Abstract
Electrospun materials made from biodegradable polycaprolactone are used widely in various tissue engineering and regenerative medicine applications because of their morphological similarity to the extracellular matrix. However, the main prerequisite for the use of such materials in clinical practice consists of the selection of the appropriate sterilization technique. This study is devoted to the study of the impact of traditional sterilization and disinfection methods on a nanofibrous polycaprolactone layer constructed by means of the needleless electrospinning technique. It was determined that hydrogen peroxide plasma treatment led to the loss of fibrous morphology and the creation of a foil. However, certain sterilization (ethylene oxide, gamma irradiation, and peracetic acid) and disinfection techniques (ethanol and UV irradiation) were found not to lead to a change in morphology; thus, the study investigates their impact on thermal properties, molecular weight, and interactions with a fibroblast cell line. It was determined that the surface properties that guide cell adhesion and proliferation were affected more than the bulk properties. The highest proliferation rate of fibroblasts seeded on nanofibrous scaffolds was observed with respect to gamma-irradiated polycaprolactone, while the lowest proliferation rate was observed following ethylene oxide sterilization.
Introduction
Biomaterials used in the field of medicine must fulfill a wide range of requirements including sterility, which is defined as a process that destroys or eliminates all forms of microbial life and is accomplished in health-care facilities via the application of both physical and chemical methods.1 The sterility of the final material is usually achieved by means of the use of one of two methods, that is aseptic fabrication in the case of, for example, bioprinted scaffolds or terminal sterilization.2 Disinfection is sometimes mistakenly confused with the term sterilization; however, it is important to bear in mind that disinfection describes a process that eliminates many or all pathogenic microorganisms, with the exception of bacterial spores, on inanimate objects. The efficacy of both sterilization and disinfection procedures is dependent on the purity of the material, its chemical and physical properties, microbial contamination, and the sterilization/disinfection process parameters such as the concentration of germicide, exposure time, temperature, pH, and relative humidity.1
The autoclaving, ethylene oxide (EtOx), gamma radiation, peracetic acid (PAA), and hydrogen peroxide plasma techniques all constitute commonly applied approaches to the sterilization of tissue engineering scaffolds. Although autoclaving can be used for the sterilization of thermoresistant polymers such as silk fibroin3,4 and polyvinylidene fluoride,5 as the majority of polymeric materials are thermosensitive, low-temperature sterilization approaches must be employed such as the widely used EtOx chemical low-temperature sterilization method. However, even though the EtOx technique is seen as particularly suitable for the sterilization of heat- and moisture-sensitive materials, its disadvantages lie in its relatively high cost and the potential hazard it poses to medical staff, that is, EtOx is known to be a human carcinogen; thus, it is particularly important that EtOx residuals be provided with a sufficient period of aeration as defined by ISO 10993-7:2008. Gamma irradiation is also widely used for the sterilization of biomaterials at a minimum recommended dose of 25 kGy; however, its sterilization parameters must be optimized for each material separately.6 No FDA recommendations yet exist with respect to ionizing radiation sterilization. The advantage of gamma sterilization lies in its being a large-scale low temperature process,1 however, its disadvantages consist of its high cost and the change it exerts in the polymeric structure in terms of chain scission and/or crosslinking.7 PAA is used for sterilization purposes in concentrations of 50–10,000 ppm (0.05–1%) and a time exposure of 15–30 min1 The advantages of PAA sterilization lie in its low cost and the rapid and effective processing of heat-sensitive polymers.8 Hydrogen peroxide gas plasma can also be used for the sterilization of thermosensitive polymers because the temperature attained during the sterilization cycle varies between 37 °C and a maximum of 44 °C; moreover, aeration is not necessary because the byproducts of the cycle are nontoxic. Disinfection techniques, particularly ethanol soaking and ultraviolet (UV) radiation, are widely applied in the development of novel biomaterials prior to in vitro testing; ethanol solutions in the concentration range 60–90% in water and UV radiation in wavelengths of between 240 and 280 nm are known to possess bactericidal properties.1
Tissue engineering and the development of the appropriate scaffolds for the regeneration of the target tissues requires the mimicking of the native structure, including via the use of nanofibrous materials produced by means of the electrospinning method. Such materials possess special properties such as a high surface to volume ratio and porosity that may exert an unpredictable effect on the sterilization technique used, that is, with respect to the efficacy thereof and the impact on the final properties of the material. A review by Rediguieri et al. provides a summary of the impact of sterilization and disinfection techniques on nanofibrous materials in terms of their morphology, surface wettability and other physicochemical properties, mechanical performance, and interactions with cell lines.2 Recently, the use of portable devices capable of the immediate production of nanofibers on the wound bed has been reported, for example by Xu,9 Aydogdu,10 and Yan.11 Such an approach does not count with the sterilization of the produced materials. On the other hand, the translational process of these materials into clinical practice will be more difficult because sterility constitutes a key factor in terms of the use of medical devices.
This study focused on the investigation of electrospun polycaprolactone (PCL), an FDA-approved polymer used in various applications, such as wound dressings,12−14 vascular grafts,15 cartilage tissue-engineering scaffolds,16 sealing of gastrointestinal anastomoses, thus preventing the occurrence of life-threatening complications,17 and many others. As the melting temperature of PCL is in the range 59–64 °C, it is essential that low-temperature sterilization methods be applied to this material.18 Therefore, the consideration of autoclaving was omitted from the study because of the low melting point of PCL. The effect of EtOx sterilization has been studied by Horakova et al.19 and Bhaskar et al.20 in the form of a comparison with ethanol solution soaking. It was found that EtOx sterilization does not affect the mechanical or degradation properties of electrospun PCL and that the proliferation rate of fibroblasts seeded on EtOx-sterilized materials was delayed compared to that on the ethanol-treated samples.19 However, the examination of the in vivo environment via the implantation of such scaffolds into a murine tendon model revealed no effect on cell infiltration and proliferation and other healing characteristics with respect to both the EtOx- and ethanol-treated samples.20 Studies of various doses of gamma irradiation and the impact on electrospun PCL conducted by Augustine et al.6 and Bosworth et al.21 revealed a shift toward a lower molecular weight in a dose-dependent manner accompanied by an increase in both the melting point and crystallinity; no effect was observed with concern to cell response.21 A study by Augustine et al. concluded that gamma irradiation led to improved hydrophilicity resulting in increased fibroblast proliferation.6 A study by Dai et al. compared hydrogen peroxide gas plasma, ethanol soaking, and UV radiation. The results revealed the enhanced hydrophilicity of the plasma-sterilized samples, which led to a higher degree of osteoblastic differentiation than that of the other investigated methods.22
As the above-mentioned studies addressed only the limits of the various sterilization and disinfection procedures available without providing a mutual comparison, we decided via this study to compare the effects of widely used sterilization techniques such as EtOx sterilization, gamma irradiation, PAA treatment, hydrogen peroxide plasma sterilization, and disinfection techniques (ethanol and UV irradiation) on electrospun PCL with respect to changes in the fibrous morphology, molecular weight, crystallinity, and interaction with a fibroblast cell line.
