ABSTRACT
Knee Osteoarthritis is a considerable public health concern, both in terms of life quality and treatment financial impacts. To investigate this disease, animal models are deemed a promising alternative. In fact, although a perfect model is generally farfetched, the creation of models that simulate human disease as accurately as possible remains an important research stake. This study aims to highlight the usefulness of the model induced by injected Mono-Iodo-Acetate and to standardize it for the rabbit species. Osteoarthritis was induced by an infra-patellar injection of 0.2 ml of an MIA solution in the left knee of 24 female New Zealand rabbits. The right knee served as a control by receiving an injection of physiological serum. The rabbits were divided into 4 groups of 6 individuals each according to the dose of MIA received per knee. All rabbits were euthanized 30 days after the injection. After sacrifice, the knees were carefully dissected and macroscopic and microscopic scores of cartilage, meniscal and synovial lesions were attributed to each group. Our study followed the laboratory animal care and management guideline published in 2017 by the Canadian Council of Animal Care. The control knees of all rabbits showed no macroscopic or microscopic lesions. The macroscopic lesions: osteophytes, meniscal lesions, fibrillation and erosion of the cartilage and microscopic lesions: disorganization of the chondrocytes, decrease in proteoglycans and synovial inflammation clinically diagnosed in human pathology were all detected and were similarly reproducible among the knees of the same group. Through this work, we highlighted the merits of the arthritis model induced by MIA, namely its simulation of several aspects of human pathology. Further advantages are low cost, speed, reproducibility. This model notably avoids delicate and risky surgical operations.
KEYWORDS: Osteoarthritis, animal model, cartilage, histological score, macroscopic score
1. Introduction
With the aging of society, knee osteoarthritis has become a serious public health concern, both in terms of quality of life and the financial burden of the disease [1]. Currently, it is no longer defined as a mere disease of worn cartilage, but rather a multifactorial mechanical and metabolic pathology [2,3]; which makes the investigation of pathophysiology and therapeutic targets more intricate.
Numerous lines of research have been issued in order to define the pathogenesis of the disease and therefore the optimal therapeutic approach. Within this translational framework, animal models are essential alternatives for investigation. Although a perfect model does not exist, creating models that simulate the characteristics of human disease as precisely and as quantitatively as possible remains an interesting research issues [4].
Animal models of osteoarthritis are whether induced or spontaneous. The induced models are further segregated into surgical and chemical models, according to the protocol used to induce the disease. Each type has advantages and limits with regard to cost, time, reproducibility, risks and the human pathology aspect that it reflects [5].
This works aims to evaluate the interest of an osteoarthritis model induced by an intra-articular injection of Mono-Iodo-Acetate (MIA) in rabbit species, and characterize the different pathology aspects that it reflects by applying the latest recommendations from the Osteoarthritis Research Society International (OARSI).
2. Materials and methods
2.1. Ethics
All the experiments in this study followed the laboratory animal care and management guideline published in 2017 by the Canadian Council of Animal Care (CCAC) [6].
2.2. Animals
Twenty-four healthy female New Zealand rabbits weighing around 3500 g (3000 g-4000 g) were kept in a thermally regulated animal house (24°C ± 1°C) with a humidity of 55% ± 15%, with renewed air, and regularly alternated dark/light cycles.
Each animal was placed in a metal cage (35 X 50 × 25 cm) that allows easy access to standardized food and ad libitum water.
2.3. Induction of osteoarthritis
Sterile MIA powder (MIA- Sigma Aldrich, Saint-Louis, Missouri, USA) was dissolved in a sterile physiological serum solution (UNIMED, Tunisia) to prepare 2 solutions with respective concentrations of 15 mg/ml and 25 mg/ml.(Figure 1)
Figure 1.
