Abstract
The auxiliary CaVα2δ-1 subunit is an important component of voltage-gated Ca2+ (CaV) channel complexes in many tissues and of great interest as a drug target. Nevertheless, its exact role in specific cell functions is still unknown. This is particularly important in the case of the neuronal L-type CaV channels where these proteins play a key role in the secretion of neurotransmitters and hormones, gene expression, and the activation of other ion channels. Therefore, using a combined approach of patch clamp recordings and molecular biology, we studied the role of the CaVα2δ-1 subunit on the functional expression and the pharmacology of recombinant L-type CaV1.3 channels in HEK-293 cells. Co-expression of CaVα2δ-1 significantly increased macroscopic currents and conferred the CaV1.3α1/CaVβ3 channels sensitivity to the antiepileptic/analgesic drugs gabapentin and AdGABA. In contrast, CaVα2δ-1 subunits harboring point mutations in N-glycosylation consensus sequences or the proteolytic site as well as in conserved cysteines in the transmembrane δ domain of the protein, reduced functionality in terms of enhancement of CaV1.3α1/CaVβ3 currents. In addition, co-expression of the δ domain drastically inhibited macroscopic currents through recombinant CaV1.3 channels possibly by affecting channel synthesis. Together these results provide several lines of evidence that the CaVα2δ-1 auxiliary subunit may interact with CaV1.3 channels and regulate their functional expression.
Keywords: Ca2+ channels, CaVα2δ subunit, L-type calcium channels, gabapentin
1. Introduction
L-type voltage-gated Ca2+ (CaV) channels are expressed in many different cell types and tissues. In myocytes they are essential for excitation-contraction coupling, whereas in neurons and endocrine cells they regulate neurotransmitter and hormone release, gene expression, and the activity of other ion channels (Lipscombe et al. 2004; Yang & Berggren, 2006). Biochemical evidence suggests that L-type CaV channels are comprised of five subunits. A principal transmembrane a1 subunit is predicted to associate with a disulfidelinked α2δ dimer, an intracellular b subunit, and a transmembrane g subunit (Catterall et al. 2000; Lacinova, 2005; Yang & Berggren, 2006). The a1 subunit is the ion-conducting element in the channel protein complex (Catterall et al. 2000; Lacinova, 2005; Yang & Berggren, 2006).
Four mammalian genes encode L-type CaV channel α1 subunits: CaV1.1 (also known as α1S), CaV1.2 (α1C), CaV1.3 (α1D), and CaV1.4 (α1F). CaV1.1α1 and CaV1.4α1 subunits are enriched in skeletal muscle and retina, respectively, whereas CaV1.2α1 and CaV1.3α1 subunits are expressed in many cells including neurons and endocrine cells (Lipscombe et al. 2004; Yang & Berggren, 2006). CaV1.2 and CaV1.3 channels underlie the majority of L-type currents in neuronal, endocrine, and cardiovascular systems. CaV1.3 channels have relatively low activation thresholds, are less sensitive to dihydropyridine antagonists, and activate with fast kinetics when compared to CaV1.2 L-type currents (Lipscombe et al. 2004). There is growing interest in CaV1.2 and CaV1.3 channels because of their link to neurodegenerative disorders including autism, bipolar disorder, and Parkinson’s disease (Rogawski & Loscher, 2004; Splawski et al. 2004; Day et al. 2006; Krey & Dolmetsch, 2007; Surmeier, 2007). Understanding the mechanisms that regulate L-type calcium channel activity and surface expression is of major importance.
In the central nervous system, L-type Ca2+ channels (CaV1.2 and CaV1.3) apparently do not to support synaptic transmission, but seem to play an important role in the excitation-transcription coupling. It has been reported that Ca2+ entry via postsynaptic L-type channels activates transcription factors pCREB (Bito et al. 1996; Dolmetsch et al. 2001) and NFATc4 (Graef et al. 1999). Phosphorylation of CREB, acting in conjunction with nuclear translocation and co-activator proteins promotes transcription of multiple genes (Zhang et al. 2005a; 2005b). Likewise, CaV1.3 channels play a unique role for hearing. Inner hair cells (IHCs), the primary sensory receptors of the mature mammalian cochlea, are responsible for relaying acoustic information transduced by mechano-sensitive channels to the central nervous system via afferent auditory nerve fibres. This is driven by Ca2+ entering IHCs through L-type channels of the CaV1.3 class (Johnson and Marcotti 2008) activated in response to depolarizing receptor potentials initiated by hair bundle deflection. In addition, it has been reported that CaV1.3 L-type channels are important for the sinoatrial node function. Using gene-targeted deletion of the CaV1.3α1 subunit, Zhang and colleagues (2002) found a decrease in the rate of firing associated with a diminished rate of diastolic depolarization. Last, CaV1.3 L-type channels are expressed at high density and play a role in the control of hormone secretion in a variety of endocrine cells including pancreatic β- and adrenal chromaffin cells where they control insulin and catecholamine release (Yang and Berggren 2006; Marcantoni et al. 2007).
In contrast to the functional studies, the molecular architecture of the L-type CaV1.3 channels is largely unknown. Though a role for the CaVβ subunits in determining a functional interaction with protein kinase A (Liang and Tavalin 2007) and arachidonic acid (Roberts-Crowley and Rittenhouse 2009) has been reported, little is known regarding the role of the Ca2+ channel auxiliary subunits in the regulation of the CaV1.3 channel activity. On the other hand, it has been reported that CaVα2δ promotes surface expression of different Caα2δ subunits and it speeds channel activation and inactivation kinetics (Singer et al. 1991; Itagaki et al., 1992; Welling et al. 1993; Felix et al. 1997; Qin et al. 1998; Klugbauer et al. 1999; Yasuda et al. 2004; Canti et al. 2005; Andrade et al. 2007a; Dickman et al. 2008; Tuluc et al. 2007; Hendrich et al. 2008). However, investigations of the CaVα2δ subunit effects on CaV1.3 channels are lacking. Here, we show that CaVα2δ augments surface expression of recombinant CaV1.3 channels in HEK-293cells. We also show that co-expression of the CaVα2δ subunit renders the CaV1.3 channels sensitive to antiepileptic/analgesic gabapentinoid drugs (GBP and AdGABA) and that co-expression of the transmembrane δ domain alone, together with the CaV1.3/CaVβ3 Ca2+ channel combination in absence or presence of CaVα2δ, results in an important inhibition of the whole cell current.
2. Materials and methods
2.1. Materials
Chloroquine (C-6628) and Fillipin III (F-4767) were obtained from Sigma-Aldrich (St. Louis, MO) and prepared as stock according to the manufacturer’s instructions. Gabapentin (1-(aminomethyl) cyclohexane acetic acid; Neurontin®; Pfizer; New York, NY) and AdGABA (a generous gift of Drs. G. Zoidis and N. Kolocouris, Univ. of Athens) were prepared as stock in distilled water and aliquots were store at −20 °C. All other chemicals were of reagent grade and obtained from different commercial sources.
2.2. cDNA clones
Cell expression constructs were made by standard techniques and their fidelity was verified by DNA sequencing. The rat neuronal CaV1.3α1 (GenBank accession number AF370009; Helton et al. 2005) was cloned into the pcDNA6/His vector (Invitrogen; Carlsbad, CA). The rabbit CaVβ1a subunit (M25817) was cloned in the pKCRH2 vector (Mishina et al. 1984) while the cDNAs coding the rat brain CaVβ2a (M80545), CaVβ3 (M88751) and CaVβ4 (L02315) subunits were cloned into the pcDNA3 vector (Invitrogen). We also used the recombinant bicistronic expression plasmid PIRES/α2δ (Sandoval et al. 2004), which carried the entire protein-coding region for the rat brain CaVα2δ-1b Ca2+ channel auxiliary subunit (M86621), or its mutant constructs (Sandoval et al. 2004; Andrade et al. 2007a), and for the green fluorescent protein (GFP) coupled by an internal ribosomal entry site (IRES) sequence. The cDNA coding the CaVd subunit, which was made by assembling a PCR fragment after the CaVα2δ-1b signal sequence (Felix et al. 1997), and the CD8 surface marker were cloned in the pcDNA3 expression plasmid (Invitrogen). The PERK mutant constructs (Harding et al. 1999) were cloned into the pcDNAI/Amp vector (Invitrogen).
