Abstract
Objectives
Determine whether auditory brainstem response (ABR) wave I amplitude is associated with measures of auditory perception in young people with normal distortion product otoacoustic emissions (DPOAEs) and varying levels of noise exposure history.
Design
Tinnitus, loudness tolerance, and speech perception ability were measured in 31 young military Veterans and 43 non-Veterans (19–35 years of age) with normal pure tone thresholds and DPOAEs. Speech perception was evaluated in quiet using NU-6 word lists and in background noise using the words in noise (WIN) test. Loudness discomfort levels were measured using 1, 3, 4, and 6 kHz pulsed pure tones. DPOAEs and ABRs were collected in each participant to assess outer hair cell (OHC) and auditory nerve function.
Results
The probability of reporting tinnitus in this sample increased by a factor of 2.0 per 0.1 μV decrease in ABR wave I amplitude (95% Bayesian confidence interval = 1.1 to 5.0) for males and by a factor of 2.2 (95% confidence interval = 1.0 to 6.4) for females after adjusting for sex and DPOAE levels. Similar results were obtained in an alternate model adjusted for pure tone thresholds in addition to sex and DPOAE levels. No apparent relationship was found between wave I amplitude and either loudness tolerance or speech perception in quiet or noise.
Conclusions
Reduced ABR wave I amplitude was associated with an increased risk of tinnitus, even after adjusting for DPOAEs and sex. In contrast, wave III and V amplitudes had little effect on tinnitus risk. This suggests that changes in peripheral input at the level of the inner hair cell (IHC) or auditory nerve may lead to increases in central gain that give rise to the perception of tinnitus. Although the extent of synaptopathy in the study participants cannot be measured directly, these findings are consistent with the prediction that tinnitus may be a perceptual consequence of cochlear synaptopathy.
INTRODUCTION
Tinnitus, hyperacusis, and difficulty understanding speech in background noise are three of the most common complaints reported to audiologists. These perceptual changes are often associated with elevated hearing thresholds, but most puzzling are the cases when patients with normal audiograms present with these problems (Barnea et al. 1990; Anari et al. 1999; Sanchez et al. 2005; Zhao & Stephens 2007; Sheldrake et al. 2015). Cochlear synaptopathy, the selective damage to afferent auditory nerve synapses onto the inner hair cells (IHCs), offers a potential explanation for the presence of these perceptual deficits in individuals who appear audiometrically normal. Cochlear synaptopathy was originally described in animal models in response to noise exposure and aging (Kujawa & Liberman 2009; Lin et al. 2011; Sergeyenko et al. 2013), but recent physiological data and temporal bone studies suggest that synaptopathy may also affect humans (Makary et al. 2011; Konrad-Martin et al. 2012; Viana et al. 2015; Stamper & Johnson 2015; Bramhall et al. 2017). In animal models, the amplitude of wave I of the auditory brainstem response (ABR) is correlated with the number of IHC-afferent fiber synapses, with smaller amplitudes associated with partial loss of nerve fibers (Kujawa & Liberman 2009; Earl & Chertoff 2010; Lin et al. 2011; Sergeyenko et al. 2013; Fernandez et al. 2015). Smaller ABR wave I amplitudes have been observed in humans with normal pure tone thresholds and a significant history of noise exposure compared with individuals with less noise exposure history, which may be an indication of noise-induced synaptopathy (Stamper & Johnson 2015; Bramhall et al. 2017). However, other studies of young people with normal audiometric thresholds and varying levels of noise exposure have not shown a noise-related decrease in ABR wave I amplitude (Prendergast et al. 2017; Guest et al. 2017). Age-related reductions in ABR wave I amplitude have been demonstrated in humans (Konrad-Martin et al. 2012; Bramhall et al. 2015) and are consistent with temporal bone studies showing auditory neuronal and synaptic loss with age (Makary et al. 2011; Viana et al. 2015).
Animal and human studies suggest that the perception of tinnitus and hyperacusis may arise from excess neural activity in the central auditory system in response to decreased peripheral input, such as seen with cochlear synaptopathy (Auerbach et al. 2014; Brotherton et al. 2015). Note that the term hyperacusis used here refers specifically to loudness hyperacusis rather than pain hyperacusis (see Tyler et al. 2014 for a detailed description of the difference between these terms). A study in a mouse model of noise-induced synaptopathy found both reduced ABR wave I amplitude and hyperacusis-like behavior (Hickox & Liberman 2014). In addition, an association has been shown between decreased ABR wave I amplitude and tinnitus in humans with normal auditory thresholds or thresholds matched to the control group (Schaette & McAlpine 2011; Gu et al. 2012). In both animal and human studies where decreased wave I amplitude was observed, wave V amplitude was either unchanged or was increased compared with animals/individuals with more normal wave I amplitudes (Schaette & McAlpine 2011; Gu et al. 2012; Sergeyenko et al. 2013; Stamper & Johnson 2015; Bramhall et al. 2017). Given that wave V of the ABR reflects contributions from central auditory nuclei, the absence of a decrease in wave V amplitude in individuals with decreased wave I amplitude has been interpreted as evidence of either central hyperactivity or loss of inhibition in response to the decreased input from the auditory nerve (Schaette & McAlpine 2011; Gu et al. 2012). Central compensatory changes resulting from cochlear synaptopathy may explain the seemingly paradoxical complaints of tinnitus and hyperacusis in patients with normal audiograms. This is supported by the fact that tinnitus and hyperacusis often occur together. An estimated 86% of patients who report hyperacusis also experience tinnitus and 27–40% of people with tinnitus report hyperacusis (Sheldrake et al. 2015). However, a recent study of young people with and without tinnitus who were matched for age, sex, and audiometric thresholds up to 14 kHz did not reveal a relationship between ABR wave I amplitude and tinnitus (Guest et al. 2017). One possible explanation for these differing results is that ABR wave I amplitude group differences observed in previous human studies were related to extended high frequency threshold differences between the groups rather than synaptopathy. Using derived narrowband ABR responses, Don and Eggermont showed that frequencies above 2 kHz have a large contribution to wave I amplitude, while wave V amplitude is driven primarily by lower frequencies (1978). Therefore, high frequency cochlear damage may lead to a decrease in wave I/V amplitude ratio by increasing the influence of lower frequency regions of the cochlea, whose relative contribution to the ABR response is greater for wave V than for wave I.
In humans, depending on age and cochlear region, approximately 5–15 auditory nerve fibers synapse with each IHC (Viana et al. 2015). In animal models, these fibers vary in terms of their spontaneous firing rate and response threshold. Fibers with low spontaneous rates have high thresholds (i.e. they only respond at high intensity levels), whereas high spontaneous rate fibers have low thresholds (Liberman 1978). Although individual auditory nerve fibers have a limited dynamic range of ~10 to 40 dB (Guinan & Stankovic 1996; Taberner & Liberman 2005), the diverse range of thresholds across fibers enables the auditory system to respond to sounds over a much larger dynamic range. High threshold fibers appear to be particularly vulnerable to noise- and age-related cochlear synaptopathy (Schmiedt et al. 1996; Furman et al. 2013). However, because high threshold fibers do not respond at low stimulus levels, their loss does not affect auditory thresholds (Furman et al. 2013). The high thresholds of these fibers make them less susceptible to the effects of background noise than low threshold fibers (Costalupes et al. 1984). An intact population of high threshold fibers is required to maintain stimulus coding fidelity in the presence of any background noise that saturates the responses of the low threshold fibers (Reiss et al. 2011). This may lead to diminished speech-in-noise perception in individuals suffering from cochlear synaptopathy. This hypothesis is supported by the finding that the combination of smaller ABR wave I amplitudes and elevated pure tone thresholds is associated with poorer performance on the QuickSIN (Bramhall et al. 2015). A study of young music students with high levels of reported noise exposure and normal audiograms showed decreased speech intelligibility in noise compared to controls with less noise exposure (Liberman et al. 2016). In this study, speech-in-noise perception was correlated with the ratio between the summating potential and ABR wave I amplitude, but the music students also had significantly poorer extended high frequency thresholds than the control group, so this may have impacted the results. In contrast, Hoben et al. did not observe a relationship between ABR wave I amplitude and speech-in-noise perception among individuals with normal OHC function (2017).
Although it has generally been assumed that noise induced cochlear synaptopathy will primarily impact low spontaneous rate/high threshold fibers, it has also been suggested that there may be a relationship between the loss of high spontaneous rate/low threshold fibers and tinnitus. In noise-exposed rats with behavioral evidence of tinnitus, Bauer et al. showed minimal IHC and OHC loss, but significant loss of high threshold auditory nerve fibers 6 months after exposure (2007). Paul et al. measured the envelope following response of young people with and without tinnitus and then used an auditory nerve model to predict the auditory nerve fiber loss that would be necessary to generate the measured responses for each group (2017). Based on this analysis, they concluded that a loss confined to high threshold fibers was insufficient to explain their results and that there must be an additional loss of low threshold fibers in the individuals with tinnitus.
In some cases, complaints of tinnitus, hyperacusis, and difficulty with speech-in-noise perception among individuals with normal audiograms may be related to sub-clinical cochlear damage. In people with normal audiograms, lower distortion product otoacoustic emission (DPOAE) levels and poorer extended high frequency thresholds have been associated with deficits such as tinnitus and difficulty with speech-in-noise perception (Badri et al. 2011; Modh et al. 2014). This highlights the importance of measuring otoacoustic emissions (OAEs) and extended high frequency thresholds in human studies of synaptopathy.
This study investigated the relationship between ABR wave I amplitude and measures of auditory perception among young military Veterans and non-Veterans with normal auditory thresholds and varying degrees of self-reported lifetime noise exposure. Lower ABR wave I amplitudes were strongly associated with the perception of tinnitus. In contrast, speech perception in quiet, performance on the words in noise (WIN) test, and loudness discomfort levels (LDLs) appeared unrelated to ABR wave I amplitude. Without temporal bone analysis, it cannot be confirmed that the study participants who reported tinnitus suffer from cochlear synaptopathy. However, the observed reduction in ABR wave I amplitude in combination with normal DPOAEs is consistent with that interpretation. These findings support the prediction that some forms of tinnitus may be associated with cochlear synaptopathy.
MATERIALS AND METHODS
Participants
One hundred sixteen participants age 19–35 were recruited as part of a larger study previously described by Bramhall et al. (2017). All participants received an audiometric evaluation from a licensed audiologist including tympanometry and air and bone conduction thresholds, screening of cognitive status with the MiniMental Status Examination (MMSE, Par Lutz, FL), and screening of DPOAE levels in response to moderate level stimuli. All participants had normal audiometric thresholds (≤ 20 dB HL) from 0.25–8 kHz, normal middle ear function, normal DPOAEs (criteria described below), and were in good general health with no significant history of otologic or neurologic disorder (including traumatic brain injury). See Bramhall et al. (2017) for additional screening criteria. Data from 10 additional participants are included in this report. A total of 74 participants were enrolled in the study (17 Veterans with a history of significant noise exposure, 14 Veterans with less noise exposure, 27 non-Veteran controls with very limited noise exposure, and 16 non-Veterans with a history of firearm use). Three participants (all non-Veterans) were excluded from the speech perception analysis because they were not native English speakers. After the screening evaluation, all subsequent study measures were taken only in a single ear. If only one ear met the study criteria (based on audiometric air and bone conduction thresholds, DPOAE screening, and tympanometry), that ear was tested. Thirty participants qualified for the study only in a single ear. The reasons for screen failing in the contralateral ear are summarized in a table in the supplemental data (see Supplemental Data Table 1). If both ears qualified for the study, the ear with higher level DPOAEs was tested to minimize the effects of outer hair cell (OHC) loss. An overview of the participant characteristics is shown in Table 1.