Materials and Methods
Preparation of the Materials
Fibrous materials were prepared from poly-ε-caprolactone (PCL, Mn 45,000 g/mol; Merck KGaA, Germany); granules of the polymer were dissolved in a solvent system composed of chloroform/ethanol/acetic acid (8/1/1, v/v/v, Penta s.r.o., Czech Republic) in a final concentration of 16 wt %. The solution was stirred at room temperature until complete dissolution was achieved. The solution was then immediately electrospun using a Nanospider 1WS500U (Elmarco s.r.o., Czech Republic) device. The conditions had been optimized previously in the preliminary experiments that led to the fabrication of a macroscopically homogeneous nanofibrous layer. The conditions applied to the needleless electrospinning of the PCL are listed in Table 1. The resulting fibrous layer was collected on a nonwoven textile layer (polypropylene spunbond—40 g/m2).
Table 1. Electrospinning Conditions Applied for the Fabrication of Nanofibrous PCL.
| PCL | |
| distance between electrodes [mm] | 180 |
| high voltage [kV] | –10/+40 |
| rewinding speed [mm/min] | 40 |
| steel orifice diameter [mm] | 0.6 |
| temperature [°C] | 22 |
| relative humidity [%] | 50 |
Sterilization of the Nanofibrous Samples
The samples intended for EtOx, gamma irradiation, and plasma treatment were packed into the appropriate packages with specific indicators and subsequently sterilized by means of the various techniques listed in Table 2. These sterilization techniques were conducted with electrospun PCL together with a spunbond layer in order to ensure enhanced handling for further analysis purposes (the spunbond layer was removed prior to the characterization of the material—morphology, molecular weight, thermal properties). Sterilization/disinfection techniques, including soaking (ethanol and PAA treatment) and UV light irradiation, were applied with the nanofibrous material without the spunbond layer.
Table 2. List of the Sterilization and Disinfection Methods Used in the Study.
| sterilization/disinfection technique | abbreviation used |
|---|---|
| EtOx | EtOx |
| gamma irradiation | gamma |
| PAA | PAA |
| hydrogen peroxide plasma | plasma |
| ethanol 70% | EtOH |
| UV light | UV |
EtOx sterilization was performed at the Military University Hospital, Prague, applying a low-temperature cycle (37 °C). The samples were sterilized by means of 3M Steri-Vac 5XL (3M Česko s.r.o., Czech Republic). Electrospun nanofibrous PCL with a spunbond layer was placed in an appropriate package with a specific indicator that proved the suitability of the EtOx concentration and the duration.
The samples subjected to gamma irradiation (PCL + spunbond layer) were placed in a specific container and sterilized by Bioster Ltd. (Czech Republic). The required dosage was >25 kGy, and the samples were irradiated at 29 ± 3.4 kGy.
PAA (36%, Delta Chem s.r.o., Czech Republic) was diluted with 20% ethanol to 5000 volume ppm as recommended by Yoganarasimha et al.8 The samples were soaked in a PAA solution for 15 min whereupon they were rinsed twice in phosphate-buffered saline (PBS, pH 7.4) for in vitro assessment purposes and in distilled water with respect to material characterization. Finally, the samples were allowed to dry prior to further testing.
Plasma sterilization was performed at the Military University Hospital, Prague, using the STERRAD 100S (Advanced Sterilization Products; Johnson & Johnson, USA) device; sterilization was based on hydrogen peroxide plasma, the direct injection of which ensured the sterilization of the porous materials. The sterilization cycle ran for 47 min, and the temperature was maintained between 47 and 56 °C. The samples (PCL + spunbond) were placed in specific packages with an indicator that proved the accuracy of the sterilization parameters.
Ethanol (Penta s.r.o., Czech Republic) was diluted to 70% and the samples soaked in the resulting solution for 30 min. The samples were then rinsed twice in PBS for in vitro assessment purposes and in distilled water with respect to material characterization. Finally, the samples were allowed to dry prior to further testing.
Disinfection via UV light was conducted in a Topsafe (Bioair, USA) laminar flow box. Samples were placed in the laminar box and UV light was applied for 30 min on each side.
Morphology Assessment of the Fibrous Mats
The samples were sputter-coated with a 7 nm layer of gold and imaged by means of a TESCAN Vega 3SB Easy Probe (TESCAN s.r.o., Czech Republic) scanning electron microscope. Images of both the inner (in contact with the underlying spunbond layer) and outer sides of the fibrous mats prior to and following sterilization were taken in three independent positions. The fibers (500 nos.) were assessed by means of NIS Elements software (LIM s.r.o., Czech Republic) for fiber diameter measurement purposes. The data is shown in box plot form with the representation of the upper and lower quartiles and the median as boxes and the variability showing the maximum and minimum measured values.
