Abstract
The treatment by Advanced Oxidation Processes (AOPs) of wastewater polluted with dyes is of particular interest in the field of environmental engineering, especially for the removal azo-dyes, representing over 50% of the global annual production of dyes. Unfortunately, most azo-dyes are non-biodegradable and can be toxic to aquatic organisms. This is the first data article that applies the methodology of response surface for the optimization of decolorization of an azo-compound using cobalt in a homogeneous medium as the catalyst of a bicarbonate activated hydrogen peroxide (BAP) system which, in turn, is an emerging technology for wastewater treatment. The Response Surface Methodology (RSM) based on a Central Composite Design (CCD) was used to evaluate and optimize the influence of three experimental variables (stoichiometric dosage of H2O2, molar ratio H2O2/NaHCO3 and cobalt concentration) on the decolorization of Ponceau 4R. Reactions were performed at 25 °C, pH 8.3 with a reaction time of 2 h. Analysis of variance (ANOVA) showed values of R2 and adjusted-R2 of 0.9815 and 0.9648, and experimental data were fit to a second-order regression model. The optimal conditions to achieve a maximum decolorization (96.31%) of a Ponceau 4R aqueous solution of 20 mg/l were: 4.73 times stoichiometric dosage of H2O2, molar ratio H2O2/NaHCO3 of 1.70 and cobalt concentration of 11.16 µM. Under the optimal reaction conditions, the influence of temperature (20, 25, 30 and 35 °C) on decolorization was evaluated and data were adjusted to second order kinetics. To verify the efficiency of the BAP system on the decolorization of Ponceau 4R, under the optimal conditions of reaction, UV–Vis spectra, at different reaction times, were measured. Additionally, blank experiments in order to evaluate the effect of individual factors in the Ponceau 4R decolorization, using BAP system, were carried out. Data showed that the Co(II)-NaHCO3-H2O2 system is a suitable technology for the decolorization of azo-dyes aqueous solutions.
Keywords: Ponceau 4R, Azo-dye, Central composite design – CCD, Bicarbonate activated hydrogen peroxide – BAP, Decolorization
Specifications table
| Subject | Chemical Engineering Environmental Science |
| Specific subject area | Catalysis AOPs are treatment technologies designed to oxidized recalcitrant organic matter in water and wastewater through reaction with hydroxyl radicals |
| Type of data | Table Image Figure |
| How data were acquired | Oxidation reactions were performed at 25 °C for 2 h and atmospheric pressure of 78 kPa in a jacketed glass batch reactor (500 ml) under continuous stirring at 300 rpm. The reactor was loaded with 200 ml aqueous solution of Ponceau 4R at 20 mg/l and specific amounts of Cl2Co·6H2O and NaHCO3. The reaction started when H2O2 was added (t = 0). All experimental data were manually recorded. Decolorization was calculated from the initial and final concentrations of Ponceau 4R. The dye concentration was determined by using a UV–Vis spectrophotometer (Mapada UV-1200) at 507 nm wavelength. A Central Composite Design (CCD) was used to evaluate and optimize three variables on decolorization of Ponceau 4R: times the stoichiometric dosage of H2O2, molar ratio H2O2/NaHCO3 and cobalt concentration. 20 experiments with 3 factors and 5 levels for each factor were established. The interaction effects and optimal parameters were obtained by using a Design-Expert 8.0 software (StatEase, Inc., Minneapolis, USA). An analysis of variance (ANOVA) with 95% confidence level was carried out to identify the significance of independent variables (factors) and their interactions. The kinetic parameters of decolorization were determined by using the BAP system at four different temperatures (20, 25, 30 y 35 °C). Experimental data was fitted to a second-order model with a SciLab-6.0.2 (SciLab Entreprises SAS) function “lsqsolve”. All graphics were obtained by using an OriginPro 8.0Ⓡ software (OriginLab Corporation, USA) and Microsoft Excel. |
| Data format | Raw Analyzed |
