Abstract
Oxidative-alkaline leaching of vanadium from vanadium-chromium-reducing residues with K2Cr2O7 was investigated in this paper. The effects of processing parameters including dosage of NaOH, dosage of K2Cr2O7, reaction time, and reaction temperature on the leaching efficiency of vanadium were studied. The results simulated by response surface methodology indicated that vanadium leaching was affected significantly by the dosage of K2Cr2O7 and NaOH, and the processing parameters that affected the leaching efficiency of vanadium followed the order m(NaOH)/m(residue) > m(K2Cr2O7)/sssssm(residue) > reaction temperature > reaction time. The leaching efficiency of vanadium was up to 99.92% under optimal conditions: reaction temperature of 90 °C, reaction time of 60 min, liquid-to-solid ratio of 5:1 mL g–1, m(K2Cr2O7)/m(residue) = 0.10, and m(NaOH)/m(residue) = 0.30. The kinetics analysis indicated that diffusion through the product layer was the controlling step and the apparent activation energy for vanadium leaching was calculated to be 58.275 kJ·mol–1.
1. Introduction
Vanadium and its compounds are widely used in many fields due to their excellent properties.1−5 After recovery of vanadium from a vanadium-producing plant, a large amount of toxic wastewater containing vanadium (V) and chromium (VI) remains to be treated. Usually, some reductants are added followed by neutralization to form a vanadium-chromium-reducing residue (VCRR) in which low-valence vanadium (IV) accounts for 3–10 wt % and low-valence chromium (III) accounts for about 10 to 20 wt %. In China, every year, more than 500,000 tons of the vanadium-chromium-reducing residue is produced.6−8 The ineffective treatment of the VCRR results in wastage of vanadium and chromium resources and environmental pollution.
In order to leach out low-valence vanadium and chromium, some oxidative leaching technologies were used. Oxidants like KClO3, H2O2, and MnO2 were applied to enhance the leaching process.9−11 During the leaching process, the low-valance vanadium was brought into contact with an oxidant and was oxidized to high-valence vanadium in order to leach out. High-valence vanadium (99.80%) and 97.93% low-valence chromium were leached out during oxidative acid leaching with MnO2, but they were very hard to filter due to the high content of Si. H2O2 alkaline leaching is a very environment-friendly technology for vanadium and chromium leaching, except for its high cost. Submolten technology is used to recover vanadium with high alkalinity and energy consumption.12,13 Meanwhile, some supplementary methods have attracted more attention. Electro-oxidation technology was applied to enhance the leaching process both in acidic and alkaline media,14,15 which offered selectivity for vanadium leaching, although it was hard to industrialize. Roasting technology was the most common technology, including sodium-roasting, calcium-roasting, and blank-roasting; most vanadium and chromium were leached out, but some poisonous gases were emitted and the processes require high energy. Microwave-roasting and ultrasound-assisted leaching have also received more attention.16−21 Ion-exchange technology22−24 and solvent extraction technology25−27 were also investigated to further improve the recovery efficiency of vanadium. However, these processes were noneconomical and were only applied on a small scale.
This paper introduced a novel process for the recovery of vanadium from the vanadium-chromium-reducing residue. In this process, K2Cr2O7, which has high oxidizability, was introduced as an efficient oxidant for oxidation leaching of vanadium in alkaline medium. The redox product (Cr(III)) retained in the leaching residue could be used as a chromium source for chromium production. The effect of experimental parameters including reaction time, reaction temperature, mass ratio of NaOH to residue, and mass ratio of K2Cr2O7 to residue was studied. The leaching kinetics and also the optimal conditions for vanadium leaching were also investigated.
2. Results and Discussion
Vanadium exists as a low-valence element in the VCRR, and it is hard to leach out in alkaline medium directly.9 It is known that ECr2O7/Cr3+0 = 1.33 V and EVO2+/VO2+ = 1.00 V; therefore, K2Cr2O7 was used to oxidize low-valence vanadium during the leaching process. The ΔG of the main reactions were calculated at 298 K.28,29 The results displayed in Figure 1 show that the ΔG was negative, indicating that the oxidative leaching of low-valence vanadium with K2Cr2O7 was feasible.
Figure 1.
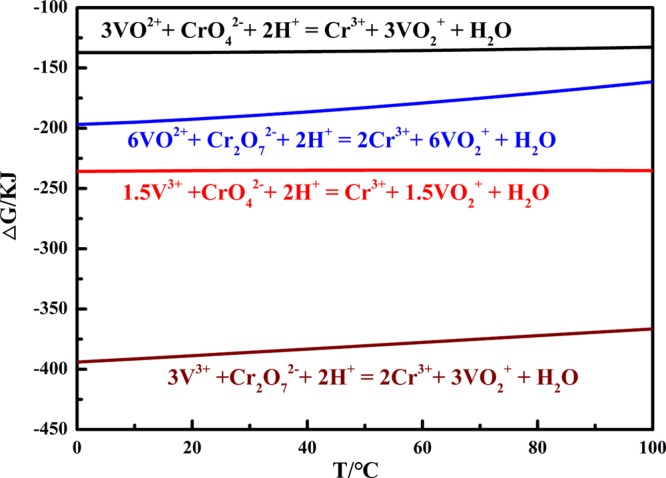
Relationship between ΔG and temperature for reaction equations.
