What you need to know.
Consider ANCA associated vasculitis (AAV) in people with chronic systemic symptoms and evidence of renal, pulmonary, ear, nose, and throat, ophthalmic, or peripheral nerve disease
Perform urinalysis in people presenting with persistent systemic symptoms and in those with specific features of vasculitis (scleritis, chronic dyspnoea, cough, haemoptysis, foot drop) because these individuals have a high probability of multi-system disease
Patients with haemoptysis plus other features of AAV warrant same day hospital assessment to evaluate for pulmonary haemorrhage
Nicola, 44, goes to her general practitioner with a one month history of fatigue and night sweats. Physical examination is unremarkable. The duration of her symptoms prompts her GP to consider systemic inflammatory disease. Urinalysis is positive for blood and protein, and blood tests show new renal impairment (serum creatine 327 μmol/L, estimated glomerular filtration rate (eGFR) 13 mL/min/1.73m2) and evidence of an inflammatory response (C-reactive protein 50 mg/L, haemoglobin 103 g/L, platelets 461×109/L, albumin 33 g/L). Nicola is referred urgently to her local renal unit and undergoes kidney biopsy and testing for anti-neutrophil cytoplasmic antibodies (ANCA). She is diagnosed with ANCA-associated vasculitis.
What is ANCA associated vasculitis?
ANCA associated vasculitis (AAV) is an umbrella term for a group of multi-system autoimmune small vessel vasculitides that can present at any age and affect 20-25 people per million per year in Europe.1 A typical GP practice with 8000 patients can expect to see one new case approximately every five years.
AAV diseases include microscopic polyangiitis, granulomatosis with polyangiitis (GPA, previously “Wegener’s granulomatosis”), and eosinophilic granulomatosis with polyangiitis (EGPA, previously “Churg-Strauss syndrome”).2 The conditions are characterised by formation of granulomas and inflammation of small arteries, arterioles, venules, and capillaries.3 Inflamed vessels may rupture (for example, causing alveolar haemorrhage or a purpuric rash) or become occluded (for example, causing segmental glomerular infarction), giving rise to a broad array of clinical symptoms and signs related to a systemic inflammatory response, end organ microvascular injury, or the mass effect of granulomas.
What symptoms do patients develop?
AAV may present with constitutional symptoms suggestive of chronic inflammatory disease (fatigue, weight loss, fever, night sweats, myalgia, or polyarthralgia) or with specific features of end-organ involvement. Almost any part of the body can be affected, but the most commonly affected systems are the upper airways, lungs, kidneys, eyes, and peripheral nerves. Therefore, presenting symptoms include sinus pain, nasal discharge, or crusting, ear pain, or deafness (from upper airways involvement), cough, shortness of breath, wheeze or haemoptysis (from lung involvement), and painful, red eyes (from scleritis). Some patients may have weakness, numbness, or difficulty walking, but many patients do not mention these symptoms, and signs of peripheral neuropathy—such as foot drop or wrist drop—should be specifically sought on examination. A classic “vasculitic” purpuric skin rash is present in a minority of patients. Common presenting symptoms are listed in box 1.
Box 1. When to consider ANCA associated vasculitis.
Consider a diagnosis of AAV in the following clinical circumstances.4 Any of these features should prompt consideration of AAV; multiple features are strongly suggestive of AAV.5 Figures in parentheses are the approximate proportion of incident cases exhibiting each feature.5 6
• Vasculitic rash with systemic features (<20%)
Purpuric (“vasculitic”) rash accompanied by features of systemic disease (eg, fever, flu like symptoms, abnormal urinalysis, etc.). Note that an isolated rash in the absence of these features is unlikely to be AAV and a prominent rash at presentation is often more suggestive of immune complex vasculitis such as IgA vasculitis (previously Henoch-Schönlein purpura)
• Respiratory symptoms (~45%)
Haemoptysis (or other features suggestive of alveolar haemorrhage—eg, shortness of breath and acute drop in haemoglobin)
Progressive shortness of breath/cough (particularly if accompanied by systemic symptoms or fine crackles suggestive of pulmonary fibrosis)
Refractory/steroid dependent asthma
• Ear, nose, and throat/upper airways symptoms (~45%)
Long standing/persistent sinusitis or otitis
Hearing loss/earache
Nasal bridge collapse (a late sign of very advanced disease; see fig 1)
Subglottic tracheal stenosis (which may be discovered through investigation of dyspnoea, stridor, or incidentally at the time of elective intubation for general anaesthetic)
• Eye symptoms (<20%)
Painful, red eye (scleritis)
Diplopia with proptosis (caused by retro‐orbital granulomatous mass)
• Nerve symptoms (~30%)
Paraesthesia/weakness in keeping with mononeuritis multiplex or other peripheral neuropathy; consider AAV in the absence of an alternative explanation (eg, diabetes, B12 deficiency) and particularly in wrist drop or foot drop
• Renal disease (~65%)
Rising serum creatine (or falling eGFR) with more than a trace of blood and protein on urinalysis (ie, suspected glomerulonephritis); in the early phases of disease there may be no symptoms and eGFR may be near normal
Who gets vasculitis?
