Abstract
OBJECTIVES
Proponents of open thoracotomy (OPEN) and robot-assisted thoracic surgery (RATS) claim its oncological superiority over video-assisted thoracic surgery (VATS) in terms of the accuracy of lymph node staging.
METHODS
The National Cancer Database was queried for patients with non-small-cell lung cancer (NSCLC) undergoing lobectomy without neoadjuvant therapy from 2010 to 2014. Nodal upstaging rates were compared using a surgical approach. Overall survival adjusted for confounding variables was examined using the Cox proportional hazards model.
RESULTS
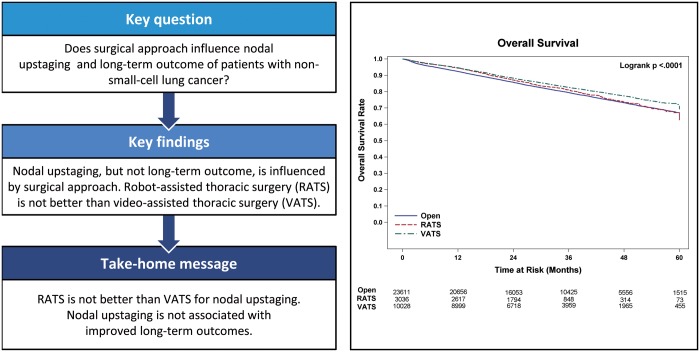
A total of 64 676 patients fulfilled the selection criteria. The number of patients who underwent lobectomy by RATS, VATS and OPEN approaches was 5470 (8.5%), 17 545 (27.1%) and 41 661 (64.4%), respectively. The mean number of lymph nodes examined for each of these approaches was 10.9, 11.3 and 10 (P < 0.01) and upstaging rates were 11.2%, 11.7% and 12.6% (P < 0.01), respectively. For patients with clinical stage I disease (N = 46 826; RATS = 4338, VATS = 13 416 and OPEN = 29 072), the mean lymph nodes examined were 10.6, 10.8 and 9.4 (P < 0.01), and upstaging rates were 10.8%, 11.1% and 12.1% (P < 0.01), respectively. A multivariable analysis suggested an association with improved survival with RATS and VATS compared with OPEN surgery [hazard ratio (HR) = 0.89 and 0.89, respectively; P < 0.01] for patients with all stages. In stage I disease, VATS but not RATS was associated with increased overall survival compared with the OPEN approach (HR = 0.81; P < 0.01).
CONCLUSIONS
RATS lobectomy is not superior to VATS lobectomy with respect to lymph node yield or upstaging of NSCLC. Increased nodal upstaging by the OPEN approach does not confer a survival advantage in any stage of NSCLC and may be associated with decreased overall survival.
Keywords: Lung cancer, Mediastinal staging, Video-assisted thoracic surgery
INTRODUCTION
Accurate assessment of lymph nodes for the presence of the metastatic disease is essential for effective treatment planning and prognostication for patients diagnosed with non-small-cell lung carcinoma (NSCLC). An equally critical component of surgical resection of the primary tumour is an intraoperative evaluation of N1 and N2 lymph nodes, regardless of negative preoperative invasive mediastinal staging results. Debate exists regarding the need for full lymph node dissection versus lymph node sampling, and whether surgical approach affects the results. Retrospective reports have shown improved survival of patients undergoing full lymph node dissection [1–4], yet results from ACOSOG Z0030 demonstrated no survival benefit for patients undergoing mediastinal lymph node dissection compared with those who underwent systematic lymph node sampling [5]. Variation undoubtedly exists in how lymph nodes are removed, assessed and reported. Unfortunately, sometimes lymph node sampling is not performed at all [6].
The impact of the surgical approach on the effectiveness of lymph node assessment is not clear. Since its introduction over 25 years ago, video-assisted thoracic surgery (VATS) lobectomy has become a well-accepted approach for the resection of early-stage NSCLC, and increasingly, more for advanced stages of the disease and complex tumour pathology. Despite evidence suggesting comparable oncologic outcomes for thoracoscopic approaches to lobectomy [6–8], advocates for traditional open approaches, as well as newer minimally invasive approaches using robotic platforms, claim superiority in the accuracy and thoroughness of lymph node assessment. Studies comparing traditional open approaches with VATS approaches have shown VATS to have a lower number of lymph nodes evaluated [9] and decreased rates of upstaging compared with thoracotomy [9–11].
As VATS lobectomy gained more widespread acceptance, questions regarding oncologic soundness diminished. Reports showed that the number of lymph nodes dissected by VATS approaches equalled that of traditional open approaches [12–14], though rates of nodal upstaging lagged behind in certain cases. As institutional and surgeon experience matured, the effectiveness of lymph node staging was shown to improve [15]. The introduction of robotic platforms into minimally invasive thoracic surgery approaches improved 3-dimensional viewing, and claims of superiority in lymph node sampling for lobectomy. Single institution reports demonstrating superior lymph node sampling compared with VATS approaches began to surface suggesting the need for additional studies with larger cohorts of patients [16].
Herein, we report results using a large multi-institutional database to retrospectively examine the rates of nodal upstaging associated with multiple surgical approaches for lobectomy for NSCLC.