Molecular Weight Analysis
Shifts in molecular weight following sterilization were assessed by means of gel permeation chromatography (GPC). The samples were dissolved in tetrahydrofuran (THF) prior to analysis so as to obtain a final concentration of 1 mg/mL. The Dionex Ultimate 3000 HPLC system with a diode array and a Varian LC-385 ELSD detector was used for analysis purposes with a polymeric Phenomenex Phenogel 1E4 GPC column with a length of 30 cm with an i.d. of 4.6 mm and particle size of 5 μm. Pure THF of HPLC quality was used as the mobile phase, the flow rate of which was set at 1 mL/min for each sample. The chromatographs were recorded using a Varian LC-385 ELSD detector for a total of 23 min. The temperature of the nebulizer and the evaporator was set at 80 °C and the nitrogen flow rate at 1.1 mL/min. The injection volume of the dissolved polymers was set at 30 μL.
The change in the molecular weight was evaluated from the shift of the maximum peak of the chromatogram representing the most numerous molecular weight in the sample. A peak shift toward longer elution times corresponded with decreasing molecular weight.
Thermal Behavior
Differential scanning calorimetry (DSC) was employed for the evaluation of changes in the thermal properties of the materials following sterilization/disinfection using a DSC 1/700 METTLER TOLEDO (Metler-Toledo GmbH, Germany) device. The materials were first tempered for 120 s and then heated in two cycles (the rate of temperature change was set at 10 °C/min). Measurements were taken in an inert nitrogen atmosphere. The PCL samples were analyzed within a temperature range of −20 to 100 °C. The resulting values were taken from the first heating cycle because this cycle reflected the sterilization changes. The thermal properties of the PCL such as the melting temperature (Tm) and the enthalpy of fusion (ΔHm) were determined from the DSC curves. A constant of 135.44 J/g (corresponding to 100% crystalline PCL) was employed for the calculation of the crystallinity (ΔΧ) of PCL according to a study by Crescenzi et al.23 The samples were measured in triplicate; the data was presented in the form of the mean ± standard deviation.
In Vitro Testing
Sterile materials were cut into spherical shapes with a diameter of 15.6 mm (so as to fit in 24-well plates); the spunbond layer was removed prior to testing. The resulting materials were seeded with 3T3 mouse fibroblasts (ATCC, USA) at a concentration of 104 cells/well. The cells were cultured in Dulbecco’s modified Eagle medium (Lonza Biotec s.r.o., Czech Republic) supplemented with 10% fetal bovine serum (Lonza Biotec s.r.o., Czech Republic), 1% glutamine (Biosera, Czech Republic), and 1% penicillin/streptomycin/amphotericin B (Lonza Biotec s.r.o., Czech Republic). Cell adhesion and proliferation were evaluated 1 and 7 days following cell seeding by means of metabolic MTT assay (n = 4) and fluorescence microscopy (n = 1), which captured the cells and the spreading thereof.
The colorimetric MTT test is based on the reduction of yellow MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] to violet crystals of formazan in the presence of viable cells. The materials were transferred into new wells containing 250 μL of MTT and 750 μL of the complete media and incubated for 4 h at 37 °C. The crystals produced as a result were subsequently dissolved using acidified isopropanol and the final absorbance of the resulting solution was measured in two wavelengths (λsample 570 nm, λreference 690 nm). The difference between the sample and reference wavelength was plotted on a graph which compared the viability of the cells on the samples. Four materials were assessed by means of metabolic testing on each testing day.
The cells were captured via fluorescence microscopy after the double staining of the cellular actin filaments and nuclei at the same time points of 1 and 7 days following cell seeding. The materials were rinsed twice in PBS and fixed in 2.5% glutaraldehyde in PBS for 15 min at 4 °C following which a blocking solution composed of 0.1% Triton X-100 in 0.1% bovine serum albumin solution in PBS (10 min, room temperature) was applied. Furthermore, the cells were stained using phalloidin-FITC (Merck KGaA, Germany, dilution 1:1000, 1 h), which resulted in the actin filaments of the cells being colored green. Following double rinsing in PBS, DAPI (Merck KGaA, Germany, dilution 1:1000, 5 min) was used for the counterstaining of the cell nuclei in blue. The surfaces of the materials with the stained cells were observed using a Nikon Eclipse-Ti-E (Nikon Imaging, Czech Republic) inverted fluorescence microscope. The cell nuclei stained with DAPI were quantified from 10 fields of view as described in Horakova et al.19 The cell density was then quantified.
The statistical analysis of cell viability and cell quantification was processed by means of GraphPad Prism software (USA). The data thus obtained was assessed using 2way ANOVA with the Bonferonni multiple comparison test.
Results
Needleless Electrospinning of the PCL
PCL was successfully electrospun into the form of planar sheets according to the aforementioned conditions that had previously been optimized based on the research team’s long-term experience with such polymeric layer fabrication. The morphology of the resulting layers is depicted in Figure 1. As the structure of the layer replicates its underlying material (spunbond textile material), both elevations and dips are present as can be seen in the first SEM image in Figure 1B. Higher magnifications revealed fibers with very small diameters of mere tens of nanometers (minimum value of 73 nm) as well as thicker fibers with diameters in the order of a few micrometers (maximal measured value of 4.0 μm).
Figure 1.
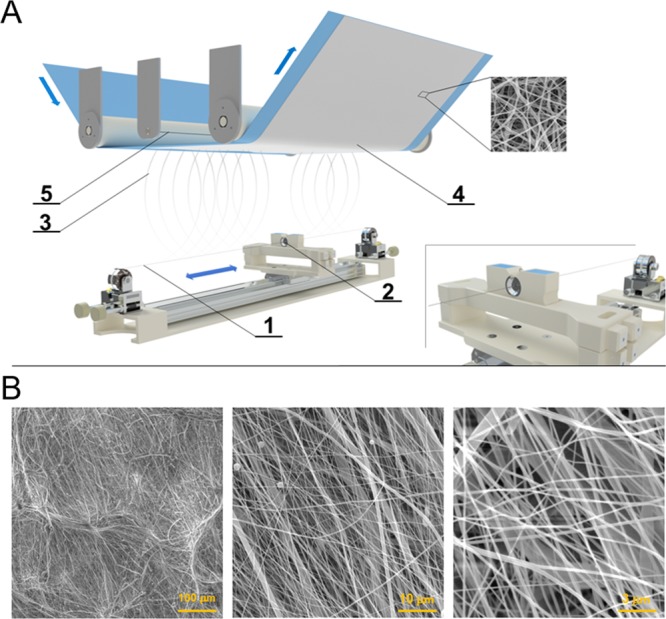
(A) Scheme of the Nanospider technology: 1—steel wire as the positive electrode, 2—steel orifice with a polymer solution reservoir, 3—fiber formation, 4—nanofiber layer collected on a supportive textile material, 5—steel wire as the negative electrode. (B) SEM images of an electrospun PCL layer: magnification bars—100, 10, and 3 μm.
