Abstract
Realizing the diagnosis of lung cancer at an inchoate stage is significant to get valuable time to conduct curative surgery. In this work, we relied on a density functional theory (DFT)-proposed Ru–SnS2 monolayer as a novel, promising biosensor for lung cancer diagnosis through exhaled gas analysis. The results indicated that the Ru–SnS2 monolayer has admirable adsorption performance for three typical volatile organic compounds (VOCs) of lung cancer patients, which therefore results in a remarkable change in the electronic behavior of the Ru-doped surface. As a consequence, the conductivity of the Ru–SnS2 monolayer increases after gas adsorption based on frontier molecular orbital theory. This provides the possibility to explore the Ru–SnS2 monolayer as a biosensor for lung cancer diagnosis at an early stage. In addition, the desorption behavior of three VOCs from the Ru–SnS2 surface is studied as well. Our calculations aim at proposing novel sensing nanomaterials for experimentalists to facilitate the progress in lung cancer prognosis.
1. Introduction
As one of the commonest cancers in our human, lung cancer has received great attention in recent years given its high incidence and mortality rates.1,2 At present, bold proteomic patterns, nuclear magnetic resonance, and chest tomography are widely applied technologies for the diagnosis of lung cancer.3,4 However, these methods are somewhat time-consuming, expensive, or invasive and what is worse is that lung cancer in most cases can only be diagnosed at an advanced stage. In other words, these so-called progressive methods cannot effectively increase the survival rate of lung cancer.
To realize the diagnosis of lung cancer at an early stage, exhaled breath analysis, based on analyzing the exhaled gas of a possible patient daily, is proposed aiming at improving the whole survival.5,6 As reported, human exhaled gas contains more than 200 volatile organic compounds (VOCs), which can reflect the potential dysfunction of human organs.7,8 VOCs that are recognized as lung cancer biomarkers are hydrocarbons such as isoprene (C5H8) and methyl cyclopentane (C6H12), hydrocarbon derivatives such as 1-propanol (C3H8O) and 2-propenal (C3H4O) as well as aromatic hydrocarbons such as benzene (C6H6) and styrene (C8H8).3,9 That is, if the existence of these typical gases can be detected, the subjects are possible victims of lung cancer. To this end, surface-enhanced Raman spectroscopy was reported as a good candidate for the detection of VOCs. Apart from that, chemical resistance-type sensors with high sensitivity and rapid response10−12 could also be a prospective method for the diagnosis of lung cancer through exhaled breath analysis. The sensing mechanism of such a sensor is based on the change in the conductivity of the sensing material after interaction with targeted gases.13 During the gas interaction, electrons will transfer between the adsorbent surface and gas molecules, changing the carrier concentration of the sensor and therefore modifying its electronic behavior.14 In fact, some scholars have proposed the application of resistivity-type sensors as promising devices in this field as it definitely provides a quite easy manner for the early diagnosis of lung cancer without pain and invasion.5,15 From this aspect, the materials with desirable chemical reactivity and high carrier mobility would be on priority to be exploited as potential sensors for VOCs.
Transition-metal dichalcogenides (TMDs) very recently become the focus of attention for sensing applications due to their strong chemical reactivity and high carrier mobility, especially when they are in two-dimensional (2D) structures. Among them, monolayer MoX2 (X = S, Se, and Te) are first explored and reported to have strong potential for gas sensing applications.16−18 However, a SnS2 monolayer thaT has a similar structure and property to those of a MoS2 monolayer faces a lack of attention. In fact, the SnS2 monolayer has an admirable indirect semiconducting property with a band gap of 2.60 eV19 and a superior carrier mobility of 50 cm2·V–1·s–1,2020 which confers its application for gas sensors, lithium-ion batteries, and water splitting.21−23 Moreover, through n-doping or p-doping of the SnS2 monolayer, much more desirable properties could be obtained.24 Ma et al.25 reported a Pd-doped SnS2 monolayer for gas sensing in transformer oil, which reveals the good performance of a transition-metal-doped SnS2 monolayer as a gas sensor. Moreover, it also stimulates us to utilize a Ru-doped SnS2 (Ru–SnS2) monolayer for VOC sensing to exploit its potential in lung cancer diagnosis. The Ru dopant has been demonstrated with good catalytic behavior for gas interaction25 and we believe that it can bring expected performance in VOC sensing. All of the reported results, including the optimization of the Ru–SnS2 monolayer and the adsorption process, were obtained using the density functional theory (DFT) method. Our theoretical calculations can provide the sensing mechanism of the Ru–SnS2 monolayer, and with the successful synthesis of large-scale atomic-layer SnS2,26,27 we are hopeful that the Ru–SnS2 monolayer could be explored as a novel chemical gas sensor in many fields.