METHODS
Data source/patient selection criteria
The National Cancer Database (NCDB) was used as the data source for this study. This large, nationwide database captures incident cancer cases diagnosed or treated at over 1500 Commission on Cancer-accredited facilities in the USA (ACS). The NCDB captures ∼70% of all newly diagnosed cancer cases yearly. The American College of Surgeons and the Commission on Cancer are not responsible for the statistical methodology employed, or the conclusions drawn by investigators analysing the de-identified data received in the form of a Participant User File document.
This research study was deemed exempt by the Roswell Park Institutional Review Board. The selection criteria used to finalize the patient study population are listed in Table 1. Nodal upstaging rates in the whole population and by surgical approach were compared. The approaches examined included open thoracotomy (OPEN), VATS and robot-assisted thoracic surgery (RATS). A subset analysis of patients with stage I disease was performed. Variables included in the NCDB that are able to be analysed include patient age, race, gender, grade, histology, analytic stage, Charlson–Deyo Score, number of lymph nodes examined (LNE), the extent of resection (R0, R1 or R2) and the year of diagnosis. The analytic stage refers to the stage coded using the AJCC Cancer Staging Manual edition in use during the year in which the case was diagnosed. Continuous variables were summarized with means, medians and standard deviations. Frequencies and cumulative frequencies were used to summarize categorical data.
Table 1:
Criteria for selecting the study population from the NCDB database
| Selection criteria | Patient number |
|---|---|
| All patients with NSCLC from 2010 to 2014 | 122 950 |
| Patients in whom this cancer was the first | 84 832 |
| Patients with lobectomy without neoadjuvant therapy | 79 185 |
| Exclude patients with unknown surgical approach | 73 470 |
| Exclude stage 0, unknown stage and occult cancer | 64 676 |
NCDB: National Cancer Database; NSCLC: non-small-cell lung cancer.
Statistical analyses
Associations between potential covariates and treatment were analysed using the Pearson χ2 test for categorical variables and Wilcoxon Rank Sum test for continuous variables. The primary endpoints of this study were the lymph node yield and nodal upstaging rate based on the surgical approach. Secondary outcome variables analysed included 30-day and 90-day mortality, as well as overall survival (OS). Univariable and multivariable logistic models were used to assess the effect of surgical approach on 30-day and 90-day mortality. Model results were summarized using estimates and 95% confidence limits for the odds ratio. Univariable and multivariable proportional hazards models were used to assess the effect of surgical approach on OS. The relative prognosis was summarized using estimates and 95% confidence limits for the hazard ratio (HR). Unadjusted differences in overall mortality based on surgical approach are shown using a Kaplan–Meier method. The log-rank test was used for comparison of survival distributions.
Propensity-weighted analyses
For confirmation of the multivariable results, propensity score (PS) methods were used to adjust for baseline covariate imbalances between the surgical approaches. The PS model was specified in advance and included fixed effects for sex (male/female), Charlson–Deyo Score (0/1/2), race (black/white/other), radiation (none/adjuvant/unknown), grade (I and II/III and IV/unknown), histology (adeno/squam/others), age at dx (continuous), number of LNE (continuous) and stage (TNM_CLIN_STAGE_GROUP 1, 2, 3, 4).
The PS was estimated with a multivariable logistic regression model for the probability of any surgical approach. Three weighted models were run to test for a difference between all three surgical approaches. For each pairing of surgical approaches, PS adjustment was done with an inverse probability of treatment weight. The whole data set (all stages) was analysed as well as the subset of patients with stage I disease. In total 6 propensity weight analyses were performed. All analyses were conducted using SAS v9.4 (Cary, NC, USA) at a significance level of 0.05.
RESULTS
During this time period, 64 676 patients fulfilled the selection criteria and were included in the study. Patient descriptive characteristics for the entire study population are given in Table 2. The median age of the cohort was 67 years. The number of patients who underwent lobectomy by RATS, VATS and OPEN approaches was 5470 (8.5%), 17 545 (27.1%) and 41 661 (64.4%), respectively. The number of events (deaths) in this cohort during follow-up was 12 205. The mean number of LNE for each of these approaches was 10.9, 11.3 and 10 (P < 0.01), and upstaging rates were 11.2%, 11.7% and 12.6% (P < 0.01), respectively. For the subset of patients with clinical stage I disease (N = 46 826; RATS = 4338, VATS = 13 416 and OPEN = 29 072), mean LNE were 10.6, 10.8 and 9.4 (P < 0.01), and upstaging rates were 10.8%, 11.1% and 12.1% (P < 0.01), respectively (Table 3). The number of events (deaths) in this cohort during follow-up was 7132. The univariate analysis examining the association between surgical approach and OS in the total population and stage I are shown in Figs. 1 and 2 respectively. Lymph node upstaging rates were higher in academic and integrated network cancer programmes when compared to community cancer programmes (Table 4). Lymph node upstaging was also associated with a greater number of LNE (12.5 vs 10.3; P < 0.001). The multivariable analysis suggested an association with improved survival of RATS and VATS when compared to OPEN surgery (HR = 0.89 and 0.89, respectively; P < 0.01) for patients with all stages (Table 5). For patients with stage I disease, VATS, but not RATS was associated with the increased OS when compared with OPEN approach (HR = 0.81; P < 0.01).