Table 1.
Participant Characteristics by Noise Exposure Group
| Non-Veteran | Non-Veteran Firearms | Veteran Low Noise | Veteran High Noise | |
|---|---|---|---|---|
| Age in years | 25.4 (4.2) | 25.8 (4.2) | 29.7 (4.2) | 26.7 (2.3) |
| Number of males | 7 | 7 | 6 | 15 |
| PTA (0.5, 1, & 2 kHz) – in dB HL | 6.9 (4.1) | 6.7 (3.0) | 9.4 (4.8) | 10.3 (5.6) |
| High frequency PTA (3, 4, & 6 kHz) – in dB HL | 2.1 (4.5) | 4.1 (5.0) | 4.5 (3.6) | 9.2 (4.9) |
| LENS-Q score | 4.3 (0.6) | 13.2 (2.4) | 10.8 (3.4) | 15.9 (0.8) |
| Speech in quiet (60 dB HL) | 98.3% (1.7) | 98.8% (1.4) | 97.7% (2.2) | 97.3% (2.4) |
| Speech in quiet (90 dB HL) | 96.7% (2.2) | 97.4% (2.0) | 97.7% (1.7) | 97.3% (3.5) |
| ABR wave I amplitude (μV) | 0.41 (0.11) | 0.33 (0.10) | 0.38 (0.13) | 0.30 (0.09) |
| WIN (dB SNR) | 4.0 (1.1) | 3.5 (1.1) | 4.6 (1.4) | 4.8 (1.4) |
| LDL – 1 kHz (dB HL) | 89.3 (10.4) | 88.4 (9.5) | 89.9 (8.3) | 88.3 (9.2) |
| LDL – 3 kHz (dB HL) | 84.2 (9.1) | 83.1 (8.2) | 83.1 (5.5) | 82.3 (8.8) |
| LDL – 4 kHz (dB HL) | 81.8 (9.9) | 81.1 (9.5) | 81.1 (5.9) | 80.2 (10.1) |
| LDL – 6 kHz (dB HL) | 78.0 (13.7) | 75.3 (10.1) | 72.6 (8.6) | 74.6 (10.9) |
| Report tinnitus | 0 | 0 | 1 | 14 |
| Total participants | 27 | 16 | 14 | 17 |
Participant characteristics are shown for each of the 4 noise exposure groups. Except for “number of males”, “report tinnitus”, and “total participants”, all values are group means with standard deviations in parentheses. The pure tone average (PTA) is the average of the pure tone thresholds at 0.5, 1, and 2 kHz, while the high frequency PTA consists of the thresholds at 3, 4, and 6 kHz. The score on the Lifetime Exposure of Noise and Solvents Questionnaire (LENS-Q) provides a measure of lifetime noise exposure to occupational, military, and recreational noise sources. The LENS-Q is scored on a log scale where lower scores indicate less noise exposure and higher scores indicate more exposure. Auditory brainstem response (ABR) wave I amplitude is reported for a 4 kHz 110 dB p-pe SPL toneburst. Perceptual measures include speech in quiet at 60 and 90 dB HL, the words in noise (WIN) test, loudness discomfort levels (LDLs) at 4 frequencies, and report of tinnitus. SNR is signal-to-noise ratio.
Procedures
All study procedures were approved by the Institutional Review Board of the VA Portland Health Care System.
• Audiometric Testing
Pure tone thresholds for the standard audiometric frequencies (0.25–8 kHz) were assessed in all potential participants as part of the screening evaluation. In addition, audiometric thresholds from 9–16 kHz were measured in 48 out of 74 qualifying participants using Sennheiser HDA 200 headphones (Old Lyme, CT). Thresholds from 9–16 kHz were obtained from 8 additional participants (7 Veterans and 1 non-Veteran) when they participated in other research studies. Two of these participants were tested 2–4 months before the study described here and the other 6 were tested 2–2.5 years later.
• Electrophysiological Testing
Electrophysiological testing was completed using an Intelligent Hearing Systems SmartEP system (Miami, FL) and Etymotic Research gold foil ER3–26A tiptrode electrodes (Elk Grove Village, IL) placed in the ear canal. The reference electrode was placed on the high forehead and the ground on the low forehead. Waveforms were generated using alternating polarity 4 kHz toneburst stimuli at 80, 90, 100, and 110 dB peak-to-peak equivalent SPL (dB p-pe SPL). As described in Bramhall et al. (2017), responses to 1, 3, and 6 kHz tonebursts were also collected, although this report will focus on the 4 kHz data. The 4 kHz stimuli were 2 ms in duration with a rise/fall time of 0.5 ms and a Blackman envelope. The ABR response was band-pass filtered from 10–1500 Hz and averaged across 2048 presentations of the 80, 90 and 100 dB p-pe SPL stimuli and 1024 presentations of the 110 dB p-pe SPL stimluli. A stimulus repetition rate of 11.1/sec was used and 2 replications of each waveform were obtained. Electrode impedance was less than 5 kOhms, with the exception of 2 participants who had impedance values of less than 12 kOhms. The positive peak and following negative trough for waves I, III, and V were initially identified with an automated Python-based peak picking program (adapted from Buran 2015) and then evaluated by an experienced audiologist and reassigned if appropriate. Wave I and III amplitudes were defined as the difference between the voltage at the positive peak and the voltage at the following negative trough. The amplitude of wave V was calculated as the difference between the peak voltage and the average prestimulus baseline voltage calculated for the 1 ms time period before stimulus presentation. Waves I, III, and V were easily identified at the 100 and 110 dB p-pe SPL stimulus levels for all participants, and for all but 4 participants at 90 dB p-pe SPL. For some participants, wave I could not be identified at 80 dB p-pe SPL, resulting in less data at that level.
• Otoacoustic Emissions Testing
DPOAE testing was conducted using a custom system that includes an ER-10 B+ probe microphone and EMAV software from Boys Town National Research Hospital (Neely & Liu 1993). DPOAEs were screened in all participants as part of study candidacy using a primary frequency sweep (DP-gram) from f2 =1 – 8 kHz in 1/6-octave increments at stimulus frequency levels of L1= 65 and L2=55 dB SPL. Measurement based stopping rules were employed in which averaging continued until 48 seconds of artifact-free data were collected, until the noise floor was below −30 dB SPL, or the signal noise ratio was ≥ 30 dB. Responses from 1.5–6 kHz were compared with the 90th and 95th percentile of a large study of DPOAEs gathered from individuals with abnormal pure tone thresholds (Gorga et al. 1997). Only individuals at or above the 90th percentile at all tested frequencies and below the 95th percentile at no more than one tested frequency were included in the study.
• Assessment of Noise Exposure History
All potential participants were asked several questions about their lifetime noise exposure history (occupational, military, and recreational) and use of hearing protection during a short interview (as described in Bramhall et al. 2017). The Lifetime Exposure of Noise and Solvents Questionnaire (LENS-Q) was completed by all non-Veteran participants (Gordon et al. 2016; Bramhall et al. 2017), but was completed as part of another study for many of the Veterans and the data was only available for 20 out of 31 Veterans (11 assigned to the Veteran High Noise group and 9 assigned to Veteran Low Noise group). The interview and questionnaire were scored as described previously to assign participants to noise exposure groups (Bramhall et al. 2017). An overview of the participant characteristics in each noise exposure group is shown in Table 1. Additional details about the noise exposure groups, such as audiometric thresholds and DPOAE levels, are described in Bramhall et al.
• Speech Perception Testing
Speech perception performance was assessed in quiet at both 60 and 90 dB HL using recorded 50 token Northwestern University Auditory Test 6 (NU-6) word lists 2A and 3A. Speech-in-noise perception was evaluated with the WIN (Wilson et al. 2007). Multitalker babble was presented at 80 dB HL and the target speech varied in 4 dB steps for a range of signal-to-noise ratios from 0–24 dB. WIN lists 1 and 2 were presented to each participant. All speech tests were presented from the VA Speech Recognition and Identification Materials, Disc 4.0 (Auditory Research Laboratory, VA Medical Center, Mountain Home, Tennessee) through an audiometer.
• Loudness Discomfort Level (LDL)
An up-down adaptive procedure was used to measure LDL for pulsed pure tones at 1, 3, 4, and 6 kHz using 2 dB steps. Participants were given the following instructions adapted from the Contour Test (Cox et al. 1997): “You will hear sounds that increase and decrease in volume. Pretend you are listening to the radio at that volume. Tell me if the sound is comfortable or uncomfortable. Keep in mind that an uncomfortably loud sound is louder than you would ever choose on your radio no matter what mood you are in.” LDL was defined as the lowest level at which participants reported the volume was uncomfortable at least 50% of the time on ascending trials. A minimum of two responses out of three presentations was required to determine LDL.
• Tinnitus Perception
All participants completed a short hearing and health history questionnaire, which included a question about the perception of tinnitus. This question asked, “Do you have constant or frequent ringing in the ears?” Participants who responded “yes” to this question were rated as having tinnitus.
Analysis
Given that a relationship between noise exposure group and ABR wave I amplitude was previously demonstrated in this cohort (Bramhall et al. 2017), all analyses used wave I amplitude rather than noise exposure group. Comparing perceptual measures with ABR wave I amplitude, rather than noise exposure group, also offers a more direct assessment of the relationship between auditory nerve activity and any perceptual deficits. The relationship between ABR wave I and each auditory perceptual measure was plotted in an initial analysis. ABR wave I amplitude for a 4 kHz toneburst was used because a large effect of noise exposure group on ABR wave I amplitude was observed at 4 kHz in Bramhall et al. A 110 dB p-pe SPL toneburst was chosen because in animal models of synaptopathy and in Bramhall et al., the effects of noise exposure on wave I amplitude were largest at higher stimulus levels. Based on this preliminary analysis, further investigation was completed only for the perceptual measure (tinnitus) that was observed to have a strong association with wave I amplitude.
The probability of reporting tinnitus was modeled using Bayesian logistic regression. The regression model included ABR wave I, III, and V amplitudes in response to a 110 dB p-pe SPL 4 kHz toneburst, DPOAE level averaged from 3–8 kHz, and 2- and 3- way interactions of these factors (eg. the interaction between wave I and wave III amplitudes). In addition, an interaction between sex and all ABR and DPOAE effects was included to model the effect of sex on the probability of reporting tinnitus. Although it was not expected that males and females would differ in their probability of reporting tinnitus after accounting for peripheral auditory function, sex was included as a factor in the model because differences in ABR wave I amplitude between males and females have been reported in the literature, with smaller amplitudes for males than females (Trune et al. 1988; Mitchell et al. 1989). The full equation for the model is included in the supplemental data (see Text, Supplemental Digital Content 1). Average DPOAE levels were used in the model rather than pure tone thresholds to specifically adjust for differences in OHC function between participants. Both animal and human studies suggest that tinnitus may be associated with loss of high spontaneous rate/low threshold auditory nerve fibers (Bauer et al. 2007; Paul et al. 2017). Given their low thresholds, it is reasonable to expect that partial loss of these fibers may impact pure tone thresholds to some degree. Therefore, matching for pure tone thresholds or adjusting for small threshold differences could prevent the detection of a synaptopathic reduction in ABR wave I amplitude. To fit the model, ABR wave I, III, and V amplitudes were averaged over the 2 replicate ABR runs measured on each participant. Due to the method of calculating wave V amplitude (peak to prestimulus baseline), 12 participants had a negative ABR wave V amplitude for 1 run of their ABR waveform. Given that a negative ABR peak amplitude is meaningless and will bias the amplitude value when averaged with the replicate, in instances where a negative amplitude was calculated, only the positive ABR amplitude value from the replicate was used. To facilitate computation, ABR and DPOAE values were standardized to a mean of zero and standard deviation of one. However, results are shown on the original scales.
