Abstract
During an investigation of the fungi from the Aspergillaceae family obtained from different environmental sources in Korea, we isolated six strains, including CNUFC WJC9-1, CNUFC BPM36-33, CNUFC MSW6, CNUFC ESW1, CNUFC TM6-2, and CNUFC WD17-1. The morphology and phylogeny of these isolates were analyzed based on their partial β-tubulin (BenA) and calmodulin (CaM) gene sequences. Based on the morphological characteristics and sequence analyses, the isolates CNUFC WJC9-1, CNUFC BPM36-33, CNUFC TM6-2, and CNUFC WD17-1 were identified as A. europaeus, A. pragensis, Penicillium fluviserpens, and P. scabrosum, respectively, and isolates CNUFC MSW6 and CNUFC ESW1 were identified as A. tennesseensis. To the best of our knowledge, the species A. europaeus, A. pragensis, A. tennesseensis, P. fluviserpens, and P. scabrosum have not been previously reported in Korea.
Keywords: Aspergillus, Aspergillaceae, morphology, Penicillium, phylogeny
1. Introduction
Aspergillus and Penicillium are genera within the phylum Ascomycota (class: Eurotiomycetes; order: Eurotiales; family: Aspergillaceae). Species belonging to these two genera are mainly environmental saprobes, which act as decomposers of organic materials [1,2]. They can be found in water, soil, vegetation, fruits, foods, indoor environments, and air [2–4]. Several species are considered beneficial for their commercial, economic, or medical uses; they are used in enzyme production, and in the fermentation of foods such as soy sauce (e.g., Aspergillus oryzae and A. sojae), cheese (e.g., Penicillium roqueforti), and sausages (e.g., P. nalgiovense). These species also produce a wide range of secondary metabolites that can be used as drugs and antibiotics [5–7], while others can cause diseases in both humans and animals and can also act as plant pathogens [8–10].
The genus Aspergillus was first described by Micheli in 1729 [11] as asexual fungi whose conidiophores resemble an aspergillum. This genus consists of 339 species, which are classified into four subgenera (Aspergillus, Circumdati, Fumigati, and Nidulantes) and 20 sections [2,3,12,13]. Identification of Aspergillus species has been revised, and now relies on standardized methods based on morphological characteristics, extrolite characterization, and multi-locus DNA sequence analyses. Molecular DNA markers used for Aspergillus involved sequencing of the internal transcribed spacer (ITS), calmodulin (CaM), β-tubulin (BenA), and the RNA polymerase II second largest subunit (RPB2) sequences. Due to the well-established CaM database, and the relative ease of locus amplification and adequate polymorphism, the CaM marker is being used for the identification of Aspergillus species [2,14]. About 56 species of Aspergillus have been reported from Korea [15]. Recently, a new Aspergillus species, A. koreanus, has been described [16]. Six more species were recorded recently from Korea, A. allahabadii and A. caninus from soil, A. sojae from meju, and A. montenegroi, A. rhizopodus, and A. tabacinus from tidal mudflats and sea sand [17–20].
The genus Penicillium was first described by Link in 1809 [21]. This genus is subdivided into two subgenera (Aspergilloides and Penicillium) and 26 sections [1,22]. Species of Penicillium can be isolated from different environmental sources including air, soil, indoor environments, and food products [1,23]. Penicillium species are also identified in a manner similar to Aspergillus species, through the use of morphological characteristics, multi-locus DNA sequencing, and extrolite analyses. The BenA marker appears to be suitable for their identification [2,14]. This genus includes 354 accepted species according to Visagie et al. [24]. Approximately 100 Penicillium species have been reported from Korea [15,16,25–27]. Twelve species of Penicillium are currently reported as new from Korea (Source: www.indexfungorum.org as of July 2019).
The aims of this study were to identify five previously unrecorded fungal species in Korea, A. europaeus, A. pragensis, A. tennesseensis, P. fluviserpens, and P. scabrosum based on morphological and molecular analyses and to contribute to the knowledge about biodiversity in Korea.
2. Materials and methods
2.1. Sampling and isolation
Commercial corn grain was collected from Wanju, Korea in August 2016. Tomato (Solanum lycopersicum L.) fruits were purchased from markets in Gwangju, Korea in July 2017. Death moths (Lepidoptera; Sphingidae) were collected from a garden at Chonnam National University located in Gwangju, Korea in January 2018. By-products of rice bran were collected from Daejeon, Korea in August 2017. Water samples were collected from Eulsukdo Island located in Busan and from a reservoir at Wando island, Korea in August 2017 and 2018, respectively. The samples were collected in sterile plastic bags or sterile 50-mL Falcon tubes and transferred to the laboratory.
To isolate fungi from corn grain, 7–10 corn grains were plated directly onto malt extract agar (MEA) (Difco™, Sparks, MD) adjusted with NaCl, glycerol, or glucose to a water activity range of 0.9–0.85. The plates were incubated at 25 °C in the dark for 7–21 d. Hyphal tips were transferred to potato dextrose agar (PDA; Difco™, Sparks, MD) media using the tips of heat-stretched capillary tubes under a stereomicroscope.
For death moths and tomato fruits, samples were examined under a stereomicroscope to detect any fungal infection. Hyphal tips or spore were transferred to PDA media using the tips of heat-stretched capillary tubes. The plates were incubated at 25 °C in the dark for 7 d.
For by-products of rice bran and water samples, we used the serial dilution plating method as described by Nguyen and Lee [28] and Nguyen et al. [29]. Individual colonies with various morphologies were collected, transferred to PDA, and subcultured until pure mycelia were obtained.
For stock storage, pure isolates were maintained in PDA slant tubes in 20% glycerol at −80 °C at the Environmental Microbiology Laboratory Fungarium, Chonnam National University, Gwangju, Korea as CNUFC WJC9-1, CNUFC BPM36-33, CNUFC MSW6, CNUFC ESW1, CNUFC TM6-2, and CNUFC WD17-1. CNUFC WJC9-1, CNUFC BPM36-33, CNUFC MSW6, and CNUFC TM6-2 were also deposited at the Collection of National Institute of Biological Resources (NIBR), Incheon, Korea. CNUFC WD17-1 was deposited at the Culture Collection of the Nakdonggang National Institute of Biological Resources (NNIBR), Sangju, Korea. Information on all isolates used in this study was shown in Table 1.
Table 1.
Information on all isolates used in this study.
| Species | Culture no. | Substrate | Geographic origin |
|---|---|---|---|
| A. europaeus | CNUFC WJC9-1 | Corn grain | Wanju, Korea (35°40′0.12′′N 126°00′0.00′′E) |
| A. pragensis | CNUFC BPM36-33 | By-product rice bran | Daejeon, Korea (36°19′17.00′′ N 127°25′ 10.99′′E) |
| A. tennesseensis | CNUFC MSW6 | Death moth | Gwangju, Korea (35°09′60.00′′N 126°54′ 59.99′′E) |
| A. tennesseensis | CNUFC ESW1 | Sea water | Eulsukdo, Busan, Korea (35°06′10.01′′N 129°02′25.01′′E) |
| P. fluviserpens | CNUFC TM6-2 | Tomato fruit | Gwangju, Korea (35°09′60.00′′N 126°54′ 59.99′′E) |
| P. scabrosum | CNUFC WD17-1 | Freshwater | Wando, Korea (34°19′1.20′′ N 126°45′0.00′′ E) |
2.2. Morphological studies
The strains were three-point inoculated onto Czapek yeast extract agar (CYA), MEA, yeast extract sucrose agar (YES), and PDA [25]. The plates were incubated at 25 °C in the dark for 7 d. Fragments of mycelia were removed from the cultures and placed on microscope slides with lactic acid (60%). An Olympus BX51 microscope with differential interference contrast optics (Olympus, Tokyo, Japan) was used to capture digital images. The size and shape of the microscopic features were recorded.
2.3. DNA extraction, PCR, and sequencing
Fungal isolates were cultured on PDA overlaid with cellophane at 25 °C for 5–7 d. Genomic DNA was extracted using the Solg TM Genomic DNA preparation Kit (Solgent Co. Ltd., Daejeon, Korea). The BenA was amplified using the primer pairs Bt2a/Bt2b, and T10/Bt2b [30]. CaM gene was amplified using the primer pairs CMD5/CMD6, and CF1/CF4 [31,32], respectively. PCR amplification was performed according to the conditions described in Visagie et al. [24] and Yilmaz et al. [33]. PCR products were purified with an Accuprep PCR Purification Kit (Bioneer Corp., Daejeon, South Korea). Sequencing was performed using the same primers pairs and analyzed using the ABI PRISM 3730XL Genetic Analyzer (Applied Biosystems, Foster City, CA).
