Abstract
Background
To examine the tumor characteristics, treatments and survival outcomes of prostate cancer (PCa) patients with a prostate-specific antigen (PSA) level < 4 ng/ml.
Methods
Of 205,913 men with primary prostate adenocarcinoma in the Surveillance, Epidemiology and End Results (SEER) database (2010 to 2015), 24,054 (11.68%) patients were diagnosed with a PSA level < 4 ng/ml. Comparisons of categorical variables among different groups were performed by using the Chi square test. Multivariate Cox regression analysis was adjusted for age, ethnicity, marital status, insurance status, TNM stage, Gleason grade, treatment and survival. Kaplan-Meier survival curves were constructed for overall mortality and tested by the log-rank test.
Results
PCa patients with a PSA level < 4 ng/ml generally had more favorable tumor characteristics: younger, lower T stage, lower Gleason grade and lower lymph node metastasis rate. However, there were more patients in stage M1 in the group of PSA level < 4 ng/ml than that in the groups of PSA level of 4–10 ng/ml, 10–20 ng/ml and > 20 ng/ml. The multivariate Cox regression model revealed that overall mortality was associated with age, marital status, race, Gleason grade, M stage and treatment approach.
Conclusions
In conclusion, PCa patients with a PSA level < 4 ng/ml have more favorable tumor characteristics at diagnosis and receive more benefit from active treatment. However, those patients with advanced TNM stage and high Gleason grade should be paid more attention in clinical application.
Keywords: Prostate cancer, PSA < 4 ng/ml, Tumor characteristics, SEER program
Background
Prostate cancer (PCa) is the most commonly diagnosed cancer in men worldwide, with an estimated 1.6 million cases and 0.366 million deaths annually [1, 2]. Due to the widespread use of serum prostate-specific antigen (PSA) measurement [3], most PCa patients are now diagnosed with clinically localized disease. PSA is a glycoprotein that is expressed in normal prostate tissue and prostate tumor tissue. Due to lack of basal cells in PCa, the level of PSA is lower in PCa cells than that in normal prostate gland cells, resulting in the disruption of the basement membrane and lumenal structure. Thus, a large number of PSA is released into the circulation [4, 5].
Historical literature suggests that increasing serum PSA level is associated with advanced TNM stage and worse outcomes [6, 7]. Therefore, the pretreatment serum PSA level is an important factor in PCa risk stratification [8]. Several studies have focused on PCa patients with a “normal” PSA level (< 4 ng/ml), who should have better disease characteristics and better outcomes, but were discovered with some biologically aggressive characteristics [9–14]. Doctors may underestimate the aggressiveness of PCa patients under normal PSA level and fail to choose an appropriate treatment method for these patients. Our study aims to use the Surveillance, Epidemiology, and End Results (SEER) database to examine initial tumor characteristics and treatment modalities, and identify prognostic factors in PCa patients with a PSA level < 4 ng/ml.
Methods
Data source
The SEER program of the National Cancer Institute collects cancer patient data from population-based cancer registries in several geographic regions in the United States. The SEER database covers approximately 28% of the population in the United States. Available data include patient demographics, tumor characteristics (histology, grading, and TNM stage), treatment and vital status. SEER*Stat 8.3.5 software was used to extract information from the database.
Study cohort
Data from patients diagnosed with prostate adenocarcinoma (site code C61.9, histological type according to ICD.0.3 code 8140) from January 1, 2010 to December 31, 2015 were extracted from the SEER-18 database with additional treatment fields. PSA information was available in the SEER database beginning in 2017. TNM stage was classified according to the American Joint Committee on Cancer, 6th edition. The following SEER variables were collected: age, year of diagnosis, ethnicity, marital status, insurance status, TNM stage, Gleason score, treatment and survival. Patients with an incomplete pathological diagnosis, a missing Gleason score, missing survival details, multiple primary tumors or missing records for metastatic data were excluded. The PSA value was categorized into the following groups: < 4 ng/ml, 4–10 ng/ml, 10–20 ng/ml and > 20 ng/ml. Treatment methods consisted of radical prostatectomy (RP), brachytherapy (BT) and external beam radiation (EBRT). The treatment approaches were divided into following three groups: no RP or radiotherapy (RT), receiving RP with or without RT and receiving RT without RP.
