Abstract
Intestinal ischemia-reperfusion (I/R) injury is a life-threatening vascular emergency and has long been a disturbing problem for surgeons. Oxidative stress is considered a vital factor in I/R injury. Metformin has anti-oxidative properties and protects against I/R injury. The present study aimed to investigate whether Metformin protects against intestinal I/R injury and reveal the protective mechanism of Metformin. I/R injury was induced in mice by temporary superior mesenteric artery occlusion, and Caco-2 cells were subjected to OGD/R to establish an in vitro model. Different doses of Metformin were administered in vivo and in vitro. We found that I/R injury led to intestinal barrier disruption and cell death by examining histopathological results and the intestinal barrier index, including TER, tight junction proteins and serum biomarkers. We confirmed the existence of pyroptosis in intestinal I/R injury. Moreover, we confirmed the role of pyroptosis in intestinal I/R injury by silencing the gasdermin D (GSDMD). Then, we confirmed that Metformin treatment protected barrier function against intestinal I/R injury and reduced oxidative stress and the inflammatory response. Importantly, Metformin reduced pyroptosis-related proteins, including NLRP3, cleaved caspase-1, and the N-terminus of GSDMD. Knocking down the GSDMD could reversed the protective effects of Metformin, which showed pyroptosis was one of the major cell death pathways controlled by Metformin treatment in setting of intestinal I/R injury. We also discovered that Metformin suppressed the expression of TXNIP and the interaction between TXNIP and NLRP3. We performed siRNA knockdown and found that the protective effects were abolished, which further confirmed our findings. In conclusion, we believe that Metformin protects against intestinal I/R injury in a TXNIP-NLRP3-GSDMD-dependent manner.
Keywords: Intestinal I/R injury, Metformin, Pyroptosis, TXNIP
Abbreviations: I/R, Ischemia-reperfusion; OGD/R, oxygen-glucose deprivation/reperfusion; SMA, superior mesenteric artery; NLRP3, NOD-, LRR- and pyrin domain-containing 3; TXNIP, Thioredoxin-interacting protein; GSDMD, gasdermin D; TER, transepithelial electrical resistance; I-FABP, intestinal fatty acid binding protein; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione; TNF-α, tumor necrosis factor α; IL-6, interleukin 6; IL-1β, interleukin 1β; IL-18, interleukin 18; ROS, Reactive Oxygen Species; DHE, Dihydroethidium
Highlights
-
•
Pyroptosis plays an important role in intestinal I/R injury.
-
•
Metformin protects against intestinal I/R injury in mice.
-
•
Metformin protects Caco-2 cells subjected to OGD/R.
-
•
Metformin inhibits pyroptosis, inflammation and oxidative stress during I/R injury.
-
•
Metformin exerts protective effect through TXNIP-NLRP3-GSDMD pathway.
1. Introduction
Ischemia-reperfusion (I/R) injury occurs when the blood supply is temporarily interrupted and leads to organ damage or even organ failure. I/R injury of the intestine is a life-threatening vascular emergency that often occurs during localized interventions, such as incarcerated hernias, volvulus, and small bowel transplantation, and can also occur as a severe complication of some systematic diseases, such as septic shock and cardiopulmonary disease [1,2]. Ischemia of the intestine leads to an increase in microvascular permeability and a disruption of the mucosal barrier, and reperfusion brings about an increase in oxidative products and infiltration of inflammatory cells. Once the disruption of the intestinal barrier exceeds its regenerative ability, the intestinal flora trigger life-threatening sepsis [3,4]. The mechanism of intestinal I/R injury is complex and involves many signaling pathways. Since intestinal ischemia is rarely preventable, most studies have concentrated on the strategy for the reperfusion period and the development of novel therapeutic approaches for reducing ROS, limiting the overloaded inflammatory response and attenuating cellular damage [5].
Pyroptosis is a newly discovered programmed cell death process that occurs in multiple tissues, including the liver and intestine, during several stress conditions. Pyroptosis not only induces cell death but also causes excessive inflammatory damage. Activation of the NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome transforms pro-caspase-1 into cleaved caspase-1 and then cleaves gasdermin (GSDM) family proteins, leading to pyroptosis [6]. Recent studies have revealed that NLRP3-related pyroptosis plays an important role in intestinal diseases, particularly in DSS-induced colitis and inflammatory bowel disease [7,8]. However, few studies have revealed the importance of pyroptosis in intestinal I/R injury.
Oxidative stress is considered a crucial factor in the development of intestinal I/R injury. Thioredoxin-interacting protein (TXNIP), an endogenous negative modulator of thioredoxin (TXN), plays a vital role in maintaining the redox balance in the cell [9]. Recent studies have reported that TXNIP contributes to I/R injury and is regarded as a considerable target for treatment [10]. Interestingly, previous studies have shown that TXNIP is not only involved in exacerbating oxidative stress but also leads to an increased inflammatory response in an NLRP3 inflammasome-dependent manner [[11], [12], [13], [14]]. However, the role of TXNIP and NLRP3-related pyroptosis in intestinal I/R injury is still not well understood.
Metformin is widely used for the treatment of type 2 diabetes and metabolic syndrome due to its strong ability to enhance insulin sensitivity and its satisfactory safety. A previous study demonstrated that Metformin mediates glucose homeostasis by reducing TXNIP expression. Metformin also has multiple protective effects in a hepatic I/R injury model via directly reducing the inflammatory response and oxidative stress [15,16]. Based on the fact that Metformin has robust anti-oxidative effects by decreasing TXNIP levels, we hypothesized that Metformin also protects the intestine from I/R injury. The aim of this study was to clarify the protective effect of Metformin in intestinal I/R injury and to demonstrate the underlying mechanism.
2. Materials and methods
2.1. Experimental animals
Healthy C57BL/6 mice (male, 7 weeks old, weighing 22–25 g) were used in the experimental to establish the animal model and all purchased from Animal Feeding Center of Xi'an Jiaotong University Health Science Center. All mice were maintained under an optimal condition (25 °C, 50% humidity and a 12:12 day/night cycle) for 7 days before the experiments. Standard diet and free access of tap was offered. The animal experiment procedures were performed in accordance with the Guide of Laboratory Animal Care and Use from the United States National Institution of Health and were approved by the Institutional Animal Care and Use Committee (IACUC) of Xi'an Jiaotong University, China.