2.3. Site-directed mutagenesis
The Pfu DNA polymerase was used in all PCRs to generate the CaVα2δ-1 mutations and all constructs were verified by sequencing. The glycosylation and proteolysis mutant constructs were generated following standard procedures in use in the laboratory (Sandoval et al. 2004; Andrade et al. 2007a). The CaVα2δ-1 subunit in which all cysteines in the extracellular region of δ were mutated to serines, was made using the pIRES-hrGFP-1a-based construct encoding the rat CaVa2δ-1 (Andrade et al. 2007a) as a template and standard PCR techniques. In all cases, the mutations were introduced with 40-mer synthetic oligonucleotides using the Quick-Change XL II mutagenesis kit (Stratagene).
2.4. Cell culture and transfection
Human embryonic kidney (HEK)-293 cells (American Type Culture Collection, ATCC; Manassas, VA) were grown in Dulbecco’s modified Eagle’s medium (DMEM)-high glucose supplemented with 10% horse serum, 2 mM L-glutamine, 110 mg/l sodium pyruvate, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in a 5% CO2/95% air humidified atmosphere. After splitting on the previous day and seeding at ~60% confluence, cells were transfected with the cDNA clones mentioned earlier using the Lipofectamine Plus reagent (Invitrogen) according to the manufacturer’s instructions. After DNA-lipid complexes were allowed to form, cells were transfected with either cDNAs encoding CaV1.3a1 alone (1 μg DNA/35-mm culture dish) or co-transfected with cDNAs for CaVβ, CaVα2δ-1 and CaVδ subunits in a 1:1 molar ratio (except where indicated).
The HEK-293 cell line stably expressing the CaV3.2 channel (GenBank accession number AF051946; Cribbs et al. 1998) was grown as previously described (Avila et al. 2006). Likewise, the RIN-m5F insulinoma β-cells (ATCC) were cultured in RPMI-1620 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. Both cell lines were plated on poly-L-lysine (0.05%)-precoated glass coverslips placed into 35-mm culture plates, and 24 h later were transfected with the CaVα2δ-1 or δ subunit cDNA constructs using Lipofectamine Plus reagent (Invitrogen). 48 h after transfection, cells were subjected to electrophysiological recording.
2.5. Electrophysiology
Ionic currents were recorded using the whole-cell configuration of the patch-clamp technique (Hamill et al. 1981), using an Axopatch 200B patch-clamp amplifier (Molecular Devices, Foster City, CA) and acquired on-line using a Digidata 1320A interface with pClamp8 software (Molecular Devices) as described elsewhere (Sandoval et al. 2004; Andrade et al. 2007a). The offset potential between the pipette and bath solutions was zeroed prior to seal formation. After establishing the whole-cell mode, capacitive transients were cancelled with the amplifier. Series resistance values were typically 2–10 MΩ, and no records were used in which the voltage error (as defined by Ver = Imax × Ra) was greater than 5 mV. Leak and residual capacitance currents were subtracted on-line by a P/4 protocol. Current signals were filtered at 2 kHz (internal 4 pole Bessel filter) and digitized at 5.71 kHz. Membrane capacitance (Cm) was determined as described previously (Avila et al. 2004) and used to normalize currents. The recording solutions are given in Table 1. Experiments were performed at room temperature (~22ºC).
Table 1.
Recording solutions. Units are in mM. The pH was adjusted to 7.3 with KOH (A-C) and CsOH (D, E).
| Sols | BaCl2 | CaCl2 | TEA-Cl | NaCl | CsCl | MgCl2 | KCl | K-Asp | HEPES | EGTA | Glucose | Na-ATP | Na-GTP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 5 | 125 | 10 | 15 | |||||||||
| B | 5 | 125 | 10 | 15 | |||||||||
| C | 5 | 140 | 3 | 10 | 5 | ||||||||
| D | 110 | 5 | 10 | 10 | 4 | 0.1 | |||||||
| E | 0.2 | 8 | 130 | 10 | 10 | 5 |
2.6. Semi-quantitative Western-blot
Cells were mechanically detached from culture dishes, washed twice with PBS pH 7.4 (137 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4, 1.8 mM KH2PO4) and lysed in triple-detergent buffer containing proteases inhibitors (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 0.1% SDS, 1.0% NP-40, 0.5% sodium deoxycholate, 1 mM PMSF, complete 1X; Roche Diagnostics). Lysis was performed in ice for 20 min vortexing each 5 min. The extracts were centrifuged to remove insoluble debris (10 min: 7500 × g) and protein concentration in the supernatants was determined using Bradford assays. Samples with 50 μg of protein were boiled for 5 min in protein loading buffer (50 mM Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.01% bromophenol blue). Proteins were resolved in 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Amersham Biosciences; Piscataway, NJ). After blocking with 6% non-fat dry milk in TBS-T (10 mM Tris-HCl, 0.15 M NaCl, 0.05% tween 20), membranes were incubated overnight with the primary anti-CaV1.30α1 antibody (Alomone Labs; Jerusalem, Israel) (1:600 in blocking solution). Membranes were then washed and incubated with horseradish peroxidase goat anti-rabbit secondary antibody (Invitrogen) diluted in TBS-T with 6% non-fat dry milk and developed with the ECL reagent (Amersham Biosciences). For b3, proteins were blotted onto nitrocellulose membranes and developed with enhanced chemiluminescence as previously described (Felix et al. 1997; Gurnett et al. 1997). As a protein loading control, membranes were stripped and incubated with a mouse monoclonal anti-β-actin antibody (Andrade et al. 2007b). Semi-quantitative analysis was carried out by densitometry using the Kodak digital Science ID v.2.0 system program.
2.7. Data analysis
Curve fitting and statistical analyses were carried out using the SigmaPlot 10 software package (SPSS Inc.; Chicago, IL). The significance of observed differences was evaluated by Student’s unpaired t test. A probability less that 5% was considered to be significant. All experimental values are given as means ± S.E.M. The peak current values were converted to peak conductance values using the expression:
| eqn (1) |
where I is current, G is conductance, Vm is the test potential and Vrev is the extrapolated reverse potential. Conductance-voltage (G-V) curves for activation were fit with a Boltzmann equation of the form:
| eqn (2) |
where Gmax is maximum conductance, Vm is the test potential, V1/2 is the potential for half-maximal activation of Gmax and k is a slope factor.
Steady-state inactivation curves were fitted with a Boltzmann function:
| eqn (3) |
where the current amplitude I has decreased to a half-amplitude at V1/2 with an e-fold change over k mV.
Current activation and decay were fitted with a second order exponential equation of the form:
| eqn (4) |
where t represents the time after the onset of the test pulse, Afast and Aslow are the contribution of a fast and a slow component to the total current amplitude, and tfast and tslow are the time constants associated with each component.
3. Results
3.1. The CaVα2δ-1 subunit increases macroscopic Ca2+ currents in cells expressing recombinant CaV1.3 channels
We co-expressed CaVα2δ-1 and CaV1.3 subunits together with different CaVβ subunits in HEK-293 cells to establish if CaVα2δ-1 plays a role in regulating expression levels of CaV1.3 L-type channels. Ca2+ channel currents in cells expressing CaVα2δ-1 were substantially greater (3–5-fold) than those in cells lacking exogenous CaVα2δ-1. This effect of CaVα2δ-1 occurred regardless of CaVβ subunit type and was independent of test voltage (Fig. 1). Given that Western blot experiments have shown that untransfected HEK-293 cells do not express endogenous CaVα2δ-1 (Sandoval et al., 2004), CaV1.3/CaVβ current enhancement detected after transfection may be therefore considered to be mediated by the heterologously expressed CaVα2δ-1 subunit. Likewise, our subsequent investigations of CaVα2δ −1 focused on the CaV1.3/CaVβ3 combination because these two subunits tend to coexist in neuronal and neuroendocrine tissue (Scholze et al. 2001; Singh et al. 2008).