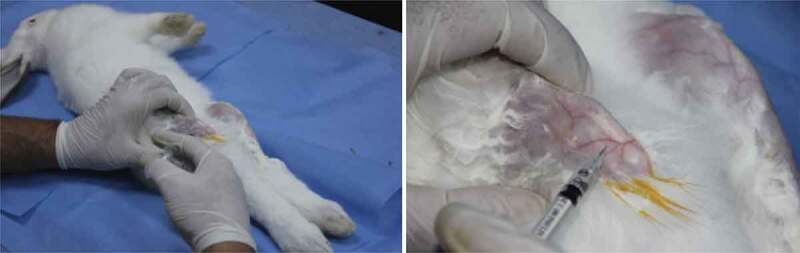
Induction of osteoarthritis by intra-articular injection of MIA
After anesthesia by intramuscular injection of a mixture of ketamine hydrochloride (30 mg/kg) and Midazolam (1 mg/kg), both knees of the animals were shaved and then disinfected with a Chlorhexidine solution. The left knee received, by infra-patellar injection, 0.2 ml of an MIA solution while the same volume of a physiological serum solution was injected into the right knee using a 29 G needle syringe.
In order to optimize the intra-articular injection method, we used a few rabbit corpses to select the optimal angle of attack for the needle: a methylene blue solution was injected from different angles to check the presence of the dye in the joint capsule area.
The rabbits were divided into 4 groups of 6 rabbits each according to the dose of MIA received per knee respectively: G1 (3 mg); G2 (5 mg); G3 (2* 3 mg with a 4 day interval); G4 (2* 5 mg with a 4 day interval).
All animals were euthanized after 30 days of induction by injecting a lethal dose of phenobarbital (150 mg/kg).
2.4. Macroscopic examination
After sacrifice, all the knees were carefully dissected, the joint cartilage of the two femoral condyles, the tibial plates and the menisci were isolated to obtain a macroscopic score of two observers, following Laverty et al. [7] and as recommended by OARSI. The joint surface of the 4 compartments (the two condyles and the tibial plates) was stained with a blue India ink solution (Quink Parker, Saint Herblain, France) diluted with phosphate buffered saline (ratio 1: 5). The ink adheres only to the cracked cartilage and helps improve visualization of the lesions by providing contrast with the surrounding normal cartilage and erosions. The excess dye was removed with a pre-moistened tissue, and then all the joints were photographed, scanned, and analyzed.
A score ranging from 1 to 7 was assigned to each compartment and an average was calculated for each knee. (Table1). The menisci were scored according to OARSI as follows: 1 (normal); 2 (minimal surface cracking); 3 (moderate cracking); 4 (severe cracking or incomplete transverse lesion); 5 (complete lesion or several incomplete lesions).
Table 1.
Macroscopic score of degenerative lesions in cartilage as recommended by OARSI
| 1 | Intact surface: surface normal in appearance and does not retain India ink |
| 2 | 0 mm<Fissures < 4 mm |
| 3 | 4 mm<Fissures < 8 mm |
| 4 | 8 mm<Fissures |
| 5 | 0 mm<Full depth erosion < 2 mm |
| 6 | 2 mm< Full depth erosion < 5 mm |
| 7 | 5 mm< Full depth erosion |
Laverty et All [7]
2.5. Histological examination
Immediately after macroscopic examination, all specimens were fixed with 10% buffered formalin for 24 hours, decalcified with 5% formic acid for 5 days and then incorporated into paraffin blocks. A series of 3 sagittal sections (4 µm thick) was made for each of the 4 compartments, through the large diameter of the cartilage lesion.
The sections were then stained with Safranin_O and light_green and then examined under a light microscope. The severity of osteoarthritis was rated on a scale of 0 to 24 by two observers, using the histological/histochemical scale proposed by OARSI. (Appendix A). The final score for each sample was the average of two independent scores.
Osteoarthritic synoviopathy was also assessed on the OARSI scale, marked from 0 to 30 after staining of sections of the synovium with eosin-hematoxylin. (Appendix B)
2.6. Radiological score
Before the dissection and macroscopic examination, all the knees were X-rayed with two orthogonal views. The severity of osteoarthritis was rated on a scale of 0 to 13 according to Mével et al. [8] (Appendix C) based on the number of osteophytes, joint space, calcifications of the menisci and calcifications of the tendons and ligaments.
2.7. Data analysis
The data for each group were presented as a mean, and compared by the ANOVA factor analysis of variance test to assess the intra-group reproducibility and the inter-group difference. The software used was Microsoft EXCEL 2016. A p value less than 0.05 was considered significant.