Statistical analysis
All the data collected in this study were analyzed by SPSS 25.0 (SPSS Inc., Chicago, IL, USA) and Intercooled Stata SE 15.0 (Stata Corporation, College Station, TX, USA). Demographic data, clinical information and tumor features were summarized with descriptive statistics. Comparisons of categorical variables among different PSA level groups were performed by using the Chi square test. The multivariate Cox proportional hazards model was used to assess the relative impacts of risk factors for cancer-specific survival (CCS) and overall survival (OS) on PCa patients with a PSA level < 4 ng/ml. Kaplan-Meier survival curves were constructed for overall mortality; the differences between the curves were tested by the log-rank test. A two-sided P < 0.05 was considered statistically significant.
Results
PCa patients with a PSA level < 4 ng/ml had more favorable tumor characteristics
Two hundred five thousand nine hundred thirteen prostate adenocarcinoma patients from January 1, 2010 to December 31, 2015 were identified in this study, and 24,054 (11.68%) of these patients were diagnosed with a PSA level < 4 ng/ml. As shown in Table 1, in the group of PSA level < 4 ng/ml, there were more patients aged< 65 (57.4%) compared with the patients aged≥65 (42.6%). Beides, the proportions of patients aged< 65 in the group of the PSA level of 4–10 ng/ml, 10–20 ng/ml and > 20 ng/ml were 49.8, 38.7 and 37.3%, respectively (p < 0.001). Interestingly, the PSA < 4 ng/ml group contained a significantly higher proportion of married men (69.3%) compared with groups of PSA level of 4–10 ng/ml, 10–20 ng/ml and > 20 ng/ml (p < 0.001). The Caucasian patients were more likely to have a lower PSA value, whereas the African-American patients had a higher PSA value. Furthermore, in the group of PSA level < 4 ng/ml, there were more patients in stage T2 than that in stage T1, which was opposite of the trend in the groups of PSA level of 4–10 ng/ml, 10–20 ng/ml and > 20 ng/ml. For the patients in stage T3 and T4, the proportion was found to increase with the increase of PSA value. In addition, the lymph node metastasis rate and metastatic stage at diagnosis were significantly higher in the patients with a PSA level > 10 ng/ml than that with a PSA level < 10 ng/ml. However, in the group of M1 stage, there were patients with a PSA level < 4 ng/ml (0.9%) compared with the patients with a PSA level of 4–10 ng/ml (0.5%). The patients with higher PSA values had higher Gleason grades (p < 0.001).
Table 1.
Clinical characteristics of prostate cancer patients
| Features | PSA | P value | |||
|---|---|---|---|---|---|
| < 4 | 4–10 | 10–20 | > 20 | ||
| Age | |||||
| < 65 | 13,817 (57.4) | 63,034 (49.8) | 12,405 (38.7) | 8629 (37.3) | < 0.001 |
| ≥ 65 | 10,237 (42.6) | 63,629 (50.2) | 19,680 (61.3) | 14,482 (62.7) | |
| Married status | |||||