| Parameters for data collection | The effects of experimental parameters on decolorization by the BAP system were examined with CCD. 3 factors (H2O2, nH2O2/nNaHCO3 and evaluation of Co(II) concentration). The ranges of variables were: from 1.5 to 4.5 times the stoichiometric dosage of H2O2, molar ratio H2O2/NaHCO3 from 0.8 to 2.0 and cobalt concentration from 5 to 15 µM. Kinetic parameters for the decolorization under optimal condition at four different temperatures (20, 25, 30 y 35 °C) were determined. |
| Description of data collection | Data has information about Ponceau 4R decolorization using the Co(II)-NaHCO3-H2O2 system, to identify the significance and interactions of three factors of the decolorization process using a CCD. Reactions were carried out with an initial dye concentration of 20 mg/l and pH 8.3 under mild conditions (atmospheric pressure and 25 °C) for 2 h of reaction time, using the BAP system catalysed by cobalt. The supplementary material contains an Excel File with all data of Ponceau 4R decolorization using BAP system. |
| Data source location | Universidad Nacional de Colombia sede Manizales Manizales city Colombia 5° 01′ 45′’ N, 75° 28′ 21′’ W |
| Data accessibility | With the article |
Value of the data
-
•
This is the first experimental design applying a bicarbonate activated hydrogen peroxide (BAP) system that allowed the development of an empiric model for decolorization of an azo dye (Ponceau 4R). The quadratic model obtained through RSM is adequate to predict the catalytic decolorization of Ponceau 4R in the range of experimental conditions used.
-
•
These experimental data can be useful for the development and application of advanced oxidation processes for water and wastewater treatment, with the advantage that the BAP system is a simple, inexpensive alternative and can be used at neutral or basic pH.
-
•
Data of the optimal decolorization conditions of Ponceau 4R can be used in equilibrium, kinetics, and mechanism studies of the oxidation of azo-dyes using the BAP system. Additionally, the use of a surface response methodology to establish the effect of variables governing the BAP system, can useful for new research on water and wastewater decolorization.
-
•
Data will be useful to researchers and the scientific community interested in the development and application of BAP technology for the treatment of wastewater containing azo-dyes.
1. Data description
The dataset contains eight Tables and six Figures. Data in Table 1 gives information about some properties of Ponceau 4R dye. The experimental conditions reported in literature, for the decolorization of organic colorants, using the BAP system are shown Table 2. Table 3 shows the levels of independent variables (factors) used in the experimental design for the decolorization of Ponceau 4R. The codified and experimental values of runs performed in the experimental design, with the decolorization obtained, are shown in Table 4. Table 5 summarizes ANOVA for the fitted quadratic model of Ponceau 4R decolorization. Experimental and predicted decolorization data of Ponceau 4R are shown in Fig 1. Fig. 2(a)–(c) display, by 3D graphics, the effect of interactions on the process variables of decolorization. Validation data of the empirical model for the Ponceau 4R decolorization, using the BAP system, are presented in Table 6. UV–Vis absorption spectra of aqueous solution of dye as a function of the reaction time are shown in Fig. 3. Fig. 4 illustrates the decolorization data at optimal reaction conditions and blank tests. Total organic carbon (TOC) and total nitrogen (TN) removals for decolorization of Ponceau 4R at optimal conditions and blank tests are summarized in Table 7. The monitoring of decolorization as a function of reaction time under optimal conditions, at four different temperatures are shown in Fig. 5. Fig. 6 represents Arrhenius linear relationship between ln(k) and 1/T(K). Table 8 shows the kinetic parameters of the second-order model fit and the coefficient of determination (R2) for the Ponceau 4R decolorization using BAP system at different temperatures.
Table 1.