Impact of Sterilization on the Fibrous Morphology
The electrospun layers were subjected to various sterilization techniques which resulted in the layer morphologies depicted in Figure 2. As described above, PCL layers are composed of very small as well as thicker fibers; therefore, minor changes can be attributed to the non-homogeneity of the PCL fibers rather than the sterilization technique. The most visible changes were observed following hydrogen peroxide plasma sterilization; the fibers were found to have completely melted and a foil was formed in place of the fibers. Further assessment of the materials addressed only those layers which exhibited the preservation of the fibrous morphology.
Figure 2.
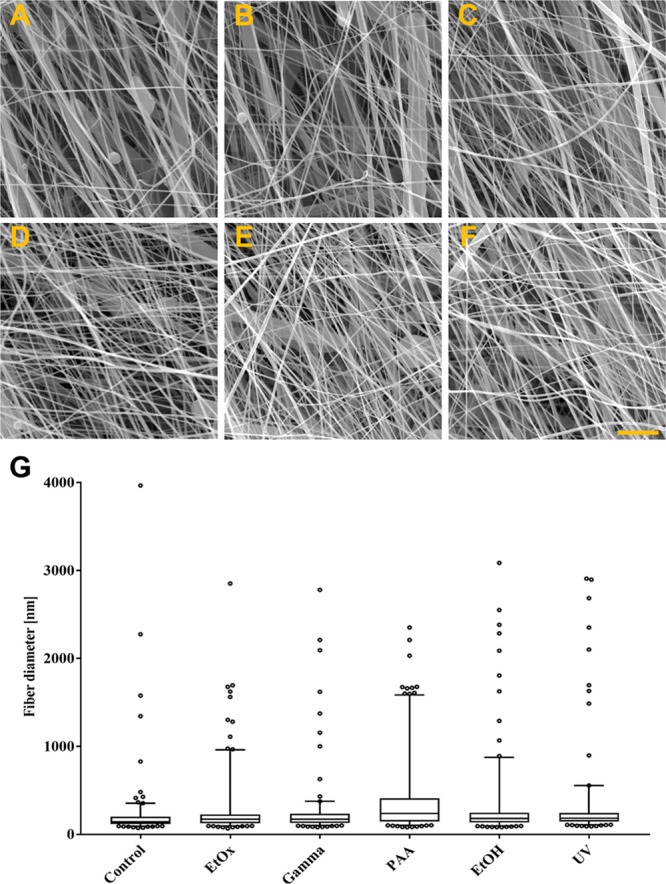
SEM pictures of electrospun PCL after sterilization by EtOx (A), gamma irradiation (B), PAA (C), disinfected by ethanol (D), UV irradiation (E), and nonsterile control (F); scale bar is 5 μm. Box plot graph of the fiber diameter characteristics (G).
The fiber diameter characteristics can be seen in Figure 3G in the form of a box plot. The mean fiber diameter of the control PCL layer was around 210 nm. In addition to these nanofibers, occasional microfibers with fiber diameters of up to 4 μm were detected within the structure (marked as outliers in the boxplot and depicted in the SEM pictures in Figures 1B and 2A). Similarly, following the sterilization/disinfection process, layers composed of nanofibers (200–400 nm) as well as microfibers were detected; thus, no visible effect on the fibrous layer morphology was confirmed.
Figure 3.
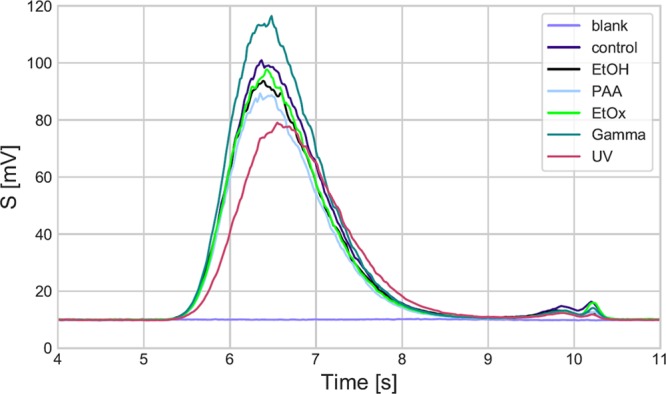
GPC chromatograms of the PCL electrospun layers: blank (pure solvent), control (prior to sterilization), following EtOx, PAA, gamma sterilization (Gamma), following ethanol (EtOH), UV disinfection.
Molecular Weight Analysis
The shift in molecular weight following the application of the various sterilization and disinfection techniques was assessed by means of GPC chromatography, and the results are summarized in Table 3 and Figure 3. The tested materials were eluted after similar periods of time as the elution of the control sample, that is after 6.4 min. A slightly higher elution time of 6.5 min was attained following EtOx sterilization and 6.6 min after gamma sterilization, which suggested slight decreases in the molecular weight following the application of these two sterilization techniques. None of the other tested methods (PAA, ethanol, UV) resulted in a shift in retention times to greater than 6.4 min.