Table 2:
Comparison of patients with all stages undergoing lobectomy by RATS, VATS and OPEN
| Variables | Groups | RATS | VATS | OPEN | P-value |
|---|---|---|---|---|---|
| Overall count, N (%) | 5470 (8.5) | 17 545 (27.1) | 41 661 (64.4) | ||
| Age (years), mean (SD) | 66.8 (9.8) | 66.6 (10.2) | 66.3 (10.3) | 0.001 | |
| Gender | Male (%) | 2421 (44.3) | 7696 (43.9) | 19 895 (47.8) | <0.01 |
| Race, N (%) | White | 4700 (85.9) | 15 235 (86.8) | 36 216 (86.9) | <0.01 |
| Black | 532 (9.7) | 1446 (8.2) | 3833 (9.2) | ||
| Other | 238 (4.4) | 864 (4.9) | 1612 (3.9) | ||
| Histology, N (%) | Squamous | 1214 (22.2) | 3877 (22.1) | 10 998 (26.4) | <0.01 |
| Adeno | 3880 (70.9) | 12 372 (70.5) | 27 163 (65.2) | ||
| Others | 376 (6.9) | 1296 (7.4) | 3500 (8.4) | ||
| Stage, N (%) | I | 4389 (80.4) | 13 678 (78.2) | 29 623 (71.3) | <0.01 |
| II | 730 (13.4) | 2599 (14.9) | 7998 (19.2) | ||
| III | 267 (4.9) | 945 (5.4) | 3137 (7.5) | ||
| IV | 70 (1.3) | 265 (1.5) | 813 (2.0) | ||
| Grade, N (%) | I/II | 3443 (62.9) | 10 747 (61.3) | 24 685 (59.3) | <0.01 |
| III/IV | 1677 (30.7) | 5569 (31.7) | 14 435 (34.6) | ||
| Unknown | 350 (6.4) | 1229 (7.0) | 2541 (6.1) | ||
| Conversion, N (%) | 515 (9.4) | 2878 (16.4) | <0.01 | ||
| CDS, N (%) | 0 | 2674 (48.9) | 9035 (51.5) | 21 045 (50.5) | <0.01 |
| 1 | 2018 (36.9) | 6153 (35.1) | 14 635 (35.1) | ||
| 2 | 778 (14.2) | 2357 (13.4) | 5981 (14.4) | ||
| LNE, mean (SD) | 10.9 (7.9) | 11.3 (8.9) | 10 (7.5) | <0.01 | |
| N stage, N (%) | Upstaged | 595 (11.2) | 1967 (11.7) | 4960 (12.6) | <0.01 |
| Downstaged | 156 (2.9) | 588 (3.5) | 1631 (4.1) | ||
| Same | 4550 (85.8) | 14 220 (79.4) | 32 869 (83.3) | ||
| 30/90-Day mortality (%) | 1.8/3.2 | 1.8/3.1 | 2.4/4.6 | <0.01 | |
| LOS, median (days) | 4 | 5 | 6 | <0.01 | |
| 5-Year survival (HR) | 0.65 (0.61–0.68) | 0.66 (0.65–0.67) | 0.60 (0.60–0.61) | <0.01 |
CDS: Charlson–Deyo Score; HR: hazard ratio; LNE: lymph nodes examined; LOS: length of stay; OPEN: open thoracotomy; RATS: robot-assisted thoracic surgery; SD: standard deviation; VATS: video-assisted thoracic surgery.
Table 3:
Comparison of patients with stage I undergoing lobectomy by RATS, VATS and OPEN
| Variables | Groups | RATS | VATS | OPEN | P-value |
|---|---|---|---|---|---|
| Overall count, N (%) | 4338 (9.3) | 13 416 (28.7) | 29 072 (62.1) | ||
| Age (years), mean (SD) | 66.7 (9.8) | 66.6 (10.2) | 66.4 (10.3) | 0.001 | |
| Gender | Male (%) | 1864 (43.0) | 5597 (41.7) | 13 152 (45.2) | <0.01 |
| Race, N (%) | White | 3731 (86.0) | 11 690 (87.1) | 25 378 (87.3) | <0.01 |
| Black | 413 (9.5) | 1053 (7.8) | 2580 (8.9) | ||
| Other | 194 (4.5) | 673 (5.0) | 1114 (3.8) | ||
| Histology, N (%) | Squamous | 954 (22.0) | 2724 (20.3) | 6967 (24.0) | <0.01 |
| Adeno | 3108 (71.6) | 9822 (73.2) | 19 986 (68.7) | ||
| Others | 276 (6.4) | 870 (6.5) | 2119 (7.3) | ||
| Grade, N (%) | I/II | 2843 (65.5) | 8704 (64.9) | 18 524 (63.7) | <0.01 |
| III/IV | 1222 (28.2) | 3771 (28.1) | 8760 (30.1) | ||
| Unknown | 273 (6.3) | 941 (7.0) | 1788 (6.2) | ||
| Conversion, N (%) | 389 (9.0) | 2030 (15.1) | <0.01 | ||
| CDS, N (%) | 0 | 2050 (47.3) | 6921 (51.6) | 14 584 (50.2) | <0.01 |
| 1 | 1647 (38.0) | 4697 (35.0) | 10 290 (35.4) | ||
| 2 | 641 (14.8) | 1798 (13.4) | 4198 (14.4) | ||
| LNE, mean (SD) | 10.6 (7.4) | 10.8 (8.4) | 9.4 (7.0) | <0.01 | |
| N stage, N (%) | Upstaged | 460 (10.8) | 1451 (11.1) | 3380 (12.1) | <0.01 |
| 30/90-Day mortality (%) | 1.6/2.6 | 1.4/2.3 | 2.1/3.8 | <0.01 | |
| LOS, median (days) | 4 | 5 | 6 | <0.01 | |
| 5-Year survival (HR) | 0.67 (0.63–0.71) | 0.72 (0.71–0.74) | 0.67 (0.66–0.68) | <0.01 |
CDS: Charlson–Deyo Score; HR: hazard ratio; LNE: lymph nodes examined, LOS: length of stay, OPEN: open thoracotomy; RATS: robot-assisted thoracic surgery; SD: standard deviation; VATS: video-assisted thoracic surgery.