2.1. Oxidation Leaching of Vanadium in Alkaline Medium
2.1.1. Effect of the Mass Ratio of K2Cr2O7 to the Residue
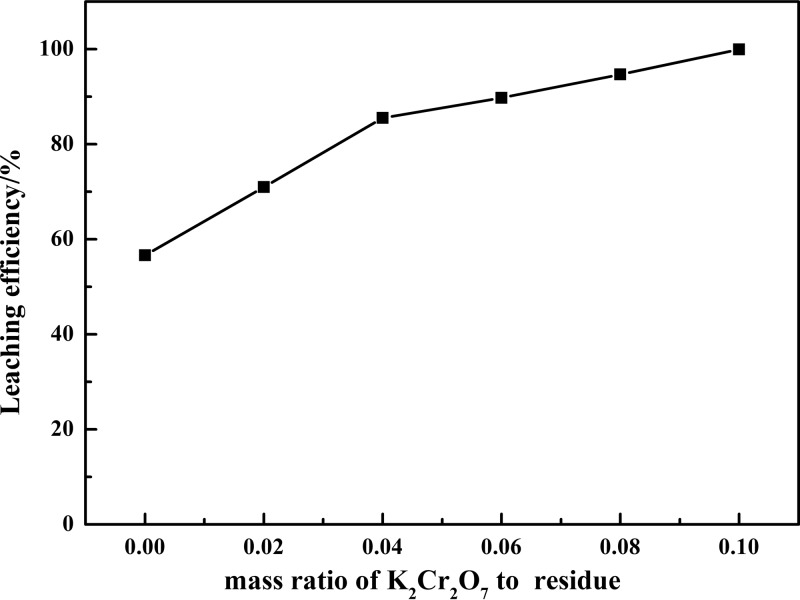
Based on the above analysis, the oxidation of low-valence vanadium with K2Cr2O7 was feasible thermodynamically. Thus, the dosage of K2Cr2O7 had a significant effect on the leaching efficiency of vanadium. Figure 2 shows the results of the effect of the mass ratio of K2Cr2O7 to residue (m(K2Cr2O7)/m(residue)) on the leaching efficiency of vanadium while other reaction conditions are kept constant: reaction time of 60 min, reaction temperature of 90 °C, liquid-to-solid ratio of 5:1 mL g–1, and mass ratio of NaOH to residue (m(NaOH)/m(residue)) = 0.3.
Figure 2.
Effect of the mass ratio of K2Cr2O7 to residue on the leaching efficiency of vanadium.
The results shown in Figure 2 indicate that the leaching efficiency increased with the increase of the dosage of K2Cr2O7. Only a small amount of vanadium leached out in alkaline medium without oxidation. During the leaching process, the low-valence vanadium made contact with K2Cr2O7 and was oxidized to high-valence vanadium (VO43–), which was easy to dissolve in alkaline medium and contributed to the high leaching efficiency of vanadium. Only 56.65% of the vanadium leached out without K2Cr2O7 and the leaching efficiency was up to 99.92% at m(K2Cr2O7)/m(residue) = 0.10. As the leaching solution had strong alkalinity, the Cr(III) in the VCRR and reduced K2Cr2O7 were retained in the leaching residue, and it acted as an efficient chromium source. Compared with oxidative leaching with H2O2,10,11,30 it offered selectivity for vanadium and separation of vanadium and chromium. The above results confirmed that K2Cr2O7 was an efficient oxidant for low-valence vanadium leaching, and m(K2Cr2O7)/m(residue) = 0.10 was chosen as the optimal condition for further experiments.
2.1.2. Effect of the Dosage of NaOH
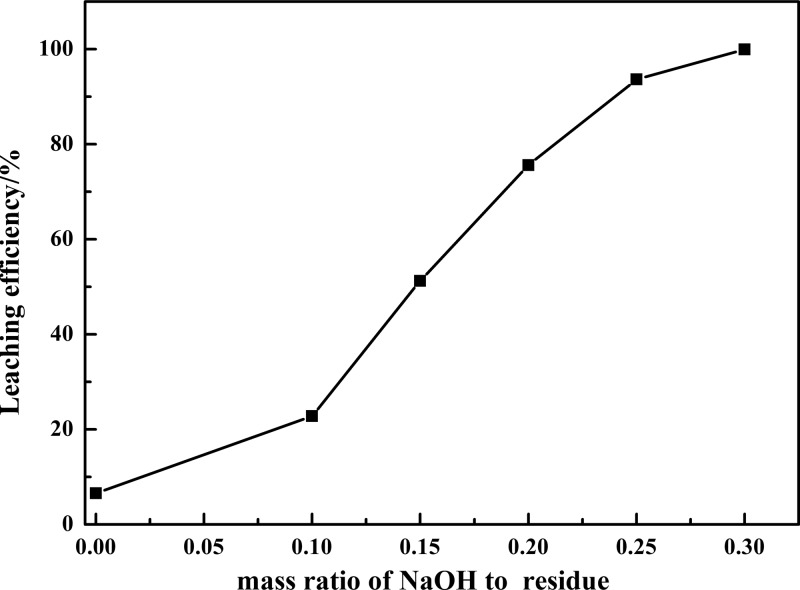
Alkaline leaching technology was preferred to acidic leaching technology in this paper as 11.30 wt % Si was present in the VCRR. The effect of mass ratio of NaOH to residue on the leaching efficiency of vanadium was investigated with the following reaction conditions: reaction time of 60 min, reaction temperature of 90 °C, liquid-to-solid ratio of 5:1 mL g–1, and m(K2Cr2O7)/m(residue) = 0.10. The m(NaOH)/m(residue) was set as 0.00, 0.10, 0.15, 0.20, 0.25, and 0.30.
Figure 3 shows that just 6.54% vanadium leached out in neutral medium and it increased up to 99.92% at m(NaOH)/m(residue) = 0.30. Low-valence vanadium was oxidized to high-valence vanadium by K2Cr2O7 in concentrated NaOH solution and achieved high leaching efficiency.
Figure 3.
Effect of the mass ratio of NaOH to residue on the leaching efficiency of vanadium.