| Married | 16,661 (69.3) | 84,764 (66.9) | 19,799 (61.7) | 12,530 (54.2) | < 0.001 |
| Unmarried | 4388 (18.2) | 25,624 (20.2) | 8142 (25.4) | 7540 (32.6) | |
| Unknown | 3005 (12.5) | 16,275 (12.8) | 4144 (12.9) | 3041 (13.2) | |
| Insurance | |||||
| Insured | 21,608 (89.8) | 114,316 (90.3) | 28,918 (90.1) | 20,417 (88.3) | < 0.001 |
| Uninsured | 210 (0.9) | 1578 (1.2) | 606 (1.9) | 830 (3.6) | |
| Unknown | 2236 (9.3) | 10,769 (8.5) | 2561 (8.0) | 1864 (8.1) | |
| Race | |||||
| Caucasian | 19,460 (80.9) | 96,796 (76.4) | 23,140 (72.1) | 15,639 (67.7) | < 0.001 |
| African-American | 3066 (12.7) | 19,717 (15.6) | 5875 (18.3) | 5309 (23.0) | |
| Other | 801 (3.3) | 6578 (5.2) | 2210 (6.9) | 1619 (7.0) | |
| Unknown | 727 (3.0) | 3572 (2.8) | 860 (2.7) | 544 (2.4) | |
| T stage | |||||
| T1 | 8243 (34.3) | 56,428 (44.5) | 14,252 (44.4) | 8916 (38.6) | < 0.001 |
| T2 | 13,705 (57.0) | 56,084 (44.3) | 11,943 (37.2) | 7968 (34.5) | |
| T3 | 1803 (7.5) | 12,825 (10.1) | 5305 (16.5) | 4234 (18.3) | |
| T4 | 74 (0.3) | 270 (0.2) | 247 (0.8) | 1158 (5.0) | |
| Unknown | 229 (1.0) | 1056 (0.8) | 338 (1.1) | 835 (3.6) | |
| N stage | |||||
| N0 | 23,083 (96.0) | 121,322 (95.8) | 29,675 (92.5) | 17,942 (77.6) | < 0.001 |
| N1 | 266 (1.1) | 1565 (1.2) | 1287 (4.0) | 3374 (14.6) | |
| Unknown | 705 (2.9) | 3776 (3.0) | 1123 (3.5) | 1795 (7.8) | |
| M stage | |||||
| M0 | 23,847 (99.1) | 125,996 (99.5) | 31,213 (97.3) | 16,996 (73.5) | < 0.001 |
| M1 | 207 (0.9) | 667 (0.5) | 872 (2.7) | 6115 (26.5) | |
| Gleason grade | |||||
| 1 | 13,865 (57.6) | 58,851 (46.5) | 9635 (30.0) | 2548 (11.0) | < 0.001 |
| 2 | 6020 (25.0) | 37,375 (29.5) | 8660 (27.0) | 3712 (16.1) | |
| 3 | 1922 (8.0) | 15,144 (12.0) | 5403 (16.8) | 3649 (15.8) | |
| 4 | 1261 (5.2) | 9783 (7.7) | 4604 (14.3) | 5401 (23.4) | |
| 5 | 986 (4.1) | 5510 (4.4) | 3783 (11.8) | 7801 (33.8) | |
Abbreviations: PSA, prostate-specific antigen
Overall mortality of patients with a PSA level < 4 ng/ml was associated with age, marital status, race, Gleason grade, M stage and treatment approach
During the follow-up, cancer-specific death and overall death were detected in the PCa patients with a PSA level < 4 ng/ml. In this study, 488 cancer-specific deaths (2.03%) and 691 overall deaths (2.87%) were recorded. The multivariate Cox regression model revealed that cancer-specific mortality and overall mortality were associated with age, marital status, race, T stage, Gleason grade, and treatment approach (Table 2). The multivariate Cox regression model revealed that the M stage was also an important risk factor for overall mortality. As shown in Table 2, the patients who received RP had a significant prolongation of survival. The patients who underwent RP with or without RT had the lowest risk of mortality in the Cox model (vs. none, CCS: HR =0.346, 95% CI: 0.255–0.470, P < 0.001; OS: HR =0.316, 95% CI: 0.242–0.412, P < 0.001).
Table 2.