Figure 1:
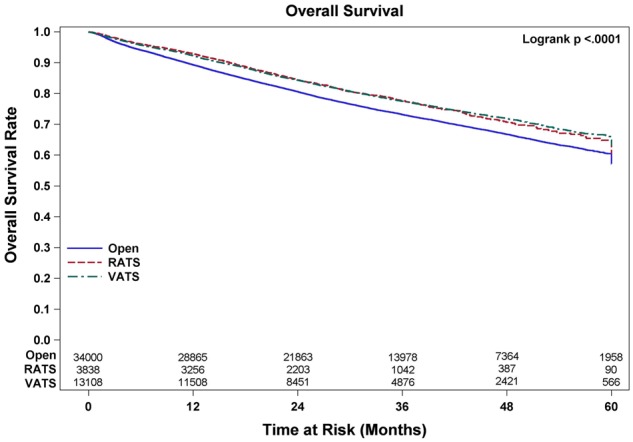
Overall survival of patients undergoing lobectomy for non-small-cell lung cancer. OPEN: open thoracotomy; RATS: robot-assisted thoracic surgery; VATS: video-assisted thoracic surgery.
Table 4:
Percentages of nodal upstaging by type of centre
| Approach | Community cancer programme | Comprehensive community cancer programme | Academic research programme | Integrated network cancer programme | P-value |
|---|---|---|---|---|---|
| All stages | |||||
| RATS | 10.29 | 9.67 | 12.11 | 12.82 | 0.03 |
| VATS | 9.76 | 11.27 | 12.14 | 12.40 | 0.12 |
| OPEN | 10.93 | 12.12 | 13.18 | 13.93 | <0.01 |
| Stage I | |||||
| RATS | 8.18 | 9.71 | 11.31 | 12.82 | 0.11 |
| VATS | 9.28 | 10.33 | 11.81 | 11.63 | 0.04 |
| OPEN | 10.42 | 11.54 | 12.68 | 13.54 | <0.01 |
All numbers are in percentages.
OPEN: open thoracotomy; RATS: robot-assisted thoracic surgery; VATS: video-assisted thoracic surgery.
Table 5:
Univariate and multivariate analyses of variables associated with overall survival
| Variables | Reference | Univariate model estimate (95% CI) | Multivariate model estimate (95% CI) |
|---|---|---|---|
| All stages | |||
| N (upstaged) | N (same) | 1.92 (1.84–2.01) | Not included in model |
| N (downstaged) | 1.64 (1.52–1.78) | ||
| RATS | Open | 0.82 (0.76–0.88) | 0.89 (0.83–0.97) |
| VATS | 0.81 (0.77–0.84) | 0.89 (0.86–0.93) | |
| Race (black) | Race (white) | 0.91 (0.86–0.98) | 0.99 (0.93–1.06) |
| Race (other) | 0.72 (0.65–0.80) | 0.77 (0.69–0.85) | |
| Grade (III/IV) | Grade (I/II) | 1.70 (1.64–1.76) | 1.40 (1.34–1.45) |
| Unknown | 0.82 (0.74–0.90) | 0.86 (0.78–0.94) | |
| Squamous | Adenocarcinoma | 1.61 (1.55–1.68) | 1.18 (1.13–1.23) |
| Histology (others) | 1.56 (1.47–1.66) | 1.28 (1.20–1.36) | |
| Female | Male | 0.60 (0.58–0.63) | 0.70 (0.67–0.72) |
| CDS 1 | CDS 0 | 1.26 (1.21–1.31) | 1.18 (1.14–1.23) |
| CDS 2 | 1.68 (1.59–1.76) | 1.48 (1.41–1.56) | |
| Clinical stage | |||
| II | I | 2.01 (1.92–2.09) | 1.80 (1.72–1.88) |
| III | 2.61 (2.46–2.76) | 2.22 (2.10–2.36) | |
| IV | 4.21 (3.83–4.64) | 3.92 (3.58–4.30) | |
| LNE | 1.00 (1.00–1.01) | 1.00 (0.99–1.00) | |
| Age | 1.03 (1.03–1.03) | 1.03 (1.03–1.04) | |
| Stage I | |||
| N (upstaged) | N (same) | 2.16 (2.04–2.29) | Not included in model |
| RATS | Open | 0.93 (0.84–1.02) | 0.95 (0.86–1.04) |
| VATS | 0.81 (0.77–0.86) | 0.86 (0.82–0.91) | |
| Race (black) | Race (white) | 0.90 (0.83–0.99) | 1.02 (0.94–1.12) |
| Race (other) | 0.66 (0.57–0.76) | 0.74 (0.64–0.85) | |
| Grade (III/IV) | Grade(I/II) | 1.69 (1.61–1.77) | 1.49 (1.42–1.57) |
| Unknown | 0.70 (0.61–0.79) | 0.78 (0.69–0.89) | |
| Squamous | Adenocarcinoma | 1.72 (1.64–1.81) | 1.24 (1.18–1.31) |
| Histology (others) | 1.49 (1.37–1.62) | 1.26 (1.15–1.37) | |
| Female | Male | 0.58 (0.55–0.61) | 0.66 (0.63–0.69) |
| CDS 1 | CDS 0 | 1.37 (1.30–1.44) | 1.24 (1.18–1.31) |
| CDS 2 | 1.91 (1.79–2.03) | 1.60 (1.50–1.70) | |
| LNE | 1.00 (1.00–1.00) | 1.00 (0.99–1.00) | |
| Age | 1.04 (1.03–1.04) | 1.04 (1.03–1.04) |
CDS: Charlson–Deyo Score; CI: confidence interval; LNE: lymph nodes examined; RATS: robot-assisted thoracic surgery; VATS: video-assisted thoracic surgery.
Propensity-weighted analyses
