Abstract
We have derived single-chain variable fragments (scFv) from tau antibody hybridomas and previously shown their promise as imaging diagnostic agents. Here, we examined the therapeutic potential of anti-tau scFv in transgenic Drosophila models that express in neurons wild-type (WT) human tau (htau) or the human tauopathy mutation R406W. scFv expressing flies were crossed with the tauopathy flies and analyzed. Overall, the survival curves differed significantly (p<0.0001). Control flies not expressing htau survived the longest, whereas R406W expressing flies had the shortest live span, which was greatly prolonged by co-expressing the anti-tau scFv (p<0.0001). Likewise, htau WT expressing flies had a moderately short live span, which was prolonged by co-expressing the anti-tau scFv (p<0.01). In addition, the htau expression impaired wing expansion after eclosion (p<0.0001), and caused progressive abdomen expansion (p<0.0001). These features were more severe in htau R406W flies than in htau WT flies. Importantly, both phenotypes were prevented by co-expression of the anti-tau scFv (p<0.01–0.0001). Lastly, brain analyses revealed scFv-mediated tau clearance (p<0.05–0.01), and its prevention of tau-mediated neurotoxicity (p<0.05–0.001).
In summary, these findings support the therapeutic potential of an anti-tau scFv, including as gene therapies, and the use of Drosophila models for such screening.
Introduction
Immunotherapies targeting various protein aggregates such as amyloid-β (Aβ), tau and α-synuclein are in different stages of clinical development, and are collectively the most common approach taken by the pharmaceutical industry to tackle diseases characterized by such depositions 11,36,39,44. The majority of these approaches involve whole antibodies and much less attention has been paid to antibody fragments, which have certain advantages that justify further exploration of their therapeutic and diagnostic potential.
The majority of tau-targeting therapies in clinical trials are immunotherapies. Of the nine ongoing clinical trials (for review see 11,39), seven are passive (whole antibodies) and two are active (peptide immunogens). This approach was originally based on successful studies in mouse tauopathy models 3,4, which were confirmed and extended by multiple laboratories (for review see 11,39). The use of antibody fragments in this context is less developed but these entities have certain advantages that supports their further development. Previously, we proposed a unique approach to image tau aggregates in vivo, using single chain variable antibody fragments (scFvs), administered intravenously 24. Importantly, the degree of brain signal correlated very well with tau pathology, indicating the diagnostic promise of this approach. Published findings from us and others show that tau antibodies are primarily taken up into neurons by receptor-mediated uptake, whereas antibody fragments (Fabs and scFvs) are taken up by bulk-mediated endocytosis 3,9,19,22–24. All co-localize with tau aggregates within the neurons and more of the fragments get into neurons than antibodies, presumably because of their smaller size 3,7,9,19,23,24.
The similarities in target engagement between antibodies and their fragments, namely binding to intraneuronal tau aggregates in the brain after peripheral injection, suggests that the fragments may also have therapeutic potential. However, this idea has not been well explored. Most of the studies testing the therapeutic potential of scFvs in Alzheimer’s models have targeted Aβ. Several reports show positive effects of therapeutic targeting of Aβ with scFvs 5,13–18,25,26,29,31,35,46,50. Interestingly, two of these articles show the feasibility of screening anti-Aβ scFvs for efficacy in Drosophila 13,29. Less work has been done targeting tau with scFvs. Initial work showed their in vivo and in vitro binding to pathological tau 24,42, and more recent reports have shown their therapeutic potential 21,32,41,45.
Here we demonstrate in Drosophila models the therapeutic potential of a particular scFv, that we have previously reported to have a diagnostic imaging potential 24. Its parent antibody, 6B2, with identical complementarity-determining regions (CDRs) is ineffective in clearing tau or preventing its toxicity in culture or in in vivo models 10,49. Transgenic expression of anti-tau scFvs is an efficient way to determine their efficacy in relevant tauopathy models and can support future gene therapy approaches to target pathological tau aggregates.
Results
Transgene expression in fly models
We had previously reported on the diagnostic potential of the anti-tau antibody fragment scFv235 24. To test the efficacy of this fragment in Drosophila melanogaster models of tauopathy, we used previously described transgenic lines 47. In these models, human tau (htau) genes either of wild type sequence or with an R406W mutation that underlies a familial form of frontotemporal dementia 20 are expressed in Drosophila neurons through the neuronal specific elav-GAL4 driver [34]. We generated transgenic UAS-scFv235 flies and crossed them with the elav-GAL4 driven tauopathy flies for co-expression to assess scFv efficacy in preventing tauopathy-induced toxicity and mortality.
First, we examined on western blots of fly heads expression of total tau (Tau-5) and hyperphosphorylated tau (PHF-1) in control flies, and in flies expressing wild type or R406W mutated human tau (Figure 1). As expected, control flies had no tau expression whereas the tauopathy models showed strong tau expression and tau hyperphosphorylation. Likewise, analysis of the scFv235 flies verified their scFv235 expression.
Figure 1: Verification of tau and scFv235 expression in Drosophila.
(A) Western blots of total tau (Tau-5) and hyperphosphorylated tau (PHF1) in (1) naïve control flies, and flies expressing wild-type (2) and R406W mutated (3) human tau in neurons (neuronal promoter elav-Gal4). Naïve flies have no tau expression whereas the tauopathy models show strong tau expression. (B) Anti-tau scFv235 expressed in Drosophila. This Tg model was crossed to the tauopathy models to assess scFv efficacy in preventing tauopathy-induced mortality.
To further examine neuronal expression of tau and scFv235, confocal imaging of brain sections from 3 weeks old flies stained for total tau (tau-5), hyperphosphorylated tau (PHF-1) and the scFv revealed neuronal cells with tau accumulation and scFv evenly distributed throughout the neuronal soma (Figure 2). Tau and scFv partially co-localized in the neurons as evident in the merged images (0.4 µm optical sections), further confirmed by z-stack image (Supplemental Figure 1), and at the ultrastructural level as per transmission electron microscopy (Figure 3).