2.4. Phylogenetic analysis
Sequences for selected strains were aligned with reference sequences obtained from GenBank using Clustal_X version 2.1 [34] and were edited manually with Bioedit version 7.2.6.0 [35]. Maximum likelihood (ML) phylogenies were constructed using MEGA version 6 [36]. The sequence of Talaromyces flavus was used as an out group. The sequences of the isolates in this study were deposited in the NCBI database under the accession numbers shown in Table 2.
Table 2.
Accession numbers for fungal strains used for the phylogenetic analysis.
| GenBank accession no. |
|||
|---|---|---|---|
| Species | Collection no. | BenA | CaM |
| Aspergillus amoenus | NRRL 4838 (T) | EF652304 | EF652392 |
| A. austroafricanus | NRRL 233 (T) | JN853963 | JN854025 |
| A. brunneo-uniseriatus | NRRL 4273 (T) | EF652123 | EF652138 |
| A. campestris | CBS 348.81 (T) | EU014091 | EF669535 |
| A. candidus | CBS 566.65 (NT) | EU014089 | EF669550 |
| A. chrysellus | NRRL 5084 (T) | EF652109 | EF652136 |
| A. creber | NRRL 58592 (T) | JN853980 | JN854043 |
| A. cvjetkovicii | NRRL 227 (T) | EF652264 | EF652352 |
| A. dimorphicus | NRRL 3650 (T) | EF652111 | EF652135 |
| A. dobrogensis | CBS 143370 (T) | LT627027 | LT558722 |
| A. europaeus | CBS 140936 | LN909018 | LN909019 |
| A. europaeus | CBS 134392 | LN909004 | LN909005 |
| A. europaeus | NRRL 66252 (T) | LN909006 | LN909007 |
| A. europaeus | CNUFC WJC9-1 | MN337608 | MN894576 |
| A. europaeus | CNUFC WJC9-2 | MN337609 | MN894577 |
| A. flaschentraegeri | NRRL 5042 (T) | EF652113 | EF652130 |
| A. flavus | NRRL 1957 (T) | EF661485 | EF661508 |
| A. fructus | NRRL 239 (T) | EF652273 | EF652361 |
| A. fruticans | CBS 486.65 (T) | EF652307 | EF652395 |
| A. griseoaurantiacus | CBS 138191 (T) | KJ775086 | KJ775357 |
| A. jensenii | NRRL 58600 | JN854007 | JN854046 |
| A. penicillioides | NRRL 4548 (T) | EF651928 | EF652024 |
| A. pragensis | CBS 135591 (T) | HE661604 | FR751452 |
| A. pragensis | CCF 4654 | HG916673 | HG916680 |
| A. pragensis | CNUFC BPM36-33 | MN337604 | MN337610 |
| A. pragensis | CNUFC BPM36-34 | MN337605 | MN337611 |
| A. protuberus | NRRL 3505 (T) | EF652284 | EF652372 |
| A. pseudonomius | NRRL 3353 | EF661495 | EF661529 |
| A. pulvinus | CBS 578.65 (T) | FJ531013 | FJ531086 |
| A. puulaauensis | NRRL 35641 (T) | JN853979 | JN854034 |
| A. restrictus | NRRL 154 (T) | EF651880 | EF652029 |
| A. subalbidus | CBS 567.65 (T) | KP987050 | EF669551 |
| A. subversicolor | NRRL 58999 (T) | JN853970 | JN854010 |
| A. sydowii | NRRL 250 (T) | EF652274 | EF652362 |
| A. tabacinus | NRRL 4791 (T) | EF652302 | EF652390 |
| A. taichungensis | IBT 19404 (T) | EU076297 | HG916679 |
| A. tamarii | NRRL 20818 | EF661474 | EF661526 |
| A. tritici | CBS 266.81 (T) | EU076293 | HG916678 |
| A. tennesseensis | NRRL 13150 (T) | JN853976 | JN854017 |
| A. tennesseensis | LEMI875 | KJ766999 | KJ766995 |
| A. tennesseensis | LEMI917 | KJ766998 | KJ766994 |
| A. tennesseensis | CNUFC ESW1 | MN337606 | MN337612 |
| A. tennesseensis | CNUFC MSW6 | MN337607 | MN337613 |
| A. venenatus | NRRL 13147 (T) | JN854003 | JN854014 |
| A. versicolor | CBS 583.65 (T) | EF652266 | EU076368 |
| A. wentii | NRRL 375 (T) | EF652106 | EF652131 |
| Penicillium alfredii | CBS138224 (T) | KJ775177 | KJ775411 |
| P. atrovenetum | CBS241.56 (T) | JX140944 | KJ867004 |
| P. astrolabium | CBS122427 (T) | DQ645793 | DQ645808 |
| P. brevicompactum | NRRL 2011 | DQ645784 | AY484817 |
| P. cinnamopurpureum | NRRL162 (T) | EF626948 | EF626949 |
| P. colei | NRRL13013 (T) | KF932926 | KF932942 |
| P. coralligerum | CBS123.65 (T) | KJ834444 | KJ866994 |
| P. crystallinum | CBS479.65 (T) | EF669682 | FJ530973 |
| P. cvjetkovicii | NRRL35841 (T) | KF932931 | KF932948 |
| P. ellipsoideosporeum | CBS112493 (T) | JQ965104 | AY678559 |
| P. fluviserpens | NRRL35838 (T) | KF932929 | KF932946 |
| P. fluviserpens | NRRL35844 | KF932933 | KF932950 |
| P. fluviserpens | CNUFC TM6-2 | MN894578 | MN317092 |
| P. fluviserpens | CNUFC TM6-3 | MN894579 | MN317093 |
| P. gravinicasei | NRRL66733 (T) | MG600565 | MG600570 |
| P. idahoense | NRRL5274 (T) | EF626953 | EF626954 |
| P. incoloratum | CBS101753 (T) | KJ834457 | KJ866984 |
| P. jamesonlandense | CBS102888 (T) | DQ309448 | KJ866985 |
| P. janczewskii | CBS221.28 (T) | KJ834460 | KJ867001 |
| P. kojigenum | CBS345.61 (T) | KJ834463 | KJ867011 |
| P. lanosum | CBS106.11 (T) | DQ285627 | FJ530974 |
| P. lemhiflumine | NRRL35843 (T) | KF932932 | KF932949 |
| P. lenticrescens | CBS138215 (T) | KJ775168 | KJ775404 |
| P. malacaense | NRRL35754 (T) | EU427268 | KJ866997 |
| P. mexicanum | CBS138227 (T) | KJ775178 | KJ775412 |
| P. monsgalena | NRRL22302 (T) | KF932927 | KF932943 |
| P. monsserratidens | NRRL35884 (T) | KF932930 | KF932947 |
| P. nodulum | CBS227.89 (T) | KJ834475 | KJ867003 |
| P. novae-zeelandiae | CBS137.41 (T) | KJ834477 | KJ866996 |
| P. paradoxum | NRRL2162 (T) | EF669683 | EF669692 |
| P. parvulum | NRRL35504 (T) | EF506218 | EF506225 |
| P. pusillum | NRRL2498 (T) | KF932925 | KF932941 |
| P. raistrickii | CBS261.33 (T) | KJ834485 | KJ867006 |
| P. ribeum | CBS127809 (T) | DQ285625 | KJ866995 |
| P. sajarovii | CBS277.83 (T) | KJ834489 | KJ867007 |
| P. salmoniflumine | NRRL35837 (T) | KF932928 | KF932945 |
| P. scabrosum | CBS683.89 (T) | DQ285610 | FJ530987 |
| P. scabrosum | CNUFC WD17-1 | MN317088 | MN317090 |
| P. scabrosum | CNUFC WD17-2 | MN317089 | MN317091 |
| P. shennangjianum | CBS228.89 (T) | KJ834491 | AY678561 |
| P. simile | CBS129191 (T) | FJ376595 | GQ979710 |
| P. soppii | CBS226.28 (T) | DQ285616 | KJ867002 |
| P. swiecickii | CBS119391 (T) | KJ834494 | KJ866993 |
| P. virgatum | CBS114838 (T) | KJ834500 | KJ866992 |
| Talaromyces flavus | NRRL2098 (T) | EU021663 | EU021694 |
Bold letters indicate isolates and accession numbers determined in our study.
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; CCF: Culture Collection of Fungi, Charles University, Prague, Czech Republic; CNUFC: Chonnam National University Fungal Collection (Gwangju, South Korea); NRRL: ARS culture collection, Peoria, IL, USA; T: ex-type strain; NT: neo-type.
