Abstract

Large-grained and well-oriented methylammonium lead tribromide (MAPbBr3) perovskite was formed from the conversion of amorphous lead bromide (PbBr2) doped with phenethylamine (PEA). The addition of PEA ions (with an optimized molar ratio of 0.008%) to the PbBr2 solution assisted the formation of a smooth PEA-doped PbBr2 layer by spin-coating. Then, the PEA-doped PbBr2 thin film would convert into large-grained and well-oriented MAPbBr3 with the help of a solid–vapor reaction under a vaporized methylammonium bromide (MABr) and choline chloride (CC) atmosphere. Furthermore, both PEA and CC would passivate the defects of perovskite to improve the crystal quality of perovskite. By applying this perovskite layer in perovskite light-emitting diodes (PeLEDs), the maximum luminance and current efficiency of PeLEDs could reach 20,869 cd/m2 and 3.99 cd/A, respectively; these values are approximately five and three times larger than those of PeLEDs without PEA. The perovskite converted from spin-coated PbBr2 with a PEA dopant remarkably improved the luminance and current efficiency of its PeLEDs.
Introduction
Organometallic trihalide perovskite materials (CH3NH3PbX3, MAPbX3, X = I, Br, and Cl) and optoelectronic devices have attracted considerable research attention because of their high-power conversion efficiency (>20%) in solar cells.1−5 Among these materials, MAPbX3 perovskites show excellent optoelectronic characteristics6−9 and have great potential for luminescent devices, such as light-emitting diodes (LEDs) and lasing devices.10−16 The improved light-emitting efficiency of these devices is related to the morphology and the grain size of MAPbX3 perovskite layers, which are very sensitive to the synthesis method, composition, and structural details.13,17,18 Cho et al.13 introduced the nanocrystal pinning method to produce fully covered MAPbX3 perovskite nanocrystals and dramatically increased the current efficiency of MAPbX3 perovskite LEDs (PeLEDs). Chih et al.18 introduced a NiOx hole injection layer and methylamine gas treatment to, respectively, improve the carrier injection and the quality of MAPbX3 perovskites, and found increases in the emission efficiency of the resulting PeLEDs.
Considering that small grain-sized MAPbX3 perovskite is beneficial for the emission efficiency of PeLEDs, large organic ammonium cations or long alkyl chains as organic capping ligands have recently been introduced as a replacement for methyl ammonium (MA) cations to modify the conventional three-dimensional crystal structure of MAPbX3 perovskite into two- or zero-dimensional crystal structures.19−27 Low-dimensional MAPbX3 perovskites such as quasi-two-dimensional (quasi-2D) perovskites could enhance the light-emitting efficiency of PeLEDs.19−27 Quan et al.24 reported the improved efficiency of quasi-2D MAPbX3 perovskite-based PeLEDs by engineering the crystal domain of the quasi-2D perovskite. A similar concept could also be applied to the CsPbX3-based PeLEDs to improve the emitting efficiency.25 Moreover, Liang et al.26 demonstrated a near-ultraviolet (UV) (PEA)2PbBr4 2D-perovskite PeLED and showed that the external quantum efficiency of near-UV PeLEDs reached 0.038% by controlling the size of the (PEA)2PbBr4 2D-perovskite.
Most MAPbX3 PeLEDs are prepared by a solution process and demonstrate high emission luminance. A vapor process may be desirable in perovskite LED applications for many reasons, such as the ease of patterning, improved uniformity, and material compatibility. However, published works on the chemical vapor deposition (CVD) of perovskites for LED applications are limited,28−31 and the performance of PeLEDs prepared by CVD remains inefficient with the reported maximum luminance close to 10,000 cd/m2.28−31 In this study, MAPbBr3 perovskite was converted from a spin-coated lead bromide (PbBr2) layer doped with phenethylamine (PEA) via synthesis with precursor vapors made of methylammonium bromide (MABr) and choline chloride (CC) at a low reaction temperature of 60 °C. Zheng et al.32 reported that quaternary ammonium halide anions and cations could effectively passivate perovskite defects. Thus, we introduced CC as a precursor to passivate crystal defects during perovskite synthesis. We found that doping of the spin-coated PbBr2 layer with PEA can modify the crystal formation of perovskite during reaction with MABr and CC precursor vapors. The presence of both PEA and CC in the CVD process could greatly enhance the optoelectronic characteristics of perovskite and the performance of the resulting PeLEDs. The optoelectrical properties of perovskite converted from the PEA-doped PbBr2 layer with MABr and CC vapors were evaluated, and the performance of the resulting perovskite PeLEDs was examined in the following sections.
Results and Discussion
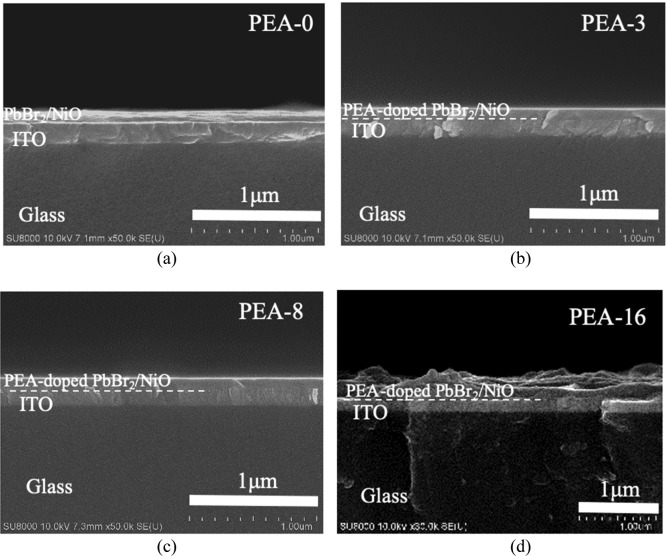
Top view scanning electron microscopy (SEM) images in Figure 1 show the surface morphology of the spin-coated PbBr2 layers with different PEA molar ratios of 0 (PEA-0), 0.003% (PEA-3), 0.008% (PEA-8), and 0.016% (PEA-16) in PbBr2 solution. The surface morphology of the spin-coated PbBr2 layer without PEA doping (PEA-0) in Figure 1a reveals polycrystal-like domains with sizes ranging from 0.9 to 0.6 μm. Unlike the case without PEA doping, the spin-coated PbBr2 with PEA doping (samples PEA-3 and PEA-8) reveals a very smooth surface as shown in Figure 1b,c when the PEA doping level does not exceed 0.008% molar ratio in PbBr2 solution. When the PEA molar ratio in PbBr2 solution further increased to 0.016% (sample PEA-16), many regions on the surface of sample PEA-16 shown in Figure 1d developed a nanosized wire structure, and the rest of the area remained flat. The enlarged SEM image of the nanowire region in the inset of Figure 1d shows that the diameter of the nanowire structure was less than 200 nm, while the flat area shows a smooth surface, as seen in the enlarged SEM image of the flat region in the inset of Figure 1d. The thicknesses of PEA-0, PEA-3, PEA-8, and PEA-16 were determined from their cross-section SEM images in Figure 2. The thickness of PEA-0 was about 65 nm. PEA-3 and PEA-8 presented almost the same thickness of approximately 80 nm, which was larger than that of PEA-0. However, the thicknesses of the nanowire structure region and the smooth region of PEA-16 reached approximately 200 and 130 nm, respectively, which are the largest thickness of the spin-coated PEA-doped PbBr2 layer in all samples. From the results of layer thickness of spin-coated PEA-doped PbBr2, the amount of PEA doping caused the increase of the layer thickness. However, the PEA molar ratio of 0.016% led to the creation of a nanowire structure area on the surface of PEA-16. It caused dramatic changes in the layer thickness.