Figure 2:
scFv235 partially co-localizes with tau in the adult brain. (A – H) Immunohistochemistry of adult Drosophila brains expressing in neurons (elav-GAL4 neuronal specific driver) scFv235 together with either tau WT (A, B, E, F) or tau R406W. (C, D, G, H). All adult brains are oriented in the same way, with two optic lobes on the lateral side and the brain in the middle. High power images of the antennal lobes of the brain marked within the insets of (A, C, E, G) are shown in panels (B, D, F, H). In all images, scFv235 was detected through anti-HA labeling (red). Total tau protein was detected through Tau-5 antibody (green in A, B, C, D) and phospho-tau protein was detected through PHF-1 antibody labeling (green in E, F, G, H). Single channel images of scFv235 are shown in (B’, D’, F’, H’) and single channels of tau proteins are shown in (B’’, D’’, F’’, H’’). While the pattern of scFv235 and tau distribution was distinct as expected by the presence of a signal peptide sequence in scFv235, some areas in the brain showed co-localization of both proteins. The representative cells showing colocalization are indicated by white arrows. (I, J).
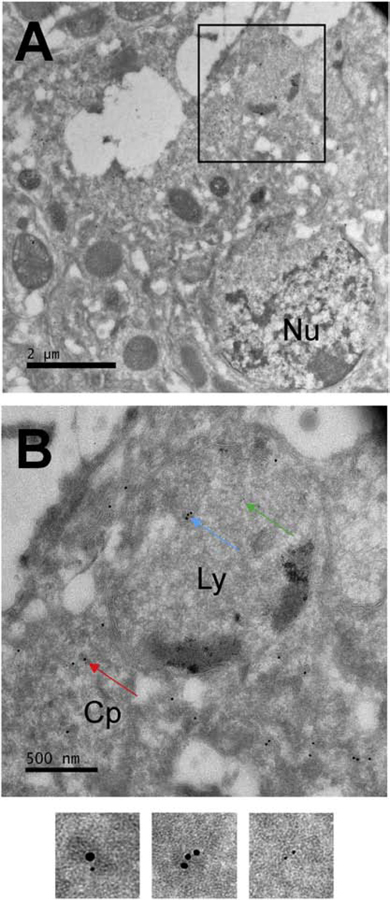
Figure 3: Ultrastructural location of scFv235 and tau in the adult brain.
(A, B) Transmission electron microscopy of adult tau WT brain showing scFv235 location within lysosome-like structure (Ly) and in the cytoplasm (Cp), sometimes colocalized with tau, as shown here with tau-5 staining. Co-localization is likely more prominent than shown here but not visible because the larger HA-scFv bound gold particle may cover the smaller tau bound gold particle. B is a magnification of the box in A. Arrows in B point at scFv235 (blue), tau (green) and their colocalization (red) with these regions shown below at higher magnification. Nu = nucleus.
Anti-tau scFv expression attenuates tau-induced mortality in tauopathy fly models
Both tauopathy models have been reported to have a shortened lifespan compared to naïve flies, with the R406W mutation conferring a more severe phenotype 47, as confirmed by us (Figure 4). “Wild type or mutant tau expression caused an accelerated mortality of adult flies, with all the flies dead by day 70 (Figure 4). This was in contrast to the survival of about 75% of control flies expressing an unrelated protein EGFP at that timepoint (not shown), which is comparable to the control and scFv groups (Figure 4).” In preliminary studies, we noticed a severe developmental toxicity in male tauopathy flies, resulting in a very low number of hatched males, compared to females, in particular in flies expressing the R406W mutation. A likely reason for the male toxicity is because elav-Gal4 is on the X chromosome. In Drosophila, it is well-established that males enhance the expression of genes in the X chromosome to compensate for only having one X chromosome 28. Therefore, we focused on analyzing therapeutic benefits of the scFv in females. For all phenotypic studies, the effect of ScFvs expression was compared with control flies that instead expressed a neutral transgene, GFPNLS (see Materials and Methods). Importantly, and as we had hypothesized, anti-tau scFv expression attenuated tau-induced mortality in the neuronal tauopathy Drosophila models (Figure 4). Overall, the survival curves were very significantly different (Log-rank test, p<0.0001). Control flies with the neuronal promoter elav-Gal4 alone survived the longest. On the other hand, flies expressing tau mutation R406W (co-expressed with a negative control uas-GFP) had the shortest lifespan. To examine the effect of scFv on tau pathology, we expressed wild type or R406W human tau with the uas-scFv transgene. The lifespan of tau R406W expressing flies was greatly prolonged by co-expressing anti-tau scFv235 (p<0.0001), when compared with those co-expressing control GFP. Likewise, flies expressing wild-type (WT) human tau with GFP had moderately short lifespans, which was prolonged by co-expressing the anti-tau scFv235 (p=0.0035).
Figure 4: scFv235 attenuates mortality in tauopathy Drosophila models.
Overall, the survival curves were very significantly different between the groups (Log-rank test, p<0.0001). Control flies with the neuronal promoter elav-Gal4 survived the longest. On the other hand, flies expressing tau mutation R406W (together with a negative control uas-GFP) had the shortest lifespan. To examine the effect of scFv on tau pathology, we expressed wild type or R406W tau together with either a control uas-GFP, or with uas-scFv transgene. Life span of tau R406W expressing flies was greatly prolonged by co-expressing anti-tau scFv235 (p<0.0001), when compared with those co-expressing control GFP. Likewise, flies expressing wild-type (WT) human tau with GFP had moderately short life span, which was prolonged by co-expressing the anti-tau scFv235 (p=0.0035). The number of flies analyzed per group were as follows: controls (n=37); scFv (n=142); Tau-WT (n=35), Tau-WT + scFv (n=51), Tau-R406W (n=145); Tau-R406W + scFv (n=80).