General properties of Ponceau 4R [1].
| Characteristic/Property | Value |
|---|---|
| IUPAC name | Trisodium (8Z)−7-oxo-8-[(4-sulfonatonaphthalen-1-yl)hydrazinylidene]naphthalene-1,3-disulfonate |
| Synonym | Red Ponceau 4R, Acid Red 18, New Coccine, Ponceau 4 R |
| C.I. number | 16,255 |
| CAS number | 2611–82–7 |
| Molecular structure | 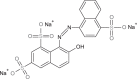 |
| Molecular formula | C20H11N2Na3O10S3 |
| Molar mass | 604.473 g/mol |
| λmax (nm) | 507 |
| Classification | Azo (monoazo) |
Table 2.
BAP system parameters used by other authors in the literature for the decolorization of organic colorants.
| Dye | H2O2, (mM) | H2O2, (SD) | NaHCO3, (mM) | nH2O2/nNaHCO3 | Co(II), (µM) | Ref. |
|---|---|---|---|---|---|---|
| Methylene blue | 20 | 3 | 25 | 0.8 | 20 | [3] |
| Reactive brilliant red X-3B | 4 | 1 | 10 | 0.4 | 5 | [4] |
| Methylene blue, X-3B, methyl orange, rodhamine B | 10 | 4 | 10 | 1.0 | 10 | [5] |
| Orange II | 4 | 2 | 10 | 0.4 | 5 | [6] |
| Orange II | 10 | 0.5 | 2.5 | 4.0 | 5 | [7] |
SD = Times the stoichiometric dosage of H2O2.
Table 3.
Levels of independent variables used in the CCD.
| Independent variable | Factor coded | Level |
||||
|---|---|---|---|---|---|---|
| −1.682 | −1 | 0 | +1 | +1.682 | ||
| Times the stoichiometric dosage of H2O2, (SD) | X1 | 0.477 | 1.5 | 3 | 4.5 | 5.523 |
| Molar ratio of H2O2 and NaHCO3, (nH2O2/nNaHCO3) | X2 | 0.391 | 0.8 | 1.4 | 2 | 2.409 |
| Cobalt concentration, (µM) | X3 | 1.591 | 5 | 10 | 15 | 18.409 |
Table 4.
Codified and experimental values of runs performed in the experimental design.
| Run Number | Codified Values |
Experimental Values |
Decolorization, (%) | ||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | H2O2, (SD) | nH2O2/nNaHCO3 | Co(II), (µM) | ||
| 1 | 1.682 | 0 | 0 | 5.52 | 1.4 | 10 | 96.31 |
| 2 | 0 | 1.682 | 0 | 3 | 2.41 | 10 | 77.64 |
| 3 | 0 | 0 | −1.682 | 3 | 1.4 | 1.59 | 64.74 |
| 4 | 0 | 0 | 1.682 | 3 | 1.4 | 18.41 | 85.83 |
| 5 | 0 | 0 | 0 | 3 | 1.4 | 10 | 84.55 |
| 6 | −1 | 1 | −1 | 1.5 | 2 | 5 | 40.85 |
| 7 | 0 | 0 | 0 | 3 | 1.4 | 10 | 89.66 |
| 8 | 0 | 0 | 0 | 3 | 1.4 | 10 | 86.13 |
| 9 | 1 | 1 | −1 | 4.5 | 2 | 5 | 87.73 |
| 10 | −1 | 1 | 1 | 1.5 | 2 | 15 | 54.60 |
| 11 | 0 | 0 | 0 | 3 | 1.4 | 10 | 87.41 |
| 12 | 1 | 1 | 1 | 4.5 | 2 | 15 | 91.72 |
| 13 | −1 | −1 | −1 | 1.5 | 0.8 | 5 | 51.65 |
| 14 | −1 | −1 | 1 | 1.5 | 0.8 | 15 | 65.81 |
| 15 | 0 | 0 | 0 | 3 | 1.4 | 10 | 85.07 |
| 16 | −1.682 | 0 | 0 | 0.48 | 1.4 | 10 | 28.28 |
| 17 | 0 | 0 | 0 | 3 | 1.4 | 10 | 88.73 |
| 18 | 1 | −1 | −1 | 4.5 | 0.8 | 5 | 85.28 |
| 19 | 1 | −1 | 1 | 4.5 | 0.8 | 15 | 85.99 |
| 20 | 0 | −1.682 | 0 | 3 | 0.39 | 10 | 70.13 |
SD = Times the stoichiometric dosage of H2O2.