2. Results and Discussion
2.1. Ru Doping Behavior on the SnS2 Monolayer
We defined the adsorption of one Ru dopant on the pristine SnS2 monolayer to form a Ru–SnS2 counterpart. The parameter of binding energy (Eb) was used to evaluate the chemical stability of the Ru–SnS2 monolayer, the Eb is calculated by the energy of Ru–SnS2 subtracting the total energies of pristine SnS2 and the Ru atom. Three doping sites were considered, namely, TS1 (on top of the S atom of the first layer), TSn (on top of the Sn atom of the second layer), and TS2 (on top of the S atom of the second layer). After full optimization, the most stable configuration for Ru doping is through the TS1 site, which possesses lower Eb compared with that of the TSn site. Interestingly, after the optimization of Ru doping on the TS2 site, the Ru dopant experiences somewhat displacement, making the structure the same as that of the TS1 site. Figure 1 plots the geometric structure and electron deformation density (EDD) of the identified Ru–SnS2 monolayer.
Figure 1.
Geometric structure (a) and EDD (b) of the Ru–SnS2 monolayer. In EDD, the green (purple) areas denote electron accumulation (depletion); the isosurface is set to 0.008 e/Å3.
As shown in Figure 1a, the Eb of the Ru–SnS2 monolayer through the TS1 site is −3.98 eV, much higher than the cohesive energy of the Ru atom (0.85 eV). Moreover, it is also larger than the Eb in the TSn site of 3.64 eV and in the TS2 site of 2.85 eV. These findings mean that Ru doping on the SnS2 monolayer through the TS1 site is quite thermodynamically favorable. After doping, the Ru adatom is captured by the surrounding S atoms, forming Ru–S bonds accordingly with a uniform bond length of 2.24 Å, slightly shorter than the sum of covalent radii of Ru and S atoms.28 This finding manifests the strong binding force in the Ru–S bond with high chemical stability. In Figure 1b, one can see that the Ru dopant is surrounded by electron depletion, indicating its electron-donating behavior, in agreement with the Hirshfeld analysis that indicates that 0.223e transfers from the Ru dopant to the SnS2 monolayer. On the other hand, the electron accumulation is mainly localized on the SnS2 monolayer and the Ru–S bonds, suggesting the strong electron hybridization between the Ru dopant and S atoms.
To further confirm the chemical stability of a single Ru atom on the SnS2 monolayer, the diffusion of the Ru atom from the TS1 site to the TS2 site is investigated. As shown in Figure 2, the energy barrier for Ru diffusion is as large as 4.11 eV, much higher than that of the critical barrier of 0.91 eV for reaction to occur energy-favorably at room temperature.29 Therefore, we believe that the single Ru atom could be stably adsorbed on the SnS2 monolayer through the TS1 site with a cluster-free problem.
Figure 2.
Ru atom diffusion on the SnS2 monolayer: (a) initial state (IS) TS1 site, (b) transition state (TS), and (c) final state (FS) TS2 site.
After confirmation of the most stable configuration of the Ru–SnS2 monolayer, its electronic behavior should be analyzed. Figure 3a plots the total density of state (DOS) of the pure and Ru-doped SnS2 monolayer. One can see that pure SnS2 monolayer performs semiconducting property without magnetic behavior. The band gap of the pristine SnS2 monolayer is calculated as 1.56 eV based on its band structure (not shown here), close to the reported one of 1.61 eV based on generalized gradient approximation (GGA) calculations,30 indicating the accuracy of our work. While after Ru doping, the DOS is obviously left-shifted to a lower region by about 2 eV, which suggests the strong electron-donating property of the Ru adatom, leading to the n-doping of the Ru–SnS2 system. At the same time, the states of the Ru dopant contribute largely to the whole DOS of the system, in which some novel states appears within the band gap of the pure SnS2 system around the Fermi level. Therefore, the conductivity of the Ru–SnS2 monolayer would be significantly reduced compared with that of the pure counterpart. From Figure 3b, it is seen that the Ru 4d orbital is highly overlapped by the S 3p, which manifests the strong orbital interaction between Ru and S atoms. These findings also explain the strong electron hybridization and binding force during the formation of the Rh–S bond.
Figure 3.
DOS of (a) pure and (b) Ru-doped SnS2 systems. The dashed line indicates the Fermi level.
Figure 4 exhibits the optimized molecular structure of three typical VOCs of lung cancer. Through analysis of bond length in different molecules, it is found that the C–H bond is measured as the same, while the C=O bond is longer than that of the C–H bond and the C=C bond is shorter than the C–C bond, indicating the stronger binding force for two C atoms in the double bond format. These results match well with the previous report.31
Figure 4.
Molecular structures of (a) C3H4O, (b) C6H6, and (c) C5H8. The black values are bond lengths.
