Abstract
A series of magnetic composites of sodium polyacrylate and polyacrylamide copolymer [Fe3O4@SiO2@P(AANa-co-AM)] were prepared. The investigation showed that the adsorption efficiency of Pb(II) was the best when the acrylamide/acrylic acid (AM/AA) mass ratio of composites was 5:5. Therefore, the composite of this ratio was selected as the adsorbent to systematically adsorb Pb(II) in aqueous solution. Static adsorption of Pb(II) to the magnetic composites in aqueous solutions was investigated by varying the solution pH and the concentration of Pb(II). The adsorption kinetics and isotherms model of Pb(II) on the Fe3O4@SiO2@P(AANa-co-AM) composites followed a pseudo-second-order model and the Langmuir isotherm model, respectively. When the temperatures were 298.15, 308.15, and 318.15 K, the maximum adsorption capacities of Fe3O4@SiO2@P(AANa-co-AM) composites were 237.53, 248.14, and 255.10 mg/g, respectively. The thermodynamic study of adsorption showed that the adsorption of Pb(II) on Fe3O4@SiO2@P(AANa-co-AM) composites was a spontaneous endothermic process. The X-ray photoelectron spectroscopy (XPS) analysis showed that the adsorption of Pb(II) was due to the chelation between −COO– and Pb(II). After four adsorption–desorption cycles, the adsorbent can still maintain a high adsorption capacity.
1. Introduction
Pb(II) is a common heavy metal that is widely distributed and exists in many forms. It has a high content in wastewater from nonferrous metal smelting, mineral processing, nuclear fuel industry, printing, leather, textile, battery, building materials, electroplating, and alloy manufacturing industries.1,2 Pb(II) is highly toxic, non-biodegradable, and often accumulated in organisms, resulting in diseases affecting the human nervous, renal, gastrointestinal, and hematopoietic systems, and is even carcinogenic, posing a serious threat to human health.3,4 Therefore, lead-containing wastewater must be treated effectively before discharging. The common methods used in practical application are chemical precipitation, membrane separation, ion exchange, electrodialysis, reverse osmosis, biological treatment, adsorption, and so on.5,6 For high-concentration heavy-metal wastewater, reverse osmosis, chemical treatment, or electrochemical treatment can be used to reduce the concentration. However, at lower concentrations, the application cost of these methods is higher. At low concentration, the adsorption method has the advantages of low cost, good adsorption effect, fast adsorption rate, no sludge production, energy-saving, simple operation, and source of a range of materials, such as graphene oxide,7 chitosan,8 activated carbon,9 and silica,10 which have been widely used in heavy-metal wastewater treatment. The hydrogels have unique chemical and physical properties such as a cross-linked three-dimensional network structure, obvious hydrophilicity, expansibility, and modifiability. As a new adsorbent, hydrogels have attracted more and more attention to the adsorption properties of dyes and heavy metals.11,12
Although a large number of adsorbents have been developed for the treatment of heavy metal wastewater, the preparation of adsorbents with no secondary pollution, high adsorption efficiency, and good reuse performance is still a challenge.13 In recent years, magnetic adsorption materials used in water treatment can achieve rapid separation and recovery of adsorbents that reach saturation adsorption, effectively prevent secondary pollution, and achieve multiple regenerations and reuse of adsorbents.13,14 Magnetic adsorbents are usually produced by binding to Fe3O4. However, the bare magnetic Fe3O4 is prone to agglomeration and easily oxidized in the air. To protect magnetic Fe3O4, many researchers have further modified magnetic materials.15 Zargoosh et al. modified magnetic Fe3O4 with salicyl hydrazide (TSH) and polyacrylic acid (PAA) as adsorbents to adsorb Pb(II), the maximum adsorption capacity was 188.7 mg/g.16 Ren et al. synthesized Fe3O4@SiO2@CS composites with a maximum adsorption capacity of 123.37 mg/g for Pb(II).17 However, the adsorption effect and reusability of these adsorbents are not very good.
The polymers containing polycarboxyl and polyamino groups exhibit excellent adsorption properties for heavy-metal ions.18 In this paper, Fe3O4@SiO2@poly(acrylate-acrylamide) composites with more carboxylate groups and amino groups on the surface were prepared by solution dispersion polymerization and the performance of Pb(II) adsorption were studied systematically.
2. Results and Discussion
2.1. Characterization
Figure S1 shows the X-ray diffraction patterns of Fe3O4, Fe3O4@SiO2-M, and Fe3O4@SiO2@P(AANa-co-AM) (Figure S1 in the Supporting Information). The six characteristic peaks at 30.4, 35.9, 43.5, 54.0, 57.4, and 63.2° (Figure S1a) were assigned to the (220), (311), (400), (422), (511), and (440) planes of pure Fe3O4 respectively.19 The peak intensity of the Fe3O4@SiO2-M decreased compared with that of Fe3O4 due to the coating of amorphous SiO2 layer (Figure S1b). It can be found from Figure S1 that the Fe3O4 characteristic peaks were observed in the XRD map of Fe3O4@SiO2@P(AANa-co-AM), indicating that the crystal structure of Fe3O4 remains unchanged (Figure S1c).
The Fourier transform infrared (FT-IR) spectra of Fe3O4, Fe3O4@SiO2-M, Fe3O4@SiO2@P(AANa-co-AM), and Pb-Fe3O4@SiO2@P(AANa-co-AM) magnetic particles are shown in Figure 1. The peak at 576 cm–1 was Fe–O vibration, as shown in Figure 1a. The two new peaks appearing at 955 and 1068 cm–1 belong to the Si–OH stretching vibration and the Si–O–Si symmetric stretching vibration, respectively (Figure 1b). This indicates that Fe3O4 particles were successfully coated by SiO2.20,21 As shown in Figure 1, the spectral lines a, b, c, and d show broad and blunt peaks at 3393–2922 cm–1, indicating the presence of association −OH. From Figure 1c, it can be seen that the peaks at 1410 and 1569 cm–1 are consistent with the symmetric and antisymmetric telescopic peaks of COO–, and the peaks at 1666 cm–1 are consistent with the telescopic vibration of C=O bond in −COO–.22 After Pb(II) adsorption by Fe3O4@SiO2@P(AANa-co-AM), due to the strong interaction between −COO– and Pb(II), as shown in Figure 1d, a symmetric telescopic peak and an antisymmetric telescopic peak of the COO– shift from 1569 and 1410 cm–1 to 1563 and 1405 cm–1, respectively.
