Abstract
Over the past few decades, many pathogenic bacteria have become resistant to existing antibiotics, which has become a threat to infectious disease control worldwide. Hence, there has been an extensive search for new, efficient, and alternative sources of antimicrobial agents to combat multidrug-resistant pathogenic microorganisms. Numerous studies have reported the potential of both essential oils and metal/metal oxide nanocomposites with broad spectra of bioactivities including antioxidant, anticancer, and antimicrobial attributes. However, only monometallic nanoparticles combined with essential oils have been reported on so far with limited data. Bi- and tri-metallic nanoparticles have attracted immense attention because of their diverse sizes, shapes, high surface-to-volume ratios, activities, physical and chemical stability, and greater degree of selectivity. Combination therapy is currently blooming and represents a potential area that requires greater attention and is worthy of future investigations. This review summarizes the synergistic effects of essential oils with other antimicrobial combinations such as mono-, bi-, and tri-metallic nanocomposites. Thus, the various aspects of this comprehensive review may prove useful in the development of new and alternative therapeutics against antibiotic resistant pathogens in the future.
Keywords: essential oil, bi-metallic nanoparticles, tri-metallic nanoparticles, synergistic effect, antimicrobial activities, multidrug-resistant pathogens
1. Introduction
Infectious diseases and foodborne illnesses are the leading cause of severe health problems worldwide and can even lead to death. Antibiotics and other antimicrobial agents are the major strategies used to combat pathogenic bacteria in human medicine [1]. However, inappropriate over prescription and irrational use of antibiotics in the treatment of infectious diseases has led to favorable conditions, exposure, and spread of resistant strains of different pathogens [2]. These negative health trends require urgent attention from scientific institutions and pharmaceutical researchers to develop novel therapeutics with different strategies for the prevention and treatment of infectious diseases [3]. Infectious diseases resulting from multidrug-resistant bacteria have become abundant, especially vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, penicillin-resistant Streptococcus pneumoniae, ceftazidime-resistant Klebsiella pneumonia and Escherichia coli, and Pseudomonas aeruginosa resistant to fluoroquinolones [4]. In addition, various types of foodborne pathogens associated with Gram-positive and Gram-negative bacteria such as Bacillus cereus, E. coli, Listeria monocytogenes, Salmonella enteritidis, and S. aureus present a major threat to public health and safety [5]. Owing to these problems, there has been renewed interest in alternative research for more effective, less toxic antimicrobial agents among natural bioactive compounds, which are found in aromatic plants, and have been used in cosmetics, aromatherapy, and folk medicine for many years, such as essential oils and plant extracts [6]. Traditionally, medicinal and aromatic plants have received significant attention for the treatment of human diseases because of their pharmacological activities, low toxicity, and economic viability [7]. The presence of active phytochemicals or bioactive compounds and their secondary metabolites, or essential oils in plants play an important role against the problem of antibiotic resistance in bacteria. Some of these alternative antimicrobial therapies may reduce the further spread and development of resistance in these pathogens.
Essential oils (EOs) are natural mixtures of volatile secondary metabolites extracted from different parts of plants, such as the flowers, buds, seeds, leaves, twigs, bark, herbs, wood, fruits, and roots [8]. EOs may have received their name because they were the essence of odor and flavor or at one time, they were considered essential to life processes. The most common chemical constituents of EOs include phenols, polyphenols, flavonols, tannins, quinones, terpenoids, flavonoids, flavones, coumarins, alkaloids, lectins, and polypeptides that exhibit potential biological activities such as anti-inflammatory, antioxidant, insecticidal, anti-allergic, antiseptic, antiviral, anti-parasitic, anticancer, and antimicrobial properties [9]. In addition, many plants’ EOs are useful as aroma in aromatherapy, flavor in food and its additives, and enhancers in cosmetics, soaps, plastics, resins, and perfumes [10]. EOs are obtained through various methods such as vacuum distillation, fermentation, solvent extraction, simultaneous distillation, microwave-assisted extraction, supercritical fluid extraction, microwave hydrodistillation, steam distillation, and static, dynamic, and high-concentration capacity headspace sampling methods [11]. The EOs obtained contain a complex mixture of 20–60 bioactive compounds at different concentrations. The yield can vary between different bioactive compounds and may differ among different plant species and plant parts depending on chemically derived compounds such as aromatic, aliphatic, and phenolic acids, and terpenes [12]. The differences in the chemical composition of EOs are due to exogenous and endogenous factors, which may lead to chemotypes or ecotypes. The exogenous factors depend on environmental factors such as light, temperature, weather conditions, precipitation, growing site, and soil. The endogenous factors are associated with anatomical and physiological characteristics of the plants like chemical variation and genetically related factors [13,14].
EOs contain various components which have been screened for their antimicrobial activities. Among these, terpenes—such as carvacrol, geraniol, menthol, and thymol—have higher antimicrobial properties. Compared to the individual components, the whole or multicomponent EOs appear to have greater antibacterial activity. However, each single chemical component in EOs plays an important role, such as in the density, fragrance, color, texture, cell penetration ability, fixation on cell walls, and bioavailability. Therefore, it is assumed that the other molecules present in EOs regulate the function of the main components to enhance synergistic effects [15]. Thus, the combination of antimicrobials with other antimicrobial agents provides many benefits, including higher biological activity, and reduces the adverse effects and toxicity of the combined components. In synergistic activity, one antimicrobial agent enhances the activity of the other, and finally they may act together more effectively [16]. This could be a new approach to solving the problem of bacterial resistance and reduced susceptibility. Most importantly, association of other antimicrobial agents with EOs targeting resistant bacteria may have different mechanisms of action, and it may lead to new choices to overcome the assault of microbial resistance. Various in vitro studies have confirmed the antimicrobial activities of some essential/volatile oils, plant extracts, and antibiotics against different microorganisms using different methods, and the synergistic effects of EOs/EOs, plant extract/plant extract, plant extract/EOs, plant extract/conventional antibiotics, and phytochemical/antibiotics [16], with a significant reduction in minimum inhibitory concentration.
Nanotechnology has proved to be a powerful tool for solving various biomedical and technological problems using its predefined structures. This field provides the power of transforming the structures of atoms or molecules into desired geometry and properties. It has both health and environmental applications, which includes effective drug delivery, cancer treatment, food packaging, harvesting solar energy, and water purification, and reduces the use of industrial chemicals, thereby making the environment healthier and safer [17]. Over the past few decades, many metal and metal oxide nanoparticles (NPs) have been studied extensively due to their distinctive properties, and their potential applications in various fields such as biomedical, biosensors, catalysis, electronics, optoelectronics, information storage, and surface-enhanced Raman spectroscopy (SERS) [18]. As the name indicates, monometallic NPs contain a single metal, which can be prepared either by biological or chemical methods whereas bi- and tri-metallic NPs are formed by the combination of two or more metals, which exhibit fascinating properties. Compared to monometallic NPs, bi- and tri-metallic NPs have drawn greater interest because of their importance in optical, electrical, and catalytic applications in various fields [19]. Transition bi- and tri-metallic NPs are used as catalysts in many organic reactions and show higher catalytic activities compared to monometallic NPs [20,21,22,23]. Bi-metallic NPs such as FePd, AuPd, PtPd, and CuPd [24,25,26,27], and tri-metallic NPs such as AuFeAg and FeAgPt [19,23] are used as heterogeneous catalysts with excellent selectivity and activity compared to monometallic NPs. It has been proven that it is possible to tune the shape, size, and properties of NPs as heterodimers [28], nano-alloys [23], and core–shell [29] for further catalytic applications. However, only monometallic NPs along with EOs have so far been reported to have limited data to target resistant bacteria.
This review highlights the most recent literature on EOs, with special focus on mono, bi-and tri-metallic NPs, along with their antimicrobial potential. We have discussed the extraction methods, chemical composition, antimicrobial effects of EOs, nano-encapsulation of EOs, and their synergistic effects on infection resistant pathogens. Potential effects and antimicrobial activities of mono-, bi-, and tri-metallic NPs will also be discussed. Additionally, the future prospects of the synergistic effects of EOs and bi- and tri-metallic NPs have also been discussed herein. Some important and widely used medicinal plants act as antimicrobial activities are depicted in Figure 1.
Figure 1.
Widely used medicinal plants with high antimicrobial activities against human pathogens.
2. Methods of Extraction of Essential Oils
EOs can be extracted from different parts of plants using several methods with appropriate solvents and techniques. Based on the characteristics of different plant materials, some specific extraction techniques are used for extracting the volatile fraction from aromatic plants. The techniques include solvent extraction, solvent flavor evaporation, Soxhlet extraction, maceration, CO2 extraction, hydrodistillation, steam distillation, dry distillation, mechanical cold pressing, microwave-assisted extraction, supercritical fluid extraction, simultaneous distillation extraction, vacuum distillation, solid-phase microextraction, direct thermal desorption, dynamic headspace, static headspace, and high-concentration capacity headspace sampling [30,31]. The yield and success of extraction depends on the type and length of extraction period, solvent, climate, the plant organ, age, temperature, pH, soil composition, and vegetative cycle stage [32,33,34,35]. Generally, dried plant material and water are used as a solvent for the extraction of EOs. However, some components of EOs are not soluble in water due to low polarity, therefore, to increase the polarity, organic solvents may be used, such as acetone, ether, petroleum ether, ethanol, chloroform, hexane, and ethyl acetate [36,37]. EOs produced using organic solvents are not considered by the National Cancer Institute as the oil residues can alter the quality of the EOs and lead to impurity [38].
Modern methods such as supercritical fluid extraction and microwave-assisted extraction were achieved at the laboratory scale, but they are intuitively difficult to expect a reliable scale-up. Solvent extraction is one of the most convenient and frequently used method to extract bioactive compounds from plant materials. Solvent extraction method is a simple and efficient method used to separate a compound into its components based on the solubility of the components when it is mixed with a solvent. It is important to choose a good solvent since a residue of the solvent could be present in the final product due to its polarity, viscosity, and vapor pressure. High solvent requirement, long extraction period, and unsatisfactory reproducibility are some few disadvantages of this method [39,40].
Hydrodistillation is a simple and traditional method for extracting EOs from plant samples and is further classified into the subcategories of water distillation, steam distillation, and direct steam distillation [41]. In this method, samples are packed into a still compartment, sufficient quantity of water is added, and the mixture is boiled by applying mild heat (water distillation); alternatively, live steam (steam distillation) followed by direct steam (direct steam distillation) can also be injected into the plant material. Hot water and steam act as the main influential factors that liberate EOs from the plant tissues. The vapor, which is a mixture of water and oil, is condensed with cooling water. The condensed mixture flows from the condenser to a separator, where the bioactive compounds and oils are separated from the water [42].
Soxhlet extraction is a common conventional method that involves solid–liquid contact for extracting several compounds. This extraction method uses chemical solvents to extract oils; the solvent is heated in a distillation flask and the resulting vapor is condensed. The condensed solvent from the condenser flows into the thimble that contains the sample. When the solution reaches an overflow, a siphon pulls the solution in the thimble back into the distillation flask, thus carrying dissolved solute into the bulk liquid [39,43]. This procedure should be repeated by washing with an organic solvent until extraction is completed.
Maceration is the simplest process of extraction; the whole or coarsely powdered sample is mixed with solvent and left to macerate for a known period with frequent agitations at room temperature. After maceration, the sample mixture is pressed and filtered through an appropriate filter [39]. Cold pressing method is one of the best methods used to extract oils. In this method, the whole plant is pressed at low temperature and pressure to squeeze the material from the pulp to release the EO. Supercritical fluid extraction is the process of separation of bioactive components using CO2 as an ideal solvent. This technique requires low pressure, moderate temperature, and CO2 as solvent for a wide variety of applications such as EO and metal cation extraction. CO2 is non-toxic, non-explosive, noncorrosive, readily available, safe, inexpensive, and easily eliminated from the extract [44,45].
Ultrasound-assisted extraction provides a cutting edge with higher yields, superior quality, clean process, and less energy. Sonication process was carried out as a solvent- or water-based method to penetrate into the plant cells via bubble implosion generated by ultrasonic cavitation [46]. The bubble implosion creates micro-jets, which pulverize the lipid glands in the plant cell tissue and the process prevents the degradation of extracts [47]. Microwave-assisted extraction technique is more efficient and bio-sustainable, and is an alternative to conventional heating because it reduces extraction time, costs, energy, solvent consumption, and CO2 emissions [48,49]. In this method, plant materials are dispersed in solvents, and the mixture exposed to microwaves, then the interaction between the microwave irradiation and solvent releases the EOs [50]. Infusion and decoction are the popular traditional methods for the preparation of aqueous extracts with cold or boiling water for a fixed time duration [51]. Some different plant EOs and its extraction methods are summarized in Table 1.
Table 1.