Table 5.
ANOVA for response surface quadratic model for decolorization of Ponceau 4R using the BAP system.
| Source | Sum of square | Df | Mean square | F value | p-value |
|---|---|---|---|---|---|
| X1 | 4658.18 | 1 | 4658.18 | 378.385 | <0.0001 |
| X2 | 0.11 | 1 | 0.11 | 0.009 | 0.9281 |
| X3 | 339.37 | 1 | 339.37 | 27.567 | 0.0004 |
| X1X2 | 113.93 | 1 | 113.93 | 9.255 | 0.0124 |
| X1X3 | 67.34 | 1 | 67.34 | 5.470 | 0.0414 |
| X2X3 | 1.03 | 1 | 1.03 | 0.084 | 0.7783 |
| X12 | 1042.42 | 1 | 1042.42 | 84.676 | <0.0001 |
| X22 | 279.92 | 1 | 279.92 | 22.738 | 0.0008 |
| X32 | 220.58 | 1 | 220.58 | 17.917 | 0.0017 |
| Model | 6517.77 | 9 | 724.20 | 58.827 | <0.0001 |
| Residual | 123.11 | 10 | 12.31 | ||
| Lack of Fit | 102.42 | 5 | 20.48 | 4.951 | 0.052 |
| Pure Error | 20.69 | 5 | 4.14 |
Fig. 1.
Correlation between the experimental and predicted data for decolorization of Ponceau 4R using BAP system.
Fig. 2.
3D surface plot for interaction effect of catalytic decolorization on (a) H2O2 dosage vs nH2O2/nNaHCO3 (b) H2O2 dosage vs cobalt concentration (c) nH2O2/nNaHCO3 vs cobalt concentration.
Table 6.
Tests for the validation of the experimental design.
| Experimental Values |
Decolorization |
Error, (%) | |||
|---|---|---|---|---|---|
| H2O2, (SD) | nH2O2/nNaHCO3 | Co(II), (µM) | Predicted, (%) | Experimental, (%) | |
| 1.98 | 1.56 | 5 | 58.45 | 61.37 ± 0.97 | 5.06 |
| 4.00 | 1.00 | 5 | 85.12 | 89.42 ± 0.08 | 5.04 |
| 4.50 | 1.40 | 5 | 90.86 | 91.94 ± 0.04 | 1.18 |
| 4.50 | 2.00 | 10 | 96.14 | 94.11 ± 0.30 | 2.11 |
Fig. 3.
UV–Vis absorption spectra of Ponceau 4R solution during the reaction time under the optimal conditions.
Fig. 4.
Decolorization data at optimal reaction conditions and blank tests for Ponceau 4R decolorization.
Table 7.
TOC and TN removals for decolorization of Ponceau 4R at optimal conditions and blank tests.
| Test | Reaction conditions | Removal, (%) |
|
|---|---|---|---|
| TOC | TN | ||
| Co(II) + NaHCO3 + H2O2 (optimal conditions) |
BAP system under optimal conditions: Co(II) concentration of 11.16 µM, 4.73 times the stoichiometric H2O2 dosage and 1.69 of molar ratio H2O2/NaHCO3. | 13.91±1.04 | 19.63±0.78 |
| Co(II) + NaOH + H2O2 (Blank test) |
This test was performed in the absence of NaHCO3 adjusting the pH of dye solution (20 mg/l) to 8.3 through the addition of 0.1 M NaOH. Co(II) concentration and H2O2 dosage were 11.16 µM and 4.73 times the stoichiometric dosage. | 0.64±0.13 | 1.2 ± 0.47 |
| Co(II) + H2O2 (Blank test) |
This test was performed in the absence of NaHCO3 (pH was not controlled). The dye solution (20 mg/l) was added with a Co(II) concentration of 11.16 µM and 4.73 times the stoichiometric dosage of H2O2. | ND | ND |
| H2O2 + NaHCO3 (Blank test) |
This test was performed in the absence of Co(II). The dye solution (20 mg/l) was added with 4.73 times the stoichiometric dosage of H2O2 and an amount of NaHCO3 that guaranteed an nH2O2/nNaHCO3 of 1.70. | ND | ND |
ND: Not detected.
