reported chronic symptoms following head trauma, with an incidence as high as 83%.90, 91, 92 Similar to the auditory system, many historical clinical studies and animal experiments have made attempts to better define this topic,16, 23, 44, 93, 94, 95, 96 but the pathogenesis remains unclear with a wide variation of clinical descriptions and histopathologic findings, including degeneration of the cristae and maculae sensory epithelia, disruption of otolithic membranes, loss of vestibular ganglion neurons, as well as the presence of fibrous tissue and new bone in perilymphatic spaces. Further studies on vestibular injury from head trauma are needed to elucidate its pathophysiology.
6. CONCLUSION
Over the past century a host of human and animal studies have explored peripheral and central audiovestibular pathology after head trauma. Although progress has been made to define abnormal otopathologic findings in head trauma, we are far from a mature understanding of how trauma results in audiovestibular symptoms. Otopathological studies that further contextualize the historical theories behind labyrinthine concussion with more contemporary explanations for auditory symptomology, such as cochlear synaptopathy, are needed. Additionally, clinical studies better characterizing the type, acuity, and severity of auditory injury following direct head impact may provide valuable tools for the accurate diagnosis and potential management of this patient population.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGMENTS
We would like to acknowledge Sachie Shishido and Louise Collins of the Leroy A. Schall Library of Otolaryngology at Massachusetts Eye and Ear Infirmary for their expert support of this historical study. We would also like to thank Anays Murillo for her help with translation of French language articles. Finally, we would like to thank Barbara Burgess, Diane Jones, and Meng Yu Zhu for locating the Harold Schuknecht's original slides at the Massachusetts Eye and Ear Infirmary.
Bartholomew RA, Lubner RJ, Knoll RM, et al. Labyrinthine concussion: Historic otopathologic antecedents of a challenging diagnosis. Laryngoscope Investigative Otolaryngology. 2020;5:267–277. 10.1002/lio2.360
Funding information American Academy of Otolaryngology CORE Grant
REFERENCES
- 1. Amberg E. Injuries to the head and ear disturbances. J Mich State Med Soc. 1914;13(84). [Google Scholar]
- 2. Helidonis ES. The history of otolaryngology from ancient to modern times. Am J Otolaryngol. 1993;14(6):382‐393. [DOI] [PubMed] [Google Scholar]
- 3. Breasted JH. New‐York historical society. Library The Edwin Smith Surgical Papyrus. Chicago, IL: The University of Chicago Press; 1930. [Google Scholar]
- 4. Cannon CR, Jahrsdoerfer RA. Temporal bone fractures. Review of 90 cases. Arch Otolaryngol. 1983;109(5):285‐288. [DOI] [PubMed] [Google Scholar]
- 5. Maillot O, Attye A, Boutet C, et al. The relationship between post‐traumatic ossicular injuries and conductive hearing loss: a 3D‐CT study. J Neuroradiol. 2017;44(5):333‐338. [DOI] [PubMed] [Google Scholar]
- 6. Healy GB. Hearing loss and vertigo secondary to head injury. N Engl J Med. 1982;306(17):1029‐1031. [DOI] [PubMed] [Google Scholar]
- 7. Merchant SN, Nadol JB. Schuknecht's pathology of the ear. PMPH‐USA. 2010;3:391‐392. [Google Scholar]
- 8. Chen JX, Lindeborg M, Herman SD, et al. Systematic review of hearing loss after traumatic brain injury without associated temporal bone fracture. Am J Otolaryngol. 2018;39(3):338‐344. [DOI] [PubMed] [Google Scholar]