2.2. Adsorption of VOC Molecules
First, we initiate the analysis of the C3H4O adsorption system, as displayed in Figure 5. We can find that the C3H4O molecule prefers to be parallel to the Ru−SnS2 surface on the top of the Ru dopant. However, the deformation of C3H4O is evident after adsorption, which is no longer a plane molecule on the Ru–SnS2 monolayer, implying the geometric activation of the adsorbed gas molecules. Besides, one C atom is captured by the Ru dopant, forming a new Ru–C bond measured as 2.19 Å. That is, the Ru dopant exerts strong binding force upon the C atom of C3H4O, which therefore leads to the large Ead of −1.42 eV. From the EDD, we find that electron accumulation is mainly localized on the gas molecule, while electron depletion is on the Ru–SnS2 monolayer, manifesting the electron-withdrawing property of C3H4O that agrees with the Hirshfeld analysis (QT = −0.085e). Besides, the Ru dopant is positively charged by 0.195e after adsorption. These results mean that 0.028e of the C3H4O molecule are accepted from the Ru dopant, whereas 0.057e are from the SnS2 surface. In other words, Ru bridges the charge transfer from the gas molecule to the SnS2 layer, thus facilitating electron redistribution in the adsorbed system. Overall, based on all of these findings, we identify the C3H4O adsorption on the Ru–SnS2 surface as chemisorption.
Figure 5.
Adsorption configuration of the C3H4O system (a) and EDD (b). In EDD, the rosy areas indicate electron accumulation and the green areas indicate electron depletion; the isosurface is set to 0.008 e/Å3.
As for the C6H6 adsorption in Figure 6, one can see that the most stable configuration is similar to that of the C3H4O system, in which the C6H6 molecule is parallel with the SnS2 layer right on top of the Rh dopant. After adsorption, the C6H6 molecule is slightly deformed, making the molecule a little bend toward the Ru dopant. This finding manifests the strong binding force between the Ru dopant and C6H6 molecule, as further confirmed by the large Ead of −2.07 eV. Based on the Hirshfeld method, the Ru is positively charged by 0.218e and the C6H6 molecule is positively charged by 0.212e after the interaction. In other words, the Ru dopant behaves as an electron bridge, enhancing the charge transfer between the Ru–SnS2 monolayer and the gas molecule, which is similar to that in the C3H4O system. After Ru doping, the charge transfer can be remarkably intensified due to the large electron mobility and chemical reactivity of the Ru dopant, which therefore results in larger Ead. From the EDD, one can see that electron accumulation is mainly localized on the Ru–SnS2 monolayer, while electron depletion is mainly localized on the C6H6 molecule, which is consistent with the Hirshfeld analysis. Apart from that, the overlap of electron accumulation and electron depletion at the area between the Ru–SnS2 layer and C6H6 molecule manifests the electron hybridization between two interaction species, which further verifies the strong adsorption performance of the Ru–SnS2 monolayer toward the C6H6 molecule.
Figure 6.
Adsorption configuration of the C6H6 system (a) and EDD (b). In EDD, the rosy areas indicate electron accumulation and the green areas indicate electron depletion; the isosurface is set to 0.008 e/Å3.
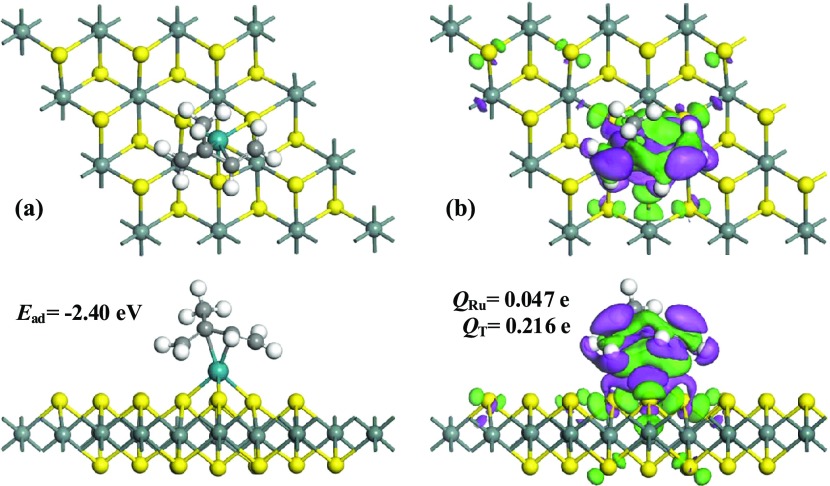
When it comes to the C5H8 system, the most stable configuration and the related EDD are depicted in Figure 7. It could be found that C5H8 prefers to be adsorbed on the Ru–SnS2 surface through the molecule-parallel position with a small slope to the plane. Rather than the little deformation of the C6H2 molecule after adsorption, the C5H8 molecule is afflicted with dramatic decomposition after being trapped by the Ru dopant with the formation of two Ru–C bonds. Originally, the five C atoms in the C5H8 molecule are in a same plane. However, after adsorption, the C atoms approach the Ru dopant, making the molecule bend accordingly. Specifically, the C–C and C=C bonds becomes elongated to 1.53 and 1.41 Å on the Ru–SnS2 surface and the newly formed Ru–C bonds are measured to be 2.15 and 2.17 Å, respectively. These results indicate not only the strong activation of C5H8 during adsorption32 but also the strong binding force between the Ru dopant and C5H8 molecule. Combined with the calculated Ead of −2.40 eV, chemisorption nature in this system could be identified. Besides, according to the Hirshfeld analysis, the C5H8 molecule as a whole transfers 0.216e to the Ru–SnS2 surface, while the Ru dopant is positively charged by 0.047e. That is to say, the Ru dopant behaves as an electron acceptor, withdrawing 0.176e from the C5H8 molecule and only 0.040e are accepted by the SnS2 monolayer. From the EDD, one can observe that electron accumulation is mainly around the Ru center and electron depletion is mainly located on the C5H8 molecule. Considering the large charge transfer between the C5H8 molecule and Ru–SnS2 monolayer, we presume that the ionic-bonding nature dominates the formation of Ru–C bonds in this system,33 as supported by the overlap of electron accumulation and electron depletion on the Ru–C bonds, which indicates electron hybridization during their formation.