Fig. 5.
Normalized concentration of Ponceau 4R (Ct/C0) as a function of reaction time (t) at four different temperatures (a) 20 °C (b) 25 °C (c) 30 °C (b) 35 °C.
Fig. 6.
Arrhenius plot for the apparent second order rate constant.
Table 8.
Kinetic parameters obtained after fitting for the second order model.
| T(°C) | k (l mol−1 s−1) | R2 |
|---|---|---|
| 20 | 44.147 | 0.9860 |
| 25 | 73.051 | 0.9762 |
| 30 | 96.182 | 0.9951 |
| 35 | 116.160 | 0.9711 |
2. Experimental design, materials and methods
2.1. Materials
Ponceau 4R (89 wt%) was a reagent food-grade purchased from Retema S.A.S.-Colombia, whose properties are summarized in Table 1. A stock solution of Ponceau 4R (20 mg/l) was made up by accurately dissolving a weighed quantity of the dye in double-distilled water. Cl2Co•6H2O, NaOH and NaHCO3 were of analytical grade, obtained from Merck KGaA (Darmstadt, Germany), while H2O2 (30 wt%) were obtained from Sigma–Aldrich (Saint Louis, MO, USA). 100 ml a stock solution of Co(II) (4000 µM) was made up by dissolving 95.2 mg of Cl2Co•6H2O in double-distilled water, and aliquots of this solution (between 250 and 920 µl) were added to the reactor to obtain the required concentration of cobalt.
2.2. Catalytic decolorization tests
The decolorization catalytic reaction was performed in a batch glass reactor, open to atmosphere, thermostated at 25 °C, under constant magnetic stirring at 300 rpm. For each test, the reactor was loaded with 200 ml of aqueous solution at 20 mg/l, plus specific amounts of NaHCO3 and Co(II). Then, the total H2O2 dosage was added to start the reaction (t = 0). The dosage of H2O2 was varied in multiples of stoichiometry amount, which is theoretically required to completely oxidize one mole of Ponceau 4R into CO2, water (H2O) and mineral acids, according to Eq. (1):
| (1) |
Decolorization was measured by monitoring the absorbance of dye in the aqueous medium at its respective maximum absorption wavelength (λmax = 507 nm), using a UV–Vis spectrophotometer (Mapada UV-1200, China). The concentration interval went from 0 to 20 mg/l, with a correlation coefficient (R2) of 0.9993. Detection limit (DL) and quantification limit (QL) were 0.12 mg/l and 0.36 mg/l, respectively. The decolorization was calculated from Eq. (2):
| (2) |
where C0 is the dye concentration at t = 0 and Ct is the dye concentration at time t.
2.3. Experimental design
The central composite design (CCD) is the most popular class of response surface design methodology used for fitting second-order models in the design of experiments [2]. The CCD was used in this work, considering the minimum and maximum levels for H2O2 (from 1.5 to 4.5 times the stoichiometric dosage -SD-), molar ratio of H2O2/NaHCO3 (from 0.8 to 2) and cobalt concentration (from 5 to 15 µM). The ranges considered for the three independent variables were chosen from data reported by others authors in the literature (Table 2).
Table 3 shows the description of experimental ranges and the relationship between codified and real values [8]. Low and high levels are denoted by −1 and +1, respectively, and the central points as 0. The ±α value depends on the number of variables and, for three variables, it is ±1.682 [8].
The list of the 20 experimental runs and decolorization values are shown in Table 4. The run corresponding to the central point was performed six times (run 5, 7, 8, 11, 15 and 17).