Various extraction methods for different essential oils
| Common Name | Scientific Name | Plant Parts | Extraction Method | References |
|---|---|---|---|---|
| Rosemary | Rosmarinus officinalis | Leaves | Hydrodistillation | [52] |
| Pu-erh ripe tea | Camellia sinensis | Leaves | Soxhlet extraction | [53] |
| Chokeberry | Aronia melanocarpa | Fruits | Maceration | [54] |
| Lemon | Citrus limon | Fruits | Cold pressing | [55] |
| Lavandin | Lavandula angustifolia | Flowers | Supercritical fluid | [56] |
| Lavender | Lavandula angustifolia | Flowers | Ultrasound-assisted | [57] |
| Black cumin | Nigella sativa | Seeds | Microwave-assisted | [58] |
| Oregano | Origanum vulgare | Leaves | Infusion and decoction | [59] |
3. Chemical Composition of Essential Oils
The chemical composition of EOs are attributed to various factors, e.g., plant species, climatic conditions, soil type, temperature, humidity, ecotype, phenophase, photoperiod, irradiance, genotype, harvesting seasons, age of leaves, agronomic conditions, geographic region, and extraction process [60,61,62]. EOs are complex mixtures of aromatic and volatile compounds with many single compounds, but the number can vary in different plant materials. Most EOs are composed of aromatic, aliphatic constituents; terpenes, terpenoids with low molecular weights; lipophilic, highly volatile, secondary plant metabolites; mono- and sesquiterpenes; and allyl and isoallyl phenols. Terpenes are formed by condensation of two or more isoprene units represented by the chemical formula (C5H8)n through the mevalonic acid pathway, which occurs in the cytoplasm of the cell [63]. Based on the number of carbon atoms present in the structure, terpenes are classified as mono-, sesqui-, di-, ses-, tri-, and tetra-terpenes (carotenoids), and alternative hemi-terpenes. Many terpenes are hydrocarbons, but alcohols, aldehydes, or ketones are oxygen-containing compounds and these terpenes are known as terpenoids. Monoterpenes are the most delegate structures composed of two isoprene units covering a wide range of oxidation states like monocyclic, bicyclic, and acyclic forms, and organic functional groups including hydrocarbons (myrcene, camphene, 𝛼-pinene, 𝛼-terpinene, and p-cimene) and alcohols (menthol, nerol, borneol, and linalool). There are other functional groups like aldehydes (geranial and citronellal), esters (citronellyl acetate, linalyl acetate, and menthyl), ketones (camphor, pulegone, and carvone), peroxides (ascaridole), phenols (carvacrol and thymol), and ethers (1,8-cineole and menthofurane), which are also main constituents of EOs [64]. Sesquiterpenes are major types of terpenes formed from the combination of three isoprene units. Sesquiterpenes provide the spicy note, and are unsaturated compounds, which include hydrocarbons (azulene, 𝛽-bisabolene, cadinenes, germacrene D, humulene, farnesenes, zingiberene, and 𝛽-caryophyllene), oxygenated sesquiterpenes (caryophyllene oxide, spathulenol, and nerolidol), alcohols (patchoulol, bisabolol, 𝛽-nerolidol, farnesol, 𝛽-santalol, and patchoulol), acids (benzoic acid and geranic acid), aldehydes (citral), ketones (germacrone, benzophenone, acetophenone, 𝛽-vetinone, and turmerones), epoxides (caryophyllene oxide and humulene epoxides), and lactones (bergapten) [65,66].
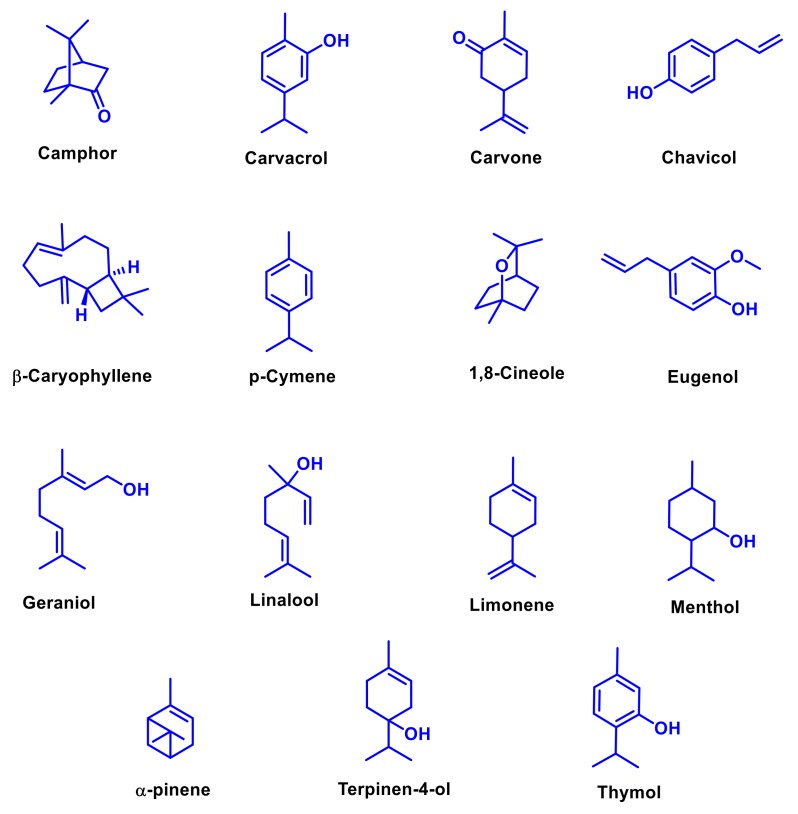
Some different kinds of organic compounds—such as sterols [67], alkaloids [68], tannins [69], and flavonoids [70]—are also present in EOs. Aromatic compounds occur as relatively small part of EOs when compared to terpenes. They contain alcohol (cinnamyl alcohol); benzene (styrene); aldehydes (cynnamaldehyde); phenols (chavicol, eugenol, vaniline, and cinnamaldehyde); methoxy compounds (methyleugenol, elemicine, estragole, and anethole), and methylene dioxy derivatives (safrole, myristine, and apiole) [71]. The other compounds like nitrogen and sulfur-containing compounds are also present as aglycones or glucosinolates in EOs [72]. The sulfur-containing compounds—namely dimethyl sulfide, allyl sulfide, diallyl sulfide, and dimethylthiophene—are mainly responsible for the characteristic odor and taste [73]. Nitrogen-containing compounds like indole, pyridine, methyl anthranilate, and pyrazine, are found in only a few EOs [74]. The major and biologically important chemical constituents of EOs and its structures are depicted in Figure 2.
Figure 2.
Major and biologically important bioactive constituents present in essential oils.
4. Antimicrobial Effects of Essential Oils
At present, many new antimicrobial agents or antibiotics have been developed from various sources for treating different microbial pathogens. However, the increased use of antibiotics has resulted in the emergence of multidrug resistance bacteria, which has led to the severity of diseases caused by bacterial pathogens. Some of the main multidrug resistance bacteria are E. coli, S. aureus, P. aeruginosa, Enterococcus spp., Salmonella spp., and coagulase-negative Staphylococcus, and are included in the category of community and hospital acquired pathogens, which affects public health [12].
Natural resources like plant extracts widely used as medicinal plants with high antimicrobial activities against human pathogens (Figure 1). EOs and their major bioactive constituents are potential candidates for antibacterial, antifungal, antiviral, antiseptic, antioxidant, anti-parasitic, and insecticidal agents for promoting food preservation, and as alternatives for treating infectious diseases (Figure 2) [75,76]. EOs exhibit wide-ranging inhibitory activities against various bacterial pathogens [77] by easily penetrating the lipids of the bacterial cell membrane and disrupting their cell wall structure [78]. Association of EOs constituents with lipids causes loss of integrity and cellular contents, and finally leads to bacterial cell death [79]. Some of the constituents of EOs such as carvone, a member of terpenoids, split in the lipid membrane, while terpinen-4-ol, an isomer of terpineol, prevent cellular respiration, and both destroy the function of the cell membrane as a permeable barrier [80,81]. Several bioassays are well known and commonly used, such as well diffusion, disk-diffusion, and agar dilution methods, but others such as bioluminescent and flow cytofluorometric methods are not widely used because they require selected equipment [82].
The minimum inhibitory concentration (MIC) is the lowest concentration of antimicrobial agent that completely inhibits growth of the organism in micro-dilution wells or tubes as detected by the unaided eye [83]. However, the determination of minimum bactericidal concentration (MBC) or minimum fungicidal concentration (MFC), also known as minimum lethal concentration (MLC), is the most common estimation of bactericidal or fungicidal activity, which is defined as the lowest concentration of antimicrobial agent needed to kill 99.9% of the final inoculum [84].
The various bioactive components present in different EOs play an individual role; for instance, the EOs of cinnamon and black pepper damaged the cell membrane and decreased the metabolic activity of E. coli and S. aureus [85,86]. Similarly, EO from Dipterocarpus gracilis inhibited the growth of P. mirabilis and B. cereus by infecting cytoplasmic membrane. The differences in antimicrobial activity of EOs may be associated with the chemical constituents, geographical location, seasons, and extraction methods [87]. Some important plants, which are the most-active EOs and their antimicrobial activity against pathogenic microorganisms, are summarized in Table 2.
Table 2.
Major chemical composition of various EOs and their antimicrobial activity against pathogenic microorganisms
| Plant Source | Plant Part | Major Chemical Compounds | Microorganisms | References |
|---|---|---|---|---|
| Fortunella margarita | Leaves | Gurjunene, eudesmol, muurolene | B. subtilis, S. aureus, Sarcina luta, S. faecalis, E. coli, K. pneumonia, P. aeruginosa | [88,89] |
| Eremanthus erythropapps | Leaves | Germacrene D, p-cymene, 𝛾-terpinene | S. epidermidis | [90] |
| Euphrasia rostkoviana | Commercial EOs | n-Hexadecanoic acid, thymol, myristic acid, linalool |
E. faecalis, E. coli, K.
pneumoniae, S. aureus, S. epidermidis, P. aeruginosa |
[91] |
| Pogostemon cablin | Leaves | Patchoulol | H. pylori | [92,93] |
| Plectranthus neochilus | Leaves | 𝛼-Pinene, trans-caryophyllene | S. mutans | [94,95] |
| Ocimum basilicum | Arial parts | Linalool, methyl chavicol | M. flavus | [96,97] |
| Salvia sclarea | Arial parts | Linalool, linalyl acetate | E. coli, S. aureus, B. subtilis, S. typhimurium, K. pneumonia, P. Aeruginosa, B. pumilus | [98] |
| Thymus kotschyanus | Arial part | Carvacrol, 1,8 cineole, thymol, borneol |
S. aureus, S. epidermidis, B.
cereus, E. coli |
[99,100] |
| Glechon marifolia | Leaves | 𝛽-Caryophyllene, bicyclogermacrene | herpes simplex virus type 1 | [101] |
| Myrtus communis | Leaves | α-Pinene, 1,8-cineole | C. albicans, A. flavus | [102,103] |
| Origanum vulgare | Leaves | Carvacrol | T. tonsurans, T. violaceum, T. floccosum, T. mentagrophytes | [104,105] |
| Syzygium aromaticum | Leaves | Eugenol, eugenylacetate | C. albicans, Candida spp. | [106,107] |
| Pelargonium graveolens | Leaves | Citronellol, geraniol | C. tropicalis | [108,109] |
| Trachyspermum ammi | Leaves | Thymol, 𝛼-pinene, | Japanese encephalitis virus | [110,111] |
| Lepechinia salviifolia | Leaves | Germacrene D | Herpes simplex virus type 1 | [112] |
| Lavandula x intermedia | EOs | Linalool, camphor and 1,8-cineole | L. monocytogenes | [113,114] |
| Thymus vulgaris | Leaves | Carvacrol | M. furfur | [115] |
| Mentha piperita L. | Leaves | Menthol | C. albicans, C. tropicalis, P. anomala and S. cerevisiae | [116,117] |
| Melaleuca cajuputi | Leaves | 1,8-Cineole, linalool, terpinen-4-ol | Aspergillus spp. A. niger | [118] |
| Cinnamomum zeylanicum | Bark | Carvacrol | Borrelia Burgdorferi | [119,120] |
| Eugenia caryophyllata | Clove buds | Eugenol, 𝛽-caryophyllene | S. aureus. | [121] |
| E. loxophleba | Leaves | 1,8 Cineole | S. aureus and E. coli | [122] |
| Salvia officinalis L. | Leaves | 1,8-Cineole, α-thujone, camphor | B. subtillis and S. epidermidis | [123,124] |
| Melaleuca alternifolia | Leaves | Terpinen-4-ol | C. albicans | [125,126] |
| Coriandrum sativum L. | Fruits | Linalool | E. coli B. bronchiseptica | [127,128] |
| B. dracunculifolia | Leaves, flowers | Spathulenol, nerolidol, |
S. aureus, B. cereus, and
P. aeruginosa. |
[60] |
| Ocimum basilicum | Arial parts | Linalool | C. albicans, S. aureus | [129] |
| Rosmarinus officinalis | Leaves | 1,8-Cineole, camphor | C. perfringens | [130] |
| Epilobium parviflorum | Arial parts | Oenothein B, myricitrin | E. fecalis, S.aureus | [131] |
5. Antioxidant Activity of Essential Oils
Free radicals play an important role in origin of life and biological evolution, leaving beneficial effects on the organisms and involved in many biochemical activities of cells such as signal transduction, gene transcription, and regulation. The human body produces oxygen free radicals and other reactive oxygen species (ROS) as byproducts through several physiological and biochemical processes. However, over production of free radicals can cause oxidative damage to biomolecules leading to many chronic diseases such as cancer, cardiovascular, diabetics, chronic inflammation, and atherosclerosis in humans. Therefore, much attention has been focused on the use of antioxidants to inhibit lipid peroxidation due to free radicals by using synthetics or natural antioxidants. Synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) are suspected to be harmful to human health. On the other hand, natural products present in medicinal plants like EOs shows significant antioxidants performance from different plant source. The EOs obtained from Zanthoxylum alatum, Ammodaucus leucotrichus, Marrubium globosum, Citrus sinensis and Citrus latifolia, Lawsonia inermis, Thymus fontanesii, Artemisia herba-alba and Rosmarinus officinalis, Syzygium aromaticum L., Origanum vulgare L., Mentha spicata L., and Eremanthus erythropappus M showed promising antioxidant activity [132,133,134,135,136,137].
6. Potential Impacts of Bi-Metallic and Tri-Metallic Nanoparticles
Over the past decades, several mono-, bi- and tri-metallic NPs have drawn significant attention due to their catalytic, optical, and magnetic properties in a wide array of fields such as catalysis, medical, imaging, remote sensing, environmental, and energy applications. As the name suggests, monometallic NPs are composed of only a single metal (Au, Pt, or Pd), whereas, bi- and tri-metallic NPs are composed of two and three different metals (Au/Ag, Pt/Pd, Fe/Ag/Pt, and Au/Fe/Ag), respectively. Bi- and tri-metallic NPs have tunable and better properties due to the addition of a second and third metal of the nanoparticle combination, and can improve catalytic activity and selectivity when compared to monometallic NPs [138]. The catalytic activity of the external metal (secondary metal) may increase by the bi-metallization of NPs.