Figure 7.
Adsorption configuration of the C5H8 system (a) and EDD (b). In EDD, the rosy areas indicate electron accumulation and the green areas indicate electron depletion; the isosurface is set to 0.008 e/Å3.
In short, the Ru–SnS2 monolayer possesses the strongest interaction with the C5H8 molecule, followed by the C6H6 molecule and the last being the C3H4O molecule. Besides, strong chemisorption is determined in three systems. At the same time, the electron hybridization could also be identified through EDD analysis, which verifies the orbital interaction in these systems. In addition, it is worth noting that when the results of charge transfer based on the Hirshfeld method are compared with Mulliken population the values are a little smaller with the same trend, but we assume that it would be more reliable because the Hirshfeld method does not rely on the basis set of the calculations. Thus, the electronic property of the Ru–SnS2 monolayer is supposed to be changed due to gas adsorption, which will be analyzed in the next section.
2.3. DOS of the Ru–SnS2 Monolayer upon Gas Adsorption
Figure 8 exhibits the total DOS and molecular DOS before and after gas adsorption, as well as the atomic DOS of bonding atoms to comprehensively understand the electronic behavior of the Ru–SnS2 monolayer for gas adsorption. It is seen from the total DOS that after adsorption the states of the Ru–SnS2 monolayer undergoes a different level of deformation, which is attributed to the DOS states of the adsorbed gas molecules whose states are activated to some extent. Specifically, one can see that the DOS peaks of three isolated gases split into several small peaks near the Fermi level. In that case, the electronic behavior of the gas adsorbed systems would be remarkably impacted because of the contribution of the adsorbed gas molecules around the Fermi level. On the other hand, although there are some states located at the deep valence band, we assume that they have little effect on the electronic behavior of the whole system due to their weak activation.
Figure 8.
DOS in various adsorption systems: (a) C3H4O, (b) C5H8O, and (c) C6H6.
Moreover, from the atomic DOS of Ru and C atoms, it is found that the Ru 4d orbital is highly overlapped by the C 2p orbital, ranging from −5 to 3 eV for the C3H4O system and from −5 to 2.5 eV for C5H8 and C6H6 systems. These findings illustrate the strong electron hybridization and binding force of the Rh–C bond; this is in agreement with the previous EDD analysis. It is worth noting that the significant changes in the electronic behavior of the Ru–SnS2 monolayer will accordingly change its electrical conductivity after adsorption. Based on this evidence, the sensing mechanism of a resistance-type sensor for detecting typical gases of lung cancer could be explored.
2.4. Frontier Molecular Orbital Theory Analysis
To explore the possibility of the Ru–SnS2 monolayer as a resistance-type gas sensor for detecting typical gases of lung cancer, we, in this section, emphasize the analysis of frontier molecular orbitals to give an insight into the potential sensing mechanism of such a chemical gas sensor. Frontier molecular orbitals include highest molecular occupied orbital (HOMO) and lowest molecular unoccupied orbital (LUMO). It is well known that the energy gap (Eg) between HOMO and LUMO is an effective parameter to evaluate the electrical conductivity of certain surfaces.34−36 Specifically, large Eg reveals small electrical conductivity and small Eg reveals large electrical conductivity.
Figure 9 exhibits the HOMO and LUMO distributions, their related energies, and the calculated Eg in various systems. It could be seen in the isolated Ru–SnS2 system that HOMO and LUMO are all mainly located at the Ru dopant, while there also exist some HOMOs and LUMOs on the SnS2 surface.
Figure 9.
HOMO and LUMO distributions and Eg of different systems: (a) isolated Ru–SnS2, (b) C3H4O system, (c) C6H6 system, and (d) C5H8 system.