Figure 1.

FT-IR spectra of Fe3O4 (a), Fe3O4@SiO2-M (b), Fe3O4@SiO2@P(AANa-co-AM) (c), and Pb-Fe3O4@SiO2@P(AANa-co-AM) (d).
The scanning electron microscopy (SEM) image of Fe3O4@SiO2@P(AANa-co-AM) (Figure 2A) shows that Fe3O4@SiO2@P(AANa-co-AM) has a relatively fluffy plushlike structure, which gives a good platform for the adsorption of heavy metals. Figure 2B is a transmission electron microscopy (TEM) image of Fe3O4@SiO2-M, and the core–shell structure can be observed. The edge of the gray part was the silica shell, the middle part of the black part was the Fe3O4 core. This indicates that Fe3O4 particles are successfully coated by SiO2. When coated with silica, Fe3O4 particles tend to aggregate into larger particles. As the surface of the electrostatic repulsion of silica, the particles have a certain degree of dispersion.
Figure 2.
SEM images of Fe3O4@SiO2@P(AANa-co-AM) (A) and TEM images of Fe3O4-M (B).
Figure S2 shows the thermogravimetric curve of Fe3O4@SiO2@P(AANa-co-AM) before and after the adsorption of Pb(II). As can be seen from Figure S2, a small weightlessness step appears in the temperature range of 50–215 °C, mainly due to the evaporation of free water contained in the sample. Before adsorption, Fe3O4@SiO2@P(AANa-co-AM) shows obvious weightlessness of about 30 wt % at 215–450 °C, which was mainly due to the oxidative decomposition of oxygen-containing functional groups in Fe3O4@SiO2@P(AANa-co-AM) molecules and the carbonation of polymer chains.23 Then, the weight loss was 24.68% at 451–582 °C, which was due to the decomposition and carbonization of the polymer skeleton, while the slow weight loss at 582 °C was attributed to the continued carbonization of the residual substances.23,24 After the adsorption, Pb(II) reacted with oxygen at high temperature to form PbO. Due to the large atomic weight of Pb(II), the percentage of residues in mass increases, resulting in an increase in the final residual. In the range of 50–800 °C, the weightlessness of composites before adsorption and after adsorption were 74.63 and 62.45 wt %, respectively.
2.2. Magnetic Separation Performance
As shown in Figure 3, Fe3O4@SiO2@P(AANa-co-AM) is well dispersed in aqueous solutions. The separation of Fe3O4@SiO2@P(AANa-co-AM) from the aqueous solution can be achieved in 30 s under an external magnetic field, which indicates that the application of the adsorbent in the field of water treatment is conducive to avoiding secondary pollution and recovering adsorbent. Two videos (MP4) are provided in the Supporting Information. Video 1 of the Supporting Information is the demo adsorption experiment of adsorbent and magnet, which shows that our adsorbent (Fe3O4@SiO2@P(AANa-co-AM)) has good magnetic properties. Video 2 of the Supporting Information is the demo adsorption experiment of adsorbent and magnet in solution (V2), which shows that our adsorbent (Fe3O4@SiO2@P(AANa-co-AM)) in solution has good magnetic properties and the adsorbent can be collected and recycled.
Figure 3.

Photographs of Fe3O4@SiO2@P(AANa-co-AM) before (A) and after (B) magnetic separation.
2.3. Adsorption of Pb(II)
2.3.1. R and Swelling Ratio (SR) Effect of Fe3O4@SiO2@P(AANA-co-AM) Composites with Different Acrylamide/Acrylic Acid (AM/AA) Ratio on Pb(II)
In general, the composition of adsorbents has a great influence on its ability to adsorb heavy metals. Figure S3 shows the R and SR influence of Fe3O4@SiO2@P(AANa-co-AM) composites with different AM/AA weight ratios. The results show that when the mass ratio of AM/AA decreases from 9:1 to 1:1, the removal rate of Pb(II) on the composites increases from 69.21 to 99.31%, which may be because the chelating effect of the −COO– group on heavy-metal ions was stronger than that of the −CONH2 group.25 When the mass ratio of AM/AA decreased from 5:5 to 3:7, the removal rate of Pb(II) remained almost unchanged. Combined with the SR analysis, when the mass ratio of AM/AA was 5:5, the swelling ratio of magnetic composites was lower (SR = 128.3 g/g), which was more suitable as an adsorbent, so the mass ratio of AM/AA was fixed at 5:5.
2.3.2. Effect of pH on Adsorptions
The pH of a solution is an important parameter that affects the adsorption performance of adsorbents. It not only affects the existence of heavy-metal ions but also determines the surface charge of the adsorbents.26,27 As shown in Figure 4, the adsorption capacity of Pb(II) by Fe3O4@SiO2@P(AANa-co-AM) composite is lower when the pH value is low and the adsorption capacity qe of Pb(II) increased obviously when the pH increases from 2.0 to 3.5. When the pH value is about 4.5, the adsorption capacity reaches the maximum. As the pH value is greater than 4.5, the adsorption capacity decreases gradually. When the pH value is low, too much H+ in the solution causes the amino and carboxyl matrix in the Fe3O4@SiO2@P(AANa-co-AM) composite to form −NH3+ and −COOH, which, in turn, weakens its chelating ability with Pb(II). At the same time, H+ also competes with Pb(II) for adsorption and occupies more adsorption points. When the pH value increases gradually, the −NH3+ and −COOH deprotonation on the composite, and a large number of −NH2 and −COOH chelate with Pb(II), which makes the adsorption capacity of Pb(II) increase greatly. However, when the pH is more than 5, −OH in aqueous solution is easy to hydrolyze with Pb(II) and form Pb(OH)2 precipitation. Therefore, the following adsorption experiments were carried out under a pH of 4.5.