The properties of bimetallic NPs include electronic, catalytic, thermal, size, and shape, which may differ from those of the monometallic NPs. In tri-metallic NPs, the addition of a third metal modifies the electronic structure, reduces the lattice, and increases the charge shift, catalytic performance, and selectivity when compared to monometallic and bimetallic NPs [139,140]. The preparation conditions regulate the structure, size, and shape of the particles such as alloy, core–shell, and heterodimer of two or more metals in bi- and tri-metallic NPs [23,28,29]. The catalytic performance of mono-, bi-, and tri-metallic NPs was also investigated individually and in combination. However, in our previous reports, bi- and tri-metallic NPs showed superior catalytic activity when compared with monometallic NPs [23]. When one metal alloy is combined with other metals to form bi or tri-metallic NPs, the catalytic properties of the consequent material become better when compared to pure metals.
Bi- and tri-metallic NPs contain a few tens to several thousand of atoms, which are excellent catalysts, with improved selectivity and efficiency due to their highly active surfaces, and act as green catalysts by recyclability [17]. Multi-metallic structures like bi- and tri-metallic NPs furnish many active inter-metallic interfaces to change the electronic structure [141] and allow tuning of the catalytic activity via composition ratios. So far, bi- and tri-metallic NPs interfaces are more active because of fast electron interchange and the presence of crystal defects, which assures substantial implementation [142]. Several different methods are used to prepare bi- and tri-metallic NPs in the required size, composition, and shape, which influence the properties of the material, including electrochemical reduction, microwave, microemulsion, co-precipitation, pyrolysis, hydrothermal, selective catalytic reduction, sol–gel, solvothermal processes, and combustion [143,144,145,146,147,148,149,150,151,152].
7. Antimicrobial Activities of Metallic Nanoparticles
Bacterial resistance has become a severe problem due to the substantial application of antibiotics preferred for the treatment of infectious disease without proper medical indications. In order to solve this problem, the use of alternative antibacterial agents to treat infectious diseases have attracted great interest, due to their heat resistance, sustainability, and improved stability under harsh processing conditions. Due to their smaller dimensions and large surface area to volume ratios, metallic NPs provide strong, targeted, and extended antimicrobial interaction with bacteria and biofilms at smaller doses. In the last few decades, new and novel antimicrobial agents—such as metallic NPs and macromolecules—have been found to be the most effective agents to combat pathogenic bacteria.
Some metal NPs such as silver (Ag), gold (Au), gallium (Ga), copper (Cu), zinc (Zn), iron (Fe), and palladium (Pd) have potential antimicrobial activities. Metal oxide NPs like aluminum oxide (Al2O3), iron oxide (Fe3O4), titanium dioxide (TiO2), copper oxide (CuO), zinc oxide (ZnO), cobalt oxide (Co3O4), manganese oxide (Mn2O3), magnesium oxide (MgO), indium oxide (In2O3), silicon dioxide (SiO2), nickel oxide (Ni2O3), zirconium dioxide (ZrO2), and chromium oxide (Cr2O3) have also shown potential antimicrobial activities (Table 3). Metal and metal oxide-based NPs damage the cell membrane by binding and releasing metallic ions into proteins and enzymes of the bacterial cell wall. NPs can attack the bacterial cell wall through several modes of action like electrostatic attraction, van der Waals forces, and hydrophobic interactions [153,154,155]. However, different types of NPs have different mechanisms to combat bacteria by forming pores on the surface of bacterial cell membrane, which in turn causes radical formation, generates reactive oxygen species, inhibits enzyme activity, deactivates proteins and DNA, induces oxidative stress, and modifies gene expression levels [156,157].
Table 3.
Antimicrobial activity of mono, bi-, and tri-metallic and metal oxide nanoparticles, specifically highlighting size, shape, bacterial strains tested, and mode of action
| NPs | Size and Shape | Bacteria Pathogens | Mode of Action | Ref. |
|---|---|---|---|---|
| Ag | 15 nm, triangular | P. aeruginosa and E. coli | Deactivation of enzymes and cellular proteins | [158] |
| 23 nm, | S. typhimurium | Interaction of NPs with membrane proteins | [159] | |
| 20 nm, triangular | E. coli and S. aureus | Destruction of outer and inner membrane | [160] | |
| 7.1 nm, spherical | E. coli and P. aeruginosa | Permeabilized membrane | [161] | |
| 25 nm, spherical | S. aureus, and E. coli | Structural changes in the cell wall and nuclear membrane | [162] | |
| Au | 10 nm, spherical | S. aureus and P. aeruginosa | Disruption of cell membrane | [163] |
| 20 nm, spherical | S. pneumoniae | Disruption of cell membrane | [164] | |
| 1-3 nm, spherical | E. coli, P. aeruginosa, S. epidermidis and B. subtilis | Interaction between NPs and bacteria could induce a metabolic imbalance | [165] | |
| 50 nm, spherical | S. oneidensis | Interaction of NPs with membrane proteins | [166] | |
| Ga | 305 nm, rod | M. smegmatis and HIV | Disruption of cell membrane | [167] |
| Ag/Au | 30 nm, triangular, | B. subtilis, E. coli, S. typhi, and S. aureus | Interaction between NPs and vital components leads to enzyme inactivation | [168] |
| Cu/Pt | 30 nm, spherical | E. coli, S. aureus, P. aeruginosa, and C. albicans | Permeabilized membrane | [169] |
| Al/Ag | 200 nm, spherical | E. coli, and S. aureus | Adsorption and inactivation of bacterial strains | [170] |
| Fe/Cu | 68 and 82 nm, spherical | S. aureus, and P. aeruginosa | Structural changes in the cell wall and nuclear membrane | [171] |
| Cu/Cr/Ni | 100 and 200 nm, plate | E. coli and S. aureus | Rupture of the membrane and denaturation of bacterial proteins | [172] |
| Cu/Zn/Fe | 42 nm, spherical | E. coli and E. faecalis. | Disruption of cell membrane | [173] |
| Au/Pt/Ag | 20-40 nm, spherical, triangle, ellipsoidal | E. coli, S. typhi, Klebsiella, E. coli and, and E. faecalis | Interaction with the cell components such as DNA and enzymes | [174] |
| ZnO | 20 nm, spherical | S. typhimurium, and S. aureus | Cell wall damage | [175] |
| CuO | 198 nm, | B. cereus, P. mirabilis and A. caviae | Loss of membrane integrity and increased permeability | [176] |
| MgO | 24 nm, | S. epidermidis | Disruption of cell membrane | [177] |
| TiO2 | 50 nm | P. fluorescens and E. coli | Destruction of membrane, DNA and proteins | [178] |
Higher prevention of bacteria is attained by a large surface area-to-mass ratio, zeta potential, surface morphology, crystal structure, smaller and tunable size and shape of particles, which allows close interaction with microbial membranes. Moreover, other factors that influence antibacterial effects of nanoparticles include bacterial strain, environmental conditions, and the exposure time. Metal and metal oxide nanoparticles were studied for their well-known antimicrobial activities against Gram-positive bacteria like S. aureus and B. subtilis, and Gram-negative bacteria like P. aeruginosa and E. coli including antibiotic-resistant strains. In particular, silver NPs alone or in combination with other nanomaterials have great potential antimicrobial activities owing to their unique chemical stability, catalytic activity, and better contact with microorganisms. Similarly, gold NPs also exhibit significant effects of antimicrobial activity but the chemicals used as precursor are expensive. In addition, metal oxide nanoparticles—such as ZnO, CuO, TiO2, Al2O3, and Fe2O3—have also demonstrated significant effects of antimicrobial activities against both Gram-positive and Gram-negative bacteria.
8. Efficiency of Nano-Encapsulated Essential Oils
It is well known that EOs have shown excellent antimicrobial activity against pathogenic microorganisms. However, their utilization is very limited because of low water solubility and their high sensitivity to oxygen, moisture, heat, and light. To increase their stability, water solubility, and protect EOs from degradation, many modification technologies have emerged as solutions to these existing challenges. Encapsulation of essential oils into nano-based delivery systems such as nanoemulsions, microemulsions, solid-lipid nanoparticles, and liposomes are models for the encapsulation of natural bioactive compounds to improve antimicrobial activities. Currently, the application of nanoencapsulation technology has increased rapidly in the food industry, especially in the EOs industry due to its interesting parameters such as size, zeta potential, and the polydispersity index.
Nanoemulsions are colloidal dispersions consisting of two immiscible solvents, oil (globules) and water (liquid), in which one is dispersed in the other with the help of a surfactant that stabilizes emulsions. Surfactants are required to formulate nanoemulsions and decrease the size of the droplet and increment the inflexibility and quality of interfacial layer. The combination of Span 20 with Tween 40 was seen to be sophisticated by delivering ideal mineral oil emulsions. For instance, the clove, cinnamon, and thyme oil nanoemulsions which were formulated with nonionic surfactants (Spans and Tweens) were having droplet size less than 100nm. Nanoemulsions are a promising nanocarrier widely used in drug delivery, which helps in improving biodistribution of drugs and minimizing toxicity. To protect EOs from extrinsic and intrinsic factors such as pH, temperature, water, humidity, activity, storage environment, and enzymatic degradation, some different delivery systems have been used as carriers including chitosan cyclodextrin, alginate, albumin, globulin, maltodextrin, and starch. Among the many nanocarriers, naturally occurring polymers such as chitosan and alginate are widely used in the biomedical and pharmaceutical fields as emulsions.
Many studies in academia and industry have continued to enhance the physical stability of EOs by encapsulating them into nanocarrriers. Recently, nanoemulsions were achieved by using low concentrations of EOs, pectin, and surfactants with the help of pseudo-ternary diagrams [179]. Similarly, nanoencapsulation of lime EOs with chitosan showed enhanced antibacterial activity against S. aureus, L. monocytogenes, Shigella dysenteriae, and E. coli as nanoemulsion [180]. Soybean oil with sodium dodecyl sulfate against S. aureus as nanoemulsion [181], Schinus molle with chitosan against Aspergillus parasiticus as nanoprecipitation [182], Zataria multiflora with lipid phase (glyceryl monostearat) and precirol against Aspergillus ochraceus, Aspergillus niger, Aspergillus flavus, Alternaria solani, Rhizoctonia solani, and Rhizopus stolonifer as solid-lipid nanoparticles [183]. In addition, Gaultheria procumbens with chitosan-cinnamic acid microgel against A. flavus as microencapsulation [184], Thymus capitatus with sodium dodecyl sulfate against E. coli and B. subtilis as nanoencapsulation [185], cardamom EO with chitosan against E. coli and S. aureus as nanocomposites [186], and Siparuna guianensis with chitosan against larvicide Aedes aegypti as nanoencapsulation [187].
9. Interaction of Essential Oils and Metallic Nanoparticles
Nanotechnology emerged significantly in pharmaceutical industry up to a considerable extent. Nanoparticles is a combined name for any colloidal carrier like nanospheres and nanocapsules. EOs can be enhanced by encapsulating with several nanomaterials such as polymeric NPs, liposomes, solid lipid NPs and nano-emulsions which consists of inner liquid core surrounded by an outer polymeric membrane called nanocapsule. Nanocapsules possess a polymeric membrane with a liquid nucleus, in which the active compounds of EOs is confined to a cavity [188]. Similarly, nanospheres are solid colloidal fragments in which bioactive components of EOs are diffuse, captured, encapsulated, or adsorbed into the polymer matrix [189]. Nano-encapsulation increases the physical stability of EOs, enhance bioactivity, reduce toxicity, and protect it from environmental interactions such as moisture, light, oxygen, pH, and controlling the release of EO. However, only nano-encapsulation, nano-emulsion, and monometallic nanoparticles with EOs has reported so far with limited data. Thus, EOs with their antimicrobial activity when blended with other potent antimicrobial agents like bi- and tri-metallic nanoparticles might be a probable source of alternative antimicrobial agents to combat multidrug resistant pathogens.
The constituents of EOs along with bi- and tri-metallic NPs with their diverse sizes, shapes, high surface-to-volume ratios, physical and chemical stability, and degree of selectivity can unlock the cell membrane channels, thus opening the passage of EOs/multimetallic to reach their target sites. These metal NPs or nanoemulsions/nanoencapsulations enclosing EOs can be adhered via electrostatic, hydrogen bonding, and covalent interactions to produce antimicrobial packaging systems. Once NPs adhere to cell walls, they directly effects toxicity due to larger concentration of NPs release more ions and distributed in the environment surrounding the bacterial cell wall. The larger concentration of generated ions disrupt cell membrane and ROS production and further helps to penetrate the cells [190]. When NPs enter inside the cell along with EOs cause damages in the structure of cell membranes, protein dysfunction causes oxidative stress and DNA damage as shown in the schematic representation in Figure 3.
Figure 3.
Proposed antibacterial mechanisms of mono, bi-, tri-metallic NPs with EOs. Combination of NPs and EOs can attack bacteria cell through multiple mechanisms; direct interaction with cell membrane by generating metal ions, disruption of cell membrane, protein dysfunction, DNA damage, inhibition of the electron transport chain, and the regulation of bacterial metabolic processes.
There are two possible ways to combat multidrug resistant pathogens along with EOs either by nano-encapsulation method or combination with bi- and tri-metallic NPs with EOs. Recently, Wu et al., prepared chitosan NPs embedded with Torreya grandis aril EOs and studied their antibacterial properties against S. aureus found stronger antibacterial activities than chitosan NPs alone [191]. Synergistic antibacterial activity of silver NPs with EOs of Kelussia odoratissima and Teucrium polium investigated their enhanced effect against multidrug-resistant bacteria [192]. Chitosan NPs encapsulated with Cymbopogon martinii EOs shows efficient and enhanced antifungal and antimycotoxin activities against Fusarium graminearum [193]. Similarly, silver NPs with eucalyptus EO showed the synergistic effect on the growth inhibition of E. coli, MRSA, S. enteric, and B. subtilis [194] and Nigella sativa EO coated with gold nanoparticles effectively controlled the growth and biofilm formation of S. aureus [195]. Whereas, rosemary and oregano EOs with silver and ZnO nanoparticles incorporated into pullulan films were effective against S. aureus, L. monocytogenes, E. coli, and S. Typhimurium [196].