Bayesian analysis, in contrast with classical statistical approaches, operates under the principle that all knowledge about any quantity (such as the effects of ABR wave I amplitude on the probability of reporting tinnitus) can be expressed as a probability distribution, with greater probability density concentrated around more plausible parameter values. All inferences, such as effect size estimates and confidence intervals are derived from these probability distributions. ‘Knowledge’ from this perspective is the combination of subject matter expertise and previous publications, collectively referred to as ‘prior probabilities’, and data collected in new studies. Bayesian analysis is the method of combining prior probabilities and new experimental data into revised ‘posterior probabilities’ via Bayes theorem. The relative weight of the priors and the experimental data on the posterior distribution depends on the amount of experimental data and the certainty of the prior belief, which is reflected in the standard deviation of the prior distribution. Priors with a tighter distribution will exert more influence on the posterior distribution, whereas those with a broader distribution will be less influential.
The published prevalence of tinnitus in the study population was used to generate priors on the intercept of the model. This prior for the prevalence of tinnitus was approximately 7.1% (Folmer et al. 2011) with a 90% prior certainty between 1.5–31.3%, which was transformed to the log-odds scale for the logistic regression model. This was chosen to reflect uncertainty about the degree of similarity between the National Health and Nutrition Examination Study (NHANES) subject pool used by Folmer et al. and the current study population. The NHANES data did not include females and had slightly different age groups than the current study. The prior for the main effect of wave I amplitude on the probability of reporting tinnitus was chosen based on published data from Guest et al. (2017) and Schaette and McAlpine (2011) where wave I amplitude was measured in participants with and without tinnitus. The sample mean and standard error for wave I amplitude in tinnitus and control participants from each study was used to simulate 100 datasets. A logistic regression model was then fit to each modeled dataset. Regression coefficients and standard errors were averaged over the simulated datasets for each publication, which provided a prior mean and standard deviation for the effect of wave I amplitude on the probability of reporting tinnitus. This ‘meta-analysis’ prior induces a 68% prior probability that increasing wave I amplitude decreases the probability of reporting tinnitus. As a sensitivity analysis, the model was refit using a ‘skeptical’ prior that is centered on zero effect of wave I amplitude on the probability of reporting tinnitus and allows only a 5% prior probability that the effect of wave I amplitude on tinnitus is as strong as that observed by Schaette and McAlpine. Further detail on the calculation of the prior for the effect of wave I amplitude on probability of reporting tinnitus and plots of the meta-analysis and skeptical prior distributions are included in the supplemental data (see Text, Supplemental Digital Content 1). The effects of wave III and V amplitude, DPOAE effects, and interaction effects were given 0-mean priors with larger standard deviations reflecting greater uncertainty about the wave III, wave V, and DPOAE effects on tinnitus. The female-specific effects were given Cauchy priors centered at 0 with a scale parameter of 0.25, allowing for the possibility of unexpectedly large effects of sex on tinnitus. A summary of the prior distributions used for the analysis is included in the supplemental data (see Text, Supplemental Digital Content 1).
The model was fit using the MCMC procedure in SAS software version 9.4. The posterior was evaluated using 3 chains of 1,000 iterations of the No-U-Turn sampler after a 1,000 sample burn-in period. All parameters had Gelman-Rubin convergence diagnostics below 1.01. Model fit was evaluated using replicate datasets, cumulative residuals, and binned residuals. There was no gross lack of fit of the model. The raw data used in the model can be found in the supplemental data (Supplemental Data Table 2).
RESULTS
DPOAE Levels and ABR Wave I Amplitude
There was no correlation between average DPOAE level from 3–8 kHz and ABR wave I amplitude for a 4 kHz 110 dB p-pe SPL toneburst (Fig. 1). This suggests that ABR wave I amplitude differences between participants were not due to differences in OHC function.
Figure 1. Relationship between average DPOAE level and ABR wave I amplitude.
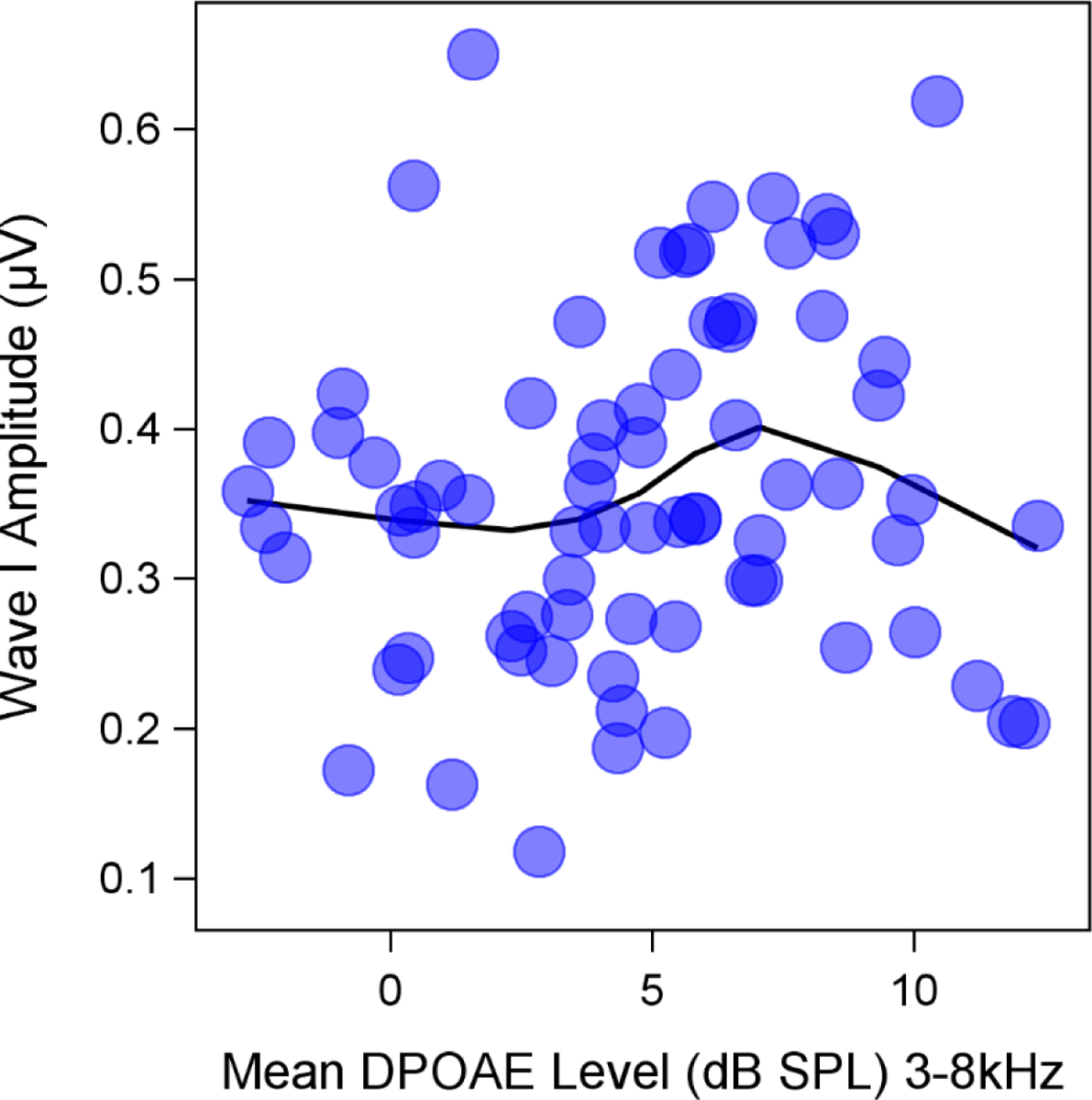
Auditory brainstem response (ABR) wave I amplitude for a 110 dB p-pe dB SPL 4 kHz stimulus was not correlated with average distortion product otoacoustic emission (DPOAE) level from 3–8 kHz. Mean ABR wave I amplitudes (across replications) and average DPOAE levels from 3–8 kHz are plotted for individual participants. A LOESS smooth line was fit to the data.
ABR Wave I Amplitude and Perceptual Measures
As tinnitus was the only perceptual measure that showed a clear relationship with ABR wave I amplitude (Fig. 2A), this measure will be discussed in more detail in the subsequent sections. All participants performed close to 100% for the speech perception in quiet task at both a conversational (60 dB HL) and a high intensity level (90 dB HL) regardless of their wave I amplitude (Fig. 2B–C). ABR wave I amplitude did not appear correlated with speech perception in noise, as measured by the WIN test (Fig. 2D). WIN scores are based on the total number of correct responses and are reported as a signal-to-noise ratio threshold, with higher values indicating poorer performance. Normal performance on the WIN is defined as a score between −2 and 6 dB. Pure tone LDLs were measured as an indicator of loudness intolerance and hyperacusis. No clear association between ABR wave I amplitude and LDL was observed (Fig. 2E). Only LDLs for the 4 kHz stimulus are plotted because LDLs across frequencies were highly correlated.
Figure 2. Relationship between ABR wave I amplitude and perceptual measures.
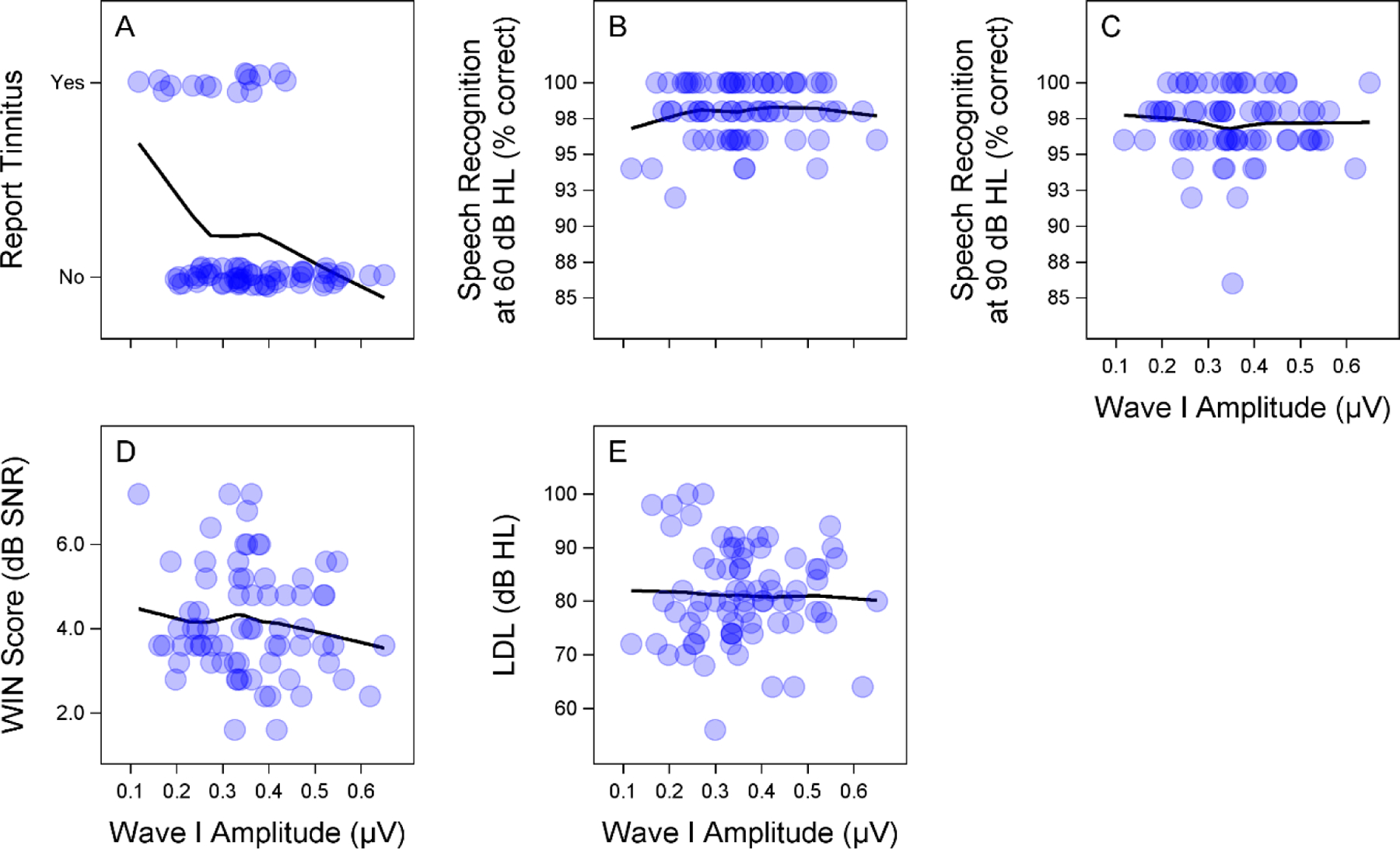
Auditory brainstem response (ABR) wave I amplitude (for a 110 dB p-pe SPL 4 kHz stimulus) is associated with perception of tinnitus, but does not appear correlated with the other perceptual measures. Scatterplots show the relationship between ABR wave I amplitude and self-report of tinnitus (A), speech perception in quiet at 60 (B) and 90 (C) dB HL, performance on the words in noise (WIN) test (D), and loudness discomfort level (LDL) (E) at 4 kHz. In each subplot, a LOESS smooth line was fit to the data.
