Abstract
Context
Matrine is a well-known anti-inflammatory quinolizidine alkaloid derived from leguminous plant Sophora flavescens Ait. (Leguminosae).
Objective
This study was designed to uncover the potential application of matrine in treating spinal cord injury (SCI).
Materials and methods
Neuron-like PC12 cells in experimental groups were pre-treated with/without matrine (200 μM) for 24 h and then stimulated by lipopolysaccharide (LPS, 5 μg/mL) for 12 h. PC12 cells in control group were cultured in complete medium. CCK-8 assay, flow cytometry, qRT-PCR, western blot and ELISA were performed to evaluate cell damage. Moreover, after cells were transfected with miR-9 inhibitor for 48 h, above indicators were tested again. qRT-PCR and western blot were also conducted to uncover the downstream effectors and signalling pathways for matrine.
Results
LPS (5 μg/mL) decreased cell viability about 50%. Matrine (200 μM) decreased cell viability about 0%, 13.8% and 30% at 24 h, 48 h and 72 h, respectively. The loss of viability, stimulation of apoptosis, and release of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) evoked by LPS were attenuated by the pre-treatment of matrine partly. Meanwhile, LPS reduced miR-9 expression about 60%, but matrine completely reversed LPS-decreased miR-9 level. By silencing miR-9 expression, the protective properties of matrine towards PC12 cells were impeded. Besides, matrine inhibited the activation of JNK and NF–κB pathways even under the condition of LPS. And the impact of matrine on the signalling were attenuated by miR-9 silencing.
Discussion and Conclusion
This paper provided in vitro evidence that matrine was able to protect PC12 cells against LPS-evoked damage. The neuroprotective properties of matrine may be due to its regulation of miR-9 expression as well as JNK and NF–κB pathways.
Keywords: Spinal cord injury (SCI), JNK pathway, NF–κB pathway
Introduction
Spinal cord injury (SCI) is a common disease caused by fracture or dislocation of spine vertebral. SCI mostly occurs in young adults and it seriously threatens patients’ health and quality of life (Lynch and Cahalan 2017). The progression of SCI contains two important segments: primary injury and secondary injury (Mourelo Farina et al. 2017). The primary injury is irreversible. So, a large proportion of studies is focussed on investigating how to control secondary injury. Currently, corticosteroids, gangliosides, hyoscine, neurotrophic drugs are recommended to treat SCI in the clinic. However, side-effects of these medications always plague neurosurgeons. For instance, administration of high-dose methylprednisolone is used to treat secondary injury, while it also induced a series of side-effects, such as gastrointestinal bleeding, sepsis, pneumonia and infection (Rouanet et al. 2017). Therefore, it is sorely needed for SCI to find more effective drugs.
Matrine is a main alkaloid component in a traditional Chinese herb, derived from leguminous plant Sophora flavescens Ait (Leguminosae) (Cai et al. 2018). It has been found to possess a wide range of pharmacological effects, including immunity-regulation (Kan et al. 2013), anti-arrhythmia (Zhou et al. 2014), antibacterial (Feng et al. 2018), analgesic (Haiyan et al. 2013), antihepatic fibrosis (Yu et al. 2014), and antitumor activities (Rashid et al. 2019). Due to these pharmacological activities, matrine has been considered as a promising candidate for the management of various diseases, like acute lung injury (Liou et al. 2016), non-alcoholic steatohepatitis (Mahzari et al. 2019), acute lymphoblastic leukaemia (Aghvami et al. 2018), etc. More recently, it was found that matrine possessed neuroprotective functions, for example, matrine had promise in treating Parkinson’s disease (Meng et al. 2017). Moreover, matrine was able to promote the growth of axon and the recovery of spinal cord following SCI (Tanabe et al. 2018). Nonetheless, the impact of matrine on SCI is still incompletely disclosed.