Figure 4.

Effect of initial pH on qe.
2.3.3. Influence of Contact Time and Adsorption Kinetics
The influence of contact time on adsorption capacity is shown in Figure 5. The results showed that the adsorbance of Pb(II) on Fe3O4@SiO2@P(AANA-co-AM) composites increased greatly within the first 100 min and reached equilibrium at about 140 min. For Fe3O4@SiO2@P(AANa-co-AM), the adsorption rate is very high, owing to its excellent water permeability and three-dimensional (3D) networks, which are highly accessible to heavy-metal ions.24
Figure 5.

Effect of contact time on the adsorption capacity of Fe3O4@SiO2@P(AANa-co-AM).
To better understand the control steps of Pb(II) adsorption rate by Fe3O4@SiO2@P(AANa-co-AM), the adsorption kinetics data were fitted with quasi-first-order model (eq 1) and quasi-second-order model (eq 2).28
| 1 |
| 2 |
where t is the adsorption time (min) and qt (mg/g) is the adsorption capacity of the adsorbent at time t. k1 (min–1) and k2 (g/(mg·min)) are the adsorption rate constants of the pseudo-first-order and the pseudo-second-order kinetic models, respectively. The values of qe and k1 were calculated from the intercept and slope of the plot of ln(qe– qt) versus t. The values of k2 and qe were calculated from the slope and intercept of the plot of t/qt versus t. The adsorption equilibrium data on Fe3O4@SiO2@P(AANa-co-AM) were fitted with the quasi-first-order model and quasi-second-order model at 298.15 K and listed in Table 1.
Table 1. Kinetic Model Parameters of Pb(II) Adsorption on Fe3O4@SiO2@P(AANa-co-AM).
| first-order
rate constants |
second-order
rate constants |
|||||
|---|---|---|---|---|---|---|
| C0 (mg/L) | qe (mg/g) | k1 (min–1) | R2 | k2 (g/(mg·min)) | qe (mg/g) | R2 |
| 250 | 135.5 | 0.02396 | 0.8033 | 0.0002174 | 266.7 | 0.9952 |
It can be seen from Figure S4A,B and Table 1 that the curve fitted by the pseudo-second-order model has a high correlation with the actual adsorption kinetics of Pb(II), and the correlation coefficient R2 > 0.999, while the curve fitted by the pseudo-first-order model has a poor correlation with the actual adsorption kinetics of Pb(II) (R2 < 0.95). Therefore, the surface chemical reaction is the main step to control the adsorption rate of Pb(II) by Fe3O4@SiO2@P(AANa-co-AM).23,29
2.3.4. Adsorption Isotherm and Thermodynamic Analysis
Figure 6 shows the relation curves between the equilibrium adsorbance and equilibrium concentration of Pb(II) at three different temperatures. The equilibrium adsorption capacities of Pb(II) are 232.97, 242.17, and 249.69 mg/g at 298.15, 308.15, and 318.15 K, respectively; the change shows that the equilibrium adsorption capacity increases with the increasing temperature, which means that raising the temperature from 298.15 to 318.15 K favors the adsorption of Pb(II).
Figure 6.

Adsorption isotherm of Pb(II) at three different temperatures.
To obtain the saturated adsorption capacity (qm) of Pb(II) on Fe3O4@SiO2@P(AANa-co-AM) composites, Langmuir and Freundlich models were used to fit the adsorption isotherm data of this work,30 and the linearized forms of these models are expressed in eqs 3 and 4,(31,32) respectively.
| 3 |
where qm (mg/g) and KL (L/mg) are the maximum adsorption capacity and the Langmuir equilibrium constant, respectively.
| 4 |
where 1/n and KF (L/g) are the Freundlich equilibrium constants.
The adsorption equilibrium data obtained from the Langmuir and Freundlich isotherm models are listed in Figure S5A,B and Table 2. The results showed that the R2 value of the Langmuir model at three different temperatures was larger than that of the Freundlich model and closer to 1. Therefore, the adsorption of Pb(II) by Fe3O4@SiO2@P(AANa-co-AM) is more in accordance with the Langmuir model, which is monolayer adsorption. According to the Langmuir isotherm equation, the calculated maximum adsorption capacities for Pb(II) by Fe3O4@SiO2@P(AANa-co-AM) are 237.53, 248.14, and 255.10 mg/g at 298.15, 308.15, and 318.15 K, respectively. The KL value of the Langmuir model and the 1/n value of the Freundlich model between 0 and 1 indicate that the adsorption is easy to proceed.33Table 3 summarizes the maximum adsorption capacities of some adsorbents reported in recent years for Pb(II), indicating that the adsorbance of Fe3O4@SiO2@P(AANa-co-AM) for Pb(II) is comparable or exceeds that of other adsorbents. So, Fe3O4@SiO2@P(AANa-co-AM) can be used for treating wastewater containing Pb(II).
Table 2. Parameters of Langmuir and Freundlich Isotherm Models.
| Langmuir |
Freundlich |
|||||
|---|---|---|---|---|---|---|
| T (K) | qm(mg/g) | KL(L/mg) | R2 | KF (L/g) | n–1 | R2 |
| 298.15 | 237.53 | 0.3935 | 0.9963 | 84.49 | 0.2677 | 0.8713 |
| 308.15 | 248.14 | 0.5255 | 0.9972 | 94.84 | 0.2671 | 0.8965 |
| 318.15 | 255.10 | 0.8150 | 0.9977 | 109.99 | 0.2570 | 0.8708 |
Table 3. Maximum Adsorption Capacities (qm) for Pb(II) by Some Adsorbents.