ABR Wave I Amplitude and Perception of Tinnitus
An overview of the characteristics of participants with and without tinnitus are shown in Table 2. Audiograms and DPOAE levels for both groups are plotted in Figures 3 and 4. Mean pure tone thresholds from 250–8000 Hz were 3–7 dB lower for participants with tinnitus compared to those without tinnitus. These differences may be due to slightly poorer OHC function in the tinnitus group, as can be observed in the mean DPOAE level differences (3.5–7 dB) between groups from 5000–8000 Hz. Poorer pure tone thresholds in the tinnitus group may also be related to the partial loss of low threshold auditory nerve fibers, which has been associated with tinnitus in previous studies (Bauer et al. 2007; Paul et al. 2017). Extended high frequency thresholds were similar between the 2 groups, with mean differences at all frequencies within 5 dB. Extended high frequency thresholds were not obtained for 3 participants with tinnitus (2 males, 1 female) and 15 participants without tinnitus (5 males, 10 females). Only participants who reported tinnitus in the test ear were included in the tinnitus group. All of these participants reported tinnitus in both ears. Perception of constant or frequent tinnitus was reported at a very high rate in the Veteran High Noise group (82%), compared with the Veteran Low Noise group (7%), and the two Non-Veteran groups (0%). Lower ABR wave I amplitudes were strongly associated with report of tinnitus (Fig. 5). In contrast, wave III (Fig. 5A) and wave V (Fig. 5B) amplitudes did not appear associated with tinnitus or wave I amplitude. In response to a 4 kHz toneburst, a mean reduction in ABR wave I amplitude was observed in participants with tinnitus, with the greatest reduction at the highest stimulus levels (Fig. 6A). Results from previous studies of ABR wave I amplitude in audiometrically normal young people with or without tinnitus are also plotted to show the relative effect sizes between studies (Schaette & McAlpine 2011; Guest et al. 2017). A similar reduction in mean amplitude was not observed for wave V (Fig. 6B). Participants with tinnitus showed a mean reduction in wave I/V ratio compared with participants without tinnitus (Fig. 6C). When comparing the current data with the prior studies, note that there are some differences in terms of the ABR measurements, namely the stimulus in the current study was a 4 kHz toneburst versus a click and the current study used a tiptrode rather than an electrode placed on the mastoid.
Table 2.
Participant Characteristics by Tinnitus Group
| Tinnitus | ||
|---|---|---|
| No | Yes | |
| Total participants | 59 | 15 |
| Number of males | 22 | 13 |
| Age in years | 26.7 (4.5) | 26.3 (2.1) |
| PTA | 7.2 (3.9) | 11.4 (5.6) |
| High frequency PTA (3, 4, & 6 kHz) | 3.2 (4.5) | 10.1 (4.4) |
| Extended high frequency PTA | 3.6 (9.2) | 4.2 (6.6) |
| ABR wave I amplitude (μV) – 1 kHz | 0.15 (0.10) | 0.10 (0.06) |
| ABR wave I amplitude (μV) – 3 kHz | 0.28 (0.12) | 0.21 (0.09) |
| ABR wave I amplitude (μV) – 4 kHz | 0.38 (0.11) | 0.29 (0.10) |
| ABR wave I amplitude (μV) – 6 kHz | 0.38 (0.11) | 0.32 (0.08) |
| DPOAE average level from 3–8 kHz | 5.38 (3.7) | 1.9 (2.3) |
Overview of participant characteristics by tinnitus group. Except for “total participants” and “number of males”, all values are group means with standard deviations in parentheses. The pure tone average (PTA) is the average of the pure tone thresholds at 0.5, 1, and 2 kHz (in dB HL), while the high frequency PTA consists of the thresholds at 3, 4, and 6 kHz, and the extended high frequency PTA is the average of all pure tone thresholds from 9–16 kHz. Auditory brainstem response (ABR) wave I amplitude is reported for 110 dB p-pe SPL tonebursts. DPOAE average level for a DP-gram is reported in dB SPL.
Figure 3. Audiometric pure tone thresholds by tinnitus group.
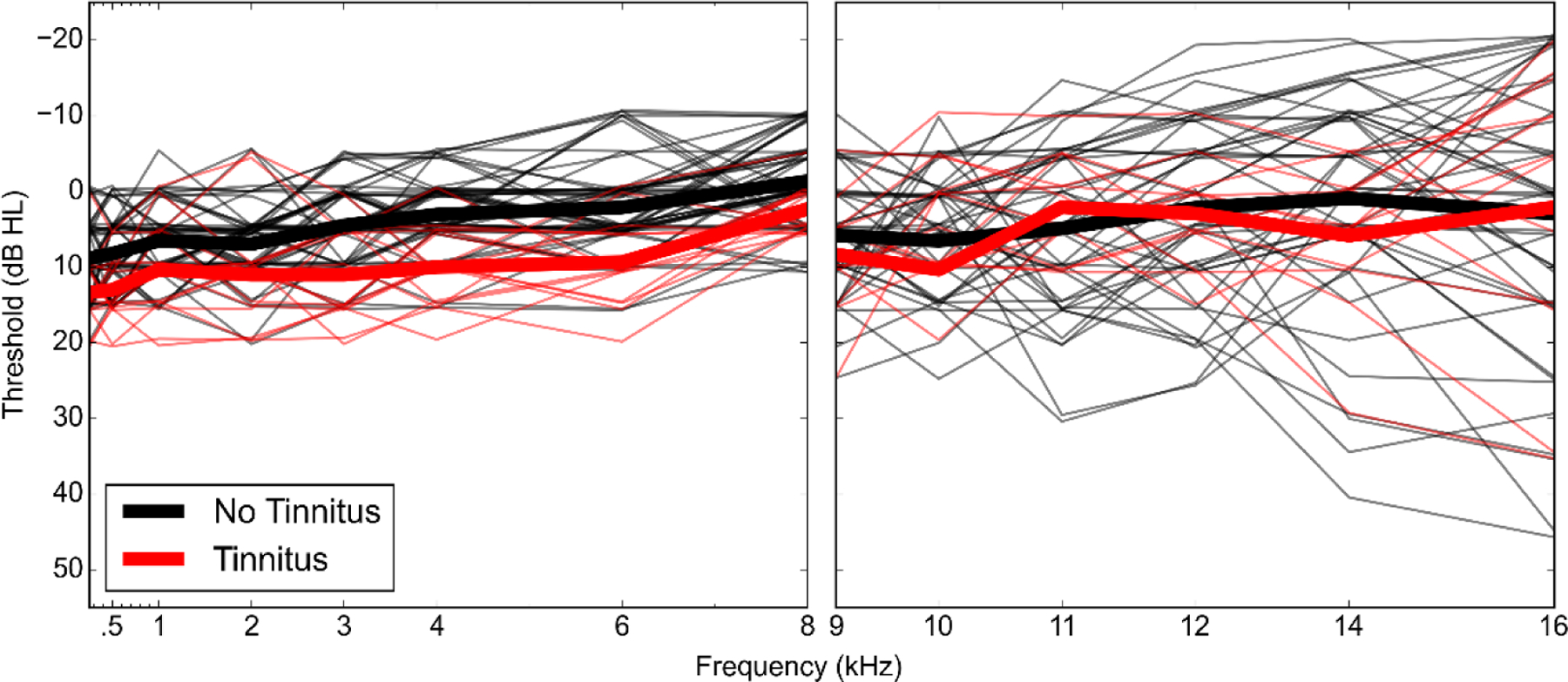
Audiometric pure tone thresholds for the test ear are shown for individual study participants (thin lines) as well as the mean thresholds for the tinnitus and no tinnitus groups (thick lines). Pure tone thresholds were measured for all participants from 0.25–8 kHz (A) and in 56 participants from 9–16 kHz (B).
Figure 4. DPOAE levels across frequency by tinnitus group.