3. Results
3.1. Phylogenetic analysis
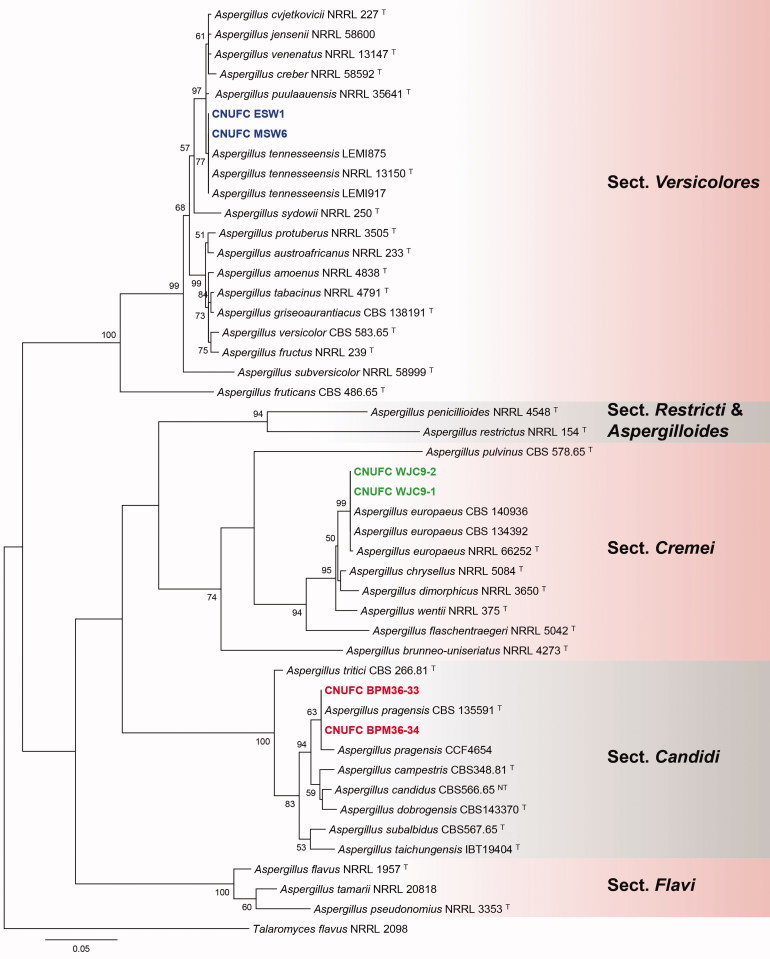
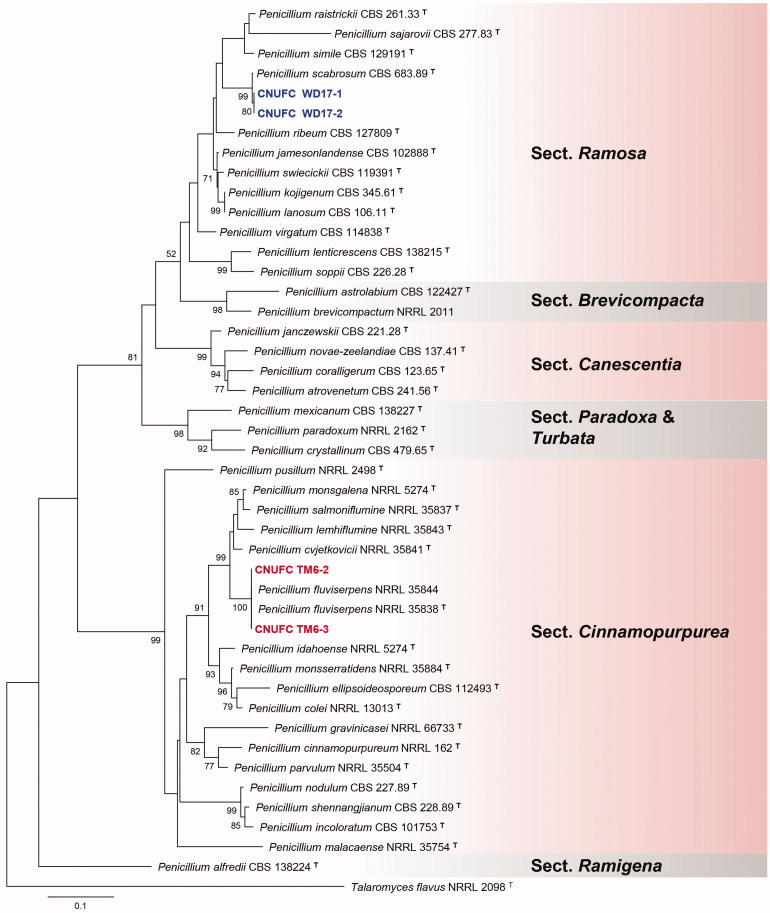
A BLASTn search of the BenA regions of CNUFC WJC9-1, CNUFC BPM36-33, CNUFC ESW1, CNUFC TM6-2, and CNUFC WD17-1 showed similarities of 100% (405/405 bp), 100% (447/447 bp), 100% (399/399 bp), 99.6% (559/561 bp), and 99.6% (551/553 bp), with A. europaeus (LN909018), A. pragensis (HG916673), A. tennesseensis (KJ76999), P. fluviserpens (KF932929), and P. scabrosum (DQ285610), respectively. Similarly, CaM regions of CNUFC WJC9-1, CNUFC BPM36-33, CNUFC ESW1, CNUFC TM6-2, and CNUFC WD17-1, showed similarities of 99.6% (507/509 bp), 100% (590/590 bp), 100% (642/642 bp), 100% (632/632 bp), and 99.3% (421/424 bp), with A. europaeus (LN909019), A. pragensis (FR751452), A. tennesseensis (KJ766995), P. fluviserpens (KF932946), and P. scabrosum (FJ530987), respectively. ML gene trees for BenA and CaM revealed that the strains, CNUFC WJC9-1, CNUFC BPM36-33, CNUFC ESW1, CNUFC TM6-2, and CNUFC WD17-1 were identical to A. europaeus, A. pragensis, A. tennesseensis, P. fluviserpens, and P. scabrosum, respectively (Figures 1 and 2).
Figure 1.
Phylogenetic tree of Aspergillus europaeus CNUFC WJC9-1 and CNUFC WJC9-2, A. pragensis CNUFC BPM36-33 and CNUFC BPM36-34, A. tennesseensis CNUFC ESW1 and CNUFC MSW6, and related species based on maximum likelihood analysis of the combined BenA and CaM sequences. The sequence of Talaromyces flavus was used as an out group. Numbers at the nodes indicate the bootstrap values (>50%) from 1000 replicates. The bar indicates the number of substitutions per nucleotide. The study isolates are shown in bold blue, green, and red.
Figure 2.
Phylogenetic tree of Penicillium fluviserpens CNUFC TM6-2 and CNUFC TM6-3, P. scabrosum CNUFC WD17-1 and CNUFC WD17-2 and related species, based on the maximum likelihood analysis of the combined BenA and CaM sequences. Sequence of Talaromyces flavus was used as an out group. Numbers at the nodes indicate the bootstrap values (>50%) from 1000 replicates. The bar indicates the number of substitutions per nucleotide. The study isolates are shown in bold blue and red.
3.2. Taxonomy
3.2.1. Taxonomy of CNUFC WJC9-1
A. europaeus Hubka, A. Nováková, Samson, Houbraken, Frisvad, M. Kolařík, Plant Systematics and Evolution 302: 645 (2016) (Table 3; Figure 3).
Table 3.
Morphological characteristics of CNUFC WJC9-1 compared with those of the reference strain, Aspergillus europaeus.
| Character | CNUFC WJC9-1 | Aspergillus europaeusa |
|---|---|---|
| Conidiophores | Smooth-walled, 6.5–11.5 μm wide | 300–750 × 7–13(−15) µm |
| Vesicle | Pyriform or globose, 11–32 μm diam., | Pyriform or globose, 11–44 μm diam., |
| Metuale | Broadening toward the top, 6–13.5 × 3–4.5 μm | Broadening toward the top, 6–25 × 5–9 μm |
| Phialides | Ampulliform, 6–9.2 × 2.4–3.9 μm | Ampuliform, 6–11.5 × 3–6 μm |
| Conidia | Globose to subglobse, 3.1–4.6 × 3.2–4.2 μm | Globose to subglobse, 3.5–5 × 3–4.5 μm |
From the description by Hubka et al. [37].