MicroRNAs (miRNAs) are newly discovered small non-coding RNAs with about 22 nucleotides. They are processed from stem-loop structure of a single strand RNA by Dicer enzyme and act through regulating the post-transfection of target genes. During SCI, a broad range of miRNAs is temporally altered, thereby participating in regulating neurons growth, apoptosis, inflammation, as well as axonal regeneration (Shi et al. 2017). So, some miRNAs are recognised as novel diagnostic and therapeutic approaches for SCI. MiR-9 is one miRNA and it is less expressed in SCI tissues compared to normal tissues (Xu et al. 2016). Besides, miR-9 was found to participate in neuronal survival and regeneration following ischaemic injury (Nampoothiri and Rajanikant 2019).
This study was designed to explore the impact of matrine in preventing second injury of the spinal cord. To further uncover the deep mechanisms of matrine’s function, its regulation on miR-9 expression and the following pathways were studied.
Materials and methods
Cell treatment
PC12 cell line (ATCC® CRL-1721™, ATCC, Manassas, VA, USA) was cultivated in RPMI-1640 medium (ATCC). The complete growth medium was made by adding 10% heat-inactivated horse serum (Gibco, Grand Island, NY, USA) and 5% foetal bovine serum (Gibco). The cells were cultured at 37 °C in an atmosphere with 95% air and 5% CO2.
To induce inflammatory injury in PC12 cells, 5 μg/mL of LPS derived from E. coli O111:B4 (Beyotime, Shanghai, China) was utilised to treat cells for 12 h (Xie et al. 2018). Matrine with purity greater than 95% was purchased from Sigma-Aldrich (St. Louis, MO, USA). Matrine was dissolved in DMSO (Sigma-Aldrich) to make a storage solution with concentration of 200 mM. Before use, the storage solution was diluted with culture medium to 200 μM. Cells were treated by 200 μM matrine for 24 h.
MiRNA transfection
MiR-9 inhibitor (anti-miR-9) and its negative control (anti-NC) were purchased from GenePharma (Shanghai, China). Transfection was conducted by utilising Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) under serum- and antibiotic-free conditions. Transfection procedure was lasted for 48 h in 6-well plates.
CCK-8 assay
With or without transfection, cells were seeded in 96-well plates (5000 cells/well) and treated as indicated. Later, 20 μL CCK-8 (Dojindo Molecular Technologies, Kyushu, Japan) was supplemented into the culture medium and the plates were cultured for 3 h at 37 °C. The absorbance was analysed by a Microplate Reader (Bio-Rad, Hercules, CA, USA) at 450 nm for calculating relative cell viability.
Apoptosis assay
With or without transfection, cells were seeded in 6-well plates (5 × 105 cells/well) and treated as indicated. Later, cells were harvested in the Eppendorf tube and stained by using the Annexin V-FITC/PI Detection Kit (Sangon Biotech, Shanghai, China) according to the manufacturer’s instructions. Flow cytometry was done by using a FACS can (Beckman Coulter, Fullerton, CA, USA).
ELISA
With or without transfection, cells were seeded in 6-well plates (5 × 105 cells/well) and treated as indicated. The culture supernatant was harvested and the concentration of IL-1β, IL-6 and TNF-α was analysed by utilising the respective ELISA kit (Abcam, Cambridge, MA, USA).
qRT-PCR
With or without transfection, cells were seeded in 6-well plates (5 × 105 cells/well) and treated as indicated. Then, cells were lysed in Trizol reagent (Sangon Biotech) for collecting total RNAs. For testing expression of IL-1β, IL-6 and TNF-α, PrimeScript RT Master Mix and TB Green® Fast qPCR Mix (Takara, Dalian, China) were utilised. For testing the expression of miR-9, Mir-X™ miRNA First Strand Synthesis Kit together with Mir-X™ miRNA qRT-PCR TB Green™ Kit (Takara) were utilised. GAPDH and U6, respectively, acted as the reference controls of normalising the relative expression of mRNA and miRNA.