2.1.3. Effect of Reaction Temperature
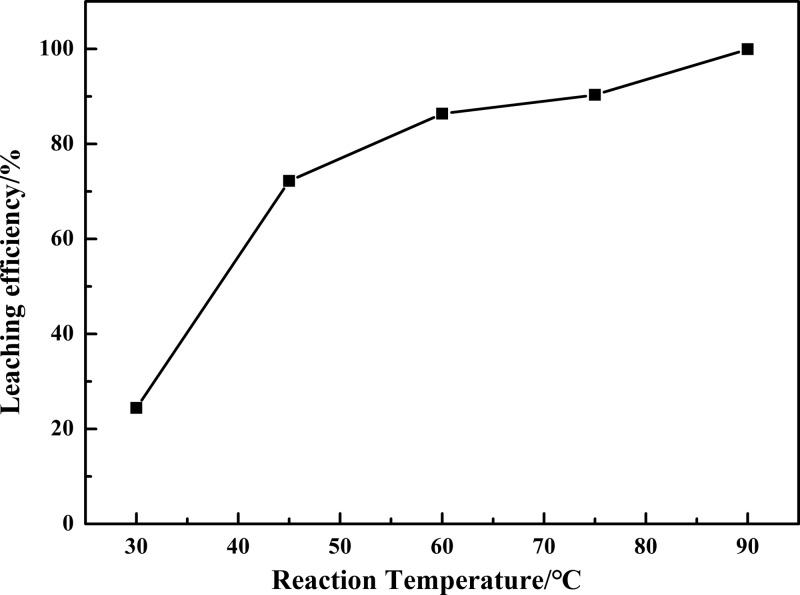
Higher reaction temperature often increases the activity of reagents, decreases the medium viscosity, and enforces the reaction intensity. Figure 4 shows the effect of reaction temperature on the leaching process under standard conditions: reaction time of 60 min, liquid-to-solid ratio of 5:1 mL g–1, m(K2Cr2O7)/m(residue) = 0.10, and m(NaOH)/m(residue) = 0.30. The leaching efficiency of vanadium was significantly improved at higher reaction temperature. The leaching efficiency increased from 24.39 to 99.92% as the reaction temperature increased from 30 to 90 °C. Therefore, 90 °C was selected for further experiments.
Figure 4.
Effect of reaction temperature on the leaching efficiency of vanadium.
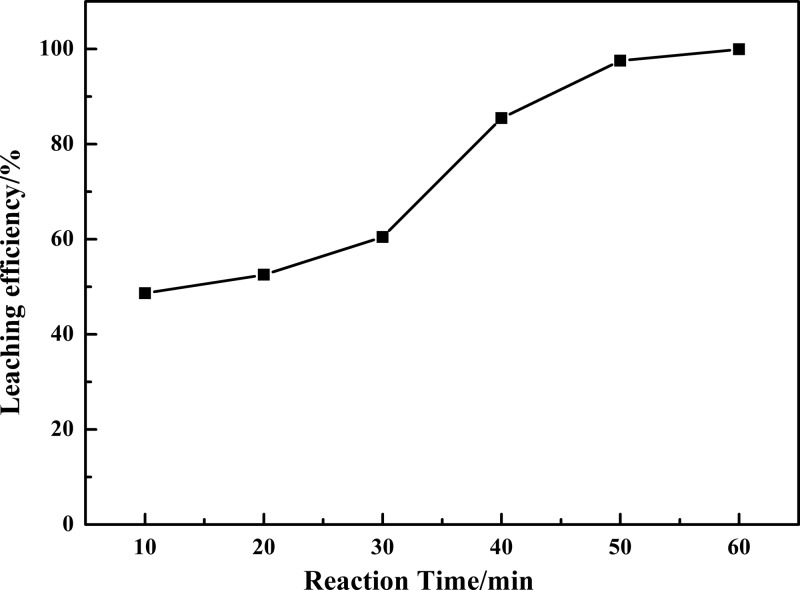
2.1.4. Effect of Reaction Time
Figure 5 shows the leaching efficiency of vanadium by varying the reaction time ranging from 10 to 60 min under selected conditions: reaction temperature of 90 °C, liquid-to-solid ratio of 5:1 mL g–1, m(K2Cr2O7)/m(residue) = 0.10, and m(NaOH)/m(residue) = 0.30. Further increasing the reaction time could increase the chances of contact of the VCRR with NaOH and K2Cr2O7, giving the low-valence vanadium in the VCRR enough time to be oxidized. Almost all of the vanadium was leached out within 60 min, and the leaching efficiency of vanadium increased from 48.64 to 99.92% as the reaction time increased from 10 to 60 min. Therefore, 60 min was sufficient to leach out vanadium.
Figure 5.
Effect of reaction time on the leaching efficiency of vanadium.
Above all, K2Cr2O7 was an efficient oxidant for low-valence vanadium leaching from the VCRR in an alkaline medium, and the leaching efficiency of vanadium was up to 99.92% under the optimal conditions: reaction temperature of 90 °C, reaction time of 60 min, liquid-to-solid ratio of 5:1 mL g–1, m(K2Cr2O7)/m(residue) = 0.10, and m(NaOH)/m(residue) = 0.30.
2.2. Response Surface Analysis
A single factor experiment was not enough to investigate the optimal reactions as it ignored the effects of the interactions between different factors.11 An effective method called response surface methodology was used to study the effects of interactions between different factors.31−33 In this study, response surface methodology simulated in Design-Expert software was used to optimize the reaction conditions. The operating parameters were set as: A: m(K2Cr2O7)/m(residue); B: m(NaOH)/m(residue); C: reaction temperature; D: reaction time, and the leaching efficiency of vanadium was set as a response. The actual values for each factor are detailed in Table 1.
Table 1. Levels for Parameters in Actual Values.
| level |
||||
|---|---|---|---|---|
| independent parameter | unit | –1 | 0 | 1 |
| A: m(K2Cr2O7)/m(residue) | 1 | 0 | 0.05 | 0.1 |
| B: m(NaOH)/m(residue) | 1 | 0.01 | 0.15 | 0.3 |
| C: reaction temperature | °C | 30 | 60 | 90 |
| D: reaction time | min | 10 | 30 | 60 |
The square root was used to express the simulated results for the leaching efficiency of vanadium after insignificant terms and it is presented in eq 1:
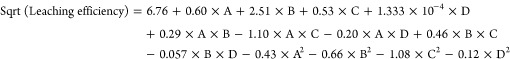 |
1 |
The analysis of variance results are detailed in Table 2. The F value and p value for the mode1 were 12.07 and <0.0001, respectively, which indicated that the model was significant and could be used to describe the optimization process.