Besides, the energies of HOMO and LUMO for the Ru–SnS2 monolayer are calculated to be −5.87 and −4.96 eV, respectively, and therefore, the Eg is 0.91 eV. This finding indicates its semiconducting behavior and manifests its suitability for gas sensing applications.37
When it comes to the gas adsorbed systems, one can see that the HOMO and LUMO distributions of the Ru–SnS2 monolayer experience pronounced changes. In the C3H4O system, one can see that HOMO is mainly around the Ru dopant and LUMO is localized on the SnS2 layer, while there only has a few HOMOs and LUMOs on the C3H4O molecule; in the C6H6 system, the HOMO is mainly on the Ru dopant and in the area between the Ru adatom and C6H6 molecule, whereas the LUMO is largely localized on the Ru dopant; in the C5H8 system, the HOMO and LUMO are both mainly localized on the Ru dopant and a few are on the gas molecule. Along with these, changes in their distribution are the related changes in their energies and subsequently their Eg that finally determine the electrical conductivity of the system. Detailedly, the Eg in the C3H4O system declines to 0.29 eV; in the C6H6 system, it declines to 0.53 eV; and in the C5H8 system, it declines to 0.30 eV. As mentioned, the reduced Eg would result in an increase in conductivity of the Ru–SnS2 monolayer after the adsorption of three typical gases. Moreover, the significant change in Eg will lead to evident changes in electrical conductivity. In this regard, we assume that the Ru–SnS2 monolayer is a promising candidate for C3H4O, C6H6, and C5H8 sensing given the admirable change in its Eg after their adsorption. Moreover, for exhale gas analysis, using a Ru–SnS2-based gas sensor can realize an effective detection and as a result make an accurate diagnosis for possible lung cancer.
2.5. Recovery Property
To further characterize the possible use of the Ru–SnS2 monolayer as a chemical sensor for lung cancer diagnosis, the recovery property is analyzed to determine the reusability of the typical sensor. The recovery behavior is the required minimum time for a sensor to desorb the adsorbed gases from its surface, in which the recovery time (τ) is explained by the transition state theory and the van’t Hoff–Arrhenius expression38
| 1 |
where A is the attempt frequency defined as 1012 s–1,39,4039,40T is the temperature, and KB is the Boltzmann constant (8.318 × 10–3 kJ/(mol·K)). Also, the Ea (potential barrier) of desorption is equal to the value of Ead due to the inverse process between the adsorption and desorption. It could be deduced that a larger Ead would increase the difficulty for gas desorption and a temperature increase can accelerate that process largely. Based on our calculated Ead in this work, however, the recovery time for such three typical gases of lung cancer from Ru–SnS2 surface would be too long and even unrealistic at room temperature. On the other hand, when the temperature is higher than 600 K, the desorption becomes possible in one day. Considering the heat loss and the sanitary safety of the devices, we recommend the one-off operation of the gas sensors for the diagnosis of lung cancer. From this aspect, it is hopeful that Ru–SnS2-based gas sensors with high sensitivity for typical gases of lung cancer could be further explored in the laboratory to extend their applications in the field of clinical medicine.
3. Conclusions
Using DFT, we theoretically investigated the Ru doping behavior on the SnS2 monolayer and simulated the adsorption behavior of Ru–SnS2 for three typical gases of lung cancer to explore its potential as a resistance-type sensor for lung cancer diagnosis. The main conclusions are as follows:
-
(i)
A single Ru atom could be stably doped on the SnS2 monolayer with high chemical stability and strong binding force without a cluster problem.
-
(ii)
The Ru–SnS2 monolayer possesses high chemisorption of three gasses that brings a significant change in its electronic property.
-
(iii)
After adsorption of typical gases of lung cancer, the conductivity of the Ru–SnS2 monolayer could be increased, which provides the possibility for its exploration as biosensors for the diagnosis of lung cancer at an early stage.
Based on these, we suggest to explore Ru–SnS2 as a resistance-type gas sensor in the clinical medicine. We are hopeful that our calculation could provide some guidance for its further exploration in other fields as well.
4. Computational Details
The DFT calculations were completed in the D mol3 package41 in a spin-polarized manner. To deal with the electron exchange–correlation terms, the Perdew–Burke–Ernzerhof (PBE) function with generalized gradient approximation (GGA) was considered.42 The DFT-D2 method was adopted to understand the Van der Waals force and long-range interactions, as proposed by Grimme.43 Double numerical plus polarization (DNP) was selected as the atomic orbital basis set,44 and the energy tolerance accuracy, maximum force, and displacement were determined as 10–5 Ha, 2 × 10–3 Ha/Å, and 5 × 10–3 Å,45 respectively. The Brillouin zone Monkhorst–Pack grid was sampled with a k-point mesh of 5 × 5 × 1 for the supercell geometric optimizations and of 10 × 10 × 1 for related electronic calculations.46 For static electronic structure calculations, a self-consistent loop energy of 10–6 Ha, a global orbital cutoff radius of 5.0 Å, and a smearing of 0.005 Ha were determined to ensure the accurate results of the total energy.47
A 3 × 3 supercell containing 16 Sn atoms and 18 S atoms was established as the SnS2 monolayer, on which one Ru atom was adsorbed to form the Ru–SnS2 monolayer. The lattice constants of the pristine and Ru-doped SnS2 monolayer were both calculated as 3.7 Å, in line with the previous report.19 For gas adsorption, the vacuum layer of 15 Å was used to prevent the interaction between adjacent units.48 The adsorption energy (Ead) is introduced to evaluate the interaction strength of adsorbing processes, calculated by the formula Ead = Esurf/gas – Esurf – Egas. In this formula, Esurf/gas, Esurf, and Egas represent the energies of the gas adsorbed system, the pure Ru–SnS2 surface, and pure gas species, respectively. In the meanwhile, Hirshfeld analysis was considered for charge transfer (QT) during gas adsorptions. The positive QT means the electron-donating behavior of the adsorbed gas molecule, while the negative QT indicates the electron-accepting behavior of the adsorbed gas molecule.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Grant No. 51907165) and the Fundamental Research Funds for the Central Universities (Grant No. XDJK2020B024),
The authors declare no competing financial interest.