3. Results
3.1. Macroscopic and radiological alterations
The acuteness of macroscopic lesions varied according to the dose of MIA injected. The high doses
(6 mg and 10 mg) caused lesions affecting the entire articular surface with large chondral erosions and exposures of the subchondral bone (Figure 2), dominant mainly in the internal compartment and reflected by a macroscopic average score of G3 and G4 respectively recorded at 5.4 and 6.6 (Table 2). Large osteophytes were present at the periphery of the tibial plate and at the femoral trochlea (Figure 3). The joint capsule was thick, irregular and dull in appearance, compared to that of the control knees, which was white, smooth and shiny.
Figure 2.
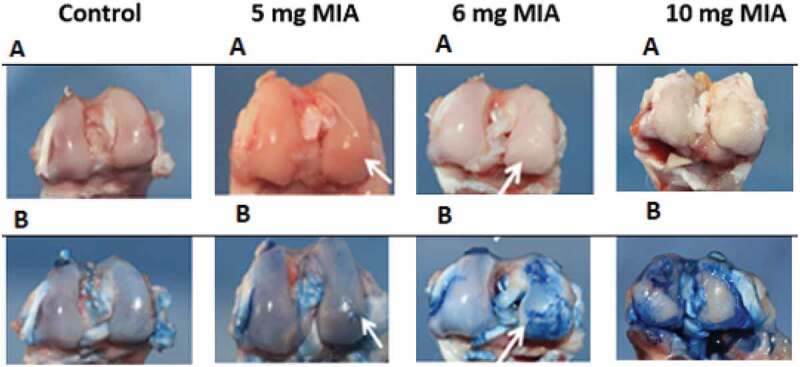
Osteoarthritis induced by MIA: macroscopic appearance of cartilage with (a) and without Indian ink (b)
Table 2.
Summary of the means, variances and probabilities of the different groups for the macroscopic, radiological and microscopic parameters
| Groups | Number of samples | Total | mean | Variance | P value | |
|---|---|---|---|---|---|---|
| Macroscopic Score | G 1 | 6 | 10. 4 | 1. 73 | 0. 122 | |
| G 2 | 6 | 20. 7 | 3. 45 | 0. 215 | < 0. 05 | |
| G 3 | 6 | 32. 4 | 5. 4 | 0. 168 | ||
| G 4 | 6 | 39. 6 | 6. 6 | 0. 248 | ||
| Radiological Score | G 1 | 6 | 3. 5 | 0. 583 | 0. 441 | |
| G 2 | 6 | 18. 25 | 3. 041 | 0. 635 | <0. 05 | |
| G 3 | 6 | 37 | 6. 166 | 0. 591 | ||
| G 4 | 6 | 49. 75 | 8. 29 | 0. 235 | ||
|
icroscopic Score of cartilage |
G 1 | 6 | 18 | 3 | 0. 5 | |
| G2 | 6 | 46 | 7. 66 | 2. 16 | < 0. 05 | |
| G3 | 6 | 83 | 13. 83 | 1. 066 | ||
| G 4 | 6 | 121. 5 | 20. 25 | 1. 075 |
Figure 3.
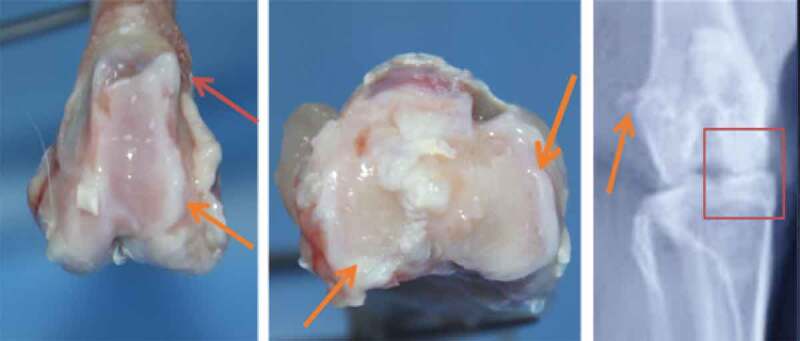
Macroscopic and radiological appearance of osteophytes and degenerative alterations
The radiological alterations were marked for these doses by a pinching of the articular space, osteophytes of the fabella and sclerosis of the subchondral bone (Figure 3). The radiological mean scores for G3 and G4 were 6.16 and 8.29 respectively. (Table 2)
The lesions were less severe in the knees of G2 injected with 5 mg of MIA. The cartilage on the central bearing areas of each joint surface was thin and yellowish, with focal cracks and erosions. Osteophytes and subchondral bone changes were minor as reflected by macroscopic and radiological scores.