Multivariate Cox regression analysis of prognostic factors for CSS and OS among primary prostate cancer patients with PSA < 4 ng/ml (diagnosed 2010–2015)
| Variables | CSS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | ||||||
| < 65 | 1 | 1 | ||||
| ≥ 65 | 2.949 | 2.378–3.659 | < 0.001 | 2.318 | 1.934–2.779 | < 0.001 |
| Married status | ||||||
| Married | 1 | 1 | ||||
| Unmarried | 1.784 | 1.451–2.194 | < 0.001 | 1.626 | 1.366–1.936 | < 0.001 |
| Insurance | ||||||
| Insured | 1 | 1 | ||||
| Uninsured | 0.836 | 0.131–6.690 | 0.836 | 1.481 | 0.614–3.574 | 0.570 |
| Race | ||||||
| Caucasian | 1 | 1 | ||||
| African-American | 1.361 | 1.069–1.733 | 0.012 | 1.260 | 1.018–1.558 | 0.033 |
| Other | 0.662 | 0.364–1.207 | 0.178 | 0.950 | 0.634–1.424 | 0.805 |
| T stage | ||||||
| T1 | 1 | 1 | ||||
| T2 | 0.830 | 0.601–1.148 | 0.061 | 0.861 | 0.733–1.011 | 0.001 |
| T3 | 2.249 | 1.423–3.553 | 0.003 | 1.007 | 0.733–1.384 | 0.440 |
| T4 | 49.213 | 30.094–80.478 | < 0.001 | 15.321 | 10.237–22.932 | < 0.001 |
| N stage | ||||||
| N0 | 1 | 1 | ||||
| N1 | 0.761 | 0.269–2.150 | 0.606 | 1.294 | 0.891–1.880 | 0.176 |
| M stage | ||||||
| M0 | 1 | 1 | ||||
| M1 | 0.627 | 0.221–1.773 | 0.379 | 4.198 | 3.065–5.751 | < 0.001 |
| Gleason grade | ||||||
| 1 | 1 | 1 | ||||
| 2 | 1.385 | 1.111–1.727 | 0.004 | 1.383 | 1.120–1.709 | 0.003 |
| 3 | 1.017 | 0.699–1.481 | 0.929 | 1.135 | 0.806–1.597 | 0.469 |
| 4 | 1.770 | 1.240–2.525 | 0.002 | 2.960 | 2.256–3.883 | < 0.001 |
| 5 | 2.402 | 1.648–3.499 | < 0.001 | 6.059 | 4.725–7.770 | < 0.001 |
| Therapy | ||||||
| No | 1 | 1 | ||||
| RP with or without RT | 0.346 | 0.255–0.470 | < 0.001 | 0.316 | 0.242–0.412 | < 0.001 |
| RT without RP | 0.599 | 0.470–0.764 | < 0.001 | 0.608 | 0.493–0.750 | < 0.001 |
Abbreviations: PSA, prostate-specific antigen; CSS, cancer-specific survival; OS, overall survival; RP, radical prostatectomy; RT, radiation therapy (brachytherapy and/or external beam radiation); HR, hazard ratio; 95% CI, 95% confidence interval
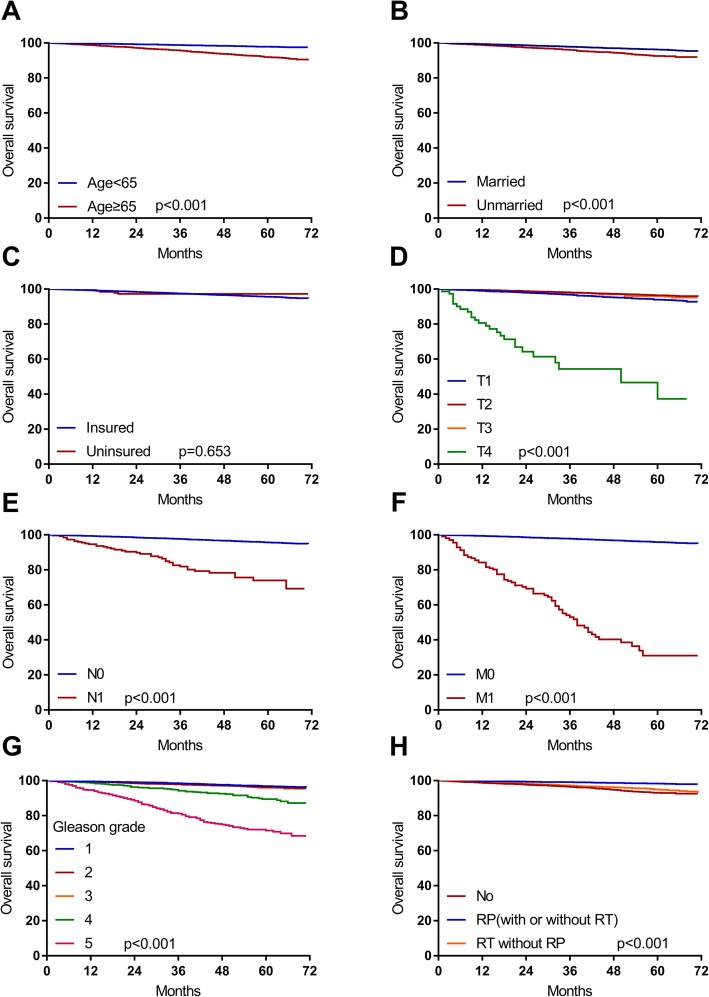
The OS estimates were classified by age, marital status, insurance status, T stage, N stage, M stage, Gleason grade, and treatment approach (Fig. 1). The OS of patients aged≥65 years old was significantly shorter than that of patients aged<65 years old (p < 0.001). In the group of PSA < 4 ng/ml, the married patients had shorter OS compared with those who were unmarried (p < 0.001). However, there was no difference in OS between insured and uninsured patients (p>0.05). Among the patients with a PSA level < 4 ng/ml, the median survival of those in stage of M1 (median survival = 38 months, 95% CI: 31.9–44.1) or T4 (median