Anti-tau scFv expression prevents tau-induced developmental toxicity in tauopathy fly models
Careful examination of the flies at 0–3 days revealed pronounced group differences in wing phenotypes (Figure 5). Specifically, all the scFv flies (n=245) had expanded wings, whereas both models of tauopathy flies showed abnormal wing morphology, which was more severe in the tau mutant flies (Tau-WT: 78 expanded, 28 unexpanded; Tau-R406W: 92 unexpanded, 59 expanded). Importantly, the tau-induced wing phenotype was completely prevented in both models with co-expression of the anti-tau scFv (Fisher exact or Chi-square test, p<0.0001 for both). Additionally, pronounced group differences were seen in abdomen appearance (Figure 6; Kruskal Wallis, p<0.0001). The tauopathy flies had an extended abdomen that progressively developed up to 2–3 weeks of age (p<0.0001), and remained at that plateau through the rest of the lifespan of the flies. This developmental phenotype was also prevented by scFv235 in both models (p<0.0001). Analysis through 21 days is shown. At later time points, a large portion of the tau-R406W flies had died but the pattern for all groups remained the same.
Figure 5: scFv235 prevents unexpanded wing phenotype in tauopathy Drosophila models.
A: The left panel depicts a normal fly with expanded wings (rated as 1). The three panels to its right show increasing severity of unexpanded wings (rated as 2–4). B: Anti-tau scFv235 prevents development of abnormal wing phenotype in tauopathy flies. Both tauopathy fly models presented with a blistered wing phenotype that was evident at an early age, and was more severe in the R406W mutant. It was completely prevented in both models with co-expression of the anti-tau scFv235 (p<0.0001). ****:p< 0.0001, comparing tau model with the same model co-expressing the scFv. Arrows in 2 point to abnormally bent wing blades.
Figure 6: Anti-tau scFv235 prevents development of extended abdomen phenotype in tauopathy flies.
A: The left panel depicts a normal control fly with an unextended abdomen (rated as 1). The four panels to its right show increasing severity of an extended abdomen (rated as 2–5). B: Anti-tau scFv235 prevents development of extended abdomen phenotype in tauopathy flies (Kruskal Wallis, p<0.0001). Both tauopathy fly models presented with a progressive abdomen extension that appeared earlier the tau mutant model (p=0.0157–0.0001). As with the wing phenotype, it was prevented in both models with co-expression of the anti-tau scFv235 (p<0.0001). Analysis through 21 days is shown. At later time points, a large portion of the Tau-R406W flies had died but the overall effects in all groups remained the same through the lifespan of the flies. ****:p< 0.0001, comparing tau model with the same model co-expressing the scFv. #, ##, ####: p<0.05, 0.01, 0.0001, comparing each tau model with control flies. The number of flies analyzed per group were as follows: WT (n=17–13 (day 0 – day 21); scFv (n=22–21); Tau-WT (n=38–36), Tau-WT + scFv (n=18–15), Tau-R406W (n=31=20); Tau-R406W + scFv (n=36–30).
Anti-tau scFv expression prevents neurotoxicity and reduces pathological tau protein in tauopathy fly models
To examine if the reduced mortality in the scFv-tauopathy flies was associated with prevention of tau-induced neurotoxicity and tau clearance, we examined Elav and tau protein levels in flies with or without scFv expression. We noticed from the, immunohistochemical analysis of tau expression, as detailed above, that neurons with high scFv expression generally had lower levels of intracellular Tau (Figure 2).
Western blot analysis of brain homogenate supernatant at different time points revealed extensive age-associated neurotoxicity, as defined by reduced levels of the neuronal marker Elav on western blots, in both of the tauopathy fly models that was prevented by the scFv (Figure 7). Expression of Armadillo, a housekeeping cell-adhesion protein expressed in all epithelial cells, did not differ between the groups and it was used to normalize the data. Specifically, two-way ANOVA analysis revealed treatment and age effects (p<0.0001 for both), and an interaction between the two factors (p=0.0007). Post-hoc analysis showed significant neurotoxicity in the tauopathy models at 21 days (WT: 51% reduction, p=0.0187; R406W: 60% reduction, p=0.0018) and 30 days (WT: 59% reduction, p<0.0001; R406W: 63% reduction, p<0.0001) of age, compared to controls. Co-expression of the anti-tau scFv prevented neurotoxicity at the same ages (WT: p=0.0004 (30 days); R406W: p=0.0472 (21 days), p=0.0012 (30 days)). Likewise, scFv-induced clearance of phospho-tau (PHF-1) was evident in the R406W tauopathy flies but not in the WT tauopathy flies (Figures 8–9). It is possible that in the latter model, the scFv neutralizes tau but does not promote its clearance. Analysis of young flies (0–3 days old), normalized for Elav protein levels to take into account neuronal loss, revealed a 78% reduction in phospho-tau in scFv-treated tau R406W flies (p=0.0156), and a strong trend for reduction in total tau (54%, p=0.075). More variance was seen in tau levels in older flies (30 days) but quantitative analysis revealed a 90% reduction in phospho-tau normalized for Elav in scFv-treated tau R406W flies (p=0.0377), and a strong trend for a similar reduction in total tau (87%, p=0.0667). At this age, a large number of the R406W flies were already dead, presumably those with the most severe tau pathology. Hence, the scFv-mediated benefits in that group on tau clearance are likely underestimated.
Figure 7: scFv235 prevents neurotoxicity in tauopathy Drosophila models.