Fig. 1.
CaV1.3 channel regulation by CaVβ and CaVα2δ-1 auxiliary subunits. The upper panels show representative traces of macroscopic Ba2+ current (IBa) recorded from HEK-293 cells that expressed CaV1.3 channels in association with the CaVβ subunit (β1a, β2a, β3 or β4) in absence and presence of the CaVα2δ-1 subunit, using solutions A (external) and D (internal) (Table 1). Currents were elicited by a depolarizing pulse to −30 mV from a Vh of −80 mV. The lower panels show average I-V relationships for IBa recorded from cells expressing CaV1.3α1/β channels in absence and presence of the CaVα2δ-1 subunit (n = 9–50 cells). Currents were evoked by 10-mV depolarizing steps from a Vh of −80 mV to potentials between −70 and +60 mV.
We next used more physiological recording conditions to test if CaVα2δ-1 augmented CaV1.3 currents when calcium is the charge carrier (Supplemental Fig. 1). L-type currents recorded with Ca2+ inactivate during the test pulse because they undergo pronounced Ca2+-dependent inactivation (Xu & Lipscombe, 2001; Yang et al. 2006). In cells expressing the CaVα2δ-1 subunit, L-type currents exhibited more prominent Ca2+-dependent inactivation at voltages > −40 mV reflecting greater levels of Ca2+ entry (Imredy & Yue, 1994; De Leon et al. 1995). Under these recording conditions, CaVα2δ-1 still increased L-type current density over a range of voltages and without affecting the voltage-dependence of channel activation and steady-state inactivation.
3.2. Effect of over-expression of CaVα2δ-1 mutant constructs on recombinant CaV1.3α1/CaVβ3 channels
We were interested in knowing if the stimulatory effects of CaVα2δ-1 on CaV1.3 channels are similar to its reported effects on CaV2.2. As mentioned earlier, CaVα2δ-1 is a glycosylated polypeptide that possesses a single transmembrane domain (δ) with a short intracellular C terminus and a long extracellular portion (Fig. 2A) which serves as an anchor in the cell membrane (Catterall, 2000; Lacinova, 2005; Yang and Berggren, 2006). We know that N-glycosylation at N136 and N184 and proteolytic processing at amino acid residues 941 to 946 of CaVα2δ-1 are important for its stimulatory effects on neuronal CaV2.2 channels (Sandoval et al. 2004; Andrade et al. 2007a). We therefore asked if the same sites play a role in CaVα2δ-1 mediated stimulation of CaV1.3 channels using different mutant constructs (Fig. 2B). As can be seen in Fig. 2C, the stimulatory effects of CaVα2δ-1 were partially lost in the double N-glycosylation CaVα2δ-1 mutant, N136Q and N184Q (Sandoval et al. 2004), and completely abolished in the proteolytic-site truncated CaVα2δ-1 mutant, P6 (Andrade et al. 2007a). It is worth noting that previous results in our laboratory indicate that both mutant constructs are expressed in the HEK-293 cells at similar levels than the wild-type CaVα2δ-1 protein (Sandoval et al., 2004; Andrade et al., 2007).
Fig. 2.
CaVα2δ-1 mutant subunits are unable to increase current amplitude through CaV1.3α1/CaVβ3 channels. (A) The CaVα2δ-1 subunit is synthesized in the endoplasmic reticulum as a pro-form that consists of a signal sequence, the α2 and the δ domains, and its posttranslational processing includes the removal of the signal sequence, glycosylation of the α2 domain and disulfide bond formation between α2 y δ and proteolytical cleavage to acquire its mature form. (B) Three different CaVα2δ-1 mutant subunits were used in this work: α2δ (DM) has two point mutations in N-glycosylation sites; α2δ (P6) has a mutation in the putative site of proteolytic processing and a construct lacking the conserved cysteines of the extracellular region of δ (C6). (C) Average I-V relationships for ICa recorded from HEK-293 cells expressing CaV1.3α1/β3 channels in presence of the wild-type CaVα2δ-1 subunit or its mutant constructs P6 and DM. n = 9–18 recorded cells. (D) Average I-V relationships for IBa recorded from HEK-293 cells expressing CaV1.3α1/β3 channels in presence of the wild-type α2δ-1 or the α2δ (C6) construct. Currents were recorded using solutions B/D (panel A) and A/D (panel B), respectively (see Table 1), and were elicited by 10-mV depolarizing steps from a Vh of −80 mV to potentials between −70 and +60 mV (n = 30–60 recorded cells).
We also tested a CaVα2δ-1 construct in which we mutated all six conserved cysteine residues in the δ domain (C6), to prevent association with α2. The C6 CaVα2δ-1 mutant was also unable to augment CaV1.3 channel currents (Fig. 2D) implying that association between α2 and δ subunits is needed for its effects on current density. As with the double N-glycosylation and proteolytic-site mutated versions of CaVα2δ-1, the C6 mutant is not express at a significantly different level than wild-type in the HEK-293 cells (data not shown).
3.3. The δ domain attenuates CaV1.3α1/CaVβ3 channel expression
Although the actions of the CaVα2δ-1 subunit have been probed using various glycosylation and deletion mutants, the effect of the δ domain in isolation from α2 is still virtually unexplored (Gurnett et al. 1996; Felix et al. 1997). We therefore assessed the effect of the δ domain on CaV1.3 channel currents. L-type currents in cells expressing the δ subunit were significantly smaller compared to currents in control cells over a range of test voltages (Figs. 3A and B). We also compared currents in cells co-expressing δ with those lacking δ and those expressing an unrelated control protein CD-8 (Fig. 3C). Likewise, we found that the inhibitory effects of δ were independent of recording conditions and observed when Ca2+ or Ba2+ were used as charge carriers (Supplemental Fig. 2).
Fig. 3.
The δ subunit inhibits the functional expression of CaV1.3α1/β3 channels. (A) Representative traces of IBa recorded from HEK-293 cells expressing CaV1.3a1/β3 channels in absence and presence of δ using solutions A and D (Table 1). Currents were elicited by a 140 ms depolarizing pulse to −30 mV from a Vh of −80 mV. (B) Average I-V relationships for IBa recorded from HEK-293 cells expressing CaV1.3α1/CaVβ3 channels with or without δ. Currents were evoked by 10-mV depolarizing steps from a Vh of −80 mV to potentials between −70 and +60 mV. (C) Relative IBa densities obtained from cells expressing CaV1.3α1/CaVβ3 channels alone (control; solid bar), plus the empty vector pcDNA3, the transmembrane protein CD8 or the δ subunit as listed. n = 5–18 recorded cells. (D) Mean IBa density obtained of HEK-293 cells expressing CaV1.3α1/β3 channels in absence and presence of δ and after co-transfection with the PERK negative dominant construct cDNAs (K618A and ΔC), or after treatment with chloroquine (100 μM), filipin III (5 μg/ml) or MG-132 (25 μM). n = 9–31 recorded cells. The asterisk denotes significant differences (p < 0.05) respect to the control value.
The effects of δ reported here may be the result of a long-term regulation. It is possible that the CaVδ subunit affect processes that control surface targeting and/or overall levels of CaV1.3 protein. To test if the inhibitory effects of δ involved internalization, we used chloroquine, a lysosomal inhibitor, filipin III, a raft/caveolae-dependent endocytosis inhibitor and MG-132, a selective inhibitor of the 26S proteasome. Neither internalization inhibitor interfered with the actions of δ on L-type current density (Fig. 3D).
