Abstract
The green alga Klebsormidium flaccidum var. zivo is a rich source of proteins, polyphenols, and bioactive small-molecule compounds. An approach involving chromatographic fractionation, anti-inflammatory activity testing, ultrahigh performance liquid chromatography-mass spectrometry profiling, chemometric analysis, and subsequent MS-oriented isolation was employed to rapidly identify its small-molecule anti-inflammatory compounds including hydroxylated fatty acids, chlorophyll-derived pheophorbides, carotenoids, and glycoglycerolipids. Pheophorbide a, which decreased intracellular nitric oxide production by inhibiting inducible nitric oxide synthase, was the most potent compound identified with an IC50 value of 0.24 µM in lipopolysaccharides-induced macrophages. It also inhibited nuclear factor kappaB activation with an IC50 value of 32.1 µM in phorbol 12-myristate 13-acetate-induced chondrocytes. Compared to conventional bioassay-guided fractionation, this approach is more efficient for rapid identification of multiple chemical classes of bioactive compounds from a complex natural product mixture.
Keywords: Klebsormidium flaccidum var. zivo, Klebsormidiaceae, anti-inflammatory, iNOS, NF-κB, chemometrics
1. Introduction
Classical approaches for identification of bioactive compounds from complex natural products involve time-consuming, multi-step bioassay-guided isolation procedures [1,2]. Without appropriate chemical and biological detection methods, often times bioactive compounds may be lost during the isolation process. Over the past decade, however, the application of advanced analytical and chromatographic instrumentation represented by hyphenated techniques such as LC-MS and LC-MS-NMR has greatly improved the efficiency of bioactive natural products discovery [2,3,4]. In addition, data analysis tools utilized in metabolomics and chemometrics have efficiently facilitated the identification of robust chemical markers for differentiation of plant species [5], screening of active compounds [6], and searching for appropriate chemical markers for quality control [7].
Klebsormidium (Klebsormidiaceae) is a genus of filamentous green algae distributed in terrestrial habitats worldwide [8]. Little is known about the chemistry of the entire genus except for a report on the analysis of fatty acids [9]. Klebsormidium flaccidum var. zivo is an optimized, non-GMO (genetically modified organism), proprietary algal strain that produces a unique blend of proteins, polyphenols, and small molecules [10]. The dried algal biomass of K. flaccidum var. zivo has been registered as KALGAETM; and a 90-day dietary toxicity study of KALGAETM in CRL Sprague-Dawley CD IGS rats and a genotoxicity evaluation in Swiss albino mice did not show adverse effects, supporting its safe use as a potential food ingredient to improve human health [10]. However, its small-molecule metabolites associated with biological activities remain unknown. Using an approach involving chromatographic fractionation, anti-inflammatory activity testing, UHPLC-qMS profiling, chemometric analysis, and selective isolation and purification, four chemotypes of compounds including hydroxylated fatty acids, chlorophyll-derived pheophorbides, carotenoids, and glycoglycerolipids, were identified to contribute to the anti-inflammatory activity of this alga. This study highlights the utility of a chemometrics-assisted approach involving orthogonal partial least squares discriminant analysis (OPLS-DA) that can facilitate rapid identification of multiple bioactive compounds from a complex natural product mixture.
2. Results and Discussion
2.1. Anti-Inflammatory Activities of Extracts and Column Fractions
To identify anti-inflammatory compounds from the algal biomass of K. flaccidum var. zivo, different extracts using organic solvents and water were prepared to obtain chemical constituents across a wide polarity range. Anti-inflammatory activity of extract was determined in terms of the decrease of inducible nitric oxide (NO) production through inhibition of nitric oxide synthase (iNOS) in lipopolysaccharides (LPS)-induced macrophages (RAW 264.7) and inhibition of nuclear factor kappaB (NF-κB) activation in phorbol 12-myristate 13-acetate (PMA)-induced human chondrosarcoma cells (SW1353) [11]. Preliminary testing results showed that the ethyl acetate extract exhibited highest inhibitory activity in the aforementioned two cell models, indicating that the anti-inflammatory constituents present in this alga are relatively lipophilic small-molecule compounds.
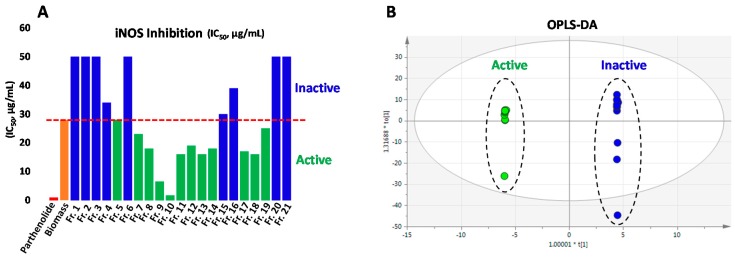
An ethyl acetate extract (IC50 28.0 μg/mL for iNOS) was thus fractionated into 21 fractions, with increasing polarity from Fr. 1 to Fr. 21, by normal-phase column chromatography (Table S1 in Supplemental Material). Assay for the inhibition of iNOS was used to monitor the anti-inflammatory activity of all 21 fractions. As shown in Figure 1A, the activity is distributed across multiple fractions, indicating the presence of multiple compounds contributing to the activity. The most active column fraction (Fr.10) gave an IC50 value of 1.7 μg/mL compared against the IC50 value of 0.2 μg/mL of the positive control parthenolide, suggesting potent anti-inflammatory compounds are present in the algal biomass.
Figure 1.