A: Representative blots of neuronal marker (Elav) and control housekeeping protein marker Armadillo (Arm) in control-, tau-WT- and tau-R406W flies. B: Quantitation of the neuronal marker Elav, normalized to Arm levels, in the different groups at 0, 14, 21, and 30 days. Progressive and comparable neuronal loss is evident in both of the tauopathy models at 21 and 30 days, which is prevented by co-expression of the anti-tau scFv. **, ***:p< 0.01, 0.001, comparing each tau model with the same model co-expressing the scFv. #, ##, ####: p<0.05, 0.01, 0.0001, comparing each tau model with control flies. Each data point for each group was obtained from 3–4 batches of 5 fly heads. Arm levels did not differ significantly between the groups (data not shown). Of the two Arm bands, the lower band was quantified to normalize Tau levels.
Figure 8: scFv235 clears neuronal tau in young tau-R406W tauopathy Drosophila model.
A. Representative blots of tau antibodies, Tau-5 and PHF-1 and control housekeeping protein marker Armadillo (Arm) in control, scFv, tau-WT, tau-WT + scFv, tau-R406W and tau-R406W + scFv flies at 0–3 days, B-C. Quantitation of Tau-5 and PHF-1 reactive blots, normalized to Arm levels, and those values normalized to the respective Elav/Arm values depicted for the corresponding group in Figure 7. scFv-mediated clearance of total (Tau-5, p=0.075) and phospho-tau (PHF-1, p=0.0156) was detected in the tau-R406W group but not in the tau-WT group. * p<0.05. Each data point was obtained from 3–5 batches of 5 fly heads. Arm levels did not differ significantly between the groups (data not shown). Of the two Arm bands, the lower band was quantified to normalize Tau levels.
Figure 9: scFv235 clears neuronal tau in old tau-R406W tauopathy Drosophila model.
A. Representative blots of tau antibodies, Tau-5 and PHF-1 and control housekeeping protein marker Armadillo (Arm) in tau-WT, tau-WT + scFv, tau-R406W and tau-R406W + scFv flies at 30 days, B-C. Quantitation of Tau-5 and PHF-1 reactive blots, normalized to Arm levels, and those values normalized to the respective Elav/Arm values depicted for the corresponding group in Figure 5. scFv-mediated clearance of total (Tau-5, p=0.0667) and phospho-tau (PHF-1, p=0.0377) was detected in the tau-R406W group but not in the tau-WT group. * p<0.05. Each data point was obtained from 3 batches of 5 fly heads. Arm levels did not differ significantly between the groups (data not shown). Of the two Arm bands, the lower band was quantified to normalize Tau levels.
Discussion
Our findings show the therapeutic potential of an anti-tau scFv in two different tauopathy Drosophila models. Specifically, neuronal co-expression of scFv235 with mutant (R406W) or normal human tau (WT), substantially improved survival in both tauopathy models, prevented developmental and progressive neurotoxicity in both models, and promoted tau clearance in the R406W model.
Drosophila melanogaster is an ideal model for conducting genetic screens and has been used previously for antibody efficacy studies targeting various protein aggregates 6,13,29,48. It has not been shown prior to this report to be useful for examining efficacy of tau antibodies or their derivatives. These particular tauopathy fly models were previously characterized and shown to have increased mortality, neurodegeneration as evident by silver staining, and tau immunoreactivity on histological sections and western blots, with a more severe phenotype in the mutant model 43,47.
Confocal and electron microscopy analysis of the expression of tau and the anti-tau scFv revealed a partial colocalization (Figures 2–3). Interestingly, neurons with strong scFv immunoreactivity often had limited tau immunoreactivity and vice versa supporting scFv-mediated clearance of pathological tau. Neurons with colocalization likely reflect ongoing scFv-induced tau clearance. Comparable patterns were seen with antibodies staining non-phosphorylated tau and hyperphosphorylated tau in both tauopathy models. We and others have previously reported on similar intraneuronal colocalization of tau and administered mAb or this scFv in culture and mouse models 3,7–10,19,22–24,30,37,38,49. Here, the scFv is expressed with a signal sequence that promotes its secretion from the neuron. Secreted scFv may be taken up again into neurons to neutralize pathological tau. Extrapolating from previous studies of this scFv in vivo administration in mice, the secreted scFv is then likely taken up again into the neuron via bulk endocytosis 24. Within the neuron of mammals, the scFv then colocalizes with the aggregated human tau protein within the endosomal-lysosomal system or possibly within the cytosol as well following the rupture of these vesicles. If the tau clearance mechanism is conserved between Drosophila and mammals, the binding of the scFv to tau may facilitate lysosomal clearance by loosening up the aggregates to allow better access of lysosomal enzymes. Within the cytosol, scFv binding to tau aggregates may prevent further seeding of aggregates and possibly promote proteasomal degradation of the scFv-tau complex. scFv-mediated tau clearance is well supported by the tau analysis of fly brains in the tau-R406W model (Figures 8–9), which then prevents the development of tau pathology-induced wing and abdomen phenotype (Figures 5–6), prevents neuronal loss (Figure 7), and enhances survival (Figure 4). The link between tau clearance and the other pathological phenotypes was not clear in the tau-WT model. In those flies, scFv did not appear to clear tau, even though it improved survival, prevented tau-induced neurotoxicity, wing blistering and abdomen extension (Figures 4–9). This likely reflects lesser sensitivity of western blot tau analysis than the other measures in detecting beneficial effects of the scFv. It is also possible that the scFv neutralizes pathological tau in the tau-WT model but does not necessarily promote its clearance.
A strong trend for clearance of total tau was observed in the R406W group. In our previous mouse studies, phospho-selective whole tau antibodies or related immunogens did not affect total tau levels 3,4,10. A key reason for a strong trend for such decrease may be the continuous neuronal expression of the scFv, which likely results in greater efficacy than when the antibody is administered periodically peripherally. In addition, the total tau antibody used for staining recognizes normal and pathological tau as well as mouse and human tau, of which the former should mostly be normal. The endogenous tau-like protein in the fly is not detected by the antibody. Therefore, the ratio of pathological tau to normal tau is likely lower in the mouse than in the fly, which explains why total tau in the mouse is not altered following antibody-mediated clearance of pathological tau. Furthermore, as discussed previously, therapeutic tau antibodies will primarily react with pathological tau within degradation pathways in the cell, regardless of which tau epitope they recognize 39,40.