Propensity-weighted analyses for all patients comparing OS based on surgical approach revealed no differences in OS between RATS when compared to VATS (log-rank P = 0.802), but statistically improved survival when comparing RATS and VATS to OPEN (log-rank P = 0.0074 and <0.0001). For the subset of patients with stage I disease, the propensity-weighted analysis revealed improved OS only for VATS compared to OPEN (logrank P < 0.0001). No differences were noted when comparing RATS to VATS or RATS to OPEN (log-rank P = 0.0651 and P = 0.3980).
Additionally, propensity-weighted analyses for 30- and 90-day mortality for all stages revealed statistically improved outcomes for both VATS and RATS compared to OPEN (30-day mortality P < 0.001; P < 0.001 and 90-day mortality P < 0.001; P < 0.001). No difference was noted when RATS is compared with VATS (P = 0.432 for 30-day mortality and P = 0.530 for 90-day mortality). For stage I patients, similar results were noted comparing RATS and VATS to OPEN, and again, no difference when comparing RATS to VATS.
DISCUSSION
Our findings from this analysis utilizing a large, multi-institution database suggest that robotic-assisted (RATS) approaches to lobectomy are not superior to thoracoscopic (VATS) approaches for lymph node evaluation at the time of lobectomy for NSCLC. Consistent with previous reports [9–11], a higher rate of nodal upstaging was noted with standard OPEN approaches. However, this did not confer a survival advantage as patients in our overall study population who underwent RATS or VATS had improved survival. The increased rate of upstaging was also seen without an increase in the number of lymph nodes sampled, as the lymph node yield was statistically higher in the VATS group compared to patients treated with RATS or OPEN approaches. These trends were also seen in patients with clinical stage 1 disease.
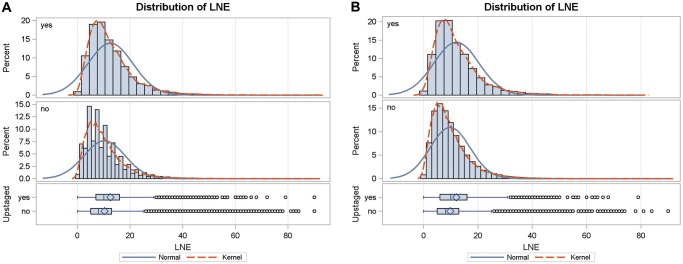
In direct contrast to results which report significantly higher lymph node yield with lobectomy via thoracotomy than with VATS lobectomy [10], our analysis showed that patients undergoing VATS had significantly more lymph nodes retrieved through dissection than patients in the RATS or OPEN groups. In these previous studies, the difference was largely due to the improved retrieval of N2 nodes with OPEN [9, 17]. Despite the statistically significant differences noted in this study, the mean number of lymph nodes harvested in each group ranged from 10 to 11.3 lymph nodes, and given the large patient numbers, are likely not clinically significant. Mean lymph node harvest in previous studies included in the meta-analysis by Zhang ranged from 6.1 up to 31. These numbers may suggest a higher proportion of lymph node ‘sampling’ approaches reported in this study as opposed to full mediastinal lymph node dissection, but data pertaining to the specifics of the surgical lymph node evaluation are not included in the NCDB. Though these average numbers meet the recommendation for minimum number of lymph nodes to be harvested during resection of lung cancer (10 nodes) based on American College of Surgeons Commission on Cancer guidelines (https://www.facs.org/-/media/files/quality-programs/cancer/ncdb/measure-specs-nscl.ashx?la=en), they fall short of targets for lymph node harvest at the time of lobectomy put forth in previous reports by Wen et al. (recommended 12 lymph nodes) and Liang et al. who recommend at least 16 lymph nodes be sampled [18, 19]. Figure 3 depicts the distribution of LNE in all stages and stage I NSCLC in this analysis. The distribution of LNE shows that there is significant room for improvement for this metric in the USA.