Table 2. Analysis of Variance.
| source | S | Df | mean square | F value | p value probability |
|---|---|---|---|---|---|
| model | 106.62 | 14 | 7.62 | 12.07 | <0.0001 |
| residual | 8.84 | 14 | 0.63 | ||
| lack-of-fit | 8.27 | 10 | 0.83 | 5.89 | 0.0511 |
| pure error | 0.56 | 4 | 0.14 |
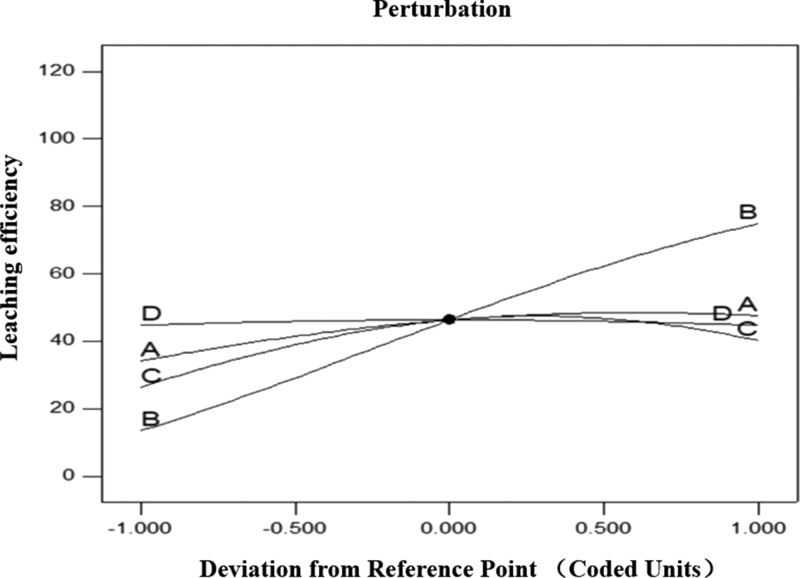
The coefficients before A, B, C, and D represent the direction and influence of the parameters on the response (Figure 6). Their coefficients were 0.60, 2.51, 0.53, and 1.333 × 10–4, respectively, which confirmed that all the processing factors had a positive effect on the leaching efficiency. Also, the influence of the processing factor on the obtained leaching efficiency response followed the order: m(NaOH)/m(residue) (B) > m(K2Cr2O7)/m(residue) (A) > reaction temperature (C) > reaction time (D). The increase in m(NaOH)/m(residue) increased the reaction activity of the hydroxide ions and enabled the oxidation reactions to be more thermodynamically favorable.10 It also intensified the oxidation solubility and mass transfer efficiency during the leaching process. In the H2O2-alkaline leaching process, the most significant factor was the dosage of H2O2, while the dosage of alkali occupied the third place.11 For the oxidative-leaching process, the influence order of the factors were not unalterable but dependent on the oxidant.
Figure 6.
Perturbation plot for the leaching efficiency of vanadium (A: m(K2Cr2O7)/m(residue); B: m(NaOH)/m(residue); C: reaction temperature; and D: reaction time).
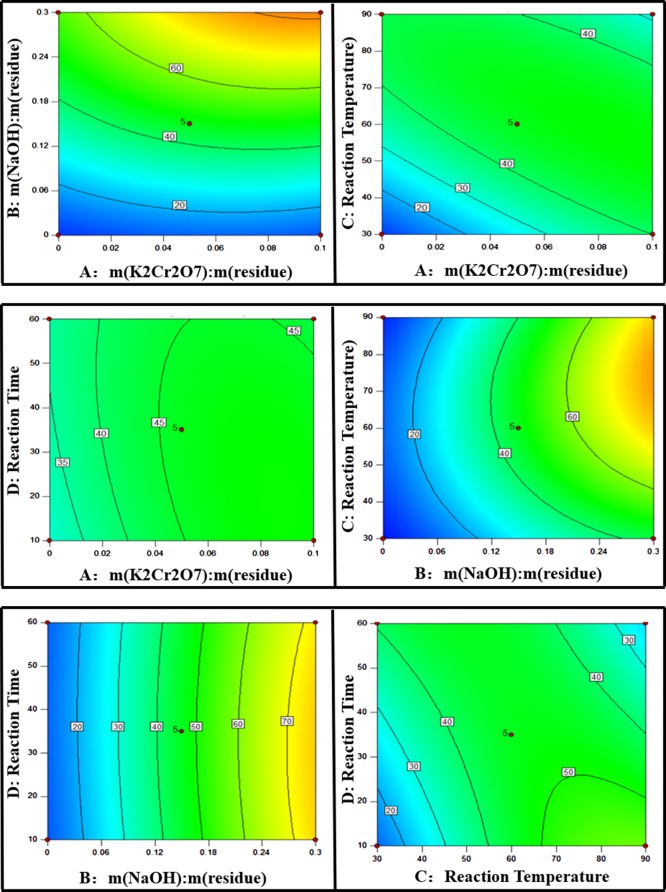
The contour plots were used to investigate the interaction between processing factors. The results detailed in Figure 7 show that the leaching efficiency of vanadium increased with the increase in the dosage of NaOH and K2Cr2O7.
Figure 7.
Response surface plots for factors.
2.3. Adsorption of Vanadium
Many technologies have been used to recover vanadium from solutions, such as hydrolysis, ammonium precipitation,34,35 solvent extraction,26,36−39 and ion exchange.23,24,40−44 In this work, adsorption with melamine was introduced to recover vanadium.45,46 The recovery of vanadium from the leaching solution was conducted by selective adsorption with melamine; almost all of the vanadium was adsorbed. The detailed adsorption conditions could be obtained from refs (50) and (51). Lastly, V2O5 was obtained by roasting the adsorption precipitate at 550 °C for 150 min in a muffle furnace.