Similar abnormal wing phenotype has been described in different tauopathy Drosophila models expressing unmutated tau isoforms, in which wing tau expression was driven by the engrailed neuronal driver 12. However, the progressive tau-induced abdomen extension has to our knowledge not been shown previously in these or other tauopathy fly models. Since we directed the tau expression to neurons and detect extensive neuronal loss in these models, these phenotypes likely relate to neurodegeneration in the peripheral nerves that innervate the wings and abdomen. Its prominence, which is easily rated, adds convenient parameters to analyze the functional consequences of tau-mediated neurodegeneration and its prevention by various therapeutic approaches.
Expression of the scFv did not appear to have any detrimental effects as reflected in comparable survival curves of WT and scFv expressing flies, normal wing and abdomen appearance and no signs of neurotoxicity as measured by Elav levels (Figures 4–7). This bodes well for its possible use as a gene therapy to treat tauopathies. It is interesting to note that the parent whole monoclonal antibody, 6B2, from which this scFv was derived, is ineffective in various culture and in vivo tauopathy models 10,49, despite having a sub-nanomolar affinity for its phospho-tau immunogen 24, and the same binding regions (CDRs) as the scFv, which has about 2500 times lower affinity for the immunogen than 6B2 24. Importantly, 6B2 is ineffective even though it is readily detected within brain neurons or neuron-like cells in culture or following an intravenous injection in tauopathy mouse model 10,19,24,37,38,49. This indicates that strength of binding to this tau epitope correlates negatively with efficacy, and the therapeutic benefits may also be influenced by antibody size and mode of delivery. These features need to be taken into account in ongoing clinical development of this approach. To further clarify this, future studies should compare the efficacy of 6B2 and its scFv using the same delivery approach in the same model. We had previously shown that the charge of another whole anti-tau antibody against the same phospho-tau region, 4E6, can substantially affect its neuronal uptake and efficacy even though the CDRs have the same sequence 8. Also, 4E6, is very effective in various culture and in vivo assays, despite having much lower affinity for the immunogen than 6B2 8,10,49. Hence, counterintuitively, lower affinity against this tau region is associated with greater efficacy. This is also supported by the fact that a chimeric engineered version of 4E6 has substantially increased binding to aggregated tau compared to the parental 4E6 but less efficacy in neutralizing tau toxicity 8. In addition, the unmodified 4E6 preferably binds to soluble tau, compared to 6B2 and chimeric 4E6, which primarily recognize aggregated tau 8,10. Together, these findings suggest that the low affinity antibody against this tau region may loosen up the tau aggregates within these vesicles, allowing better access of lysosomal degrading enzymes. Conversely, the high affinity antibody may make these aggregates more compact and therefore more difficult to degrade. Concurrently, the low affinity antibodies against this region primarily bind to soluble pathological tau, which may be more toxic than aggregated tau 8,10.
At present time, there are no treatments available that slow, halt or reverse tau pathology or affect in any way the progression of the associated clinical symptoms. Alzheimer’s disease is the most common tauopathy but there are numerous other diseases that are in part or wholly defined by age-related tau lesions, mostly in neurons but sometimes in glia. Tau immunotherapies are currently the most common approach in clinical trials to attempt to treat these diseases, with at least eleven therapies in clinical trials 1,2,11,39. Nine of these are passive immunizations using whole antibodies and two are active immunizations that seek to generate an anti-tau antibody response. A trial with one antibody was discontinued, presumably for pharmacokinetic reasons 11,39. This approach is supported by numerous preclinical studies from multiple laboratories that have repeatedly shown the efficacy of tau immunotherapies in clearing pathological tau and preventing associated functional deficits 11,39. The efficacy of antibody fragments has not been extensively examined but the findings to date are supportive indicating tau clearance and when assessed, functional improvements as well 21,32,41,45. Specifically, peripheral administration or lentiviral expression of anti-tau scFvs leads to clearance of pathological tau within the brain in mouse models 32,41, and similar benefits are seen when scFvs are expressed in the brain via AAV-vectors 21,45.
In summary, our findings show for the first time that anti-tau scFv expression in Drosophila tauopathy models prevents or attenuates the development of various tau-induced phenotypes, including unexpanded wings, extended abdomens, neurotoxicity and age-associated mortality. Drosophila is an ideal model to further examine the mechanisms of these beneficial effects to improve this promising therapeutic approach, which has led to nine ongoing clinical trials.
Materials and Methods
Drosophila genetics
All Drosophila stocks were cultured in standard cornmeal medium at 25 °C. All transgenes were expressed through the Gal4/UAS system. Specifically, the indicated UAS lines were crossed to flies containing Elav-Gal4 c155, a neuronal Gal4 driver on the X chromosome (Bloomington Stock Center # 458). To express mutant and wild type htau, we used the previously described UAS-tauWT and UAS-tauR406W lines, which express the longest isoform of tau, at levels estimated to be 0.5 and 0.4 fold, respectively, of tau levels per protein in normal human brain homogenate 47.
To study the in vivo efficacy of anti-tau scFv, the scFv235 transgene with an HA tag fused to the C-terminus was subcloned into the pUAST plasmid and sent out to generate a stable transgenic line (Bestgene Inc.) through standard P-element transgenesis techniques 34. A transgenic line inserted in the 2nd chromosome was used for our analysis. As a negative control for UAS-ScFv235, we used a control UAS-GFPNLS (Bloomington Stock Center # 4775). Using this approach, we generated flies expressing the following transgenes: 1) controls (naïve and elav-GAL4), and flies expressing: 2) scFv235; 3) wt human tau (htau) + GFP (control); 4) R406W human tau (R406W htau) + GFP (control); 5) scFv235 + htau; and; 6) scFv235 + R406W htau.