Figure 1.
SEM images of the top view of the spin-coated PbBr2 layer (a) without PEA in PbBr2 solution (PEA-0), and with PEA molar ratios of (b) 0.003% (PEA-3), (c) 0.008% (PEA-8), and (d) 0.016% (PEA-16) in PbBr2 solution. Insets of (d) are enlarged SEM images of the nanowires and the flat region of PEA-16.
Figure 2.
SEM images of the cross-section view of the spin-coated PbBr2 layer (a) without PEA in PbBr2 solution (PEA-0), and with PEA molar ratios of (b) 0.003% (PEA-3), (c) 0.008% (PEA-8), and (d) 0.016% (PEA-16) in PbBr2 solution.
The optical absorbances and crystallinities of PEA-0, PEA-3, PEA-8, and PEA-16 were determined by measurement of optical absorption and X-ray diffraction (XRD), respectively, which are shown in Figure 3. The absorbance spectrum of PEA-0 shown in Figure 3a increased rapidly at wavelengths less than 360 nm, which corresponds to the absorption of PbBr2. However, the XRD spectrum of PEA-0 in Figure 3b does not show any PbBr2-related peaks except the indium tin oxide (ITO) peaks. The results of absorbance and XRD spectra of PEA-0 suggest that the spin-coated PbBr2 without PEA doping may be an amorphous PbBr2 layer. Looking back at the polycrystal-like domains on the surface of PEA-0 in Figure 1a may indicate an agglomeration of amorphous PbBr2.
Figure 3.

(a) Absorbance and (b) XRD spectra of spin-coated PbBr2 layers with and without PEA doping. Inset of (a) is the enlarged absorbance spectra around the wavelength of 400 nm.
The absorbance spectra of PEA-3 and PEA-8 in Figure 3a are nearly identical to that of PEA-0, which only showed a rapid increase in PbBr2 absorbance around the wavelength of 360 nm. The XRD spectra of PEA-3 and PEA-8 in Figure 3b do not show PbBr2- or (PEA)2PbBr4-related peaks, which were similar to the XRD spectrum of PEA-0. This indicates that the spin-coated PEA-doped PbBr2 retains its amorphous property when the molar ratio of PEA in PbBr2 solution does not exceed 0.008%. The absorbance and XRD spectra of spin-coated PEA-doped PbBr2 do not show the presence of (PEA)2PbBr4 when the molar ratio of PEA in PbBr2 solution reaches as high as 0.008%. Besides, when we compared the surface morphology of PEA-0, PEA-3, and PEA-8 in Figure 1, we found that the PEA molar ratio less than 0.008% would turn the agglomerated surface of amorphous PbBr2 to smooth amorphous PbBr2. This indicates that a small amount of PEA-related ligand in PbBr2 solution may suppress the agglomeration of amorphous PbBr2, smoothen the surface of the spin-coated PEA-doped PbBr2 layer, and increase the thickness of the spin-coated PEA-doped PbBr2. The smooth morphology of these spin-coated PEA-doped PbBr2 might be beneficial for the next-step synthesis of perovskite with MABr and CC vapors.
When the PEA molar ratio in PbBr2 solution further increased to 0.016%, the absorbance and XRD spectra of spin-coated PEA-doped PbBr2 (PEA-16) in the inset of Figure 3a,b reveal a (PEA)2PbBr4-related absorption peak at a wavelength of 400 nm and a diffraction peak at 2θ of 5.2°, respectively. Besides the (PEA)2PbBr4-related absorption, the sample PEA-16 still revealed PbBr2-related absorption around the wavelength of 360 nm. However, the PbBr2-related diffraction peaks were not observed in the XRD spectrum of the sample PEA-16. Therefore, we consider this PEA-doped PbBr2 layer with 0.016% molar ratio consists of amorphous PbBr2 and (PEA)2PbBr4. The (PEA)2PbBr4-related absorbance and diffraction peaks of PEA-16 in the absorption and XRD spectra, respectively, might be attributed to the nanowire structure areas on the surface of PEA-16, as shown in Figure 1d. The main absorption peak of PbBr2 in the absorbance spectrum of PEA-16 should be attributed to the flat areas, which should correspond to amorphous PbBr2, on the surface of PEA-16 in Figure 1d. Although the high PEA doping created (PEA)2PbBr4 in PEA-doped PbBr2, Chiang et al.31 reported that (PEA)2PbBr4 could also be converted to MAPbBr3 perovskite by reacting with MABr. However, the rough surface of PEA-16 obtained from such a high PEA doping level may degrade the material quality or surface coverage of the synthesized perovskite.
Next, the spin-coated PbBr2 thin films fabricated with different PEA doping levels were reacted with precursor vapors of MABr and CC to convert them into perovskite. The vapors of MABr at 60 °C would first react with the surface of spin-coated PEA-doped PbBr2 and form a perovskite/PEA-doped PbBr2 interface and perovskite on the top. Once the perovskite fully covered the surface of PEA-doped PbBr2, the MABr vapor might diffuse through the top perovskite layer to react with unreacted spin-coated PEA-doped PbBr2. Besides, the MA ions, Pb ions, and Br ions in the top perovskite and the unreacted PEA-doped PbBr2 would mutually diffuse through the perovskite/PEA-doped PbBr2 interface to assist the formation of perovskite. Therefore, the perovskite/PEA-doped PbBr2 interface could continually move into the PEA-doped PbBr2 layer and finally turn the entire PEA-doped PbBr2 layer into a perovskite layer. Zheng et al.32 reported that quaternary ammonium halide anions and cations could effectively passivate perovskite defects. Thus, the CC as a precursor could provide quaternary ammonium halide anions and cations in the vapor–solid reaction process of perovskite to passivate the defects of perovskite and improve the luminance of PeLEDs. In our experiment, we found that CC indeed cooperated in the vapor–solid reaction process of perovskite at a reaction temperature of 60 °C. The participation of CC would ramp up the formation velocity of perovskite, and the formation velocity of perovskite was proportional to the amount of CC. Besides, the incorporation of CC in perovskite would lead to the blue shift of the absorption wavelength of perovskite. Comprehensive studies on the effects of the precursor CC for perovskite formation are still underway. The details of study results of perovskite formation with the CC precursor will be discussed elsewhere. Here, we study only the effects of PEA doping in the PbBr2 precursor layer upon the formation of the perovskite and the performance of PeLEDs. Therefore, we fix the weights of precursor CC and MAI at 206.3 and 550 mg for the formation of perovskite in this study.