2.4. Kinetics Analysis
The shrink core model is widely used to describe the kinetics behavior of the leaching process between solid and liquid reagents.14,47−50 The typical three kinetics models are represented in Table 3.
Table 3. Kinetics Models and Equationsa.
| controlling step | equations | ||
|---|---|---|---|
| diffusion through a liquid film |
|
||
| diffusion through a product layer |
|
||
| surface chemical reaction |
|
η is the leaching efficiency, K1, K2, and K3, are the apparent rate constants for three different models, min–1, and t is the reaction time, min.
The experimental data were simulated with the above three equations and the apparent rate constant and correlation coefficients are provided in Table 4. The results indicated that the regression coefficient of diffusion through the product layer was maximum, and it meant that diffusion through the product layer was the rate-controlling step. Therefore, eq 4 was selected to describe the leaching kinetics behavior of vanadium from the VCRR. The results shown in Figure 8 indicate that the apparent activation energy for vanadium leaching was 58.275 kJ·mol–1, which proposed that the leaching process was controlled mainly by diffusion of the solution through the surface layer, which was consistent with the results discussed above.
| 5 |
where Ea is the apparent activation energy, A is the pre-exponential factor, and R is the molar gas constant.
Table 4. Apparent Rate Constants K1, K2, and K3 for the Kinetics Models and Correlation Coefficients.
| diffusion
through the liquid film |
diffusion through the product layer |
surface chemical reaction |
||||
|---|---|---|---|---|---|---|
| η |
1 – 2/3η – (1 – η)2/3 |
1 – (1 – η)1/3 |
||||
| parameter (°C) | K1 (min–1) | R2 | K2 (min–1) | R2 | K3 (min–1) | R2 |
| 30 | 0.0032 | 0.9596 | 0.00013 | 0.9950 | 0.01807 | 0.9377 |
| 45 | 0.0090 | 0.9699 | 0.00067 | 0.9960 | 0.02306 | 0.9273 |
| 60 | 0.0101 | 0.9428 | 0.00280 | 0.9846 | 0.02247 | 0.9203 |
| 75 | 0.0108 | 0.9524 | 0.00348 | 0.9928 | 0.02279 | 0.9344 |
| 90 | 0.0119 | 0.9207 | 0.00635 | 0.9843 | 0.02311 | 0.8866 |
Figure 8.
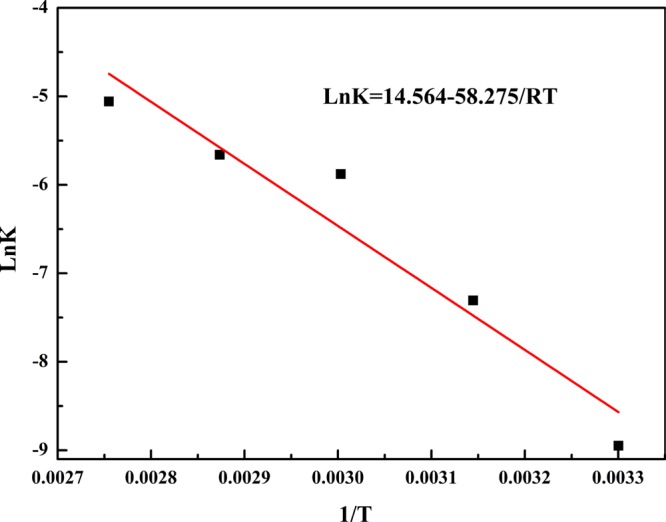
Natural logarithm of the reaction rate constant versus reciprocal temperature for the leaching process.
3. Conclusions
This paper focused on the oxidative leaching of vanadium with K2Cr2O7 from vanadium-chromium-reducing residues in alkaline medium. The conclusions are given as follows:
-
(1)
The optimal processing factors were obtained from response surface methodology, and the influence of processing parameters on the leaching efficiency of vanadium followed the order m(NaOH)/m(residue) (B) > m(K2Cr2O7)/m(residue) (A) > reaction temperature (C) > reaction time (D). Under optimal conditions, leaching efficiency of vanadium was up to 99.92% (reaction temperature of 90 °C, reaction time of 60 min, liquid-to-solid ratio of 5:1 mL g–1, m(K2Cr2O7)/m(residue) = 0.10, and m(NaOH)/m(residue) = 0.30).
-
(2)
The diffusion through the product layer was the rate-controlling step, and the leaching kinetics behaviors followed the shrink core model. The apparent activation energy for vanadium leaching was calculated to be 58.275 kJ·mol–1.
4. Materials and Methods
4.1. Materials
The VCRR was dried and ground to a suitable particle size before each experiment. The X-ray fluorescence (XRF) (XRF-1800, Shimadzu, Japan) and X-ray diffraction (XRD) (XRD-6000, Shimadzu, Japan) results indicated that the VCRR contained 18.80% Cr, 11.30% Si, and 3.11% V, and the main phases were FeCr2O4, NaCr(SO4)2, Na2(Cr,V)Si2O9, and VOSO4.14,30 All the reagents used in the experiments were of analytical grade, which were purchased from Kelong Co., Ltd., Chengdu, China, and used as received without purification.