The amino acid sequence of ScFv235 construct is as follows with the scFv sequence in bold: M K K T A I A I A V A L A G F A T V A Q A A E L D V V M T Q T P L T L S V T I G Q P A S I S C K S S Q S L L Y S N G K T Y L N W L L Q R P G Q S P K R L I Y L V S K L D S G V P D R F T G S G S G T D F T L K I S R V E A E D L G V Y Y C V Q G T H S P L T F G A G T K L E L K S S G G G G S G G G G G G S S R S S L E V Q L Q Q S G P E L V K P G A S V K I S C K T S E Y T F T E Y T K H W V K Q S H G K S L E W I G S I N P N N G D T Y Y N Q K F T D K A T L T V D K S S T T A S M E L R S L T F E D S A V Y Y C A M G D S A W F A Y W G Q G T L V T V S A A K T T P P S V T S G Q A G Q H H H H H H G A Y P Y D V P D Y A S
Preceding the scFv sequence is a leader sequence with a signal peptide directing its secretion. The scFv sequence is composed of a variable light chain that is connected to a variable heavy chain via a linker, S S G G G G S G G G G G G S S R S S. Following the scFv sequence is an antibody constant domain, G T L V T V S A A K T T P P S V T S G Q A G Q followed by a His tag (H H H H H H H) and an HA tag (G A Y P Y D V P D Y A S).
Phenotypic analysis
Age-dependent toxicity was assessed by survival curves. Specifically, newly eclosed flies were collected for a period of two days, and their survival was monitored over the indicated time period. The flies were passed into new vials with fresh food every three days. Since htauR406W expressing male flies failed to eclose to adulthood in 25 °C (likely because Elav-Gal4 is on the X chromosome), the survival studies were done with female adult flies only.
The wing expansion phenotype was scored after one day of adult eclosion. Newly eclosing wild type flies emerge with unexpanded wings that appear small and dark in color. After a few hours, CCAP-expressing neurons trigger the expansion of the wings, which then appear larger and largely transparent 27,33. Possible defects in this process was assessed under the light microscope.
To score the abdomen phenotype, we developed a system where a normal abdomen morphology was given a score of 1, and the most bloated-appearing abdomens were given a score of 5. The scores were given at each time point when fly survival was assessed.
Six groups of flies were employed for the phenotypic analysis. For the WT control, an oregon-R stock was used. To obtain the other five groups of flies, crosses were set up as follows: scFv: elav-Gal4 x scFv235, TauWT: elav-Gal4 x uas-GFP/Cyo;uas-tauWT, TauWT + scFv: elav-Gal4 x uas-scFv/Cyo;uas-tauWT, TauR406W: elav-Gal4 x uas-GFP;uas-tauR406W, TauR406W + scFv: elav-Gal4 x uas-scFv;uas-tauR406W. The progeny resulting from this WT stock and these crosses discussed above eclosed over a period of three days. When 0–3 day old flies were collected on the third day, Day 0 was determined as the midpoint of this range. Flies were scored for wing and abdominal phenotype the day the progeny were collected, and then rated on their abdomens once a week over a span of five weeks. Wing phenotype was rated as expanded or unexpanded and abdominal phenotype was scored from 1–5. This scoring was repeated with different groups of flies of the same genotypes. For graphing purposes, a range of four days was created to compile all scoring data. These ranges allowed for a Day 0 point for both the wing and abdominal graphs, as well as the plotting of abdominal values on the week mark.
Immunohistochemistry and Western Blots
For immunohistochemistry, adult brains were dissected and fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) for 20 minutes before incubation with antibody. For subsequent steps, antibody labeling was performed in PBS-Tx (PBS containing 0.4% Triton x-100). The fixed tissues were incubated with the indicated primary antibody for 1 hour at room temperature, followed by incubation with a secondary antibody for another hour. These samples were washed in PBS-Tx three times for ten minutes each before mounting and visualization. All fluorescent images were obtained using an LSN700 confocal microscope (Zeiss). For co-localization studies in Figure 2, 40x water immersion lens was used, with an optical section of 0.4 µm. A representative image in Figure 2G confirming colocalization was reconstructed vertically using z-stacked images in Image J (shown in Supplementary Figure 1).
For western blots, five adult fly heads were lysed in 50 µl of the lysis buffer (10 mM Tris-HCl pH 7.5, 150 mM NaCl, protease inhibitor cocktail (Roche), 1 mM EDTA, and 1% SDS). After centrifugation for 20 min at 5400 x g, seven µl of such lysates were loaded into each well of SDS-PAGE gels (12%) for electrophoresis. The proteins on gels were transferred to PVDF membranes using standard western blot protocols. After blocking the membranes with a blocking solution (5% dry milk in PBS with 0.1% Triton x-100), the membranes were subsequently incubated with primary antibodies for 1 hour followed by peroxidase-conjugated secondary antibodies (Jackson Immuno Research). The bands were visualized on X-ray films after incubation with the ProtoGlow ECL detection kit (National Diagnostics). The band intensities were measured by quantifying the pixel intensities using Image J. The following antibodies were used: Rat anti-Elav (Elav is a neuronal marker, DSHB Univ. of Iowa; 7E8A10; 1:1000 for western blot); mouse anti-armadillo (Armadillo is a housekeeping gene. Its protein is the Drosophila homolog of β-catenin and has roles in cell adhesion and cell signaling, DSBH Univ of Iowa, N2 7A1; 1:10,000 for western blot); mouse anti-tau (Tau-5 (ThermoFisher, non-phosphorylated tau), 1:1000 for westerns, 1:100 for immunohistochemistry, and; PHF-1 (Peter Davies, Feinstein Institute, NY, phosphorylated tau), 1:2000 for western blot, 1:250 for immunohistochemistry); rabbit monoclonal anti-human influenza hemagglutinin (anti-HA, Cell Signaling Technology, C29F4, 1:250 for immunohistochemistry) and rat anti-HA antibody (Sigma, 3F10, 1:250 used for western blots) were used to detect the anti-tau scFv235.