Table 3. Molecular Weight of the Electrospun PCL Prior to (Control) and Following Sterilization/Disinfection: Retention Time Tm, Number Average Molecular Weight Mn.
| Tr [min] | Mn [g/mol] | |
|---|---|---|
| control | 6.38 | 21,000 |
| EtOH | 6.35 | 21,700 |
| PAA | 6.43 | 19,900 |
| EtOx | 6.48 | 18,900 |
| gamma | 6.55 | 17,600 |
| UV | 6.43 | 19,900 |
Thermal Behavior
The effect of the sterilization/disinfection of nanofibrous PCL was studied by means of DSC. The resulting melting temperature, melting enthalpy, and degree of crystallization were recorded (see Table 4) following the assessment of the measured thermographs. The DSC curves were found to have the same appearance in all tested samples. None of the treatments resulted in changes in the thermal behavior of the electrospun PCL. The melting temperature was 61–63 °C and the degree of crystallinity 61–67%.
Table 4. Thermal Characteristics of Electrospun PCL Prior to (Control) and Following Sterilization/Disinfection: Melting Temperature Tm, Melting Enthalpy ΔHm, and Crystallinity Degree ΔΧ.
| Tm [°C] | ΔHm [J/g] | ΔΧ [%] | |
|---|---|---|---|
| control | 62 ± 2 | 84 ± 6 | 62 ± 4 |
| EtOx | 62 ± 1 | 86 ± 6 | 64 ± 4 |
| gamma | 61 ± 2 | 86 ± 2 | 63 ± 2 |
| PAA | 63 ± 2 | 91 ± 11 | 67 ± 8 |
| EtOH | 62 ± 2 | 82 ± 11 | 61 ± 8 |
| UV | 62 ± 2 | 86 ± 2 | 63 ± 2 |
In Vitro Testing
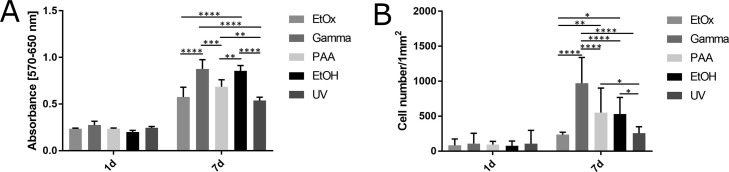
Fibroblasts were seeded on the PCL samples following sterilization by means of EtOx, gamma irradiation, and PAA and disinfection via ethanol and UV light. The metabolic assay and cell density were then evaluated 1 and 7 days following cell seeding as depicted in Figure 4. No statistically significant differences were observed between the samples exposed to the various sterilization/disinfection techniques during day 1. After 7 days, however, the highest number of cells and highest level of metabolic activity was determined on the PCL that had been sterilized by means of gamma irradiation followed by the samples disinfected using ethanol. The lowest metabolic activity and cell density were determined on those PCL samples that had been exposed to EtOx sterilization and UV light disinfection.
Figure 4.
Graphs of fibroblast metabolic activity measured via MTT assay (A) and cell density on PCL samples (B) exposed to EtOx, PAA, and gamma sterilization and ethanol (EtOH) and UV disinfection.* denotes p < 0.0332, **p < 0.0021, ***p < 0.0002, ****p < 0.0001 (2 way ANOVA, Bonferonni).
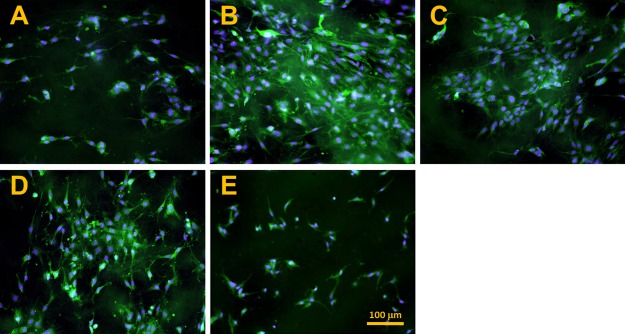
The morphologies of the fibroblasts after a week of culturing are depicted in Figure 5. The coverage of the cell surface correlates with the proliferation rate of the fibroblasts. An almost confluent layer of cells is evident following gamma sterilization (Figure 5B). The lowest cell number can be seen on those samples exposed to EtOx sterilization and UV irradiation disinfection (Figure 5A,E). The cell number also correlates with cellular spreading—the best spread morphology of the fibroblasts was observed on the PCL following gamma irradiation, PAA and ethanol treatment, while the lowest rate of fibroblast spreading was captured on those materials subjected to EtOx sterilization and UV irradiation.
Figure 5.
Fluorescence microscopy images of the fibroblasts following 7 days of culturing on nanofibrous PCL samples sterilized using EtOx (A), gamma irradiation (B) and PAA (C) and disinfected using ethanol (D) and UV irradiation (E); scale bar 100 μm.
Discussion
Various sterilization techniques have been researched worldwide with concern to electrospun PCL, all of which are subject to limitations such as availability and high cost. With respect to electrospun materials, terminal sterilization rather than fabrication under aseptic conditions is considered the preferred option. However, the application of terminal sterilization during the development of medical devices and tissue engineering scaffolds must be considered very carefully due to its non-negligible influence on the final material properties of such devices. Thus, the study provides a summary of the impact of the most commonly applied sterilization and disinfection methods on nanofibrous PCL in terms of its morphology and molecular weight and the analysis of its thermal properties and cell material interaction. The materials used as scaffolds in the field of tissue engineering differ in terms of their material compositions and structures. Our study focused on electrospun PCL with a molecular weight of 45,000 because of its utilization for wound dressings as reported in previous studies.24,25 The use of other polymeric materials (even in their nanofibrous forms) will have to be tested and verified separately as summarized in a review by Rediguieri.2 Although it is possible that similar chemical structures, for example, within the biodegradable polyesters group, such as polylactide, may behave in a similar way, research will have to be conducted to confirm this supposition. The PCL structure studied consisted of nanofibers with diameters of around 200 nm as well as microfibers of up to 4 μm in diameter. This combined structure was found to provide benefits from the mechanical point of view as well as in terms of facilitating cell material interactions.26,27 The impact of sterilization techniques on PCL-based scaffolds prepared via differing fiber-forming methods such as centrifugal spinning28,29 and ac spinning30 will have to be further confirmed. If similar fibrous structures are obtained, the results presented herein will be useful in terms of their transfer to the consideration of such materials.