4.2. Experimental Procedure
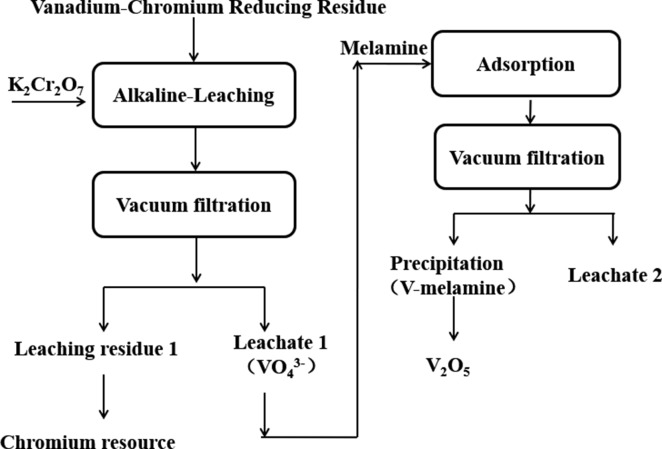
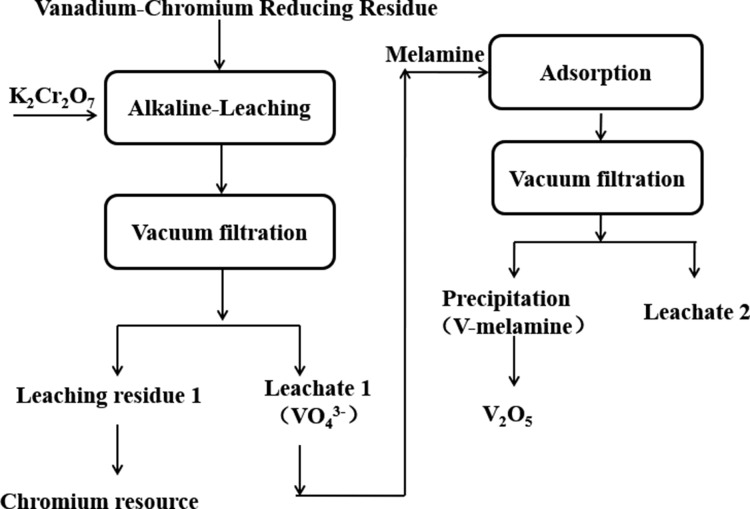
The experiments were performed according to the flow sheet as shown in Figure 9, which includes the procedures of oxidation leaching of vanadium and adsorption of vanadium. All experiments were performed in a glass beaker (250 mL) with a thermostatic mixing water bath pot. The detailed experimental procedure could be seen in references (10)(11), and (51).
Figure 9.
Flow sheet of recovery of vanadium.
Acknowledgments
This work was supported by the Science and Technology Research Program of Chongqing Municipal Education Commission (no. KJQN201901403) and Chongqing Science and Technology Commission (no. cstc2018jcyjAX0018).
The authors declare no competing financial interest.
References
- Wen J.; Ning P.; Cao H.; Zhao H.; Sun Z.; Zhang Y. Novel method for characterization of aqueous vanadium species: A perspective for the transition metal chemical speciation studies. J. Hazard. Mater. 2019, 364, 91–99. 10.1016/j.jhazmat.2018.09.069. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Luo D.; Deng C.; Lv L.; Liang B.; Li C. Simultaneous extraction of vanadium and titanium from vanadium slag using ammonium sulfate roasting-leaching process. J. Alloys Compd. 2018, 742, 504–511. 10.1016/j.jallcom.2018.01.300. [DOI] [Google Scholar]
- Yan B.; Wang D.; Wu L.; Dong Y. A novel approach for pre-concentrating vanadium from stone coal ore. Miner. Eng. 2018, 125, 231–238. 10.1016/j.mineng.2018.06.005. [DOI] [Google Scholar]
- Choi C.; Kim S.; Kim R.; Choi Y.; Kim S.; Jung H.-Y.; Yang J. H.; Kim H.-T. A review of vanadium electrolytes for vanadium redox flow batteries. Renew. Sust. Energ. Rev. 2017, 69, 263–274. 10.1016/j.rser.2016.11.188. [DOI] [Google Scholar]
- Cai Z.; Zhang Y.; Liu T.; Huang J. Mechanisms of Vanadium Recovery from Stone Coal by Novel BaCO3/CaO Composite Additive Roasting and Acid Leaching Technology. Minerals 2016, 6, 26. 10.3390/min6020026. [DOI] [Google Scholar]
- Peng H. A literature review on leaching and recovery of vanadium. J. Environ. Chem. Eng. 2019, 7, 103313. 10.1016/j.jece.2019.103313. [DOI] [Google Scholar]
- Wang X.; Gao D.; Chen B.; Meng Y.; Fu Z.; Wang M. A clean metallurgical process for separation and recovery of vanadium and chromium from V-Cr-bearing reducing slag. Hydrometallurgy 2018, 181, 1–6. 10.1016/j.hydromet.2018.08.008. [DOI] [Google Scholar]
- Wang M.; Chen B.; Huang S.; Wang X.; Liu B.; Ge Q.; Xie S. A novel technology for vanadium and chromium recovery from V-Cr-bearing reducing slag. Hydrometallurgy 2017, 171, 116–122. 10.1016/j.hydromet.2017.05.007. [DOI] [Google Scholar]
- Yang K.; Zhang X.; Tian X.; Yang Y.; Chen Y. Leaching of vanadium from chromium residue. Hydrometallurgy 2010, 103, 7–11. 10.1016/j.hydromet.2010.02.006. [DOI] [Google Scholar]
- Peng H.; Liu Z.; Tao C. A green method to leach vanadium and chromium from residue using NaOH-H2O2. Sci. Rep. 2018, 8, 426. 10.1038/s41598-017-18918-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H.; Wang F.; Li G.; Guo J.; Li B. Highly Efficient Recovery of Vanadium and Chromium: Optimized by Response Surface Methodology. ACS Omega 2019, 4, 904–910. 10.1021/acsomega.8b02708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.-B.; Zheng S.-L.; Zhang Y.; Li Z.-H.; Wang Z.-K. Oxidative leaching of a Vietnamese chromite ore in highly concentrated potassium hydroxide aqueous solution at 300°C and atmospheric pressure. Miner. Eng. 2005, 18, 527–535. 10.1016/j.mineng.2004.08.002. [DOI] [Google Scholar]
- Liu B.; du H.; Wang S.-N.; Zhang Y.; Zheng S.-L.; Li L.-J.; Chen D.-H. A novel method to extract vanadium and chromium from vanadium slag using molten NaOH-NaNO3binary system. AIChE J. 2013, 59, 541–552. 10.1002/aic.13819. [DOI] [Google Scholar]
- Peng H.; Liu Z.; Tao C. Selective leaching of vanadium from chromium residue intensified by electric field. J. Environ. Chem. Eng. 2015, 3, 1252–1257. 10.1016/j.jece.2015.03.031. [DOI] [Google Scholar]
- Liu Z.; Nueraihemaiti A.; Chen M.; Du J.; Fan X.; Tao C. Hydrometallurgical leaching process intensified by an electric field for converter vanadium slag. Hydrometallurgy 2015, 155, 56–60. 10.1016/j.hydromet.2015.04.005. [DOI] [Google Scholar]