2.2. Experimental model
Healthy C57BL/6 mice were anesthetized with 1–2% isoflurane and laparotomy was performed to expose the superior mesenteric artery (SMA). To observe the effect of I/R injury, ischemia was induced in mice by a temporary occlusion of SMA with an atraumatic vascular clamp for 30,60 and 90 min (n = 16). Then, releasing the clamp to allow a reperfusion for 120 min and harvesting the blood samples and small intestine tissues under an anesthetized condition from 6 of them and the other 10 were fed for 72 h to calculate the survive rate. The successful establishment of the model could be revealed by the color change of small intestinal segment. To discover the protective properties of the Metformin, another set of animals were randomly divided into different groups:Sham group, different doses treatment groups (20 and 40 mg/kg), and control group(n = 6). As mentioned, the Metformin was applied as a pretreatment of reperfusion period. Thus, Metformin (TopScience, USA, T0740) was intraperitoneal injected at the beginning of reperfusion at 40 mg/kg or 20 mg/kg and then applied an optimum I/R injury model as previous mentioned in Metformin treatment group while saline would be intraperitoneal injected in control group. And in order to reveal the importance of NLRP3 in intestinal I/R injury, we also treated model animal and sham animal with CY-09 (TopScience, USA, T4164) at dose of 20 mg/kg in another group(n = 6) [17].
To establish an I/R model in vitro, Caco-2 cells were subjected to oxygen-glucose deprivation/reperfusion (OGD/R) to induce an I/R injury. Briefly, the Caco-2 cells were washed with PBS then cultured with glucose-free medium. The cells were kept in an incubator chamber (Phcbi, Japan,MCO-5M) under a condition of 95% N2 and 5% CO2 for 2,4 or 6 h then moved to 95% O2, 5% CO2, and still to be cultured in the glucose-containing medium for 2 h [18]. Caco-2 cells were incubated with or without Metformin at the dose of 1 mM/2 mM for 30 min before the model set-up. The Caco-2 cells were incubated with CY-09 (10 μM) for 30 min before the model set-up to clarify the role of NLRP3 in I/R injury.
2.3. Histopathological examination
Intestine tissue samples were gathered 120 min after reperfusion. Samples were embedded in paraffin after fixed in 10% formalin solution and sectioned into 5-μm-thick. To examine the histopathological injury, we stained sections with hematoxylin and eosin (H&E) stain. Histological injuries in the mucosa were evaluated by quantitative measured of tissue damage by a blinded observer. The Chiu's score classification of small intestine injury was applied to evaluate the damage in small intestine samples [19] (Table 1).
Table 1.
The Chiu's core classification of small intestine injury.
| Classification | Pathological change |
|---|---|
| Level 0 | Mucosa without changes. |
| Level 1 | Well-constituted villosities, no cellular lysis or inflammatory process, although there is formation of Grunhagen's sub-epithelial space. |
| Level 2 | Presence of cellular lysis, formation of Grunhagen's sub-epithelial space and increased spacing among the villosities. |
| Level 3 | Destruction of the free villosities section, presence of dilated capillaries and inflamed cells. |
| Level 4 | Structural destruction of the villosities, only traces of some villosities, formed by inflamed cells and necrotic material, with hemorrhage and basal glandular ulceration. |
| Level 5 | Destruction of all the mucosa, no glandular structure can be seen, only the amorphous material laying on the sub-mucosa tissue. |
2.4. Detection of oxidative stress
The intestinal samples were gathered 120 min after reperfusion to detect the level of oxidative stress. The activities of glutathione (GSH), superoxide dismutase (SOD) and the level of malondialdehyde (MDA) in intestine tissues were detected by commercial biochemical kits (Nanjing Jiancheng, China) as previously mentioned [20]. To detect the level of ROS, the Caco-2 cells were incubated with Dihydroethidium (DHE) dye (Sigma-Aldrich, USA) for 30 min at a concentration of 3 μM after I/R model establishment and Metformin treatment. The results were observed with a fluorescence microscope (Carl Zeiss, Germany), and representative fields were chosen for application.
2.5. Enzyme-linked immunosorbent assay (ELISA)
Small intestine tissues were homogenized with PBS at 4 °C, and then centrifuged at 4 °C for 0 min at 13,000 rpm. Then, the level of IL-1β, IL-6 and TNF-αwere detected with ELISA kits (Nanjing Jiancheng, China) according to the manufacturer's instructions. The level of I-FABP in serum, a special biomarker for intestinal barrier function, was also detected via corresponding ELISA kit (Nanjing Jiancheng, China, H265).
2.6. Transepithelial electrical resistance
Intestinal barrier integrity was evaluated by measuring transepithelial electrical resistance (TER). Briefly, the intestinal mucosae were collected as soon as the mice were sacrificed and then TER were detected with a Ussing chamber(EM-CSYS,PI,USA) as described[21].
2.7. Cell culture
Caco-2 was purchased from the cell Bank of Shanghai Institutes for Biological Science (Shanghai, China). Cells were maintained in complete Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum, penicillin (100U/mL) and ciprofloxacin (10 μg/mL) as well as 1% nonessential amino acids, and was kept under a controlled condition (37 °C,5% CO2). 0.25% trypsin solution containing ethylene diamine tetraacetic acid (EDTA) wasused for cell passage.
2.8. Cell viability assay and LDH detection
The cell viability was evaluated with the Cell Counting Kit‐8 (CCK‐8, Abcam, the US) following the manufacturer's description. Simply speaking, 1 × 104 Caco-2 cells were seeded into 96-well plates and cells were kept in an incubator chamber under a condition of 95% N2 and 5% CO2 for different time (0,2,4,6 h) and then moved to 95% O2, 5% CO2 for 2 h as mentioned. Then, 10 μL CCK‐8 was mixed with 90 μL medium and added to each well and incubated at 37 °C for 2 h. The Optical density (OD) values were measured at 450 nm.
Lactate dehydrogenase (LDH) activity was tested with the commercial kit (Nanjing Jiancheng, China) according to the manufacturer's descriptions.
2.9. Knockdown of TXNIP and GSDMD in Caco-2 cells
In order to knock TXNIP and GSDMD down, Caco-2 cells were transfected with 100 nM of either TXNIP or GSDMD target siRNA or control nonspecific siRNA (Genechem, China) with Lipofectamine 2000 reagent (Genechem, China) following the manufacturer's protocol. After 24 h, the cells were subjected to OGD/R model as previously mentioned. The efficiency of depletion was confirmed by Western blot.