survival = 50 months, 95% CI: 24.2–75.8) was the shortest. Furthermore, in the group of PSA < 4 ng/ml, patients in N1 stage and Gleason grade4 stage had shorter OS (p < 0.001). Thus, these results suggested patients with advanced TNM stage and high Gleason grade might had worse outcomes. Additionly, our results showed the 5-year OS rates of patients receiving no RP or radiotherapy (RT), receiving RP with or without RT and receiving RT without RP were 93.07, 98.30 and 94.89%, respectively (p < 0.001).
Fig. 1.
The overall survival curve of patients with a PSA level < 4 ng/ml associated with different factors. a Age < 65 and Age ≥ 65; (b) Marital status; (c) Insurance status; (d) T stage; (e) N stage; (f) M stage; (g) Gleason grade;(h) Treatment approach
Discussion
The serum PSA determination is the most commonly used method to detect PCa. Usually, the PSA level > 4 ng/ml is considered abnormal. Over the past few decades, a high frequency of PCa patients with a PSA level < 4 ng/ml has been reported [9]. Autopsy studies have shown that there was a high incidence of occult cancer in the prostate of men who die without being diagnosed with PCa [15]. Thus, the incidence of PCa patients with a PSA level < 4 ng/ml may be underestimated at initial diagnosis. Based on a large population analysis, we found that 11.68% of PCa patients were initially diagnosed with a PSA level < 4 ng/ml. Several novel findings have been identified in our study as follows.
First, the 24,054 PCa patients with a PSA level < 4 ng/ml had more favorable clinical characteristics than those with other PSA level(4–10 ng/ml, 10–20 ng/ml and > 20 ng/ml): the patients with a PSA level < 4 ng/ml were younger and diagnosed with lower T stages (T1–2), lower Gleason grades and lower lymph node metastasis rates. This is consistent with contemporary studies [10, 16, 17]. However, in our study, there were more cases in stage M1 among patients with a PSA level < 4 ng/ml compared with those with a PSA level of 4–10 ng/ml. PCa patients with a PSA level < 4 ng/ml should have undergone abnormal digital rectal examination or magnetic resonance imaging [18], so according to stage, the T1 stage patients comprised the lowest proportion of the patients among the different PSA level groups. Therefore, the PCa patients with a PSA level < 4 ng/ml may be biologically aggressive in some respects, and physicians should appropriately pay attention to prostate patients with a low PSA level. Besides, married patients and Caucasian patients were more likely to have lower PSA values; whereasAfrican-American men without PCa tended to have higher PSA values than Caucasian men without PCa [19].