The inhibitory effects of the δ domain on current density depended on cDNA concentrations. Using fixed levels of CaV1.3α1 and CaVβ3 cDNAs (1:1 molar ratio) we varied δ cDNA levels and show a dose dependent decrease in L-type current densities with an increase in the relative molar ratio of δ (Figs. 4A and B). Interestingly, wild-type CaVα2δ-1 could not compete away the inhibitory effects of δ (Figs. 4C and D). L-type currents measured in cells co-expressing (CaV1.3α1/CaVβ3/CaVα2δ) were attenuated greatly in the presence of δ, suggesting a possible interaction of δ with the channel complex. Likewise, the inhibitory action of δ was channel specific inhibiting CaV2.2 (Supplemental Fig. 3) and CaV1.3 channels (Figs. 4A–4C) but not affecting low voltage activated CaV3.2 (T-type) currents or endogenous K+ currents recorded in untransfected HEK-293 cells (Figs. 4E and F).
Fig. 4.
The inhibitory actions of the δ subunit on recombinant CaV1.3α1/β3 channel are specific. (A) Representative traces of IBa current evoked in HEK-293 cells expressing CaV1.3α1/CaVβ3 channels, transfected with various concentrations of δ. Currents were elicited by depolarizing pulses to −30 mV from a Vh of −80 mV. (B) Comparison of IBa densities obtained in cells transfected with the different concentrations of the δ plasmid. Asterisks denote significant differences (p < 0.05) with respect to the control. n = 8–21 recorded cells. (C) Representative traces of IBa evoked in cells expressing CaV1.3α1/CaVβ3/CaVα2δ-1 channels in absence and presence of δ in equal (1:1) or double molar relationship (2:1) with respect to the other channel subunits. Currents were evoked as in A. (D) Comparison of mean IBa density obtained of HEK-293 cells as in C. n = 19–60 recorded cells. Asterisks denote significant differences (p < 0.05) with respect to the control. (E) Average I-V relationships for IBa recorded from HEK-293 cells stably expressing CaV3.2 channels in absence (pIRES empty vector) and presence of δ cloned into the mammalian expression pIRES vector. Currents were elicited by 100 ms depolarizing pulses in 10 mV steps from a Vh of −80 mV. (B) Average I-V relationships for macroscopic endogenous K+ currents (IK) recorded from HEK-293 cells in absence (pIRES empty vector) and presence of δ using solutions C and E (Table 1). Currents were elicited by 200 ms depolarizing pulses in 10 mV steps from a Vh of −80 mV (n = 10–14 cells).
3.4. δ decreases CaV1.3 channel expression
We next quantified the levels of CaV1.3α1 and CaVβ3 subunits in cells expressing and lacking δ by Western blotting using CaV1.3 and CaVβ3 specific antibodies. Levels of both CaV1.3α1 and CaVβ3 subunits, but not control CD8 protein were significantly lower in cells expressing δ compared to control cells (Fig. 5). The δ-dependent decrease in CaV1.3 and CaVβ3 protein levels might not involve the unfolded protein response (UPR) pathway which has been implicated in the mechanism of action of hemi-Ca2+ channels and Ca2+ channel-related subunits (Raghib et al. 2001, Page et al. 2004, Sandoval et al. 2007), given that two mutant kinase-lacking PERK constructs, PERK DC and PERK K618A (Harding et al. 1999), that interfere with this pathway apparently did not prevent the inhibitory effects of δ on CaV1.3 L-type currents (Fig. 3D).
Fig. 5.
δ domain-induced inhibition is related to decreased CaV subunits expression. (A) The left panel shows the Western blot analysis of membranes from untransfected HEK-293 cells (lane 1) or cells expressing CaV1.3α1/CaVβ3 channels (lane 2) in presence of the δ domain (lane 3) or the transmembrane protein CD8 (lane 4), using an antibody that recognizes the CaV1.3a1 protein. A ~200 kDa band, the expected molecular mass of rat CaV1.3α1 is detected in cells expressing recombinant CaV1.3α1/CaVβ3 channels both in presence and absence of δ. The right panel shows a densitometric analysis of the bands. (B) The left panel shows the Western blot analysis of membranes from HEK-293 cells as in (A) using an antibody that recognizes the CaVb3 protein (~60 kDa). The right panel shows a densitometric analysis of the bands. In both cases bars represent averaged data (± S.E.M.) from 3 independent experiments; the relative levels of the CaV1.3α1 and the CaVβ3 protein expression were analyzed after normalization to those of β-actin. Mean values for the cells that did not express δ were set at 100%.
3.5. Native L-type current are regulated by wild-type α2δ-1 and isolated δ subunits
We next asked if CaVα2δ-1 could also influence native CaV1.3 L-type channels and used RIN-m5F rat insulinoma β-cells. Transient expression of exogenous wild-type CaVα2δ-1 and δ in RIN-m5F cells resulted in significantly larger and smaller currents densities, respectively, when compared to control L-type currents supporting a role for CaVα2δ-1 in controlling overall activity of CaV1.3 L-type channels (Fig. 6).
Fig. 6.
The α2δ-1 subunit regulates native L-type CaV channels in RIN-m5F cells. (A) Representative traces of native IBa recorded from rat insulinoma RIN-m5F cells mock transfected with the pIRES empty vector (control), or the cDNAs coding for the CaVα2δ-1 subunit or the δ domain. Currents were elicited by voltage steps to 0 mV from a Vh of −80 mV using solutions A and D (Table 1). (B) Mean IBa density obtained from RIN-m5F control cells and in presence of the CaVα2δ-1 or the δ constructs as in (A). The asterisk denotes significant difference (p < 0.05). The number of recorded cells is indicated in parentheses.
Although the role of the CaVγ subunit as a component of neuronal CaV channels is still a matter of debate (Moss et al., 2003; Sandoval et al., 2007), we considered important to examine whether the actions of CaVγ could alter the inhibitory of CaVγ. To this end, we performed Western blot experiments in the RIN-m5F cell line to investigate if CaVγ was expressed. The result of this analysis indicates that the CaVγ auxiliary subunit is absent in these cells (Supplemental Fig. 4). Given that the macroscopic Ca2+ currents in the RIN-m5F cells are indeed affected by CaVδ expression, it is reasonable to conclude that this inhibition is independent of CaVγ.
3.6. CaVα2δ-1 modifies the pharmacology of CaV1.3 L-type channels
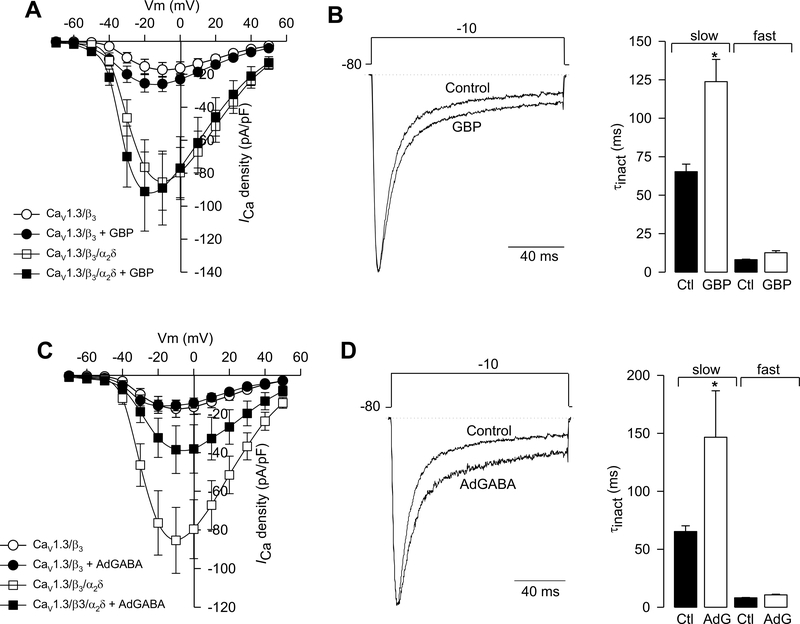
The CaVα2δ subunit modifies the pharmacological sensitivity of neuronal Ca2+ channels to drugs. Most notable, gabapentin (GBP) inhibits N- and P/Q-type channels but only when they associate with CaVα2δ-1 and CaVα2δ−2 (Vega-Hernandez & Felix, 2002; Zoidis et al. 2005; Hendrich et al. 2008; Mich & Horn, 2008). There is evidence that the CaVα2δ-dependent inhibitory actions of GBP and its analogs are important for its therapeutically beneficial actions as analgesics (Field et al. 2006). We were therefore interested in knowing if CaVα2δ-1 could also modify the pharmacological sensitivity of CaV1.3 L-type currents to GBP. Chronic exposure (48 h) to 1 mM GBP, had a small effect on peak L-type current amplitudes in cells expressing CaVCaV1.3α1/CaVβ3 with and without CaVα2δ-1 (Fig. 7A). Average peak L-type current density at −10 mV was −89 ± 22 in absence and −85 ± 17 pA/pF in presence of the drug. However, in consistency with previous reports showing inhibition of recombinant N-type CaV2.2 channel activity (Vega-Hernandez & Felix, 2002; Hendrich et al. 2008), in our hands GBP strongly decreased CaV2.2/CaVβ3 current density when co-expressed with CaVα2δ-1 (Fig. 8), suggesting differential responses of distinct channel types to the drug.