- 9. Gradenigo G. Krankheiten des Labyrinths und des Nervus acusticus In: Schwartze H, ed. Handbuch der Ohrenheilkunde. Vol 2 Leipzig: Verlag von F.C.W. Vogel; 1893:352‐555. [Google Scholar]
- 10. Barnick O. Uber Brüche des Schadelgrundes und die durch sie bedingten Blutungen in das Ohrlabyrinth. Archiv für Ohrenheilkunde. 1897;43:23‐52. [Google Scholar]
- 11. Moos S. Four cases of gunshot wounds of the ear Archives of Opthalmology and Otology. Vol 2 New York: William Wood & Co; 1871:343‐356. [Google Scholar]
- 12. Wright W. An Essay on the Human Ear: its Anatomical Structure and Incidental Complaints. London: Printed for Longman, Hurst, Rees, Orme, and Brown, Paternoster‐Row, and T. and G. Underwood, Fleet‐Street; 1817. [Google Scholar]
- 13. Toynbee J. The Disease of the Ear: Their Nature, Diagnosis, and Treatment. London: H.K. Lewis; 1860. [Google Scholar]
- 14. Politzer A. Traumen des inneren Ohres Lehrbuch der Ohrenheilkunde. 4th ed. Stuttgart: Enke; 1901:645‐655. [Google Scholar]
- 15. Passow A. Verletzungen des schallempfindenden Apparates Die Verletzungen des Gehörorganes; Wiesbaden, Germany: Bergmann. 1905:118‐181. [Google Scholar]
- 16. Stenger P. Beitrag zur Kenntniss der nach Kopfverletzungen Auftretenden Veranderungen im inneren Ohr. Archiv Fur Ohrenheilkunde. 1909;79:43‐69. [Google Scholar]
- 17. Theodore E. Beitrag zur pathologie der labyrintherschütterung. Zeitsch f Ohrenheilk. 1910;61:299‐307. [Google Scholar]
- 18. Voss O. Gibt es eine labyrintherschütterung. Archiv Ohrenheilkunde‐Nasen Und Kehlkopf. 1934;137:264‐288. [Google Scholar]
- 19. Grove W. Skull fractures involving the ear: a clinical study of 211 cases. Laryngoscope. 1939;49(9):833 ‐ 870. [Google Scholar]
- 20. Ramadier C. Traumatismes de l'Oreille. Paris: Masson and Cie; 1937. [Google Scholar]
- 21. Mauthner O. Die traumatische Erkrangungen des inneren Ohres. Arch f Ohrenheilk. 1911;87(146). [Google Scholar]
- 22. Mellenger W. The base of the skull with particular reference to fractures. Ann Otol Rhinol Laryngol. 1938;47(291):291‐305. [Google Scholar]
- 23. Wittmaack K. Uber die traumatische Labyrinthdegeneration. Archiv Ohrenheilkunde Nasen Und Kehkopfheil. 1932;131:59‐124. [Google Scholar]
- 24. Brunner H. Disturbances of the function of the ear after concussion of brain. Laryngoscope. 1940;50(10):921‐949. [Google Scholar]
- 25. Oppenheim H. Lehrbuch d. Nervenkrank. 3rd ed.; Berlin, Germany: S Karger; 1902:1001. [Google Scholar]
- 26. Sakai K. Anatomische Befunde am menschlichen Gehörorgan nach basisfraktur. Archiv f Ohrenheilkunde. 1911;85:188‐197. [Google Scholar]
- 27. Schoenbauer B. Schaedelbasisbrueche. Handb d Neur d Ohres. 1928;2(1):327. [Google Scholar]
- 28. Cheatle A. Nine specimens of fracture through the temporal bone. Proc R Soc Med. 1908;1:10‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grove W. Otologic observations in trauma of the head: a clinical study based on forty‐two cases. Arch Otolaryngol. 1928;8(3):249‐299. [Google Scholar]
- 30. Brunner H. Pathologie und Klinik der Erkrankungen des inneren Ohres nach Stumpfen Schadeltraumen. Monatschrift Fur Ohrenheilkunde. 1925;59:697. [Google Scholar]
- 31. Koch J. Studien über Veränderungen des Gehörorgans, insbesondere Störungen der Innenohrfunktion nach Schadelunfallen mit und ohne Verletzung des Schlafenbeins. Archiv Ohrenheilkunde. 1933;137:105‐160. [Google Scholar]
- 32. Ricker G. Die Entstehung der pathologisch‐anatomischen Befunde nach Hirnerschutterüng im Abhängigkeit vom Gefässnervensystems des Hirns. Virchow's Arch f Path Anat. 1919;226. [Google Scholar]
- 33. Knauer AEE. Die Pathologische Physiologie der Hirnerschütterung. J F Psychol U Neurol. 1922;29. [Google Scholar]