10. Synergistic Antimicrobial Activity of Essential Oils
The development of resistance to different antimicrobial agents by bacteria, fungi, viruses, and parasites is a great challenge to the effective treatment of infectious diseases, and hence there is a need for more intensive, new, and novel antimicrobials. Some plant extracts and EOs have been used as medicine for the treatment of infectious diseases traditionally. However, these plant-based medicines are not effective for severe systemic infections due to the absence of clinically applicable pharmaceutical forms. Subsequently, many antibiotics have been developed as synthetic antimicrobial agents, but these drugs are complicated by their high toxicity, low tolerability, and ineffectiveness against new emerging microbes. One probable way to enhance the range and scope of current antimicrobial therapy is the use of a combination of antimicrobials.
The combination of antimicrobial agents such as essential/volatile oils, plant extract/EOs, plant extracts/antibiotics, EOs/EOs, EOs/antibiotics, plant extract/plant extract, EOs/monometallic NPs, and phytochemical/antibiotics [16] has confirmed the significant effects of antimicrobial activities. In addition, nanoencapsulation of many EOs and their antimicrobial activities have also been discussed in the previous section. Synergy can be assessed by combining two antimicrobial compounds and conducting antibacterial activity, whereby the sum of the antibacterial activities is greater than antibacterial activity of the individual components due to several substances, which improves solubility. According to Hossain et al. [197], combination of eight EOs of plants showed enhanced antimicrobial activity against A. niger, P. chrysogenum, A. flavus, and A. parasiticus when compared to the antimicrobial activity of a single EO. Similarly, in a study conducted by Knezevic et al. [198], it was reported that the combination of EOs of Eucalyptus camaldulensis with conventional antibiotics such as gentamycin, ciprofloxacin, and polymyxin B showed synergistic antibacterial effect against Acinetobacter baumannii.
Very few researchers have studied the synergistic antimicrobial effects of metal and metal oxide NPs (monometallic NPs) along with EOs and conventional antibiotics [199,200]. Scandorieiro et al. [201] reported that the combination of silver NPs with Origanum vulgare EO resulted in synergistic antimicrobial activities against E. coli, A. baumannii, and S. aureus. Similarly, hydroxyapatite NPs with peppermint EO against S. aureus, E. faecium, E. coli, P. aeruginosa, and C. parapsilosis [202], olive EO with lipid nanoparticles against P. pyogenes and S. aureus [203], Siparuna guianensis EO with chitosan NPs against A. aegypti [187] showed enhanced antimicrobial effects. In addition, rosemary and oregano EOs, with silver and zinc oxide NPs incorporated into pullulan films were effective against pathogenic microorganisms such as S. aureus, L. monocytogenes, E. coli, and S. typhimurium [196], cinnamon EO with chitosan NPs against E. coli and S. aureus [204] also had synergistic antimicrobial activities.
11. Challenges and Future Directions
EOs have great potential for the promotion of health and preventing and treating infectious diseases. However, EOs have some drawbacks due to their low solubility, stability, and high volatility in medicinal applications. Hence, the encapsulation of EOs increases their solubility and stability, and maintains controlled release, which makes them more bioavailable; protects them from air, humidity, light; and lead to volatilization, increasing their biological activities. To date, there are limited studies on synergistic antimicrobial effects of metal and metal oxide NPs along with EOs. Bi-and tri-metallic NPs have recently attracted more attention because of their unique catalytic, optical, electronic, and magnetic properties [19], and their utilization is associated with their well-defined properties when compared to their monometallic counterparts [205,206].
Bi- and tri-metallic NPs are formed by the combination of two or more different metals or materials into a single system, and they exhibit special properties which are not observed in the case of the monometallic forms [207]. Moreover, these bi- and tri- metallic NPs are used as catalysts for many organic transformations [23] with excellent selectivity and activity. In addition, altering the shape, size, and/or surface chemistry of NPs as core–shell, heterodimer, and nano-alloys [23,28,29] enhance their catalytic performance. As discussed earlier in the previous section, several studies on of the nanoencapsulated EOs and metal and metal oxide NPs have demonstrated potential antimicrobial activities and synergistic effects, which have already been documented.
The present study acknowledged more findings on in vitro studies of EOs, and nanoencapsulation of EOs, however, more efforts are required to conduct studies on the synergistic effects of bi- and tri-metallic NPs along with different EOs. Tuning into different size, shape, and/or surface chemistry of NPs allows their functionalities to be more enhanced for better applications. Furthermore, the use of multi-component nanoparticles like bi- and tri-metallic NPs comprised of three or more metals have also intervened as hybrid materials that can upgrade optical and magnetic properties. However, the inertness of these materials with respect to the environment, health, and safety concerns must be considered due to their potential hazards and toxicity. Thus, EOs along with mono, bi-, and tri-metallic NPs might be a prospective source of alternative antimicrobial agents, and may play an important synergistic role in the discovery of new drugs for the treatment of a wide range of pathogenic infections in the near future. Finally, we will continue to see creative advances in the synthesis of these unique dual-component and multi-component nanomaterials and their antimicrobial activities along with EOs individually or in combinations.
12. Conclusions
The increasing number of clinical complications related to multidrug resistant microorganisms has inspired researchers to focus their interest on alternative antimicrobial agents, which are an appropriate solution to treating infections that are more serious to human health. There are several types of EOs, which have individual components with diverse bioactivities including antimicrobial potential. However, EOs have certain limitations, including low solubility and stability, and currently they have reduced clinical applications owing to the development of resistance. Nanotechnology is expected to have a significant impact on the therapeutics of antimicrobial agents at the nanoscale level to develop nanomedicines. As the size of a particle decreases, the specific surface area, reactivity, and bioavailability increases, allowing greater interaction with the surrounding environment that enhances the antimicrobial effects. Indeed, nanoparticles are able to solve the major inconvenience of EO components by increasing the chemical stability in the presence of moisture, air, light, and high temperatures, factors which can lead to the rapid evaporation and to the degradation of the active components. Encapsulation simultaneously increases the antimicrobial potency of EOs by controlled or sustained release and facilitating close interaction with the microorganisms.
Metal and metal oxide NPs like Ag, Au, Ga, FeO, ZnO, TiO, MgO, and CuO act as promising antimicrobial agents. Bi-metallic or multimetallic nanostructures such as hybrid, core–shell, or alloy structures like FePd, AuPd, CuPd, PtPd, AuFeAg, and FeAgPt enhance antimicrobial activities and catalytic performance. For this reason, it is necessary to find an alternative method to combat multidrug resistance pathogens by using EO encapsulation with suitable metal or metal oxide NPs or combination of EOs with different bi- and tri-metallic NPs. The present study revealed more information on the extraction methods, chemical composition, nano-encapsulation, and antimicrobial activities of EOs and antimicrobial potential of some metal and metal oxide NPs. The future biotechnological perspective to stabilize EOs using nanostructured materials might establish a valuable prerequisite for obtaining functionalized materials with modified surface, inhibitory effects and ability to adhere on the microorganisms for targeting and/or controlled release. However, more research is required to explore the mechanism of individual essential oil components along with bi- and tri-metallic NPs with an initiation in systematically conduct experiments on the synergistic mechanisms among different components. Therefore, new or alternative strategies for EOs with bi- and tri-metallic NPs and synergistic studies can provide an interesting platform in the future to combat multidrug resistance pathogens with greater activity. In addition, it is necessary to control the toxicity, risk assessment, and safety aspects, and to avoid the passage of such nanomaterials into the human body.
Acknowledgments
The authors are grateful for the funding (NRF-2019R1F1A1052625) support by the National Research Foundation of Korea, Republic of Korea.
Author Contributions
Collection of data: N.B., J.K.P., and K.B.; Writing—original draft preparation: N.B. and J.K.P.; Writing—review and editing: K.-H.B. All authors have read and agreed to publish the final version of the manuscript.
Funding
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2019R1F1A1052625).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gyles C. The growing problem of antimicrobial resistance. Can. Veter. J. Rev. Veter. Can. 2011;52:817–820. [PMC free article] [PubMed] [Google Scholar]
- 2.Livermore D.M. Has the era of untreatable infections arrived? J. Antimicrob. Chemother. 2009;64:i29–i36. doi: 10.1093/jac/dkp255. [DOI] [PubMed] [Google Scholar]
- 3.Mahady G. Medicinal plants for the prevention and treatment of bacterial infections. Curr. Pharm. Des. 2005;11:2405–2427. doi: 10.2174/1381612054367481. [DOI] [PubMed] [Google Scholar]
- 4.Kon K., Rai M. Combining Essential Oils with Antibiotics and other Antimicrobial Agents to Overcome Multidrug-Resistant Bacteria. Elsevier BV; London, UK: 2013. pp. 149–164. [Google Scholar]
- 5.Abad M.J., Bedoya L.M., Bermejo P. Essential Oils from the Asteraceae Family Active against Multidrug-Resistant Bacteria. Elsevier BV; London, UK: 2013. [Google Scholar]
- 6.Holmes C., Hopkins V., Hensford C., MacLaughlin V., Wilkinson D., Rosenvinge H. Lavender oil as a treatment for agitated behaviour in severe dementia: a placebo controlled study. Int. J. Geriatr. Psychiatry. 2002;17:305–308. doi: 10.1002/gps.593. [DOI] [PubMed] [Google Scholar]
- 7.Auddy B., Ferreira M., Blasina F., Lafon L., Arredondo F., Dajas F., Tripathi P.C., Seal T., Mukherjee B. Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J. Ethnopharmacol. 2003;84:131–138. doi: 10.1016/S0378-8741(02)00322-7. [DOI] [PubMed] [Google Scholar]
- 8.Prabuseenivasan S., Jayakumar M., Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006;6:39. doi: 10.1186/1472-6882-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J., Jang M., Seo J., Kim G.-H. Evaluation For Antibacterial Effects of Volatile Flavors from Chrysanthemum indicum Against Food-borne Pathogens and Food Spoilage Bacteria. J. Food Saf. 2011;31:140–148. doi: 10.1111/j.1745-4565.2010.00277.x. [DOI] [Google Scholar]
- 10.Pandey A.K., Kumar P., Singh P., Tripathi N.N., Bajpai V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017;7:45. doi: 10.3389/fmicb.2016.02161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Chouhan S., Sharma K., Guleria S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines. 2017;4:58. doi: 10.3390/medicines4030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barra A. Factors affecting chemical variability of essential oils: a review of recent developments. Nat. Prod. Commun. 2009;4:1147–1154. doi: 10.1177/1934578X0900400827. [DOI] [PubMed] [Google Scholar]
- 14.Edwards-Jones V. Alternative Antimicrobial Approaches to Fighting Multidrug-Resistant Infections. Elsevier BV; Oxford, UK: 2013. pp. 1–9. [Google Scholar]
- 15.Bassole I.H.N., Lamien-Meda A., Bayala B., Tirogo S., Franz C., Novak J., Nebié R.C., Dicko M.H. Composition and Antimicrobial Activities of Lippia multiflora Moldenke, Mentha piperita L. and Ocimum basilicum L. Essential Oils and Their Major Monoterpene Alcohols Alone and in Combination. Molecules. 2010;15:7825–7839. doi: 10.3390/molecules15117825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rakholiya K.D., Kaneria M.J., Chanda S.V. Medicinal Plants as Alternative Sources of Therapeutics against Multidrug-Resistant Pathogenic Microorganisms Based on Their Antimicrobial Potential and Synergistic Properties. Elsevier BV; Oxford, UK: 2013. pp. 165–179. [Google Scholar]
- 17.Sharma G., Kumar A., Sharma S., Naushad M., Dwivedi R.P., Alothman Z.A., Mola G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019;31:257–269. doi: 10.1016/j.jksus.2017.06.012. [DOI] [Google Scholar]
- 18.Srinoi P., Chen Y.-T., Vittur V., Marquez M.D., Lee T.R. Bimetallic Nanoparticles: Enhanced Magnetic and Optical Properties for Emerging Biological Applications. Appl. Sci. 2018;8:1106. doi: 10.3390/app8071106. [DOI] [Google Scholar]
- 19.Mishra K., Basavegowda N., Lee Y.R. Biosynthesis of Fe, Pd, and Fe–Pd bimetallic nanoparticles and their application as recyclable catalysts for [3 + 2] cycloaddition reaction: a comparative approach. Catal. Sci. Technol. 2015;5:2612–2621. doi: 10.1039/C5CY00099H. [DOI] [Google Scholar]
- 20.Yu W., Porosoff M., Chen J.G. Review of Pt-Based Bimetallic Catalysis: From Model Surfaces to Supported Catalysts. Chem. Rev. 2012;112:5780–5817. doi: 10.1021/cr300096b. [DOI] [PubMed] [Google Scholar]
- 21.Yao T., Cui T., Wang H., Xu L., Cui F., Wu J. A simple way to prepare Au@polypyrrole/Fe3O4 hollow capsules with high stability and their application in catalytic reduction of methylene blue dye. Nanoscale. 2014;6:7666–7674. doi: 10.1039/C4NR00023D. [DOI] [PubMed] [Google Scholar]
- 22.Fang Y., Wang E. Simple and direct synthesis of oxygenous carbon supported palladium nanoparticles with high catalytic activity. Nanoscale. 2013;5:1843. doi: 10.1039/c3nr34004j. [DOI] [PubMed] [Google Scholar]
- 23.Basavegowda N., Mishra K., Lee Y.R. Trimetallic FeAgPt alloy as a nanocatalyst for the reduction of 4-nitroaniline and decolorization of rhodamine B: A comparative study. J. Alloy. Compd. 2017;701:456–464. doi: 10.1016/j.jallcom.2017.01.122. [DOI] [Google Scholar]
- 24.Guisbiers G., Khanal S., Ruiz-Zepeda F., De La Puente J.R., Yacaman M.J. Cu–Ni nano-alloy: mixed, core–shell or Janus nano-particle? Nanoscale. 2014;6:14630–14635. doi: 10.1039/C4NR05739B. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L.F., Zhong S., Wang Y. Highly Branched Concave Au/Pd Bimetallic Nanocrystals with Superior Electrocatalytic Activity and Highly Efficient SERS Enhancement. Angew. Chem. Int. Ed. 2012;52:645–649. doi: 10.1002/anie.201205279. [DOI] [PubMed] [Google Scholar]
- 26.Lim B., Lu X., Jiang M., Camargo P., Cho E.C., Lee E.P., Xia Y. Facile Synthesis of Highly Faceted Multioctahedral Pt Nanocrystals through Controlled Overgrowth. Nano Lett. 2008;8:4043–4047. doi: 10.1021/nl802959b. [DOI] [PubMed] [Google Scholar]
- 27.Boucher M.B., Zugic B., Cladaras G., Kammert J., Marcinkowski M.D., Lawton T.J., Sykes E.C.H., Flytzani-Stephanopoulos M. Single atom alloy surface analogs in Pd0.18Cu15 nanoparticles for selective hydrogenation reactions. Phys. Chem. Chem. Phys. 2013;15:12187. doi: 10.1039/c3cp51538a. [DOI] [PubMed] [Google Scholar]
- 28.Gu H., Yang Z., Gao J., Chang C., Xu B. Heterodimers of Nanoparticles: Formation at a Liquid−Liquid Interface and Particle-Specific Surface Modification by Functional Molecules. J. Am. Chem. Soc. 2005;127:34–35. doi: 10.1021/ja045220h. [DOI] [PubMed] [Google Scholar]
- 29.Liu X., Liu X. Bimetallic nanoparticles: kinetic control matters. Angew. Chem. Int. Ed. 2012;51:3311–3313. doi: 10.1002/anie.201108661. [DOI] [PubMed] [Google Scholar]
- 30.Rubiolo P., Sgorbini B., Liberto E., Cordero C., Bicchi C. Essential oils and volatiles: sample preparation and analysis. A review. Flavour Fragr. J. 2010;25:282–290. doi: 10.1002/ffj.1984. [DOI] [Google Scholar]
- 31.Schmidt E. Production of Essential Oils. In: Baser K.H.C., Buchbauer G., editors. Handbook of Essential Oils: Science, Technology and Applications. 2nd ed. CRC Press; Boca Raton, FL, USA: 2015. [Google Scholar]
- 32.Cowan M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/CMR.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils – A review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 34.Masotti V., Juteau F., Bessiere J.M., Viano J. Seasonal and Phenological Variations of the Essential Oil from the Narrow Endemic Species Artemisia molinieri and Its Biological Activities. J. Agric. Food Chem. 2003;51:7115–7121. doi: 10.1021/jf034621y. [DOI] [PubMed] [Google Scholar]
- 35.Angioni A., Barra A., Coroneo V., Dessi S., Cabras P. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J. Agric. Food Chem. 2006;54:4364–4370. doi: 10.1021/jf0603329. [DOI] [PubMed] [Google Scholar]
- 36.Eloff J.N. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 1998;60:1–8. doi: 10.1016/S0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 37.Parekh J., Jadeja D., Chanda S. Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk. J. Biol. 2005;29:203–210. [Google Scholar]
- 38.National Cancer Institute. Aromatherapy and Essential Oils (PDQ®) - General Information. [(accessed on 14 July 2012)]; Available online: http://www.cancer.gov/cancertopics/pdq/cam/aromatherapy/Health%20Professional/page2.