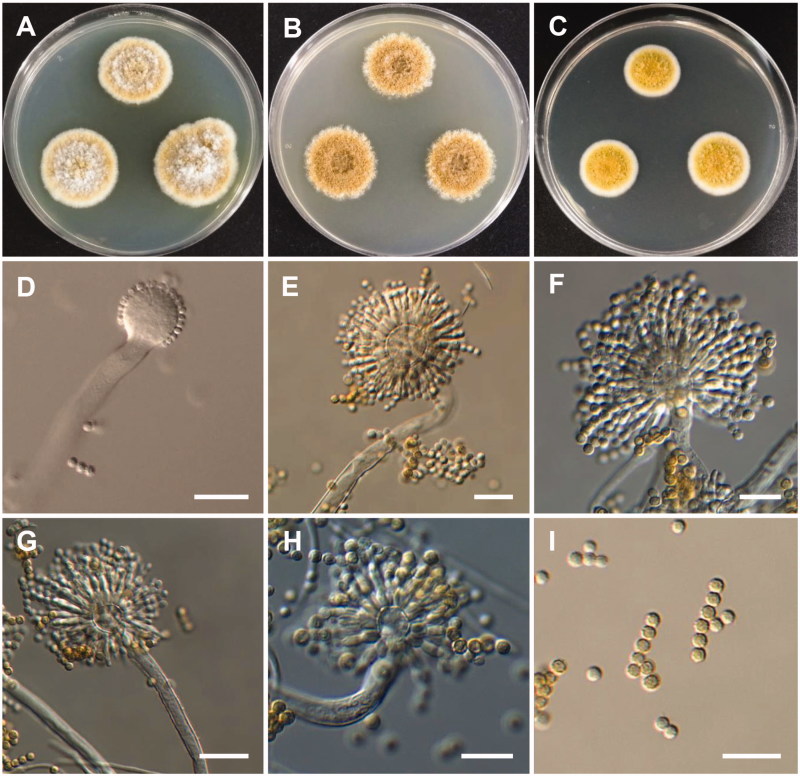
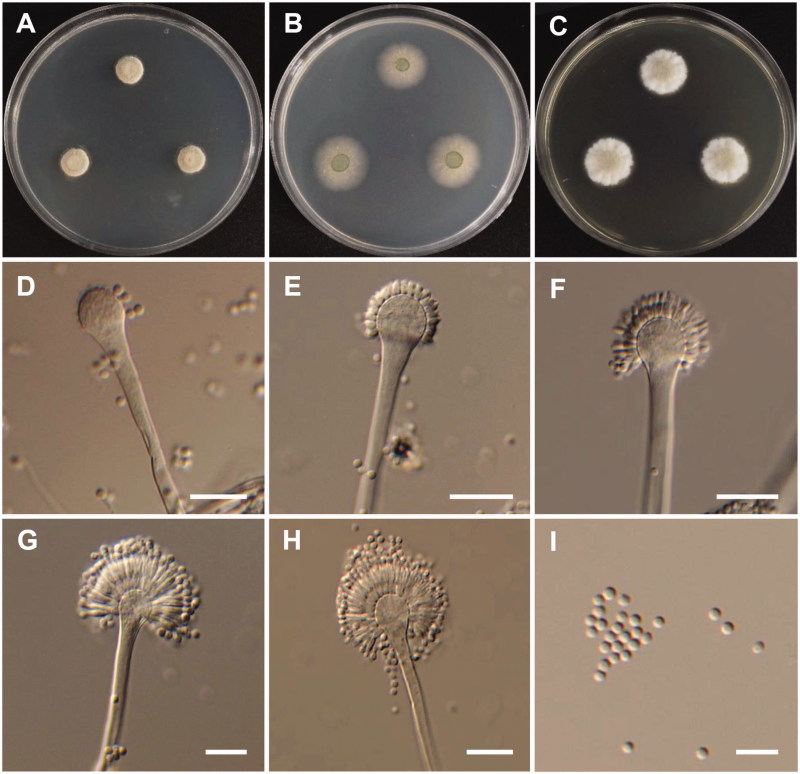
Figure 3.
Morphology of Aspergillus europaeus. (A) Colonies on Potato dextrose agar (PDA); (B) Colonies on Blakeslee’s malt extract agar (MEA); (C) Czapek yeast autolysate agar (CYA); (D–H) Conidiophores; (I) Conidia (scale bars: D–I = 20 μm).
Colony characteristics: Colonies on CYA were floccose, pale yellow to dark brown, with yellowish white mycelium, no soluble pigment, moderate sporulation, reverse yellow, and reached 11–13 mm in diameter after 7 d at 25 °C. On MEA, colonies were floccose, with a raised colony center, no soluble pigment, the reverse was pale yellow, and reached 20–22 mm in diameter after 7 d at 25 °C. On PDA, colonies were plane, floccose in the colony center, with strong sporulation, no soluble pigment, reverse light olive, and reached 21–23 mm in diameter after 7 d at 25 °C.
Micromorphology: Conidiophores were smooth-walled, 6.5–11.5 µm wide. Vesicles were pyriform or globose, and were 11–32 µm in diameter. Metulae were broadened toward the top, 6–13.5 × 3–4.5 µm. Phialides were ampulliform, 6–9.2 × 2.4–3.9 µm. Conidia were globose to subglobose, roughened, and yellow-brown to brown at maturity, 3.1–4.6 × 3.2–4.2 µm.
3.2.2. Taxonomy of CNUFC BPM36-33
A. pragensis V. Hubka, J. C. Frisvad & M. Kolařík, Medical Mycology 52: 565–576 (2014) (Table 4; Figure 4).
Table 4.
Morphological characteristics of CNUFC BPM36-33 compared with those of the reference strain Aspergillus pragensis.
| Character | CNUFC BPM36-33 | Aspergillus pragensisa |
|---|---|---|
| Conidiophores | Smooth-walled, 89–400 μm long | Hyaline, smooth-walled, usually 90–600 μm (but up to 1200 μm) |
| Vesicle | Pyriform or globose, 9–23 μm | Predominantly globose, 9–21 μm |
| Metuale | Cylindrical or wedge-shaped, measured 3.8–11.4 × 2.6–3.3 μm | Wedge-shaped or cylindrical, 4.5–10.5 × 3.5–5.5 μm |
| Phialides | Ampulliform, 4–8 × 2.6–4.1 μm | Ampuliform, 6–8.5 × 2.5–3.5 μm |
| Conidia | Globose with rough echinulate walls, 2.6–3.4 μm | Globose, 2.5–3.5 μm, smooth |
From the description by Hubka et al. [41].
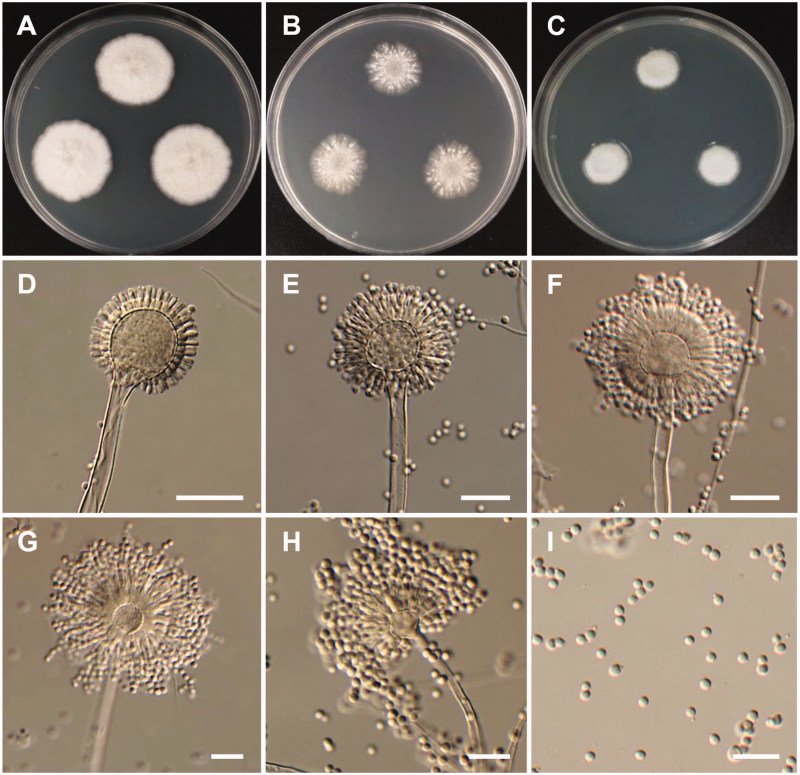
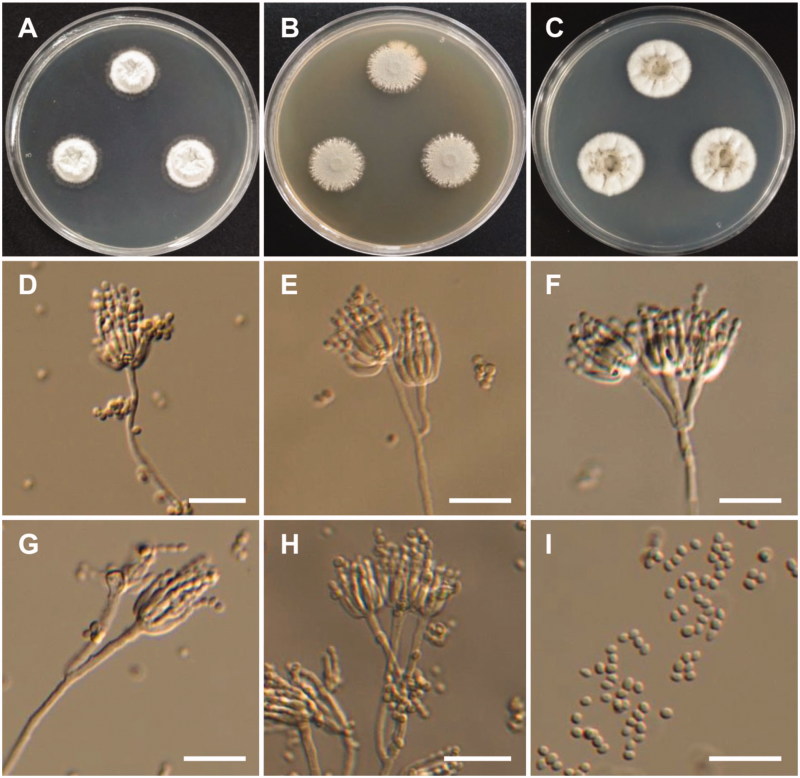
Figure 4.