Data analysis of variance (ANOVA), using Design Expert software version 8.0 (StatEase, Inc., Minneapolis, USA) for Ponceau 4R decolorization with 95% confidence level are show in Table 5.
The quadratic model for catalytic decolorization of Ponceau 4R can be described by Eq. (3):
| (3) |
The coefficients of the response model R2 and adjusted-R2 were 0.9815 and 0.9648, respectively.
Fig. 1 shows the correlation between the experimental and predicted data for decolorization of Ponceau 4R using BAP system. Fig. 2 shows the 3D surface generated by Eq. (3) and the influence of variables analyzed in the decolorization. By means mathematical optimization of the model (maximization of a Eq. (3) occurs where its derivative is equal to zero), the values of variables to achieve the maximum decolorization (98.13%) were determined, corresponding to 4.73 times the stoichiometric dosage of H2O2, 1.70 of nH2O2/nNaHCO3 and 11.16 µM of cobalt concentration. After carrying out the decolorization reaction under optimal conditions, a decolorization experimental of 96.46 ± 0.166% (error 1.70%) was obtained.
Additional catalytic decolorization tests, under the optimal operating conditions, were carried out in order to validate the quadratic model. The experimental and predicted values with Eq. (3) are shown in Table 6.
2.4. Decolorization monitoring using UV–Vis spectra
The efficiency of the Co(II)-NaHCO3-H2O2 system for Ponceau 4R decolorization under the optimal conditions was evaluated by measuring the changes of absorption UV–Vis spectra as a function of the reaction time, and the data are displayed in Fig. 3.
2.5. Blank tests
Blank tests, without H2O2, NaHCO3 and Co(II), were performed in order to evaluate the effect of individual factors in the Ponceau 4R decolorization (Fig. 4) under the optimal conditions of the experimental design. Blank tests descriptions are summarized in Table 7. Besides, the total organic carbon (TOC) and total nitrogen (TN) removals were estimated for each test. The TOC and TN removals were calculated using the Eqs. (4) and (5):
| (4) |
| (5) |
where TOC0, TOCf, TN0 and TNf are the TOC and TN contents at the beginning and end of the reaction (Fig. 4). The contents of TOC and TN were determined by using a TOC/TN analyzer (Multi N/C 3100, Analytik Jena AG, Germany).
2.6. Kinetics of decolorization
Data obtained for the normalized concentration of Ponceau 4R (Ct/C0) versus time (t), at four different temperatures (20, 25, 30 and 35 °C), are summarized in Fig. 6. Such data were adjusted to second order kinetics [9], [10]–11], according to Eqs. (5) and (6):
| (5) |
Rearranging Eq. (5):
| (6) |
were k is apparent second order rate constant.
Experimental data Ct/C0 was fitted to the model described in Eq. (5). The values of constant k as a function temperature (T) (Table 8) were obtained by using the Levenberg-Marquardt algorithm [12]. The Scilab-6.0.2Ⓡ function “lsqrsolve” that minimizes the sum of square differences between experimental and predicted values of the nonlinear kinetic function, for each temperature evaluated, was used. The dependence of k values from the reciprocal of absolute temperature (1/T) is shown in Fig. 6. The calculated apparent activation energy (Ea) from the Arrhenius plot regression (Fig. 6) was 47.88 kJ/mol, a value similar to that obtained in the degradation of Acid Orange 7 by catalytic wet hydrogen peroxide oxidation (Ea = 47.30 kJ/mol) [12].
Acknowledgments
Authors gratefully acknowledge the Administrative Department of Science, Technology and Innovation (Colciencias) and Universidad Nacional de Colombia sede Manizales (project DIMA-UNAL Code 45478) for supplying the resources for this scientific research.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dib.2020.105463.
Appendix. Supplementary materials
References
- 1.National Center for Biotechnology Information, PubChem Database. Compound summary Ponceau 4R. https://pubchem.ncbi.nlm.nih.gov/compound/Ponceau-4R, 2020(accessed 17 January 2020).