Fig. 7.
The α2δ-1 auxiliary subunit renders the CaV1.3α1/CaVβ3 channels sensitive to gabapentinoids. (A) Average I-V relationships for ICa recorded from HEK-293 cells expressing CaV1.3α1/CaVβ3 channels, with or without CaVα2δ-1, in absence and presence of 1 mM gabapentin (GBP) for 48 h. Currents were evoked by 10-mV depolarizing steps from a Vh of −80 mV to potentials between −70 and +50 mV. n = 9–22 recorded cells. (B) Superimposed normalized current traces in absence (control) and presence of the drug. Currents were elicited by a 140 ms depolarizing pulse to −10 mV from a Vh of −80 mV. (C) Comparison of slow and fast components of inactivation (τinact) in absence and presence of GBP. The values of τinact were obtained by fitting the decaying phase of current traces with eqn 4. The asterisk denotes significant differences (p < 0.05) compared with control. (D) Average I-V relationships for ICa recorded from HEK-293 cells expressing CaV1.3α1/CaVβ3 channels, with or without CaVα2δ-1, in absence and presence of 1 mM AdGABA for 48 h. Currents were evoked by 10-mV depolarizing steps from a Vh of −80 mV to potentials between −70 and +50 mV. (E) Superimposed normalized typical current traces in absence (control) and presence of the drug. Currents were elicited by a 140 ms depolarizing pulse to −10 mV from a Vh of −80 mV. (F) Comparison of slow and fast components of inactivation (τinact) in absence and presence of AdGABA. The asterisk denotes significant difference (p < 0.05) compared with control (n = 15–17 cells).
Fig. 8.
Inhibition of recombinant N-type CaV channels by gabapentinoids. (A) Mean IBa density obtained from HEK-293 cells expressing CaV1.3α1/CaVβ3-1 channels in the control condition and after chronic treatment (48 h) with 1 mM GBP or AdGABA using solutions A and D (Table 1). Currents were elicited by a 140 ms depolarizing pulse to +10 mV from a Vh of −80 mV. n = 7–17 recorded cells. (B) Comparison of the time constants of inactivation (τinact) in absence after the exposure to GBP and AdGABA as indicated. The values of τinact were obtained by fitting the decaying phase of current traces with eqn 4. The asterisk denotes significant differences (p < 0.05) compared with control.
On the other hand, GBP did influence the voltage-dependence of CaV CaV1.3α1/CaVβ3/CaVα2δ-1 channel activation as well as the kinetics of activation and inactivation. GBP induced ~10 mV right shift in the voltage-dependence of L-channel activation, increased the rate of channel activation, and decreased the rate of the slow component of channel inactivation (Fig. 7B). The effect of GBP on the slow component of L-channel inactivation is consistent with its effects on other (N- and P/Q-type) Ca2+ channels (Kang et al. 2002; Hendrich et al. 2008).
Finally, we examined the effects of 2-aminomethyl-2-tricyclo[3.3.1.11,7]decaneacetic-acid hydrochloride 5 (AdGABA), a novel adamantine derivative of GABA that also has strong inhibitory effects on recombinant N-type channels (Zoidis et al. 2005). It is worth noting that although the pharmacological evaluation of AdGABA has demonstrated anticonvulsive and antinociceptive properties, these properties were detectable only at high (sedative) doses, which may limit its potential clinical use. However, both gabapentin (GBP) and AdGABA seem to be acting via the same mechanism (Zoidis et al. 2005).
Chronic exposure (48 h) to 1 mM AdGABA strongly inhibited peak L-type current amplitudes in cells expressing CaV CaV1.3α1/CaVβ3 with CaVα2δ-1 (~2.2-fold inhibition) but not in cells without CaVα2δ-1 (Fig. 7C). Ad GABA did not affect the voltage-dependence or rate of channel activation but like GBP, it lengthened the slow component of channel inactivation in cells expressing CaVα2δ-1 (Fig. 7D).
The pharmacological changes in CaV1.3 L-channels mediated by CaVα2δ-1 are mechanistically similar to those on CaV2.2 N-type channels, but the channels differ in their pharmacological specificity for GBP and AdGABA. Preferential action on CaV2.2 channels by GBP (Fig. 8) might explain why this drug is an effective analgesic.
4. Discussion
Although neuronal L-type Ca2+ channels are thought to open too slowly to contribute to action potential-dependent Ca2+ entry, they seems to play an essential role in regulating activity-dependent gene expression. A complication of studying native L-type channels is that they represent a minor fraction of the whole-cell Ca2+ current in most neurons. A common approach to overcome this problem is the use of cellular systems overexpressing the CaV1.3α1 channel protein (Scholze et al., 2001; Safa et al., 2001; Helton et al., 2005).
Diverse effects of the auxiliary CaVα2δ-1 subunit have been reported on the properties of cloned high voltage-activated Ca2+ channels. Heterologous co-expression of this protein with neuronal CaV2.1α1 (De Waard & Campbell, 1995; Gurnett et al. 1996; Yasuda et al. 2004), CaV2.2α1 (Sandoval et al. 2004; Andrade et al. 2007a), CaV2.3α1 (Parent et al. 1997; Yasuda et al. 2004) or cardiac CaV1.2α1 (Singer et al. 1991; Felix et al. 1997; Itagaki et al., 1992; Shistik et al. 1995; Bangalore et al. 1996; Yasuda et al. 2004) and various combinations of CaVβ subunits resulted in a significant increase in current amplitude. The CaVα2δ-1 subunit has been also shown to mediate hyperpolarizing shifts in the voltage dependence of Ca2+ channel activation (Yasuda et al. 2004) and inactivation (Singer et al. 1991; Felix et al. 1997; Yasuda et al. 2004), in addition to regulating the kinetics of current activation (Singer et al. 1991; Bangalore et al. 1996) and inactivation (Singer et al. 1991; De Waard & Campbell, 1995; Felix et al. 1997). The increase in current amplitude could be attributed to enhanced targeting of expressed CaVa1 subunits to the plasma membrane, while the effects on the time course and/or voltage dependence of current activation and inactivation suggest a more specific modulation of the channel’s gating.
Very recently, the first successful attempt at knocking out the CaVα2δ-1-subunit has been reported. A comparison of the electrophysiological properties in isolated cardiomyocytes from the CaVα2δ-1 (−/−) and wild-type mice showed that the absence of the CaVα2δ-1 gene results in an attenuated Ca2+ current amplitude, a decrease in Ca2+ density, an increase in the time constants (fast and slow), and a depolarizing shift in activation and inactivation (Fuller-Bicer et al., 2009). Interestingly, the ablation of the CaVα2δ-1 subunit resulted in reduced GBP binding in the knock-out animals compared with wild-type in either brain or skeletal muscle (Fuller-Bicer et al., 2009).