Morphology of Aspergillus pragensis. (A) Colonies on Czapek yeast autolysate agar (CYA); (B) Colonies on Blakeslee’s malt extract agar (MEA); (C) Colonies on Potato dextrose agar (PDA); (D–H) Conidiophores; (I) Conidia (scale bars: D–I = 20 μm).
Colony characteristics: Colonies on CYA were white, with a floccose surface, reverse light brown, and no diffusible pigment, and reached 10–13 mm in diameter after 7 d at 25 °C. On MEA, colonies were pinkish white, with a floccose surface, slightly raised at the center, no diffusible pigment, the reverse was puff brown, and reached 5–8 mm in diameter after 7 d at 25 °C. On PDA, colonies were white, slightly raised at the center, no sporulation, and reached 4–6 mm in diameter after 7 d at 25 °C.
Micromorphology: Conidiophores were smooth-walled, and were 89–400 µm in diameter. Vesicles were pyriform or globose, and were 9–23 µm in diameter. Metulae were cylindrical or wedge-shaped, 3.8–11.4 × 2.6–3.3 µm. Phialides were ampulliform, 4–8 × 2.6–4.1 µm. Conidia were globose with rough echinulate walls, and were 2.6–3.4 µm in diameter.
3.2.3. Taxonomy of CNUFC ESW1
A. tennesseensis Jurjević, S.W. Peterson & B.W. Horn, IMA Fungus 3 (1): 73 (2012) (Table 5; Figure 5).
Table 5.
Morphological characteristics of CNUFC ESW1 compared with those of the reference strain Aspergillus tennesseensis.
| Character | CNUFC ESW1 | Aspergillus tennesseensisa |
|---|---|---|
| Conidiophores | Greenish smooth walled, yellow to brown, sometimes hyaline to brownish shades 18.5–413.5 × 4.2–7.3 μm | Smooth walled, hyaline to yellowish with brownish shades (35–)100–300(–400) × 4–7 μm |
| Vesicle | Pyriform, 7.5–17.6 μm | Pyriform (7–)10–16(–18) μm |
| Metulae | 4–7.1 × 2.4–4.1 μm | (4–)6– (–8) × 2.5–4 μm |
| Phialides | Forming chains resembling penicillate fructifications, 5.5–11.4 × 2.1–3 μm | Fragmentary heads resembling penicillate fructifications, 5–8(–11) × 2–3 μm |
| Conidia | Conidia globose, spherical, finely roughened, 2.4–5.1 μm | Spherical to subspherical, ellipsiodal to pyriform (2.5–)3–4(–8) μm, roughened wall |
From the description by Jurjevic et al. [50].
Figure 5.
Morphology of Aspergillus tennesseensis. (A) Colonies on Czapek yeast autolysate agar (CYA); (B) Colonies on Blakeslee’s malt extract agar (MEA); (C) Colonies on yeast malt extract agar (YES); (D–H) Conidiophores. (I) Conidia (scale bars: D–I = 20 μm).
Colony characteristics: Colonies on CYA were radially sulcate, centrally raised, pea-green in color, with central sporulation, no soluble pigments, and exudates were observed in some isolates, the reverse was brown-yellow in color, and reached 19–22 mm in diameter after 7 d at 25 °C. On MEA, colonies were plane, mycelia green white at the margins to dark green color at the centers, no soluble pigments or exudates, reverse gray-green or pale lemon yellow, and reached 12–14 mm in diameter after 7 d at 25 °C. On YES, colonies were floccose, mycelial pale white at margins to gray-green at the center, centrally sparse sporulation, no soluble pigments, no exudates, reverse dull orange to pale brown, and reached 23–26 mm in diameter after 7 d at 25 °C.
Micromorphology: Conidial heads biseriate, conidiophores greenish, smooth-walled stipes typically yellow to brown, sometimes hyaline to brownish shades, 18.5–413.5 × 4.2–7.3 µm. Vesicles were pyriform, 7.5–17.6 µm in diameter. Metulae were 4–7.1 × 2.4–4.1 µm. Phialides forming chains resembling penicillate fructifications, 5.5–11.4 × 2.1–3 µm. Conidia were globose, spherical, and finely roughened, and were 2.4–5.1 µm in diameter.
3.2.4. Taxonomy of CNUFC TM6-2
P. fluviserpens S. W. Peterson, Z. Jurjevic & J.C. Frisvad, PloS One 10: 1–28 (2015) (Table 6; Figure 6).
Table 6.
Morphological characteristics of CNUFC TM6-2 compared with those of the reference strain Penicillium fluviserpens.
| Character | CNUFC TM6-2 | Penicillium fluviserpensa |
|---|---|---|
| Conidiophores | Monoverticillate, 17–110 μm | Smooth to finely roughened, monoverticillate (5–) 30–130 (–180) μm |
| Phialides | Ampulliform, 5.5–9 × 2–3.2 μm | Ampulliform (5–) 6–8 (–32) × 2–3.5 μm |
| Conidia | Ellipsoidal to sub-spherical, 2.5–3.3 µm in diameter | Ellipsoidal to sub-spherical 2.5–3.5(–7) μm |
From the description by Peterson et al. [63].
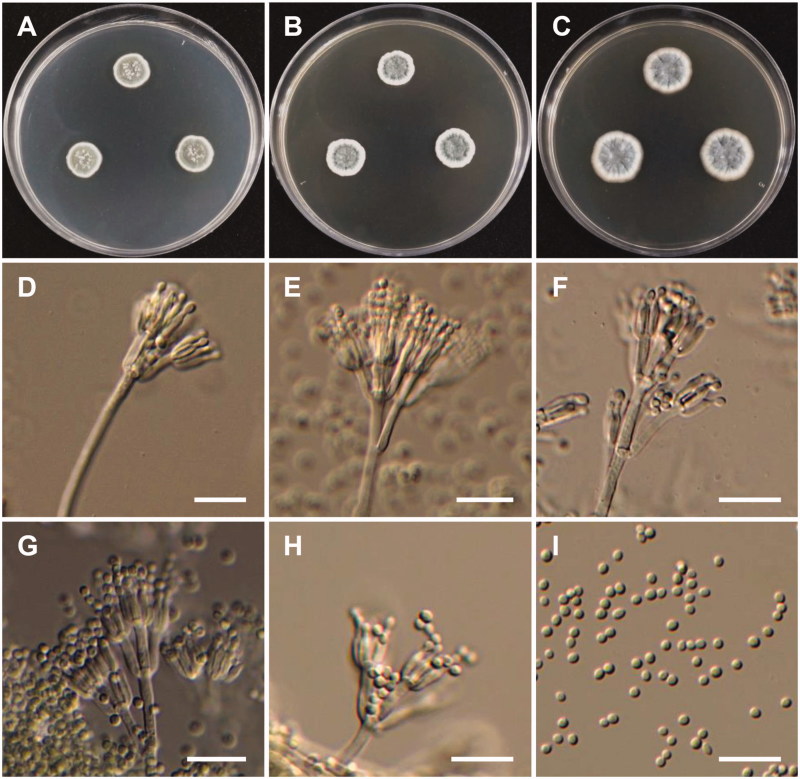
Figure 6.