The research determined that it is not possible to employ hydrogen peroxide plasma techniques for the sterilization of nanofibrous PCL because they lead to the complete loss of the fibrous structure. With respect to hydrogen peroxide plasma, however, the temperature during the cycle should not exceed 55 °C (during the sterilization process between 10 and 40 °C). Dai et al.22 also investigated the impact of low-temperature hydrogen peroxide plasma on electrospun PCL; however, in contrast to our results, the morphology of the electrospun mats exhibited no change, that is, the samples retained their fibrous morphology following plasma sterilization. We explain this apparently conflicting outcome by the fact that the input material differed with concern to its molecular weight; our study involved the use of PCL with an Mn of 45,000 g/mol, while Dai et al. used PCL with a higher Mn of 110,000 g/mol. Moreover, the study by Dai et al. proved the effectiveness of hydrogen plasma sterilization along with a lower level of surface wettability than that of the corresponding non-sterilized sample.22 A study by Franklin et al.31 which studied the application of the hydrogen peroxide plasma sterilization technique to a porous PCL scaffold prepared via fused deposition modeling concluded that this sterilization method was significantly cytotoxic for canine chondrocytes.31
EtOx treatment is suitable for the sterilization of thermosensitive polymers such as PCL. The application device allows for the running of two cycles under different temperatures, that is 37 and 55 °C. Our study used the low-temperature application because our previous research (data not shown) proved that higher temperatures lead to the melting of fibrous structures. Although no changes were observed in the fibrous morphology or molecular weight following the sterilization of the electrospun PCL, it was noted that fibroblast proliferation occurred later on the samples sterilized with EtOx than on those exposed to the other sterilization and disinfection techniques applied in the study. This outcome was reported in our previous study which involved the investigation of more material characteristics than were covered by the research described herein and which included the observation that EtOx sterilization did not influence mechanical properties in terms of ultimate tensile strength and elongation at break. Although the initial Young’s modulus was observed to increase following EtOx sterilization (by around 2.5 times), the degradation rate of the electrospun PCL mats sterilized via this method showed no variation from the non-sterilized samples with respect to the accelerated enzyme environment.19 However, as this sterilization technique evinced no cytotoxic effects, it can be concluded that it provides a suitable method for the purpose.
Gamma treatment represents the most extensively studied sterilization method for electrospun PCL, with respect to which gamma irradiation of 29 kGy did not lead to a change in morphology. One week following cell seeding, the fibroblasts seeded on the gamma-irradiated samples exhibited the highest level of viability and cell density per unit area of all tested techniques, the explanation for which may lie in a change in the wettability of the surface as reported by Augustine et al. After applying a dosage of 25 kGy, the water contact angle decreased from 106 ± 5 to 84 ± 0.6° and the cells were found to prefer slightly hydrophilic to hydrophobic surfaces.6 Cell interactions are mediated by proteins adsorbed from the cell culture medium and with respect to hydrophobic substrates; these adhesion proteins are bound in their rigid or denaturated state.32 This assumption was confirmed by the same study. Increased doses of gamma irradiation supported fibroblast cell proliferation due to the decreased water contact angle.7 Bosworth et al.21 also studied the effect of the gamma irradiation dose on electrospun PCL. They discovered that the molecular weight changed when a dose of 25 kGy was applied, which was not the case in our study which revealed only a slight switch toward a lower molecular weight (the retention time was shifted by 0.1 min compared to the non-sterilized sample).21 However, it is important to mention that the input polymer in the study by Bosworth et al. had a molecular weight of 80,000 g/mol as opposed to 45,000 g/mol as used in our study. Cottam et al.,33 when investigating gamma-irradiated films made of PCL, observed a decreased number average molecular weight, indicating polymeric chain scission and an increased weight average molecular weight, which was explained via cross linking and the increased polydispersity index. The full explanation for changes in the molecular structure following gamma irradiation remains to be determined. Moreover, the dosage applied may differ in each cycle; although it is recommended that the value should exceed 25 kGy, it never attained the same value (in our study a dose of 29 ± 3.4 kGy was applied).
Sterilization using PAA, which was performed based on the results obtained by Yoganarasimha et al.,8 did not exert an impact on the fibrous morphology and molecular weight. Fibroblast proliferation on the PAA-sterilized mat was at a lower level than that on the gamma-treated samples but higher than on the EtOx-sterilized materials, which could have been due, again, to the decreased surface wettability, as reported by Yoganarasimha et al.8 Although PAA soaking can be used for immediate sterilization purposes prior to implantation, this technique is not applied at the same scale as are gamma irradiation and EtOx sterilization. The necessity for the soaking of materials for a period of 15 min may be problematic if active substances are incorporated within the structure, that is it may lead to the occurrence of the unwanted effect of the burst release of such substances.
Disinfection methods are used routinely prior to the in vitro assessment of electrospun scaffolds. However, as the use of these methods has not been approved by regulatory agencies for further translation to clinical use, their usage is necessarily limited. This study compared the impact of the application of 70% ethanol soaking and UV irradiation for disinfection purposes. It was determined that neither of these methods influenced the morphology of the fibers, the molecular weight, or the thermal properties of electrospun PCL. In terms of the fibroblast proliferation rate, ethanol soaking proved to be a more effective disinfection method than UV in terms of higher cell viability and cell number per area.
All factors considered, the most suitable methods for the sterilization of electrospun PCL with a molecular weight of 45,000 g/mol were found to be the application of EtOx and gamma irradiation, and it is strongly recommended that both approaches be further investigated and compared and that future studies should be devoted to the detailed research of the impact of both these sterilization techniques on surface wettability, mechanical properties, degradation rate, and so forth. Moreover, sterilization efficacy will be verified against all types of microorganisms in order to prove the suitability of these sterilization methods in terms of their translation to clinical applications.
Conclusions
Samples of an electrospun PCL mat were subjected to various sterilization and disinfection techniques in order to compare their impact on the surface and bulk properties of the samples. As hydrogen peroxide plasma sterilization techniques led to changes in the morphology of the fibrous layers, their use was excluded with respect to this material. The most promising results were achieved with concern to the gamma irradiation and EtOx sterilization methods, and it is reasonable to conclude that they be considered for future clinical application. Improved fibroblast interactions were achieved following gamma irradiation. On the other hand, EtOx sterilization led to decreased cell proliferation with no observed cytotoxic effect.