- 39.Voon H.C., Bhat R., Rusul G. Flower Extracts and Their Essential Oils as Potential Antimicrobial Agents for Food Uses and Pharmaceutical Applications. Compr. Rev. Food Sci. Food Saf. 2011;11:34–55. doi: 10.1111/j.1541-4337.2011.00169.x. [DOI] [Google Scholar]
- 40.Wijekoon M.J.O., Bhat R., Karim A.A. Effect of extraction solvents on the phenolic compounds and antioxidant activities of bunga kantan (Etlingera elatior Jack.) inflorescence. J. Food Compos. Anal. 2011;24:615–619. doi: 10.1016/j.jfca.2010.09.018. [DOI] [Google Scholar]
- 41.Dilworth L.L., Riley C.K., Stennett D.K. Plant. Constituents. Elsevier BV; Oxford, UK: 2017. pp. 61–80. [Google Scholar]
- 42.Silva L., Nelson D., Drummond M., Dufossé L., Glória M. Comparison of hydrodistillation methods for the deodorization of turmeric. Food Res. Int. 2005;38:1087–1096. doi: 10.1016/j.foodres.2005.02.025. [DOI] [Google Scholar]
- 43.Mahadagde P. Techniques Available for the Extraction of Essential Oils from Plants: A Review. Int. J. Res. Appl. Sci. Eng. Technol. 2018;6:2931–2935. doi: 10.22214/ijraset.2018.3643. [DOI] [Google Scholar]
- 44.Rozzi N., Phippen W., Simon J., Singh R. Supercritical Fluid Extraction of Essential Oil Components from Lemon-Scented Botanicals. LWT. 2002;35:319–324. doi: 10.1006/fstl.2001.0873. [DOI] [Google Scholar]
- 45.Pourmortazavi S.M., Saghafi Z., Ehsani A., Yousefi M. Application of supercritical fluids in cholesterol extraction from foodstuffs: A review. J. Food Sci. Technol. 2018;55:2813–2823. doi: 10.1007/s13197-018-3205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexandru L., Cravotto G., Giordana L., Binello A., Chemat F. Ultrasound-assisted extraction of clove buds using batch- and flow-reactors: A comparative study on a pilot scale. Innov. Food Sci. Emerg. Technol. 2013;20:167–172. doi: 10.1016/j.ifset.2013.07.011. [DOI] [Google Scholar]
- 47.Roldán-Gutiérrez J.M., Jimenez J.R., Luquedecastro M., De Castro M.L. Ultrasound-assisted dynamic extraction of valuable compounds from aromatic plants and flowers as compared with steam distillation and superheated liquid extraction. Talanta. 2008;75:1369–1375. doi: 10.1016/j.talanta.2008.01.057. [DOI] [PubMed] [Google Scholar]
- 48.Leonelli C., Veronesi P., Cravotto G. Microwave-assisted Extraction for Bioactive Compounds. Springer Science and Business Media; New York, NY, USA: 2013. p. 238. [Google Scholar]
- 49.Kahriman N., Yayli B., Yücel M., Karaoglu S.A., Yayli N. Chemical constituents and antimicrobial activity of the essential oil from Vicia dadianorum extracted by hydro and microwave distillations. Rec. Nat. Prod. 2012;6:49–56. [Google Scholar]
- 50.Vian M.A., Fernandez X., Visinoni F., Chemat F. Microwave hydrodiffusion and gravity, a new technique for extraction of essential oils. J. Chromatogr. A. 2008;1190:14–17. doi: 10.1016/j.chroma.2008.02.086. [DOI] [PubMed] [Google Scholar]
- 51.Handa S.S., Khanuja S.P.S., Longo G., Rakesh D.D. Extraction technologies for medicinal and aromatic plants. ICS-UNIDO; Trieste, Italy: 2008. pp. 93–106. [Google Scholar]
- 52.Elyemni M., Louaste B., Nechad I., Elkamli T., Bouia A., Taleb M., Chaouch M., Eloutassi N. Extraction of Essential Oils of Rosmarinus officinalis L. by Two Different Methods: Hydrodistillation and Microwave Assisted Hydrodistillation. Sci. World J. 2019:3659432–3659436. doi: 10.1155/2019/3659432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao X., Lv S., Wu Y., Li J., Zhang W., Meng W., Wang C., Meng Q. Volatile components of essential oils extracted from Pu-erh ripe tea by different extraction methods. Int. J. Food Prop. 2017;20:S240–S253. doi: 10.1080/10942912.2017.1295256. [DOI] [Google Scholar]
- 54.Ćujić N., Savikin K., Janković T., Pljevljakusic D., Zdunić G., Ibrić S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016;194:135–142. doi: 10.1016/j.foodchem.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Ferhat M.A., Boukhatem M.N., Hazzit M., Meklati B.Y., Chemat F. Cold pressing hydrodistillation and microwave dry distillation of citrus essential oil from Algeria: A comparative study. Electron. J. Biol. 2016;S1:30–41. [Google Scholar]
- 56.Nematollahi A., Kamali H., Aminimoghadamfarouj N., Golmakani E. The optimization of essential oils supercritical CO2extraction from Lavandula hybrida through static-dynamic steps procedure and semi-continuous technique using response surface method. Pharmacogn. Res. 2015;7:57–65. doi: 10.4103/0974-8490.147209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen P., Liu B., Liu X., Fu J. Ultrasound-assisted extraction and dispersive liquid–liquid microextraction coupled with gas chromatography-mass spectrometry for the sensitive determination of essential oil components in lavender. Anal. Methods. 2019;11:1541–1550. doi: 10.1039/C8AY02687D. [DOI] [Google Scholar]
- 58.Abedi A.-S., Rismanchi M., Shahdoostkhany M., Mohammadi A., Mortazavian A.M. Microwave-assisted extraction of Nigella sativa L. essential oil and evaluation of its antioxidant activity. J. Food Sci. Technol. 2017;54:3779–3790. doi: 10.1007/s13197-017-2718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martins N., Barros L., Santos-Buelga C., Henriques M., Silva S., Ferreira I.C. Decoction, infusion and hydroalcoholic extract of Origanum vulgare L.: Different performances regarding bioactivity and phenolic compounds. Food Chem. 2014;158:73–80. doi: 10.1016/j.foodchem.2014.02.099. [DOI] [PubMed] [Google Scholar]
- 60.Cazella L.N., Glamoclija J., Soković M., Gonçalves J.E., Linde G.A., Colauto N.B., Gazim Z.C. Antimicrobial Activity of Essential Oil of Baccharis dracunculifolia DC (Asteraceae) Aerial Parts at Flowering Period. Front. Plant. Sci. 2019;10:27. doi: 10.3389/fpls.2019.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dhifi W., Bellili S., Jazi S., Bahloul N., Mnif W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines. 2016;3:25. doi: 10.3390/medicines3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Regnault-Roger C., Vincent C., Arnason J. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu. Rev. Èntomol. 2012;57:405–424. doi: 10.1146/annurev-ento-120710-100554. [DOI] [PubMed] [Google Scholar]
- 63.Lalit M. Errata for Plant Growth and Development. Plant. Growth Dev. 2002:ibc1–ibc2. [Google Scholar]
- 64.Eslahi H., Fahimi N., Sardarian A.R. In: Chemical composition of essential oils. In Essential oils in food processing: Chemistry, safety and applications. 1st ed. Hashemi S.M.B., Khaneghah A.M., de Souza Sant’ Ana A., editors. IFT Press, Wiley Blackwell; Hoboken, NJ, USA: 2018. [Google Scholar]
- 65.Swamy M.K., Akhtar M.S., Sinniah U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evidence Based Complement. Altern. Med. 2016;2016:1–21. doi: 10.1155/2016/3012462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bilia A.R., Guccione C., Isacchi B., Righeschi C., Firenzuoli F., Bergonzi M.C. Essential Oils Loaded in Nanosystems: A Developing Strategy for a Successful Therapeutic Approach. Evidence Based Complement. Altern. Med. 2014;2014:1–14. doi: 10.1155/2014/651593. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Manayi A., Nabavi S.M., Daglia M., Jafari S. Natural terpenoids as a promising source for modulation of GABAergic system and treatment of neurological diseases. Pharmacol. Rep. 2016;68:671–679. doi: 10.1016/j.pharep.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 68.Silva L.N., Zimmer K.R., Macedo A.J., Trentin D. Plant Natural Products Targeting Bacterial Virulence Factors. Chem. Rev. 2016;116:9162–9236. doi: 10.1021/acs.chemrev.6b00184. [DOI] [PubMed] [Google Scholar]
- 69.Salini R., Pandian S.K. Interference of quorum sensing in urinary pathogen Serratia marcescens by Anethum graveolens. Pathog. Dis. 2015;73 doi: 10.1093/femspd/ftv038. [DOI] [PubMed] [Google Scholar]
- 70.Martín-Rodríguez A.J., Ticona J.C., Jiménez I.A., Flores N., Fernandez J.J., Bazzocchi I.L. Flavonoids from Piper delineatum modulate quorum-sensing-regulated phenotypes in Vibrio harveyi. Phytochemistry. 2015;117:98–106. doi: 10.1016/j.phytochem.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 71.Grayson D.H. Monoterpenoids (mid-1997 to mid-1999) Nat. Prod. Rep. 2000;17:385–419. doi: 10.1039/a804437f. [DOI] [PubMed] [Google Scholar]
- 72.Shireman R. Essential Fatty Acids. Elsevier BV; London, UK: 2003. pp. 2169–2176. [Google Scholar]
- 73.Iranshahi M. A review of volatile sulfur-containing compounds from terrestrial plants: biosynthesis, distribution and analytical methods. J. Essent. Oil Res. 2012;24:393–434. doi: 10.1080/10412905.2012.692918. [DOI] [Google Scholar]
- 74.Morsy N. Chemical Structure, Quality Indices and Bioactivity of Essential Oil Constituents. Act. Ingred. Aromat. Med. Plants. 2017:175–206. [Google Scholar]
- 75.Safaei-Ghomi J., Ahd A.A. Antimicrobial and antifungal properties of the essential oil and methanol extracts of Eucalyptus largiflorens and Eucalyptus intertexta. Pharmacogn. Mag. 2010;6:172–175. doi: 10.4103/0973-1296.66930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Astani A., Reichling J., Schnitzler P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytotherapy Res. 2009;24:673–679. doi: 10.1002/ptr.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Teixeira B., Marques A., Ramos C., Neng N.R., Nogueira J., Saraiva J.A., Nunes M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crop. Prod. 2013;43:587–595. doi: 10.1016/j.indcrop.2012.07.069. [DOI] [Google Scholar]
- 78.Cosentino S., Tuberoso C., Pisano M.B., Satta M., Mascia V., Arzedi E., Palmas F. In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999;29:130–135. doi: 10.1046/j.1472-765X.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- 79.Edris A. Pharmaceutical and therapeutic Potentials of essential oils and their individual volatile constituents: A review. Phytotherapy Res. 2007;21:308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 80.Oosterhaven K., Poolman B., Smid E. S-carvone as a natural potato sprout inhibiting, fungistatic and bacteristatic compound. Ind. Crop. Prod. 1995;4:23–31. doi: 10.1016/0926-6690(95)00007-Y. [DOI] [Google Scholar]
- 81.Cox S.D., Mann C.M., Markham J.L., Bell H.C., Gustafson J.E., Warmington J.R., Wyllie S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil) J. Appl. Microbiol. 2001;88:170–175. doi: 10.1046/j.1365-2672.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 82.Balouiri M., Sadiki M., Ibnsouda S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2015;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weinstein M.P., Patel J.B., Burnham C.A., Campeau S., Conville P.S., Limbago B., Mathers A., Mazzulli T., Munro S., Eliopoulos G.M., et al. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically: Approved Standard—8th Edition; CLSI Document M07-A8; Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2009. [Google Scholar]
- 84.Canillac N., Mourey A. Antibacterial activity of the essential oil of Picea excelsa on Listeria, Staphylococcus aureus and coliform bacteria. Food Microbiol. 2001;18:261–268. doi: 10.1006/fmic.2000.0397. [DOI] [Google Scholar]
- 85.Zhang J., Ye K.-P., Zhang X., Pan D.-D., Sun Y.-Y., Cao J.-X. Antibacterial Activity and Mechanism of Action of Black Pepper Essential Oil on Meat-Borne Escherichia coli. Front. Microbiol. 2017;7:4168. doi: 10.3389/fmicb.2016.02094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y., Liu X., Wang Y., Jiang P., Quek S.Y. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016;59:282–289. doi: 10.1016/j.foodcont.2015.05.032. [DOI] [Google Scholar]