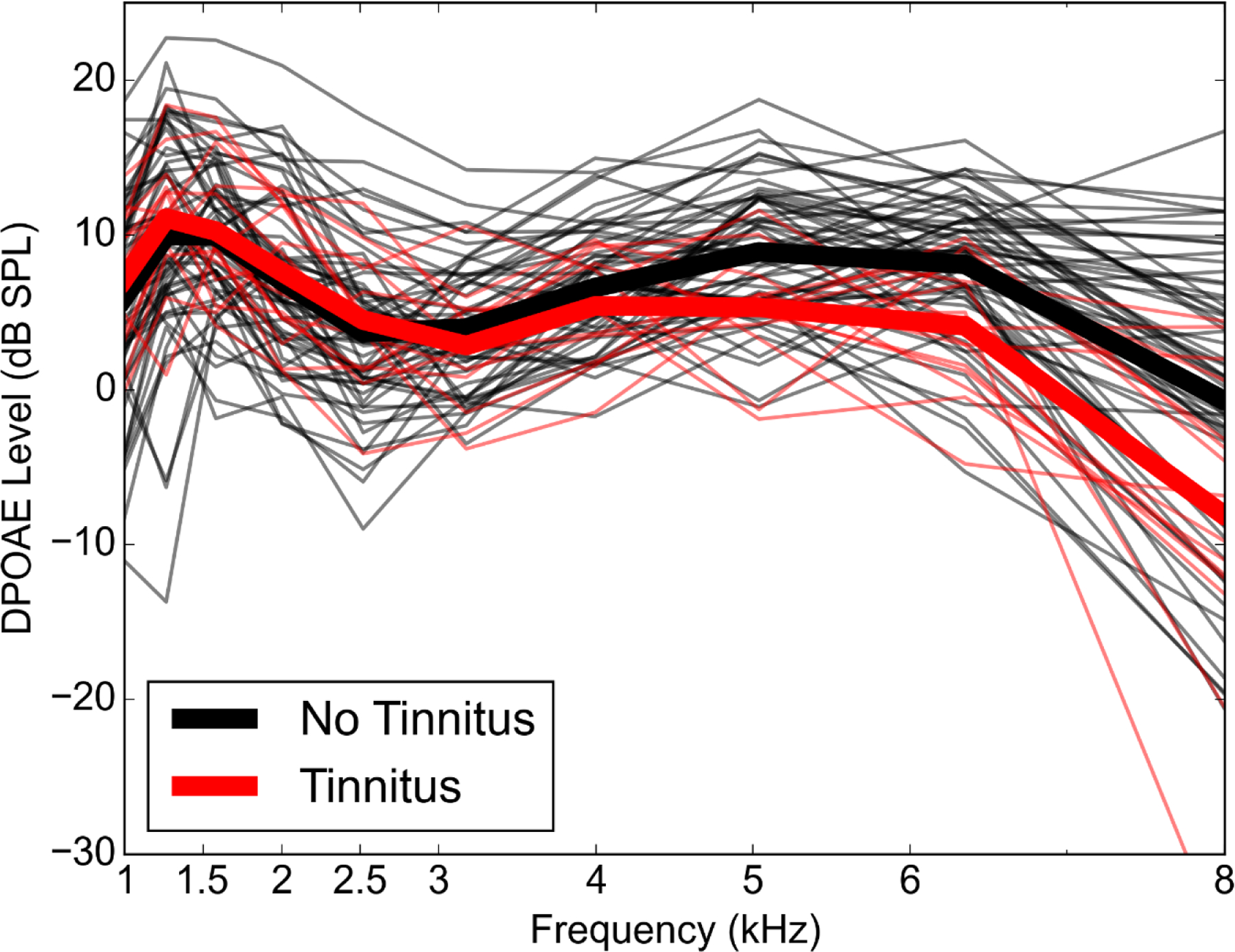
DPOAE levels are shown for individual study participants (thin lines) in response to a 65/55 dB SPL stimulus at f2 frequencies of 1–8 kHz. Mean DPOAE levels for the tinnitus and no tinnitus groups are shown with thick lines.
Figure 5. Relationship between ABR peak amplitudes for individual participants.
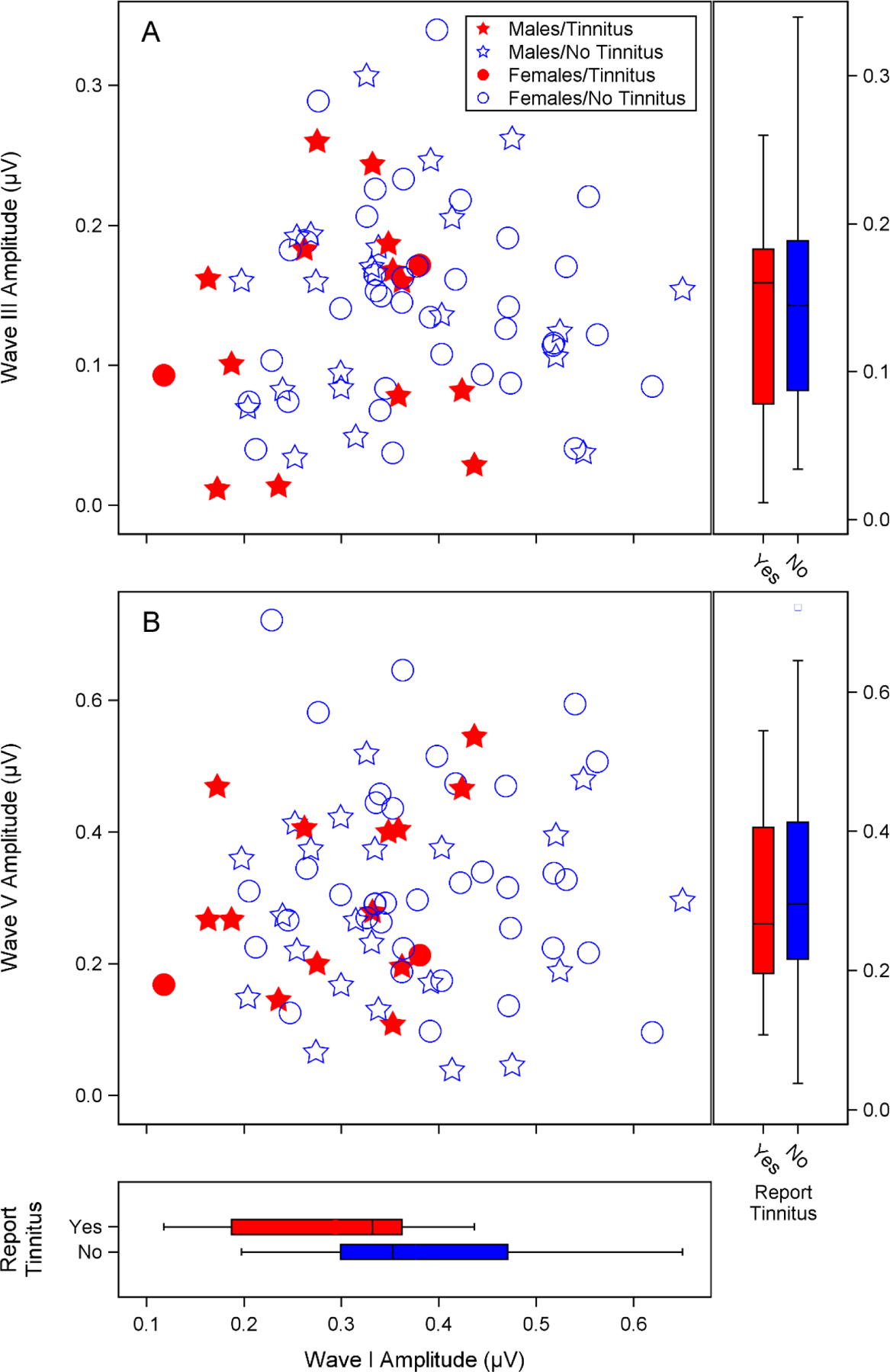
Auditory brainstem response (ABR) wave I amplitude was reduced in participants with tinnitus, but wave III and V amplitudes appeared independent of wave I amplitude and tinnitus perception. Combined scatterplots and boxplots show individual ABR peak amplitudes for a 4 kHz 110 dB p-pe SPL stimulus from males and females with and without tinnitus. Replicate ABR runs for each participant were averaged together to generate a mean amplitude. The scatterplots show mean ABR wave I amplitude versus mean wave III (A) or wave V (B) amplitude. Wave I and III amplitudes were calculated as the voltage difference between the wave peak and the following trough. Wave V amplitude was calculated as the difference between the wave peak and the prestimulus baseline. Males are indicated with stars and females with circles. Filled symbols indicate participants who reported tinnitus. Boxplots show the relationship between wave I, III, and V amplitudes and report of tinnitus. The bottom, midline, and top of the boxes represent the 1st quartile, median, and 3rd quartile respectively, while the end of the whiskers indicate the points furthest from the box that still fall within 1.5 interquartile ranges from the edges of the box. Open circles indicate values outside of this range.
Figure 6. Relationship between ABR peak amplitudes and tinnitus across level.
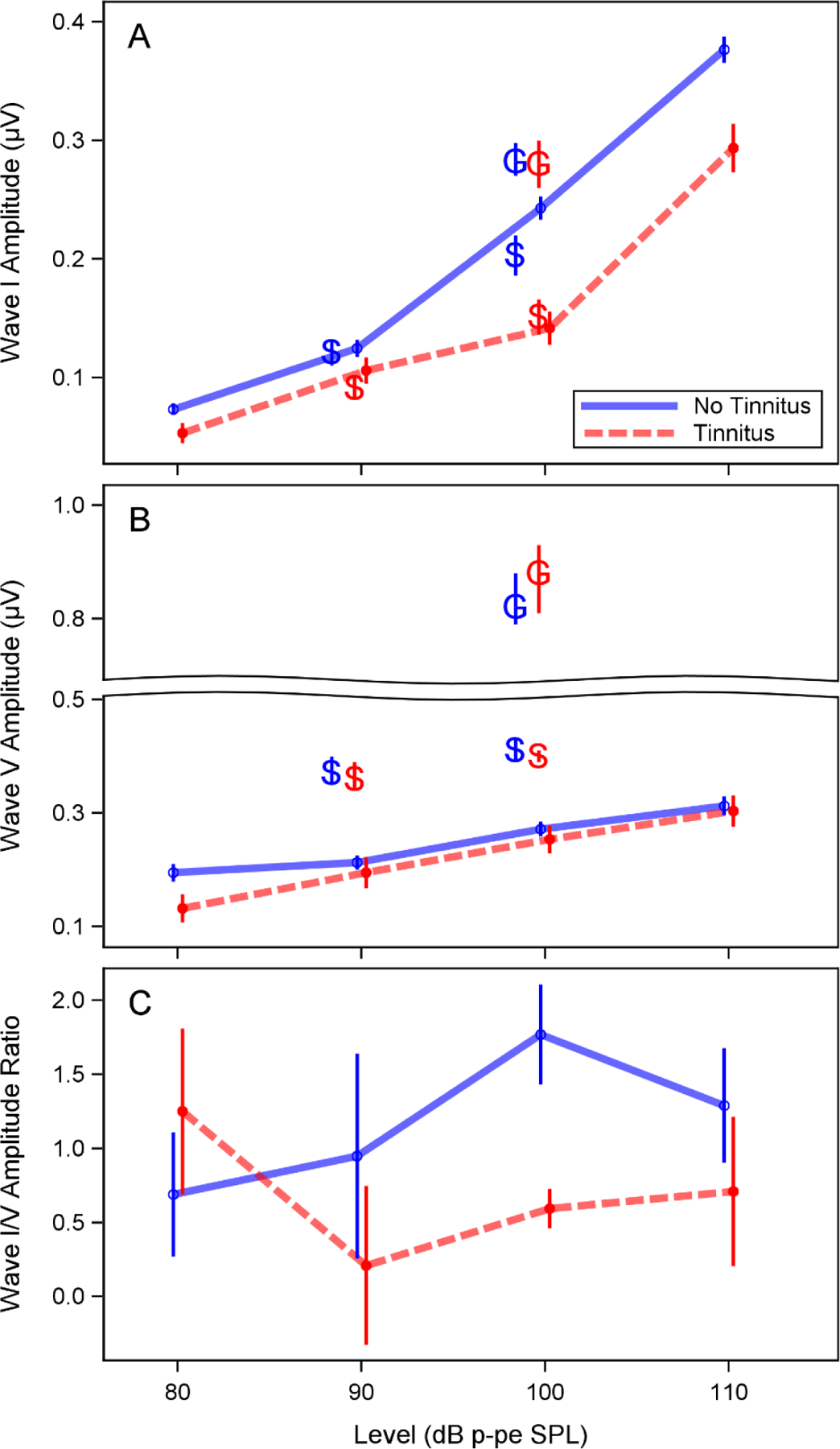
Auditory brainstem response (ABR) wave I amplitude was reduced in the participants with tinnitus, but a similar reduction was not observed for wave V amplitude. ABR amplitudes were measured in response to a 4 kHz toneburst stimulus at 80, 90, 100, and 110 dB p-pe SPL. Mean wave I amplitude (A), wave V amplitude (B), and wave V/I amplitude ratio (C) were calculated for study participants with and without tinnitus. Wave I amplitude was calculated as the voltage difference between the wave peak and the following trough. Wave V amplitude was calculated as the difference between the wave peak and the prestimulus baseline. Error bars indicate the standard error of the mean. The wave I amplitudes reported by Schaette and McAlpine (2011) and Guest et al. (2017) in response to a click at 90 and/or 100 dB p-pe SPL are also plotted in panels A and B (indicated by “S” and “G”) for comparison. A split axis is used in panel B to facilitate this comparison. Note that in the previous studies, mastoid electrodes were used rather than a tiptrode.