Transmission Electron Microscopy
Young adult Drosophila heads were cut off and fixed with 2% paraformaldehyde and 0.05% glutaraldehyde in 0.1M phosphate buffer (pH 7.2) for 15 mins. Each brain was then dissected and further fixed in the same fixative for 30 mins before change to 2% paraformaldehyde and subsequently stored overnight at 4°C. The brain tissue was then washed with 50 mM glycine, embedded with 10% gelatin, infused with 2.3 M sucrose and cryosectioned at 90 nm on carbon/formvar coated copper grids. The grids were incubated with rabbit anti-HA-scFv antibody (Cell Signaling Technology, Danvers, MA), followed by application of 18 nm colloidal gold conjugated goat anti-rabbit IgG (Jackson ImmunoReasearch Laboratories Inc., West Grove, PA). After 5 min fixation with 2% paraformaldehyde and a wash with 50 mM glycine, grids were then incubated with Tau-5 or PHF1 anti-mouse antibody, followed by 6 nm colloidal gold conjugated goat anti-mouse antibody (Jackson ImmunoReasearch Laboratories Inc.). The grids were subsequently fixed in 1% glutaraldehyde for 5 min, washed with distilled water, contrasted and embedded in a mixture of 3% uranyl acetate and 2% methylcellulose. All stained grids were examined under a Talos120C electron microscope (Thermo Fisher Scientific, Hillsboro) and photographed with a Gatan (4Kx4k) OneView camera.
Statistics
All data were analyzed with GraphPad Prism 8. Survival was analyzed with the Log rank test. Wing phenotype was analyzed by Chi-square and Fisher exact test, which gave the same results. Abdomen extension was analyzed at the individual time points by Kruskal Wallis test followed by Dunn’s post-hoc test. Neurotoxicity (Elav levels) over time was analyzed by two-way ANOVA, followed by Tukey’s post hoc test. Tau levels were analyzed by a Student’s t-test, two-tailed. Differences were considered significant for p<0.05.
Supplementary Material
A representative image in Figure 2G confirming colocalization of scFv and tau was reconstructed vertically using z-stacked images in Image J.
Acknowledgements:
This work was supported by NIH grants NS077239, AG032611 and AG058282. We thank Peter Davies, Feinstein Institute, for providing the PHF-1 antibody for tau staining. We thank NYU Langone Health DART Microscopy Lab Alice Liang, Chris Petzold and Kristen Dancel-Manning for their assistance with TEM work. This core is partially funded by NYU Cancer Center Support Grant NIH/NCI P30CA016087.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
E.M.S. is an inventor on patents on tau immunotherapies and related diagnostics, which are assigned to New York University, and some of these are licensed to and are being co-developed with H. Lundbeck A/S.
References:
- 1.“Safety and Tolerability of PNT001 in Healthy Adults,” Https://Clinicaltrials.Gov/Ct2/Show/NCT04096287?Term=PNT001&Draw=2&Rank=1 (2020).
- 2.“Study With Lu AF87908 in Healthy Subjects and Patients With Alzheimer’s disease,” Https://Clinicaltrials.Gov/Ct2/Show/NCT04149860?Term=Lu+AF87908&Draw=2&Rank=1 (2020).
- 3.Asuni AA, et al. , “Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements,” J. Neurosci 27(34), 9115 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutajangout A, et al. , “Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain,” J. Neurochem 118(4), 658 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cattepoel S, et al. , “Chronic intranasal treatment with an anti-Abeta(30–42) scFv antibody ameliorates amyloid pathology in a transgenic mouse model of Alzheimer’s disease,” PLoS. One 6(4), e18296 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chartier A, et al. , “Prevention of oculopharyngeal muscular dystrophy by muscular expression of Llama single-chain intrabodies in vivo,” Hum. Mol. Genet 18(10), 1849 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Collin L, et al. , “Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer’s disease,” Brain 137(Pt 10), 2834 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Congdon EE, et al. , “Tau antibody chimerization alters its charge and binding, thereby reducing its cellular uptake and efficacy,” 42, 157 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Congdon EE, et al. , “Antibody Uptake into Neurons Occurs Primarily via Clathrin-dependent Fcgamma Receptor Endocytosis and Is a Prerequisite for Acute Tau Protein Clearance,” J. Biol Chem 288(49), 35452 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Congdon EE, et al. , “Affinity of Tau antibodies for solubilized pathological Tau species but not their immunogen or insoluble Tau aggregates predicts in vivo and ex vivo efficacy,” Mol. Neurodegener 11(1), 62 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Congdon EE and Sigurdsson EM, “Tau-targeting therapies for Alzheimer disease,” Nat. Rev. Neurol 14(7), 399 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dourlen P, et al. , “Functional screening of Alzheimer risk loci identifies PTK2B as an in vivo modulator and early marker of Tau pathology,” Mol. Psychiatry 22(6), 874 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Funez P, et al. , “Anti-Abeta single-chain variable fragment antibodies exert synergistic neuroprotective activities in Drosophila models of Alzheimer’s disease,” Hum. Mol. Genet 24(21), 6093 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frenkel D and Solomon B, “Filamentous phage as vector-mediated antibody delivery to the brain,” Proc. Natl. Acad. Sci. U. S. A 99(8), 5675 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frenkel D, Solomon B, and Benhar I, “Modulation of Alzheimer’s beta-amyloid neurotoxicity by site-directed single-chain antibody,” J. Neuroimmunol 106(1–2), 23 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Fukuchi K, et al. , “Amelioration of amyloid load by anti-Abeta single-chain antibody in Alzheimer mouse model,” Biochem. Biophys. Res. Commun 344(1), 79 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuchi K, et al. , “Anti-Abeta single-chain antibody delivery via adeno-associated virus for treatment of Alzheimer’s disease,” Neurobiol. Dis 23(3), 502 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gimenez-Llort L, et al. , “Early intervention in the 3xTg-AD mice with an amyloid beta-antibody fragment ameliorates first hallmarks of Alzheimer disease,” MAbs 5(5), 665 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu J, Congdon EE, and Sigurdsson EM, “Two novel Tau antibodies targeting