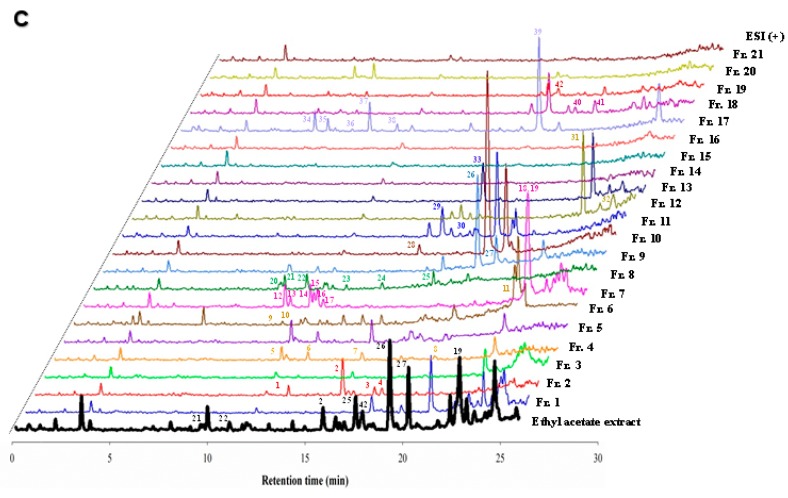
(A) Anti-inflammatory activities of 21 column fractions derived from K. flaccidum var. zivo against iNOS, in comparison with the parent ethyl acetate extract and the positive control parthenolide; (B) Orthogonal partial least squares discriminant analysis (OPLS-DA) discriminating active and inactive fractions; and (C) UHPLC-qMS profiling of the ethyl acetate extract and its 21 column fractions with compound numbers labeled beside respective peaks.
2.2. Chemical Profiling of Small-Molecule Compounds by UHPLC-qMS-DAD
Reversed-phase ultrahigh performance liquid chromatography (UHPLC) coupled with a single quadrupole mass spectrometer (qMS) and a multi-channel UV detector was utilized to generate chemical profiles of the 21 fractions derived from the ethyl acetate extract with the understanding that the response of different classes of compounds vary considerably under different MS detection methods. Taking advantage of qMS that operates in both positive and negative ionization mode (both ESI and APCI) simultaneously in a single run that facilitates identification of true molecular ion for a specific compound, four classes of compounds, including 21 fatty acid derivatives (1–10, 12–17, and 20–24), seven pheophorbides (11, 26–28, 33, 39, and 42), five carotenoids (18, 19, 25, 29, and 30), and nine glycoglycerolipids (31, 32, 34–38, 40, and 41), were detected (Table 1, Figure 1C) [12,13,14,15,16]. A rapid structural identification or prediction of these compounds was conducted as follows.
Table 1.
Compounds 1–42 detected by UHPLC-qMS-UV in column fractions.
| Compound | m/z (+) a | m/z (-) b | RT c (min) | UVmax (nm) | Location d | Identification/Prediction | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 251 | 295 | 13.18 | \ | Fr. 2 | 7, 10, 13-Hexadecatrienoic acid e | [12] |
| 2 | 279 | 323 | 15.92 | \ | Fr. 2,3,4,5 | α-Linolenic acid e | [13] |
| 3 | 305 | 349 | 17.60 | \ | Fr. 2 | 8, 11, 14-Eicosadienoic acid e | [12] |
| 4 | 281 | 325 | 17.98 | \ | Fr. 2 | -Linoleic acid e | [12] |
| 5 | 277 | 293 | 11.77 | \ | Fr. 4,5 | Hydroxy-C18:3 FA f | |
| 6 | 279 | 295 | 13.16 | \ | Fr. 4,5 | Hydroxy-C18:2 FA f | |
| 7 | 281 | 301 | 14.96 | \ | Fr. 4 | Hydroxy-C18:1 FA f | |
| 8 | 283 | 299 | 19.74 | \ | Fr. 4 | Hydroxy-C18:0 FA f | |
| 9 | 305 | 321 | 9.67 | \ | Fr. 6 | Hydroxy-C20:3 FA f | |
| 10 | 307 | 323 | 10.83 | \ | Fr. 6 | Hydroxy-C20:2 FA f | |
| 11 | 607 | 605 | 22.51 | 408 | Fr. 6 | Pheophorbide a methyl ester | [14] |
| 12 | 293 | 309 | 10.47 | \ | Fr. 7 | Dihydroxy-C18:3 FA f | |
| 13 | 293 | 309 | 10.71 | \ | Fr. 7 | Dihydroxy-C18:3 FA f | |
| 14 | 295 | 311 | 11.78 | \ | Fr. 7 | Dihydroxy-C18:2 FA f | |
| 15 | 295 | 311 | 11.98 | \ | Fr. 7 | Dihydroxy-C18:2 FA f | |
| 16 | 295 | 311 | 12.16 | \ | Fr. 7 | Dihydroxy-C18:2 FA f | |
| 17 | 295 | 311 | 12.42 | \ | Fr. 7 | Dihydroxy-C18:2 FA f | |
| 18 | 568 | \ | 22.95 | 454; 478 | Fr. 6,7 | Zeaxanthin | [15] |
| 19 | 568 | \ | 23.18 | 448; 474 | Fr. 6,7 | Lutein | [15] |
| 20 | 277 | 293 | 9.71 | \ | Fr. 8,9 | Hydroxy-C18:3 FA f | |
| 21 | 277 | 293 | 9.92 | \ | Fr. 8 | (10E,12Z,15Z)-9-hydroxyoctadecadienoic acid | [16] |
| 22 | 279 | 295 | 11.05 | \ | Fr. 8,9 | (10E,12Z)-9-hydroxyoctadecadienoic acid | [16] |
| 23 | 278 | \ | 13.12 | \ | Fr. 8 | C18:3 FAA g | |
| 24 | 280 | \ | 14.98 | \ | Fr. 8 | (9Z,12Z)-octadecadienamide | [17] |
| 25 | 584 h | \ | 17.59 | 448; 474 | Fr. 8,9 | Capsanthin | [18] |
| 26 | 593 | 591 | 19.36 | 410 | Fr. 9,10,11 | Pheophorbide a | [19] |
| 27 | 593 | 591 | 20.32 | 410 | Fr. 9,10,11 | 15-Epimer of Pheophorbide a | [19] |
| 28 | 607 | 605 | 15.86 | 436 | Fr. 10 | Pheophorbide b | [14] |
| 29 | 600 h | \ | 16.55 | 438; 466 | Fr. 11,12 | Neoxanthin | [15] |
| 30 | 600 h | \ | 17.00 | 440; 471 | Fr. 11,12 | Violaxanthin | [15] |
| 31 | 767 | 789 | 23.29 | \ | Fr. 12,13 | MGDG i(16:4/18:3) | [20] |
| 32 | 769 | 791 | 24.85 | \ | Fr. 12 | MGDG (16:3/18:3) | [21] |