Morphology of Penicillium fluviserpens. (A) Colonies on Czapek yeast autolysate agar (CYA); (B) Colonies on Blakeslee’s malt extract agar (MEA); (C) Colonies on Potato dextrose agar (PDA); (D–H) Conidiophores; (I) Conidia (scale bars: D–I = 20 μm).
Colony characteristics: Colonies on CYA were velutinous, pale gray-green, radially sulcate at margins and sulcate to wrinkled centrally, white mycelium, abundant sporulation, no soluble pigment, reverse pale caramel brown, and reached 21–23 mm in diameter after 7 d at 25 °C. On MEA, colonies were calendine green, velutinous, lightly sulcate, with moderate sporulation, reverse was pale green, and reached 24–25 mm in diameter after 7 d at 25 °C. On PDA, colonies were moderately deep, with white mycelia, pale gray-green at the center, no sporulation, no soluble pigment, and reached 23–27 mm in diameter after 7 d at 25 °C.
Micromorphology: Conidiophores were monoverticillate, 17–110 µm. Phialides were ampulliform, 5.5–9 × 2–3.2 µm. Conidia were sub-spherical to ellipsoidal, and were 2.5–3.3 µm in diameter.
3.2.5. Taxonomy of CNUFC WD17-1
P. scabrosum Frisvad, Samson & Stolk, Persoonia 14 (2): 177 (1990) (Table 7; Figure 7).
Table 7.
Morphological characteristics of CNUFC WD17-1 compared with those of the reference strain Penicillium scabrosum.
| Character | CNUFC WD17-1 | Penicillium scabrosuma |
|---|---|---|
| Conidiophores | Biverticillate, 2.9–4.5 μm | Biverticillate, 200–400 × 3–4 μm |
| Metulae | 2–4, 10.3–18.5 × 2.3–4.1 μm | 10–20 × 2.5–4.0 μm |
| Phialides | Ampulliform, 4–8 per metula, 8.6–11.8 × 2.1–3.1 μm. | 5–12 per metula, 7–11 × 2.0–2.5 μm |
| Conidia | Globose to subglobose, 2.3–3.2 µm | Globose to subglobose, 2.4–3.2 µm |
From the description by Frisva et al. [67].
Figure 7.
Morphology of Penicillium scabrosum. (A) Colonies on Czapek yeast autolysate agar (CYA); (B) Colonies on Blakeslee’s malt extract agar (MEA); (C) Colonies on yeast malt extract agar (YES); (D–H) Conidiophores; (I) Conidia (scale bars: D–I = 20 μm).
Colony characteristics: Colonies on CYA were plane, radially wrinkled, with good sporulation, white or green mycelium, green conidia, no soluble pigment, reverse bright yellow, and reached 13–16 mm in diameter after 7 d at 25 °C. On MEA, colonies were velutinous, with good sporulation, green conidia, no soluble pigments, radially sulcate at margins, and the reverse was yellow, and reached 14–17 mm in diameter after 7 d at 25 °C. On YES, colonies were radially wrinkled, centrally floccose, no soluble pigment, reverse yellow, and reached 24–27 mm in diameter after 7 d at 25 °C.
Micromorphology: Conidiophores were biverticillate, 2.9–4.5 µm. Metulae were 10.3–18.5 × 2.3–4.1 µm, 2–4. Phialides were ampulliform, 4–8 per metula, 8.6–11.8 × 2.1–3.1 µm. Conidia were globose to subglobose, and were 2.3–3.2 µm in diameter.
4. Discussion
Species of Aspergillus and Penicillium belonging to the sections Cremei, Candidi, Versicolores, Cinnamopurpurea, and Ramosa, were discovered during a survey on the biodiversity of Aspergillaceae inhabiting different substrates. Here, three Aspergillus and two Penicillium species in five different sections were identified and compared to their most closely related species.
Analysis of the combined BenA and CaM datasets showed that the strains CNUFC WJC9-1 and CNUFC WJC9-2 were clustered within the same clade as A. europaeus NRRL 66252 (ex-type strain), belonging to the section Cremei (Figure 1). The isolate CNUFC WJC9-1 is morphologically similar to A. europaeus previously described by Hubka et al. [37] with respect to producing globose to subglobose coarsely roughened conidia, with a yellow-brown to brown color at maturity. However, the sizes of metuale reported in the literature (6–25 × 5–9 µm) are bigger than those of our isolate. Aspergillus section Cremei (known as the A. cremeus group) was first described by Raper and Fennell [38] and included five species, A. itaconicus, A. flaschentraegeri, A. stromatoides, A. chrysella, and A. cremea. The species belonging to this section are characterized by their yellowish-brown to brown or gray-green colony color, with biseriate conidial heads and long conidiophores [2]. Several fungal species belonging to the section Cremi are frequently found in soil and foods where they can cause spoilage of cereals and nuts; they are found less frequently in indoor environments or in clinical material [37,39]. A. europaeus was earlier reported from soil samples in European caves and several steppe-like localities in the Czech Republic [37]. In this study, A. europaeus was isolated from corn grains. A. europaeus shares the production of 3-O-methylsulochrin and 3-O-demethylsulochrin with A. wentii [37,40].
The strains CNUFC BPM36-33 and CNUFC BPM36-34 reside in a well-supported clade with A. pragensis CBS 135591 (ex-type strain), belonging to the section Candidi (Figure 1). The morphological characteristics of the isolate A. pragensis in this study were similar to those previously described by Hubka et al. [41]. Colony diameter on CYA was similar to that of the previously described A. pragensis type species (CYA: 22–24 mm after 14 d); however, differences in colony diameter were observed on MEA (MEA: 16–18 mm after 14 d). No growth was observed for the isolate CNUFC BPM36-33 on MEA at 37 °C. The Aspergillus section Candidi was established by Gams et al. [42] for the previous A. candidus group based on the criterion proposed by Thom and Raper [43]. Currently, this section includes seven species [41,44], isolated from dust, cave air, carpet, mouse dung, herbivore dung, cave sediment, bat droppings and guano, indoor environments, and clinical samples [44,45]. These are economically significant species, which are used in biotechnology sectors; these species are used as starter cultures for the production of food sauces, alcoholic beverages, production of extracellular enzymes, and waste degradation. They are also known to produce many bioactive compounds including antimicrobial, anti-oxidative, antitumor, and cytotoxic compounds [44]. In addition, these species are also known to cause human infection, namely, onychomycosis, invasive aspergillosis, otomycosis, and pulmonary aspergilloma [46,47]. Two species, A. candidus and A. tritici were isolated from Meju samples in Korea [48]. A. pragensis was recovered from human clinical material (nail) and was found to be responsible for causing onychomycosis in the Czech Republic [41]; it has also been isolated from rock samples from unnamed Karst caves in Suiyang located beside the Kuankuoshui National Natural Reserve, China [49]. In this study, A. pragensis was isolated from a by-product of rice bran.
The strains CNUFC MSW6 and CNUFC ESW1 were clustered within the same clade as A. tennesseensis NRRL 13150 (ex-type strain) in the section Versicolores (Figure 1). The isolate CNUFC ESW1 was morphologically most similar to A. tennesseensis as described by Jurjevic et al. [50], although there were differences in the length and color of conidiophores. The conidiophores described by Jurjevic et al. [50] were (35–)100–300(–400) µm in length, while our isolates were 18.5–413.5 µm in length. The Aspergillus section Versicolores was first described by Thom and Church [51]. Members of this section are found in soil, foods items [45], toxic dairy feed [50], and indoor environments [4,52,53], and can cause diseases in humans and animals [54,55]. Some species of this section produce kipukasins, nucleoside derivates, and the mycotoxin, sterigmatocystin [56–58]. A. tennesseensis has been reported to produce various compounds such as versicoamides F–H, prenylated indole alkaloids, diorcinol L, and (R)-diorcinol B [59,60]. In Korea, there are only six species reported to belong to the section Versicolores, including A. creber, A. jensenii, A. nidulans, A. sydowii, A. tabacinus, and A. versicolor. These were isolated from different sources including meju [48], chronic granulomatous patient [61], poultry farming soil [62], tidal mudflats, and sea sand [17]. In this study, A. tennesseensis was isolated from sea water and dead moths.