The lowest dose, 3 mg, induced no macroscopic or radiological changes under our experimental conditions.
3.2. Microscopic alterations (Figure 4)
Figure 4.
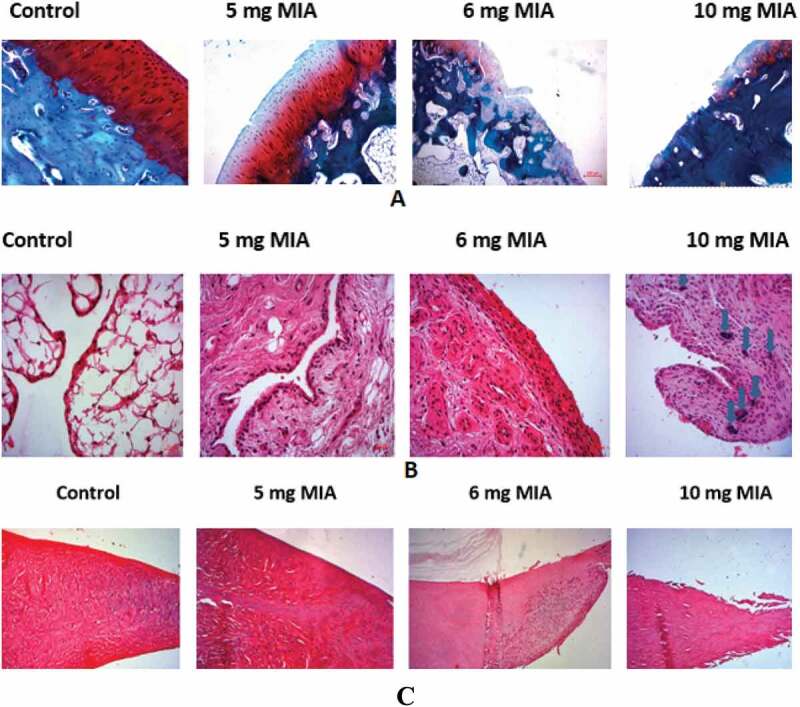
Osteoarthritis induced by MIA: (a) cartilage (b) synovium (c) menisci: microscopic appearance of cartilage
In groups G3 and G4, the microscopic changes were marked by the total destruction of the articular surface including areas of damaged cartilaginous frame with the bone under sclerotic chondral exposed and areas where the cartilage was severely cracked with cluster of condrocytes. Also, the Sfranin_O coloration was reduced, indicating a depletion of the extra cellular matrix in glycosaminoglycans. The synovial membrane was hyperplastic and fibrous, with an inflammatory infiltration made of mononuclear leukocytes.
G2 presented less severe lesions with a decrease in the thickness of the cartilage which presented moderate cracks, a better glycosaminoglycans content and an inflammatory but less hyperplastic synovial. G1 showed only slight cracking of the cartilaginous surface with a moderate inflammatory infiltrate of the synovium.
Histological evaluation of the menisci shows surface fibrillation and cell depletion which are proportional to the dose of MIA administered
The mean histological scores are summarized in Table 2.
4. Discussion
It is increasingly established that osteoarthritis is not only due to mechanical stress but also to its coexistence with a metabolic disorder that promotes chondrocytic degeneration at the expense of its regeneration [3,9]. This multifactorial pathogenesis makes the choice between different animal models (spontaneous, surgical or chemical) a critical issue, since none of these models reflects all aspects of human pathology [10,11].
Since metabolic disorders are involved in the early stages of human osteoarthritis [12], cartilage metabolism is a promising therapeutic target; which promotes the interest of the model of osteoarthritis induced by MIA. Injecting MIA interferes with cartilage metabolism by inhibiting glyceraldehydes-3 phosphate dehydrogenase, a key enzyme in glycolysis. As chondrocytes depend on an anaerobic metabolism (cartilage is avascular), the inhibition of this enzyme leads to a decrease in intracellular ATP, the main source of energy for the cell [13]. This metabolic disturbance leads to progressive cellular apoptosis, a decrease in glycosaminoglycans and sclerosis of the subchondral bone in the bearing areas of the cartilage [14,15], as observed in humans.