| 33 | 609 | 607 | 17.64 | 408 | Fr. 13 | 15-Hydroxy-pheophorbide a j | [14] |
| 34 | 507 | 529 | 6.99 | \ | Fr. 17 | MGMG k (C16:4) | [22] |
| 35 | 509 | 531 | 7.66 | \ | Fr. 17 | MGMG (C16:3) | [23] |
| 36 | 511 | 533 | 8.96 | \ | Fr. 17 | MGMG (C16:2) | [23] |
| 37 | 537 | 559 | 9.86 | \ | Fr. 17 | MGMG (C18:3) | [24] |
| 38 | 539 | 561 | 11.24 | \ | Fr. 17 | MGMG (C18:2) | |
| 39 | 609 | 607 | 18.50 | 408 | Fr. 17,18 | 15-Hydroxy-pheophorbide a j | [14] |
| 40 | 929 | 951 | 19.90 | \ | Fr. 18 | DGDG l(16:4/18:3) | [12] |
| 41 | 931 | 953 | 20.89 | \ | Fr. 18 | DGDG (16:3/18:3) | [12] |
| 42 | 625 | 623 | 17.99 | 400 | Fr. 19,20 | Hydro-pheophorbide-lactone a | [25] |
a Precursor ion in ESI (+); 1, 2, 3, 4, 11, 24, 26, 27, 28, 33, 39, 42 exhibit [M + H]+ in ESI (+); 5, 6, 7, 8, 9, 10, 12, 13, 14, 15, 16, 17, 20, 21, 22, 23, 24 exhibit [M − H2O + H]+ in ESI (+); 31, 32, 34, 35, 36, 37, 40, 41 exhibit [M + Na]+ in ESI (+). b Precursor ion in ESI (−); 1, 2, 3, 4, 31, 32, 34, 35, 36, 37, 40, 41 exhibit [M + HCOO]− in ESI (+); 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 20, 21, 22, 26, 27, 28, 33, 39, 42 exhibit [M − H]− in ESI (+). c Retention time. d Column fraction(s) in which compounds are located. e Identification was confirmed by GC-MS analysis. f Fatty acid. g Fatty acid amide. h Compound forming a radical molecular ion [M]+. i Monogalactosyldiacylglycerol. j 33 and 39 were identified as diastereoisomers. k Monogalactosylmonoacylglycerol. l Digalactosyldiacylglycerol.
The presence of fatty acids in Klebsormidium spp. [9] prompted a dereplication analysis of such compounds in K. flaccidum var. zivo. Typical chromatographic behaviors of major compounds in Fr.1−9 and their precursor ions identified from ESI-MS spectra were clearly associated with fatty acids or their derivatives. Generally, fatty acids are better ionized in negative ESI mode [26]. Hydroxylated fatty acids show de-protonated quasi-molecular ion [M − H]− and de-hydrated protonated quasi-molecular ion [M + H − H2O]+ in the ESI (−) and ESI (+) MS spectra, respectively. Dihydroxylated fatty acids may produce a fragment ion at m/z [M + H − 2H2O]+ (see Figure S1). Four fatty acids, 7,10,13-hexadecatrienoic acid (1), α-linolenic acid (2), 8, 11, 14-eicosadienoic acid (3), and linoleic acid (4), which are present in the least polar Fr. 1 and Fr. 2 were readily determined by the LC-MS data. This was further confirmed by orthogonal GC-MS analysis, which characterized the four compounds and additional non-hydroxylated fatty acids that were not detected by LC-MS (see Table S2 and Figure S2). The remaining 14 hydroxylated fatty acids or their derivatives were characterized at structural type only because LC-MS cannot provide exact structural information of such compounds in terms of positional and configurational double bonds and hydroxyl groups.
The strong green color of this algal material certainly suggests the presence of chlorophyll-derived compounds. Analysis of the ESI-MS and UV data of the dominant peaks in Fr. 9−11 indicated that pheophorbide a (26) and epi-pheophorbide a (27) [27] were present in these fractions (Table 1), which was further confirmed by direct comparison of LC-MS-UV data with an authentic sample of pheophorbide a. Partial conversion of 26 into 27 was observed to occur in solution (e.g., methanol) stored at room temperature for a couple of days due to inherent structural tautomerism. Compounds 26 and 27 (m/z 593 [M + H]+) are characteristic for a strong UV absorption around 410 nm [14]. Additional five analogues with similar UV characteristics were identified in Fr. 6, Fr. 10, Fr. 13, Fr. 17/18, and Fr. 19/20 (Table 1, Figure 1C). Based on their mass data and chromatographic behaviors associated with polarity and retention time, the five compounds were predicted to be pheophorbide a methyl ester (11) [14], pheophorbide b (28) [14], hydroxyl-pheophorbide a (33) [14], isomer of hydroxyl-pheophorbide a (39) [14], and hydro-pheophorbide-lactone a (42) [25].