Western blot
With or without transfection, cells were seeded in 6-well plates (5 × 105 cells/well) and treated as indicated. Then, cells were lysed in RIPA buffer (Beyotime) for collecting total proteins. In the Western blot procedure, the following primary antibodies against Bcl-2 (LS-C675297), Bax (LS-C63137), cleaved-caspase-3 (LS-C12499), IL-1β (LS-C104781), IL-6 (LS-C746886), TNF-α (LS-C132747), c-Jun (LS-C747156), p-c-Jun (LS-C368401), JNK (LS-C169083), p-JNK (LS-C84066), IκBα (LS-C331335), p-IκBα (LS-C416376), p65 (LS-C413576), p-p65 (LS-C154220), β-actin (LS-C82919, LifeSpan BioSciences, Seattle, WA, USA) and pro-caspase-3 (orb310041, Biorbyt, San Francisco, CA, USA) were utilised. Finally, the target bands were developed by ECL Plus Western Blotting Substrate (Biorbyt). The grey level of each band was examined by Image Lab™ Software (Bio-Rad).
Statistical analysis
Results were represented as mean ± SD. Statistical analysis was done in SPSS 19.0 software (Chicago, IL, USA). One-way ANOVA and Student t-test were utilised for the comparison between groups and among groups. Statistical differences were set at p < 0.05.
Results
The viability of PC12 cells was not affected by 200 μM matrine at 24 h
A study has indicated that 200 μM matrine protects lung cancer cells from LPS-induced injury (Liou et al. 2016), so, 200 μM matrine was used to treat PC12 cells in this study. As shown in Figure 1, compared with control group, cell viability was invariable at 24 h (p > 0.05). However, cell viability was significantly decreased at 48 and 72 h (p < 0.05). So, we chose 24 h as the treatment time of matrine.
Figure 1.
The viability of PC12 cells was not affected by 200 μM matrine at 24 h. PC12 cells were treated by 200 μM matrine for 0, 24, 48, 72 h, the viability of cell was examined by CCK-8 assay. ns, no significant, *p < 0.05 versus Control group.
Matrine protected PC12 cells against LPS-provoked damage
As seen in Figure 2(A), cell viability was not significantly changed between matrine group and the control (p > 0.05). Then, cells were pre-treated with matrine and followed by LPS stimulation. Figure 2(B,C) showed that, the viability and apoptosis rate induced by LPS were attenuated by matrine (p < 0.05). Moreover, the repression of Bcl-2 expression, elevation of Bax expression and cleavage of caspase-3 evoked by LPS were all attenuated by matrine (p < 0.05, Figure 2(D,E)). Also, the mRNA and protein expression as well as release of pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α, driven by LPS were impeded by the pre-treatment of matrine (p < 0.05, Figure 2(F–H)). These data demonstrated that matrine possessed abilities in protecting PC12 cells against LPS-provoked damage.
Figure 2.
Matrine protected PC12 cells against LPS-provoked damage. (A) PC12 cells were treated by 200 μM matrine for 24 h, and the viability of cell was examined by CCK-8. ns, no significant. The matrine-treated cells were stimulated with 5 μg/mL LPS for 12 h. (B) Cell viability, (C) apoptosis rate, (D, E) expression of apoptosis-associated proteins, (F) mRNA and (G) protein expression of pro-inflammatory cytokines, as well as (H) release of these cytokines in culture supernatant were examined by CCK-8 assay, flow cytometry, qRT-PCR, western blot and ELISA. *p < 0.05 versus Control group. #p < 0.05 versus LPS group.
Matrine elevated miR-9 expression
Meanwhile, miR-9 expression following LPS or LPS plus matrine treatment was examined by qRT-PCR. Data presented in Figure 3 showed that LPS clearly suppressed miR-9 expression (p < 0.05). Nonetheless, matrine treatment remarkably elevated miR-9 expression even under LPS-treated condition (p < 0.05).
Figure 3.
Matrine elevated miR-9 expression. PC12 cells were treated by 200 μM matrine for 24 h and then stimulated with 5 μg/mL LPS for 12 h. MiR-9 expression was examined by qRT-PCR. *p < 0.05 versus Control group. #p < 0.05 versus LPS group.