2.10. Western blot assay
The protein expression in intestine tissue (n = 6) was detected by Western blot as previously mentioned [20]. Briefly, RIPA lysis buffer was used to extract the total protein and nucleoprotein were extracted with RIPA lysis at 14000g for 15 min. The protein was separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) after determining the protein concentration. Then the proteins were transferred onto polyvinylidene difluoride (PVDF) membranes. The resulting blots were blocked with 8% skim milk and incubated with anti-occludin antibody (1:1000; Proteintech, China), anti-ZO-1 antibody (1:1000; Proteintech, China), anti-TXNIP antibody (1:1000; Proteintech, China), anti-NLRP3 antibody (1:1000; Cell Signaling Technology, USA), anti-Caspase1 antibody (1:1000; Proteintech, China),anti-IL-1β antibody (1:1000; Abcam, USA), anti-IL-18 (1:1000; Abcam, USA),anti-N-terminal of GSDMD(1:1000; Abcam, USA), anti-β-actin antibody (1:10000; Santa Cruz Biotechnology, USA) overnight at 4 °C. Then, the bolts were incubated with horseradish peroxidase-conjugated secondary antibodies (1: 10000; Abcam, USA) for 1 h at 37 °C. The proteins were detected with the chemiluminescence (ECL) system. The expressions of proteins were normalized to β-actin as a reference.
2.11. Immunofluorescence staining
Immunofluorescence staining was used for expression of detecting the NLRP3 and TXNIP in the Caco-2 cells. Simply speaking, The Caco-2 cells, which had been fixed with 4% paraformaldehyde, were incubated with rabbit anti-NLRP3 or TXNIP antibody (1:200, Abcam, USA) overnight at 4° in 48-well-plate. Then the second goat anti-rabbit antibody (1:300, Servicebio, China) was incubated hour at 25 °C and counterstained with 4′-6-diamidino-2-phenylindole (DAPI) (Beyotime, China). The results were observed with a fluorescence microscope (Carl Zeiss, Germany) and representative fields were chosen for application.
2.12. NLRP3 and TXNIP colocalization
In order to revealing the interaction between NLRP3 and TXNIP in Caco-2 cells, the OGD/R-treated cells, which had been fixed with 4% paraformaldehyde, were incubated with primary antibodies against NLRP3 (1:200, R&D, USA) and TXNIP (1:200; Abcam, USA) overnight at 4 °C. Next, Alexa Fluor 488‐labeled goat anti‐mouse and FITC-labeled goat anti‐rabbit secondary antibodies (Proteintech, China) were used to binding the primary antibody visually. Cell nuclei were counterstained with DAPI (Beyotime, China). The images were observed with a fluorescence microscope (Carl Zeiss, Germany) as perviouly mentioned[12].
2.13. Statistical analysis
The measurement data were described as the mean ± standard deviation (SD). All statistical analyses were performed with the SPSS18.0 software (SPSS Inc., USA). Student's t-test and one-way analysis of variance (ANOVA) were used for the comparison among the three groups. The figure was made by GraphPad (version7) Prism software (GraphPad Software, CA). All tests were two-sided and significance was accepted at p < 0.05.
3. Results
3.1. Intestinal ischemia-reperfusion injury induced intestinal barrier disruption and epithelial cell injury
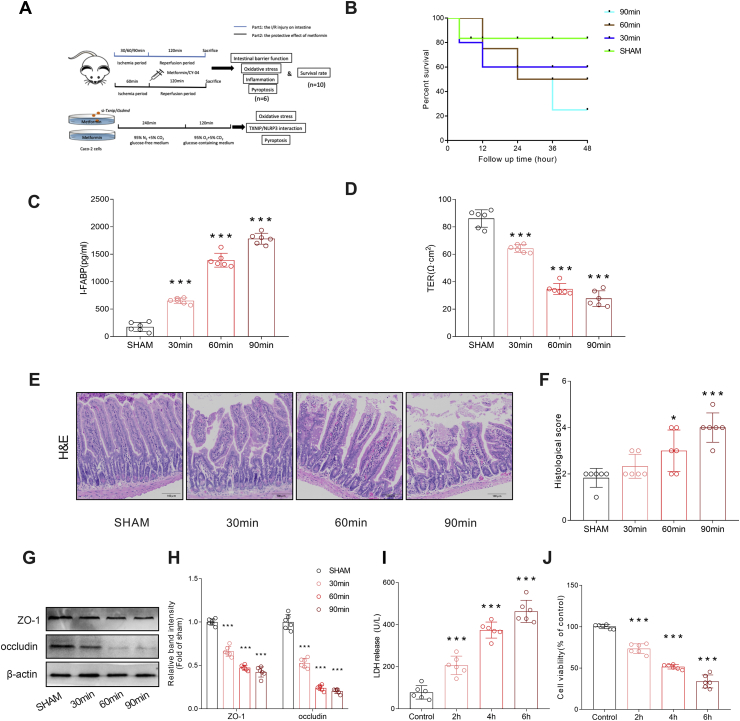
To determine the impacts of different degrees of intestinal I/R injury on the animal models, we examined the survival rates of different groups (Fig. 1B). The results showed that a longer ischemia time resulted in a lower survival rate. To confirm the influence of I/R injury on the intestinal barrier, we first detected the serum level of intestinal fatty binding protein (I-FABP) (Fig. 1C). The results showed that I-FABP increased during I/R injury, which indicates obvious damage to the intestinal barrier structure. To further determine the negative impact on intestinal barrier function, we measured TER in different groups and found that TER decreased after I/R injury in an ischemia time-dependent manner (Fig. 1D). We further confirmed intestinal barrier disruption by histopathological examination. H&E staining and histological scoring showed notable damage to the intestinal structure in I/R injury, which was consistent with the functional changes (Fig. .1E–F). The tight junctions between intestinal epithelial cells are a key part of intestinal permeability, and so we examined the expression of the tight junction protein ZO-1 and occludin. Western blot results showed that the tight junction proteins were reduced after I/R injury (Fig. .1G–H). Moreover, we noticed an elevation in LDH (Fig. 1I) and a significant decrease in cell viability (Fig. 1J), suggesting the presence of cell death during I/R injury. Overall, we demonstrated that I/R injury induced intestinal barrier disruption and cell death.