Several subsequent studies [16–18] have shown that the cascade of biochemical, histological, radiological and macroscopic changes induced by MIA simulates human pathology perfectly. Even on a molecular level, more recent works have concluded that the oxidative stress mechanism [19] and the alteration of the expression of the anabolic genes generated by this model [20] are parallel to those observed in humans. All of these changes were dose-dependent.
The similitude of the degenerative changes caused in this model is thus confirmed, both microscopically and macroscopically, with those observed in humans in a more accessible, highly reproducible way (weak intra-group variance – Table 2), in a dose-dependent manner (p < 0.05 for all parameters). The obtained results observed exclusively in rodents, might be extrapolated on a larger species (rabbit) that offers a larger cartilaginous surface and facilitates the investigation.
However, the great diversity of the studied osteoarthritis models and of the methods for evaluating degenerative lesions makes it difficult to compare the results. For this reason, OARSI sought a consensus which favors a better uniformity of the controlled means of study and the results reported in research on animal models of osteoarthritis [21].
In this sense, histological assessment, including fixing, making cuts and stains, have been well codified [22] beforehand. Pritzker et al. [23] proposed a universal system for scoring histological lesions, before OARSI published guidelines for the histological evaluation of osteoarthritis models in a variety of species [7,24].
Vinod et al. [25] in 2018 study the model of osteoarthritis induced at MIA in rabbits, but, according to the published histology figures, the fast green dye was almost absent which reduces the contrast compared to the Safranin O staining, which makes the evaluation of the loss of GAG not reliable. We encountered this problem at the start of our study, which required optimization to have interpretable histological sections.
Secondly, this study was only interested in the histological study of the cartilage, without microscopic aspects of the disease which affect the menisci and the synovium, and this will raises questions about the reflection of this model of human disease. In our study we have proven the similarities of degenerative lesions of all joint tissues with those observed in humans.
Third, the doses of MIA used in this study appear to reflect the early stages of OA, which reduces their usefulness for other therapeutic studies.
5. Conclusion
This study was undertaken to highlight the advantages of the osteoarthritis model induced by MIA, which simulates human pathology in several regards, in addition to its low cost, speed, reproducibility. Such a model can also avoid likely delicate and risky surgical operations. As for standardization, it typically adheres to the consensus established by OARSI. It is the first to show the applicability of this type of osteoarthritis model. This promising model deserves further investigation since it highlights the metabolic theory in the pathogenesis of osteoarthritis and presents it as a promising therapeutic target.
Appendices.
Appendix A. The microscopic cartilage score proposed by OARSI [6]
| Parameter |
|---|
| Safranin O-fast green staining |
| 0 = uniform staining throughout articular cartilage |
| 1 = loss of staining in superficial zone of hyaline cartilage <50% of the length of the condyle or plateau |
| 2 = loss of staining in superficial zone of hyaline cartilage >50% of the length of the condyle or plateau |
| 3 = loss of staining in the upper 2/3’s of hyaline cartilage <50% of the length of the condyle or plateau |
| 4 = loss of staining in the upper 2/3’s of hyaline cartilage >50% of the length of the condyle or plateau |
| 5 = loss of staining in all of hyaline cartilage <50% of the length of the condyle or plateau |
| 6 = loss of staining in all of hyaline cartilage >50% of the length of the condyle or plateau |
| Structure |
| 0 = Normal |
| 1 = Surface irregularities |
| 2 = Fissures in <50% surface |
| 3 = Fissures in >50% surface |