Analysis of the compounds with retention time around 23 min (Figure 1C) and characteristic UV absorptions at 430−480 nm in Fr. 6/7 (Table 1) suggested a chemical skeleton for carotenoids as such compounds were previously identified in several algal materials [28]. Lutein (19) was unequivocally determined to be present in Fr. 6/7 by direct comparison of LC-MS-UV data with an authentic sample. Zeaxanthin (18), a structural isomer of lutein, was also readily identified in Fr. 6/7 by comparison of their UV absorptions in which zeaxanthin has relatively longer UV wavelengths (Table 1, Figure S3) due to an extended conjugated system [15]. The presence of three additional carotenoids, capsanthin (25) in Fr. 8/9, and neoxanthin (29) and violaxanthin (30) in Fr. 11/12, whose structures are closely related to lutein and zeaxanthin, were indicated by their characteristic UV absorptions and molecular weight information (Table 1) [15,18].
Glycoglycerolipids are major components of chloroplast lipids in algae [29]. Our LC-MS data showed the presence of two monogalactosyldiacyglycerols (MGDGs) (31 [20] and 32 [21]) in Fr. 12/13, five monogalactosylmonoacyglycerols (MGMGs) (34–38) [22,23,24] in Fr. 17, and two digalactosyldiacylglycerols (DGDGs) (40 and 41) [12] in Fr. 18. All these galactolipids formed sodium adduct ions [M + Na]+ and formate adduct ions [M + HCOO]− in the positive and negative ESI-MS spectra, respectively. In the positive ESI-MS spectra, the fragment ion [M + H – 162 (hexose)]+ corresponding to the loss of a galactosyl moiety was observed in MGDGs and MGMGs, while the fragment ion [M + H – 162 × 2]+ corresponding to the loss of two galactosyl units were commonly present in DGDGs. For MGDGs and DGDGs, fragment ions corresponding to individual acyl groups, e.g., in compound 31, were observed as well (Figure S4).
2.3. Determination of Anti-Inflammatory Markers by Chemometric Analysis
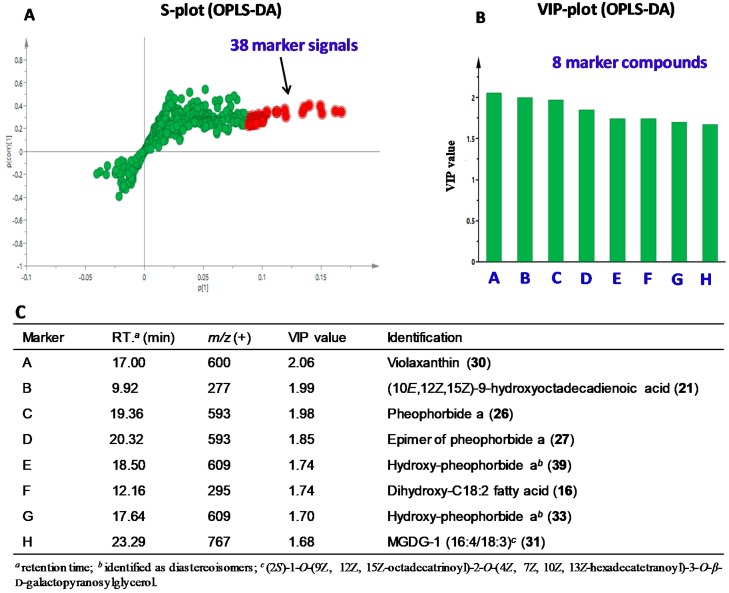
Chemometrics was applied to explore potential anti-inflammatory marker compounds in all 21 column fractions, which were classified into two groups, active and inactive, according to IC50 values of iNOS inhibition. Twelve fractions (Fr. 5, Fr. 7–14, and Fr. 17–19) that had enriched activities with IC50 values less than that of the parent ethyl acetate extract (28.0 μg/mL) were defined as active, while the remaining nine fractions were considered as inactive (Figure 1A). For each fraction, 654 chromatographic signals generated from the positive ESI-MS were used for OPLS-DA modeling, which clearly showed discrimination of respective fractions in the active and inactive groups (Figure 1B). Thirty-eight signals with variable importance in projection (VIP) values greater than 1.5 were shown from the S-plot (Figure 2A), and were defined as potential anti-inflammatory marker signals. The coefficient plot (Figure S6) indicated that these signals correlated with eight compounds shown in Figure 2B,C. It is interesting to note that the eight marker compounds derived from active fractions represent the aforementioned four classes of compounds. According to the literature, iNOS inhibitory activities of violaxanthin (30) (Marker A) [30], pheophorbide a (26) (Marker C) [19], and MGDG-1 (31) (Marker H) [20] have been reported. Fatty acids, especially hydroxylated fatty acids, have also demonstrated anti-inflammatory activity via multiple molecular mechanisms including iNOS inhibition [31,32], supporting identification of compounds 21 (Marker B) and 16 (Marker F) as anti-inflammatory markers. The remaining three marker compounds 27 (Marker D), 39 (Marker E), and 33 (Marker G) are analogues of pheophorbide a, thereby implicating potential anti-inflammatory activity as well.
Figure 2.
Prediction of anti-inflammatory marker compounds by chemometrics: (A) OPLS-DA S-plot of chromatographic signals of 21 column fractions generated from the positive ESI-MS with 38 marker signals (VIP > 1.5) highlighted in red; (B) VIP plot showing 8 marker compounds corresponding to 38 marker signals; and (C) chromatographic and MS information of the 8 marker compounds.