Fig. 1.
Intestinal ischemia-reperfusion injury induced intestinal barrier disruption and epithelial cell injury. Mice were performed with a temporary occlusion of SMA with an atraumatic vascular for different period and then release the clamp to allow a 120-min reperfusion to build an I/R model. (A) The experimental design of the present work. To evaluate the intestinal injury of different ischemia period, the animals were divided into four groups: the different ischemia period (30,60 and 90 min) group and sham group(n = 6). To determine the protective effect of metformin, another set of animals were administrated with metformin (20 or 40 mg/kg) or saline immediately at the end of a 60-min ischemia period. CY-04 (20 mg/kg), a specific inhibitor of NLRP3 inflammasomes, was used as a positive control for metformin. The OGD/R model was launched in Caco-2 cells. si-Txnip/Gsdmd were applied to revealed the roles of pyroptosis and TXNIP/NLRP3/GSDMD axis in the protective effect of metformin. (B)Survival rates were calculated in different groups (n = 10). (C-D)The integrity of the intestinal barrier was evaluated with the serum I-FABP levels and TER. (E–F) the histopathological damage was estimated with the H&E staining and the Chiu's score classification of small intestine injury (Table 1) was applied to grade the histological score. (G–H) The expressions of the tight junction protein, ZO-1 and occludin, were analyzed by Western blot. Different OGD/R models were induced in Caco-2 cells as mentioned in Methods. (I)The releasing levels of LDH were detected. (J) Cell viability was measured with CCK-8 assay. The values were showed as the mean ± SEM in Fig. 1(C–J) (n = 6) and Fig. 1B(n = 10). *p < 0.05, **p < 0.01, ***p < 0.001 compared with sham group.
3.2. Intestinal ischemia-reperfusion injury induced intestinal inflammation and activation of the NLRP3-Related pyroptosis
Inflammation is an important part of intestinal barrier damage. Therefore, we firstly determined whether excessive inflammation occurred during I/R injury. First, we detected the levels of inflammatory factors in intestinal tissue (Fig. .2A–C). All three inflammatory factors were increased in the I/R injury model, and a longer ischemia period resulted in a more significant increase in inflammatory factors. We then confirmed activation of the NLRP3 inflammasome in an I/R injury model (Fig. .2D–E). NLRP3 was notably increased after I/R injury and was consistent with damage to the intestinal barrier. We further measured the activity of caspase-1 and two downstream products, IL-1β and IL-18, by Western blot (Fig. .2D–E). We found an evident increase in caspase-1 activity and IL-1β and IL-18 in vivo. The N-terminus of GSDMD is the effector molecule of pyroptosis, and we observed an increase in the expression of GSDMD in vivo (Fig. .2F–G). Furthermore, we knocked down the GSDMD in Caco-2 to alleviate the pyroptosis and then set up the OGD/R model. We found that LDH releasing and cell viability were all ameliorated significantly, which showed that pyroptosis was one of the major form of cell death during I/R injury (Fig. .2H–I). Thus, we confirmed the important role of pyroptosis in intestinal I/R injury. Based on our results, we considered 60 min to be the proper ischemia time to establish the I/R models, and we used it in subsequent experiments.
Fig. 2.
Intestinal ischemia-reperfusion injury induced intestinal inflammation and activation of the NLRP3-related pyroptosis. The animal model was launched as mentioned. (A–C) The inflammatory factors in intestinal tissues, including IL-6, IL-1β and TNF-α, were evaluated. (D–E) Pyroptosis-related proteins in intestinal tissues, including NLRP3, cleaved Caspase-1, IL-1β and IL-18, were examined by Western blot. (F–G) GSDMD in intestinal tissues was detected by Western blot. Pyroptosis was suppressed by knockdown the GSDMD with si-Gsdmd in vitro before the OGD/R. (H) the releasing levels of LDH were detected (I) Cell viability was measured with CCK-8 assay. The values were showed as the mean ± SEM(n = 6). *p < 0.05, **p < 0.01, ***p < 0.001 compared with sham group, #p < 0.05 compared with si-Con group and &p < 0.05 compared with si-Con+OGD/R group.
3.3. Metformin protected against intestinal ischemia-reperfusion injury induced intestinal barrier disruption and cell injury
To demonstrate the protective effect of Metformin, different doses of Metformin (20 or 40 mg/kg, i.p.) were first administered before reperfusion. Then, we determined the degree of I/R injury in different groups, and H&E staining results showed reduced injury after Metformin treatment, and the high-dose (40 mg/kg) treatment showed a more powerful protective effect (Fig. .3A–B). The I-FABP and TER results were also consistent with the protective effect of Metformin treatment (Fig. .3C–D). Then, we examined whether Metformin protected intestinal barrier function and found that Metformin protected against damage to ZO-1 and occludin. (Fig. .3E–F). We further confirmed the protective effects in vitro. The cells were kept in a low-oxygen/glucose condition for 4 h then induced an OGD/R models as mentioned. The LDH level was decreased after Metformin treatment (Fig. 3 G). The Metformin treatment also increased the cell viability after I/R injury (Fig. 3 H). Therefore, we confirmed that Metformin could protect against intestinal I/R injury.
Fig. 3.
Metformin protected against intestinal ischemia-reperfusion injury induced intestinal barrier disruption and cell injury. The animal models were launched with a 60-min ischemia period as mentioned. Different doses of metformin (20 or 40 mg/kg) were used to treat the mice as pervious mentioned. (A) The histological damage was evaluated with H&E staining. (B) The histological scores were calculated with the Chiu's score classification of small intestine injury (Table 1). (C) The levels of I-FABP in serum were detected. (D) The TER of gut mucosae was measured. (E–F) The expressions of the tight junction protein, ZO-1 and occludin, were analyzed by Western blot. The cells were kept in a low-oxygen/glucose condition for 4 h then induced an OGD/R models as mentioned. Metformin were subjected at different dose (1 or 2 mM) (G) The releasing levels of LDH were detected. (H) Cell viability was measured with CCK-8 assay. The values were showed as the mean ± SEM(n = 5–6). *p < 0.05 compared with no-treatment group and #p < 0.05 compared with low dose group.