Furthermore, several prognostic factors for PCa patients with a PSA level < 4 ng/ml, which were correlated with higher mortality risk, were found, including older age (≥65 years), unmarried status, African-American ethnicity, high T stage (T4), high M stage (M1), higher Gleason grade, and lack of surgery or radiotherapy. Our result suggested that the Gleason grading system had an affirmative predictive value in the prognosis of PCa patients with a PSA level < 4 ng/ml. Initial treatment of diagnosed male patients with PCa requires evaluation of clinical staging based on a digital rectal examination, the pretreatment serum PSA value, the Gleason score of a biopsy and the percentage of cancer involvement in the biopsy core. Though our study showed that patients could benefit fromsurgery and radiotherapy, and active surveillance could be considered as the preferred option for localized low-risk PCa patients [20–25]. Based on prognostic factors, the PCa patients with a PSA level < 4 ng/ml can receive a preliminary evaluation from physicians. For PCa patients with a PSA level < 4 ng/ml, risk stratification (high Gleason grade and T stage), age, life expectancy, comorbidities and patient preferences should be considered in the choice of treatment strategies.
To our knowledge, this is the first large-sample-size population-based study focused on PCa patients with a PSA level < 4 ng/ml. Moreover, our study is based on a comprehensive design, including analysis of tumor characteristics, patient treatments, and survival outcomes. However, there were several limitations in our study. First, information on prostate volume, family history, body mass index or biochemical recurrence was not available in the SEER database. Similarly, the information on RT radiation dosage was unavailable. Last but not least, the reasons for such patients to visit doctors were not available. Besides, the data of PSA from SEER database are limited. We will enrich the conclusions drawn in our study through other data sources in future research, and make more comprehensive and in-depth analysis and summary of tumor characteristics, treatments, and survival outcomes for patients with PSA < 4 ng / ml.
Conclusion
In conclusion, PCa patients with a PSA level < 4 ng/ml have more favorable tumor characteristics at diagnosis and receive more benefit from active treatment. However, we should pay more attention to the subset of these patients with advanced TNM stage and high Gleason grade.
Acknowledgement
Not applicable
Abbreviations
- PCa
prostate cancer
- SEER
Surveillance, Epidemiology and End Results
- PSA
prostate-specific antigen
- RP
radical prostatectomy
- BT
brachytherapy
- EBRT
external beam radiation
- RT
radiotherapy
- CCS
cancer-specific survival
- OS
overall survival
- M1
high M stage
Authors’ contributions
ZZ and WY contributed to the conception and design of the study; ZZ and YZ performed the experiments, CC and HL collected and analyzed data; ZZ and WY wrote the manuscript; All authors reviewed and approved the final version of the manuscript.
Funding
No funding was received for this study.
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Peking Union Medical College Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhibo Zhen and Zhien Zhou contributed equally to this study and should be considered co-first author
References
- 1.Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2017.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US preventive services task force recommendation statement. JAMA. 2018;319:1901–1913. doi: 10.1001/jama.2018.0161. [DOI] [PubMed] [Google Scholar]
- 4.Lilja H, Christensson A, Dahlen U, et al. Prostate-specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin. Clin Chem. 1991;37:1618–1625. doi: 10.1093/clinchem/37.9.1618. [DOI] [PubMed] [Google Scholar]
- 5.Mikolajczyk SD, Marks LS, Partin AW, et al. Free prostate-specific antigen in serum is becoming more complex. Urology. 2002;59:797–802. doi: 10.1016/S0090-4295(01)01605-3. [DOI] [PubMed] [Google Scholar]
- 6.Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the prostate cancer prevention trial. J Natl Cancer Inst. 2006;98:529–534. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 7.D'Amico AV, Whittington R, Malkowicz SB, et al. Pretreatment nomogram for prostate-specific antigen recurrence after radical prostatectomy or external-beam radiation therapy for clinically localized prostate cancer. J Clin Oncol. 1999;17:168–172. doi: 10.1200/JCO.1999.17.1.168. [DOI] [PubMed] [Google Scholar]