Several genetic and environmental risk factors have been identified, but most of the variation in incidence is accounted for by patient age. Incidence rises progressively with age until the mid-late 80s, so that the incidence in the population aged over 70 is 80-90 per million per year1 and consequently AAV is the leading cause of glomerulonephritis in this age group.7 However, AAV can present at any age—including in childhood—and affects all ethnicities; the incidence is approximately the same in women and men. Environmental risk factors are absent in most cases but exposure to drugs such as cocaine, hydralazine, and propylthiouracil has been implicated.3
Why is it missed?
Diagnosis of AAV is often delayed or missed because the condition has virtually no pathognomonic features, and most of the presenting symptoms are either general—and thus may be misdiagnosed as another systemic disease8—or more specific but attributed to more common diseases such as uncomplicated sinusitis, asthma, or scleritis. For example, EGPA may present with shortness of breath and wheeze and is often initially misdiagnosed as refractory or frequently relapsing asthma.9 People with predominant myalgia may be misdiagnosed as having polymyalgia rheumatica and—because corticosteroid treatment partially treats AAV—correct diagnosis is delayed until the steroid dose is weaned and other symptoms emerge. Granulomatosis with polyangiitis with cavitating lung lesions can be misdiagnosed as lung cancer.
Diagnosis can also be delayed or missed when symptoms in disparate organs are not recognised as manifestations of a single disease.10 A patient may attend separate clinics: ear, nose, and throat (for nasal crusting), ophthalmology (for scleritis), and neurology (for peripheral neuropathy) without anybody “joining the dots” between these symptoms. Indeed, a UK case-control study found that patients had frequent healthcare encounters in the months before a new diagnosis of GPA, with nearly 20% of patients attending two or more specialist clinic appointments in the year before diagnosis (compared to ~5% of controls).10
Why does this matter?
There is a stark difference in patient outcomes when diagnosis is delayed or missed: untreated, only one in 10 patients survives beyond two years11—a prognosis that is worse than most cancers. If promptly recognised, however, AAV responds well to immunosuppressive treatment and has a prognosis akin to other chronic inflammatory diseases. In randomised controlled trials, 60-90% of patients enter disease remission,8 12 and the 2 year survival is 90-97%.13
This difference in survival stems from the degree of irreversible organ damage that occurs before the diagnosis is made (fig 1). This can also leave patients with long term symptoms—such as painful paraesthesia, dyspnoea, deafness, or facial deformity—or with life-limiting organ dysfunction such as chronic kidney disease. The consequences of late diagnosis are particularly apparent in the subset of people who have renal involvement. In this group, delayed diagnosis and worse renal function at presentation are associated with a higher risk of end-stage renal disease and early mortality.14 15
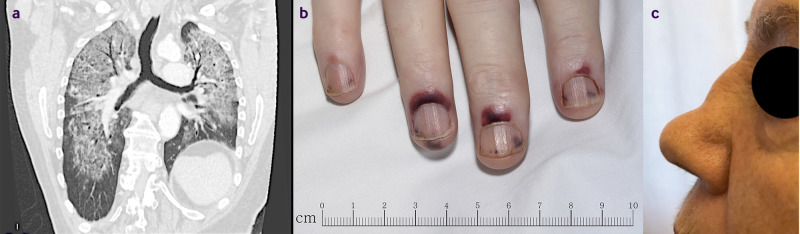
Fig 1.
Clinical consequences of AAV. (a) Diffuse alveolar haemorrhage. (b) Nail fold infarction and splinter haemorrhages. (c) Nasal bridge collapse resulting from chronic, erosive inflammation in the upper airways. Delayed diagnosis can result in permanent disability/deformity and early mortality. Manifestations of advanced disease such as a “saddle nose” deformity are relatively rare but can still occur if a diagnosis of AAV is missed (these images are not directly related to the case study in this article)
How is it diagnosed?
Clinical
The diagnosis of AAV is clinical, supported by serological and histological data. There are many possible presenting symptoms—none more important than another for making the diagnosis—and no isolated discriminating symptoms of AAV. Rather, the key to diagnosis is prompt recognition of an inflammatory disease pattern when multiple symptoms emerge, especially if more than one organ system is implicated or in combination with chronic systemic symptoms. Therefore, when two or more of these symptoms develop concurrently, the index of suspicion should be high5 (box 1).