| adsorbent | qm (mg/g) | refs |
|---|---|---|
| ECAA | 219.3 | (2) |
| AC | 58.00 | (9) |
| CMC/PAM | 312.5 | (12) |
| FMMS | 223.2 | (13) |
| Fe3O4/LDH-AM | 266.6 | (14) |
| MNPs | 188.7 | (16) |
| EDCMS | 123.5 | (17) |
| Fe3O4-P(Cys/HEA) | 38.69 | (19) |
| LLDPE-g-PAA-co-starch/OMMT | 430.0 | (22) |
| M-PAM-HA | 174.9 | (23) |
| P(AANa-co-AM)/GO | 452.3 | (25) |
| Fe3O4@SiO2@P(AANa-co-AM) | 237.53 | this work |
To analyze the thermodynamic behavior of adsorption of Pb(II) by Fe3O4@SiO2@P(AANa-co-AM), some thermodynamic parameters can be obtained from the following equations.
| 5 |
| 6 |
where R is the ideal gas constant (8.314 J/(mol·K)), K0 is the adsorption equilibrium constant, T is the absolute temperature, ΔG0 is the standard Gibbs free energy change, ΔH0 is the standard enthalpy change (J/mol), and ΔS0 is the standard entropy change (J/(mol·K)). K0 was obtained from the intercept of plotting ln(qe/Ce) versus Ce at three different temperatures by extrapolating Ce to zero according to the Singh and Khan method.34 ΔH0 and ΔS0 were obtained from the slope and intercept in the curve of ln K0 versus T–1. The values of ln K0, ΔH0, ΔS0, and ΔG0 are listed in Table 4.
Table 4. Thermodynamic Parameters for the Adsorption of Pb(II) on Fe3O4@SiO2@P(AANa-co-AM).
| T (K) | ln k0 | ΔH0 (kJ/mol) | ΔS0 (J/(mol·k)) | ΔG0 (kJ/mol) |
|---|---|---|---|---|
| 298.15 | 4.148 | 26.09 | 121.9 | –10.28 |
| 308.15 | 4.444 | –11.38 | ||
| 318.15 | 4.811 | –12.72 |
Normally, when the value of ΔH0 is between 2.1 and 20.9 kJ/mol, the electrostatic interaction between the adsorption ion and the adsorption site is consistent, which indicates that the adsorption is due to physical adsorption. When the value of ΔH0 is between 20.9 and 418.4 kJ/mol, the adsorption is transferred from the adsorbent surface to the adsorbing ions to form coordinate bonds or involves charge sharing, which indicates that adsorption is chemical adsorption.33 The value of ΔH0 in this work is 26.09 kJ/mol as shown in Table 4, indicating that the adsorption of Pb(II) on Fe3O4@SiO2@P(AANa-co-AM) is chemical adsorption. Meanwhile, the positive value of ΔH0 indicates that adsorption is a spontaneous endothermic reaction, and increasing the temperature is beneficial to the adsorption process, which is consistent with the discussion on the effect of temperature change. The negative value of ΔG0 indicates that the adsorption of Pb(II) on Fe3O4@SiO2@P(AANa-co-AM) is a spontaneous process, and the positive value of ΔS0 indicates that the adsorption process is driven by entropy rather than enthalpy.
2.3.5. X-ray Photoelectron Spectroscopy (XPS) Analysis
To explore the adsorption mechanism of Fe3O4@SiO2@P(AANa-co-AM) for Pb(II), the samples before and after adsorption were characterized by XPS. The characterization results are given in Figure 7. The characteristic peaks of C 1s, N 1s, O 1s, and Na 1s appeared at 284, 399, 531, and 1071 eV in the full-scale XPS spectra, respectively (Figure 7a). Fe3O4@SiO2@P(AANa-co-AM) of adsorption Pb(II) had strong Pb 4f peaks (Figure 7a), which indicated that Pb(II) was indeed adsorbed on the composites. The three group peaks of Fe3O4@SiO2@P(AANa-co-AM) and Fe3O4@SiO2@P(AANa-co-AM)-Pb(II) in Figure 7b belong to the C–C, C–N, and C=O binding energy of C 1s, respectively.
Figure 7.
Full-scale XPS spectra of Fe3O4@SiO2@P(AANa-co-AM) (a). High-resolution XPS spectra of Fe3O4@SiO2@P(AANa-co-AM) and Fe3O4@SiO2@P(AANa-co-AM)-Pb(II) for C 1s, (b), O 1s (c), and the N 1s (d).
Clearly, due to the reaction of adjacent N or O atoms with Pb(II), the binding energies of C 1s increases slightly after the adsorption of lead ions.27 The peaks at 530.67 and 531.27 eV correspond to the C–O and C=O bond energies in the O 1s high-resolution spectra before adsorption, respectively (Figure 7c). It can be observed that after the adsorption of Pb(II), the bond energy of the C=O changes less, while the bond energy of the C–O increases significantly from 530.67 to 532.30 eV, which indicates strong chelation between −COO– groups and Pb(II).25 The peak at 399.60 eV is due to the bond energy of the acylamino N–H in the N 1s high-resolution spectra before the adsorption of the composites (Figure 7d); the bond energy of the N–H remained basically unchanged after adsorption of Pb(II), indicating that the affinity of acylamino to Pb(II) is extremely small due to the weak activity of acylamino.35 Therefore, the adsorption mechanism of composites for Pb(II) is the chelation of −COO– for Pb(II).
2.3.6. Regeneration Study
In general, in the actual wastewater treatment process, it is necessary to prepare excellent adsorbents with high desorption efficiency and good reusability to reduce the cost.36Figure 8 shows the removal rate of Pb(II) in each cycle after desorption with 30 mL of 2% HNO3 and regeneration with 30 mL of 0.1 mol/L NaOH solution. The results showed that the removal rate of Pb(II) by Fe3O4@SiO2@P(AANa-co-AM) decreased from 99.56 to 93.28% for 100 mg/L of Pb(II) at pH 4.5. This indicates that the Fe3O4@SiO2@P(AANa-co-AM) composite exhibits excellent regeneration performance as an adsorbent for Pb(II).
Figure 8.

Removal efficiency of Pb(II) in adsorption cycles.