Figures 4 and 5 show the XRD spectra and their top views, respectively, of MAPbBr3 perovskites converted from PEA-doped PbBr2 with PEA molar ratios of 0, 0.003, 0.008, and 0.016%, which were named PEA-0 PVSK, PEA-3 PVSK, PEA-8 PVSK, and PEA-16 PVSK, respectively. First, the XRD spectra of PEA-0 PVSK, PEA-3 PVSK, and PEA-8 in Figure 4a were almost identical. All peaks of PEA-0 PVSK, PEA-3 PVSK, and PEA-8 PVSK in the XRD spectra of Figure 4a should be attributed to MAPbBr3 perovskite and ITO. However, the XRD spectrum of PEA-16 PVSK in Figure 4a shows not only the peaks of MAPbBr3 perovskite and ITO but also a clear (PEA)2PbBr4-related peak at 2θ = 5.2°. The (PEA)2PbBr4 peak of PEA-16 PVSK should be attributed to the remains of unreacted (PEA)2PbBr4 found in the precursor layer of PEA-16. This XRD spectrum indicates that PEA-16 PVSK has larger amounts of (PEA)2PbBr4 in its perovskite layer than PEA-3 PVSK and PEA-8 PVSK. To further make sure of the existence of (PEA)2PbBr4 in perovskite, we performed grazing-incidence wide-angle X-ray scattering (GIWAXS) measurements on samples of PEA-0 PVSK, PEA-8 PVSK, and PEA-16 PVSK, and their GIWAXS spectra are shown in Figure 4b. The peaks of the GIWAXS spectrum of PEA-0 PVSK should be all related to MAPbBr3 and ITO, and could serve as a reference for the GIWAXS spectrum of MAPbBr3 without any doping. We first compared the GIWAXS spectra of PEA-0 PVSK and PEA-16 PVSK. PEA-0 PVSK and PEA-16 PVSK both presented a high background signal in low diffraction angles. Therefore, it is hard to recognize the (PEA)2PbBr4 peak near the diffraction angle of 5° in the GIWAXS spectrum of PEA-16 PVSK, although the XRD spectrum of PEA-16 PVSK shows clear (PEA)2PbBr4 at 5.2°. We found the extra peaks in the GIWAXS spectra of PEA-16 PVSK other than the peaks of MAPbBr3 and ITO by comparing the GIWAXS spectra of PEA-0 PVSK and PEA-16 PVSK beside the low diffraction angle. Those extra peaks were probably attributed to (PEA)2PbBr4. By comparing the GIWAXS spectra of PEA-8 PVSK and PEA-16 PVSK, we could find peaks that corresponded to (PEA)2PbBr4 in the GIWAXS spectrum of PEA-8 PVSK. Therefore, one can say that (PEA)2PbBr4 was in PEA-8 PVSK. PEA might randomly distribute in the precursor layer of PEA-8 because there is no (PEA)2PbBr4-related peaks in the PEA-8 XRD spectrum. However, the perovskite and (PEA)2PbBr4 crystals simultaneously formed when MABr and CC vapors reacted with the amorphous PEA-8 precursor layer. The simultaneous formation of the perovskite and (PEA)2PbBr4 crystals might benefit the morphology and the quality of the perovskite layer.
Figure 4.
(a) XRD and (b) GIWAXS spectra of perovskite converted from spin-coated PbBr2 with different PEA molar ratios in PbBr2 solution.
Figure 5.
SEM images of the top view of perovskites converted from a spin-coated PbBr2 layer (a) without PEA in PbBr2 solution (PEA-0 PVSK) and with PEA molar ratios of (b) 0.003% (PEA-3 PVSK), (c) 0.008% (PEA-8 PVSK), and (d) 0.016% (PEA-16 PVSK) in PbBr2 solution. Insets of (d) are enlarged SEM images of the nanowires and the flat region of PEA-16 PVSK.
It is also important to know the surface morphologies of perovskite converted from different PEA-doped PbBr2, which were studied by SEM. The surface of PEA-0 PVSK with a thickness of 140 nm in Figure 5a shows the typical polycrystalline morphology of perovskite with an average grain size of 0.18 μm. The top view image of PEA-0 PVSK shows lots of high-angle grain boundaries between grains because the misorientations between grains were large. The surface of PEA-3 PVSK in Figure 5b shows the polycrystalline morphology of perovskite with an average grain size of 0.12 μm, which is smaller than that of the PEA-0 PVSK case. However, the misorientations between the shrunk grains remained large because PEA-3 PVSK contained many high-angle grain boundaries, which were higher than that of PEA-0 PVSK. The thickness of PEA-3 PVSK is approximately 130 nm. Zhang et al.33 reported that the presence of PEA in the perovskite precursor solution suppresses the crystal growth of perovskite and reduces the grain size of the resulting crystals. Cheng et al.34 also reported how the addition of PEABr to the CsPbBr3 precursor solution affects the growth mechanism of spin-coated CsPbBr3. Cs+ is partially replaced by PEA+ at the grain boundaries, which limits the grain growth of perovskite. We observed the same mechanisms in the present work from comparing the top view SEM images of PEA-0 PVSK and PEA-3 PVSK. Although a low amount of PEA was doped in the solid-phase PbBr2 layer, PEA+ may also replace some of the MA+ in the grain boundaries during the reaction with MABr and CC vapor, which reduces the grain size of the MAPbBr3 perovskite.
An increase of the PEA molar ratio to 0.008% in PbBr2 solution did not further reduce the grain size of perovskite converted from the spin-coated PEA-doped PbBr2 precursor layer. The morphology of PEA-8 PVSK totally differed from those of PEA-0 PVSK and PEA-3 PVSK. However, the thickness of PEA-8 PVSK was similar to those of PEA-0 PVSK and PEA-3 PVSK (130 nm). The morphology of PEA-8 PVSK in Figure 5c presents a highly condensed layer and continuous, orientation-aligned crystals with a step-flow morphology and a large grain size. PEA-8 PVSK shows much less high-angle grain boundaries than PEA-0 PVSK and PEA-3 PVSK. Increasing PEA doping in spin-coated PbBr2 not only smoothened the surface of spin-coated PbBr2 but also effectively suppressed the misorientation of polycrystals after conversion to perovskite. In previous studies, the PEA-related ligands suppressed the grain growth of perovskite;19−27,33,34 it could not improve the misorientation between the grains of the perovskite. However, in our observation here, the proper amounts of PEA doping in spin-coated PbBr2 may act as a surfactant during the reaction with MABr and CC vapors, thereby enhancing the migration of PbBr2 and MABr during the formation of perovskite at a low reaction temperature of 60 °C to improve the coalescence and alignment of orientation of crystals. Furthermore, the results of XRD and GIWAXS studies confirmed that perovskite and (PEA)2PbBr4 crystals are simultaneously formed when MABr and CC vapors reacted with the amorphous spin-coated PEA-doped PbBr2. This (PEA)2PbBr4 might be able to assist the crystal coalescence and alignment of perovskite growth. The improved surface morphology of PEA-8 PVSK may enhance the optoelectrical properties of the perovskite.