- 34. Schuknecht HF. A clinical study of auditory damage following blows to the head. Ann Otol Rhinol Laryngol. 1950;59(2):331‐358. [DOI] [PubMed] [Google Scholar]
- 35. Proctor B, Gurdjian ES, Webster JE. The ear in head trauma. Laryngoscope. 1956;66(1):16‐59. [DOI] [PubMed] [Google Scholar]
- 36. Politzer A. Menierescher Symptomencomplex infolge traumatischer Labyrinthläsionen. Arch f Ohrenh. 1896;41:165‐175. [Google Scholar]
- 37. Ulrich K, Verletzungen d. Gehörorgans bei Schädelbasisfrakturen. Acta Otolaryngol. 1926;6(1). [Google Scholar]
- 38. Ulrich K. Klinische und anatomische Untersuchungen über Verletzungen des Gehörorgans bei Schädelbasisfrakturen. Schweiz Med Wochenschr. 1921;2:566‐567. [Google Scholar]
- 39. Ulrich K. Ueber Vagus, Fazialis‐ und Akusticusverletzungen. Schweiz Med Wchnschr. 1922;21:21. [Google Scholar]
- 40. Uffenorde W. Constations histologique‐contribution a l'etude de la surdite par commotion beitrag. Anat Phys Path Des Ohres. 1924;292‐324. [Google Scholar]
- 41. Nager F. Über Spätmeningitis nach Labyrinthfraktur. Acta Otolaryngol. 1930;14(127):127‐134. [Google Scholar]
- 42. Klingenberg A. Die isolierte Schneckenfraktur bei Schaedelbasisbruechen. Z Hals‐ Usw Heilk. 1929;22:452‐463. [Google Scholar]
- 43. Schuknecht HF, Neff WD, Perlman HB. An experimental study of auditory damage following blows to the head. Ann Otol Rhinol Laryngol. 1951;60(2):273‐289. [DOI] [PubMed] [Google Scholar]
- 44. Lindsay JR, Zajtchuk J. Concussion of the inner ear. Ann Otol Rhinol Laryngol. 1970;79(4):699‐709. [DOI] [PubMed] [Google Scholar]
- 45. Nassulphis P. Die Schädigung des Innenohres und Seiner Nerven nach Schädeltrauma. Monatsschr Ohrenh. 1946;79:68‐86 and 222‐252. [Google Scholar]
- 46. Manasse P. Zur Pathologischen Anatomie der Traumatischen Taubheit. Virchow's Arch f Path Anat. 1907;189:188‐209. [Google Scholar]
- 47. Alexander G. New histopathological findings in the ear in lues and their importance in the general pathology of the ear. Laryngoscope. 1928;38(5):295‐305. [Google Scholar]
- 48. Linthicum FaR C. Neuro‐otological observations in the concussion of the brain. Arch Otolaryngol. 1931;13(6):785‐821. [Google Scholar]
- 49. Linck A. Beitrag zur klinik und pathologie der schädelbasisfrakturen. Ztschr f Ohrenh. 1921;81:265‐306. [Google Scholar]
- 50. Schuknecht H, Davison, RC . Deafness and Vertigo from Head Injury. Detroit, Canada: AMA Arch Otolaryngology. 1956. [DOI] [PubMed] [Google Scholar]
- 51. Knoll RMLR, O'Malley J, Barholomew RA, et al. Contemporary interpretation of Harold Schuknecht's feline model of labyrinthine concussion. Registry Newslett. 2019;26(2):1‐5. [Google Scholar]
- 52. Perlman H. Process of healing injuries to the capsule of the labyrinth. Arch Otolaryngol. 1939;29:287‐305. [Google Scholar]
- 53. Lurie MHDH, Hawkins VE. Acoustic trauma of the organ of Corti in Guinea pigs. Laryngoscope. 1944;54:375‐386. [Google Scholar]
- 54. Lindquist SE. Stimulation Deafness: A Study of Temporary and Permanent Hearing Losses Resulting from Exposure to Noise and to Blast Impulses. Chicago, IL: Journal of Comparative and Physiological Psychology. 1949. [DOI] [PubMed] [Google Scholar]
- 55. Makishima K, Snow JB. Pathogenesis of hearing loss in head injury. Studies in man and experimental animals. Arch Otolaryngol. 1975;101(7):426‐432. [DOI] [PubMed] [Google Scholar]
- 56. Zhou D, Xu W, He L. Histopathology of nonacoustic labyrinth following head injury in Guinea pigs. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1994;29(6):350‐352. [PubMed] [Google Scholar]