Likewise, recent studies have shown that the CaVα2δ-1 subunit might play a more pronounced role in regulating current amplitudes than the other Ca2+ channel auxiliary subunits (Yasuda et al. 2004). In spite of this, to date, there have been relatively few studies showing the effects of CaVα2δ-1 on L-type CaV1.3 channel activity (Williams et al. 1992; Scholze et al. 2001). Indeed, it has not yet been investigated whether the expression of the CaV1.3α1 subunit requires this auxiliary subunit for trafficking to the cell membrane, or for functional expression, or whether CaVα2δ-1 influences the biophysical properties of the CaV1.3 channels in mammalian cells. In the present study we show that co-expression of the CaVα2δ-1 subunit has clear effects on the functional expression of recombinant and native CaV1.3 channels. Over-expression of exogenous CaVα2δ-1 produced a ~3- to 5-fold increase in the amplitude of currents through recombinant CaVCaV1.3α1/CaVβ3 channels expressed in HEK-293 cells, but had only minor effects on their kinetics or voltage dependence of activation and inactivation. Interestingly, similar effects has been previously reported in Xenopus oocytes in which the amplitude of neuroendocrine CaV1.3α1/CaVβ3 channel currents was increased by ~2- to 5-fold upon co-expression of the CaVα2δ subunit (Scholze et al. 2001). This argues either for an effect of the auxiliary subunit on the trafficking of the nascent CaV1.3α1/CaVβ3 channels from the endoplasmic reticulum to the cell membrane, or an effect to stabilize the membrane channels in a functional conformation. Further studies will be necessary to determine whether CaVα2δ-1 affects the properties of the currents acting at the single channel level.
In order to evaluate in more detail the molecular determinants of CaV1.3 channel regulation by the CaVα2δ-1 subunit, a series of site-directed mutants was constructed and functionally analyzed. The amino acids N136 and N184 as well as the sequence between residues R941 to V946 in the protein has been described previously to be important for the subunit-induced current stimulation (Sandoval et al. 2004; Andrade et al. 2007a). The two asparagine residues seem to be glycosylated in vivo while the six amino acids localized between A941 and V946 (Arg-Leu-Leu-Glu-Ala-Val) presumably constitute the proteolytic site in CaVα2δ-1. Substitution of such amino acids renders the CaVα2δ-1 subunit non-functional as shown by patch-clamp experiments in experiments using CaV2.2α1/CaVβ3 channels. Consistent with this, electrophysiological recordings performed in cells expressing mutant constructs indicated that the stimulatory effect of CaVα2δ-1 on macroscopic currents through CaV1.3a1/CaVb3 channels was partially or completely lost. Last, the same experiments were repeated for a construct in which all cysteine residues in the extracellular region of δ were substituted by methionine or serine (C962M; C984S; C987S; C1032S; C1047S; C1059S). Mutation of the six residues also abolished the stimulatory effect of CaVα2δ-1 on functional CaV1.3α1/CaVβ3 channels, suggesting that the disulphide linkage between the α2 and the δ polypeptides is required for function. Given that the voltage dependence and time course for the activation and inactivation of the channels were practically unaltered, these effects of the CaVα2δ-1 mutant constructs may not be explained by alterations in the functional properties of the channels, but might involve a reduced number of functional channels in the surface of the membrane.
Another interesting finding of our study was that the δ domain of the CaVα2δ-1 auxiliary subunit exerts an important inhibitory effect on currents through recombinant CaV1.3 channels heterologously expressed in HEK-293 cells. This inhibition was specific given that δ co-expression did not affect endogenous K+ current or heterologously expressed low threshold T-type channels (of the CaV3.2 class), and was not mimicked by the unrelated protein CD8. An exciting issue to be clarified relates to cell pathway(s) by which this inhibition occurs. Based on our findings we could speculate that distinct mechanisms underlie the decrease in current density after δ co-expression. It has been reported that the expression of short variants of the CaV2α1 subunit as well as the over-expression of the neuronal γ2 subunit (stargazing) suppress currents through the activation of an endoplasmic reticulum resident RNA-dependent kinase (PERK) which activates components of the unfolded protein response (UPR) (Page et al. 2004; Sandoval et al. 2007). We therefore tested whether CaV1.3 current suppression by δ involved activation of the UPR using two mutant constructs that have shown to prevent the activation of endogenous PERK (Harding et al. 1999) and therefore inhibit the UPR. With the PERK ΔC and the K618A mutation the suppressive effect of δ remained unaltered, suggesting that activation of PERK may not play a role in the effects of the regulatory subunit. Likewise, to analyze the possible participation of a CaV1.3 channel internalization/degradation-dependent mechanism after δ co-expression, a series of inhibitors were used. We tested chloroquine, filipin III and MG-132, but all internalization inhibitors failed to alter the inhibitory actions of δ as current reduction persisted after treatment. However, several alternative mechanisms could be anticipated to elucidate the δ-induced regulation of CaV1.3 channel functional expression including: i) that interaction with δ strongly affects the folding of nascent CaV1.3α1 subunits and affects the interaction with the CaVα2δ and/or CaVβ subunits, ii) that δ is able to unmask retention signals that prevent the unassembled channel subunits from leaving the ER, and iii) that δ over-expression could be increasing the degradation rate of the mRNAs for other CaV subunit reducing the channel protein levels and hence altering the functional expression of the channels.
Last, the expression and functional integrity of the CaVα2δ-1 subunit in our model system was also confirmed pharmacologically by examining the chronic effects of GBP (1 mM) and AdGABA, (1 mM) two anticonvulsant drugs that bind the auxiliary subunit (Gee et al. 1996; Zoidis et al. 2005). GBP did not affect the amplitude of the currents and have a minor effect on the voltage dependence of activation. Exposure to the drug did, however, slow down the kinetics of inactivation. These results differ to that reported recently for recombinant neuronal CaV channels in which chronic incubation with GBP (1 mM) reduced currents through CaV2.1α1/CaVβ4/CaVα2δ−2 and CaV2.2α1/CaVβ1b/CaVα2δ-1 channels and shifted the voltage dependence of steady-state inactivation to more positive potentials (Hendrich et al. 2008). A possible explanation for this difference is that the affinity of GBP to the CaVα2δ subunit may be modulated by other subunits, and that the effects of the drug depend on the composition and environment of the channel. Likewise, we also found that chronic treatment with AdGABA significantly inhibited macroscopic currents through CaV1.3α1/CaVβ3/CaVα2δ-1 channels. To our knowledge, the inhibitory effect of AdGABA on CaV1.3 channel functional expression, represents a previously uncharacterized action of this drug, and is in agreement with our previous report describing the synthesis and pharmacological profile of AdGABA (Zoidis et al. 2005), in which we found that it reduces the functional expression of neuronal recombinant channels of the CaV2.2 class.
Supplementary Material
Acknowledgements
We gratefully appreciate the technical expertise of M. Urban and G. Aguilar. We also thank M. Oyadomari and D. Ron (Skirball Institute of Biomolecular Medicine, New York, NY) for the PERK cDNA constructs, J.C. Gomora (IFC-UNAM, Mexico) for the CaV3.1 channel stably expressing HEK-293 cell line and the b1a cDNA clone, as well as G. Zoidis and N. Kolocouris (Univ. of Athens) for AdGABA and M. Hernandez (Cinvestav-IPN, Mexico) for his generous gift of the β-actin antibodies. This work was supported in part by a grant from The National Council for Science and Technology (Conacyt) to R.F. Doctoral and postdoctoral fellowships from Conacyt and Instituto de Ciencia y Tecnología del Distrito Federal to A.A. and A.S., respectively, are gratefully acknowledged. We would like to thank J. Lueck (Univ. of Iowa) for helpful comments on the manuscript. K.P.C. is an Investigator of the Howard Hughes Medical Institute.