Matrine protected PC12 cells against LPS-evoked damage through elevating miR-9 expression
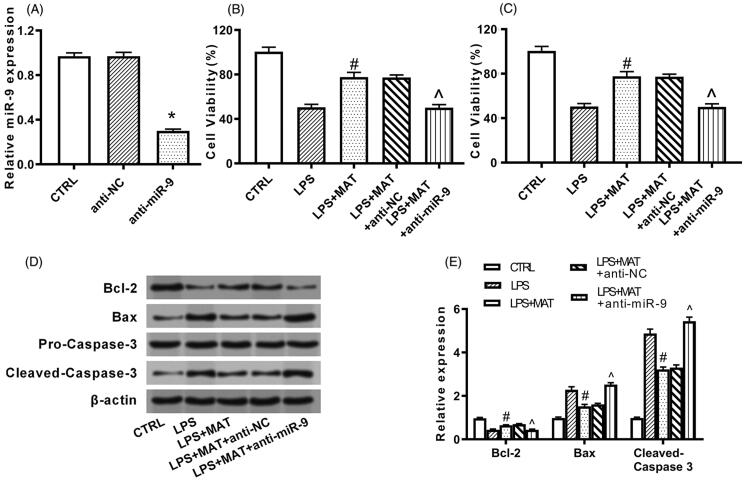
Since matrine could simultaneously protect PC12 cells and elevate miR-9 expression, following experiments were designed to uncover if matrine exerted its function through modulating miR-9. To this purpose, miR-9 expression was repressed by transfection with the specific inhibitor. Figure 4(A) showed that miR-9 expression in PC12 cells was suppressed following transfection, unsurprisingly (p < 0.05). It was found that the protective action of matrine towards PC12 cells was attenuated by miR-9 silencing. To be specific, the impact of matrine towards cell viability (p < 0.05, Figure 4(B)), apoptosis rate (p < 0.05, Figure 4(C)), apoptosis-associated protein expression (p < 0.05, Figure 4(D,E)), as well as the release of pro-inflammatory cytokines (p < 0.05, Figure 5(A–C)) were all partially or completely reversed by miR-9 silence.
Figure 4.
Matrine protected PC12 cells against LPS-provoked apoptosis through elevating miR-9 expression. (A) PC12 cells were transfected with nothing, anti-NC or anti-miR-9. MiR-9 expression was examined by qRT-PCR. *p < 0.05 versus anti-NC group. Following transfection, cells were treated by 200 μM matrine for 24 h and then stimulated with 5 μg/mL LPS for 12 h. (B) Cell viability, (C) apoptosis rate, and (D, E) expression of apoptosis-associated proteins were examined by CCK-8 assay, flow cytometry and Western blot. #p < 0.05 versus LPS group. ^p < 0.05 versus LPS + MAT + anti-NC group.
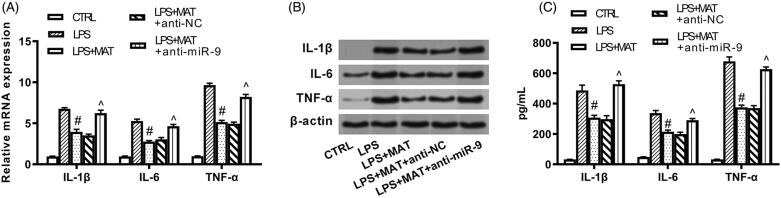
Figure 5.
Matrine protected PC12 cells against LPS-provoked inflammatory injury through elevating miR-9 expression. PC12 cells were transfected with nothing, anti-NC or anti-miR-9. Following transfection, cells were treated by 200 μM matrine for 24 h and then stimulated with 5 μg/mL LPS for 12 h. (A) MRNA and (B) protein expression of pro-inflammatory cytokines, as well as (C) release of these cytokines in culture supernatant were examined by qRT-PCR, Western blot and ELISA. #p < 0.05 versus LPS group. ^p < 0.05 versus LPS + MAT + anti-NC group.