Figure 3:
Distribution of LNE in patients with all stages (A) and stage I (B) non-small-cell lung cancer. Both normal distribution and kernel smoothed curves are depicted. LNE: lymph nodes examined.
Figure 2:
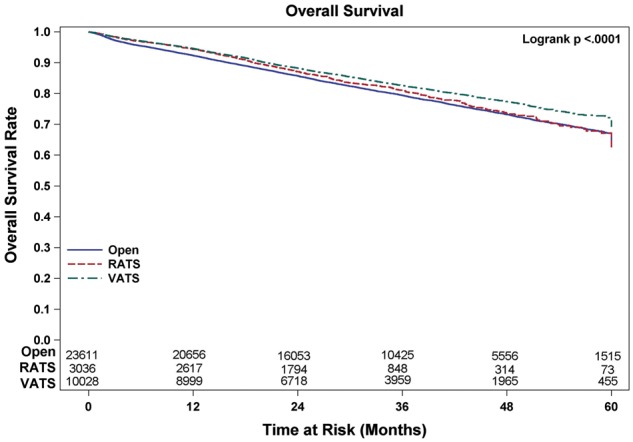
Overall survival of patients undergoing lobectomy for stage I non-small-cell lung cancer. OPEN: open thoracotomy; RATS: robot-assisted thoracic surgery; VATS: video-assisted thoracic surgery.
Other studies examining whether the surgical approach affects lymph node sampling or dissection in terms of the number of lymph nodes retrieved offer mixed results. An analysis of the patients in the ACOSOG Z0030 database showed no significant difference in the number of lymph nodes retrieved based on approach [14]. Additional studies, including an earlier randomized trial by Kirby et al., also found no difference in the number of lymph nodes sampled for biopsy in patients undergoing lobectomy by VATS versus thoracotomy [12, 13]. One recent study comparing RATS to VATS and OPEN found that the number of lymph node stations that were sampled with RATS was significantly higher than with VATS or thoracotomy [20]. However, they did not report on the total number of individual nodes sampled. While the proposed superior visualization and manoeuvrability afforded by robotic-assisted thoracoscopy may allow for more nodal stations to be accessed, our data suggest that the actual lymph node yield is not significantly different than that achieved with open or VATS approaches.
While it is important to consider how many lymph nodes or lymph node stations each surgical approach allows for assessment for occult metastasis, the clinical importance of examining this tissue is to ensure that the disease is staged properly so that the patients receive the appropriate treatment. Nodal upstaging from clinical stage 1 to pathological stage 2 disease has been reported to occur in up to 22.6% of NSCLC cases [21] and is an indication for adjuvant chemotherapy [22]. Our study found the OPEN approach to have a higher rate of nodal upstaging even though the number of LNE was less than or equal to the minimally invasive approaches. The reasons for this difference remain unclear, but an explanation may be that more nodal tissue is being removed by traditional OPEN. Unfortunately, the weight or volume of nodal tissue is not a variable that is reported in the NCDB. Another consideration, as has been pointed out previously [10, 11], is that one of the main pitfalls of the majority of studies examining this issue, including the current study, is their retrospective nature and the fact that the reasons behind the surgeons’ choices to pursue one surgical approach over the others cannot be ascertained for individual patients. For example, certain characteristics of a tumour beyond its size (central location, airway or vessel involvement) may cause a surgeon to opt for an open approach and pursue a more aggressive lymph node dissection, leading to a higher rate of nodal upstaging. Information reflective of these characteristics is not captured in the data sets used for these studies. The other issue is the perceived importance of lymph node staging. The hypothesis that this impacts the number of LNE is supported by the observation that academic and integrated network programmes show a higher number of LNE irrespective of the surgical approach.
Nonetheless, the increased upstaging we observed with the OPEN approach is in concordance with previous retrospective studies comparing VATS against traditional thoracotomy for patients with stage 1 disease [9–11]. The rates of nodal upstaging in these studies were 10–11.9% for VATS and 14.3–24.6% for thoracotomy, which is similar to our reported rates of 11.1% for VATS and 12.1% for the OPEN approach. Other studies comparing RATS to VATS also found no significant difference in the rates of nodal upstaging between these techniques [16, 23].
When considering surgical options for the treatment of lung cancer, weighing short-term morbidity against the potential for overall long-term survival is critical. While factors such as rates of nodal upstaging certainly affect the long-term survival in patients undergoing resection of NSCLC, there may be other unmeasured factors that can also affect survival. Our results suggest that minimally invasive approaches with VATS or RATS offer survival rates that are comparable to, or at least as safe as the OPEN approach regardless of disease stage. This is in agreement with several previous studies [6–8]. While the overall operative time may be longer than with open approaches, some of the advantages of minimally invasive techniques such as VATS and RATS include comparable intraoperative complication rates, lower postoperative complication rates and hospital length of stay that is about 2 days shorter on average than patients undergoing open resection [12, 14, 24]. Our findings support the assertion that VATS or RATS may be equivalent to, if not superior to, open approaches in the appropriate patient.