Acknowledgments
The study was supported by the Nanofibrous Wound Dressings project (number FV10416) commissioned by the Ministry of Industry and Trade of the Czech Republic.
The authors declare no competing financial interest.
References
- Rutala W. A.Guideline for Disinfection and Sterilization in Healthcare Facilities; CDC, 2008; Vol. 2008, p 163. [Google Scholar]
- Rediguieri C. F.; Sassonia R. C.; Dua K.; Kikuchi I. S.; de Jesus Andreoli Pinto T. Impact of Sterilization Methods on Electrospun Scaffolds for Tissue Engineering. Eur. Polym. J. 2016, 82, 181–195. 10.1016/j.eurpolymj.2016.07.016. [DOI] [Google Scholar]
- Hofmann S.; Stok K. S.; Kohler T.; Meinel A. J.; Müller R. Effect of Sterilization on Structural and Material Properties of 3-D Silk Fibroin Scaffolds. Acta Biomater. 2014, 10, 308–317. 10.1016/j.actbio.2013.08.035. [DOI] [PubMed] [Google Scholar]
- Rnjak-Kovacina J.; DesRochers T. M.; Burke K. A.; Kaplan D. L. The Effect of Sterilization on Silk Fibroin Biomaterial Properties. Macromol. Biosci. 2015, 15, 861–874. 10.1002/mabi.201500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche G.; Marois Y.; Guidoin R.; King M. W.; Martin L.; How T.; Douville Y. Polyvinylidene Fluoride (PVDF) as a Biomaterial: From Polymeric Raw Material to Monofilament Vascular Suture. J. Biomed. Mater. Res. 1995, 29, 1525–1536. 10.1002/jbm.820291209. [DOI] [PubMed] [Google Scholar]
- Augustine R.; Saha A.; Jayachandran V. P.; Thomas S.; Kalarikkal N. Dose-Dependent Effects of Gamma Irradiation on the Materials Properties and Cell Proliferation of Electrospun Polycaprolactone Tissue Engineering Scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 526–533. 10.1080/00914037.2014.977900. [DOI] [Google Scholar]
- Gonzalez M. E.; Salmoral E. M.; Traverso K.; Floccari M. E. Effects of Gamma Radiation on a Plastic Material Based on Bean Protein. Int. J. Polym. Mater. 2002, 51, 721–731. 10.1080/714975831. [DOI] [Google Scholar]
- Yoganarasimha S.; Trahan W. R.; Best A. M.; Bowlin G. L.; Kitten T. O.; Moon P. C.; Madurantakam P. A. Peracetic Acid: A Practical Agent for Sterilizing Heat-Labile Polymeric Tissue-Engineering Scaffolds. Tissue Eng., Part C 2014, 20, 714–723. 10.1089/ten.tec.2013.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S.-C.; Qin C.-C.; Yu M.; Dong R.-H.; Yan X.; Zhao H.; Han W.-P.; Zhang H.-D.; Long Y.-Z. A Battery-Operated Portable Handheld Electrospinning Apparatus. Nanoscale 2015, 7, 12351–12355. 10.1039/c5nr02922h. [DOI] [PubMed] [Google Scholar]
- Aydogdu M. O.; Altun E.; Crabbe-Mann M.; Brako F.; Koc F.; Ozen G.; Kuruca S. E.; Edirisinghe U.; Luo C.; Gunduz O.; Edirisinghe M. Cellular Interactions with Bacterial Cellulose: Polycaprolactone Nanofibrous Scaffolds Produced by a Portable Electrohydrodynamic Gun for Point-of-Need Wound Dressing. Int. Wound J. 2018, 15, 789–797. 10.1111/iwj.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X.; Yu M.; Ramakrishna S.; Russell S. J.; Long Y.-Z. Advances in Portable Electrospinning Devices for in Situ Delivery of Personalized Wound Care. Nanoscale 2019, 11, 19166–19178. 10.1039/c9nr02802a. [DOI] [PubMed] [Google Scholar]
- Augustine R.; Kalarikkal N.; Thomas S. Electrospun PCL Membranes Incorporated with Biosynthesized Silver Nanoparticles as Antibacterial Wound Dressings. Appl. Nanosci. 2016, 6, 337–344. 10.1007/s13204-015-0439-1. [DOI] [Google Scholar]
- Ahmed J.; Altun E.; Aydogdu M. O.; Gunduz O.; Kerai L.; Ren G.; Edirisinghe M. Anti-fungal bandages containing cinnamon extract. Int. Wound J. 2019, 16, 730–736. 10.1111/iwj.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B.; Augustine R.; Kalarikkal N.; Thomas S.; Seantier B.; Grohens Y. Recent Advances in Electrospun Polycaprolactone Based Scaffolds for Wound Healing and Skin Bioengineering Applications. Mater. Today Commun. 2019, 19, 319–335. 10.1016/j.mtcomm.2019.02.009. [DOI] [Google Scholar]
- Yalcin I.; Horakova J.; Mikes P.; Sadikoglu T. G.; Domin R.; Lukas D. Design of Polycaprolactone Vascular Grafts. J. Ind. Text. 2016, 45, 813–833. 10.1177/1528083714540701. [DOI] [Google Scholar]
- Alves da Silva M. L.; Martins A.; Costa-Pinto A. R.; Costa P.; Faria S.; Gomes M.; Reis R. L.; Neves N. M. Cartilage Tissue Engineering Using Electrospun PCL Nanofiber Meshes and MSCs. Biomacromolecules 2010, 11, 3228–3236. 10.1021/bm100476r. [DOI] [PubMed] [Google Scholar]