When the molar ratio of PEA in PbBr2 solution is as high as 0.016%, (PEA)2PbBr4-related nanowires appear on the surface of the spin-coated PEA-doped PbBr2 precursor layer. Figure 5d shows the surface SEM image of perovskite (PEA-16 PVSK) converted from spin-coated PEA-doped PbBr2 with (PEA)2PbBr4-related nanowires on its surface; insets of Figure 5d present the enlarged images of the nanowire and flat areas of PEA-16 PVSK. Nanosized perovskite grew on the (PEA)2PbBr4-related nanowires, as shown in the inset of the enlarged image of the nanowire area in Figure 5d. The nanosized perovskite in the nanowire region in Figure 1d might be directly converted from (PEA)2PbBr4. The flat areas of spin-coated PEA-doped PbBr2 in Figure 1d were converted into several perovskite branches in the inset of the enlarged image of the flat area in Figure 5d, which was caused by the high content of PEA, and these branches reduced the surface coverage of the perovskite film. The thicknesses of perovskite in the nanowire region and the flat surface were 600 and 180 nm, respectively. Therefore, the surface of PEA-16 PVSK presents the roughest surface morphology. Combining the results of XRD and SEM observations of PEA-16 PVSK, we concluded that the (PEA)2PbBr4-related peak in the XRD spectrum of PEA-16 PVSK could be attributed to the residual (PEA)2PbBr4-related nanowires in the nanowire area. However, the rough surface and the reduced surface coverage of PEA-16 PVSK might decline the performance of PeLEDs.
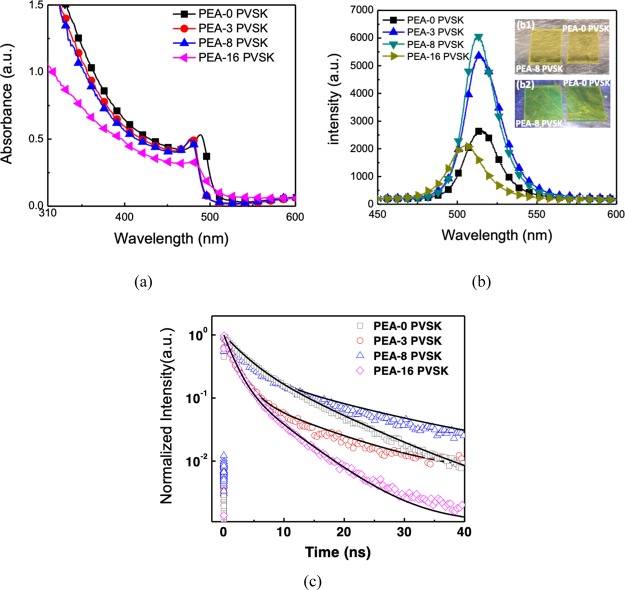
The absorption, photoluminescence (PL), and time-resolved PL (TRPL) measurements were performed on perovskite converted from different PEA doping levels of PbBr2 to understand their optoelectrical properties. Figure 6 shows the absorbance spectra, PL spectra, and TRPL decay curves of all perovskite samples. The absorbance spectrum of PEA-0 PVSK in Figure 6a shows a steep increase of absorbance around the wavelength of 510 nm. PEA-3 PVSK and PEA-8 PVSK presented almost the same absorbance spectrum in Figure 6a with the absorption edge around 490 nm, which was less than the absorption edge of PEA-0 PVSK. The blue shift of the absorption edge of perovskite converted from PEA-doped PbBr2 might be because of the crystal size reduction of perovskite. However, the absorption onset of PEA-16 PVSK was around 510 nm, and the perovskite slowly increased its absorbance around 490 nm. The low slope of absorption edge of PEA-16 PVSK could be attributed to the nanosized perovskite on (PEA)2PbBr4-related nanowires and nanosized perovskite branches, as shown in Figure 5d. Besides, the absorption curve of PEA-16 PVSK showed a very small absorbance variation at a wavelength near 400 nm, which could be considered as the absorption of (PEA)2PbBr4. The PL spectra of all perovskite samples are shown in Figure 6b. The PL wavelength of perovskite without PEA doping is 514.2 nm. The PL wavelength slightly shifted from 514 to 512 nm as the PEA molar ratio increased to 0.008%. This shift could be attributed to the formation of (PEA)2PbBr4 in perovskite and modification of the grain size of perovskite with a small PEA molar ratio. Among the samples, the PEA-16 PVSK case showed the shortest PL wavelength of 506 nm because the high PEA content causes the formation of nanosized perovskite on the (PEA)2PbBr4-related nanowires and nanosized perovskite branches, as shown in Figure 5d. In addition to the shift of the PL emission peak wavelength of perovskite, the perovskites showed maximum PL emission intensity when the PEA molar ratio was 0.008%, but this intensity sharply declined to less than that of PEA-0 PVSK when the PEA molar ratio exceeded 0.016%. The increase in the PL intensity of PEA-8 PVSK case implies improvements in the quality of the perovskite. Insets of Figure 6b show the photographs of PEA-0 PVSK and PEA-8 PVSK at ambient light and under UV flashlight exposure at a peak wavelength of 365 nm. The PEA-8 PVSK shows a stronger green emission than PEA-0 PVSK under irradiation of UV flashlight. We performed the TRPL measurement on all perovskite samples converted from PbBr2 with and without PEA doping to further understand their carrier recombination properties and fitted TRPL decay curves of all samples to extract the decay time of perovskite. The TRPL decay curves and their fitting curves of all perovskite samples are shown in Figure 6c. The PEA-0 PVSK case revealed a short TRPL decay time (τ1) and a long TRPL decay time (τ2) of 3.12 and 9.78 ns, respectively. Previous studies reported that the short TRPL decay time τ1 is caused by the bimolecular recombination of photogenerated free carriers, and the long TRPL decay time τ2 is mainly contributed by trap-assisted recombination.35,36 The τ2 of perovskite increased from 9.78 to 16.17 ns as the PEA molar ratio increased from 0 to 0.008%. Two phenomena might cause PEA-8 PVSK to have the best PL emission intensity and the longest decay time of TRPL. First, PEA-8 PVSK might have improved material quality because PEA-8 PVSK shows much less high-angle grain boundaries than those of PEA-0 PVSK and PEA-3 PVSK. Second, both PEA and CC would passivate the defects of perovskite.19−27,32 These two phenomena may be associated with the reduction of defect-assisted recombination in perovskite, thereby enhancing the PL emission intensity and prolonging the τ2 of TRPL of perovskite. However, when the PEA molar ratio reached 0.016%, the surface morphology of perovskite deteriorated, and perovskite branches formed. This phenomenon resulted in the degradation of the film quality of the perovskite and reduced its τ1 and τ2 to 1.54 and 6.05 ns, respectively. Therefore, the strongest PL intensity and the longest decay time of TRPL of PEA-8 PVSK might indicate better light-emitting properties than the rest of the perovskite samples.
Figure 6.
(a) Absorbance spectra, (b) PL spectra, and (c) TRPL decay curves of all perovskites converted from spin-coated PbBr2 with different PEA molar ratios in PbBr2 solution. Insets (b1,b2) in (b) are photographs of PEA-0 PVSK and PEA-8 PVSK without and with UV flashlight exposure at a peak wavelength of 365 nm, respectively.