The anti-inflammatory data for all active fractions in terms of IC50 values for inhibition of iNOS (as shown in Figure 1A) appear to support that pheophorbide a, the major compound in Fr. 9 and Fr. 10 (Figure 1C, Table S1), along with pheophorbide a analogues and another three classes of compounds (fatty acids, carotenoids, and glycoglycerolipids) are responsible for much of the anti-inflammatory activity of the extract. Thus, chemometrics is an excellent tool to rapidly predict multiple anti-inflammatory compounds from a complex mixture.
2.4. Isolation and Anti-Inflammatory Testing of Purified Compounds
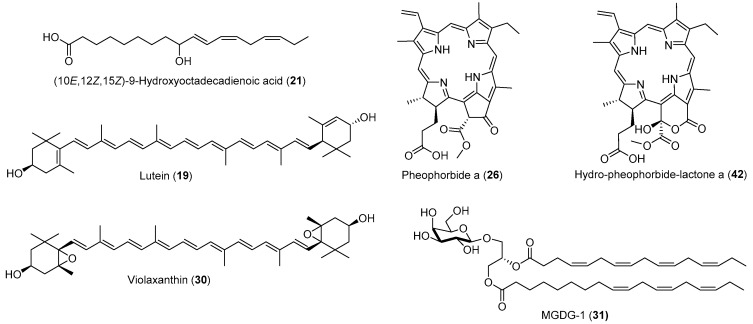
To validate the prediction of chemometric analysis, Fr. 8 containing fatty acid 21 [16], Fr. 10 containing pheophobide a (26), and Fr. 12 containing glycoglycerolipid 31 were selected for follow-up isolation of representative anti-inflammatory marker compounds, followed by structural confirmation and anti-inflammatory testing. This selection criterion was based on potent activity, significant quantity, and unique chemical profiles of the active fractions. As a result, marker compounds 21, 26, and 31 representing hydroxylated fatty acids, chrolorophyll-derived pheophobides, and glycoglycerolipids, respectively, were isolated, and their structures were confirmed by NMR spectroscopic analysis. The carotenoid violaxanthin (29) (Marker A) is a minor compound in Fr. 11 based on the LC-MS-UV profile and its anti-inflammatory activity has previously been reported [30]. Thus, isolation of this compound from Fr. 11 with a relative low activity (IC50, 16.0 μg/mL) was not pursued.
The evaluation of anti-inflammatory activity of the three purified marker compounds indicated that pheophorbide a (26) was the most active in inhibiting iNOS with an IC50 value of 0.24 µM, while (10E,12Z,15Z)-9-hydroxyoctadecadienoic acid (21) and MGDG-1 (31) were less active with IC50 values of 22.4 and 17.4 µM, respectively (Table 2). Compounds 26, 21, and 31 also demonstrated moderate inhibitory activity against NF-κB with IC50 values of 32.1, 122.3, and 47.0 µM, respectively. The NF-κB inhibitory activity of 26 had previously been reported [33]. A commercial sample of lutein was also tested, which gave an IC50 value of 26.4 µM against iNOS, but was not active against NF-kB (Table 2). These activity data, in conjunction with chemical profiling and relative contents of potential active compounds, explain well the IC50 values of relevant active column fractions (Figure 1, Table S1). Considering the low potency of other active fractions, isolation of additional active compounds from this algal material that outperform pheophorbide a in terms of iNOS inhibition is unlikely. For example, we conducted isolation of hydro-pheophorbide-lactone a (42, Figure 3), a structurally close pheophorbide a analogue, which was present in Fr. 19 with an IC50 value of 25.0 µg/mL for structural confirmation because only partial NMR data of this compound has been available in the literature [25]. This compound inhibited iNOS with an IC50 value of 20.8 µM which is approximately 87-fold less active than pheophorbide a. Thus, pheophorbide a is the most active among all identified anti-inflammatory compounds.
Table 2.
In vitro anti-inflammatory activities of compounds 19, 21, 26, and 31.
| Compound | iNOS (IC50, µM) | NF-κB (IC50, µM) |
|---|---|---|
| 19 a | 26.4 ± 5.3 | NA b |
| 21 | 22.4 ± 2.4 | 122.3 ± 20.3 |
| 26 | 0.24 ± 0.03 | 132.1 ± 3.4 |
| 31 | 17.4 ± 1.3 | 47.0 ± 6.7 |
| Parthenolide | 0.72 ± 0.08 | 3.83 ± 0.60 |
a Obtained from a commercial source. b Not active at the highest test concentration of 87.9 μM (50 µg/mL).
Figure 3.
Structures of anti-inflammatory compounds from K. flaccidum var. zivo.
In conclusion, a chemometrics-assisted approach has demonstrated its advantage in rapidly identifying anti-inflammatory compounds from K. flaccidum var. zivo with minimum isolation efforts. Our findings may facilitate product development using an optimized algal biomass with high contents of bioactive compounds. This approach can be utilized to accelerate the discovery of bioactive natural products preferably with novel chemistry.
3. Materials and Methods
3.1. General Experimental Procedures
The NMR spectra using standard pulse programs were recorded at room temperature on a Bruker Avance DPX-400 spectrometer (Bruker, Billerica, MA, USA) operating at 400 (1H) and 100 (13C) MHz. The chemical shift (δ, ppm) values were calibrated using the residual NMR solvent and coupling constant (J) was reported in Hertz (Hz). Column chromatography was done on normal-phase silica gel (230 × 400 mesh, J. T. Baker, Center Valley, PA, USA) or reversed-phase silica gel (C18, 40 μm, J. T. Baker). Silica gel 60 F254 TLC plates (Merck, Darmstadt, Germany) and reversed-phase TLC plates (C18, Merck, Darmstadt, Germany) were used for analytical TLC. The plates were visualized by spraying 10% H2SO4 followed by heating. The authentic samples pheophorbide a (>95%) and lutein (>90%) were purchased from Cayman Chemical Company (Ann Arbor, MI, USA) and Acros Organics (Pittsburgh, PA, USA), respectively. Their structure and purity were further confirmed by NMR and HPLC in our laboratory. The 37 standards of fatty acid methyl esters (Supelco® 37 components FAME mix) used for GC-MS analysis were purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Plant Material
The algal biomass KALGAETM is derived from K. flaccidum var. zivo and its general physical, chemical, and nutritional compositions has been descried in a previous paper [10].