3. Conclusions
The adsorption of Pb(II) by magnetic composites is highly dependent on the initial Pb(II) concentration and the solution pH. The adsorption kinetics study of Pb(II) on the magnetic composites can be well described by the pseudo-second-order model. The adsorption isotherm fitted well with the Langmuir model and the calculated maximum adsorption capacities are 237.53, 248.14, and 255.10 mg/g at 298.15, 308.15, and 318.15 K, respectively. Thermodynamic studies show that adsorption is chemical adsorption and a spontaneous endothermic process, which is driven by entropy. The XPS analysis reveals that the mechanism of adsorption of Pb(II) onto the magnetic composites is the chelation of −COO– with Pb(II). Furthermore, the composite exhibits good magnetic responsiveness and reusability, which give it high potential to remove Pb(II) from wastewater.
4. Experimental Section
4.1. Materials
Chemical reagents including acrylamide(AM), acrylic acid (AA), tetraethylorthosilicate (TEOS), silane coupling agent (3-aminopropyl) triethoxysilane (APTES), K2S2O8,N,N-methylene diphenylamide (MBA), CH3CH2OH, ammonium hydroxide (NH3·H2O, 25%), NaOH, and HNO3 were purchased from Chengdu Jinshan Chemical Reagent Co., Ltd, Chengdu, China. FeCl3·6H2O, Pb(NO3)2 and FeSO4·7H2O were purchased from Shanghai Qiangshun Chemical Reagent Co., Ltd, Shanghai, China. The chemical reagents used in the experiment were all analytical grade, and the water used was distilled water.
4.2. Preparation of Magnetic Fe3O4 Particles
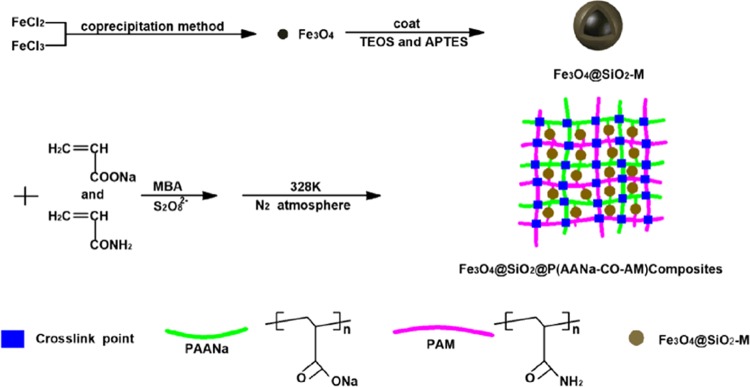
In this paper, Fe3O4 particles were prepared by the co-precipitation method.37 First, 2.50 g of FeSO4·7H2O and 4.86 g of FeCl3·6H2O were dissolved in 100 mL of distilled water to form a uniform dark orange solution under magnetic stirring. Then, the aqueous solution of NaOH (5.1 g of NaOH dissolved in 50 mL of distilled water) was injected into the above solution under intense stirring at room temperature for 5 h. Finally, the product was filtered and washed to neutral with distilled water and anhydrous ethanol and dispersed in distilled water by the ultrasonic treatment of 10 min for the next step.
4.3. Preparation of Modified Fe3O4@SiO2
The modified Fe3O4@SiO2 was prepared in two steps. According to previous studies, the core–shell Fe3O4/SiO2 was synthesized by the revised Stöber method.38 Generally, 20 mL of distilled water, 80 mL of ethanol, 5 g of TEOS, and 5 mL of ammonium hydroxide (25%) were mixed under mechanical stirring in a 313.15 K water bath for hydrolyzing and condensing of TEOS. After 10 min, 100 mL (80 mL ethanol and 20 mL water) of a Fe3O4 solution containing about 2 g of Fe3O4 was added to this mixed solution. After the reaction was carried out at room temperature for 12 h, the products were collected by a magnet, washed to neutral with distilled water and anhydrous ethanol, and then dispersed again in solutions containing 160 mL anhydrous ethanol, 40 mL of distilled water, and 4 mL of ammonium hydroxide (25%) for further use.
APTES/Fe3O4@SiO2 (Fe3O4@SiO2-M) was prepared by further modification of Fe3O4@SiO2 by silane coupling agent (APTES). The specific experimental methods were as follows: 4 mL of APTES was added to the above-mentioned Fe3O4@SiO2 solution and mechanically stirred for 24 h in a water bath at 333.15 K, and then cooled naturally to room temperature. The resulting product was then collected with a magnet, washed repeatedly with distilled water and ethanol, and dried in a vacuum oven at 333.15 K.
4.4. Preparation of Fe3O4@SiO2@P(AANa-co-AM) Composites
In a water bath of 278.15 K, sodium hydroxide was used to prepare AA with a neutralization degree of 0.8. Typical processes for preparing Fe3O4@SiO2@P(AANa-co-AM) composites with a mass ratio of AM/AA = 5:5 can be described as follows: 2 g of Fe3O4@SiO2-M was ultrasonically dissolved in 50 mL of distilled water for 30 min, then transferred to a 250 mL three-necked flask, 14.8 mL of AANa solution (contained 5.04 g AA), 5.04 g of AM, and 0.1 g of the cross-linking agent were added, and then ventilated with N2 for 15 min. Under strong mechanical stirring, 10 mL of K2S2O8 solution (containing 0.2 g of K2S2O8) was added to the three-necked flask and heated to 328.15 K for 1.5 h. Finally, the obtained Fe3O4@SiO2@P(AANa-co-AM) composites were shredded and soaked in ethanol–water mixture (5:5, v/v) for 24 h to remove unreacted monomers from the sample. Then, the product was dried in a vacuum oven at 338.15 K. When the mass ratio of AA to AM was changed and the other conditions remained unchanged, the Fe3O4@SiO2@P(AANa-co-AM) magnetic composites with AM:AA mass ratios of 9:1, 7:3, and 3:7 were prepared, respectively. The simple preparation process of the sample is shown in Scheme 1.
Scheme 1. Preparation of the Fe3O4@SiO2@P(AANa-co-AM) Composites.