Proper PEA doping in spin-coated PbBr2 helps improve the crystal quality and optical properties of perovskite after its reaction with MABr and CC precursor vapors. Therefore, PeLEDs with PEA-0 PVSK, PEA-3 PVSK, PEA-8 PVSK, and PEA-16 PVSK were produced to understand how changes in the PEA molar ratio affect the optoelectrical properties of PeLEDs. PeLEDs with PEA-0 PVSK, PEA-3 PVSK, PEA-8 PVSK, and PEA-16 PVSK were denoted PEA-0 PeLED, PEA-3 PeLED, PEA-8 PeLED, and PEA-16 PeLED, respectively. The scheme of the PeLED structure is shown in the inset of Figure 7b. Figure 7 presents the current density–brightness–voltage (J–L–V) curves and the current efficiency–current density curves of the PeLED samples. PEA-0 PeLED, PEA-3 PeLED, and PEA-8 PeLED showed similar typical J–V characteristics. At a forward bias below 2.5 V, the current density of the PeLEDs decreased with increasing PEA molar ratios. Increases in the PEA molar ratio may result in highly dense perovskite films and improve the quality of perovskite to effectively suppress the forward leakage current density of PeLEDs under a small forward bias. However, when the PEA molar ratio was as high as 0.016%, the surface morphology of perovskite deteriorated, and perovskite branches were formed; thus, J–V characteristic of PEA-16 PeLED in Figure 7a revealed a very high forward leakage current density under a forward bias less than 2.5 V. PEA-8 PeLED has the optimized J–V characteristic. The electroluminescence (EL) spectra of all PeLEDs showed nearly the same peak wavelength of 516 nm. The PL peak wavelength of perovskite showed a blue shift with increasing PEA molar ratio, but the EL peak wavelength of PeLEDs remained at approximately 516 nm despite the increase in the PEA molar ratio. The reason behind this phenomenon remains unknown. The possible reason for this phenomenon might be that the injected carrier flowed through a low energy level MAPbBr3 region such as the branched MAPbBr3 region of the PEA-16 PeLED and recombined in that region to emit a wavelength of 516 nm.
Figure 7.

(a) J–L–V and (b) current efficiency–current density curves of all PeLED samples. The scheme of the PeLED structure is shown in the inset of (b).
From observation of the EL properties of all PeLEDs, it was found that the PEA-3 PeLED showed lower luminance and current efficiency than the PEA-0 PeLED at all applied biases, as shown in Figure 7. A PEA molar ratio of 0.003% in PbBr2 reduced the grain size of perovskite, but the misorientation between grains is still large and then induced lots of high-angle boundaries. In this case, the small crystal size of the perovskite created a large number of grain boundaries in the perovskite, but the total amounts of PEA in spin-coated PbBr2 and CC in precursor vapors may be insufficient to passivate defects in perovskite grains and grain boundaries, which would also have resulted in a shorter TRPL decay time than that of perovskite converted from PbBr2 without PEA doping. Therefore, the PEA-3 PeLED showed a maximum luminance and current efficiency of only 3840 cd/m2 and 0.86 cd/A, respectively, at an applied voltage of 8 V; these values, however, are less than those of PEA-0 PeLED (4070 cd/m2 and 1.26 cd/A, respectively), at the same bias. When the PEA molar ratio was as high as 0.016%, the surface morphology of perovskite deteriorated, and perovskite branches formed. This phenomenon deteriorates the intensity of PL and the decay time of TRPL decay of PEA-16 PVSK. Besides, the reduced surface coverage of perovskite in the perovskite branch region of PEA-16 PVSK caused a severe leakage current in the low bias region. It implies that most of the injected carrier flowed through those partially covered perovskite regions of PEA-16 PVSK. Consequently, among the samples, the PEA-16 PeLED showed the lowest luminance and current efficiency at all applied biases. In the best scenario, the perovskite was transformed into a low misorientation of polycrystals with large grains in Figure 5c when the PEA molar ratio was at an optimized value of 0.008% in our study. The large grains and the low misorientation of polycrystals of perovskite may effectively reduce the presence of defects. Moreover, the optimized PEA in the spin-coated PbBr2 film and CC in precursor vapors may be sufficient to passivate defects in the grains and grain boundaries of perovskite. The strongest PL intensity and the longest TRPL decay time presented remarkable enhancements in the optical properties of the PEA-8 PVSK case. Therefore, the PEA-8 PeLED demonstrates great improvements in luminance and current efficiency at all applied biases compared with the PEA-0 PeLED. The maximum luminance and current efficiency of the PEA-8 PeLED were 20,869 cd/m2 and 3.99 cd/A, respectively; which are approximately five and three times larger than those of the PEA-0 PeLED. Table 1 lists several results of reported PeLEDs prepared by the solution process and the CVD process to compare with our PeLED results. The luminance and current efficiency of the PEA-8 PeLED are still less than the PeLEDs prepared by the solution process. However, the performances of the PEA-8 PeLED were better than those of CVD-prepared PeLEDs.
Table 1. Several Collections of the Reported Performances of PeLEDs fabricated by the Solution Process and the CVD Process.
| material | synthesized method | EL wavelength (nm) | current efficiency (cd/A) | Maximum luminance (cd/m2) | ref. no. |
|---|---|---|---|---|---|
| MAPbBr3 | Solution | 540 | 15.9 | 70,000 | (18) |
| MAPbBr3 | Solution | 535 | 55.5 | 55,400 | (37) |
| MAPbBr3 | Solution | 530 | 16.4 | 17,600 | (38) |
| MAPbBr3 | Solution | 520 | 15.1 | 30,100 | (39) |
| MAPbBr3 | Solution | 540 | 28.9 | 22,800 | (40) |
| MAPbBr3 | CVD | 530 | 0.08 | 560 | (29) |
| MAPbBr3 | CVD | 532 | 8.16 | 6530 | (30) |
| MAPbBr3 | CVD | 531 | 1.3 | 6200 | (31) |
| MAPbBr3 | CVD | 516 | 3.99 | 20,896 | this work |
Conclusions
In summary, we prepared spin-coated PbBr2 with different levels of PEA doping by varying the PEA molar ratio in PbBr2 solution to modify the growth of perovskite and then reacting the resultant products with MABr and CC precursor vapors. The spin-coated PbBr2 became very smooth when PEA molar ratios were not larger than 0.008% in PbBr2 solution. However, the surface of the spin-coated PbBr2 revealed (PEA)2PbBr4-related nanowires when the PEA molar ratio was as high as 0.016%. Therefore, the surface morphology of perovskite deteriorated, and perovskite branches formed when the perovskite was converted from this PEA-doped spin-coated PbBr2 with (PEA)2PbBr4-related nanowires. Consequently, among the samples prepared, the PEA-16 PeLED revealed the lowest luminance and current efficiency at all applied biases. In contrast, the smooth PbBr2 with a PEA molar ratio of 0.008% could be converted into large-grained and with low misorientation of polycrystals of perovskite via reaction with precursor vapors made of MABr and CC. This phenomenon could effectively improve the crystal quality of the perovskite. The amounts of PEA in the spin-coated PbBr2 film at a PEA molar ratio of 0.008% in PbBr2 solution and CC in the precursor vapor together may be sufficient to passivate defects in the grains and grain boundaries of perovskite. Therefore, the optical properties of perovskite could be improved by introducing a PEA molar ratio of 0.008% to the PbBr2 solution. The PEA-8 PeLED showed great improvements in the luminance and current efficiency at all applied biases compared with the PEA-0 PeLED. The maximum luminance and current efficiency of PEA-8 PeLED were 20,869 cd/m2 and 3.99 cd/A, respectively; which are approximately five and three times larger than those of PEA-0 PeLED. By using a PEA dopant in the PbBr2 layer, we successfully pushed the maximum luminance of perovskite-based LEDs to over 20,000 cd/m2 at a low reaction temperature of 60 °C.