the 396/404 region are primarily taken up by neurons and reduce Tau protein pathology,” J. Biol Chem 288(46), 33081 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutton M, et al. , “Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17,” 393(6686), 702 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Ising C, et al. , “AAV-mediated expression of anti-tau scFvs decreases tau accumulation in a mouse model of tauopathy.,” J Exp. Med 214(5), 1227 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo A, et al. , “Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy,” 523(7561), 431 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnamurthy PK, Deng Y, and Sigurdsson EM, “Mechanistic Studies of Antibody-Mediated Clearance of Tau Aggregates Using an ex vivo Brain Slice Model,” Front Psychiatry 2, 59 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnaswamy S, et al. , “Antibody-derived in vivo imaging of tau pathology,” J. Neurosci 34(50), 16835 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levites Y, et al. , “Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid beta, amyloid beta 40, and amyloid beta 42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice,” J. Neurosci 26(46), 11923 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu R, et al. , “Single chain variable fragments against beta-amyloid (Abeta) can inhibit Abeta aggregation and prevent abeta-induced neurotoxicity,” 43(22), 6959 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Luan H, et al. , “Functional dissection of a neuronal network required for cuticle tanning and wing expansion in Drosophila,” J. Neurosci 26(2), 573 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucchesi JC and Kuroda MI, “Dosage compensation in Drosophila,” Cold Spring Harb. Perspect. Biol 7(5) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin-Pena A, Rincon-Limas DE, and Fernandez-Funez P, “Anti-Abeta single-chain variable fragment antibodies restore memory acquisition in a Drosophila model of Alzheimer’s disease,” Sci. Rep 7(1), 11268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McEwan WA, et al. , “Cytosolic Fc receptor TRIM21 inhibits seeded tau aggregation,” Proc. Natl. Acad. Sci. U. S. A 114(3), 574 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meli G, et al. , “Direct in vivo intracellular selection of conformation-sensitive antibody domains targeting Alzheimer’s amyloid-beta oligomers,” J. Mol. Biol 387(3), 584 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Nisbet RM, et al. , “Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model,” Brain 140(5), 1220 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park JH, et al. , “Targeted ablation of CCAP neuropeptide-containing neurons of Drosophila causes specific defects in execution and circadian timing of ecdysis behavior,” Development 130(12), 2645 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Rubin GM and Spradling AC, “Genetic transformation of Drosophila with transposable element vectors,” 218(4570), 348 (1982). [DOI] [PubMed] [Google Scholar]
- 35.Ryan DA, et al. , “Abeta-directed single-chain antibody delivery via a serotype-1 AAV vector improves learning behavior and pathology in Alzheimer’s disease mice,” Mol. Ther 18(8), 1471 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schilling S, et al. , “Passive Abeta Immunotherapy: Current Achievements and Future Perspectives,” Molecules 23(5) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shamir DB, Deng Y, and Sigurdsson EM, “Live Imaging of Pathological Tau Protein and Tau Antibodies in a Neuron-Like Cellular Model,” Methods Mol. Biol 1779, 371 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Shamir DB, et al. , “Internalization of tau antibody and pathological tau protein detected with a flow cytometry multiplexing approach,” Alzheimers. Dement 12(10), 1098 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sigurdsson EM, “Tau Immunotherapies for Alzheimer’s Disease and Related Tauopathies: Progress and Potential Pitfalls,” J. Alzheimers. Dis 64(s1), S555–S565 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sigurdsson EM, “Alzheimer’s therapy development: A few points to consider,” Prog. Mol. Biol. Transl. Sci 168, 205 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Spencer B, et al. , “Selective targeting of 3 repeat Tau with brain penetrating single chain antibodies for the treatment of neurodegenerative disorders,” Acta Neuropathol 136(1), 69 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian H, et al. , “Isolation and characterization of antibody fragments selective for toxic oligomeric tau,” Neurobiol. Aging 36(3), 1342 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trotter MB, et al. , “The Drosophila model system to study tau action,” Methods Cell Biol 141, 259 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Valera E, Spencer B, and Masliah E, “Immunotherapeutic Approaches Targeting Amyloid-beta, alpha-Synuclein, and Tau for the Treatment of Neurodegenerative Disorders,” Neurotherapeutics 13(1), 179 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vitale F, et al. , “Anti-tau conformational scFv MC1 antibody efficiently reduces pathological tau species in adult JNPL3 mice,” Acta Neuropathol. Commun 6(1), 82 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang XP, et al. , “Conformation-dependent single-chain variable fragment antibodies specifically recognize beta-amyloid oligomers,” FEBS Lett 583(3), 579 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Wittmann CW, et al. , “Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles,” 293(5530), 711 (2001). [DOI] [PubMed] [Google Scholar]
- 48.Wolfgang WJ, et al. , “Suppression of Huntington’s disease pathology in Drosophila by human single-chain Fv antibodies,” Proc. Natl. Acad. Sci. U. S. A 102(32), 11563 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Q, et al. , “Dynamic assessment of tau immunotherapies in the brains of live animals by two-photon imaging,” EBioMedicine 35, 270 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang J, et al. , “Muscle-directed anti-Abeta single-chain antibody delivery via AAV1 reduces cerebral Abeta load in an Alzheimer’s disease mouse model,” J. Mol. Neurosci 49(2), 277 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A representative image in Figure 2G confirming colocalization of scFv and tau was reconstructed vertically using z-stacked images in Image J.