References
- Henschke C. I.; Yankelevitz D. F. CT Screening for Lung Cancer. Radiol. Clin. North Am. 2009, 49 (4), 477–490. [DOI] [PubMed] [Google Scholar]
- Herbst R. S.; Heymach J. V.; Lippman S. M. Lung cancer. N. Engl. J. Med. 2008, 359, 1367–1380. 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran V. H.; Chan H. P.; Thurston M.; Jackson P.; Lewis C.; Yates D.; Bell G.; Thomas P. S. Breath Analysis of Lung Cancer Patients Using an Electronic Nose Detection System. IEEE Sens. J. 2010, 10, 1514–1518. 10.1109/JSEN.2009.2038356. [DOI] [Google Scholar]
- Varellagarcia M.; Kittelson J.; Schulte A. P.; Vu K. O.; Wolf H. J.; Zeng C.; Hirsch F. R.; Byers T.; Kennedy T.; Miller Y. E.; et al. Multi-target interphase fluorescence in situ hybridization assay increases sensitivity of sputum cytology as a predictor of lung cancer. Cancer Detect. Prev. 2004, 28, 244–251. 10.1016/j.cdp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Itoh T.; Nakashima T.; Akamatsu T.; Izu N.; Shin W. Nonanal gas sensing properties of platinum, palladium, and gold-loaded tin oxide VOCs sensors. Sens. Actuators, B 2013, 187, 135–141. 10.1016/j.snb.2012.09.097. [DOI] [Google Scholar]
- Altintas Z.; Tothill I. Biomarkers and biosensors for the early diagnosis of lung cancer. Sens. Actuators, B 2013, 188, 988–998. 10.1016/j.snb.2013.07.078. [DOI] [Google Scholar]
- Itoh T.; Miwa T.; Tsuruta A.; Akamatsu T.; Izu N.; Shin W.; Park J.; Hida T.; Eda T.; Setoguchi Y. Development of an Exhaled Breath Monitoring System with Semiconductive Gas Sensors, a Gas Condenser Unit, and Gas Chromatograph Columns. Sensors 2016, 16, 1891. 10.3390/s16111891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T.; Carbone D. P. Proteomics analysis in lung cancer: Challenges and opportunities. Respirology 2007, 12, 22–28. 10.1111/j.1440-1843.2006.00957.x. [DOI] [PubMed] [Google Scholar]
- Hakim M.; Broza Y. Y.; Barash O.; Peled N.; Phillips M.; Amann A.; Haick H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012, 112, 5949–66. 10.1021/cr300174a. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Xu Z.; Yang Z.; Song X. High-performance flexible self-powered tin disulfide nanoflowers/reduced graphene oxide nanohybrid-based humidity sensor driven by triboelectric nanogenerator. Nano Energy 2020, 67, 104251 10.1016/j.nanoen.2019.104251. [DOI] [Google Scholar]
- Zhang D.; Zong X.; Wu Z. Fabrication of tin disulfide/graphene oxide nanoflower on flexible substrate for ultrasensitive humidity sensing with ultralow hysteresis and good reversibility. Sens. Actuators, B 2019, 287, 398–407. 10.1016/j.snb.2019.01.123. [DOI] [Google Scholar]
- Zhang D.; Zong X.; Wu Z.; Zhang Y. Ultrahigh-performance impedance humidity sensor based on layer-by-layer self-assembled tin disulfide/titanium dioxide nanohybrid film. Sens. Actuators, B 2018, 266, 52–62. 10.1016/j.snb.2018.03.007. [DOI] [Google Scholar]
- Cui H.; Zhang X.; Chen D.; Tang J. Pt & Pd decorated CNT as a workable media for SOF2 sensing: A DFT study. Appl. Surf. Sci. 2019, 471, 335–341. 10.1016/j.apsusc.2018.12.016. [DOI] [Google Scholar]
- Zhang D.; Li Q.; Li P.; Pang M.; Luo Y. Fabrication of Pd-decorated MoSe 2 nanoflowers and density functional theory simulation toward ammonia sensing. IEEE Electron Device Lett. 2019, 40, 616–619. 10.1109/LED.2019.2901296. [DOI] [Google Scholar]
- Kim K. Y.; Yu H. Y.; Baek I. B.; Ahn C. G.; Lee B. K.; Kim Y.; Yoon Y. S.; Lim J. E.; Lee B. J.; Jang W. I.; Park J. H. Use of Gas-Sensor Array Technology in Lung Cancer Diagnosis. J. Sens. Sci. Technol. 2013, 22, 249–255. 10.5369/JSST.2013.22.4.249. [DOI] [Google Scholar]
- Cui H.; Zhang X.; Zhang G.; Tang J. Pd-doped MoS2 monolayer: A promising candidate for DGA in transformer oil based on DFT method. Appl. Surf. Sci. 2019, 470, 1035–1042. 10.1016/j.apsusc.2018.11.230. [DOI] [Google Scholar]
- Cui H.; Zhang G.; Zhang X.; Tang J. Rh-doped MoSe2 as toxic gas scavenger: A first-principles study. Nanoscale Adv. 2019, 1, 772–780. 10.1039/C8NA00233A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.; Wang X.; Yang A.; Chu J.; Lv P.; Liu Y.; Rong M. MoTe2: A Promising Candidate for SF6 Decomposition Gas Sensors With High Sensitivity and Selectivity[J]. IEEE Electron Device Lett. 2018, 39, 292–295. 10.1109/LED.2017.2786322. [DOI] [Google Scholar]
- Bacaksiz C.; Cahangirov S.; Rubio A.; Senger R.; Peeters F.; Sahin H.. Bilayer SnS2: Easy-tunable Stacking Sequence by Charging and Loading Pressure arXiv:1602.01824. arXiv.org e-Print archive. https://arxiv.org/abs/1602.01824 (accessed February 4, 2016).