Experiments
An ITO/glass with a thickness of 140 nm ITO was prepared as the PeLED substrate. We dripped NiO solution (prepared using nickel (II) formate dihydrate, ethanolamine, ethylenediamine, and ethylene glycol) on the ITO/glass and then spun it at 4500 rpm for 90 s. The spin-coated NiO was then post-annealed at 400 °C for 10 min to form a 10 nm-thick NiO hole transfer layer on the ITO/glass. Prior to deposition of the PEA-doped PbBr2 layer, we prepared a PbBr2 and PEA mixed solution by dissolving PbBr2 and PEA in dimethyl sulfoxide (DMSO; 99.9%; Sigma-Aldrich) for spin-coating PEA-doped PbBr2 on the NiO layer. We added 183.5 mg of PbBr2 with a purity of 99.999%, which was purchased from Sigma-Aldrich, to 500 μL DMSO. Liquid PEA (99%; Sigma-Aldrich) with volumes of 2, 5, and 10 μL was then mixed with the PbBr2 solution to form PEA-doped PbBr2 solutions with PEA/PbBr2 molar ratios of 0.003, 0.008, and 0.016%, respectively. PbBr2 layers with different amounts of PEA doping were prepared from those PEA-doped PbBr2 solutions with PEA molar ratios of 0, 0.003, 0.008, and 0.016%. We dripped PEA-doped PbBr2 solutions with different PEA molar ratios on the NiO/ITO/glass. We initially rotated the NiO/ITO/glass covered with PEA-doped PbBr2 solution at 500 rpm for 7 s and then ramped up the rotation speed to 7000 rpm for 70 s. The PEA-doped spin-coated PbBr2/NiO/ITO/glass was then baked at 60 °C for 10 min to form a PEA-doped PbBr2 layer with thickness ranging from 65 to 200 nm.
After deposition of PbBr2 with different PEA doping concentrations, samples of PEA-doped spin-coated PbBr2/NiO/ITO/glass were transferred to a quartz furnace to synthesize the perovskite film by using mixed precursor vapors of MABr (Greatcell Solar Materials) and CC (>99%; Sigma-Aldrich) via the vapor–solid reaction of the spin-coated PbBr2 layer with different PEA doping amounts. The MABr (550 mg) and CC (206.3 mg) powders were placed near the samples on a quartz boat to achieve a temperature identical to the synthesis temperature. MAPbBr3 perovskite was synthesized at a furnace temperature of 60 °C and a working pressure of 1 Torr for 3 h. A MAPbBr3 layer with a thickness of approximately 130 nm was formed on glass/ITO/NiO. The perovskites converted from PEA-doped PbBr2 with PEA molar ratios of 0, 0.003, 0.008, and 0.016% were denoted PEA-0 PVSK, PEA-3 PVSK, PEA-8 PVSK, and PEA-16 PVSK, respectively. 2,2′,2″-(1,3,5-Benzinetriyl)-tris(1-phenyl-1-H-benzimidazole) (70 nm; >99%; Solenne, the Netherlands), lithium fluoride (1 nm), and Al (80 nm) were thermally deposited on the CH3NH3PbBr3 layer inside a vacuum chamber (10–6 Torr) to complete the PeLED structure. All fabricated PeLEDs had an active area of 0.06 cm2. PeLEDs with perovskite layers converted from spin-coated PbBr2 with PEA molar ratios of 0, 0.003, 0.008, and 0.016% were designated PEA-0 PeLED, PEA-3 PeLED, PEA-8 PeLED, and PEA-16 PeLED, respectively.
The material and optoelectrical properties of the spin-coated PbBr2 layers with different PEA doping amounts and resulting perovskites, such as their morphology, material quality, and PL, were characterized by SEM (HITACHI SU8000), XRD, GIWAXS [D8 DISCOVER with the General Area Detector Diffraction System (GADDS), Bruker AXS GmbH], absorption, and PL measurements. Current density–brightness–voltage (J–L–V) measurements were carried out using a Keithley 2400 source measurement unit and a Keithley 2000 digital multimeter. The intensity of EL of PeLEDs was recorded using a silicon photodiode (Hamamatsu S2387, Japan), calibrated with a PR655 spectrophotometer (Photo Research, USA). Measurements of the J–L–V curves were carried out inside a nitrogen-filled glove box with oxygen and moisture levels <1 ppm.
Acknowledgments
We extend our gratitude to the Ministry of Science and Technology (MOST), Taiwan for their financial support under grant nos. MOST 107-2221-E-006-186-MY3, MOST 104-2221-E-006-069-MY3, NSC 101-2221-E-006-066-MY3, and NSC 102-3113-P-009-007-CC2. The Advanced Optoelectronic Technology Center, the National Cheng Kung University (as a project of the Ministry of Education of Taiwan), the Bureau of Energy, and Ministry of Economic Affairs of Taiwan also supported this research project under grant no. 102-E0603. P.C. thanks the research grant from the Ministry of Science and Technology of Taiwan (MOST 107-2221-E-006-190-MY3, MOST 107-2119-M-006-002, MOST 108-3116-F-006-001, and 108-2218-E-006-043-MY3). This work was financially supported by the Hierarchical Green-Energy Materials (Hi-GEM) Research Center, from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. This research was, in part, supported by the Ministry of Education, Taiwan, ROC., Headquarter of University Advancement to the National Cheng Kung University. We also thank Prof. W.-Y.C. from the Department of Photonics in the National Cheng Kung University for supporting the AFM measurements. Finally, we would like to thank our colleague Prof. Shih-Hui Chang for assisting the manuscript editing.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Financial support for this research was provided by the Ministry of Science and Technology (MOST), Taiwan under grant nos. MOST 107-2221-E-006-186-MY3, MOST 104-2221-E-006-069-MY3, NSC 101-2221-E-006-066-MY3, NSC102-3113-P-009-007-CC2, MOST 107-2221-E-006-190-MY3, MOST 107-2119-M-006-002, MOST 108-3116-F-006-001, and 108-2218-E-006-043-MY3. This work was also financially supported by the Hierarchical Green-Energy Materials (Hi-GEM) Research Center from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
The authors declare no competing financial interest.