- 8.Partin AW, Kattan MW, Subong EN, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997;277:1445–1451. doi: 10.1001/jama.1997.03540420041027. [DOI] [PubMed] [Google Scholar]
- 9.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 10.Catalona WJ, Smith DS, Ornstein DK. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/mL and benign prostate examination. Enhancement of specificity with free PSA measurements. JAMA. 1997;277:1452–1455. doi: 10.1001/jama.1997.03540420048028. [DOI] [PubMed] [Google Scholar]
- 11.Partin AW, Carter HB, Chan DW, et al. Prostate specific antigen in the staging of localized prostate cancer: influence of tumor differentiation, tumor volume and benign hyperplasia. J Urol. 1990;143:747–752. doi: 10.1016/S0022-5347(17)40079-6. [DOI] [PubMed] [Google Scholar]
- 12.Oefelein MG, Smith N, Carter M, et al. The incidence of prostate cancer progression with undetectable serum prostate specific antigen in a series of 394 radical prostatectomies. J Urol. 1995;154:2128–2131. doi: 10.1016/S0022-5347(01)66713-2. [DOI] [PubMed] [Google Scholar]
- 13.Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883–895. doi: 10.1001/jama.2018.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giacalone NJ, Wu J, Chen MH, et al. Prostate-specific antigen failure and risk of death within comorbidity subgroups among men with unfavorable-risk prostate cancer treated in a randomized trial. J Clin Oncol. 2016;34:3781–3786. doi: 10.1200/JCO.2016.68.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delongchamps NB, Singh A, Haas GP. The role of prevalence in the diagnosis of prostate cancer. Cancer Control. 2006;13:158–168. doi: 10.1177/107327480601300302. [DOI] [PubMed] [Google Scholar]
- 16.Freedland SJ, Aronson WJ, Kane CJ, et al. Biochemical outcome after radical prostatectomy among men with normal preoperative serum prostate-specific antigen levels. Cancer. 2004;101:748–753. doi: 10.1002/cncr.20390. [DOI] [PubMed] [Google Scholar]
- 17.Lange PH, Ercole CJ, Lightner DJ, et al. The value of serum prostate specific antigen determinations before and after radical prostatectomy. J Urol. 1989;141:873–879. doi: 10.1016/S0022-5347(17)41037-8. [DOI] [PubMed] [Google Scholar]
- 18.Naji L, Randhawa H, Sohani Z, et al. Digital rectal examination for prostate cancer screening in primary care: a systematic review and meta-analysis. Ann Fam Med. 2018;16:149–154. doi: 10.1370/afm.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan TO. Jacobsen SJ, McCarthy WF, et al. Age-specific reference ranges for serum prostate-specific antigen in black men. N Engl J Med. 1996;335:304–310. doi: 10.1056/NEJM199608013350502. [DOI] [PubMed] [Google Scholar]
- 20.Chen RC, Rumble RB, Loblaw DA, et al. Active surveillance for the management of localized prostate cancer (cancer care Ontario guideline): American society of clinical oncology clinical practice guideline endorsement. J Clin Oncol. 2016;34:2182–2190. doi: 10.1200/JCO.2015.65.7759. [DOI] [PubMed] [Google Scholar]
- 21.Dahabreh IJ, Chung M, Balk EM, et al. Active surveillance in men with localized prostate cancer: a systematic review. Ann Intern Med. 2012;156:582–590. doi: 10.7326/0003-4819-156-8-201204170-00009. [DOI] [PubMed] [Google Scholar]
- 22.Dall'Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62:976–983. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 23.Lao C, Edlin R, Rouse P, et al. The cost-effectiveness of active surveillance compared to watchful waiting and radical prostatectomy for low risk localised prostate cancer. BMC Cancer. 2017;17:529. doi: 10.1186/s12885-017-3522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barocas DA, Alvarez J, Resnick MJ, et al. Association between radiation therapy, surgery, or observation for localized prostate cancer and patient-reported outcomes after 3 years. JAMA. 2017;317:1126–1140. doi: 10.1001/jama.2017.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bokhorst LP, Valdagni R, Rannikko A, et al. A decade of active surveillance in the PRIAS study: an update and evaluation of the criteria used to recommend a switch to active treatment. Eur Urol. 2016;70:954–960. doi: 10.1016/j.eururo.2016.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.