The relationship between ABR wave I amplitude (for a 4 kHz 110 dB p-pe SPL stimulus) and perception of tinnitus remained when using a Bayesian logistic regression model to adjust for DPOAE and sex differences between participants with and without tinnitus (Fig. 7). After adjusting for sex and DPOAE level, the probability of reporting tinnitus increased by a factor of 2.0 per 0.1 μV decrease in ABR wave I amplitude (95% Bayesian confidence interval (CI) = 1.1–5.0) for males and by a factor of 2.2 (95% CI = 1.0–6.4) for females. One unique characteristic of Bayesian statistical models is that they generate a posterior distribution of the factor of interest. To aid in interpretation of the certainty of the model results described here, the posterior probability that a 0.1 μV decrease in ABR wave I amplitude is associated with an increase in the probability of reporting tinnitus was calculated by integrating over the portion of the posterior distribution where the probability of reporting tinnitus was greater than zero. This calculation indicated a 98% posterior probability in males and a 97% posterior probability in females that a 0.1 μV decrease in ABR wave I amplitude is associated with an increase in the probability of reporting tinnitus. This effect can be visualized in Figure 7A. When wave III amplitude, wave V amplitude, and average DPOAE level from 3–8 kHz are set to the sample means, the probability of tinnitus is close to 0% for large wave I amplitudes and increases as wave I amplitude decreases. For example, when wave III amplitude, wave V amplitude, and average DPOAE level from 3–8 kHz are set to the mean values for the whole cohort and sex is set to male, we can compare the mean risk of tinnitus for a low (0.25 μV) versus a high (0.5 μV) ABR wave I amplitude. A wave I amplitude of 0.25 μV is associated with a mean risk of tinnitus of 35% (95% Bayesian CI = 17–58%). In contrast, a wave I amplitude of 0.5 μV is associated with a mean risk of tinnitus of 8% (95% CI = 1–28%). This translates to a relative risk of 4.1 (95% CI = 1.1–29.0) for a wave I amplitude of 0.25 μV versus 0.5 μV or an absolute risk difference of 26% (95% CI = 3–51%). In contrast, changes in wave III or wave V amplitude had little effect on the probability of tinnitus when DPOAE maximum amplitude at 4 kHz, and the amplitudes of the remaining 2 ABR waves were set to the mean (Fig. 7B–C).
Figure 7. ABR wave I, III, and V amplitudes and the probability of reporting tinnitus.
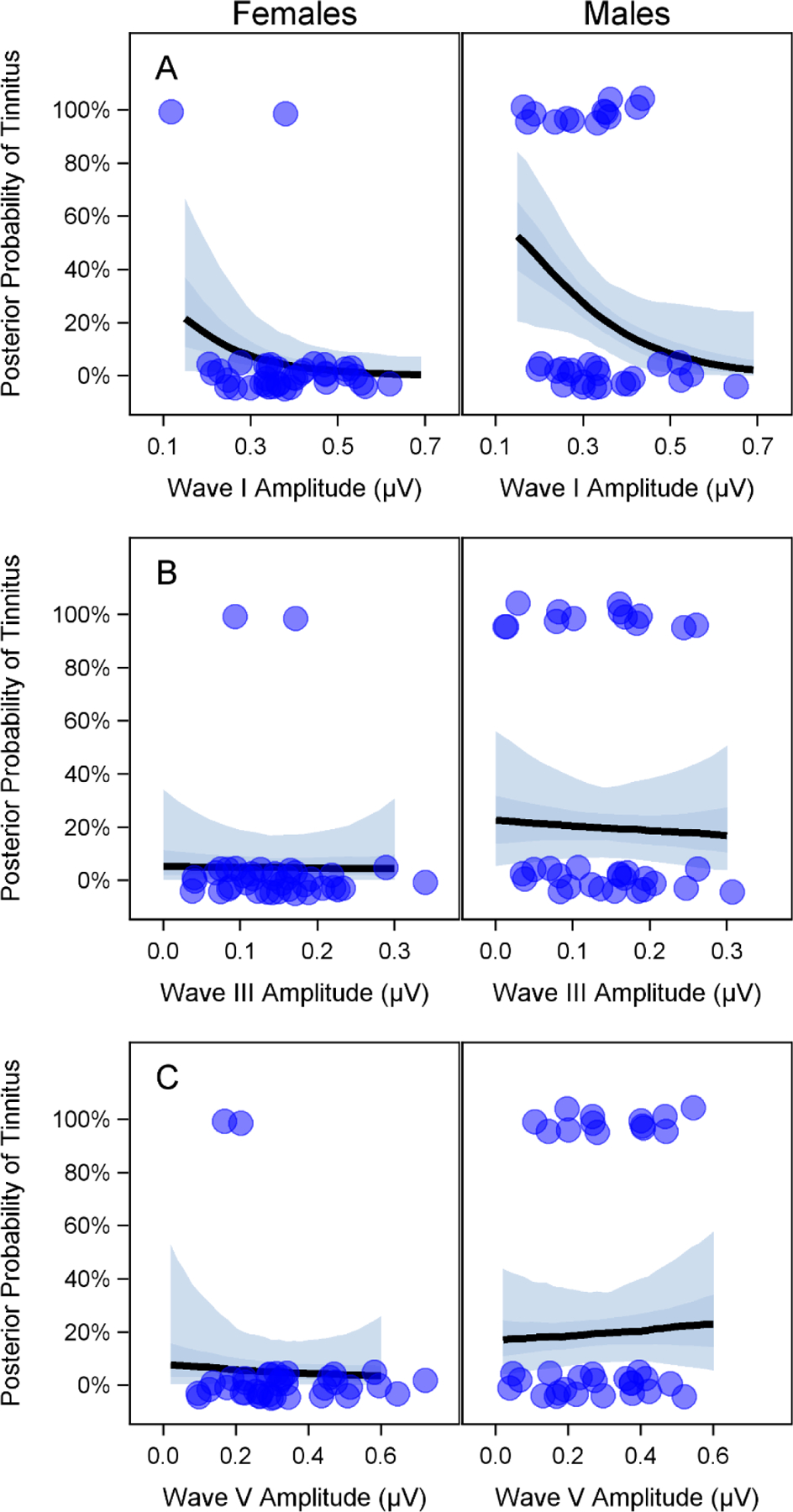
The amplitude of auditory brainstem response (ABR) wave I, but not waves III or V, can be used to predict the probability of reporting tinnitus. Results from a Bayesian logistic regression model are plotted to show the relationship between ABR wave I (A), III (B), and V (C) amplitudes (for a 110 dB p-pe SPL 4 kHz stimulus) and the posterior probability of reporting tinnitus. For each subplot, average distortion product otoacoustic emission (DPOAE) levels from 3–8 kHz and the 2 ABR waves not shown on the x-axis of the subplot are set to the mean value for the whole data set. For example, in panel A, average DPOAE level is set to 4.7 dB SPL, wave III amplitude is set to 0.14 μV, and wave V amplitude is set to 0.31 μV. The dark and light shaded regions indicate the interquartile range and 95% Bayesian confidence interval respectively. Dots indicate the ABR wave amplitudes for participants with (top) and without (bottom) tinnitus. Dots are jittered vertically to make them easier to visualize.
Given the abundance of data (74 participants were used in the analysis), using the skeptical prior for the relationship between ABR wave I amplitude and tinnitus in the Bayesian model had little effect on the posterior inferences. Model results using the skeptical prior can be found in the supplemental data (see Text, Supplemental Digital Content 1).
The model suggests a similar relationship between ABR wave amplitude and tinnitus in both males and females, although the predicted probability of reporting tinnitus for a given ABR amplitude and DPOAE level is higher in males than females. One possible explanation for this result is that men are at greater propensity for tinnitus at any given level of peripheral auditory function. This explanation is theoretically ungrounded at present. Alternatively, men may appear at greater risk of tinnitus at a given level of peripheral auditory function as a consequence of sex-related measurement error in the ABR wave I amplitude. In this analysis, the chances of reporting tinnitus are reduced as wave I amplitude (W1) increases, so that:
Compared to females, the ABR wave I amplitude measurement, which is an indirect measure of auditory nerve activity (θ), is biased small in males by a factor ε:
Therefore, the probability of reporting tinnitus at a particular wave I amplitude can be rewritten for males and females as:
The expected probability for males (top row) is bigger than the expected probability for females (bottom row) by a factor of b·ε since both b and ε are positive. This means that at any given level of observed wave I amplitude, males will have a higher probability of tinnitus than females due to a biased relationship between wave I amplitude and auditory nerve activity.
An alternate male-only Bayesian model reveals similar results to the male-specific analysis of the whole dataset (see Text, Supplemental Digital Content 1), confirming that the observed association between ABR wave I amplitude and tinnitus is not an artifact of sex-related differences in ABR wave I amplitude. Given that only 2 female participants in this study reported tinnitus in the test ear, additional data from females with tinnitus will be necessary to confirm the relationship between ABR wave I amplitude and the probability of reporting tinnitus in females.
DISCUSSION
Noise exposure history was associated with tinnitus probability
The high probability of reporting tinnitus in the Veteran High Noise group compared with the other lower noise exposure groups suggests an association between noise exposure history and tinnitus, with only the individuals with the highest exposures developing tinnitus. Although the relationship between noise exposure and tinnitus is not a new finding, this relationship is usually associated with abnormal pure tone thresholds. Guest et al. found a relationship between noise exposure history and tinnitus in young people with normal audiograms who were matched for pure tone thresholds out to 14 kHz, but they did not measure OAEs (2017). The clear association between tinnitus and high intensity noise exposure in the current study is remarkable because it is observed in individuals with normal pure tone thresholds and normal DPOAEs. This suggests that the origin of tinnitus in this sample is not related to OHC dysfunction.
Perception of tinnitus was associated with reduced amplitude of ABR wave I, but not wave V
Mean ABR wave I amplitudes were reduced for study participants with tinnitus versus those without tinnitus, but this was not the consequence of an overall reduction in all ABR peak amplitudes. An increase in the gain of the central auditory system in response to reduced peripheral input has been proposed as a possible mechanism of tinnitus generation (Wang et al. 2011; Auerbach et al. 2014; Brotherton et al. 2015). Tinnitus-related central neuronal hyperactivity in response to cochlear damage has also been illustrated through computational modeling studies (Schaette & Kempter 2006; Schaette & Kempter 2009; Norena 2011). Although reduced peripheral input is generally assumed to result from OHC loss and be associated with auditory threshold elevation, loss of cochlear synaptic connections would also decrease peripheral input. In the present and previous studies, individuals with tinnitus and normal auditory thresholds show a reduction in wave I amplitude compared with individuals without tinnitus, but do not demonstrate a similar reduction in wave V amplitude (Schaette & McAlpine 2011). In fact, Gu et al. found a reduction in wave I amplitude and an increase in wave V amplitude when comparing individuals with tinnitus to age-, sex-, and threshold-matched (out to 8 kHz) individuals without tinnitus, although extended high frequency thresholds were somewhat poorer in the tinnitus group compared to the non-tinnitus group (2012). This suggests hyperactivity or loss of inhibition may develop in the auditory brainstem after a reduction in input from the auditory nerve and lead to the perception of tinnitus. A recent study showing increased spontaneous activity in the inferior colliculus of mice with noise-induced cochlear synaptopathy also suggests that tinnitus may be a consequence of cochlear synaptic degeneration (Hesse et al. 2016). Alternatively, rather than an indication of central gain, the decrease in ABR wave I/V amplitude ratio observed in this and previous studies may be driven by the relative insensitivity of wave V amplitude to changes in peripheral function, particularly in the high frequencies, as compared to wave I amplitude (Don & Eggermont 1978).