References
- Kojima A.; Teshima K.; Shirai Y.; Miyasaka T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- Lee M. M.; Teuscher J.; Miyasaka T.; Murakami T. N.; Snaith H. J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 33 8, 643–647. 10.1126/science.1228604. [DOI] [PubMed] [Google Scholar]
- Burschka J.; Pellet N.; Moon S.-J.; Humphry-Baker R.; Gao P.; Nazeeruddin M. K.; Grätzel M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. 10.1038/nature12340. [DOI] [PubMed] [Google Scholar]
- Zhou H.; Chen Q.; Li G.; Luo S.; Song T.-b.; Duan H.-S.; Hong Z.; You J.; Liu Y.; Yang Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. 10.1126/science.1254050. [DOI] [PubMed] [Google Scholar]
- Yang W. S.; Noh J. H.; Jeon N. J.; Kim Y. C.; Ryu S.; Seo J.; Seok S. I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. 10.1126/science.aaa9272. [DOI] [PubMed] [Google Scholar]
- Luo J.; Im J. H.; Mayer M. T.; Schreier M.; Nazeeruddin M. K.; Park N. G.; Tilley S. D.; Fan H. J.; Grätzel M. Water photolysis at 12.3% efficiency via perovskite photovoltaics and earth-abundant catalysts. Science 2014, 345, 1593–1596. 10.1126/science.1258307. [DOI] [PubMed] [Google Scholar]
- Green M. A.; Ho-Baillie A.; Snaith H. J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. 10.1038/nphoton.2014.134. [DOI] [Google Scholar]
- Song J.; Li J.; Li X.; Xu L.; Dong Y.; Zeng H. Quantum dot light-emitting diodes based on inorganic perovskite cesium lead halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. 10.1002/adma.201502567. [DOI] [PubMed] [Google Scholar]
- Deschler F.; Price M.; Pathak S.; Klintberg L. E.; Jarausch D.-D.; Higler R.; Hüttner S.; Leijtens T.; Stranks S. D.; Snaith H. J.; Atatüre M.; Phillips R. T.; Friend R. H. High photoluminescence efficiency and optically pumped lasing in solution-processed mixed halide perovskite semiconductors. J. Phys. Chem. Lett. 2014, 5, 1421–1426. 10.1021/jz5005285. [DOI] [PubMed] [Google Scholar]
- Kim Y.-H.; Cho H.; Heo J. H.; Kim T.-S.; Myoung N.; Lee C.-L.; Im S. H.; Lee T.-W. Multicolored organic/inorganic hybrid perovskite light-emitting diodes. Adv. Mater. 2015, 27, 1248–1254. 10.1002/adma.201403751. [DOI] [PubMed] [Google Scholar]
- Hoye R. L. Z.; Chua M. R.; Musselman K. P.; Li G.; Lai M.-L.; Tan Z.-K.; Greenham N. C.; MacManus-Driscoll J. L.; Friend R. H.; Credgington D. Enhanced performance in fluorene-free organometal halide perovskite light-emitting diodes using tunable, low electron affinity oxide electron injectors. Adv. Mater. 2015, 27, 1414–1419. 10.1002/adma.201405044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Wang N.; Jin Y.; Si J.; Tan Z.-K.; Du H.; Cheng L.; Dai X.; Bai S.; He H.; Ye Z.; Lai M. L.; Friend R. H.; Huang W. Interfacial control toward efficient and low-voltage perovskite light-emitting diodes. Adv. Mater. 2015, 27, 2311–2316. 10.1002/adma.201405217. [DOI] [PubMed] [Google Scholar]
- Cho H.; Jeong S.-H.; Park M.-H.; Kim Y.-H.; Wolf C.; Lee C.-L.; Heo J. H.; Sadhanala A.; Myoung N.; Yoo S.; Im S. H.; Friend R. H.; Lee T.-W. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science 2015, 350, 1222–1225. 10.1126/science.aad1818. [DOI] [PubMed] [Google Scholar]
- Zhu H.; Fu Y.; Meng F.; Wu X.; Gong Z.; Ding Q.; Gustafsson M. V.; Trinh M. T.; Jin S.; Zhu X.-Y. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015, 14, 636–642. 10.1038/nmat4271. [DOI] [PubMed] [Google Scholar]
- Tan Z.-K.; Moghaddam R. S.; Lai M. L.; Docampo P.; Higler R.; Deschler F.; Price M.; Sadhanala A.; Pazos L. M.; Credgington D.; Hanusch F.; Bein T.; Snaith H. J.; Friend R. H. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692. 10.1038/nnano.2014.149. [DOI] [PubMed] [Google Scholar]
- Jaramillo-Quintero O. A.; Sanchez R. S.; Rincon M.; Mora-Sero I. Bright visible-infrared light emitting diodes based on hybrid halide perovskite with spiro-OMeTAD as a hole-injecting layer. J. Phys. Chem. Lett. 2015, 6, 1883–1890. 10.1021/acs.jpclett.5b00732. [DOI] [PubMed] [Google Scholar]
- Yu J. C.; Kim D. B.; Jung E. D.; Lee B. R.; Song M. H. High-performance perovskite light-emitting diodes via morphological control of perovskite films. Nanoscale 2016, 8, 7036–7042. 10.1039/c5nr05604g. [DOI] [PubMed] [Google Scholar]
- Chih Y.-K.; Wang J.-C.; Yang R.-T.; Liu C.-C.; Chang Y.-C.; Fu Y.-S.; Lai W.-C.; Chen P.; Wen T.-C.; Huang Y.-C.; Tsao C.-S.; Guo T.-F. NiOx electrode interlayer and CH3NH2/CH3NH3PbBr3 interface treatment to markedly advance hybrid perovskite-based light-emitting diodes. Adv. Mater. 2016, 28, 8687–8694. 10.1002/adma.201602974. [DOI] [PubMed] [Google Scholar]
- Byun J.; Cho H.; Wolf C.; Jang M.; Sadhanala A.; Friend R. H.; Yang H.; Lee T.-W. Efficient visible quasi-2D perovskite light-emitting diodes. Adv. Mater. 2016, 28, 7515–7520. 10.1002/adma.201601369. [DOI] [PubMed] [Google Scholar]
- Ban M.; Zou Y.; Rivett J. P. H.; Yang Y.; Thomas T. H.; Tan Y.; Song T.; Gao X.; Credgington D.; Deschler F.; Sirringhaus H.; Sun B. Solution-processed perovskite light emitting diodes with efficiency exceeding 15% through additive-controlled nanostructure tailoring. Nat. Commun. 2018, 9, 3892. 10.1038/s41467-018-06425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; Zhang X.; Deng J.; Chu Z.; Jiang Q.; Meng J.; Wang P.; Zhang L.; Yin Z.; You J. Efficient green light-emitting diodes based on quasi-two-dimensional composition and phase engineered perovskite with surface passivation. Nat. Commun. 2018, 9, 570. 10.1038/s41467-018-02978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N.; Cheng L.; Ge R.; Zhang S.; Miao Y.; Zou W.; Yi C.; Sun Y.; Cao Y.; Yang R.; Wei Y.; Guo Q.; Ke Y.; Yu M.; Jin Y.; Liu Y.; Ding Q.; Di D.; Yang L.; Xing G.; Tian H.; Jin C.; Gao F.; Friend R. H.; Wang J.; Huang W. Perovskite light-emitting diodes based on solution-processed self-organized multiple quantum wells. Nat. Photonics 2016, 10, 699–704. 10.1038/nphoton.2016.185. [DOI] [Google Scholar]