Two clinical scenarios pose particular diagnostic difficulty. The first is alveolar haemorrhage: it can be hard to recognise and is devastating if missed. Alveolar haemorrhage may present with haemoptysis, but this symptom is absent in up to half of cases.16 Some patients with extensive airspace bleeding will report only of shortness of breath or cough and have a near normal chest examination. Patients with haemoptysis plus other features of AAV warrant same day hospital assessment to rule out pulmonary haemorrhage without further primary care investigations if these would delay referral (box 2). All other patients with suspected vasculitis and dyspnoea or cough should have a full blood count; if there is a recent drop in haemoglobin (or a low haemoglobin with no historic comparator) then we advise urgently discussing the possibility of pulmonary haemorrhage with a specialty service.
Box 2. First line investigations.
First line investigations support a diagnosis of AAV by evaluating for a chronic inflammatory response or renal failure or by testing for differential diagnoses such as infection and cancer. Given the multi-system nature of AAV, and the broad differential diagnosis, it is usually appropriate to request all of these investigations in cases of suspected AAV. The only exception is the ANCA test which would usually be requested by a specialist service.
Urinalysis (consider AAV causing glomerulonephritis if more than a trace of either blood or protein is detected; if abnormal then consider quantifying proteinuria with a urine protein:creatine or albumin:creatine ratio)
Full blood count (normocytic anaemia/thrombocytosis/eosinophilia due to chronic inflammation; microcytic anaemia in chronic covert blood loss in low grade alveolar haemorrhage or gut involvement; rapidly falling haemoglobin in acute alveolar haemorrhage)
Urea and electrolytes (rising serum creatine/falling eGFR)
Serum albumin (may be low due to inflammation or nephrotic range proteinuria)
C reactive protein or erythrocyte sedimentation rate (both measurements are high in inflammation)
Liver function tests, calcium (typically normal or near-normal in AAV, so abnormalities might suggest an alternative diagnosis such as infection or cancer)
Chest radiography (may have nodular, fibrotic, or infiltrative lesions in AAV; exclude lung cancer or infection)
ANCA testing in liaison with specialty service
The second difficult scenario is AAV presenting as organ limited disease, without systemic symptoms. For example, most patients with isolated glomerulonephritis have no symptoms until renal failure advances to the point of uraemia. Thus, consider AAV in patients with falling eGFR with blood and protein on urine dipstick even if they have no constitutional symptoms and full blood count and C reactive protein results are normal.
The differential diagnosis of AAV includes cancer, chronic infection (particularly bacterial endocarditis), and other autoimmune conditions. Because of the difficulties in diagnosis, discuss patients with possible or suspected AAV early with a specialty service.
Investigations
Investigations can further support a diagnosis of AAV and refute the major differential diagnoses of infection and cancer. As in the case study, urinalysis can rapidly screen for renal involvement in a multi-system disease. Patients with haematuria or proteinuria in this context have a high probability of having AAV. This probability is ~2% if the dipstick findings occur in isolation, rising to 85% if there is concomitant sinus and pulmonary disease.17 18
ANCA testing improves diagnostic certainty for patients with a high pre-test probability of disease.18 However, up to 10% of patients with small vessel vasculitis clinically test negative for ANCA. Conversely, false positive results can occur in the general population and in association with infections, malignancy, and autoimmune gastrointestinal and renal disease.4 Given this complexity, ANCA tests are not usually requested in primary care. Kidney or lung biopsies may be taken by specialty services to confirm a diagnosis of AAV or exclude differential diagnoses such as cancer.
How is it managed?
AAV is managed by a specialty service, which may be a rheumatology, renal, respiratory, or dedicated vasculitis service. It is treated with immunosuppressive therapies such as glucocorticoids, cyclophosphamide, rituximab, azathioprine, methotrexate, and mycophenolate mofetil. Adjunctive treatments aim to reduce the risk of infections—particularly pneumocystis, osteoporosis, diabetes, and cardiovascular disease.19
Case history outcome
Nicola was treated with glucocorticoids, cyclophosphamide, plasma exchange, and rituximab. Her renal function initially deteriorated before her disease entered remission, but she avoided the need for dialysis. She now enjoys a good quality of life and her renal function has improved and stabilised to a serum creatine of 188 μmol/L/eGFR 25 mL/min/1.73m2. Without prompt consideration of a multi-system illness in primary care, Nicola would almost certainly have developed irreversible end stage renal failure within weeks.
Education into practice.
• Which investigations would you request in a patient who presented—like Nicola—with a month of fatigue and night sweats?
• What would be the significance of blood and protein on urinalysis in such a patient?
• How would you manage a patient with a new multi-system illness who reported shortness of breath and haemoptysis?
How patients were involved in the creation of this article.
Nicola Welsh is an author of this article. She was involved in planning the content, writing her perspective and case history, and reviewing the final manuscript.
Additional resources.