3.4. Metformin protected against intestinal ischemia-reperfusion injury induced intestinal inflammation and pyroptosis
We verified that Metformin protects against I/R-induced intestinal barrier disruption. Then, we determined whether Metformin exerted a protective effect by suppressing NLRP3-induced pyroptosis. We administered Metformin (20 mg/kg) to the model group and used CY-09 (20 mg/kg), an NLRP3 inhibitor, as a positive control as previously described and then detected inflammatory factors in the different groups. The results showed that IL-6 and IL-1β were decreased after treatment with either Metformin or CY-09, while the level of TNF-α was not affected by Metformin or CY-09 (Fig. .4A–C). Then, we examined the activity of the NLRP3 inflammasome. Western blot analysis revealed that components of the NLRP3 inflammasome, including NLRP3 and cleaved caspase-1, were suppressed after Metformin and CY-09 treatment (Fig. .4D–E). Moreover, the pyroptosis-related inflammatory products IL-1β and IL-18 were also decreased after Metformin administration (Fig. .4D–E). The N-terminus of GSDMD, the major effector of pyroptosis, was obviously reduced in the Metformin and CY-09 groups (Fig. .4F–G). Then, we examined the changes in NLRP3 in vitro and found that Metformin suppresses the expression of NLRP3 in OGD/R-treated cells (Fig. .4H–I). Metformin has been proved to regulate many forms of cell death. In order to confirm that the pyroptosis was a major form of cell death controlled by Metformin in I/R injury, we knockdown the GSDMD in Caco-2 to alleviate the pyroptosis and then set up the OGD/R model. We found that suppressing the pyroptosis could also ameliorate LDH releasing and cell viability in absence of Metformin. No differences in cell viability and LDH were detected between si-Gsdmd+Metformin and si-Gsdmd groups, which means a reverse of the protective property of Metformin which reversed the protective property of Metformin (Fig. .4J–K). Thus, we determined that NLRP3-induced pyroptosis was one of the major cell death pathways controlled by Metformin treatment in setting of intestinal I/R injury.
Fig. 4.
Metformin protected against intestinal ischemia-reperfusion injury induced intestinal inflammation and pyroptosis. The I/R models were established as mentioned and the CY-09 was administered at 20 mg/kg as a positive control. (A–C) The inflammatory factors in intestinal tissues, including IL-6, IL-1β and TNF-α, were evaluated. (D–E) Pyroptosis-related proteins in intestinal tissue, including NLRP3, cleaved Caspase-1, IL-1β and IL-18, were examined by Western blot. (F–G) The expression of GSDMD in vivo was detected by Western blot. (H–I) The expression of NLRP3 was also detected in vitro by immunofluorescence. si-Gsdmd was used to inhibit the pyroptosis and revealed the relationship between metformin and pyroptosis (J) the releasing levels of LDH were detected (K) Cell viability was measured with CCK-8 assay. The values were showed as the mean ± SEM(n = 5–6). *p < 0.05 compared with no-treatment group and #p < 0.05 compared with metformin treatment group. $p < 0.05 compared with si-Con group and &p < 0.05 compared with si-Con+OGD/R group.
3.5. Metformin protected against intestinal ischemia-reperfusion injury induced oxidative stress and reduced the interaction between TXNIP and NLRP3
Oxidative stress is a crucial part of I/R injury. We examined whether Metformin influenced oxidative stress in I/R injury. Firstly, we detected oxidative stress in intestinal tissues. The level of MDA in intestinal tissue was decreased after Metformin treatment. In contrast, protective factors, including GSH and SOD, were all increased (Fig. .5A–C). Next, we incubated Caco-2 cells with or without Metformin at a dose of 1 mM or 2 mM for 30 min before the OGD/R model established as previously described. Then, we measured the level of ROS production in the different groups, and the Metformin-treated group showed an obvious decrease (Fig. .5D–E). We next determined the expression of TXNIP and the interaction of TXNIP-NLRP3 in vitro. The expression of TXNIP was elevated during I/R injury but decreased after Metformin treatment (Fig. .5F–I). Finally, we showed an interaction between TXNIP and NLRP3 by immunofluorescence co-localization in Caco-2 cells. Metformin (2 mM) strongly decreased the TXNIP-NLRP3 interaction (Fig. 5 J). Hence, we determined that Metformin decreases oxidative damage in I/R injury and shows a protective effect by reducing the TXNIP-NLRP3 interaction.
Fig. 5.
Metformin protected against intestinal ischemia-reperfusion injury induced oxidative stress and TXNIP-NLRP3 interaction in vitro. Caco-2 cells were induced to an OGD/R model as mentioned. Metformin treated models at different dose (1 or 2 mM). (A–C) Oxidative stress was measured by SOD, GSH and MDA. (D–E) ROS was detected with a DHE staining and the ROS-positive cells were calculated by two random observers. (F–G) the expression of TXNIP was examined by Western blot. (H–I) The expression of TXNIP was also detected in vitro by immunofluorescence. (J) the co-localization of TXNIP and NLRP3 was showed by immunofluorescence. The values were showed as the mean ± SEM(n = 5–6). *p < 0.05 compared with no-treatment group and #p < 0.05 compared with low dose group.
3.6. Silencing of TXNIP abolished metformin-inhibited pyroptosis in vitro
To confirm that Metformin could protect against intestinal I/R injury in a TXNIP-depenent way We silenced TXNIP in Caco-2 cells by using siRNA. We found that knockdown of TXNIP reversed the protective effects of Metformin. In the groups that were transfected with control siRNA, Metformin showed a similar effect as previous results (Fig. .6A–B). However, in the groups in which TXNIP was silenced, Metformin treatment showed no difference compared to that of other groups regarding I/R-induced inflammasome activity and pyroptosis. The LDH release results that there was no difference between si-Txnip+Metformin and si-Txnip groups, also revealed that knockdown of TXNIP reversed the protective effect of Metformin in vitro (Fig. 6 C). In conclusion, we confirmed that Metformin protects against intestinal I/R injury by reducing inflammation and pyroptosis via downregulating the TXNIP-NLRP3 pathway.
Fig. 6.
Knockdown of TXNIP abolished metformin-inhibited pyroptosis in vitro. Caco-2 cells were transfected with TXNIP-targeting siRNA or control siRNA. Then cells were induced as OGD/R models and received metformin treatment at different dose (1 or 2 mM). (A–B) The expression of TXNIP and pyroptosis-related proteins were detected by Western blot. (C) The levels of LDH releasing were detected. The values were showed as the mean ± SEM(n = 5–6). *p < 0.05 compared with si-Con group and #p < 0.05 compared with si-Con+OGD/R group.