Limitations
Limitations of this study include its retrospective nature and the unavailability of important descriptors of lymph node characterization such as volume and mass, which are not reported in the NCDB. Information pertaining to specific lymph node stations assessed is also not available. The overall number of lymph node harvest for each surgical approach reported here is relatively low. Given that the number of lymph nodes resected during lung cancer resection has been shown to impact overall prognosis, the results reported here could be affected by the adequacy of lymph node dissection [18]. There is variation in technique and aggressiveness amongst individual surgeons in performing lymph node dissection or sampling. This is well demonstrated in the meta-analysis by Zhang, which included mostly single-institution studies which had a range of 6.1–31 average lymph nodes harvested. A study in which the mean VATS lymph node harvest was 6.1, compared to the open approach at 7.7, compared to another where VATS harvest was 31 compared to 30 by the open approach, speaks more surgeon variability with the aggressiveness of dissection instead of a surgical approach. Additionally, surgical skill level simply cannot be accounted for in a study of this nature. For example, a highly skilled VATS or robotic surgeon with excellent technique may perform a more thorough lymph node dissection compared to an OPEN done by a surgeon with poor technique. Likewise, the differences in how pathological specimens are processed between individual pathologists and institutions, and how the lymph nodes are reported cannot be accounted for. This variable cannot be controlled and may significantly impact the conclusions of this study. Station identification is likely uniform, but how the number of lymph nodes is arrived at is not. Other factors not controlled for in this analysis include the impact of tumour location, and whether it is central or peripheral, may impact the rate of occult nodal disease and how that may affect these results [25]. While PS matched analyses control for some bias, limitations in the granularity of the data limit the extent to which these biases can be controlled for. Lastly, given the number of analyses performed on this large data set comparing three different surgical approaches, there is substantial multiplicity that can potentially impact results.
CONCLUSION
The results of our analysis suggest that VATS and RATS approaches are not inferior to OPEN for intraoperative lymph node evaluation, though they may be inferior in the ability to uncover occult lymph node metastases. However, this does not appear to affect long-term survival for patients undergoing minimally invasive approaches. Our results support previous evidence suggesting that minimally invasive approaches such as VATS and RATS are not inferior to OPEN in patients undergoing resection for early-stage NSCLC.
Funding
This work was supported by the National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Cancer Institute’s Biostatistics Shared Resources.
Conflict of interest: none declared.
Author contributions
Mark W. Hennon: Conceptualization; Writing—original draft; Writing—review & editing. Luke H. DeGraaff: Methodology; Writing—original draft; Writing—review & editing. Adrienne Groman: Formal analysis; Methodology; Writing—review & editing. Todd L. Demmy: Conceptualization; Methodology; Writing—review & editing. Sai Yendamuri: Conceptualization; Data curation; Formal analysis; Project administration; Supervision; Writing—original draft; Writing—review & editing.
Glossary
ABBREVIATIONS
- HR
Hazard ratio
- LNE
Lymph nodes examined
- NCDB
National Cancer Database
- NSCLC
Non-small-cell lung cancer
- OPEN
Open thoracotomy
- OS
Overall survival
- PS
Propensity score
- RATS
Robot-assisted thoracic surgery
- VATS
Video-assisted thoracic surgery
Presented at the 27th European Conference on General Thoracic Surgery, Dublin, Ireland, 9–12 June 2019.