4.5. Characterization
The crystal structures of Fe3O4, Fe3O4@SiO2-M, and Fe3O4@SiO2@P(AANa-co-AM) samples were investigated by X-ray diffraction (XRD, D8 Advance) spectra using Cu Kα radiation (λ = 0.154 nm). The infrared spectra of the composites in the range of 4000–400 cm–1 were measured by Fourier transform infrared (FT-IR, Nicolet iS10) spectroscopy. The morphologies of the samples were observed by scanning electron microscopy (SEM, Spectro Zeiss Evo18) and transmission electron microscopy (TEM, JEOL, JEM-2010). Thermogravimetric analysis (TGA) was performed on a TGA–differential scanning calorimetry (DSC) analyzer (Metter Toledo) under a nitrogen flow with a heating rate of 10 K/min. The functional group changes of Fe3O4@SiO2@P(AANa-co-AM) composite before and after adsorbing Pb(II) were determined by X-ray photoelectron spectroscopy (XPS, Escalab 250Xi, Al-Kα 1486.6). The sample (0.5 g) was immersed in 500 mL of water to determine the swelling weight of the sample at different contact times and its swelling ratio was calculated until the sample reached a swelling equilibrium. The swelling ratio of the sample can be calculated as SR = (ms – md)/md, where md and ms are the masses of the dry sample and the swollen sample, respectively.
4.6. Adsorption Experiments
The stock solution of Pb(II) was prepared by dissolving Pb(NO3)2 in distilled water and further diluting to the desired concentration. The pH of the solution was adjusted to the desired value with 0.1 M NaOH and 0.1 M HNO3 solution. Batch experiments were carried out in a water bath thermostatic shaker at 120 rpm for 6 h at 298.15 K. In a typical experiment, 50 mL of Pb(II) solutions and 50 mg of adsorbent were mixed in a 100 mL conical flask for adsorption. The influence of the varying initial aqueous pH values was evaluated in the range of 2.0–5.0 with an initial Pb(II) concentration of 200 mg/L at 298.15 K, and the medium with optimum pH value was adopted for all of the following experiments. The influence of the contact time on adsorption was studied by changing the contact time (0–360 min). The adsorption isotherm was obtained by varying the initial Pb(II) concentration from 50 to 300 mg/L at three different temperatures of 298.15, 308.15, and 318.15 K within 6 h, respectively. After filtration, the lead-ion concentration was determined by flame atomic adsorption spectrophotometry (FAAS, Shimadzu AA-6300C). The equilibrium adsorption capacity (qe, mg/g) and the removal rate (R) of Pb(II) were calculated by eqs 7 and 8, respectively
| 7 |
| 8 |
where C0 and Ce are the initial and the equilibrium concentrations (mg/L), respectively, V is the volume of the solution (L), and m is the dry weight of the adsorbent.
4.7. Desorption and Regeneration Studies
The adsorption was carried out in 50 mL of 100 mg/L Pb(II) solution at pH 4.5 with 50 mg of Fe3O4@SiO2@P(AANa-co-AM) at 298.15 K for 6 h. After filtration, the Pb-loaded adsorbent was immersed in 30 mL of 2% (v/v) HNO3 and shaken at 298.15 K for 2 h. After desorption, the adsorbents were regenerated with 30 mL of 0.1 M NaOH solution and then washed to neutral with distilled water for the next adsorption.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (21566010 and 31760196).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00403.
XRD spectra of Fe3O4 (a), Fe3O4@SiO2-M (b), and Fe3O4@SiO2@P(AANa-co-AM) (c). TGA curves of Fe3O4@SiO2@P(AANa-co-AM) before (A) and after (B) adsorbed Pb(II). Pb(II) removal rate and swelling rate bar diagram of Fe3O4@SiO2@P(AANA-co-AM) composites with different AA/AM ratios. Fitting of pseudo-first-order and pseudo-second-order models. Fitting of Langmuir and Freundlich models for Pb(II) adsorption (PDF)
Demo adsorption experiment of adsorbent and magnet (MP4)
Demo adsorption experiment of adsorbent and magnet in solution (MP4)
Author Contributions
H.L. performed the methodology and writing of the original, draft. F.Z. performed the review and editing. Q.W. managed the resources, formal analysis, and supporting information. All authors read and approved the final manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Huang Y.; Zeng X. F.; Guo L. L.; Lan J. H.; Zhan L. L.; Cao D. P. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 2018, 194, 462–469. 10.1016/j.seppur.2017.11.068. [DOI] [Google Scholar]
- Huang Y. G.; Wang Z. Q. Preparation of composite aerogels based on sodium alginate, and its application in removal of Pb2+and Cu2+ from water. Int. J. Biol. Macromol. 2018, 107, 741–747. 10.1016/j.ijbiomac.2017.09.057. [DOI] [PubMed] [Google Scholar]
- Li Y.; Bai P.; Yan Y.; Yan W. F.; Shi W.; Xu R. Removal of Zn2+, Pb2+, Cd2+, and Cu2+ from aqueous solution by synthetic clinoptilolite. Microporous Mesoporous Mater. 2019, 273, 203–211. 10.1016/j.micromeso.2018.07.010. [DOI] [Google Scholar]