3.3. Extraction of Algal Biomass
In total, 10.0 g of powdered biomass was extracted with 200 mL of methanol under ultrasonication (40 kHz, 300W) for 1 h, and allowed to stand overnight. The mixture was then filtered using a vacuum suction filter to obtain the organic phase. A portion of organic phase (50 mL) was evaporated to dryness to yield a methanol extract (0.41 g). The remaining 150 mL was evaporated to dryness, suspended in 150 mL of water and extracted with an equal volume of ethyl acetate five times. The combined ethyl acetate layers were concentrated to dryness to yield an ethyl acetate extract (0.36 g). Scale-up extractions with same ratios of sample and solvent produced similar yields of extracts. To prepare the water extract that may contain polysaccharides, 10.0 g of powdered biomass was extracted with 200 mL of water under ultrasonication (40 kHz, 300W) for 1 h. The mixture was then filtered using a vacuum suction filter to obtain the water phase, which was freeze dried to yield the desired extract (2.44 g).
3.4. Fractionation of Ethyl Acetate Extract
15.0 g of ethyl acetate extract, prepared from a scale-up extraction, was absorbed on normal-phase silica gel and then loaded on a normal-phase silica gel column (200 g). The column was eluted with chloroform first and then a gradient solvent system consisting of chloroform and methanol in ratios of 100:1, 50:1, 20:1, 10:1, 5:1, 1:1. Based on the TLC analysis, fractions containing similar compounds were combined to afford 21 major fractions (Fr.1−21). The detailed information on weight and solvent systems used to generate fractions is shown in the Supplementary Material (Table S1).
3.5. Chemical Profiling of Column Fractions by UHPLC-qMS-DAD
An Agilent Series 1290 UHPLC system (Agilent Technologies, Santa Clara, CA, USA) coupled with a DAD detector and an Agilent 6120 quadrupole-MS mass spectrometer were utilized for chemical profiling. Column fractions were prepared at a concentration of 1 mg/mL in methanol. Separation was achieved using an Agilent Eclipse Plus-C18 column (100 × 2.1 mm, 1.8 µm) maintained at 25 °C. Binary mobile phase was 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) at a flow rate of 0.25 mL/min under the following gradient program: 0–6 min, 30–50% (B); 6–10 min, 50–80% (B); 10–23 min, 80–95% (B); 23–25 min, 95–100% (B). The UV wavelength detection was set as 254, 400, 410 and 450 nm with a bandwidth of 4. The spectrum was stored from 190 to 600 nm. MS data was obtained under the ESI (±) mode. Optimal source parameters were set as follows: drying gas (N2) flow rate, 10.0 L/min; drying gas temperature, 300 °C; nebulizer, 35 psig; vaporizer temperature, 200 °C; capillary voltage, 4.0 kV; fragmentor voltage, 100 V. Each sample was analyzed over the mass range of m/z 100-1500. A QC sample was prepared using pooled column fractions and analyzed once every three tested samples to monitor the system stability.
3.6. Chemometric Analysis
Raw UHPLC-qMS data of 21 column fractions acquired in the ESI (+) mode were processed by Agilent ChemStation software (E.02.02) to export a data matrix containing retention times and peak intensities into Excel, in which 654 signals of each fraction were included in the retention time window between 5 and 25 min. This dataset was used as an input for SIMCA-P 13.0 to perform OPLS-DA. The corresponding VIP values were calculated. According to the distribution of signals and VIP values, the signals showing a VIP value > 1.5 were considered as potential marker signals. The marker signals were then correlated with specific compounds based on the retention time and LC-qMS identification of active compounds in column fractions.
3.7. Isolation of Anti-Inflammatory Marker Compounds from Active Column Fractions
Detailed procedures for the isolation of compounds 21, 22, and 24 from Fr. 8, 26 from Fr. 10, 31 from Fr. 12, and 42 from Fr. 19 are shown in Supplementary Material.
3.8. In Vitro Anti-Inflammatory Assays for Inhibition of iNOS and NF-κB
Extracts (20 mg/mL), fractions (20 mg/mL) and compounds (2 mg/mL) were dissolved in DMSO and diluted in serum free media prior to the assay to achieve various test concentrations. The assays for inhibition of iNOS and NF-κB activity were performed in mouse macrophage (RAW264.7) and human chondrosarcoma (SW1353) cell lines, respectively. Both cell lines were obtained from American Type Culture Collection (ATCC), Manassas, VA, USA. The detailed procedures have been described in a previous publication [34]. IC50 values were obtained from concentration response curves. Parthenolide was used as a positive control in the both assays.
Supplementary Materials
Supplementary materials, including isolation procedure for selected anti-inflammatory compounds, GC-MS analysis of fatty acids, and NMR and MS spectra of isolated compounds are available online.