Matrine inhibited JNK and NF–κB pathways through elevating miR-9 expression
Next, the further mechanisms of which matrine protected PC12 cells were studied by testing the activation of two inflammation-related signalling pathways, i.e., JNK and NF–κB pathways (Liu G and Rondinone 2005; Baker et al. 2011). Western blot results illustrated that, the ratio of p/t-c-Jun, p/t-JNK, p/t-IκBα and p/t-p65 was all enhanced by LPS (p < 0.05, Figure 6(A–D)). The above increased ratio was attenuated by the pre-treatment of matrine (p < 0.05). Additionally, the impact of matrine on these proteins were partially or completely reversed when miR-9 expression was suppressed (p < 0.05).
Figure 6.
Matrine inhibited JNK and NF–κB pathways through elevating miR-9 expression. PC12 cells were transfected with nothing, anti-NC or anti-miR-9. Following transfection, cells were treated by 200 μM matrine for 24 h and then stimulated with 5 μg/mL LPS for 12 h. Expression of core proteins in (A, B) JNK and (C, D) NF–κB pathways were examined by Western blot. #p < 0.05 versus LPS group. ^p < 0.05 versus LPS + MAT + anti-NC group.
Discussion
Following the primary injury, a series of pathological and physiological changes occur, including neuron apoptosis and inflammation. In the trauma-induced SCI, the apoptosis of neurons is largely restricted to sites near the impact zone itself and generally occurs within about 8 h after the trauma (Emery et al. 1998). However, a second apoptosis occurs within about 7 days after the injury of white matter, which leads to the necrosis and apoptosis of neurons and gliocytes (Lu et al. 2000). Besides, SCI is invariably associated with spinal cord inflammation which adversely affects the outcome of SCI (Anwar et al. 2016). Reducing neuron apoptosis and chronic inflammation in patients with SCI are considered to be pivotal in controlling secondary injury (Zhang N et al. 2012; Allison et al. 2018). In the present study, LPS was utilised to stimulate apoptosis and inflammatory injury in PC12 cells to make a cell model of SCI, as descripted elsewhere (Li R et al. 2018; Xie et al. 2018). Matrine exhibited protective functions in this cell model of SCI. Furthermore, matrine exerted its beneficial impacts possibly through its regulation on miR-9 expression as well as JNK and NF–κB pathways.
Matrine has long been known as an anti-inflammatory quinolizidine alkaloid (Kan et al. 2013). For instance, matrine was able to repress LPS-induced inflammation in intestinal cells (Wu et al. 2017) and lung epithelial cells (Liou et al. 2016). The similar function of matrine was observed in this study in LPS-damaged PC12 cells, as evidenced by the repressed release of pro-inflammatory cytokines, including IL-1β, IL-6 and TNF-α. Besides, the anti-apoptotic activity of matrine in various experimental systems was previously revealed, such as hypoxia/reoxygenation-injured cardiac microvascular endothelial cells (Zhao XB et al. 2018) and focal cerebral ischaemic mice (Zhao et al. 2015). In line with this, matrine also exerted anti-apoptotic property in LPS-damaged PC12 cells. And matrine prevented PC12 cells from initiating apoptosis possibly via a mitochondrial-dependent pathway, since the balance of Bcl-2 and Bax was disturbed. Altogether, the findings of this paper evidenced the neuroprotective property of matrine, which was consistence with findings reported elsewhere (Meng et al. 2017).
Since scholars around the world have focussed on studying the pharmacological effects of matrine, the mechanisms of which matrine exerts its function are also preliminary uncovered. A small fraction of studies revealed that, matrine acted its function through regulating miRNAs, like miR-21 (Zhao L et al. 2018), miR-19b-3p (Wei et al. 2018), miR-126 (An et al. 2016) and miR-133a (Liao et al. 2015). Our study suggested that miR-9 was another downstream effector of matrine, as its expression was clearly elevated by matrine. And the neuroprotective property of matrine was attenuated when miR-9 was silenced by the transfection of specific inhibitor. Further experiments were required to discover other miRNAs by which matrine protected neurons.