- 87.Díez J.G., Alheiro J., Falco V., Fraqueza M.J., Patarata L.A.D.S.C. Chemical characterization and antimicrobial properties of herbs and spices essential oils against pathogens and spoilage bacteria associated to dry-cured meat products. J. Essent. Oil Res. 2016;29:117–125. doi: 10.1080/10412905.2016.1212738. [DOI] [Google Scholar]
- 88.Ibrahim N.A., El-Hawary S.S., Mohammed M.M., Farid M.A., Abdel-Wahed N.A., Ali M.A., El-Abd E.A. Chemical Composition, Antiviral against avian Influenza (H5N1) Virus and Antimicrobial activities of the Essential Oils of the Leaves and Fruits of Fortunella margarita, Lour Swingle, Growing in Egypt. J. Appl. Pharm. Sci. 2015;5:6–12. [Google Scholar]
- 89.Mitropoulou G., Bardouki H., Vamvakias M., Panas P., Paraskeuas P., Rangou A., Kourkoutas I. Antimicrobial activity of Pistacia lentiscus and Fortunella margarita essential oils against Saccharomyces cerevisiae and Aspergillus niger in fruit juices. J. Biotechnol. 2018;280:S62–S63. doi: 10.1016/j.jbiotec.2018.06.204. [DOI] [Google Scholar]
- 90.Dos Santos N.O., Mariane B., Lago J.H.G., Sartorelli P., Rosa W., Soares M.G., Silva A., Lorenzi H., Vallim M.A., Pascon R. Assessing the Chemical Composition and Antimicrobial Activity of Essential Oils from Brazilian Plants—Eremanthus erythropappus (Asteraceae), Plectrantuns barbatus, and P. amboinicus (Lamiaceae) Molecules. 2015;20:8440–8452. doi: 10.3390/molecules20058440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Novy P., Davidova H., Serrano-Rojero C.S., Rondevaldova J., Pulkrabek J., Kokoska L. Composition and Antimicrobial Activity of Euphrasia rostkoviana Hayne Essential Oil. Evidence Based Complement. Altern. Med. 2015;2015:1–5. doi: 10.1155/2015/734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu X.D., Xie J.H., Wang Y.H., Li Y.C., Mo Z.Z., Zheng Y., Su J.Y., Liang Y.E., Liang J.Z., Su Z.R., et al. Selective Antibacterial Activity of Patchouli Alcohol Against Helicobacter pylori Based on Inhibition of Urease. Phytotherapy Res. 2014;29:67–72. doi: 10.1002/ptr.5227. [DOI] [PubMed] [Google Scholar]
- 93.Das P., Dutta S., Begum J., Anwar N. Antibacterial and Antifungal Activity Analysis of Essential Oil of Pogostemon cablin (Blanco) Benth. Bangladesh J. Microbiol. 2016;30:7–10. doi: 10.3329/bjm.v30i1-2.28446. [DOI] [Google Scholar]
- 94.Crevelin E.J., Caixeta S.C., Dias H.J., Groppo M., Cunha W., Martins C.H.G., Crotti A.E. Antimicrobial Activity of the Essential Oil of Plectranthus neochilus against Cariogenic Bacteria. Evidence Based Complement. Altern. Med. 2015;2015:1–6. doi: 10.1155/2015/102317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Crevelin E.J., Aguiar G.P., Lima K.E., Severiano M., Groppo M., Ambrósio S.R. Antifungal activity of the essential oils of Plectranthus neochilus (Lamiaceae) and Tagetes erecta (Asteraceae) cultivated in brazil. Int. J. Complement. Altern. Med. 2018;11:1. doi: 10.15406/ijcam.2018.11.00343. [DOI] [Google Scholar]
- 96.Beatovic D., Krstic-Milosevic D., Trifunovic S., Šiljegović J., Glamočlija J., Ristić M., Jelačić S. Chemical composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Rec. Nat. Prod. 2015;9:62–75. [Google Scholar]
- 97.Helal I., El-Bessoumy A., Al-Bataineh E., Joseph M.R.P., Rajagopalan P., Chandramoorthy H.C., Ahmed S.B.H. Antimicrobial Efficiency of Essential Oils from Traditional Medicinal Plants of Asir Region, Saudi Arabia, over Drug Resistant Isolates. BioMed Res. Int. 2019;2019:8928306–8928309. doi: 10.1155/2019/8928306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cui H., Zhang X., Zhou H., Zhao C., Lin L. Antimicrobial activity and mechanisms of Salvia sclarea essential oil. Bot. Stud. 2015;56:16. doi: 10.1186/s40529-015-0096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Antimicrobial activity of the essential oil of Thymus kotschyanus grown wild in Iran. Int. J. Biosci. IJB. 2015;6:239–248. [Google Scholar]
- 100.Mahboubi M., HeidaryTabar R., Mahdizadeh E., Hosseini H. Antimicrobial activity and chemical composition of Thymus species and Zataria multiflora essential oils. Agric. Nat. Resour. 2017;51:395–401. doi: 10.1016/j.anres.2018.02.001. [DOI] [Google Scholar]
- 101.Venturi C.R., Danielli L.J., Klein F., Apel M.A., Montanha J.A., Bordignon S.A.L., Roehe P., Fuentefria A., Henriques A.T. Chemical analysis and in vitro antiviral and antifungal activities of essential oils from Glechon spathulata and Glechon marifolia. Pharm. Biol. 2014;53:682–688. doi: 10.3109/13880209.2014.936944. [DOI] [PubMed] [Google Scholar]
- 102.Bouzabata A., Cabral C., Gonçalves M.J., Cruz M.T., Bighelli A., Cavaleiro C., Casanova J., Tomi F., Salgueiro L. Myrtus communis L. as source of a bioactive and safe essential oil. Food Chem. Toxicol. 2015;75:166–172. doi: 10.1016/j.fct.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 103.Abbaszadegan A., Nabavizadeh M., Gholami A., Sheikhiani R., Shokouhi M.M., Shams M.S., Ghasemi Y. Chemical constituent and antimicrobial effect of essential oil from Myrtus communis leaves on microorganisms involved in persistent endodontic infection compared to two common endodontic irrigants: An in vitro study. J. Conserv. Dent. 2014;17:449–453. doi: 10.4103/0972-0707.139836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Papajani V., Haloci E., Goci E., Shkreli R., Manfredini S. Evaluation of antifungal activity of Origanum vulgare and Rosmarinus officinalis essential oil before and after inclusion in 𝛽-cyclodextrine. Int. J. Pharm. Pharm. Sci. 2015;7:270–273. [Google Scholar]
- 105.Puškárová A., Bučková M., Kraková L., Pangallo D., Kozics K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017;7:8211. doi: 10.1038/s41598-017-08673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Latifah-Munirah B., Himratul-Aznita W.H., Zain N.M. Eugenol, an essential oil of clove, causes disruption to the cell wall of Candida albicans (ATCC 14053) Front. Life Sci. 2015;8:231–240. doi: 10.1080/21553769.2015.1045628. [DOI] [Google Scholar]
- 107.Radünz M., Da Trindade M.L.M., Camargo T.M., Radünz A.L., Borges C.D., Gandra E.A., Helbig E. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem. 2019;276:180–186. doi: 10.1016/j.foodchem.2018.09.173. [DOI] [PubMed] [Google Scholar]
- 108.Souza C.M.C., Junior S.A.P., Moraes T.D.S., Damasceno J.L., Mendes S.A., Dias H.J., Stefani R., Tavares D.C., Martins C.H.G., Crotti A.E., et al. Antifungal activity of plant-derived essential oils on Candida tropicalis planktonic and biofilms cells. Med. Mycol. 2016;54:515–523. doi: 10.1093/mmy/myw003. [DOI] [PubMed] [Google Scholar]
- 109.Boukhatem M.N., Kameli A., Saidi F. Essential oil of Algerian rose-scented geranium (Pelargonium graveolens): Chemical composition and antimicrobial activity against food spoilage pathogens. Food Control. 2013;34:208–213. doi: 10.1016/j.foodcont.2013.03.045. [DOI] [Google Scholar]
- 110.Roy S., Chaurvedi P., Chowdhary A. Evaluation of antiviral activity of essential oil of Trachyspermum ammi against Japanese encephalitis virus. Pharmacogn. Res. 2015;7:263–267. doi: 10.4103/0974-8490.157977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vitali L.A., Beghelli D., Nya P.C.B., Bistoni O., Cappellacci L., Damiano S., Lupidi G., Maggi F., Orsomando G., Papa F., et al. Diverse biological effects of the essential oil from Iranian Trachyspermum ammi. Arab. J. Chem. 2016;9:775–786. doi: 10.1016/j.arabjc.2015.06.002. [DOI] [Google Scholar]
- 112.Brand Y.M., Roa-Linares V.C., Betancur-Galvis L.A., Durán-García D.C., Stashenko E. Antiviral activity of Colombian Labiatae and Verbenaceae family essential oils and monoterpenes on Human Herpes viruses. J. Essent. Oil Res. 2015;28:1–8. doi: 10.1080/10412905.2015.1093556. [DOI] [Google Scholar]
- 113.Tardugno R., Serio A., Pellati F., D’Amato S., Lopez C.C., Bellardi M.G., Di Vito M., Savini V., Paparella A., Benvenuti S. Lavandula x intermedia and Lavandula angustifolia essential oils: phytochemical composition and antimicrobial activity against foodborne pathogens. Nat. Prod. Res. 2018;33:3330–3335. doi: 10.1080/14786419.2018.1475377. [DOI] [PubMed] [Google Scholar]
- 114.Garzoli S., Petralito S., Ovidi E., Turchetti G., Masci V.L., Tiezzi A., Trilli J., Cesa S., Casadei M.A., Giacomello P., et al. Lavandula x intermedia essential oil and hydrolate: Evaluation of chemical composition and antibacterial activity before and after formulation in nanoemulsion. Ind. Crop. Prod. 2020;145:112068. doi: 10.1016/j.indcrop.2019.112068. [DOI] [Google Scholar]
- 115.Vinciguerra V., Rojas F., Tedesco V., Giusiano G., Angiolella L. Chemical characterization and antifungal activity of Origanum vulgare, Thymus vulgaris essential oils and carvacrol against Malassezia furfur. Nat. Prod. Res. 2018;33:3273–3277. doi: 10.1080/14786419.2018.1468325. [DOI] [PubMed] [Google Scholar]
- 116.Almeida E.T.D.C., De Souza E.L., Guedes J.P.D.S., Barbosa I.M., De Sousa C.P., Castellano L.R.C., Magnani M., De Souza E.L. Mentha piperita L. essential oil inactivates spoilage yeasts in fruit juices through the perturbation of different physiological functions in yeast cells. Food Microbiol. 2019;82:20–29. doi: 10.1016/j.fm.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 117.Saharkhiz M.J., Motamedi M., Zomorodian K., Pakshir K., Miri R., Hemyari K. Chemical Composition, Antifungal and Antibiofilm Activities of the Essential Oil of Mentha piperita L. ISRN Pharm. 2012;2012:1–6. doi: 10.5402/2012/718645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bharat C.S., Praveen D. Evaluation of in vitro antimicrobial potential and phytochemical analysis of spruce, cajeput and jamrosa essential oil against clinical isolates. Int. J. Green Pharm. 2016;10:27–42. [Google Scholar]
- 119.Feng J., Zhang S., Shi W., Zubcevik N., Miklossy J., Zhang Y. Selective Essential Oils from Spice or Culinary Herbs Have High Activity against Stationary Phase and Biofilm Borrelia burgdorferi. Front. Med. 2017;4:169. doi: 10.3389/fmed.2017.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Condò C., Anacarso I., Sabia C., Iseppi R., Anfelli I., Forti L., De Niederhäusern S., Bondi M., Messi P. Antimicrobial activity of spices essential oils and its effectiveness on mature biofilms of human pathogens. Nat. Prod. Res. 2018;34:567–574. doi: 10.1080/14786419.2018.1490904. [DOI] [PubMed] [Google Scholar]
- 121.Mahboubi M., Mahboubi M. Chemical Composition, Antimicrobial and Antioxidant Activities of Eugenia caryophyllata Essential Oil. J. Essent. Oil Bear. Plants. 2015;18:967–975. doi: 10.1080/0972060X.2014.884779. [DOI] [Google Scholar]
- 122.Aldoghaim F.S., Flematti G., Hammer K. Antimicrobial Activity of Several Cineole-Rich Western Australian Eucalyptus Essential Oils. Microorganisms. 2018;6:122. doi: 10.3390/microorganisms6040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wei Z.-F., Zhao R.-N., Dong L.-J., Zhao X., Su J.-X., Zhao M., Li L., Bian Y.-J., Zhang L.-J. Dual-cooled solvent-free microwave extraction of Salvia officinalis L. essential oil and evaluation of its antimicrobial activity. Ind. Crop. Prod. 2018;120:71–76. doi: 10.1016/j.indcrop.2018.04.058. [DOI] [Google Scholar]
- 124.Damjanovic-Vratnica B., Ðakov T., Sukovic D., Damjanovic J. Chemical Composition and Antimicrobial Activity of Essential Oil of Wild-Growing Salvia officinalis L. from Montenegro. J. Essent. Oil Bear. Plants. 2008;11:79–89. doi: 10.1080/0972060X.2008.10643602. [DOI] [Google Scholar]