| 4 = erosion 1/3 hyalin cartilage <50% surface |
| 5 = erosion 1/3 hyalin cartilage >50% surface |
| 6 = erosion 2/3 hyalin cartilage <50% surface |
| 7 = erosion 2/3 hyalin cartilage >50% surface |
| 8 = full depth erosion hyalin cartilage <50% surface |
| 9 = full depth erosion hyalin cartilage >50% surface |
| 10 = full depth erosion hyaline and calcified cartilage to the subchondral bone <50% surface |
| 11 = full depth erosion hyaline and calcified cartilage to the subchondral bone >50% surface |
| Chondrocyte density |
| 0 = No decrease in cells |
| 1 = focal decrease in cells |
| 2 = multifocal decrease in cells |
| 3 = multifocal confluent decrease in cells |
| 4 = diffuse decrease in cells |
| Cluster formation |
| 0 = Normal |
| 1 = <4 clusters |
| 2 = >4 but <8 clusters |
| 3 = >8 clusters |
Appendix B. the microscopic score for osteoarthritic synoviopathy suggested by OARSI [6]
| Synoviocyteproliferation | 0 normal 1 slight 2 moderate 3 severe |
One layer Up to two cells deep Up to four cells deep Up to more than four cells deep |
| Hypertrophy | 0 normal 1 slight 2 moderate 3 severe |
Squamous Cuboidal Cylindrical Cylindrical withcytoplasmic protrusions |
| Inflammatory infiltrate | 0 absent 1 slight 2 moderate 3 severe |
Few cells Some foci of cells Diffuse infiltration |
| Fibrinous exudate | 0 absent 1 slight 2 moderate 3 severe |
Slight amount,focal Multifocal deposits of fibrin Diffuse deposits of fibrine |
| Lymphoplasmacytic infiltrate | 0 absent 1 slight 2 moderate 3 severe |
Single/few cells Some cells, mainly arrounvessels Many cells,diffuse infiltration |
| Lymphoplasmacytic aggregates | 0 Absent 1 single 2 many |
1 >1 |
| Synovial stroma villous hyperplasia | 0 absent 1 slight 2 moderate 3 severe |
No villi/smooth waves Some small villi Multifocal villous hyperplasia Diffusevillous hyperplasia |
| Proliferation of fibroblasts/fibrocytes | 0 absent 1 slight 2 moderate 3 severe |
Normal cellularity density Mild proliferation Moderate proliferation Extensive proliferation |
| Proliferation of blood vessels | 0 normal 1 slight 2 moderate 3 severe |
Mild proliferation Moderate proliferation Extensive proliferation |
| Cartilage/bone_detritus | 0 absent 1 few 2 many |
1–3 >3 |
| Hemosiderosis | 0 absent 2 few cells 3 many cells |
1–3 >3 |
Appendix C. The radiological score according to Mével et al. [7]
| Scoring category | Observations | Score | |
|---|---|---|---|
| Menisci calcification | None | 0 | |
| 1 | 1 | ||
| 2 | 2 | ||
| Number of visible osteophytes | Medial tibial condyle | None | 0 |
| Low- Moderate | 1 | ||
| Moderate- Severe | 2 | ||
| Medial femoral condyle | None | 0 | |
| Low- Moderate | 1 | ||
| Moderate- Severe | 2 | ||
| Medial fabella | Absence | 0 | |
| Presence | 1 | ||
| Structural modifications of subchondral bone (sclerosis) | None | 0 | |
| Low- Moderate | 1 | ||
| Moderate- High | 2 | ||
| Width of the joint space | Normal | 0 | |
| Reduced | 1 | ||
| Absent | 2 | ||
| Calcification of tendons and ligaments | None | 0 | |
| 1 site | 1 | ||
| >1 site | 2 | ||
| Total | 13 |
Disclosure statement
The authors report no conflict of interest.
References
- [1].Gore M, Tai K-S, Sadosky A, et al. Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. J Med Econ. 2011. janv;14(4):497‑507. [DOI] [PubMed] [Google Scholar]
- [2].Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009. août 1;17(8):9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vincent TL, Williams RO, Maciewicz R, et al. Mapping pathogenesis of arthritis through small animal models. Rheumatology. 2012. November 1;51(11):193141. [DOI] [PubMed] [Google Scholar]
- [4].Lampropoulou-Adamidou K, Lelovas P, Karadimas EV, et al. Useful animal models for the research of osteoarthritis. Eur J Orthop Surg Traumatol. 2014. avr 1;24(3):26371. [DOI] [PubMed] [Google Scholar]
- [5].Teeple E, Jay GD, Elsaid KA, et al. Animal models of osteoarthritis: challenges of model selection and analysis. AAPS J. 2013. avr 1;15(2):43846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Canadian Council on Animal Care . Guide to the care and use of experimental animals. Canadian Council on Animal Care; 2017.