References
- [1].Andrade A, Sandoval A, Oviedo N, De Waard M, Elias D, Felix R, Proteolytic cleavage of the voltage-gated Ca2+ channel α2δ subunit: structural and functional features, Eur. J. Neurosci 25 (2007) 1705–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Andrade A, de Leon MB, Hernandez-Hernandez O, Cisneros B, Felix R, Myotonic dystrophy CTG repeat expansion alters Ca2+ channel functional expression in PC12 cells, FEBS Lett. 581 (2007) 4430–4438. [DOI] [PubMed] [Google Scholar]
- [3].Avila G, Sandoval A, Felix R, Intramembrane charge movement associated with endogenous K+ channel activity in HEK-293 cells, Cell. Mol. Neurobiol 24 (2004) 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Avila T, Andrade A, Felix R, Transforming growth factor-b1 and bone morphogenetic protein-2 downregulate CaV3.1 channel expression in mouse C2C12 myoblasts, J. Cell. Physiol 209 (2006) 448–456. [DOI] [PubMed] [Google Scholar]
- [5].Bangalore R, Mehrke G, Gingrich K, Hofmann F, Kass RS Influence of L-type Ca channel α2/δ-subunit on ionic and gating current in transiently transfected HEK-293 cells, Am. J. Physiol 270 (1996) H1521–H1528. [DOI] [PubMed] [Google Scholar]
- [6].Bito H, Deisseroth K, Tsien RW, CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression, Cell 87 (1996) 1203–1214. [DOI] [PubMed] [Google Scholar]
- [7].Canti C, Nieto-Rostro M, Foucault I, Heblich F, Wratten J, Richards MW, Hendrich J, Douglas L, Page KM, Davies A, Dolphin AC, The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of α2δ subunits is key to trafficking voltage-gated Ca2+ channels, Proc. Natl. Acad. Sci. USA 102 (2005) 11230–11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Catterall WA, Structure and regulation of voltage-gated Ca2+ channels, Annu. Rev. Cell. Dev. Biol 16 (2000) 521–555. [DOI] [PubMed] [Google Scholar]
- [9].Cribbs LL, Lee JH, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, Perez-Reyes E, Cloning and characterization of α1H from human heart, a member of the T-type Ca2+ channel gene family, Circ Res 83 (1998) 103–109. [DOI] [PubMed] [Google Scholar]
- [10].Roberts-Crowley ML, Rittenhouse AR, Arachidonic acid inhibition of L-type calcium (CaV1.3b) channels varies with accessory CaVβ subunits, J. Gen. Physiol 133 (2009) 387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ, Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models, Nat. Neurosci 9 (2006) 251–259. [DOI] [PubMed] [Google Scholar]
- [12].de Leon M, Wang Y, Jones L, Perez-Reyes E, Wei X, Soong TW, Snutch TP, Yue DT, Essential Ca2+-binding motif for Ca2+-sensitive inactivation of L-type Ca2+ channels, Science 270 (1995) 1502–1506. [DOI] [PubMed] [Google Scholar]
- [13].De Waard M, Campbell KP, Subunit regulation of the neuronal α1A Ca2+ channel expressed in Xenopus oocytes, J. Physiol 485 (1995) 619–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dickman K, Kurshan PT, Schwarz TL, Mutations in a Drosophila α2δ voltage-gated calcium channel subunit reveal a crucial synaptic function, J. Neurosci 28 (2008) 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME, Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway, Science 294 (2001) 333–339. [DOI] [PubMed] [Google Scholar]
- [16].Felix R, Gurnett CA, De Waard M, Campbell KP, Dissection of functional domains of the voltage-dependent Ca2+ channel α2δ subunit, J. Neurosci 17 (1997) 6884–6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, Bramwell S, Corradini L, England S, Winks J, Kinloch RA, Hendrich J, Dolphin AC, Webb T, Williams D, Identification of the α2δ-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin, Proc. Natl. Acad. Sci. USA 103 (2006) 17537–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fuller-Bicer GA, Varadi G, Koch SE, Ishii M, Bodi I, Kadeer N, Muth JN, Mikala G, Petrashevskaya NN, Jordan MA, Zhang SP, Qin N, Flores CM, Isaacsohn I, Varadi M, Mori Y, Jones WK, Schwartz A, Targeted disruption of the voltage-dependent calcium channel α2/δ-1-subunit. Am. J. Physiol. Heart. Circ. Physiol 297 (2009) H117–H124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN, The novel anticonvulsant drug, gabapentin (Neurontin), binds to the α2δ subunit of a calcium channel, J. Biol. Chem 271 (1996) 5768–5776. [DOI] [PubMed] [Google Scholar]
- [20].Graef IA, Mermelstein PG, Stankunas K, Neilson JR, Deisseroth K, Tsien RW, Crabtree GR, L-type calcium channels and GSK-3 regulate the activity of NFATc4 in hippocampal neurons, Nature 401 (1999) 703–708. [DOI] [PubMed] [Google Scholar]
- [21].Gurnett CA, De Waard M, Campbell KP, Dual function of the voltage-dependent Ca2+ channel α2δ subunit in current stimulation and subunit interaction, Neuron 16 (1996) 431–440. [DOI] [PubMed] [Google Scholar]
- [22].Gurnett CA, Felix R, Campbell KP, Extracellular interaction of the voltage-dependent Ca2+ channel α2δ and a1 subunits, J. Biol. Chem 272 (1997) 18508–18512. [DOI] [PubMed] [Google Scholar]
- [23].Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ, Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches, Pflugers Arch. 391 (1981) 85–100. [DOI] [PubMed] [Google Scholar]
- [24].Harding HP, Zhang Y, Ron D, Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase, Nature 397 (1999) 271–274. [DOI] [PubMed] [Google Scholar]
- [25].Helton TD, Xu W, Lipscombe D, Neuronal L-type calcium channels open quickly and are inhibited slowly, J. Neurosci 25 (2005) 10247–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC, Pharmacological disruption of calcium channel trafficking by the α2δ ligand gabapentin, Proc. Natl. Acad. Sci. USA 105 (2008) 3628–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Imredy JP, Yue DT, Mechanism of Ca2+-sensitive inactivation of L-type Ca2+ channels, Neuron 12 (1994) 1301–1318. [DOI] [PubMed] [Google Scholar]
- [28].Itagaki K, Koch WJ, Bodi I, Klöckner U, Slish DF, Schwartz A Native-type DHP-sensitive calcium channel currents are produced by cloned rat aortic smooth muscle and cardiac α1 subunits expressed in Xenopus laevis oocytes and are regulated by α2- and β-subunits, FEBS Lett. 297 (1992) 221–225. [DOI] [PubMed] [Google Scholar]
- [29].Johnson SL, Marcotti W, Biophysical properties of CaV1.3 calcium channels in gerbil inner hair cells, J. Physiol 586 (2008) 1029–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kang MG, Felix R, Campbell KP, Long-term regulation of voltage-gated Ca2+ channels by gabapentin, FEBS Lett. 528 (2002) 177–182. [DOI] [PubMed] [Google Scholar]
- [31].Krey JF, Dolmetsch RE, Molecular mechanisms of autism: a possible role for Ca2+ signaling, Curr. Opin. Neurobiol 17 (2007) 112–119. [DOI] [PubMed] [Google Scholar]
- [32].Klugbauer N, Lacinova L, Marais E, Hobom M, Hofmann F, Molecular diversity of the calcium channel α2δ subunit, J. Neurosci 19 (1999) 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lacinova L, Voltage-dependent calcium channels, Gen. Physiol. Biophys 24 Suppl 1 (2005) 1–78. [PubMed] [Google Scholar]
- [34].Liang Y, Tavalin SJ, Auxiliary β subunits differentially determine PKA utilization of distinct regulatory sites on CaV1.3 L type Ca2+ channels, Channels (Austin) 1 (2007) 102–112. [DOI] [PubMed] [Google Scholar]