- 125.Mertas A., Garbusińska A., Szliszka E., Jureczko A., Kowalska M., Krol W. The Influence of Tea Tree Oil (Melaleuca alternifolia) on Fluconazole Activity against Fluconazole-Resistant Candida albicans Strains. BioMed Res. Int. 2015;2015:590470–590479. doi: 10.1155/2015/590470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brun P., Bernabè G., Filippini R., Piovan A. In Vitro Antimicrobial Activities of Commercially Available Tea Tree (Melaleuca alternifolia) Essential Oils. Curr. Microbiol. 2018;76:108–116. doi: 10.1007/s00284-018-1594-x. [DOI] [PubMed] [Google Scholar]
- 127.Khalil N., Mohamed L.A., Fikry S., Singab A.N., Salama O. Chemical composition and antimicrobial activity of the essential oils of selected Apiaceous fruits. Futur. J. Pharm. Sci. 2018;4:88–92. doi: 10.1016/j.fjps.2017.10.004. [DOI] [Google Scholar]
- 128.Yildiz H. Chemical Composition, Antimicrobial, and Antioxidant Activities of Essential Oil and Ethanol Extract of Coriandrum sativum L. Leaves from Turkey. Int. J. Food Prop. 2015;19:1593–1603. doi: 10.1080/10942912.2015.1092161. [DOI] [Google Scholar]
- 129.Radaelli M., Da Silva B.P., Weidlich L., Hoehne L., Flach A., Da Costa L.A.M.A., Ethur E.M. Antimicrobial activities of six essential oils commonly used as condiments in Brazil against Clostridium perfringens. Braz. J. Microbiol. 2016;47:424–430. doi: 10.1016/j.bjm.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lorenzo-Leal A.C., Palou E., López-Malo A., Bach H. Antimicrobial, Cytotoxic, and Anti-Inflammatory Activities of Pimenta dioica and Rosmarinus officinalis Essential Oils. BioMed Res. Int. 2019;2019:1639726. doi: 10.1155/2019/1639726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bajer T., Šilha D., Ventura K., Bajerová P. Composition and antimicrobial activity of the essential oil, distilled aromatic water and herbal infusion from Epilobium parviflorum Schreb. Ind. Crop. Prod. 2017;100:95–105. doi: 10.1016/j.indcrop.2017.02.016. [DOI] [Google Scholar]
- 132.Guleria S., Tiku A.K., Koul A., Gupta S., Singh G., Razdan V.K. Antioxidant and Antimicrobial Properties of the Essential Oil and Extracts of Zanthoxylum alatum Grown in North-Western Himalaya. Sci. World J. 2013;2013:1–9. doi: 10.1155/2013/790580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Manssouri M., Znini M., Majidi L. Studies on the antioxidant activity of essential oil and various extracts of Ammodaucus leucotrichus Coss. & Dur. Fruits from Morocco. J. Taibah Univ. Sci. 2020;14:124–130. [Google Scholar]
- 134.Sarikurkcu C., Tepe B., Daferera D., Polissiou M., Harmandar M. Studies on the antioxidant activity of the essential oil and methanol extract of Marrubium globosum subsp. globosum (lamiaceae) by three different chemical assays. Bioresour. Technol. 2008;99:4239–4246. doi: 10.1016/j.biortech.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 135.Toscano-Garibay J.D., Arriaga-Alba M., Sánchez-Navarrete J., Mendoza-García M., Flores-Estrada J.J., Moreno-Eutimio M.A., Espinosa-Aguirre J.J., González-Ávila M., Ruiz-Pérez N.J. Antimutagenic and antioxidant activity of the essential oils of Citrus sinensis and Citrus latifolia. Sci. Rep. 2017;7:11479. doi: 10.1038/s41598-017-11818-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Elaguel A., Kallel I., Gargouri B., Ben Amor I., Hadrich B., Ben Messaoud E., Gdoura R., Lassoued S., Gargouri A. Lawsonia inermis essential oil: extraction optimization by RSM, antioxidant activity, lipid peroxydation and antiproliferative effects. Lipids Heal. Dis. 2019;18:1–11. doi: 10.1186/s12944-019-1141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Benyoucef F., Dib M.E.A., Arrar Z., Costa J., Muselli A. Synergistic Antioxidant Activity and Chemical Composition of Essential Oils From Thymus fontanesii, Artemisia herba-alba and Rosmarinus officinalis. J. Appl. Biotechnol. Rep. 2018;5:151–156. doi: 10.29252/JABR.05.04.03. [DOI] [Google Scholar]
- 138.Khanal S., Bhattarai N., McMaster D., Bahena D., Velázquez-Salazar J.J., Yacaman M.J. Highly monodisperse multiple twinned AuCu-Pt trimetallic nanoparticles with high index surfaces. Phys. Chem. Chem. Phys. 2014;16:16278–16283. doi: 10.1039/C4CP02208D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Toshima N., Ito R., Matsushita T., Shiraishi Y. Trimetallic nanoparticles having a Au-core structure. Catal. Today. 2007;122:239–244. doi: 10.1016/j.cattod.2007.03.013. [DOI] [Google Scholar]
- 140.Tsai S.-H., Liu Y.-H., Wu P.-L., Yeh C.-S. Preparation of Au–Ag–Pd trimetallic nanoparticles and their application as catalysts Electronic supplementary information (ESI) available: UV-vis spectra and EDX analysis of Au–Ag–Pd colloidal suspensions. [(accessed on 24 February 2020)];J. Mater. Chem. 2003 13:978–980. doi: 10.1039/b300952a. Available online: http://www.rsc.org/suppdata/jm/b3/b300952a/ [DOI] [Google Scholar]
- 141.Tang S., Vongehr S., Wang Y., Cui J., Wang X., Meng X. Versatile synthesis of high surface area multi-metallic nanosponges allowing control over nanostructure and alloying for catalysis and SERS detection. J. Mater. Chem. A. 2014;2:3648–3660. doi: 10.1039/C3TA14541G. [DOI] [Google Scholar]
- 142.Li T., Vongehr S., Tang S., Dai Y., Huang X., Meng X. Scalable Synthesis of Ag Networks with Optimized Sub-monolayer Au-Pd Nanoparticle Covering for Highly Enhanced SERS Detection and Catalysis. Sci. Rep. 2016;6:37092. doi: 10.1038/srep37092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang X., Li F., Zhang Y., Bond A.M., Zhang J. Stannate derived bimetallic nanoparticles for electrocatalytic CO2 reduction. J. Mater. Chem. A. 2018;6:7851–7858. doi: 10.1039/C8TA02429D. [DOI] [Google Scholar]
- 144.Guo H., Li H., Jarvis K., Wan H., Kunal P., Dunning S.G., Liu Y., Henkelman G., He S. Microwave-Assisted Synthesis of Classically Immiscible Ag–Ir Alloy Nanoparticle Catalysts. ACS Catal. 2018;8:11386–11397. doi: 10.1021/acscatal.8b02103. [DOI] [Google Scholar]
- 145.Wu M.-L., Lai L.-B. Synthesis of Pt/Ag bimetallic nanoparticles in water-in-oil microemulsions. Colloids Surfaces A Physicochem. Eng. Asp. 2004;244:149–157. doi: 10.1016/j.colsurfa.2004.06.027. [DOI] [Google Scholar]
- 146.Allaedini G., Tasirin S.M., Aminayi P. Synthesis of Fe–Ni–Ce trimetallic catalyst nanoparticles via impregnation and co-precipitation and their application to dye degradation. Chem. Pap. 2016;70:231–242. doi: 10.1515/chempap-2015-0190. [DOI] [Google Scholar]
- 147.Ding K., Zhao Y., Liu L., Li Y., Zhao J., Chen Y., Niu J., Wang Q. Preparation of trimetallic composite nanoparticles PdxNiyFez by a pyrolysis method in the solvent of ionic liquids for ethanol oxidation reaction. Int. J. Electrochem. Sci. 2015;10:6768–6779. [Google Scholar]
- 148.Wu Y., Wang Z., Chen S., Guo X., Liu Z. One-step hydrothermal synthesis of silver nanoparticles loaded on N-doped carbon and application for catalytic reduction of 4-nitrophenol. RSC Adv. 2015;5:87151–87156. doi: 10.1039/C5RA07589K. [DOI] [Google Scholar]
- 149.Li P., Xin Y., Li Q., Wang Z., Zhang Z., Zheng L. Ce–Ti Amorphous Oxides for Selective Catalytic Reduction of NO with NH3: Confirmation of Ce–O–Ti Active Sites. Environ. Sci. Technol. 2012;46:9600–9605. doi: 10.1021/es301661r. [DOI] [PubMed] [Google Scholar]
- 150.Khodadadi B. TiO2/SiO2 prepared via facile sol-gel method as an ideal support for green synthesis of Ag nanoparticles using Oenothera biennis extract and their excellent catalytic performance in the reduction of 4-nitrophenol. Nanochem Res. 2017;2:140–150. [Google Scholar]
- 151.Bhunia K., Khilari S., Pradhan D. Trimetallic PtAuNi alloy nanoparticles as an efficient electrocatalyst for the methanol electrooxidation reaction. Dalton Trans. 2017;46:15558–15566. doi: 10.1039/C7DT02608K. [DOI] [PubMed] [Google Scholar]
- 152.Ashok A., Kumar A., Tarlochan F. Surface Alloying in Silver-Cobalt through a Second Wave Solution Combustion Synthesis Technique. Nanomaterials. 2018;8:604. doi: 10.3390/nano8080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li H., Chen Q., Zhao J., Urmila K. Enhancing the antimicrobial activity of natural extraction using the synthetic ultrasmall metal nanoparticles. Sci. Rep. 2015;5:11033. doi: 10.1038/srep11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Armentano I., Arciola C.R., Fortunati E., Ferrari D., Mattioli S., Amoroso C.F., Rizzo J., Kenny J.M., Imbriani M., Visai L. The Interaction of Bacteria with Engineered Nanostructured Polymeric Materials: A Review. Sci. World J. 2014;2014:1–18. doi: 10.1155/2014/410423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Luan B., Huynh T., Zhou R. Complete wetting of graphene by biological lipids. Nanoscale. 2016;8:5750–5754. doi: 10.1039/C6NR00202A. [DOI] [PubMed] [Google Scholar]
- 156.Prabhu S., Poulose E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012;2:32. doi: 10.1186/2228-5326-2-32. [DOI] [Google Scholar]
- 157.Dakal T.C., Kumar A., Majumdar R.S., Yadav V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016;7:720654. doi: 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Raza M.A., Kanwal Z., Rauf A., Sabri A.N., Riaz S., Naseem S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials. 2016;6:74. doi: 10.3390/nano6040074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McShan D., Zhang Y., Deng H., Ray P.C., Yu H. Synergistic Antibacterial Effect of Silver Nanoparticles Combined with Ineffective Antibiotics on Drug Resistant Salmonella typhimurium DT104. J. Environ. Sci. Heal. Part C. 2015;33:369–384. doi: 10.1080/10590501.2015.1055165. [DOI] [PubMed] [Google Scholar]
- 160.D’Agostino A., Taglietti A., Desando R., Bini M., Patrini M., Dacarro G., Cucca L., Pallavicini P., Grisoli P. Bulk Surfaces Coated with Triangular Silver Nanoplates: Antibacterial Action Based on Silver Release and Photo-Thermal Effect. Nanomaterials. 2017;7:7. doi: 10.3390/nano7010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ramalingam B., Parandhaman T., Das S.K. Antibacterial Effects of Biosynthesized Silver Nanoparticles on Surface Ultrastructure and Nanomechanical Properties of Gram-Negative Bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces. 2016;8:4963–4976. doi: 10.1021/acsami.6b00161. [DOI] [PubMed] [Google Scholar]
- 162.Aazam E.S., Zaheer Z. Growth of Ag-nanoparticles in an aqueous solution and their antimicrobial activities against Gram positive, Gram negative bacterial strains and Candida fungus. Bioprocess. Biosyst. Eng. 2016;39:575–584. doi: 10.1007/s00449-016-1539-3. [DOI] [PubMed] [Google Scholar]
- 163.Katas H., Lim C.S., Azlan A.Y.H.N., Buang F., Busra M.F.M. Antibacterial activity of biosynthesized gold nanoparticles using biomolecules from Lignosus rhinocerotis and chitosan. Saudi Pharm. J. 2018;27:283–292. doi: 10.1016/j.jsps.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Folorunso A., Akintelu S., Oyebamiji A.K., Ajayi S., Abiola B., Abdusalam I., Morakinyo A. Biosynthesis, characterization and antimicrobial activity of gold nanoparticles from leaf extracts of Annona muricata. J. Nanostructure Chem. 2019;9:111–117. doi: 10.1007/s40097-019-0301-1. [DOI] [Google Scholar]
- 165.Zheng K., Setyawati M.I., Leong D.T., Xie J. Antimicrobial Gold Nanoclusters. ACS Nano. 2017;11:6904–6910. doi: 10.1021/acsnano.7b02035. [DOI] [PubMed] [Google Scholar]
- 166.Jahnke J.P., Cornejo J.A., Sumner J.J., Schuler A.J., Atanassov P., Ista L.K. Conjugated gold nanoparticles as a tool for probing the bacterial cell envelope: The case of Shewanella oneidensis MR-1. Biointerphases. 2016;11:011003. doi: 10.1116/1.4939244. [DOI] [PubMed] [Google Scholar]