- 57. Danielidis V, Tsimpiris N, Balatsouras DG, et al. Short‐term pathophysiologic changes and histopathologic findings of the auditory pathway after closed head injury, using a rabbit model. Audiol Neurootol. 2007;12(3):145‐154. [DOI] [PubMed] [Google Scholar]
- 58. Cullen DK, Harris JP, Browne KD, et al. A porcine model of traumatic brain injury via head rotational acceleration. Meth Mol Biol (Clifton, NJ). 2016;1462:289‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chorney SR, Suryadevara AC, Nicholas BD. Audiovestibular symptoms as predictors of prolonged sports‐related concussion among NCAA athletes. Laryngoscope. 2017;127(12):2850‐2853. [DOI] [PubMed] [Google Scholar]
- 60. Assi H, Moore RD, Ellemberg D, Hebert S. Sensitivity to sounds in sport‐related concussed athletes: a new clinical presentation of hyperacusis. Sci Rep. 2018;8(1):9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after "temporary" noise‐induced hearing loss. J Neurosci. 2009;29(45):14077‐14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liberman MC, Epstein MJ, Cleveland SS, Wang H, Maison SF. Toward a differential diagnosis of hidden hearing loss in humans. PLoS One. 2016;11(9):e0162726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ceranic BJ, Prasher DK, Raglan E, Luxon LM. Tinnitus after head injury: evidence from otoacoustic emissions. J Neurol Neurosurg Psychiatry. 1998;65(4):523‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lew HL, Jerger JF, Guillory SB, Henry JA. Auditory dysfunction in traumatic brain injury. J Rehabil Res Dev. 2007;44(7):921‐928. [DOI] [PubMed] [Google Scholar]
- 65. Oleksiak M, Smith BM, St Andre JR, Caughlan CM, Steiner M. Audiological issues and hearing loss among veterans with mild traumatic brain injury. J Rehabil Res Dev. 2012;49(7):995‐1004. [DOI] [PubMed] [Google Scholar]
- 66. Kraus N, Lindley T, Colegrove D, et al. The neural legacy of a single concussion. Neurosci Lett. 2017;646:21‐23. [DOI] [PubMed] [Google Scholar]
- 67. Kraus N, Thompson EC, Krizman J, Cook K, White‐Schwoch T, LaBella CR. Auditory biological marker of concussion in children. Sci Rep. 2016;6:39009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thompson EC, Krizman J, White‐Schwoch T, Nicol T, LaBella CR, Kraus N. Difficulty hearing in noise: a sequela of concussion in children. Brain Inj. 2018;32(6):763‐769. [DOI] [PubMed] [Google Scholar]
- 69. Munjal SK, Panda NK, Pathak A. Audiological deficits after closed head injury. J Trauma. 2010;68(1):13‐18. discussion 18. [DOI] [PubMed] [Google Scholar]
- 70. Emerson LP, Mathew J, Balraj A, Job A, Singh PR. Peripheral auditory assessment in minor head injury: a prospective study in tertiary hospital. Indian J Otolaryngol Head Neck Surg. 2011;63(1):45‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Singh G, Singh B, Singh D. Prospective study of otological injury secondary to head trauma. Indian J Otolaryngol Head Neck Surg. 2013;65(Suppl 3):498‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bergemalm PO. Progressive hearing loss after closed head injury: a predictable outcome? Acta Otolaryngol. 2003;123(7):836‐845. [DOI] [PubMed] [Google Scholar]
- 73. Wasserman EB, Kerr ZY, Zuckerman SL, Covassin T. Epidemiology of sports‐related concussions in National Collegiate Athletic Association Athletes from 2009‐2010 to 2013‐2014: symptom prevalence, symptom resolution time, and return‐to‐play time. Am J Sports Med. 2016;44(1):226‐233. [DOI] [PubMed] [Google Scholar]
- 74. Mez J, Solomon TM, Daneshvar DH, Stein TD, McKee AC. Pathologically confirmed chronic traumatic encephalopathy in a 25‐year‐old former college football player. JAMA Neurol. 2016;73(3):353‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Martland HS. Punch Drunk. JAMA. 1928;91(15):1103‐1107. [Google Scholar]