The strains CNUFC TM6-2 and CNUFC TM6-3 are well placed with other species in the Penicillium section Cinnamopurpurea as shown in Figure 2. The morphological features of our isolates were in line with the description of P. fluviserpens by Peterson et al. [63]. However, the isolate CNUFC TM6-2 exhibited a colony measurement which differed from that of the description of P. fluviserpens on CYA (10–12 mm), MEA (8–11 mm), and PDA (10–12 mm) by Peterson et al. [63]. Members of this section are slow-growing, often with brown reverse on some media and mostly produce colonies with similar morphologies, subglobose to ellipsoidal, smooth to finely roughened spores, and have monoverticillate to divaricate biverticillate smooth-walled conidiophores. This section contains about 16 species [24,63,64]. Only two species, P. chermesinum (recently found to have phylogeny similar to P. cvjetkovicii) and P. malacaense were reported from meju samples in Korea with no detailed description [65]. Species in this section are known to produce the human lung tumor inhibitor compound, citreoviridin, and are commonly isolated from pecans, moldy nuts, air samples and hospital environments [63]. P. fluviserpens was previously isolated from air sampler from different locations, USA, California, Pennsylvania [63] and as endophytes from coffee plants in Colombia [66]. Interestingly, the present isolates in our study are from a tomato sample.
The strains CNUFC WD17-1 and CNUFC WD17-2 were grouped with P. scabrosum CBS 683.89 (ex-type strain) in the phylogenetic analysis of BenA and CaM sequences, and belong to the section Ramosa (Figure 2). There were differences observed with respect to the number of phialides per metula and colony diameter for the isolate P. scabrosum in comparison to previous descriptions by Frisvad et al. [67]. However, the size and shape of phialides and conidia were similar to those of the described species. Penicillium species in this section are characterized by biverticillate or terverticillate conidiophores [1]. They are commonly isolated from soil [67,68], but it is also found in sea water, fruiting bodies of Chroogomphus rutilus, and Tunisian orchard apples [69–71]. P. scabrosum has been reported to produce cyclopenin, cyclopenol, viridicatin, fumagillin, as well as a large number of unknown metabolites [67]. Larsen et al. [72] found two metabolites produced by P. scabrosum to be penigequinolone A and B. To the best of our knowledge, this is the first report of isolation of P. scabrosum from a freshwater sample.
Different species of the genus Penicillium have been reported to produce a variety of bioactive extrolites, including mycotoxins citrinin and patulin. Andersen and Frisvad [73] showed that P. tularense isolated from tomato fruit could produce janthitrems, paspalinine, paxilline, and 3-O-acetoxypaxilline. Harwig et al. [74] have reported that P. expansum is capable of producing patulin and citrinin in tomato fruit. Our strain P. fluviserpens was also isolated from tomato fruit. Therefore, it suggested that the strain may also produce mycotoxins as well as secondary metabolites. Interestingly, in this study, strains of A. tennesseensis found on the moths may be a potential as new biopesticide. Isolation and descriptions of new record from specific substrates and habitats, like freshwater, sea water, and dead moths, will be added to our knowledge on fungal diversity. Further studies are needed to better understand the ecological roles of both Aspergillus and Penicillium on different substrates. More studies on extrolites production and their ecological roles, the production of extracellular enzymes and antimicrobial compounds are needed.
Funding Statement
This study was financially supported by Chonnam National University [Grant number: 2017-2827]. This work was in part supported by the Graduate Program for the Undiscovered Taxa of Korea, the Project on Survey and Discovery of Indigenous Fungal Species of Korea funded by NIBR, and the Project on Discovery of Fungi from Freshwater and Collection of Fungarium, funded by NNIBR of the Ministry of Environment (MOE).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Houbraken J, Samson RA. Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud Mycol. 2011;70:1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samson RA, Visagie CM, Houbraken J, et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol. 2014;78:141–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houbraken J, de Vries RP, Samson RA, et al. Modern taxonomy of biotechnologically important Aspergillus and Penicillium species Adv Appl Microbiol. 2014;86:199–249. [DOI] [PubMed] [Google Scholar]
- 4.Visagie CM, Hirooka Y, Tanney JB, et al. Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Stud Mycol. 2014;78:63–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frisvad JC, Smedsgaard J, Larsen TO. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud Mycol. 2004;49:201–241. [Google Scholar]
- 6.Chávez R, Bull P, Eyzaguirre J. The xylanolytic enzyme system from the genus Penicillium. J Biotechnol. 2006;123:413–433. [DOI] [PubMed] [Google Scholar]
- 7.Ichishima E. Development of enzyme technology for Aspergillus oryzae, A. sojae, and A. luchuensis, the national microorganisms of Japan. Biosci Biotechnol Biochem. 2016;80:1681–1692. [DOI] [PubMed] [Google Scholar]
- 8.Latgé JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon-Chung KJ, Sugui JA. Aspergillus fumigatus—what makes the species a ubiquitous human fungal pathogen? PLoS Pathog. 2013;9:e1003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan JFW, Lau SKP, Yuen KY, et al. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect. 2016;5:e19–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Micheli PA. Nova Plantarum Genera juxta Tournefortii methodum disposita. Florence: (Italy): Typis Bernardi Paperinii; 1729. [Google Scholar]
- 12.Hubka V, Nováková A, Kolařík M, et al. Revision of Aspergillus section Flavipedes: seven new species and proposal of section Jani sect. nov. Mycologia. 2015;107:169–208. [DOI] [PubMed] [Google Scholar]
- 13.Gautier M, Normand AC, Ranque S. Previously unknown species of Aspergillus. Clin Microbiol Infect. 2016;22:662–669. [DOI] [PubMed] [Google Scholar]
- 14.Raja HA, Miller AN, Pearce CJ, et al. Fungal identification using molecular tools: a primer for the natural products research community. J Nat Prod. 2017;80:756–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HJ, Kim JS, Cheon KH, et al. Species list of Aspergillus, Penicillium and Talaromyces in Korea, based on ‘One Fungus One Name’ System. Kor J Mycol. 2016;44:207–219. [Google Scholar]
- 16.Hyde KD, Hongsanan S, Jeewon R, et al. Fungal diversity notes 367-491: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016;80:1–270. [Google Scholar]
- 17.Lee S, Park MS, Lim YW. Diversity of marine-derived Aspergillus from tidal mudflats and sea sand in Korea. Mycobiology. 2016;44:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KM, Lim J, Lee JJ, et al. Characterization of Aspergillus sojae isolated from Meju, Korean traditional fermented soybean brick. J Microbiol Biotechnol. 2017;27:251–261. [DOI] [PubMed] [Google Scholar]
- 19.Tagele SB, Adhikari M, Gurung SK, et al. New records of Aspergillus allahabadii and Penicillium sizovae from crop field soil in Korea. Mycobiology. 2018;46:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adhikari M, Gurung SK, Kim SW, et al. Aspergillus caninus (Syn: Phialosimplex caninus): a new isolate from field soils in Korea. Kor J Mycol. 2018;46:383–392. [Google Scholar]
- 21.Link HF. Observationes in ordines plantarum naturales. Dissertatio 1. Mag Ges Naturf Freunde Berlin. 1809;3:3–42. [Google Scholar]
- 22.Houbraken J, Wang L, Lee HB, et al. New sections in Penicillium containing novel species producing patulin, pyripyropens or other bioactive compounds. Pers Int Mycol J. 2016;36:299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diao YZ, Chen Q, Jiang XZ, et al. Penicillium section Lanata‐divaricata from acidic soil. Cladistics. 2018;35:514–549. [DOI] [PubMed] [Google Scholar]
- 24.Visagie CM, Houbraken J, Frisvad JC, et al. Identification and nomenclature of the genus Penicillium. Stud Mycol. 2014;78:343–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pangging M, Nguyen TTT, Lee HB. New records of four species belonging to Eurotiales from soil and freshwater in Korea. Mycobiology. 2019;47:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park MS, Chung D, Baek K, et al. Three unrecorded species belonging to Penicillium section Sclerotiora from marine environments in Korea. Mycobiology. 2019;47:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phookamsak R, Hyde KD, Jeewon R, et al. Fungal diversity notes 929–1035: taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers. 2019;95:1–273. [Google Scholar]