- 167.Narayanasamy P., Switzer B.L., Britigan B.E. Prolonged-acting, Multi-targeting Gallium Nanoparticles Potently Inhibit Growth of Both HIV and Mycobacteria in Co-Infected Human Macrophages. Sci. Rep. 2015;5:8824. doi: 10.1038/srep08824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Syed B., Karthik N., Bhat P., Bisht N., Prasad A., Satish S., Prasad M.N. Phyto-biologic bimetallic nanoparticles bearing antibacterial activity against human pathogens. J. King Saud Univ. Sci. 2019;31:798–803. doi: 10.1016/j.jksus.2018.01.008. [DOI] [Google Scholar]
- 169.Dobrucka R., Dlugaszewska J. Antimicrobial activity of the biogenically synthesized core–shell Cu@Pt nanoparticles. Saudi Pharm. J. 2018;26:643–650. doi: 10.1016/j.jsps.2018.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Aleksandr L., Alexander P., Bakina O.V., Sergey K., Irena G. Synthesis of antimicrobial AlOOH–Ag composite nanostructures by water oxidation of bimetallic Al–Ag nanoparticles. RSC Adv. 2018;8:36239–36244. doi: 10.1039/C8RA04173C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lozhkomoev A., Lerner M.I., Pervikov A., Kazantsev S.O., Fomenko A.N. Development of Fe/Cu and Fe/Ag Bimetallic Nanoparticles for Promising Biodegradable Materials with Antimicrobial Effect. Nanotechnol. Russ. 2018;13:18–25. doi: 10.1134/S1995078018010081. [DOI] [Google Scholar]
- 172.Vaseghi Z., Tavakoli O., Nematollahzadeh A. Rapid biosynthesis of novel Cu/Cr/Ni trimetallic oxide nanoparticles with antimicrobial activity. J. Environ. Chem. Eng. 2018;6:1898–1911. doi: 10.1016/j.jece.2018.02.038. [DOI] [Google Scholar]
- 173.Alzahrani K.E., Niazy A., Alswieleh A.M., Wahab R., El-Toni A.M., Alghamdi H. Antibacterial activity of trimetal (CuZnFe) oxide nanoparticles. Int. J. Nanomed. 2017;13:77–87. doi: 10.2147/IJN.S154218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yadav N., Jaiswal A.K., Dey K.K., Yadav V.B., Nath G., Srivastava A.K., Yadav R.R. Trimetallic Au/Pt/Ag based nanofluid for enhanced antibacterial response. Mater. Chem. Phys. 2018;218:10–17. doi: 10.1016/j.matchemphys.2018.07.016. [DOI] [Google Scholar]
- 175.Akbar A., Sadiq M.B., Ali I., Muhammad N., Rehman Z., Khan M.N., Muhammad J., Khan S.A., Rehman F.U., Anal A.K. Synthesis and antimicrobial activity of zinc oxide nanoparticles against foodborne pathogens Salmonella typhimurium and Staphylococcus aureus. Biocatal. Agric. Biotechnol. 2019;17:36–42. doi: 10.1016/j.bcab.2018.11.005. [DOI] [Google Scholar]
- 176.Nabila M.I., Kannabiran K. Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatal. Agric. Biotechnol. 2018;15:56–62. doi: 10.1016/j.bcab.2018.05.011. [DOI] [Google Scholar]
- 177.Nguyen N.-Y.T., Grelling N., Wetteland C.L., Rosario R., Liu H. Antimicrobial Activities and Mechanisms of Magnesium Oxide Nanoparticles (nMgO) against Pathogenic Bacteria, Yeasts, and Biofilms. Sci. Rep. 2018;8:16260. doi: 10.1038/s41598-018-34567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Azizi-Lalabadi M., Ehsani A., Divband B., Alizadeh-Sani M. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-54025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Artiga-Artigas M., Guerra-Rosas M., Morales-Castro J., Salvia-Trujillo L., Martin-Belloso O. Influence of essential oils and pectin on nanoemulsion formulation: A ternary phase experimental approach. Food Hydrocoll. 2018;81:209–219. doi: 10.1016/j.foodhyd.2018.03.001. [DOI] [Google Scholar]
- 180.Sotelo-Boyás M., Correa-Pacheco Z., Bautista-Baños S., Corona-Rangel M. Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. LWT. 2017;77:15–20. doi: 10.1016/j.lwt.2016.11.022. [DOI] [Google Scholar]
- 181.Ben Jemaa M., Falleh H., Serairi R., Neves M.A., Snoussi M., Isoda H., Nakajima M., Ksouri R. Nanoencapsulated Thymus capitatus essential oil as natural preservative. Innov. Food Sci. Emerg. Technol. 2018;45:92–97. doi: 10.1016/j.ifset.2017.08.017. [DOI] [Google Scholar]
- 182.Alcaraz A.G.L., Cortez-Rocha M.O., Velá Zquez-Contreras C.A., Acosta-Silva A.L., Santacruz-Ortega H.D.C., Burgos-Hernandez A., Argü Argüelles-Monal W.M. Enhanced Antifungal Effect of Chitosan/Pepper Tree (Schinus molle) Essential Oil Bionanocomposites on the Viability of Aspergillus parasiticus Spores. J. Nanomater. 2016;2016:1–10. doi: 10.1155/2016/6060137. [DOI] [Google Scholar]
- 183.Nasseri M., Golmohammadzadeh S., Arouiee H., Jaafari M.R., Neamati H. Antifungal activity of Zataria multiflora essential oil-loaded solid lipid nanoparticles in-vitro condition. Iran. J. Basic Med. Sci. 2016;19:1231. [PMC free article] [PubMed] [Google Scholar]
- 184.Kujur A., Kiran S., Dubey N., Prakash B. Microencapsulation of Gaultheria procumbens essential oil using chitosan-cinnamic acid microgel: Improvement of antimicrobial activity, stability and mode of action. LWT. 2017;86:132–138. doi: 10.1016/j.lwt.2017.07.054. [DOI] [Google Scholar]
- 185.Benjemaa M., Neves M.A., Falleh H., Isoda H., Ksouri R., Nakajima M. Nanoencapsulation of Thymus capitatus essential oil: Formulation process, physical stability characterization and antibacterial efficiency monitoring. Ind. Crop. Prod. 2018;113:414–421. doi: 10.1016/j.indcrop.2018.01.062. [DOI] [Google Scholar]
- 186.Jamil B., Abbasi R., Abbasi S., Khan S.U., Ihsan A., Javed S., Bokhari H., Imran M. Encapsulation of Cardamom Essential Oil in Chitosan Nano-composites: In-vitro Efficacy on Antibiotic-Resistant Bacterial Pathogens and Cytotoxicity Studies. Front. Microbiol. 2016;7:1277. doi: 10.3389/fmicb.2016.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ferreira T.P., Haddi K., Corrêa R.F.T., Zapata V.L.B., Piau T.B., Souza L.F.N., Santos S.-M.G., Oliveira E.E., Jumbo L.O.V., Ribeiro B., et al. Prolonged mosquitocidal activity of Siparuna guianensis essential oil encapsulated in chitosan nanoparticles. PLoS Neglected Trop. Dis. 2019;13:e0007624. doi: 10.1371/journal.pntd.0007624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rai M., Paralikar P., Jogee P., Agarkar G., Ingle A.P., Derita M., Zacchino S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Int. J. Pharm. 2017;519:67–78. doi: 10.1016/j.ijpharm.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 189.Ferreira C.D., Nunes I.L. Oil nanoencapsulation: Development, application, and incorporation into the food market. Nanoscale Res. Lett. 2019;14:9. doi: 10.1186/s11671-018-2829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tamayo L., Zapata P., Vejar N., Azocar M., Gulppi M., Zhou X., Thompson G., Rabagliati F., Páez M.A. Release of silver and copper nanoparticles from polyethylene nanocomposites and their penetration into Listeria monocytogenes. Mater. Sci. Eng. C. 2014;40:24–31. doi: 10.1016/j.msec.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 191.Wu J., Shu Q., Niu Y., Jiao Y., Chen Q. Preparation, Characterization, and Antibacterial Effects of Chitosan Nanoparticles Embedded with Essential Oils Synthesized in an Ionic Liquid Containing System. J. Agric. Food Chem. 2018;66:7006–7014. doi: 10.1021/acs.jafc.8b01428. [DOI] [PubMed] [Google Scholar]
- 192.Oroojalian F., Orafaee H., Azizi M. Synergistic antibaterial activity of medicinal plants essential oils with biogenic silver nanoparticles. Nanomed. J. 2017;4:237–244. [Google Scholar]
- 193.Kalagatur N.K., Ghosh O.S.N., Sundararaj N., Mudili V. Antifungal Activity of Chitosan Nanoparticles Encapsulated With Cymbopogon martinii Essential Oil on Plant Pathogenic Fungi Fusarium graminearum. Front. Pharmacol. 2018;9:610. doi: 10.3389/fphar.2018.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Heydari M.A., Mobini M., Salehi M. The Synergic Activity of Eucalyptus Leaf Oil and Silver Nanoparticles Against Some Pathogenic Bacteria. Arch. Pediatr. Infect. Dis. 2017;5:61654. [Google Scholar]
- 195.Manju S., Malaikozhundan B., Sekar V., Shanthi S., Jaishabanu A., Ekambaram P., Vaseeharan B. Antibacterial, antibiofilm and cytotoxic effects of Nigella sativa essential oil coated gold nanoparticles. Microb. Pathog. 2016;91:129–135. doi: 10.1016/j.micpath.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 196.Morsy M., Khalaf H.H., Sharoba A.M., Cutter C.N., El-Tanahi H.H. Incorporation of Essential Oils and Nanoparticles in Pullulan Films to Control Foodborne Pathogens on Meat and Poultry Products. J. Food Sci. 2014;79:M675–M684. doi: 10.1111/1750-3841.12400. [DOI] [PubMed] [Google Scholar]
- 197.Hossain F., Follett P., Vu K.D., Harich M., Salmieri S., Lacroix M. Evidence for synergistic activity of plant-derived essential oils against fungal pathogens of food. Food Microbiol. 2016;53:24–30. doi: 10.1016/j.fm.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 198.Knezevic P., Aleksic V., Simin N., Svirčev E., Petrović A., Mimica-Dukic N. Antimicrobial activity of Eucalyptus camaldulensis essential oils and their interactions with conventional antimicrobial agents against multi-drug resistant Acinetobacter baumannii. J. Ethnopharmacol. 2016;178:125–136. doi: 10.1016/j.jep.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 199.Biasi-Garbin R.P., Otaguiri E.S., Morey A.T., Da Silva M.F., Morguette A.E.B., Lancheros C.A.C., Kian D., Perugini M.R.E., Nakazato G., Durán N., et al. Effect of Eugenol against Streptococcus agalactiae and Synergistic Interaction with Biologically Produced Silver Nanoparticles. Evidence Based Complement. Altern. Med. 2015;2015:1–8. doi: 10.1155/2015/861497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Theophel K., Schacht V.J., Schluter M., Schnell S., Stingu C.-S., Schaumann R., Bunge M. The importance of growth kinetic analysis in determining bacterial susceptibility against antibiotics and silver nanoparticles. Front. Microbiol. 2014;5:544. doi: 10.3389/fmicb.2014.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Scandorieiro S., De Camargo L.C., Lancheros C.A.C., Yamada-Ogatta S.F., Nakamura C.V., De Oliveira A.G., Andrade C.G.T.J., Durán N., Nakazato G., Kobayashi R. Synergistic and Additive Effect of Oregano Essential Oil and Biological Silver Nanoparticles against Multidrug-Resistant Bacterial Strains. Front. Microbiol. 2016;7:3974. doi: 10.3389/fmicb.2016.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Badea M.L., Iconaru S., Groza A., Chifiriuc M.C., Beuran M., Predoi D. Peppermint Essential Oil-Doped Hydroxyapatite Nanoparticles with Antimicrobial Properties. Molecules. 2019;24:2169. doi: 10.3390/molecules24112169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Saporito F., Sandri G., Bonferoni M.C., Rossi S., Boselli C., Cornaglia A.I., Mannucci B., Grisoli P., Vigani B., Ferrari F. Essential oil-loaded lipid nanoparticles for wound healing. Int. J. Nanomed. 2017;13:175–186. doi: 10.2147/IJN.S152529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Vahedikia N., Garavand F., Tajeddin B., Cacciotti I., Jafari S.M., Omidi T., Zahedi Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B Biointerfaces. 2019;177:25–32. doi: 10.1016/j.colsurfb.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 205.Ferrando R., Jellinek J., Johnston R. Nanoalloys: From Theory to Applications of Alloy Clusters and Nanoparticles. Chem. Rev. 2008;108:845–910. doi: 10.1021/cr040090g. [DOI] [PubMed] [Google Scholar]
- 206.Burda C., Chen X., Narayanan R., El-Sayed M.A. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev. 2005;105:1025–1102. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- 207.Mishra K., Basavegowda N., Lee Y.R. AuFeAg hybrid nanoparticles as an efficient recyclable catalyst for the synthesis of α,β- and β,β-dichloroenones. Appl. Catal. A Gen. 2015;506:180–187. doi: 10.1016/j.apcata.2015.09.014. [DOI] [Google Scholar]