- [7].Laverty S, Girard CA, Williams JM, et al. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the rabbit. Osteoarthritis Cartilage. 2010. October;18:S5365. [DOI] [PubMed] [Google Scholar]
- [8].Mével E, Merceron C, Vinatier C, et al. Olive and grape seed extract prevents post-traumatic osteoarthritis damages and exhibits in vitro anti IL-1β activities before and after oral consumption. Sci Rep. 2016. September 19;6:33527. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Boyd RD, Walker ER, Wu DD, et al. Morphologic and morphometric changes in synovial membrane associated with mechanically induced osteoarthrosis. Arthritis Rheum. 1991. mai 1;34(5):51524. [DOI] [PubMed] [Google Scholar]
- [10].Kim HA, Cheon EJ.. Animal model of osteoarthritis. J Rheum Dis. 2012. October 1;19(5):23947. [Google Scholar]
- [11].Gregory MH, Capito N, Kuroki K, et al. A review of translational animal models for knee osteoarthritis. Arthritis. 2012;2012:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McCulloch K, Litherland GJ, Rai TS. Cellular senescence in osteoarthritis pathology. Aging Cell. 2017. avr;16(2):2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kalbhen D. Degenerative joints. In: Verbruggen G, Veys E, editors. Degenerative joint disease following chondrocyte injury: chemically induced osteoarthritis. Vol. 2. Amsterdam: Elsevier Science Publishers; 1985. p. 299–9. [Google Scholar]
- [14].Guingamp C, Gegout-Pottie P, Philippe L, et al. Mono-iodoacetate-induced experimental osteoarthritis. A dose-response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum. 1997;40(9):1670–1679. [DOI] [PubMed] [Google Scholar]
- [15].Saied A, Cherin E, Gaucher H, et al. Assessment of articular cartilage and subchondral bone: subtle and progressive changes in experimental osteoarthritis using 50 MHz echography in vitro. J Bone Miner Res. 1997;12(9):1378–1386. [DOI] [PubMed] [Google Scholar]
- [16].Guzman RE, Evans MG, Bove S, et al. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol Pathol. 2003;31(6):619–624. [DOI] [PubMed] [Google Scholar]
- [17].Beyreuther B, Callizot N, Stöhr T. Antinociceptive efficacy of lacosamide in the monosodium iodoacetate rat model for osteoarthritis pain. Arthritis Res Ther. 2007. févr 6;9(1):R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Al-Saffar FJ, Ganabadi S, Yaakub H, et al. Collagenase and sodium iodoacetate-induced experimental osteoarthritis model in Sprague Dawley rats. Asian J Sci Res. 2009;2:167–179. [Google Scholar]
- [19].Jiang L, Li L, Geng C, et al. Monosodium iodoacetate induces apoptosis via the mitochondrial pathway involving ROS production and caspase activation in rat chondrocytes in vitro. J Orthop Res. 2013. mars 1;31(3):3649. [DOI] [PubMed] [Google Scholar]
- [20].Nam J, Perera P, Liu J, et al. Sequential alterations in catabolic and anabolic gene expression parallel pathological changes during progression of monoiodoacetate-induced arthritis. Plos One. 2011. September 13;6(9):e24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Little CB, Zaki S. What constitutes an « animal model of osteoarthritis » – the need for consensus? Osteoarthritis Cartilage. 2012. avr 1;20(4):2617. [DOI] [PubMed] [Google Scholar]
- [22].Schmitz N, Laverty S, Kraus VB, et al. Basic methods in histopathology of joint tissues. Osteoarthritis Cartilage. 2010. October 1;18:S1136. [DOI] [PubMed] [Google Scholar]
- [23].Pritzker KPH, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006. janv 1;14(1):13‑29. [DOI] [PubMed] [Google Scholar]
- [24].Aigner T, Cook JL, Gerwin N, et al. Histopathology atlas of animal model systems – overview of guiding principles. Osteoarthritis Cartilage. 2010. October 1;18:S26. [DOI] [PubMed] [Google Scholar]
- [25].Vinod E, Boopalan PRJVC, Arumugam S, et al. Creation of monosodium iodoacetate-induced model of osteoarthritis in rabbit knee joint. Indian J Med Res. 2018;147(3):312. [DOI] [PMC free article] [PubMed] [Google Scholar]