- 76. Millspaugh JA. Dementia pugilistica. US Naval Med Bull. 1937;35(297):e303. [Google Scholar]
- 77. Mawdsley C, Ferguson FR. Neurological disease in boxers. Lancet. 1963;2(7312):795‐801. [DOI] [PubMed] [Google Scholar]
- 78. Spillane JD. Five boxers. Br Med J. 1962;2(5314):1205‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lin FR, Metter EJ, O'Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68(2):214‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu CM, Lee CT. Association of Hearing Loss with Dementia. JAMA Netw Open. 2019;2(7):e198112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hamernik RP, Turrentine G, Roberto M, Salvi R, Henderson D. Anatomical correlates of impulse noise‐induced mechanical damage in the cochlea. Hear Res. 1984;13(3):229‐247. [DOI] [PubMed] [Google Scholar]
- 82. Hickman TT, Smalt C, Bobrow J, Quatieri T, Liberman MC. Blast‐induced cochlear synaptopathy in chinchillas. Sci Rep. 2018;8(1):10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cho SI, Gao SS, Xia A, et al. Mechanisms of hearing loss after blast injury to the ear. PLoS One. 2013;8(7):e67618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Eeg‐Olofsson M, Stenfelt S, Granstrom G. Implications for contralateral bone‐conducted transmission as measured by cochlear vibrations. Otol Neurotol. 2011;32(2):192‐198. [DOI] [PubMed] [Google Scholar]
- 85. Mattingly JK, Banakis Hartl RM, Jenkins HA, Tollin DJ, Cass SP, Greene NT. A comparison of Intracochlear pressures during ipsilateral and contralateral stimulation with a bone conduction implant. Ear Hear. 2019;41(2):312‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Toh A, Ho EC, Turner N. Contralateral deafness post head injury without temporal bone fractures. Am J Otolaryngol. 2010;31(1):54‐56. [DOI] [PubMed] [Google Scholar]
- 87. Mohd Khairi MD, Irfan M, Rosdan S. Traumatic head injury with contralateral sensorineural hearing loss. Ann Acad Med Singap. 2009;38(11):1017‐1018. [PubMed] [Google Scholar]
- 88. Villarreal IM, Mendez D, Silva JM, Del Alamo PO. Contralateral Cochlear labyrinthine concussion without temporal bone fracture: unusual posttraumatic consequence. Case Rep Otolaryngol. 2016;2016:2123182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ulug T, Ulubil SA. Contralateral labyrinthine concussion in temporal bone fractures. J Otolaryngol. 2006;35(6):380‐383. [DOI] [PubMed] [Google Scholar]
- 90. Griffiths MV. The incidence of auditory and vestibular concussion following minor head injury. J Laryngol Otol. 1979;93(3):253‐265. [DOI] [PubMed] [Google Scholar]
- 91. Gottshall KR, Gray NL, Drake AI, Tejidor R, Hoffer ME, McDonald EC. To investigate the influence of acute vestibular impairment following mild traumatic brain injury on subsequent ability to remain on activity duty 12 months later. Mil Med. 2007;172(8):852‐857. [DOI] [PubMed] [Google Scholar]
- 92. Jury MA, Flynn MC. Auditory and vestibular sequelae to traumatic brain injury: a pilot study. N Z Med J. 2001;114(1134):286‐288. [PubMed] [Google Scholar]
- 93. Nager FR. Beitrage zur histologic der erworbenen taubstumrnheit. Zeitsch f Ohren‐Heilk. 1907;101:217‐244. [Google Scholar]
- 94. Manasse P. Schadelbasis Irakture. Beitr Z Anat Physiol Path u Therap d Ohres. 1924;21:230. [Google Scholar]
- 95. Fischer J, Wolfsen LE. The Inner Ear. New York: Grune and Stratton; 1943. [Google Scholar]
- 96. Schuknecht HF, Davison RC. Deafness and vertigo from head injury. AMA Arch Otolaryngol. 1956;63(5):513‐528. [DOI] [PubMed] [Google Scholar]