4. Discussion
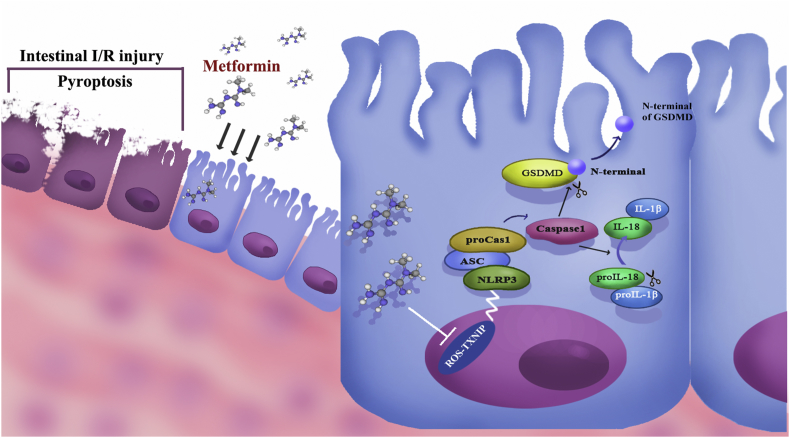
Intestinal ischemia-reperfusion injury has long been a problem for clinical surgeons. On the one hand, as a surgical emergency, I/R injury is undetected and rapidly progressive; thus, prevention is limited. On the other hand, once I/R injury occurs, the damaged intestinal barrier leads to systematic complications and sepsis, which leads to high mortality without effective therapy. Many studies have focused on suppressing oxidative stress and inflammation to attenuate I/R injury [22,23]. Metformin is a widely used diabetes drug, and many studies have demonstrated that Metformin has strong anti-oxidative and anti-inflammatory effects. Thus, Metformin has therapeutic potential in I/R injury [24]. In our study, we first determined that I/R injury leads to disruption of the intestinal barrier and intestinal cell death. Then, we confirmed the protective effect of Metformin against I/R injury, and we noticed that Metformin strong reduces I/R-induced oxidative stress in vivo and in vitro. As a key factor in cellular redox balance, TXNIP is a bridge between oxidative stress and inflammatory injury. Hence, we further determined whether Metformin suppressed oxidative stress during I/R injury and the interaction between TXNIP and NLRP3. To evaluate our hypothesis, we further knocked down TXNIP and found a reverse in the protective effect of Metformin. Based on these results, we believe that Metformin protects against intestinal I/R by decreasing cell pyroptosis via the TXNIP-NLRP3 pathway (Fig. 7).
Fig. 7.
The mechanism map of the protective properties of metformin against intestinal ischemia-reperfusion injury via a TXNIP-NLRP3-GSDMD pathway.
One of the remarkable findings of the present study is that we determined the presence of pyroptosis in intestinal I/R injury and that Metformin had a robust effect against pyroptosis. Pyroptosis is a form of programmed cell death that is associated with inflammatory product release, which is quite different from apoptosis. We found that I/R injury leads to an excessive inflammatory response in vivo and in vitro. Interestingly, we found that IL-1β and IL-18 were increased, which is commonly detected during pyroptosis. We observed that few studies revealed the role of pyroptosis during I/R injury. New research has clarified that pyroptosis is triggered by various stimuli and is initialized by activation of GSDM family proteins [25]. The accumulation of the NLRP3 inflammasome has been considered an important activator of pyroptosis. The mature NLRP3 inflammasome cleaves caspase-1 and then triggers cleavage of GSDM family proteins [26]. We detected activation of the NLRP3 inflammasome during I/R injury in the gut. Even more importantly, we found that the N-terminus of GSDMD was elevated after I/R injury, indicating the presence of pyroptosis. By knockdown the GSDMD, we confirmed that pyroptosis was a major form of cell death during the I/R injury. Because of the release of inflammatory factors such as IL-1β and IL-18, pyroptosis is not only a normal way to induce cell death but also an amplifier of tissue injury, which may explain why I/R leads to rapid and severe damage to the intestinal barrier.
Metformin was first used to treat people with type 2 diabetes, and many studies have demonstrated that Metformin protects against various diseases due to its anti-oxidative properties [24,27]. Metformin was first used to treat people with type 2 diabetes, and many studies have demonstrated that Metformin protects against various diseases due to its anti-oxidative properties [[28], [29], [30], [31]]. However, very few studies have reported the protective effects of Metformin in intestinal I/R injury. According to our work, Metformin strongly protects the intestinal barrier against I/R injury by reducing tight junction protein injury and maintaining the integrity of the intestinal barrier structure. Metformin also reduced cell death in vitro and in vivo. Notably, knocking GSDMD down will abolished the protective effect of Metformin, which means pyroptosis was one of the major forms of cell death mediated by Metformin during the I/R injury.
Most studies have focused on the anti-oxidative property of Metformin in I/R injury and revealed that the protective mechanism was related to mitochondrial maintenance through AMPK-eNOS signaling [32]. Some studies also demonstrated that Metformin prevents ER stress-induced mitochondrial dysfunction [33]. Our results also confirmed that Metformin reduced oxidative stress in intestinal I/R injury, which was consistent with previous studies. Moreover, we also observed strong inflammatory injury in vivo and in vitro, which causes severe damage to the intestinal barrier. However, few studies have revealed the mechanism by which Metformin actually stops damage to tissues, since apoptosis following mitochondrial damage cannot explain the excessive inflammatory response in intestinal I/R injury. In other words, we tried to find the bridge between oxidative stress and inflammatory injury, which may be affected by Metformin. In our study, we observed that Metformin significantly suppressed accumulation of the NLRP3 inflammasome and activation of Caspase-1. The downstream factors of pyroptosis, including IL-1β and IL-18, were decreased after Metformin treatment. Remarkably, GSDMD, a major pyroptosis effector, was also repressed by Metformin. Thus, we believe that pyroptosis plays an important role in intestinal I/R injury and is inhibited by Metformin.