Although the presence of cochlear synaptopathy in the study participants with tinnitus cannot be confirmed without post-mortem histological analysis, these findings are consistent with the hypothesis that cochlear synaptic loss is associated with the perception of tinnitus. The cross-sectional nature of this study prohibited comparing ABR wave I amplitudes within subjects before and after the onset of tinnitus, but a prospective study of individuals who will be exposed to high intensity sounds would help confirm these results.
ABR wave I amplitude and speech perception appeared unrelated
No effect of ABR wave I amplitude on speech perception in quiet was observed. This is not surprising considering the relative ease of this task for individuals with normal pure tone thresholds. Additionally, even in the presence of synaptopathy, one would expect relatively good performance on this task as both low and high threshold fibers could contribute.
ABR wave I amplitude was also not correlated with performance on the WIN test. There are several possible explanations for the lack of a wave I amplitude effect on WIN performance: 1) The participants in this cohort do not have synaptopathy and therefore do not show the predicted effects on speech-in-noise perception, 2) These participants have synaptopathy and related speech perception in noise deficits, but the WIN test does not adequately assess this problem (e.g., because it is not sufficiently difficult), 3) Synaptopathy alone (i.e., in the absence of elevated pure tone thresholds) does not have an adverse impact on speech perception in noise. As suggested by previous work, the perceptual effects of synaptopathy may become more evident when combined with elevated pure tone thresholds (Bramhall et al. 2015). Further studies of synaptopathy in humans will be necessary to distinguish between these possibilities. More complex speech-in-noise perception tasks, such as those that specifically require access to temporal fine structure may yield different results.
ABR wave I amplitude was not associated with loudness tolerance
Analysis of loudness tolerance showed no clear relationship between ABR wave I amplitude and LDLs. Mean LDLs were lower than those reported in a normative sample of young people with normal audiometric thresholds from 500–6000 Hz (Sherlock & Formby 2005). This may be due to differences in the test instructions provided to the participants. LDL was measured as an indicator of hyperacusis, but recent studies suggest LDL alone is not a reliable metric for this purpose (Sheldrake et al. 2015; Zaugg et al. 2016). Therefore, it is difficult to draw any conclusions about the relationship between ABR wave I amplitude and hyperacusis from this result. In future studies, use of hyperacusis questionnaires in combination with measures of loudness growth may help determine if hyperacusis is associated with reduced ABR wave I amplitudes.
Current clinical tests are not sensitive to all noise-related auditory changes
The results of this study in combination with previous work (Bramhall et al. 2017) indicate that high intensity noise exposure is associated with tinnitus and ABR wave I amplitude reductions even when auditory thresholds, OAEs, and clinical measures of speech perception are normal. This suggests that damage to the auditory system can occur in humans after noise exposure without showing up on a clinical audiology assessment. Although it is not clear whether this noise-induced damage adversely affects speech perception in noise or hyperacusis, the damage is clearly associated with tinnitus. The high prevalence of tinnitus highlights the importance of developing new clinical tests that are sensitive to cochlear synaptopathy.
Comparison to other studies
The relationship between reduced ABR wave I amplitude and tinnitus observed in the present study is consistent with a previous report of smaller mean ABR wave I amplitudes in individuals with normal pure tone thresholds who have tinnitus versus those without tinnitus (Schaette & McAlpine 2011). Also in agreement with this previous study is the lack of a mean decrease in wave V amplitude in the participants with tinnitus.
The absence of tinnitus in the Non-Veteran Firearms group despite the previously demonstrated reduction of ABR wave I amplitudes in this group (Bramhall et al. 2017) was surprising. This may indicate a threshold of noise exposure (and by extension reduction in ABR wave I amplitude) that must be reached before tinnitus is induced. This could translate into a percent reduction in the number of IHC-auditory nerve synapses that must be lost to initiate an increase in central gain large enough to result in the perception of tinnitus. Alternatively, as suggested by previous studies, the perception of tinnitus may be related to the loss of low threshold auditory nerve fibers (Bauer et al. 2007; Paul et al. 2017). The lack of tinnitus in the Non-Veteran Firearms group may be explained by differences in the subpopulation of auditory nerve fibers affected by synaptopathy. For example, individuals with low ABR wave I amplitudes and no tinnitus may have primarily a loss of high threshold auditory nerve fibers, whereas individuals with low ABR wave I amplitudes and tinnitus may have a combination of low and high threshold fiber loss.
Guest et al. found an association between reported lifetime noise exposure history and tinnitus in young adults with normal auditory thresholds (2017). However, unlike the results presented here and in Bramhall et al. (2017), ABR wave I amplitude was not correlated with noise exposure or tinnitus. In contrast to the present study, where participants were recruited based on their noise exposure history and screened with DPOAEs to ensure relatively normal OHC function, Guest et al. recruited participants based on the presence/absence of tinnitus and a normal audiogram. Although the tinnitus and non-tinnitus groups were matched on audiometric thresholds out to 14 kHz, OAEs were not measured. Because OAEs are pre-neural responses generated by the OHCs, it seems reasonable to assert that DPOAEs, or evoked OAEs in general, are necessary to rule out the effects of noise on OHC function. In humans, OAE level shifts are observed more often than pure tone threshold shifts when the two tests are compared at corresponding audiometric frequencies after exposure to noise (Engdahl & Kemp 1996; Seixas et al. 2005; Marshall et al. 2009) or ototoxic drugs (Mulheran & Degg 1997; Ozturan & Lam 1998; Littman et al. 1998; Katbamna et al. 1999; Stavroulaki et al. 2001; Stavroulaki et al. 2002). It is possible that noise-related OHC damage may have contributed to the perception of tinnitus in some of the Guest et al. study participants. Additionally, tinnitus is a heterogeneous disorder with many potential mechanisms other than synaptopathy (Henry et al. 2014). By recruiting based on the perception of tinnitus, they may have increased the likelihood of including a greater variety of tinnitus etiologies in their study. The presence of tinnitus unrelated to noise exposure in their sample may have obscured their results. This seems likely given that the noise exposure scores for several of the individual tinnitus participants were similar to the scores seen in the non-tinnitus controls.
Bramhall et al. (2015) investigated the relationship between speech perception in noise and ABR wave I amplitude across individuals of a variety of ages with 4 frequency pure tone averages (for 0.5, 1, 2 and 4 kHz) ranging from approximately 0 – 40 dB HL. They found that lower ABR wave I amplitudes were associated with poorer performance on the QuickSIN, but there was an interaction with pure tone average, with a greater wave I amplitude effect seen in individuals with poorer audiometric thresholds. In that study, participants with normal pure tone thresholds showed little effect of ABR wave I amplitude on their speech in noise perception. These results are consistent with the apparent lack of association between ABR wave I amplitude and the WIN in the current study.
In a study design similar to the present study, Liberman et al. divided young adults with normal pure tone thresholds from 250–8000 Hz into two groups based on their self-reported noise exposure history (2016). As in the present study, they observed similar speech perception performance in quiet for both noise exposure groups. However, they also found a significant decrease in speech-in-noise perception for males with higher reported noise exposure compared with those with lower exposure. These contradictory findings may be due to differences in study design (both studies used NU-6 word lists, but in the Liberman study words were presented at 35 dB HL with a white noise masker versus the current study where words were presented at 80 dB HL in multitalker babble), group differences in the extended high frequency thresholds and DPOAEs for the Liberman study, or simply an artifact of sampling variability.
Limitations
Studying cochlear synatopathy in humans is very difficult due to several limitations that are not a problem in animal models. The following limitations should be considered when drawing conclusions from this and other human studies of synaptopathy. 1) Synaptopathy cannot be confirmed non-invasively. In this study, ABR wave I amplitude was used as an indirect measure of auditory nerve function. While this measure is highly correlated with synaptopathy in animal models, it is only a proxy for synaptopathy and can be impacted by other factors. Although ABR wave I amplitude as measured in this study revealed an association between wave I amplitude and the probability of reporting tinnitus at a group level, the variability in wave I amplitudes observed among the participants with and without tinnitus would preclude use of this measure for detecting synaptopathy or objectively diagnosing tinnitus in individuals. Accomplishing these tasks will require the development of more sensitive metrics. 2) In most cases, individuals with synaptopathy would be expected to also have OHC loss, making it difficult to isolate the effects of OHC loss from the effects of synaptopathy. In this study we limited study participation to individuals with normal OHC function and adjusted for average DPOAE level from 3–8 kHz in our statistical model, but it’s possible the DPOAE stimulus level we used was not sensitive to all OHC damage. 3) Human studies of synaptopathy are generally confined to cross-sectional study designs because it is unethical to purposefully expose people to noise levels that would be expected to result in synaptic loss. This makes it difficult to conclusively confirm relationships between noise exposure, ABR wave I amplitudes, and perceptual changes. 4) Due to the difficult task of finding individuals with high levels of noise exposure and good OHC function, sample sizes are limited. In this study, there were only 2 female Veterans with high noise exposure, which limited the number of females with tinnitus. The effects of the small sample size can be observed in the width of the confidence intervals.
CONCLUSIONS
This study investigated the relationship between ABR wave I amplitude and three aspects of auditory perception (tinnitus, speech perception, and loudness discomfort levels) in young military Veterans and non-Veterans with normal auditory thresholds and DPOAEs. Perception of frequent or constant tinnitus was associated with a reduction in ABR wave I amplitude, but not wave V amplitude. The relationship between ABR wave I amplitude and tinnitus remained after adjusting for differences in sex and DPOAE levels between participants. Although the presence of cochlear synaptopathy cannot be confirmed in the participants with lower ABR wave I amplitudes, this suggests a link between synaptopathy and tinnitus. Speech perception, both in quiet and in noise, and loudness discomfort levels did not appear correlated with ABR wave I amplitude, but this should be interpreted with caution as it may be a consequence of the specific perceptual measures used in this study.
Supplementary Material
Acknowledgements
N.F.B. designed and performed experiments, analyzed data, and wrote the paper; D.K.M. aided in the design of experiments and provided critical revision; G.P.M. provided statistical analysis and critical revision. The authors thank Drs. Brad Buran and Charlie Liberman for helpful comments on the study and manuscript. The research described here was supported by the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service - Award #C1484-M (to N.F.B.) and #C9230-C (to NCRAR). The opinions and assertions presented are private views of the authors and are not to be construed as official or as necessarily reflecting the views of the VA or the Department of Defense.