JNK and NF–κB signalling pathways are two deterministic pathways that involved in cell apoptosis and inflammation in response to a variety of stimulis like LPS (Khan et al. 2017; Zhang H et al. 2018). JNK is started to be activated within 1 h following SCI and prolonged for 3 days (Li QM et al. 2007). c-Jun is the most important downstream factor of JNK and similar expression trend of c-Jun is also occurred during SCI (Liu X et al. 2015). Likewise, NF–κB pathway is activated following SCI and it participates in regulating neuron apoptosis and inflammation (Wang et al. 2018). Thus, inhibiting the activation of JNK and NF–κB pathways has promise in controlling the secondary injury of SCI. In the current paper, JNK and NF–κB pathways were found to be activated by LPS in PC12 cells. Moreover, matrine pre-treatment inhibited JNK and NF–κB pathways, and its impact on these two signalling pathways could be partially or completely abolished when miR-9 was silenced. This evidence collectively suggested that matrine inhibited LPS-evoked JNK and NF–κB pathways via a miR-9-dependent way.
To conclude, this paper provided in vitro evidence that matrine was able to protect PC12 cells against LPS-evoked damage. The neuroprotective properties of matrine may be due to its regulation on miR-9 expression and the following pathways, like JNK and NF–κB pathways. Nonetheless, the revealed pharmacological effect of matrine needs to be verified in vivo, which will largely improve the findings of this study.
Consent for publication
All authors are informed and agree to publish.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author contributions
Guangji Wang conceived and designed the experiments, Jinsong Jiang performed the experiments and analysed the data and Jinsong Jiang and Guangji Wang wrote the manuscript. All authors read and approved the final manuscript.
Disclosure statement
The authors declare that they have no competing interests.
References
- Aghvami M, Ebrahimi F, Zarei MH, Salimi A, Pourahmad Jaktaji R, Pourahmad J.. 2018. Matrine induction of ROS mediated apoptosis in human ALL B-lymphocytes via mitochodrial targeting. Asian Pacific J Cancer Prevention. 19:555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DJ, Beaudry KM, Thomas AM, Josse AR, Ditor DS.. 2018. Changes in nutrient intake and inflammation following an anti-inflammatory diet in spinal cord injury. J Spinal Cord Med. 42:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Q, Han C, Zhou Y, Li F, Li D, Zhang X, Yu Z, Duan Z, Kan Q.. 2016. Matrine induces cell cycle arrest and apoptosis with recovery of the expression of miR-126 in the A549 non-small cell lung cancer cell line. Mol Med Rep. 14(5):4042–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar MA, Al Shehabi TS, Eid AH.. 2016. Inflammogenesis of secondary spinal cord injury. Front Cell Neurosci. 10:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RG, Hayden MS, Ghosh S.. 2011. NF-kappaB, inflammation, and metabolic disease. Cell Metabol. 13(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai XH, Guo H, Xie B.. 2018. Structural modifications of matrine-type alkaloids. MRMC. 18(9):730–744. [DOI] [PubMed] [Google Scholar]
- Emery E, Aldana P, Bunge MB, Puckett W, Srinivasan A, Keane RW, Bethea J, Levi AD.. 1998. Apoptosis after traumatic human spinal cord injury. J Neurosur. 89(6):911–920. [DOI] [PubMed] [Google Scholar]
- Feng F, Wa MM, Luo HX, Guan CP, Zhou XZ.. 2018. Effect of matrine on reducing damage to bovine mammary epithelial cells induced by Staphylococcus aureus alpha-hemolysin. Polish J Vet Sci. 21:409–413. [DOI] [PubMed] [Google Scholar]