Author Contributions
S.Q.: chemical analysis, isolation, data analysis and manuscript preparation; S.I.K., bioassay and interpretation of data and manuscript editing; M.W., LC-MS analysis and manuscript editing; J.Z., NMR analysis and manuscript editing; S.R., chemical analysis and isolation; I.A.K., A.S., W.P.P., data analysis and manuscript editing; X.-C.L., data analysis and manuscript preparation and revision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by the USDA Agricultural Research Service Specific Cooperative Agreement No. 58-6060-6-015 and ZIVO Biosciences, INC.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Biavatti M.W., Vieira P.C., da Silva M.F., Fernandes J.B., Albuquerque A., Magalhaes C.M., Pagnocca F.C. Chemistry and bioactivity of Raulinoa echinata Cowan, an endemic Brazilian Rutaceae species. Phytomedicine. 2010;8:121–124. doi: 10.1078/0944-7113-00016. [DOI] [PubMed] [Google Scholar]
- 2.Yang J., Liang Q., Wang M., Jeffries C., Smithson D., Tu Y., Boulos N., Jacob M.R., Shelat A.A., Wu Y. UPLC-MS-ELSD-PDA as a powerful dereplication tool to facilitate compound identification from small-molecule natural product libraries. J. Nat. Prod. 2014;77:902–909. doi: 10.1021/np4009706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Della Corte A., Chitarrini G., Di Gangi I.M., Masuero D., Soini E., Mattivi F., Vrhovsek U. A rapid LC-MS/MS method for quantitative profiling of fatty acids, sterols, glycerolipids, glycerophospholipids and sphingolipids in grapes. Talanta. 2015;140:52–61. doi: 10.1016/j.talanta.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Yao C.L., Yang W.Z., Si W., Shen Y., Zhang N.X., Chen H.L., Pan H.Q., Yang M., Wu W.Y., Guo D.A. An enhanced targeted identification strategy for the selective identification of flavonoid O-glycosides from Carthamus tinctorius by integrating offline two-dimensional liquid chromatography/linear ion-trap-Orbitrap mass spectrometry, high-resolution diagnostic product ions/neutral loss filtering and liquid chromatography-solid phase extraction-nuclear magnetic resonance. J. Chromatogr. A. 2017;1491:87–97. doi: 10.1016/j.chroma.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 5.Qiu S., Yang W.Z., Yao C.L., Qiu Z.D., Shi X.J., Zhang J.X., Hou J.J., Wang Q.R., Wu W.Y., Guo D.A. Nontargeted metabolomic analysis and "commercial-homophyletic" comparison-induced biomarkers verification for the systematic chemical differentiation of five different parts of Panax ginseng. J. Chromatogr. A. 2016;1453:78–87. doi: 10.1016/j.chroma.2016.05.051. [DOI] [PubMed] [Google Scholar]
- 6.Kamal N., Viegelmann C.V., Clements C.J., Edrada-Ebel R. Metabolomics-guided isolation of anti-trypanosomal metabolites from the endophytic fungus Lasiodiplodia theobromae. Planta Med. 2017;83:565–573. doi: 10.1055/s-0042-118601. [DOI] [PubMed] [Google Scholar]
- 7.Hou J.J., Cao C.M., Xu Y.W., Yao S., Cai L.Y., Long H.L., Bi Q.R., Zhen Y.Y., Wu W.Y., Guo D.A. Exploring lipid markers of the quality of coix seeds with different geographical origins using supercritical fluid chromatography mass spectrometry and chemometrics. Phytomedicine. 2018;45:1–7. doi: 10.1016/j.phymed.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Rindi F., Guiry M.D., Lopez-Bautista J.M. Distribution, morphology, and phylogeny of klebsormidium (Klebsormidiales, Charophyceae) in urban environments in Europe (1) J. Phycol. 2008;44:1529–1540. doi: 10.1111/j.1529-8817.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- 9.Liu J.Z., Vanormelingen P., Vyverman W. Fatty acid profiles of four filamentous green algae under varying culture conditions. Bioresour. Technol. 2016;200:1080–1084. doi: 10.1016/j.biortech.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Brickel J.A., Matulka R.A., Steffek A.E. KALGAE™ (Klebsormidium flaccidum var. ZIVO) dried algal biomass - 90-day dietary toxicity study and genotoxicity studies. Toxicol. Rep. 2018;5:959–969. doi: 10.1016/j.toxrep.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li N., Khan S.I., Qiu S., Li X.-C. Synthesis and anti-inflammatory activities of phloroglucinol-based derivatives. Molecules. 2018;23:3232. doi: 10.3390/molecules23123232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao L.X., Gerde J.A., Lee S.L., Wang T., Harrata K.A. Microalgae lipid characterization. J. Agric. Food Chem. 2015;63:1773–1787. doi: 10.1021/jf5050603. [DOI] [PubMed] [Google Scholar]
- 13.Yang Q., Cao W.D., Zhou X.X., Xie Y.H., Wang S.W. Anti-thrombotic effects of α-linolenic acid isolated from Zanthoxylum bungeanum Maxim seeds. BMC Complem. Altern. Med. 2014;14:348–355. doi: 10.1186/1472-6882-14-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan P.J., Appleton D.R., Mustafa M.R., Lee H.B. Rapid identification of cyclic tetrapyrrolic photosensitisers for photodynamic therapy using on-line hyphenated LC-PDA-MS coupled with photo-cytotoxicity assay. Phytochem. Anal. 2011;23:52–59. doi: 10.1002/pca.1324. [DOI] [PubMed] [Google Scholar]