- Song H. S.; Li S. L.; Gao L.; Xu Y.; Ueno K.; Tang J.; Cheng Y. B.; Tsukagoshi K. High-performance top-gated monolayer SnS2 field-effect transistors and their integrated logic circuits. Nanoscale 2013, 5, 9666–9670. 10.1039/c3nr01899g. [DOI] [PubMed] [Google Scholar]
- Chang K.; Wang Z.; Huang G.; Li H.; Chen W.; Lee J. Y. Few-layer SnS 2 /graphene hybrid with exceptional electrochemical performance as lithium-ion battery anode. J. Power Sources 2012, 201, 259–266. 10.1016/j.jpowsour.2011.10.132. [DOI] [Google Scholar]
- Qu B.; Ma C.; Ji G.; Xu C.; Xu J.; Meng Y. S.; Wang T.; Lee J. Y. Layered SnS2-reduced graphene oxide composite--a high-capacity, high-rate, and long-cycle life sodium-ion battery anode material. Adv. Mater. 2014, 26, 3854–3859. 10.1002/adma.201306314. [DOI] [PubMed] [Google Scholar]
- Hennig R. G. Theoretical perspective of photocatalytic properties of single-layer SnS2. Phys. Rev. B 2013, 88, 3925–3938. 10.1103/PhysRevB.88.115314. [DOI] [Google Scholar]
- Xia C.; Peng Y.; Zhang H.; Wang T.; Wei S.; Jia Y. The characteristics of n- and p-type dopants in SnS2 monolayer nanosheets[J]. Phys. Chem. Chem. Phys. 2014, 16, 19674–1968025. 10.1039/C4CP02214A. [DOI] [PubMed] [Google Scholar]
- Ma S.; Jin Y.; Si Y. Adsorption behavior of Pd-doped SnS2 monolayer upon H2 and C2H2 for dissolved gas analysis in transformer oil[J]. Adsorption 2019, 25, 1587–1594. 10.1007/s10450-019-00149-8. [DOI] [Google Scholar]
- Cui H.; Liu T.; Zhang Y.; Zhang X. Ru-InN Monolayer as a Gas Scavenger to Guard the Operation Status of SF6 Insulation Devices: A First-Principles Theory. IEEE Sens. J. 2019, 19, 5249–5255. 10.1109/JSEN.2019.2899966. [DOI] [Google Scholar]
- Ye G.; Gong Y.; Lei S.; He Y.; Bo L.; Xiang Z.; Jin Z.; Dong L.; Lou J.; Vajtai R.; et al. Synthesis of large-scale atomic-layer SnS2 through chemical vapor deposition. Nano Res. 2017, 10, 2386–2394. 10.1007/s12274-017-1436-3. [DOI] [Google Scholar]
- Jiao X.; Li X.; Jin X.; Sun Y.; Xu J.; Liang L.; Ju H.; Zhu J.; Pan Y.; Yan W.; et al. Partially Oxidized SnS2 Atomic Layers Achieving Efficient Visible-Light-Driven CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 18044. 10.1021/jacs.7b10287. [DOI] [PubMed] [Google Scholar]
- Pyykkö P.; Atsumi M. Molecular single-bond covalent radii for elements 1–118. Chem. - Eur. J. 2009, 15, 186–197. 10.1002/chem.200800987. [DOI] [PubMed] [Google Scholar]
- Cui H.; Chen D.; Zhang Y.; Zhang X. Dissolved gas analysis in transformer oil using Pd catalyst decorated MoSe2 monolayer: A first-principles theory. Sustainable Mater. Technol. 2019, 20, e00094 10.1016/j.susmat.2019.e00094. [DOI] [Google Scholar]
- Li B.; Xing T.; Zhong M.; Huang L.; Lei N.; Zhang J.; Li J.; Wei Z. A two-dimensional Fe-doped SnS2 magnetic semiconductor. Nat. Commun. 2017, 8, 1958 10.1038/s41467-017-02077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G.; Li M.. Ni-doped MoS2 biosensor: a promising candidate for early diagnosis of lung cancer by exhaled breathe analysis[J]. Appl. Phys. A 124751 10.1007/s00339-018-2185-1. [DOI] [Google Scholar]
- Fan Y.; Zhang J.; Qiu Y.; Zhu J.; Zhang Y.; Hu G. A DFT study of transition metal (Fe, Co, Ni, Cu, Ag, Au, Rh, Pd, Pt and Ir)-embedded monolayer MoS2 for gas adsorption. Comput. Mater. Sci. 2017, 138, 255–266. 10.1016/j.commatsci.2017.06.029. [DOI] [Google Scholar]