- Hayati B.; Maleki A.; Najafi F.; Gharibi F.; McKay G.; Gupta V. K.; Puttaiah S. H.; Marzban N. Heavy metal adsorption using PAMAM/CNT nanocomposite from aqueous solution in batch and continuous fixed bed systems. Chem. Eng. J. 2018, 346, 258–270. 10.1016/j.cej.2018.03.172. [DOI] [Google Scholar]
- Ma J. H.; Liu Y. T.; Ali O.; Wei Y. F.; Zhang S. Q.; Zhang Y. M.; Cai T.; Liu C. B.; Luo S. L. Fast adsorption of heavy metal ions by waste cotton fabrics based double network and influencing factors insight. J. Hazard. Mater. 2018, 344, 1034–1042. 10.1016/j.jhazmat.2017.11.041. [DOI] [PubMed] [Google Scholar]
- Zhang Q. H.; Yang W. N.; Ngo H. H.; Guo W. S.; Jin P. K.; Dzakpasu M.; Yang S. J.; Wang Q.; Wang X. C.; Ao D. Current status of urban wastewater treatment plants in China. Environ. Int. 2016, 92-93, 11–22. 10.1016/j.envint.2016.03.024. [DOI] [PubMed] [Google Scholar]
- Abdi G.; Alizadeh A.; Zinadini S.; Moradi G. Removal of dye and heavy metal ion using a novel synthetic polyethersulfone nanofiltration membrane modified by magnetic graphene oxide/metformin hybrid. J. Membr. Sci. 2018, 552, 326–335. 10.1016/j.memsci.2018.02.018. [DOI] [Google Scholar]
- Huang Y. J.; Wu H. L.; Shao T. K.; Zhao X.; Peng H.; Gong Y. F.; Wan H. H. Enhanced copper adsorption by DTPA-chitosan/alginate composite beads: Mechanism and application in simulated electroplating wastewater. Chem. Eng. J. 2018, 339, 322–333. 10.1016/j.cej.2018.01.071. [DOI] [Google Scholar]
- Alguacil F. J.; Alcaraz L.; García-Díaz I.; López F. A. Removal of Pb2+ in Wastewater via Adsorption onto an Activated Carbon Produced from Winemaking Waste. Metals 2018, 8, 697 10.3390/met8090697. [DOI] [Google Scholar]
- Dutta D. P.; Nath S. Low cost synthesis of SiO2/C nanocomposite from corn cobs and its adsorption of uranium (VI), chromium (VI) and cationic dyes from wastewater. J. Mol. Liq. 2018, 269, 140–151. 10.1016/j.molliq.2018.08.028. [DOI] [Google Scholar]
- Thakur S.; Sharma B.; Verma A.; Chaudhary J.; Tamulevicius S.; Thakur V. K. Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J. Cleaner Prod. 2018, 198, 143–159. 10.1016/j.jclepro.2018.06.259. [DOI] [Google Scholar]
- Godiya C. B.; Cheng X.; Li D.; Chen Z.; Lu X. L. Carboxymethyl cellulose/polyacrylamide composite for cascaded treatment/reuse of heavy metal ions in wastewater. J. Hazard. Mater. 2019, 364, 28–38. 10.1016/j.jhazmat.2018.09.076. [DOI] [PubMed] [Google Scholar]
- Zhao Z. F.; Zhang X.; Zhou H. J.; Liu G.; Kong M. G.; Wang G. Z. Microwave-assisted synthesis of magnetic Fe3O4-mesoporous magnesium silicate core-shell composites for the removal of heavy metal ions. Microporous Mesoporous Mater. 2017, 242, 50–58. 10.1016/j.micromeso.2017.01.006. [DOI] [Google Scholar]
- Sun J. H.; Chen Y.; Yu H. Q.; Yan L. G.; Du B.; Pei Z. G. Removal of Cu2+, Cd2+ and Pb2+ from aqueous solutions by magnetic alginate microsphere based on Fe3O4/MgAl-layered double hydroxide. J. Colloid Interface Sci. 2018, 532, 474–484. 10.1016/j.jcis.2018.07.132. [DOI] [PubMed] [Google Scholar]
- Reddy K. R.; Lee K. P.; Gopalan A. I. Self-assembly approach for the synthesis of electro-magnetic functionalized Fe3O4/polyaniline nanocomposites: Effect of dopant on the properties. Colloids Surf., A 2008, 320, 49–56. 10.1016/j.colsurfa.2007.12.057. [DOI] [Google Scholar]
- Zargoosh K.; Abedini H.; Abdolmaleki A.; Molavian M. R. Effective Removal of Heavy Metal Ions from Industrial Wastes Using Thiosalicyl hydrazide-Modified Magnetic Nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 14944–14954. 10.1021/ie401971w. [DOI] [Google Scholar]
- Ren Y.; Abbood H. A.; He F. B.; Peng H.; Huang K. X. Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: Preparation, characterization, and application in heavy metal adsorption. Chem. Eng. J. 2013, 226, 300–311. 10.1016/j.cej.2013.04.059. [DOI] [Google Scholar]
- Song Y. C.; Yang L. Y.; Wang Y. G.; Yu D.; Shen J. F.; Ouyang X. K. Highly efficient adsorption of Pb(II) from aqueous solution using amino-functionalized SBA-15/calcium alginate microspheres as adsorbent. Int. J. Biol. Macromol. 2019, 125, 808–819. 10.1016/j.ijbiomac.2018.12.112. [DOI] [PubMed] [Google Scholar]
- Hua R.; Li Z. K. Sulfhydryl functionalized hydrogel with magnetism: synthesis, characterization, and adsorption behavior study for heavy metal removal. Chem. Eng. J. 2014, 249, 189–200. 10.1016/j.cej.2014.03.097. [DOI] [Google Scholar]
- Zhang Y. W.; Zhang M.; Yang J. B.; Ding L.; Zheng J.; Xu J. L.; Xiong S. L. Formation of Fe3O4@SiO2@C/Ni hybrids with enhanced catalytic activity and histidine-rich protein separation. Nanoscale 2016, 8, 15978–15988. 10.1039/C6NR05078F. [DOI] [PubMed] [Google Scholar]
- Huang B. Y.; Liu Y. G.; Li B.; Zeng G. M.; Hu X. J.; Zheng B. H.; Li T. T.; Jiang L. H.; Tan X. F.; Zhou L. Synthesis of graphene oxide decorated with core@double-shell nanoparticles and application for Cr(VI) removal. RSC Adv. 2015, 5, 106339–106349. 10.1039/c5ra22862j. [DOI] [Google Scholar]