REFERENCES
- 1. Doddoli C, Aragon A, Barlesi F, Chetaille B, Robitail S, Giudicelli R.. Does the extent of lymph node dissection influence outcome in patients with stage I non-small-cell lung cancer? Eur J Cardiothorac Surg 2005;27:680–5. [DOI] [PubMed] [Google Scholar]
- 2. Wu Y, Zhi-Fan H, Si-Yu W, Xue-Ning Y, Wei O.. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1–6. [DOI] [PubMed] [Google Scholar]
- 3. Keller SM, Adak S, Wagner W, Johnson DH.. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Ann Thorac Surg 2000;70:358–66. [DOI] [PubMed] [Google Scholar]
- 4. Lardinois D, Suter H, Hakki H, Rousson V, Betticher D, Ris HB.. Morbidity, survival, and site of recurrence after mediastinal lymph-node dissection versus systematic sampling after complete resection for non-small cell lung cancer. Ann Thorac Surg 2005;80:268–74; discussion 74–5. [DOI] [PubMed] [Google Scholar]
- 5. Darling GE, Allen MS, Decker PA, Ballman K, Malthaner RA, Inculet RI. et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 trial. J Thorac Cardiovasc Surg 2011;141:662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee PC, Nasar A, Port JL, Paul S, Stiles B, Chiu YL. et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013;96:951–60; discussion 60–1. [DOI] [PubMed] [Google Scholar]
- 7. Paul S, Isaacs AJ, Treasure T, Altorki NK, Sedrakyan A.. Long term survival with thoracoscopic versus open lobectomy: propensity matched comparative analysis using SEER-Medicare database. BMJ 2014;349:g5575.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Su S, Scott WJ, Allen MS, Darling GE, Decker PA, McKenna RJ. et al. Patterns of survival and recurrence after surgical treatment of early stage non-small cell lung carcinoma in the ACOSOG Z0030 (ALLIANCE) trial. J Thorac Cardiovasc Surg 2014;147:747–52; discussion 52–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Merritt RE, Hoang CD, Shrager JB.. Lymph node evaluation achieved by open lobectomy compared with thoracoscopic lobectomy for N0 lung cancer. Ann Thorac Surg 2013;96:1171–7. [DOI] [PubMed] [Google Scholar]
- 10. Boffa DJ, Kosinski AS, Paul S, Mitchell JD, Onaitis M.. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347–53; discussion 53. [DOI] [PubMed] [Google Scholar]
- 11. Licht PB, Jorgensen OD, Ladegaard L, Jakobsen E.. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg 2013;96:943–9; discussion 49–50. [DOI] [PubMed] [Google Scholar]
- 12. Kirby TJ, Mack MJ, Landreneau RJ, Rice TW.. Lobectomy—video-assisted thoracic surgery versus muscle-sparing thoracotomy: a randomized trial. J Thorac Cardiovasc Surg 1995;109:997–1002. [DOI] [PubMed] [Google Scholar]
- 13. Watanabe A, Koyanagi T, Obama T, Ohsawa H, Mawatari T, Takahashi N. et al. Assessment of node dissection for clinical stage I primary lung cancer by VATS. Eur J Cardiothorac Surg 2005;27:745–52. [DOI] [PubMed] [Google Scholar]
- 14. Scott WJ, Allen MS, Darling G, Meyers B, Decker PA, Putnam JB. et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976–81; discussion 81–3. [DOI] [PubMed] [Google Scholar]
- 15. Lee PC, Kamel M, Nasar A, Ghaly G, Port JL, Paul S. et al. Lobectomy for non-small cell lung cancer by video-assisted thoracic surgery: effects of cumulative institutional experience on adequacy of lymphadenectomy. Ann Thorac Surg 2016;101:1116–22. [DOI] [PubMed] [Google Scholar]
- 16. Wilson JL, Louie BE, Cerfolio RJ, Park BJ, Vallieres E, Aye RW. et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901–6; discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 17. Zhang W, Wei Y, Jiang H, Xu J, Yu D.. Thoracotomy is better than thoracoscopic lobectomy in the lymph node dissection of lung cancer: a systematic review and meta-analysis. World J Surg Oncol 2016;14:290.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wen YS, Xi KX, Xi KX, Zhang RS, Wang GM, Huang ZR. et al. The number of resected lymph nodes is associated with the long-term survival outcome in patients with T2 N0 non-small cell lung cancer. Cancer Manag Res 2018;10:6869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liang W, He J, Shen Y, Shen J, He Q, Zhang J. et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-small-cell lung cancer: a population study of the US SEER database and a Chinese Multi-Institutional Registry. J Clin Oncol 2017;35:1162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang HX, Woo KM, Sima CS, Bains MS, Adusumilli PS, Huang J. et al. Long-term survival based on the surgical approach to lobectomy for clinical stage I nonsmall cell lung cancer: comparison of robotic, video-assisted thoracic surgery, and thoracotomy lobectomy. Ann Surg 2017;265:431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heineman DJ, Ten Berge MG, Daniels JM, Versteegh MI, Marang-van de Mheen PJ, Wouters MW. et al. Clinical staging of stage I non-small cell lung cancer in the Netherlands-need for improvement in an era with expanding nonsurgical treatment options: data from the Dutch lung surgery audit. Ann Thorac Surg 2016;102:1615–21. [DOI] [PubMed] [Google Scholar]
- 22. Heineman DJ, Daniels JM, Schreurs WH.. Clinical staging of NSCLC: current evidence and implications for adjuvant chemotherapy. Ther Adv Med Oncol 2017;9:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee BE, Shapiro M, Rutledge JR, Korst RJ.. Nodal upstaging in robotic and video assisted thoracic surgery lobectomy for clinical N0 lung cancer. Ann Thorac Surg 2015;100:229–33; discussion 33–4. [DOI] [PubMed] [Google Scholar]
- 24. Oh DS, Reddy RM, Gorrepati ML, Mehendale S, Reed MF.. Robotic-assisted, video-assisted thoracoscopic and open lobectomy: propensity-matched analysis of recent premier data. Ann Thorac Surg 2017;104:1733–40. [DOI] [PubMed] [Google Scholar]
- 25. Decaluwe H, Moons J, Fieuws S, De Wever W, Deroose C, Stanzi A. et al. Is central lung tumour location really predictive for occult mediastinal nodal disease in (suspected) non-small-cell lung cancer staged cN0 on 18F-fluorodeoxyglucose positron emission tomography-computed tomography? Eur J Cardiothorac Surg 2018;54:134–40. [DOI] [PubMed] [Google Scholar]