- 15.Pop R.M., Weesepoel Y., Socaciu C., Pintea A., Vincken J.P., Gruppen H. Carotenoid composition of berries and leaves from six Romanian sea buckthorn (Hippophae rhamnoides L.) varieties. Food Chem. 2014;147:1–9. doi: 10.1016/j.foodchem.2013.09.083. [DOI] [PubMed] [Google Scholar]
- 16.Murakami N., Shirahashi H., Nagatsu A., Sakakibara J. Two unsaturated 9R-hydroxy fatty acids from the Cyanobacterium Anabaena flosaquae. Lipids. 1992;27:776–778. doi: 10.1007/BF02535848. [DOI] [Google Scholar]
- 17.Dabur R., Mittal A. Detection and qualitative analysis of fatty acid amides in the urine of alcoholics using HPLC-QTOF-MS. Alcohol. 2016;52:71–78. doi: 10.1016/j.alcohol.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Dang Y.Y., Zhang H., Xiu Z.L. Three-liquid-phase extraction and separation of capsanthin and capsaicin from Capsicum annum L. Czech. J. Food Sci. 2014;32:109–114. doi: 10.17221/96/2013-CJFS. [DOI] [Google Scholar]
- 19.Islam M.N., Ishita I.J., Jin S.E., Choi R.J., Lee C.M., Kim Y.S., Jung H.A., Choi J.S. Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents pheophorbide a and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem. Toxicol. 2013;55:541–548. doi: 10.1016/j.fct.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 20.Banskota A.H., Gallant P., Stefanova R., Melanson R., O’Leary S.J.B. Monogalactosyldiacylglycerols, potent nitric oxide inhibitors from the marine microalga Tetraselmis chui. Nat. Prod. Res. 2012;27:1084–1090. doi: 10.1080/14786419.2012.717285. [DOI] [PubMed] [Google Scholar]
- 21.Wang R., Furumoto T., Motoyama K., Okazaki K., Kondo A., Fukui H. Possible antitumor promoters in Spinacia oleracea (Spinach) and comparison of their contents among cultivars. Biol. Pharm. Bull. 2002;66:248–254. doi: 10.1271/bbb.66.248. [DOI] [PubMed] [Google Scholar]
- 22.Fang Z., Jeong S.Y., Jung H.A., Choi J.S., Min B.S., Woo M.H. Capsofulvesins A-C, cholinesterase inhibitors from Capsosiphon fulvescens. Chem. Pharm. Bull. 2013;60:1351–1358. doi: 10.1248/cpb.c12-00268. [DOI] [PubMed] [Google Scholar]
- 23.Banskota A.H., Stefanova R., Gallant P., Osborne J.A., Melanson R., O’Leary S.J.B. Nitric oxide inhibitory activity of monogalactosylmonoacylglycerols from a freshwater microalgae Chlorella sorokiniana. Nat. Prod. Res. 2013;27:1028–1031. doi: 10.1080/14786419.2012.696255. [DOI] [PubMed] [Google Scholar]
- 24.Manzo E., Ciavatta M.L., Pagano D., Fontana A. An efficient and versatile chemical synthesis of bioactive glycol-glycerolipids. Tetrahedron Lett. 2012;53:879–881. doi: 10.1016/j.tetlet.2011.12.030. [DOI] [Google Scholar]
- 25.Zhao Y.H., Wang X.M., Wang H., Liu T.X., Xin Z.H. Two new noroleanane-type triterpene saponins from the methanol extract of Salicornia herbacea. Food Chem. 2014;151:101–109. doi: 10.1016/j.foodchem.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 26.Villalobos Solis M.I.V., Patel A., Orsat V., Singh J., Lefsrud M. Fatty acid profiling of the seed oils of some varieties of field peas (Pisum sativum) by RP-LC/ESI-MS/MS: Towards the development of an oilseed pea. Food Chem. 2013;139:986–993. doi: 10.1016/j.foodchem.2012.12.052. [DOI] [PubMed] [Google Scholar]
- 27.Hynninen P.H., Sievers G.Z. Conformations of chlorophylls a and a’ and their magnesium-free derivatives as revealed by circular dichroism and proton magnetic resonance. Naturforsch. B. 1981;36:1000–1009. doi: 10.1515/znb-1981-0819. [DOI] [Google Scholar]
- 28.Wang N., Manabe Y., Sugawara T., Paul N.A., Zhao J. Identification and biological activities of carotenoids from the freshwater alga Oedogonium intermedium. Food Chem. 2018;242:247–255. doi: 10.1016/j.foodchem.2017.09.075. [DOI] [PubMed] [Google Scholar]
- 29.Harwood J.L., Jones A.L. Lipid metabolism in algae. Adv. Bot. Res. 1989;16:1–53. [Google Scholar]
- 30.Soontornchaiboon W., Joo S.S., Kim S.M. Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoids in RAW 264.7 macrophages. Biol. Pharm. Bull. 2012;200:1080–1084. doi: 10.1248/bpb.b12-00187. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y.F., Wang K., Zhang Y.Z., Zheng Y.F., Hu F.L. In vitro anti-inflammatory effects of three fatty acids from royal jelly. Mediat. Inflamm. 2016:3583684. doi: 10.1155/2016/3583684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez D.H., Fiol-deRoque M.A., Noguera-Salva M.A., Teres S., Campana F., Piotto S., Castro J.A., Mohaibes R.J., Escriba P.V., Busquets X. 2-Hydroxy arachidonic acid: A new non-steroidal anti-inflammatory drug. PLoS ONE. 2013;8:e72052. doi: 10.1371/journal.pone.0072052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinrich M., Bork P.M., Schmitz M.L., Rimpler H., Frei B., Sticher O. Pheophorbide A from Solanum diflorum interferes with NF-kappa B activation. Planta Med. 2001;67:156–157. doi: 10.1055/s-2001-11496. [DOI] [PubMed] [Google Scholar]
- 34.Zhao J.P., Khan S.I., Wang M., Vasquez Y., Yang M.H., Avula B., Wang Y.H., Avonto C., Smillie T.J., Khan I.A. Octulosonic acid derivatives from Roman chamomile (Chamaemelum nobile) with activities against inflammation and metabolic disorder. J. Nat. Prod. 2014;77:509–515. doi: 10.1021/np400780n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.