- Haiyan W, Yuxiang L, Linglu D, Tingting X, Yinju H, Hongyan L, Lin M, Yuanxu J, Yanrong W, Jianqiang Y.. 2013. Antinociceptive effects of matrine on neuropathic pain induced by chronic constriction injury. Pharm Biol. 51(7):844–850. [DOI] [PubMed] [Google Scholar]
- Kan QC, Zhu L, Liu N, Zhang GX.. 2013. Matrine suppresses expression of adhesion molecules and chemokines as a mechanism underlying its therapeutic effect in CNS autoimmunity. Immunol Res. 56(1):189–196. [DOI] [PubMed] [Google Scholar]
- Khan MA, Farahvash A, Douda DN, Licht JC, Grasemann H, Sweezey N, Palaniyar N.. 2017. JNK activation turns on LPS-and Gram-negative bacteria-induced NADPH oxidase-dependent suicidal NETosis. Sci Rep. 7(1):3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QM, Tep C, Yune TY, Zhou XZ, Uchida T, Lu KP, Yoon SO.. 2007. Opposite regulation of oligodendrocyte apoptosis by JNK3 and Pin1 after spinal cord injury. J Neurosci. 27(31):8395–8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Yin F, Guo Y, Ruan Q, Zhu Q.. 2018. Angelica polysaccharide protects PC-12 cells from lipopolysaccharide-induced injury via down-regulating microRNA-223. Biomed Pharmacother. 108:1320–1327. [DOI] [PubMed] [Google Scholar]
- Liao H, Zhao X, Qu J, Zhang J, Cai H.. 2015. Matrine suppresses invasion and metastasis of NCI-H1299 cells by enhancing microRNA-133a expression. Int J Clin Exper Med. 8:10714–10722. [PMC free article] [PubMed] [Google Scholar]
- Liou CJ, Lai YR, Chen YL, Chang YH, Li ZY, Huang WC.. 2016. Matrine attenuates COX-2 and ICAM-1 expressions in human lung epithelial cells and prevents acute lung injury in LPS-induced mice. Mediators Inflam. 2016:3630485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Rondinone CM.. 2005. JNK: bridging the insulin signaling and inflammatory pathway. Cur Opinion Investigational Drugs. 6:979–987. [PubMed] [Google Scholar]
- Liu X, Li C, Liang F, Wang Y, Li Z, Yang J.. 2015. Effects of hyperbaric oxygen on glucose-regulated protein 78 and c-Jun N-terminal kinase expression after spinal cord injury in rats. Int J Clin Exper Med. 8:3309–3317. [PMC free article] [PubMed] [Google Scholar]
- Lu J, Ashwell KW, Waite P.. 2000. Advances in secondary spinal cord injury: role of apoptosis. Spine. 25(14):1859–1866. [DOI] [PubMed] [Google Scholar]
- Lynch J, Cahalan R.. 2017. The impact of spinal cord injury on the quality of life of primary family caregivers: a literature review. Spinal Cord. 55(11):964–978. [DOI] [PubMed] [Google Scholar]
- Mahzari A, Li S, Zhou X, Li D, Fouda S, Alhomrani M, Alzahrani W, Robinson SR, Ye JM.. 2019. Matrine protects against MCD-induced development of NASH via upregulating HSP72 and downregulating mTOR in a manner distinctive from metformin. Frontiers Pharmacol. 10:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Wang J, Ding F, Xie Y, Zhang Y, Zhu J.. 2017. Neuroprotective effect of matrine on MPTP-induced Parkinson’s disease and on Nrf2 expression. Oncol Letters. 13(1):296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourelo Farina M, Salvador de la Barrera S, Montoto Marques A, Ferreiro Velasco ME, Galeiras Vazquez R.. 2017. Update on traumatic acute spinal cord injury. Part 2. Med Intensiva. 41:306–315. [DOI] [PubMed] [Google Scholar]
- Nampoothiri SS, Rajanikant GK.. 2019. MiR-9 upregulation integrates post-ischemic neuronal survival and regeneration in vitro. Cell Mol Neurobiol. 39(2):223–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid HU, Xu Y, Muhammad Y, Wang L, Jiang J.. 2019. Research advances on anticancer activities of matrine and its derivatives: an updated overview. Eur J Med Chem. 161:205–238. [DOI] [PubMed] [Google Scholar]