- Novoselov K. S.; Geim A. K.; Morozov S. V.; Jiang D.; Zhang Y.; Dubonos S. V.; Grigorieva I. V.; Firsov A. A. Electric Field Effect in Atomically Thin Carbon Films[J]. Science 2004, 306, 666. 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- Rad A. S.; Shabestari S. S.; Mohseni S.; Aghouzi S. A. Study on the adsorption properties of O3, SO2, and SO3 on B-doped graphene using DFT calculations. J. Solid State Chem. 2016, 237, 204–210. 10.1016/j.jssc.2016.02.023. [DOI] [Google Scholar]
- Zhang D.; Wu J.; Li P.; Cao Y. Room-temperature SO2 gas-sensing properties based on a metal-doped MoS2 nanoflower: an experimental and density functional theory investigation. J. Mater. Chem. A 2017, 5, 20666 10.1039/C7TA07001B. [DOI] [Google Scholar]
- Hao C.; Xiaoxing Z.; Jun Z.; Ying Z.. Nanomaterials-based gas sensors of SF6 decomposedspecies for evaluating the operation status of high-voltage insulation devices High Voltage [Online], 2019. https://digital-library.theiet.org/content/journals/10.1049/hve.2019.0130.
- YH Z.; YB C.; KG Z.; CH L.; J Z.; HL Z.; Y P. Improving gas sensing properties of graphene by introducing dopants and defects: a first-principles study. Nanotechnology 2009, 20, 185504 10.1088/0957-4484/20/18/185504. [DOI] [PubMed] [Google Scholar]
- Peng S.; Cho K.; Qi P.; Dai H. Ab initio study of CNT NO2 gas sensor. Chem. Phys. Lett. 2004, 387, 271–276. 10.1016/j.cplett.2004.02.026. [DOI] [Google Scholar]
- Hoa N. D.; Quy N. V.; Cho Y.; Kim D. Porous single-wall carbon nanotube films formed by in Situ arc-discharge deposition for gas sensors application. Sens. Actuators, B 2009, 135, 656–663. 10.1016/j.snb.2008.10.041. [DOI] [Google Scholar]
- Delley B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. 10.1063/1.1316015. [DOI] [Google Scholar]
- Cui H.; Yan C.; Jia P.; Cao W. Adsorption and sensing behaviors of SF6 decomposed species on Ni-doped C3N monolayer: A first-principles study. Appl. Surf. Sci. 2020, 512, 145759 10.1016/j.apsusc.2020.145759. [DOI] [Google Scholar]
- Tkatchenko A.; DiStasio R. A. Jr.; Head-Gordon M.; Scheffler M. Dispersion-corrected Møller-Plesset second-order perturbation theory. J. Chem. Phys. 2009, 131, 171. 10.1063/1.3213194. [DOI] [PubMed] [Google Scholar]
- Cui H.; Jia P.; Peng X. Adsorption of SO2 and NO2 molecule on intrinsic and Pd-doped HfSe2 monolayer: A first-principles study. Appl. Surf. Sci. 2020, 513, 145863 10.1016/j.apsusc.2020.145863. [DOI] [Google Scholar]
- Delley B. Hardness conserving semilocal pseudopotentials. Phys. Rev. B 2002, 66, 155125 10.1103/PhysRevB.66.155125. [DOI] [Google Scholar]
- Cui H.; Zhang X.; Li Y.; Chen D.; Zhang Y. First-principles insight into Ni-doped InN monolayer as a noxious gases scavenger. Appl. Surf. Sci. 2019, 494, 859–866. 10.1016/j.apsusc.2019.07.218. [DOI] [Google Scholar]
- Ju W.; Li T.; Su X.; Li H.; Li X.; Ma D. Au cluster adsorption on perfect and defective MoS2 monolayers: structural and electronic properties. Phys. Chem. Chem. Phys. 2017, 19, 20735. 10.1039/C7CP03062B. [DOI] [PubMed] [Google Scholar]
- Wu P.; Yin N.; Li P.; Cheng W.; Huang M. The adsorption and diffusion behavior of noble metal adatoms (Pd, Pt, Cu, Ag and Au) on a MoS2 monolayer: a first-principles study. Phys. Chem. Chem. Phys. 2017, 19, 20713–20722. 10.1039/C7CP04021K. [DOI] [PubMed] [Google Scholar]