References
- Anari M, Axelsson A, Eliasson A et al. (1999). Hypersensitivity to sound--questionnaire data, audiometry and classification. Scand.Audiol, 28(4), 219–230. [DOI] [PubMed] [Google Scholar]
- Auerbach BD, Rodrigues PV, & Salvi RJ (2014). Central gain control in tinnitus and hyperacusis. Front.Neurol, 5, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badri R, Siegel JH, & Wright BA (2011). Auditory filter shapes and high-frequency hearing in adults who have impaired speech in noise performance despite clinically normal audiograms. J.Acoust.Soc.Am, 129(2), 852–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea G, Attias J, Gold S et al. (1990). Tinnitus with normal hearing sensitivity: extended high-frequency audiometry and auditory-nerve brain-stem-evoked responses. Audiology, 29(1), 36–45. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, & Myers K (2007). Primary afferent dendrite degeneration as a cause of tinnitus. Journal of Neuroscience Research, 85(7), 1489–1498. [DOI] [PubMed] [Google Scholar]
- Bramhall N, Ong B, Ko J et al. (2015). Speech Perception Ability in Noise is Correlated with Auditory Brainstem Response Wave I Amplitude. J.Am.Acad.Audiol, 26(5), 509–517. [DOI] [PubMed] [Google Scholar]
- Bramhall NF, Konrad-Martin D, McMillan GP et al. (2017). Auditory Brainstem Response Altered in Humans With Noise Exposure Despite Normal Outer Hair Cell Function. Ear Hear, 38(1), e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton H, Plack CJ, Maslin M et al. (2015). Pump up the volume: could excessive neural gain explain tinnitus and hyperacusis? Audiol.Neurootol, 20(4), 273–282. [DOI] [PubMed] [Google Scholar]
- Buran B (2015). Auditory-wave-analysis: v1.1. Retrieved April 2017, from https://zenodo.org/record/17365#.WOUXbvnyvmg
- Costalupes JA, Young ED, & Gibson DJ (1984). Effects of continuous noise backgrounds on rate response of auditory nerve fibers in cat. J.Neurophysiol, 51(6), 1326–1344. [DOI] [PubMed] [Google Scholar]
- Cox RM, Alexander GC, Taylor IM et al. (1997). The contour test of loudness perception. Ear Hear, 18(5), 388–400. [DOI] [PubMed] [Google Scholar]
- Don M, & Eggermont JJ (1978). Analysis of the click-evoked brainstem potentials in man unsing high-pass noise masking. The Journal of the Acoustical Society of America, 63(4), 1084–1092. [DOI] [PubMed] [Google Scholar]
- Earl BR, & Chertoff ME (2010). Predicting auditory nerve survival using the compound action potential. Ear Hear, 31(1), 7–21. [DOI] [PubMed] [Google Scholar]
- Engdahl B, & Kemp DT (1996). The effect of noise exposure on the details of distortion product otoacoustic emissions in humans. J.Acoust.Soc.Am, 99(3), 1573–1587. [DOI] [PubMed] [Google Scholar]
- Fernandez KA, Jeffers PW, Lall K et al. (2015). Aging after noise exposure: acceleration of cochlear synaptopathy in “recovered” ears. J.Neurosci, 35(19), 7509–7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer RL, McMillan GP, Austin DF et al. (2011). Audiometric thresholds and prevalence of tinnitus among male veterans in the United States: data from the National Health and Nutrition Examination Survey, 1999–2006. J.Rehabil.Res.Dev, 48(5), 503–516. [DOI] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, & Liberman MC (2013). Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J.Neurophysiol, 110(3), 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JS, Griest SE, Thielman EJ et al. (2016). Audiologic characteristics in a sample of recently-separated military Veterans: The Noise Outcomes in Servicemembers Epidemiology Study (NOISE Study). Hear.Res, E-pub ahead of print [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Ohlrich B et al. (1997). From laboratory to clinic: a large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear Hear, 18(6), 440–455. [DOI] [PubMed] [Google Scholar]
- Gu JW, Herrmann BS, Levine RA et al. (2012). Brainstem auditory evoked potentials suggest a role for the ventral cochlear nucleus in tinnitus. J.Assoc.Res.Otolaryngol, 13(6), 819–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest H, Munro KJ, Prendergast G et al. (2017). Tinnitus with a normal audiogram: Relation to noise exposure but no evidence for cochlear synaptopathy. Hear.Res, 344, 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ Jr, & Stankovic KM (1996). Medial efferent inhibition produces the largest equivalent attenuations at moderate to high sound levels in cat auditory-nerve fibers. J.Acoust.Soc.Am, 100(3), 1680–1690. [DOI] [PubMed] [Google Scholar]
- Henry JA, Roberts LE, Caspary DM et al. (2014). Underlying mechanisms of tinnitus: review and clinical implications. J.Am.Acad.Audiol, 25(1), 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse LL, Bakay W, Ong HC et al. (2016). Non-Monotonic Relation between Noise Exposure Severity and Neuronal Hyperactivity in the Auditory Midbrain. Front.Neurol, 7, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickox AE, & Liberman MC (2014). Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J.Neurophysiol, 111(3), 552–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoben R, Easow G, Pevzner S, & Parker MA (2017). Outer hair cell and auditory nerve function in speech recognition in quiet and in background noise. Frontiers in Neuroscience, 11, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katbamna B, Homnick DN, & Marks JH (1999). Effects of chronic tobramycin treatment on distortion product otoacoustic emissions. Ear Hear, 20(5), 393–402. [DOI] [PubMed] [Google Scholar]
- Konrad-Martin D, Dille MF, McMillan G et al. (2012). Age-related changes in the auditory brainstem response. J.Am.Acad.Audiol, 23(1), 18–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, & Liberman MC (2009). Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J.Neurosci, 29(45), 14077–14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC (1978). Auditory-nerve response from cats raised in a low-noise chamber. J.Acoust.Soc.Am, 63(2), 442–455. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Epstein MJ, Cleveland SS et al. (2016). Toward a Differential Diagnosis of Hidden Hearing Loss in Humans. PloS One, 11(9), e0162726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG et al. (2011). Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J.Assoc.Res.Otolaryngol, 12(5), 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman TA, Magruder A, & Strother DR (1998). Monitoring and predicting ototoxic damage using distortion-product otoacoustic emissions: pediatric case study. J.Am.Acad.Audiol, 9(4), 257–262. [PubMed] [Google Scholar]
- Makary CA, Shin J, Kujawa SG et al. (2011). Age-related primary cochlear neuronal degeneration in human temporal bones. J.Assoc.Res.Otolaryngol, 12(6), 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Lapsley Miller JA, Heller LM et al. (2009). Detecting incipient inner-ear damage from impulse noise with otoacoustic emissions. J.Acoust.Soc.Am, 125(2), 995–1013. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Phillips DS, & Trune DR (1989). Variables affecting the auditory brainstem response: audiogram, age, gender and head size. Hear.Res, 40(1–2), 75–85. [DOI] [PubMed] [Google Scholar]
- Modh D, Katarkar A, Alam N et al. (2014). Relation of distortion product otoacoustic emission and tinnitus in normal hearing patients: a pilot study. Noise Health, 16(69), 69–72. [DOI] [PubMed] [Google Scholar]
- Mulheran M, & Degg C (1997). Comparison of distortion product OAE generation between a patient group requiring frequent gentamicin therapy and control subjects. Br.J.Audiol, 31(1), 5–9. [DOI] [PubMed] [Google Scholar]
- Neely S, & Liu Z (1993). EMAV: Otoacoustic emission averager In Tech Memo No. 17: Boys Town National Research Hospital Omaha. [Google Scholar]
- Norena AJ (2011). An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci.Biobehav.Rev, 35(5), 1089–1109. [DOI] [PubMed] [Google Scholar]
- Ozturan O, & Lam S (1998). The effect of hemodialysis on hearing using pure-tone audiometry and distortion-product otoacoustic emissions. ORL J.Otorhinolaryngol.Relat.Spec, 60(6), 306–313. [DOI] [PubMed] [Google Scholar]
- Paul BT, Bruce IC, & Roberts LE (2017). Evidence that hidden hearing loss underlies amplitude modulation encoding deficits in individuals with and without tinnitus. Hearing Research, 344, 170–182. [DOI] [PubMed] [Google Scholar]
- Prendergast G, Guest H, Munro KJ et al. (2017). Effects of noise exposure on young adults with normal audiograms I: Electrophysiology. Hear.Res, 344, 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss LA, Ramachandran R, & May BJ (2011). Effects of signal level and background noise on spectral representations in the auditory nerve of the domestic cat. J.Assoc.Res.Otolaryngol, 12(1), 71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez TG, Medeiros IR, Levy CP et al. (2005). Tinnitus in normally hearing patients: clinical aspects and repercussions. Braz J.Otorhinolaryngol, 71(4), 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaette R, & Kempter R (2006). Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: a computational model. Eur.J.Neurosci, 23(11), 3124–3138. [DOI] [PubMed] [Google Scholar]
- Schaette R, & Kempter R (2009). Predicting tinnitus pitch from patients’ audiograms with a computational model for the development of neuronal hyperactivity. J.Neurophysiol, 101(6), 3042–3052. [DOI] [PubMed] [Google Scholar]
- Schaette R, & McAlpine D (2011). Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J.Neurosci, 31, 13452–13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, & Boettcher FA (1996). Age-related loss of activity of auditory-nerve fibers. J.Neurophysiol, 76(4), 2799–2803. [DOI] [PubMed] [Google Scholar]
- Seixas NS, Goldman B, Sheppard L et al. (2005). Prospective noise induced changes to hearing among construction industry apprentices. Occup.Environ.Med, 62(5), 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC et al. (2013). Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J.Neurosci, 33(34), 13686–13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrake J, Diehl PU, & Schaette R (2015). Audiometric characteristics of hyperacusis patients. Front.Neurol, 6, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock LP, & Formby C (2005). Estimates of loudness, loudness discomfort, and the auditory dynamic range: normative estimates, comparison of procedures, and test-retest reliability. J.Am.Acad.Audiol, 16(2), 85–100. [DOI] [PubMed] [Google Scholar]
- Stamper GC, & Johnson TA (2015). Auditory function in normal-hearing, noise-exposed human ears. Ear Hear, 36(2), 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavroulaki P, Apostolopoulos N, Segas J et al. (2001). Evoked otoacoustic emissions--an approach for monitoring cisplatin induced ototoxicity in children. Int.J.Pediatr.Otorhinolaryngol, 59(1), 47–57. [DOI] [PubMed] [Google Scholar]
- Stavroulaki P, Vossinakis IC, Dinopoulou D et al. (2002). Otoacoustic emissions for monitoring aminoglycoside-induced ototoxicity in children with cystic fibrosis. Arch.Otolaryngol.Head.Neck.Surg, 128(2), 150–155. [DOI] [PubMed] [Google Scholar]
- Taberner AM, & Liberman MC (2005). Response properties of single auditory nerve fibers in the mouse. J.Neurophysiol, 93(1), 557–569. [DOI] [PubMed] [Google Scholar]
- Trune DR, Mitchell C, & Phillips DS (1988). The relative importance of head size, gender and age on the auditory brainstem response. Hear.Res, 32(2–3), 165–174. [DOI] [PubMed] [Google Scholar]
- Tyler RS, Pienkowski M, Roncancio ER et al. (2014). A review of hyperacusis and future directions: part I. Definitions and manifestations. Am.J.Audiol, 23(4), 402–419. [DOI] [PubMed] [Google Scholar]
- Viana LM, O’Malley JT, Burgess BJ et al. (2015). Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hear.Res, 327, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, & Caspary DM (2011). Inhibitory neurotransmission in animal models of tinnitus: maladaptive plasticity. Hear.Res, 279(1–2), 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RH, Carnell CS, & Cleghorn AL (2007). The Words-in-Noise (WIN) test with multitalker babble and speech-spectrum noise maskers. J.Am.Acad.Audiol, 18(6), 522–529. [DOI] [PubMed] [Google Scholar]
- Zaugg TL, Thielman EJ, Griest S et al. (2016). Subjective Reports of Trouble Tolerating Sound in Daily Life versus Loudness Discomfort Levels. Am.J.Audiol, 25(4), 359–363. [DOI] [PubMed] [Google Scholar]
- Zhao F, & Stephens D (2007). A critical review of King-Kopetzky syndrome: Hearing difficulties, but normal hearing? Audiogical Medicine, 5, 119–124. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