- Rosendorf J.; Horakova J.; Klicova M.; Palek R.; Cervenkova L.; Kural T.; Hosek P.; Kriz T.; Tegl V.; Moulisova V.; Tonar Z.; Treska V.; Lukas D.; Liska V. Experimental Fortification of Intestinal Anastomoses with Nanofibrous Materials in a Large Animal Model. Sci. Rep. 2020, 10, 1134. 10.1038/s41598-020-58113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff M. A.; Hutmacher D. W. The return of a forgotten polymer-Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. 10.1016/j.progpolymsci.2010.04.002. [DOI] [Google Scholar]
- Horakova J.; Mikes P.; Saman A.; Jencova V.; Klapstova A.; Svarcova T.; Ackermann M.; Novotny V.; Suchy T.; Lukas D. The Effect of Ethylene Oxide Sterilization on Electrospun Vascular Grafts Made from Biodegradable Polyesters. Mater. Sci. Eng. C 2018, 92, 132–142. 10.1016/j.msec.2018.06.041. [DOI] [PubMed] [Google Scholar]
- Bhaskar P.; Bosworth L. A.; Wong R.; O’brien M. A.; Kriel H.; Smit E.; McGrouther D. A.; Wong J. K.; Cartmell S. H. Cell response to sterilized electrospun poly(ε-caprolactone) scaffolds to aid tendon regenerationin vivo. J. Biomed. Mater. Res., Part A 2017, 105, 389–397. 10.1002/jbm.a.35911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosworth L. A.; Gibb A.; Downes S. Gamma Irradiation of Electrospun Poly(ε-Caprolactone) Fibers Affects Material Properties but Not Cell Response. J. Polym. Sci., Part B: Polym. Phys. 2012, 50, 870–876. 10.1002/polb.23072. [DOI] [Google Scholar]
- Dai Y.; Xia Y.; Chen H.-B.; Li N.; Chen G.; Zhang F.-M.; Gu N. Optimization of sterilization methods for electrospun poly(ε-caprolactone) to enhance pre-osteoblast cell behaviors for guided bone regeneration. J. Bioact. Compat. Polym. 2016, 31, 152–166. 10.1177/0883911515598795. [DOI] [Google Scholar]
- Crescenzi V.; Manzini G.; Calzolari G.; Borri C. Thermodynamics of Fusion of Poly-β-Propiolactone and Poly-ϵ-Caprolactone. Comparative Analysis of the Melting of Aliphatic Polylactone and Polyester Chains. Eur. Polym. J. 1972, 8, 449–463. 10.1016/0014-3057(72)90109-7. [DOI] [Google Scholar]
- Krchova S.; Dzan L.; Lukas D.; Mikes P.; Jencova V.; Horakova J.; Pilarova K. Nanovlákna v Hojení Kožních Ran. Čes. Dermatovenerol. Časopis Čes. Akad. Dermatovenerol. 2014, 4, 234–240. [Google Scholar]
- Horakova J.; Oulehlova Z.; Novotny V.; Jencova V.; Mikes P.; Havlickova K.; Prochazkova R.; Heczkova B.; Hadinec P.; Sehr S.; Wendel H.-P.; Bell C.-M.; Krajewski S. The Assessment of Electrospun Scaffolds Fabricated from Polycaprolactone with the Addition of L-Arginine. Biomed. Phys. Eng. Express 2020, 6, 025012. 10.1088/2057-1976/ab756f. [DOI] [PubMed] [Google Scholar]
- Amirian J.; Lee S.-Y.; Lee B.-T. Designing of Combined Nano and Microfiber Network by Immobilization of Oxidized Cellulose Nanofiber on Polycaprolactone Fibrous Scaffold. J. Biomed. Nanotechnol. 2016, 12, 1864–1875. 10.1166/jbn.2016.2308. [DOI] [PubMed] [Google Scholar]
- Kasoju N.; Bhonde R. R.; Bora U. Fabrication of a Novel Micro–nano Fibrous Nonwoven Scaffold with Antheraea Assama Silk Fibroin for Use in Tissue Engineering. Mater. Lett. 2009, 63, 2466–2469. 10.1016/j.matlet.2009.08.037. [DOI] [Google Scholar]
- Alenezi H.; Cam M. E.; Edirisinghe M. Experimental and Theoretical Investigation of the Fluid Behavior during Polymeric Fiber Formation with and without Pressure. Appl. Phys. Rev. 2019, 6, 041401. 10.1063/1.5110965. [DOI] [Google Scholar]
- Kuzelova Kostakova E.; Meszaros L.; Maskova G.; Blazkova L.; Turcsan T.; Lukas D. Crystallinity of Electrospun and Centrifugal Spun Polycaprolactone Fibers: A Comparative Study. J. Nanomater. 2017, 2017, 1–9. 10.1155/2017/8952390. [DOI] [Google Scholar]
- Lawson C.; Stanishevsky A.; Sivan M.; Pokorny P.; Lukáš D. Rapid Fabrication of Poly(ε-Caprolactone) Nanofibers Using Needleless Alternating Current Electrospinning. J. Appl. Polym. Sci. 2016, 133, 43232. 10.1002/app.43232. [DOI] [Google Scholar]
- Franklin S. P.; Stoker A. M.; Cockrell M. K.; Pfeiffer F. M.; Sonny Bal B.; Cook J. L. Effects of Low-Temperature Hydrogen Peroxide Gas Plasma Sterilization on In Vitro Cytotoxicity of Poly(ϵ-Caprolactone) (PCL). J. Biomater. Sci., Polym. Ed. 2012, 23, 1–10. 10.1163/092050611x612296. [DOI] [PubMed] [Google Scholar]
- Bacakova L.; Filova E.; Parizek M.; Ruml T.; Svorcik V. Modulation of Cell Adhesion, Proliferation and Differentiation on Materials Designed for Body Implants. Biotechnol. Adv. 2011, 29, 739–767. 10.1016/j.biotechadv.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Cottam E.; Hukins D. W. L.; Lee K.; Hewitt C.; Jenkins M. J. Effect of sterilisation by gamma irradiation on the ability of polycaprolactone (PCL) to act as a scaffold material. Med. Eng. Phys. 2009, 31, 221–226. 10.1016/j.medengphy.2008.07.005. [DOI] [PubMed] [Google Scholar]