- Rouanet C, Reges D, Rocha E, Gagliardi V, Silva GS.. 2017. Traumatic spinal cord injury: current concepts and treatment update. Arq Neuro-Psiquiatr. 75(6):387–393. [DOI] [PubMed] [Google Scholar]
- Shi Z, Zhou H, Lu L, Li X, Fu Z, Liu J, Kang Y, Wei Z, Pan B, Liu L, et al. 2017. The roles of microRNAs in spinal cord injury. Int J Neurosci. 127(12):1104–1115. [DOI] [PubMed] [Google Scholar]
- Tanabe N, Kuboyama T, Tohda C.. 2018. Matrine directly activates extracellular heat shock protein 90, resulting in axonal growth and functional recovery in spinal cord injured-mice. Frontiers Pharmacol. 9:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Dai W, Shi L, Teng H, Li X, Wang J, Geng W.. 2018. Neuroprotection by paeoniflorin against nuclear factor kappa B-induced neuroinflammation on spinal cord injury. BioMed Res Int. 2018:9865403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YP, Wang XH, Liu G, Zhang JF, Yang YX, Zhang J, Song XL, Li ZD, Zhao LD.. 2018. Matrine exerts inhibitory effects in melanoma through the regulation of miR-19b-3p/PTEN. Int J Oncol. 53:791–800. [DOI] [PubMed] [Google Scholar]
- Wu G, Zhou W, Zhao J, Pan X, Sun Y, Xu H, Shi P, Geng C, Gao L, Tian X.. 2017. Matrine alleviates lipopolysaccharide-induced intestinal inflammation and oxidative stress via CCR7 signal. Oncotarget. 8:11621–11628. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Xie Y, Zhang H, Zhang Y, Wang C, Duan D, Wang Z.. 2018. Chinese Angelica polysaccharide (CAP) alleviates LPS-induced inflammation and apoptosis by down-regulating COX-1 in PC12 cells. Cell Physiol Biochem. 49(4):1380–1388. [DOI] [PubMed] [Google Scholar]
- Xu Y, An BY, Xi XB, Li ZW, Li FY.. 2016. MicroRNA-9 controls apoptosis of neurons by targeting monocyte chemotactic protein-induced protein 1 expression in rat acute spinal cord injury model. Brain Res Bull. 121:233–240. [DOI] [PubMed] [Google Scholar]
- Yu JL, Li JH, Chengz RG, Ma YM, Wang XJ, Liu JC.. 2014. Effect of matrine on transforming growth factor beta1 and hepatocyte growth factor in rat liver fibrosis model. Asian Pacific J Trop Med. 7(5):390–393. [DOI] [PubMed] [Google Scholar]
- Zhang H, Chen S, Zeng M, Lin D, Wang Y, Wen X, Xu C, Yang L, Fan X, Gong Y, et al. 2018. Apelin-13 administration protects against LPS-induced acute lung injury by inhibiting NF-kappaB pathway and NLRP3 inflammasome activation. Cell Physiol Biochem. 49(5):1918–1932. [DOI] [PubMed] [Google Scholar]
- Zhang N, Yin Y, Xu SJ, Wu YP, Chen WS.. 2012. Inflammation & apoptosis in spinal cord injury. Indian J Med Res. 135:287–296. [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zhang X, Cui S.. 2018. Matrine inhibits TPC-1 human thyroid cancer cells via the miR-21/PTEN/Akt pathway. Oncol Lett. 16(3):2965–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Zhou R, Zhu XY, Hao YJ, Li N, Wang J, Niu Y, Sun T, Li YX, Yu JQ.. 2015. Matrine attenuates focal cerebral ischemic injury by improving antioxidant activity and inhibiting apoptosis in mice. Int J Mol Med. 36(3):633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XB, Qin Y, Niu YL, Yang J.. 2018. Matrine inhibits hypoxia/reoxygenation-induced apoptosis of cardiac microvascular endothelial cells in rats via the JAK2/STAT3 signaling pathway. Biomed Pharmacother. 106:117–124. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wu Y, Deng L, Chen L, Zhao D, Lv L, Chen X, Man J, Wang Y, Shan H, et al. 2014. The alkaloid matrine of the root of Sophora flavescens prevents arrhythmogenic effect of ouabain. Phytomedicine. 21(7):931–935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.