TXNIP is a vital factor in cellular redox balance and is involved in diabetes and insulin resistance, and TXNIP is suppressed by Metformin in diabetes [34]. Interestingly, some studies found that TXNIP not only maintained redox balance but also interacted with NLRP3 to trigger an inflammatory process, which is also called the TXNIP-NLRP3 axis [12,35,36]. In our present work, we found that the TXNIP-NLRP3 axis was involved in the protective effect of Metformin. More importantly, we discovered that the activity of the TXNIP-NLRP3 axis led to GSDMD-induced pyroptosis, which could be the reason for excessive inflammatory injury and has not been reported before. We believe that Metformin has a promising anti-pyroptosis property and is related to the TXNIP-NLRP3-GSDMD axis.
The mechanism of I/R injury is complex and involves multiple molecular pathways. Our study only revealed one of the possible mechanisms. Additionally, some limitations need to be addressed in the present work. Many prospective clinical trials are needed to confirm the effects of Metformin in humans because our results were only based on experiments. We believe that Metformin has promising therapeutic potential for treating intestinal I/R injury.
Funding
This study was supported by funding from “the National Nature Science Foundation of China” (Grant No. 81601672).
Data availability statement
The data related to mice model data, serum cytokine levels, histological staining and Western blot images used to support the findings of this study are available from the corresponding author upon request.
Contributions
Jia YF participated in the research design, animal research and writing of the paper; Cui RX participated in animal research, data analysis and revising of the paper; Wang C participated in the H&E and WB performance; Feng Y participated in the establishment of in vitro experiment; Li ZY and Tong YM participated in the animal research; Qu K, Liu C and Zhang JY provided substantial advice in designing the study and assisting in the division of labor, writing and revising the paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are indebted to all individuals who participated in or helped with this research project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101534.
Contributor Information
Kai Qu, Email: joanne8601@163.com.
Chang Liu, Email: liuchangdoctor@163.com.
Jingyao Zhang, Email: you12ouy@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lehtimaki T.T., Karkkainen J.M., Saari P., Manninen H., Paajanen H., Vanninen R. Detecting acute mesenteric ischemia in CT of the acute abdomen is dependent on clinical suspicion: review of 95 consecutive patients. Eur. J. Radiol. 2015;84(12):2444–2453. doi: 10.1016/j.ejrad.2015.09.006. PubMed PMID: 26413771. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz M.Z. Novel therapies for the management of short bowel syndrome in children. Pediatr. Surg. Int. 2013;29(10):967–974. doi: 10.1007/s00383-013-3404-7. PubMed PMID: 23989526. [DOI] [PubMed] [Google Scholar]
- 3.Koike K., Moore E.E., Moore F.A., Read R.A., Carl V.S., Banerjee A. Gut ischemia/reperfusion produces lung injury independent of endotoxin. Crit. Care Med. 1994;22(9):1438–1444. doi: 10.1097/00003246-199409000-00014. PubMed PMID: 8062567. [DOI] [PubMed] [Google Scholar]
- 4.de Groot H., Rauen U. Ischemia-reperfusion injury: processes in pathogenetic networks: a review. Transplant. Proc. 2007;39(2):481–484. doi: 10.1016/j.transproceed.2006.12.012. PubMed PMID: 17362763. [DOI] [PubMed] [Google Scholar]
- 5.Ates B., Yilmaz I., Geckil H., Iraz M., Birincioglu M., Fiskin K. Protective role of melatonin given either before ischemia or prior to reperfusion on intestinal ischemia-reperfusion damage. J. Pineal Res. 2004;37(3):149–152. doi: 10.1111/j.1600-079X.2004.00148.x. PubMed PMID: 15357658. [DOI] [PubMed] [Google Scholar]
- 6.Kovacs S.B., Miao E.A. Gasdermins: effectors of pyroptosis. Trends Cell Biol. 2017;27(9):673–684. doi: 10.1016/j.tcb.2017.05.005. PubMed PMID: 28619472; PubMed Central PMCID: PMCPMC5565696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang X., Feng Z., Jiang Y., Li J., Xiang Q., Guo S. VSIG4 mediates transcriptional inhibition of Nlrp3 and Il-1beta in macrophages. Sci. Adv. 2019;5(1):eaau7426. doi: 10.1126/sciadv.aau7426. PubMed PMID: 30662948; PubMed Central PMCID: PMCPMC6326752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X., Liu G., Yuan Y., Wu G., Wang S., Yuan L. NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-kappaB signaling. Cell Death Dis. 2019;10(12):906. doi: 10.1038/s41419-019-2157-1. PubMed PMID: 31787755; PubMed Central PMCID: PMCPMC6885517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu J., Holmgren A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. PubMed PMID: 23899494. [DOI] [PubMed] [Google Scholar]
- 10.Gao C., Wang R., Li B., Guo Y., Yin T., Xia Y. TXNIP/Redd1 signaling and excessive autophagy: a novel mechanism of myocardial ischemia/reperfusion injury in mice. Cardiovasc. Res. 2019 doi: 10.1093/cvr/cvz152. PubMed PMID: 31241142. [DOI] [PubMed] [Google Scholar]
- 11.Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11(2):136–140. doi: 10.1038/ni.1831. PubMed PMID: 20023662. [DOI] [PubMed] [Google Scholar]
- 12.Cao Z., Fang Y., Lu Y., Tan D., Du C., Li Y. Melatonin alleviates cadmium-induced liver injury by inhibiting the TXNIP-NLRP3 inflammasome. J. Pineal Res. 2017;62(3) doi: 10.1111/jpi.12389. PubMed PMID: 28099758. [DOI] [PubMed] [Google Scholar]
- 13.Jiang L., Fei D., Gong R., Yang W., Yu W., Pan S. CORM-2 inhibits TXNIP/NLRP3 inflammasome pathway in LPS-induced acute lung injury. Inflamm. Res. 2016;65(11):905–915. doi: 10.1007/s00011-016-0973-7. PubMed PMID: 27412237. [DOI] [PubMed] [Google Scholar]
- 14.Yuan X., Zheng Y., Chen C., Wang C. Anisodamine inhibits endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in rhabdomyolysis-induced acute kidney injury. Apoptosis. 2017;22(12):1524–1531. doi: 10.1007/s10495-017-1414-y. PubMed PMID: 28918467. [DOI] [PubMed] [Google Scholar]