- [35].Lipscombe D, Helton TD, Xu W, L-type calcium channels: the low down, J. Neurophysiol 92 (2004) 2633–2641. [DOI] [PubMed] [Google Scholar]
- [36].Marcantoni A, Baldelli P, Hernandez-Guijo JM, Comunanza V, Carabelli V, Carbone E, L-type calcium channels in adrenal chromaffin cells: role in pace-making and secretion, Cell Calcium. 42 (2007) 397–408. [DOI] [PubMed] [Google Scholar]
- [37].Mishina M, Kurosaki T, Tobimatsu T, Morimoto Y, Noda M, Yamamoto T, Terao M, Lindstrom J, Takahashi T, Kuno M, Numa S, Expression of functional acetylcholine receptor from cloned cDNAs, Nature 307 (1984) 604–608. [DOI] [PubMed] [Google Scholar]
- [38].Mich PM, Horne WA, Alternative splicing of the Ca2+ channel β4 subunit confers gabapentin specificity for inhibition of CaV2.1 trafficking, Mol. Pharmacol 74 (2008) 904–912. [DOI] [PubMed] [Google Scholar]
- [39].Moss FJ, Dolphin AC, Clare JJ, Human neuronal stargazin-like proteins, γ2, γ3 and γ4; an investigation of their specific localization in human brain and their influence on CaV2.1 voltage-dependent calcium channels expressed in Xenopus oocytes. BMC Neurosci. 4 (2003) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Page KM, Heblich F, Davies A, Butcher AJ, Leroy J, Bertaso F, Pratt WS, Dolphin AC, Dominant-negative calcium channel suppression by truncated constructs involves a kinase implicated in the unfolded protein response, J. Neurosci 24 (2004) 5400–5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Parent L, Schneider T, Moore CP, Talwar D, Subunit regulation of the human brain a1E calcium channel, J. Membr. Biol 160 (1997) 127–140. [DOI] [PubMed] [Google Scholar]
- [42].Qin N, Olcese R, Stefani E, Birnbaumer L, Modulation of human neuronal a1Etype calcium channel by α2δ-subunit, Am. J. Physiol 274 (1998) C1324–C1331. [DOI] [PubMed] [Google Scholar]
- [43].Raghib A, Bertaso F, Davies A, Page KM, Meir A, Bogdanov Y, A. C..Dolphin, Dominant-negative synthesis suppression of voltage-gated calcium channel CaV2.2 induced by truncated constructs, J. Neurosci 21 (2001) 8495–8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rogawski MA, Loscher W, The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions, Nat. Med 10 (2004) 685–692. [DOI] [PubMed] [Google Scholar]
- [45].Safa P, Boulter J, Hales TG Functional properties of Cav1.3 (α1D) L-type Ca2+ channel splice variants expressed by rat brain and neuroendocrine GH3 cells. J. Biol. Chem 276 (2001) 38727–38737. [DOI] [PubMed] [Google Scholar]
- [46].Sandoval A, Oviedo N, Andrade A, Felix R, Glycosylation of asparagines 136 and 184 is necessary for the α2δ subunit-mediated regulation of voltage-gated Ca2+ channels, FEBS Lett. 576 (2004) 21–26. [DOI] [PubMed] [Google Scholar]
- [47].Sandoval A, Andrade A, Beedle AM, Campbell KP, Felix R, Inhibition of recombinant N-type CaV channels by the γ2 subunit involves unfolded protein response (UPR)-dependent and UPR-independent mechanisms, J. Neurosci 27 (2007) 3317–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Scholze A, Plant TD, Dolphin AC, Nürnberg B, Functional expression and characterization of a voltage-gated CaV1.3(α1D) calcium channel subunit from an insulin-secreting cell line, Mol. Endocrinol 15 (2001) 1211–1221. [DOI] [PubMed] [Google Scholar]
- [49].Shistik E, Ivanina T, Puri T, Hosey M, Dascal N, Ca2+ current enhancement by α2/δ and β subunits in Xenopus oocytes: contribution of changes in channel gating and a1 protein level, J. Physiol 489 (1995) 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Singh A, Gebhart M, Fritsch R, Sinnegger-Brauns MJ, Poggiani C, Hoda JC, Engel J, Romanin C, Striessnig J, Koschak A, Modulation of voltage- and Ca2+-dependent gating of CaV1.3 L-type calcium channels by alternative splicing of a Cterminal regulatory domain, J. Biol. Chem 283 (2008) 20733–20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N, The roles of the subunits in the function of the calcium channel, Science 253 (1991) 1553–1557. [DOI] [PubMed] [Google Scholar]
- [52].Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT, CaV1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism, Cell 119 (2004) 19–31. [DOI] [PubMed] [Google Scholar]
- [53].Surmeier J, Calcium, ageing, and neuronal vulnerability in Parkinson’s disease, Lancet Neurol. 6 (2007) 933–938. [DOI] [PubMed] [Google Scholar]
- [54].Tuluc P, Kern G, Obermair GJ, Flucher BE, Computer modeling of siRNA knockdown effects indicates an essential role of the Ca2+ channel α2δ-1 subunit in cardiac excitation-contraction coupling, Proc. Natl. Acad. Sci. USA 104 (2007) 11091–11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Vega-Hernandez A, Felix R, Down-regulation of N-type voltage-activated Ca2+ channels by gabapentin, Cell. Mol. Neurobiol 22 (2002) 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Welling B, Bosse E, Cavalie A, Bottlender R, Ludwig A, Nastainczyk W, Flockerzi V, Hofmann F, Stable co-expression of calcium channel α1, β and α2/δ subunits in a somatic cell line, J. Physiol 471 (1993) 749–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Williams ME, Feldman DH, McCue AF, Brenner R, Velicelebi G, Ellis SB, Harpold MM, Structure and functional expression of α1, α2, and β subunits of a novel human neuronal calcium channel subtype, Neuron 8 (1992) 71–84. [DOI] [PubMed] [Google Scholar]
- [58].Xu W, Lipscombe D, Neuronal CaV1.3α1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines, J. Neurosci 21 (2001) 5944–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yang SN, Berggren PO, The role of voltage-gated calcium channels in pancreatic β-cell physiology and pathophysiology, Endocr. Rev 27 (2006) 621–676. [DOI] [PubMed] [Google Scholar]
- [60].Yang PS, Alseikhan BA, Hiel H, Grant L, Mori MX, Yang W, Fuchs PA, Yue DT, Switching of Ca2+-dependent inactivation of CaV1.3 channels by calcium binding proteins of auditory hair cells, J. Neurosci 26 (2006) 10677–10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yasuda T, Chen L, Barr W, McRory JE, Lewis RJ, Adams DJ, Zamponi GW, Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells, Eur. J. Neurosci 20 (2004) 1–13. [DOI] [PubMed] [Google Scholar]
- [62].Zhang X, Odom DT, Koo SH, Conkright MD, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, Kadam S, Ecker JR, Emerson B, Hogenesch JB, Unterman T, Young RA, Montminy M, Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc. Natl Acad. Sci. USA, 102 (2005b) 4459–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhang H, Maximov A, Fu Y, Xu F, Tang TS, Tkatch T, Surmeier DJ, Bezprozvanny I, Association of CaV1.3 L-type calcium channels with Shank, J. Neurosci 25 (2005b) 1037–1049G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhang Z, Xu Y, Song H, Rodriguez J, Tuteja D, Namkung Y, Shin HS, Chiamvimonvat N, Functional roles of CaV1.3 (α1D) calcium channel in sinoatrial nodes: insight gained using gene-targeted null mutant mice. Circ. Res 90 (2002) 981–987. [DOI] [PubMed] [Google Scholar]
- [65].Zoidis G, Papanastasiou I, Dotsikas I, Sandoval A, Dos Santos RG, Papadopoulou-Daifoti Z, Vamvakides A, Kolocouris N, Felix R, The novel GABA adamantane derivative (AdGABA): design, synthesis, and activity relationship with gabapentin, Bioorg. Med. Chem 13 (2005) 2791–2798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.