- 2.Sarrai A.E., Hanini S., Merzouk N.K., Tassalit D., Szabó T., Hernádi K., Nagy L. Using central composite experimental design to optimize the degradation of Tylosin from aqueous solution by photo-Fenton reaction. Materials (Basel) 2016;9:1–11. doi: 10.3390/ma9060428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu A., Li X., Ye S., Yin G., Zeng Q. Catalyzed oxidative degradation of methylene blue by in situ generated cobalt (II)-bicarbonate complexes with hydrogen peroxide. Appl. Catal. B-Environ. 2011;102:37–43. doi: 10.1016/j.apcatb.2010.11.022. [DOI] [Google Scholar]
- 4.Yang Z., Wang H., Chen M., Luo M., Xia D., Xu A., Zeng Q. Fast degradation and biodegradability improvement of reactive brilliant red X-3B by the cobalt(II)/bicarbonate/hydrogen peroxide system. Ind. Eng. Chem. Res. 2012;51:11104–11111. doi: 10.1021/ie301334y. [DOI] [Google Scholar]
- 5.Li X., Xiong Z., Ruan X., Xia D., Zeng Q., Xu A. Kinetics and mechanism of organic pollutants degradation with cobalt–bicarbonate–hydrogen peroxide system: investigation of the role of substrates. Appl. Catal. A-Gen. 2012;411-412:24–30. doi: 10.1016/j.apcata.2011.10.016. [DOI] [Google Scholar]
- 6.Long X., Yang Z., Wang H., Chen M., Peng K., Zeng Q., Xu A. Selective degradation of orange II with the cobalt(II)–bicarbonate–hydrogen peroxide system. Ind. Eng. Chem. Res. 2012;51:11998–12003. doi: 10.1021/ie3013924. [DOI] [Google Scholar]
- 7.Luo M., Lv L., Deng G., Yao W., Ruan Y., Li X., Xu A. The mechanism of bound hydroxyl radical formation and degradation pathway of acid orange II in fenton-like Co2+-HCO3− system. Appl. Catal. A Gen. 2014;469:198–205. doi: 10.1016/j.apcata.2013.09.045. [DOI] [Google Scholar]
- 8.Herney-Ramirez J., Lampinen M., Vicente M.A., Costa C.A., Madeira L.M. Experimental design to optimize the oxidation of orange II dye solution using a clay-based fenton-like catalyst. Ind. Eng. Chem. Res. 2008;47:284–294. doi: 10.1021/ie070990y. [DOI] [Google Scholar]
- 9.Ahmadi M., Behin J., Mahnam A.R. Kinetics and thermodynamics of peroxydisulfate oxidation of reactive yellow 84. J. Saudi Chem. Soc. 2016;20:644–650. doi: 10.1016/j.jscs.2013.07.004. [DOI] [Google Scholar]
- 10.Xu H.-Y., Liu W.-C., Qi S.-Y., Li Y., Zhao Y., Li J.-W. Kinetics and optimization of the decoloration of dyeing wastewater by a schorl-catalyzed fenton-like reaction. J. Serb. Chem. Soc. 2014;79:361–377. doi: 10.2298/JSC130225075X. [DOI] [Google Scholar]
- 11.Santana C.S., Nicodemos Ramos M.D., Vieira Velloso C.C., Aguiar A. Kinetic evaluation of dye decolorization by Fenton processes in the presence of 3-hydroxyanthranilic acid. Int. J. Environ. Res. Public Health. 2019;16:1602. doi: 10.3390/ijerph16091602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herney-Ramirez J., Silva A.M.T., Vicente M.A., Costa C.A., Madeira L.M. Degradation of acid orange 7 using a saponite-based catalyst in wet hydrogen peroxide oxidation: kinetic study with the Fermi's equation. Appl. Catal. B Environ. 2011;101:197–205. doi: 10.1016/j.apcatb.2010.09.020. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