- Rahimi G.; Rastegar S. O.; Rahmani Chianeh F.; Gu T. Ultrasound-assisted leaching of vanadium from fly ash using lemon juice organic acids. RSC Adv. 2020, 10, 1685. 10.1039/C9RA09325G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L.; Xu Z.; Chen L.; Liu Y.; Zhang T.-A. Effect of microwave heating on the pressure leaching of vanadium from converter slag. Hydrometallurgy 2019, 184, 45–54. 10.1016/j.hydromet.2018.11.004. [DOI] [Google Scholar]
- Xiao Q.; Yan H.; Wei Y.; Wang Y.; Zeng F.; Zheng X. Optimization of H2O2 dosage in microwave-H2O2 process for sludge pretreatment with uniform design method. J. Environ. Sci. 2012, 24, 2060–2067. 10.1016/S1001-0742(11)60998-4. [DOI] [PubMed] [Google Scholar]
- Ibrahim Turgut H.; Eyupoglu V.; Ali Kumbasar R. The comprehensive investigation of the room temperature ionic liquid additives in PVC based polymer inclusion membrane for Cr(VI) transport. J. Vinyl Addit. Technol. 2019, 25, E107–E119. 10.1002/vnl.21649. [DOI] [Google Scholar]
- Huang X.; Zhang J.; Peng K.; Na Y.; Xiong Y.; Liu W.; Liu J.; Lu L.; Li S. Functional magnetic nanoparticles for enhancing ultrafiltration of waste cutting emulsions by significantly increasing flux and reducing membrane fouling. J. Membr. Sci. 2019, 573, 73–84. 10.1016/j.memsci.2018.11.074. [DOI] [Google Scholar]
- Turgut H. I.; Eyupoglu V.; Kumbasar R. A.; Sisman I. Alkyl chain length dependent Cr(VI) transport by polymer inclusion membrane using room temperature ionic liquids as carrier and PVDF-co-HFP as polymer matrix. Sep. Purif. Technol. 2017, 175, 406–417. 10.1016/j.seppur.2016.11.056. [DOI] [Google Scholar]
- Zhu X.; Li W.; Tang S.; Zeng M.; Bai P.; Chen L. Selective recovery of vanadium and scandium by ion exchange with D201 and solvent extraction using P507 from hydrochloric acid leaching solution of red mud. Chemosphere 2017, 175, 365–372. 10.1016/j.chemosphere.2017.02.083. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Wang X.; Wang M. Separation and recovery of chromium and vanadium from vanadium-containing chromate solution by ion exchange. Hydrometallurgy 2013, 136, 31–35. 10.1016/j.hydromet.2013.03.008. [DOI] [Google Scholar]
- Zeng L.; Li Q.; Xiao L.; Zhang Q. A study of the vanadium species in an acid leach solution of stone coal using ion exchange resin. Hydrometallurgy 2010, 105, 176–178. 10.1016/j.hydromet.2010.07.001. [DOI] [Google Scholar]
- Ye G.; Hu Y.; Tong X.; Lu L. Extraction of vanadium from direct acid leaching solution of clay vanadium ore using solvent extraction with N235. Hydrometallurgy 2018, 177, 27–33. 10.1016/j.hydromet.2018.02.004. [DOI] [Google Scholar]
- Yang X.; Zhang Y.; Bao S.; Shen C. Separation and recovery of vanadium from a sulfuric-acid leaching solution of stone coal by solvent extraction using trialkylamine. Sep. Purif. Technol. 2016, 164, 49–55. 10.1016/j.seppur.2016.03.021. [DOI] [Google Scholar]
- Tavakoli M. R.; Dreisinger D. B. Separation of vanadium from iron by solvent extraction using acidic and neutral organophosporus extractants. Hydrometallurgy 2014, 141, 17–23. 10.1016/j.hydromet.2013.10.008. [DOI] [Google Scholar]
- Peng H.; Guo J.; Liu Z.; Tao C. Direct advanced oxidation process for chromium(III) with sulfate free radicals. SN Appl. Sci. 2019, 1, 14. 10.1007/s42452-018-0020-0. [DOI] [Google Scholar]
- Peng H.; Guo J.; Li G.; Cheng Q.; Zhou Y.; Liu Z.; Tao C. Highly efficient oxidation of chromium (III) with hydrogen peroxide in alkaline medium. Water Sci. Technol. 2019, 79, 366–374. 10.2166/wst.2019.056. [DOI] [PubMed] [Google Scholar]
- Peng H.; Liu Z.; Tao C. Leaching Kinetics of Vanadium with Electro-oxidation and H2O2 in Alkaline Medium. Energ. Fuel 2016, 30, 7802–7807. 10.1021/acs.energyfuels.6b01364. [DOI] [Google Scholar]
- Yuan Y.; Tan L.; Xu Y.; Dong J.; Zhao Y.; Yuan Y. Optimization of Processing Parameters for Lettuce Vacuum Osmotic Dehydration Using Response Surface Methodology. Pol. J. Food Nutr. Sci. 2018, 68, 15. 10.1515/pjfns-2017-0013. [DOI] [Google Scholar]
- Nawaz H.; Shad M. A.; Rauf A. Optimization of extraction yield and antioxidant properties of Brassica oleracea Convar Capitata Var L. leaf extracts. Food Chem. 2018, 242, 182–187. 10.1016/j.foodchem.2017.09.041. [DOI] [PubMed] [Google Scholar]