- 15.Chai T.F., Hong S.Y., He H., Zheng L., Hagen T., Luo Y. A potential mechanism of Metformin-mediated regulation of glucose homeostasis: inhibition of Thioredoxin-interacting protein (Txnip) gene expression. Cell. Signal. 2012;24(8):1700–1705. doi: 10.1016/j.cellsig.2012.04.017. PubMed PMID: 22561086. [DOI] [PubMed] [Google Scholar]
- 16.Cahova M., Palenickova E., Dankova H., Sticova E., Burian M., Drahota Z. Metformin prevents ischemia reperfusion-induced oxidative stress in the fatty liver by attenuation of reactive oxygen species formation. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;309(2):G100–G111. doi: 10.1152/ajpgi.00329.2014. PubMed PMID: 26045616. [DOI] [PubMed] [Google Scholar]
- 17.Jiang H., He H., Chen Y., Huang W., Cheng J., Ye J. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 2017;214(11):3219–3238. doi: 10.1084/jem.20171419. PubMed PMID: 29021150; PubMed Central PMCID: PMCPMC5679172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun S., Hu F., Wu J., Zhang S. Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol. 2017;11:577–585. doi: 10.1016/j.redox.2016.12.029. PubMed PMID: 28110213; PubMed Central PMCID: PMCPMC5247568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu C.J., McArdle A.H., Brown R., Scott H.J., Gurd F.N. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch. Surg. 1970;101(4):478–483. doi: 10.1001/archsurg.1970.01340280030009. PubMed PMID: 5457245. [DOI] [PubMed] [Google Scholar]
- 20.Jia Y., Li Z., Feng Y., Cui R., Dong Y., Zhang X. Methane-rich saline ameliorates sepsis-induced acute kidney injury through anti-inflammation, antioxidative, and antiapoptosis effects by regulating endoplasmic reticulum stress. Oxid. Med. Cell. Longev. 2018;2018:4756846. doi: 10.1155/2018/4756846. PubMed PMID: 30581532; PubMed Central PMCID: PMCPMC6276407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie S., Yang T., Wang Z., Li M., Ding L., Hu X. Astragaloside IV attenuates sepsis-induced intestinal barrier dysfunction via suppressing RhoA/NLRP3 inflammasome signaling. Int. Immunopharm. 2019;78:106066. doi: 10.1016/j.intimp.2019.106066. PubMed PMID: 31835087. [DOI] [PubMed] [Google Scholar]
- 22.Carden D.L., Granger D.N. Pathophysiology of ischaemia-reperfusion injury. J. Pathol. 2000;190(3):255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. PubMed PMID: 10685060. [DOI] [PubMed] [Google Scholar]
- 23.Granger D.N., Kvietys P.R. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. PubMed PMID: 26484802; PubMed Central PMCID: PMCPMC4625011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou J., Massey S., Story D., Li L. Metformin: an old drug with new applications. Int. J. Mol. Sci. 2018;19(10) doi: 10.3390/ijms19102863. PubMed PMID: 30241400; PubMed Central PMCID: PMCPMC6213209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi: 10.1038/nature15514. PubMed PMID: 26375003. [DOI] [PubMed] [Google Scholar]
- 26.He W.T., Wan H., Hu L., Chen P., Wang X., Huang Z. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25(12):1285–1298. doi: 10.1038/cr.2015.139. PubMed PMID: 26611636; PubMed Central PMCID: PMCPMC4670995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foretz M., Guigas B., Bertrand L., Pollak M., Viollet B. Metformin: from mechanisms of action to therapies. Cell Metabol. 2014;20(6):953–966. doi: 10.1016/j.cmet.2014.09.018. PubMed PMID: 25456737. [DOI] [PubMed] [Google Scholar]
- 28.Leech T., Chattipakorn N., Chattipakorn S.C. The beneficial roles of Metformin on the brain with cerebral ischaemia/reperfusion injury. Pharmacol. Res. 2019;146:104261. doi: 10.1016/j.phrs.2019.104261. PubMed PMID: 31170502. [DOI] [PubMed] [Google Scholar]
- 29.Higgins L., Palee S., Chattipakorn S.C., Chattipakorn N. Effects of Metformin on the heart with ischaemia-reperfusion injury: evidence of its benefits from in vitro, in vivo and clinical reports. Eur. J. Pharmacol. 2019;858:172489. doi: 10.1016/j.ejphar.2019.172489. PubMed PMID: 31233747. [DOI] [PubMed] [Google Scholar]
- 30.Saribal D., Erdem E., Gungor-Ordueri N.E., Usta A., Karakus C., Karacan M. Metformin decreases testicular damages following ischaemia/reperfusion injury in rats. Andrologia. 2019 doi: 10.1111/and.13481. PubMed PMID: 31815318. [DOI] [PubMed] [Google Scholar]
- 31.Topcu A., Balik G., Atak M., Mercantepe T., Uydu H.A., Tumkaya L. An investigation of the effects of Metformin on ovarian ischemia-reperfusion injury in rats. Eur. J. Pharmacol. 2019;865:172790. doi: 10.1016/j.ejphar.2019.172790. PubMed PMID: 31730761. [DOI] [PubMed] [Google Scholar]
- 32.Calvert J.W., Gundewar S., Jha S., Greer J.J., Bestermann W.H., Tian R. Acute Metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57(3):696–705. doi: 10.2337/db07-1098. PubMed PMID: 18083782. [DOI] [PubMed] [Google Scholar]
- 33.Chen Q., Thompson J., Hu Y., Das A., Lesnefsky E.J. Metformin attenuates ER stress-induced mitochondrial dysfunction. Transl. Res. 2017;190:40–50. doi: 10.1016/j.trsl.2017.09.003. PubMed PMID: 29040818; PubMed Central PMCID: PMCPMC5705457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massollo M., Marini C., Brignone M., Emionite L., Salani B., Riondato M. Metformin temporal and localized effects on gut glucose metabolism assessed using 18F-FDG PET in mice. J. Nucl. Med. 2013;54(2):259–266. doi: 10.2967/jnumed.112.106666. PubMed PMID: 23287574. [DOI] [PubMed] [Google Scholar]
- 35.Han Y., Xu X., Tang C., Gao P., Chen X., Xiong X. Reactive oxygen species promote tubular injury in diabetic nephropathy: the role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018;16:32–46. doi: 10.1016/j.redox.2018.02.013. PubMed PMID: 29475133; PubMed Central PMCID: PMCPMC5842313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi: 10.1038/nature09663. PubMed PMID: 21124315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data related to mice model data, serum cytokine levels, histological staining and Western blot images used to support the findings of this study are available from the corresponding author upon request.