- 28.Nguyen TTT, Lee HB. Isolation and characterization of three Zygomycetous fungi in Korea: Backusella circina, Circinella muscae, and Mucor ramosissimus. Mycobiology. 2018;46:317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen TT, Choi YJ, Lee HB. Isolation and characterization of three unrecorded Zygomycete fungi in Korea: Cunninghamella bertholletiae, Cunninghamella echinulata, and Cunninghamella elegans. Mycobiology. 2017;45:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 1995;61:1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong SB, Cho HS, Shin HD, et al. Novel Neosartorya species isolated from soil in Korea. Int J Syst Evol Microbiol. 2006;56:477–486. [DOI] [PubMed] [Google Scholar]
- 32.Peterson SW, Vega F, Posada F, et al. Penicillium coffeae, a new endophytic species isolated from a coffee plant and its phylogenetic relationship to P. fellutanum, P. thiersii and P. brocae based on parsimony analysis of multilocus DNA sequences. Mycologia. 2005;97:659–666. [DOI] [PubMed] [Google Scholar]
- 33.Yilmaz N, Visagie CM, Houbraken J, et al. Polyphasic taxonomy of the genus Talaromyces. Stud Mycol. 2014;78:175–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson JD, Gibson TJ, Plewniak F, et al. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 36.Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hubka V, NováKová A, Samson RA, et al. Aspergillus europaeus sp. nov., a widely distributed soil-borne species related to A. wentii (section Cremei). Plant Syst Evol. 2016;302:641–650. [Google Scholar]
- 38.Raper KB, Fennell DI. The genus Aspergillus. Baltimore (MD): Williams & Wilkins; 1965. [Google Scholar]
- 39.Rainer J, Peintner U, Pöder R. Biodiversity and concentration of airborne fungi in a hospital environment. Mycopathologia. 2001;149:87–97. [DOI] [PubMed] [Google Scholar]
- 40.Rabie C, Terblanche M. Influence of temperature on the toxicity of different isolates of Aspergillus wentii Wehm. S Afr J Agr Sci. 1967;10:263–266. [Google Scholar]
- 41.Hubka V, Lyskova P, Frisvad JC, et al. Aspergillus pragensis sp. nov. discovered during molecular reidentification of clinical isolates belonging to Aspergillus section Candidi. Med Mycol. 2014;52:565–576. [DOI] [PubMed] [Google Scholar]
- 42.Gams W, Christensen M, Onions AH, et al. Infrageneric taxa of Aspergillus In: Samson RA, Pitt JI, editors. Advances in Penicillium and Aspergillus systematics. New York (NY): Plenum Press; 1985. p. 55–62. [Google Scholar]
- 43.Thom C, Raper KB. A manual of the aspergilli. Baltimore(MD): Williams & Wilkins; 1945. [Google Scholar]
- 44.Hubka V, Nováková A, Jurjević Ž, et al. Polyphasic data support the splitting of Aspergillus candidus into two species; proposal of Aspergillus dobrogensis sp. nov. Int J Syst Evol Microbiol. 2018;68:995–1011. [DOI] [PubMed] [Google Scholar]
- 45.Pitt JI, Hocking AD. Fungi and food spoilage. 3rd ed New York (NY): Springer; 2009. [Google Scholar]
- 46.de Hoog GS, Guarro J, Gené J, et al. Atlas of clinical fungi. 3rd ed Utrecht (the Netherlands): CBS-KNAW Fungal Biodiversity Centre; 2009. [Google Scholar]
- 47.Hubka V, Kubatova A, Mallatova N, et al. Rare and new aetiological agents revealed among 178 clinical Aspergillus strains obtained from Czech patients and characterized by molecular sequencing. Med Mycol. 2012;50:601–610. [DOI] [PubMed] [Google Scholar]
- 48.Hong SB, Kim DH, Samson RA. Aspergillus associated with Meju, a fermented soybean starting material for traditional soy sauce and soybean paste in Korea. Mycobiology. 2015;43:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang ZF, Liu F, Zhou X, et al. Culturable mycobiota from Karst caves in China with descriptions of 20 new species. Persoonia. 2017;39:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jurjevic Z, Peterson SW, Horn BW. Aspergillus section Versicolores: nine new species and multilocus DNA sequence based phylogeny. IMA Fungus. 2012;3:59–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thom C, Church MB. The Aspergilli. Baltimore (MD): Williams and Wilkins Co; 1926. [Google Scholar]
- 52.Samson R, Houbracken K, Summerbell RC, et al. Common and important species of Actinomycetes and fungi in indoor environments In: Flannigan B, Samson R, Miller JD, editors. Microorganisms in home and indoor work environments. New York (NY): Taylor & Francis; 2001. 287 − 293. [Google Scholar]
- 53.Andersen B, Frisvad JC, Søndergaard I, et al. Associations between fungal species and water-damaged building materials. Appl Environ Microbiol. 2011;77:4180–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baddley JW, Marr KA, Andes DR, et al. Patterns of susceptibility of Aspergillus isolates recovered from patients enrolled in the transplant-associated infection surveillance network. J Clin Microbiol. 2009;47:3271–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreno G, Arenas R. Other fungi causing onychomycosis. Clin Dermatol. 2010;28:160–163. [DOI] [PubMed] [Google Scholar]
- 56.Jiao P, Mudur SV, Gloer JB, et al. Kipukasins, nucleoside derivatives from Aspergillus versicolor. J Nat Prod. 2007;70:1308–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veršilovskis A, De Saeger S. Sterigmatocystin: occurrence in foodstuffs and analytical methods–an overview. Mol Nutr Food Res. 2010;54:136–147. [DOI] [PubMed] [Google Scholar]
- 58.Kobayashi N, Kubosaki A, Takahashi Y, et al. Distribution of sterigmatocystin-producing Aspergilli in Japan. Food Safety. 2018;6:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu L, Wang L, Bao L, et al. Versicoamides F–H, prenylated indole alkaloids from Aspergillus tennesseensis. Org Lett. 2017;19:942–945. [DOI] [PubMed] [Google Scholar]
- 60.Li ZX, Wang XF, Ren GW, et al. Prenylated diphenyl ethers from the marine algal-derived endophytic fungus Aspergillus tennesseensis. Molecules. 2018;23:2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim M, Shin JH, Suh SP, et al. Aspergillus nidulans infection in a patient with chronic granulomatous disease. J Kor Med Sci. 1997;12:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim JD. Keratinolytic activity of five Aspergillus species isolated from poultry farming soil in Korea. Mycobiology. 2003;31:157–161. [Google Scholar]
- 63.Peterson SW, Jurjević Ž, Frisvad JC. Expanding the species and chemical diversity of Penicillium section Cinnamopurpurea. PLoS One. 2015;10:e0121987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anelli P, Peterson SW, Haidukowski M, et al. Penicillium gravinicasei, a new species isolated from cave cheese in Apulia, Italy. Int J Food Mcirobiol. 2018;282:66–70. [DOI] [PubMed] [Google Scholar]
- 65.Kim DH, Kim SH, Kwon SW, et al. The Mycobiota of air inside and outside the Meju fermentation room and the origin of Meju fungi. Mycobiology. 2015;43:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vega FE, Simpkins A, Aime MC, et al. Fungal endophyte diversity in coffee plants from Colombia, Hawai’i, Mexico and Puerto Rico. Fungal Ecol. 2010;3:122–138. [Google Scholar]
- 67.Frisvad JC, Samson RA, Stolk AC. A new species of Penicillium, P. scabrosum. Persoonia. 1990;14:177–182. [Google Scholar]
- 68.Frisvad JC, Larsen TO, Dalsgaard PW, et al. Four psychrotolerant species with high chemical diversity consistently producing cycloaspeptide A, P. jamesonlandense sp. nov., P. ribium sp. nov., P. soppii and P. lanosum. Int J Syst Evol Microbiol. 2006;56:1427–1437. [DOI] [PubMed] [Google Scholar]
- 69.Rong C, Ma Y, Liu Y, et al. Penicillium chroogomphum, a new species in Penicillium section Ramosa isolated from fruiting bodies of Chroogomphus rutilus in China. Mycoscience. 2016;57:79–84. [Google Scholar]
- 70.Ouhibi S, Santos C, Ghali R, et al. Penicillium tunisiense sp. nov., a novel species of Penicillium section Ramosa discovered from Tunisian orchard apples. Int J Syst Evol Microbiol. 2018;68:3217–3225. [DOI] [PubMed] [Google Scholar]
- 71.Gonçalves MFM, Santos L, Silva BMV, et al. Biodiversity of Penicillium species from marine environments in Portugal and description of Penicillium lusitanum sp. nov., a novel species isolated from sea water. Int J Syst Evol Microbiol. 2019;69:3014–3021. [DOI] [PubMed] [Google Scholar]
- 72.Larsen TO, Smedsgaard J, Frisvad JC, et al. Consistent production of penigequinolone A and B by Penicillium scabrosum. Biochem Syst Ecol. 1999;27:329–332. [Google Scholar]
- 73.Andersen B, Frisvad JC. Natural occurrence of fungi and fungal metabolites in moldy tomatoes. J Agric Food Chem. 2004;52:7507–7513. [DOI] [PubMed] [Google Scholar]
- 74.Harwig J, Scott PM, Stoltz DR, et al. Toxins of molds from decaying tomato fruit. Appl Environ Microbiol. 1979;38:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]