- Yuan M.; Quan L. N.; Comin R.; Walters G.; Sabatini R.; Voznyy O.; Hoogland S.; Zhao Y.; Beauregard E. M.; Kanjanaboos P.; Lu Z.; Kim D. H.; Sargent E. H. Perovskite energy funnels for efficient light-emitting diodes. Nat. Nanotechnol. 2016, 11, 872–877. 10.1038/nnano.2016.110. [DOI] [PubMed] [Google Scholar]
- Quan L. N.; Zhao Y.; García de Arquer F. P.; Sabatini R.; Walters G.; Voznyy O.; Comin R.; Li Y.; Fan J. Z.; Tan H.; Pan J.; Yuan M.; Bakr O. M.; Lu Z.; Kim D. H.; Sargent E. H. Tailoring the energy landscape in quasi-2D halide perovskites enables efficient green-light emission. Nano Lett. 2017, 17, 3701–3709. 10.1021/acs.nanolett.7b00976. [DOI] [PubMed] [Google Scholar]
- Ng Y. F.; Kulkarni S. A.; Parida S.; Jamaludin N. F.; Yantara N.; Bruno A.; Soci C.; Mhaisalkar S.; Mathews N. Highly efficient Cs-based perovskite light-emitting diodes enabled by energy funneling. Chem. Commun. 2017, 53, 12004–12007. 10.1039/c7cc06615e. [DOI] [PubMed] [Google Scholar]
- Liang D.; Peng Y.; Fu Y.; Shearer M. J.; Zhang J.; Zhai J.; Zhang Y.; Hamers R. J.; Andrew T. L.; Jin S. Color-pure violet-light-emitting diodes based on layered lead halide perovskite nanoplates. ACS Nano 2016, 10, 6897–6904. 10.1021/acsnano.6b02683. [DOI] [PubMed] [Google Scholar]
- Kumar G. S.; Pradhan B.; Kamilya T.; Acharya S. Enhancing performances of hybrid perovskite light emitting diodes with thickness controlled PMMA interlayer. Bull. Chem. Soc. Jpn. 2018, 91, 1241–1248. 10.1246/bcsj.20180102. [DOI] [Google Scholar]
- Gil-Escrig L.; Miquel-Sempere A.; Sessolo M.; Bolink H. J. Mixed iodide–bromide methylammonium lead perovskite-based diodes for light emission and photovoltaics. J. Phys. Chem. Lett. 2015, 6, 3743–3748. 10.1021/acs.jpclett.5b01716. [DOI] [PubMed] [Google Scholar]
- Leyden M. R.; Meng L.; Jiang Y.; Ono L. K.; Qiu L.; Juarez-Perez E. J.; Qin C.; Adachi C.; Qi Y. Methylammonium lead bromide perovskite light-emitting diodes by chemical vapor deposition. J. Phys. Chem. Lett. 2017, 8, 3193–3198. 10.1021/acs.jpclett.7b01093. [DOI] [PubMed] [Google Scholar]
- Ji H.; Shi Z.; Sun X.; Li Y.; Li S.; Lei L.; Wu D.; Xu T.; Li X.; Du G. Vapor-assisted solution approach for high-quality perovskite CH3NH3PbBr3 thin films for high-performance green light-emitting diode applications. ACS Appl. Mater. Interfaces 2017, 9, 42893–42904. 10.1021/acsami.7b13260. [DOI] [PubMed] [Google Scholar]
- Chiang K.-M.; Hsu B.-W.; Chang Y.-A.; Yang L.; Tsai W.-L.; Lin H.-W. Vacuum-deposited organometallic halide perovskite light-emitting devices. ACS Appl. Mater. Interfaces 2017, 9, 40516–40522. 10.1021/acsami.7b12805. [DOI] [PubMed] [Google Scholar]
- Zheng X.; Chen B.; Dai J.; Fang Y.; Bai Y.; Lin Y.; Wei H.; Zeng X. C.; Huang J. Defect passivation in hybrid perovskite solar cells using quaternary ammonium halide anions and cations. Nat. Energy 2017, 2, 17102. 10.1038/nenergy.2017.102. [DOI] [Google Scholar]
- Zhang M.; Yuan F.; Zhao W.; Jiao B.; Ran C.; Zhang W.; Wu Z. High performance organo-lead halide perovskite light-emitting diodes via surface passivation of phenethylamine. Org. Electron. 2018, 60, 57–63. 10.1016/j.orgel.2018.05.026. [DOI] [Google Scholar]
- Cheng L.-P.; Huang J.-S.; Shen Y.; Li G.-P.; Liu X.-K.; Li W.; Wang Y.-H.; Li Y.-Q.; Jiang Y.; Gao F.; Lee C.-S.; Tang J.-X. Efficient CsPbBr3 perovskite light-emitting diodes enabled by synergetic morphology control. Adv. Opt. Mater. 2019, 7, 1801534. 10.1002/adom.201801534. [DOI] [Google Scholar]
- Son D.-Y.; Lee J.-W.; Choi Y. J.; Jang I.-H.; Lee S.; Yoo P. J.; Shin H.; Ahn N.; Choi M.; Kim D.; Park N.-G. Self-formed grain boundary healing layer for highly efficient CH3NH3PbI3 perovskite solar cells. Nat. Energy 2016, 1, 16081. 10.1038/nenergy.2016.81. [DOI] [Google Scholar]
- Chen Q.; Zhou H.; Song T.-B.; Luo S.; Hong Z.; Duan H.-S.; Dou L.; Liu Y.; Yang Y. Controllable self-induced passivation of hybrid lead iodide perovskites toward high performance solar cells. Nano Lett. 2014, 14, 4158–4163. 10.1021/nl501838y. [DOI] [PubMed] [Google Scholar]
- Lee S.; Park J. H.; Nam Y. S.; Lee B. R.; Zhao B.; Di Nuzzo D.; Jung E. D.; Jeon H.; Kim J.-Y.; Jeong H. Y.; Friend R. H.; Song M. H. Growth of nanosized single crystals for efficient perovskite light-emitting diodes. ACS Nano 2018, 12, 3417–3423. 10.1021/acsnano.7b09148. [DOI] [PubMed] [Google Scholar]
- Prakasam V.; Di Giacomo F.; Abbel R.; Tordera D.; Sessolo M.; Gelinck G.; Bolink H. J. Efficient perovskite light-emitting diodes: effect of composition, morphology, and transport layers. ACS Appl. Mater. Interfaces 2018, 10, 41586–41591. 10.1021/acsami.8b15718. [DOI] [PubMed] [Google Scholar]
- Ji X.; Peng X.; Lei Y.; Liu Z.; Yang X. Multilayer light emitting devices with organometal halide perovskite: Polymer composite emission layer: The relationship of device performance with the compositions of emission layer and device configurations. Org. Electron. 2017, 43, 167–174. 10.1016/j.orgel.2017.01.024. [DOI] [Google Scholar]
- Lee S.; Park J. H.; Lee B. R.; Jung E. D.; Yu J. C.; Di Nuzzo D.; Friend R. H.; Song M. H. Amine-based passivating materials for enhanced optical properties and performance of organic–inorganic perovskites in light-emitting diodes. J. Phys. Chem. Lett. 2017, 8, 1784–1792. 10.1021/acs.jpclett.7b00372. [DOI] [PubMed] [Google Scholar]