- Mat Rosid S. J.; Wan Abu Bakar W. A.; Ali R. Characterization and modelling optimization on methanation activity using Box-Behnken design through cerium doped catalysts. J. Cleaner Prod. 2018, 170, 278–287. 10.1016/j.jclepro.2017.09.073. [DOI] [Google Scholar]
- Wen J.; Jiang T.; Zhou W.; Gao H.; Xue X. A cleaner and efficient process for extraction of vanadium from high chromium vanadium slag: Leaching in (NH4)2SO4-H2SO4 synergistic system and NH4+ recycle. Sep. Purif. Technol. 2019, 216, 126–135. 10.1016/j.seppur.2019.01.078. [DOI] [Google Scholar]
- Peng H.; Yang L.; Wang L.; Guo J.; Li B. Recovery of vanadium with urea in acidic medium. Environ. Chem. Lett. 2019, 1867. 10.1007/s10311-019-00902-z. [DOI] [Google Scholar]
- Navarro R.; Guzman J.; Saucedo I.; Revilla J.; Guibal E. Vanadium recovery from oil fly ash by leaching, precipitation and solvent extraction processes. Waste Manag. 2007, 27, 425–438. 10.1016/j.wasman.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Li X.-B.; Wei C.; Wu J.; Li C.-X.; Li M.-T.; Deng Z.-G.; Xu H.-S. Thermodynamics and mechanism of vanadium(IV) extraction from sulphate medium with D2EHPA, EHEHPA and CYANEX 272 in kerosene. Trans. Nonferrous Met. Soc. China 2012, 22, 461–466. 10.1016/S1003-6326(11)61199-0. [DOI] [Google Scholar]
- Cai Z. L.; Feng Y. L.; Zhou Y. Z.; Li H. R.; Wang W. D. Selective Separation and Extraction of Vanadium (V) Over Manganese (II) from Co-Leaching Solution of Roasted Stone Coal and Pyrolusite Using Solvent Extraction. J. Geodesy 2013, 65, 1492–1498. 10.1007/s11837-013-0768-z. [DOI] [Google Scholar]
- Zhao J.; Hu Q.; Li Y.; Liu H. Efficient separation of vanadium from chromium by a novel ionic liquid-based synergistic extraction strategy. Chem. Eng. J. 2015, 264, 487–496. 10.1016/j.cej.2014.11.071. [DOI] [Google Scholar]
- Zhu X.-Z.; Huo G.-S.; Ni J.; Song Q. Removal of tungsten and vanadium from molybdate solutions using ion exchange resin. Trans. Nonferrous Met. Soc. China 2017, 27, 2727–2732. 10.1016/S1003-6326(17)60301-7. [DOI] [Google Scholar]
- Nguyen T. H.; Lee M. S. Separation of molybdenum and vanadium from acid solutions by ion exchange. Hydrometallurgy 2013, 136, 65–70. 10.1016/j.hydromet.2013.03.007. [DOI] [Google Scholar]
- Mazurek K. Recovery of vanadium, potassium and iron from a spent vanadium catalyst by oxalic acid solution leaching, precipitation and ion exchange processes. Hydrometallurgy 2013, 134-135, 26–31. 10.1016/j.hydromet.2013.01.011. [DOI] [Google Scholar]
- Wang X.; Xiao C.; Wang M.; Xiao W. Removal of silicon from vanadate solution using ion exchange and sodium alumino-silicate precipitation. Hydrometallurgy 2011, 107, 133–136. 10.1016/j.hydromet.2011.02.001. [DOI] [Google Scholar]
- Wang X.; Wang M.; Shi L.; Hu J.; Qiao P. Recovery of vanadium during ammonium molybdate production using ion exchange. Hydrometallurgy 2010, 104, 317–321. 10.1016/j.hydromet.2010.06.012. [DOI] [Google Scholar]
- Peng H.; Liu Z.; Tao C. Adsorption Process of Vanadium (V) with Melamine. Water, Air, Soil Pollut. 2017, 228, 272. 10.1007/s11270-017-3452-z. [DOI] [Google Scholar]
- Peng H.; Liu Z.; Tao C. Adsorption kinetics and isotherm of vanadium with melamine. Water Sci. Technol. 2017, 75, 2316–2321. 10.2166/wst.2017.094. [DOI] [PubMed] [Google Scholar]
- Levenspiel O.Chemical Reaction Engineering; Wiley, 1962. [Google Scholar]
- Liu H.-B.; du H.; Wang D.-W.; Wang S.-N.; Zheng S.-L.; Zhang Y. Kinetics analysis of decomposition of vanadium slag by KOH sub-molten salt method. Trans. Nonferrous Met. Soc. China 2013, 23, 1489–1500. 10.1016/S1003-6326(13)62621-7. [DOI] [Google Scholar]
- Qiu S.; Wei C.; Li M.; Zhou X.; Li C.; Deng Z. Dissolution kinetics of vanadium trioxide at high pressure in sodium hydroxide–oxygen systems. Hydrometallurgy 2011, 105, 350–354. 10.1016/j.hydromet.2010.08.005. [DOI] [Google Scholar]
- Szymczycha-Madeja A. Kinetics of Mo, Ni, V and Al leaching from a spent hydrodesulphurization catalyst in a solution containing oxalic acid and hydrogen peroxide. J. Hazard. Mater. 2011, 186, 2157–2161. 10.1016/j.jhazmat.2010.11.120. [DOI] [PubMed] [Google Scholar]
- Peng H.; Yang L.; Chen Y.; Guo J.; Li B. Recovery and Separation of Vanadium and Chromium by Two-Step Alkaline Leaching Enhanced with an Electric Field and H2O2. ACS Omega 2020, 5340. 10.1021/acsomega.9b04346. [DOI] [PMC free article] [PubMed] [Google Scholar]