- Irani M.; Ismail H.; Ahmad Z.; Fan M. H. Synthesis of linear low-density polyethylene-g-poly(acrylicacid)-co-starch/organo-montmorillonite hydrogel composite as an adsorbent for removal of Pb(II) from aqueous solutions. J. Environ. Sci. 2015, 27, 9–20. 10.1016/j.jes.2014.05.049. [DOI] [PubMed] [Google Scholar]
- Zhao F. P.; Tang W. Z.; Zhao D. B.; Meng Y.; Yin D. L.; Sillanpää M. Adsorption kinetics, isotherms and mechanisms of Cd(II), Pb(II), Co(II), and Ni(II) by a modified magnetic polyacrylamide microcomposite adsorbent. J. Water Process Eng. 2014, 4, 47–57. 10.1016/j.jwpe.2014.09.003. [DOI] [Google Scholar]
- Zhou G.; Luo J. M.; Liu C. B.; Chu L.; Ma J. H.; Tang Y. H.; Zeng Z. B.; Luo S. L. A highly efficient polyampholyte hydrogel sorbent based fixed-bed process for heavy metal removal in actual industrial effluent. Water Res. 2016, 89, 151–160. 10.1016/j.watres.2015.11.053. [DOI] [PubMed] [Google Scholar]
- He S. F.; Zhang F.; Cheng S. Z.; Wang W. Synthesis of Sodium Acrylate and Acrylamide Copolymer/GO Hydrogels and Their Effective Adsorption for Pb2+ and Cd2+. ACS Sustainable Chem. Eng. 2016, 4, 3948–3959. 10.1021/acssuschemeng.6b00796. [DOI] [Google Scholar]
- Liang X. X.; Ouyang X.-K.; Wang S. Y.; Yang L.-Y.; Huang F. F.; Ji C.; Chen X. H. Efficient adsorption of Pb(II) from aqueous solutions using aminopropyltriethoxysilane-modified magnetic attapulgite@chitosan (APTS-Fe3O4/APT@CS) composite hydrogel beads. Int. J. Biol. Macromol. 2019, 137, 741–750. 10.1016/j.ijbiomac.2019.06.244. [DOI] [PubMed] [Google Scholar]
- Chu L.; Liu C. B.; Zhou G. Y.; Xu R.; Tang Y. H.; Zeng Z. B.; Luo S. L. A double network gel as low cost and easy recycle adsorbent: highly efficient removal of Cd(II) and Pb(II) pollutants from wastewater. J. Hazard. Mater. 2015, 300, 153–160. 10.1016/j.jhazmat.2015.06.070. [DOI] [PubMed] [Google Scholar]
- Zhou G. Y.; Liu C. B.; Tang Y. H.; Luo S. L.; Zeng Z. B.; Liu Y. T.; Xu R.; Chu L. Sponge-like polysiloxane-graphene oxide gel as a highly efficient and renewable adsorbent for lead and cadmium metals removal from wastewater. Chem. Eng. J. 2015, 280, 275–282. 10.1016/j.cej.2015.06.041. [DOI] [Google Scholar]
- Tan P.; Sun J.; Hu Y. Y.; Fang Z.; Bi Q.; Chen Y. C.; Cheng J. H. Adsorption of Cu2+, Cd2+ and Ni2+ from aqueous single metal solutions on graphene oxide membranes. J. Hazard. Mater. 2015, 297, 251–260. 10.1016/j.jhazmat.2015.04.068. [DOI] [PubMed] [Google Scholar]
- Madadrang C. J.; Kim H. Y.; Gao G. H.; Wang N.; Zhu J.; Feng H.; Gorring M.; Kasner M. L.; Hou S. F. Adsorption Behavior of EDTA-Graphene Oxide for Pb(II) Removal. ACS Appl. Mater. Interfaces 2012, 4, 1186–1193. 10.1021/am201645g. [DOI] [PubMed] [Google Scholar]
- Li X. L.; Qi Y. X.; Li Y. F.; Zhang Y.; He X. H.; Wang Y. H. Novel magnetic beads based on sodium alginate gel crosslinked by zirconium(IV) and their effective removal for Pb2+ in aqueous solutions by using a batch and continuous systems. Bioresour. Technol. 2013, 142, 611–619. 10.1016/j.biortech.2013.05.081. [DOI] [PubMed] [Google Scholar]
- Hui B.; Zhang Y.; Ye L. Structure of PVA/gelatin hydrogel beads and adsorption mechanism for advanced Pb(II) removal. J. Ind. Eng. Chem. 2015, 21, 868–876. 10.1016/j.jiec.2014.04.025. [DOI] [Google Scholar]
- Karthik R.; Meenakshi S. Removal of Cr(VI) ions by adsorption onto sodium alginate-polyaniline nanofbers. Int. J. Biol. Macromol. 2015, 72, 711–717. 10.1016/j.ijbiomac.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Irene Dias da Silva L.; Pontes F. V. M.; Carneiro M. C.; Monteiro M. I. C.; Almeida M. D. D.; Neto A. A. Evaluation of the chromium bioavailability in tanned leather shavings using the SM&T sequential extractions scheme. Chem. Speciation Bioavailability 2011, 23, 183–187. 10.3184/095422911X13027118597382. [DOI] [Google Scholar]
- Zhu Q.; Li Z. K. Hydrogel supported nanosized hydrous manganese dioxide: Synthesis, characterization, and adsorption behavior study for Pb2+, Cu2+, Cd2+ and Ni2+ removal from water. Chem. Eng. J. 2015, 281, 69–80. 10.1016/j.cej.2015.06.068. [DOI] [Google Scholar]
- Yu J.; Lu Q. F.; Zheng J. D.; Li Y. Chitosan/attapulgite/poly(acrylic acid) hydrogel prepared by glow-discharge electrolysis plasma as a reusable adsorbent for selective removal of Pb2+ ions. Iran. Polym. J. 2019, 28, 881–893. 10.1007/s13726-019-00751-1. [DOI] [Google Scholar]
- Li B.; Qiao Y. S.; An J. H.; Shen L. Z.; Ma Q.; Guo Y. Synthesis, characterisation, and evaluation of core-shell Fe3O4/SiO2/polypyrrole composite nanoparticles. Micro Nano Lett. 2018, 13, 902–906. 10.1049/mnl.2017.0907. [DOI] [Google Scholar]
- Lu Z. Y.; Dai J.; Song X. G.; Wang G.; Yang W. S. Facile synthesis of Fe3O4/SiO2 composite nanoparticles from primary silica particles. Colloids Surf., A 2008, 317, 450–456. 10.1016/j.colsurfa.2007.11.020. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.