RCGP eLearning hosts 5 and 30 minute online learning modules aimed at improving the recognition and diagnosis of ANCA vasculitis in primary care https://www.thelaurencurrietwilightfoundation.org/gpcampaign/
A patient’s perspective.
I initially visited my GP in August 2018 after feeling very tired and experiencing headaches and nausea. I had been having some night sweats but had self-diagnosed this as a peri-menopausal symptom. I was also caring for my mum who had terminal cancer, and my two kids alongside running a charity so put most of my symptoms down to stress and anxiety. I explained this to my GP who said he would like to check some blood tests, which surprised me. I thought he was just being thorough and fully expected the results to be fine. The next day he called to explain that I needed to be admitted to hospital as my kidneys were not working properly. My initial reaction was shock as I still felt relatively well; however, in hindsight, there were a few minor “warning signs” over the past year that I had ignored. These were very minor though, and it is worrying that there were not more obvious signs that something was wrong. I had no idea you could feel so well until things were so critical with your kidneys. After a few tests including a kidney biopsy, I was diagnosed with vasculitis. I was started on various treatments, including plasma exchange. I am now on lots of medication and have 6 monthly rituximab infusions to suppress my disease. I am back at work and although I have to take life a little slower, I am generally very well and feeling good. I will be forever grateful to my intuitive GP who really listened to me and didn’t delay in testing me. This most likely saved me from dialysis and I can never thank him or the renal team enough for their swift care. I really hope this is a reminder to other doctors that testing early, with such minor symptoms, can be lifesaving.
Competing interests The BMJ has judged that there are no disqualifying financial ties to commercial companies. The authors declare the following other interests: none.
Further details of The BMJ policy on financial interests are here: https://www.bmj.com/about-bmj/resources-authors/forms-policies-and-checklists/declaration-competing-interests
Authorship and contributorship: RWH conceived the idea for this article, wrote the first complete draft, and acts as guarantor. NW, TEF, PJG, and ND all contributed to the design of the article and wrote sections of the first draft. NW wrote “A patient’s perspective.” All authors approved the final manuscript and agree to be accountable for all aspects of the work.
Funding: RWH is supported by a Fellowship from the Wellcome Trust (209562/Z/17/Z).
Patient consent obtained.
Provenance and peer review: commissioned; externally peer reviewed.
This is one of a series of occasional articles highlighting conditions that may be more common than many doctors realise or may be missed at first presentation. The series advisers are Anthony Harnden, professor of primary care, Department of Primary Care Health Sciences, University of Oxford, and Dr Kevin Barraclough, School of Social and Community Medicine, University of Bristol. To suggest a topic for this series, please email us at practice@bmj.com.
References
- 1. Pearce FA, Lanyon PC, Grainge MJ, et al. Incidence of ANCA-associated vasculitis in a UK mixed ethnicity population. Rheumatology (Oxford) 2016;55:1656-63. 10.1093/rheumatology/kew232 [DOI] [PubMed] [Google Scholar]
- 2. Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised international Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum 2013;65:1-11. 10.1002/art.37715 [DOI] [PubMed] [Google Scholar]
- 3. Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol 2014;10:463-73. 10.1038/nrrheum.2014.103 [DOI] [PubMed] [Google Scholar]
- 4. Bossuyt X, Cohen Tervaert J-W, Arimura Y, et al. Position paper: revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol 2017;13:683-92. 10.1038/nrrheum.2017.140 [DOI] [PubMed] [Google Scholar]
- 5. Houben E, Bax WA, van Dam B, et al. Diagnosing ANCA-associated vasculitis in ANCA positive patients: a retrospective analysis on the role of clinical symptoms and the ANCA titre. Medicine (Baltimore) 2016;95:e5096. 10.1097/MD.0000000000005096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ungprasert P, Crowson CS, Cartin-Ceba R, et al. Clinical characteristics of inflammatory ocular disease in anti-neutrophil cytoplasmic antibody associated vasculitis: a retrospective cohort study. Rheumatology (Oxford) 2017;56:1763-70. 10.1093/rheumatology/kex261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Shaughnessy MM, Hogan SL, Poulton CJ, et al. Temporal and demographic trends in glomerular disease epidemiology in the southeastern United States, 1986-2015. Clin J Am Soc Nephrol 2017;12:614-23. 10.2215/CJN.10871016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stone JH, Merkel PA, Spiera R, et al. RAVE-ITN Research Group Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010;363:221-32. 10.1056/NEJMoa0909905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D’Cruz DP, Barnes NC, Lockwood CM. Difficult asthma or Churg-Strauss syndrome? BMJ 1999;318:475-6. 10.1136/bmj.318.7182.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pearce FA, Hubbard RB, Grainge MJ, Watts RA, Abhishek A, Lanyon PC. Can granulomatosis with polyangiitis be diagnosed earlier in primary care? A case-control study. QJM 2018;111:39-45. 10.1093/qjmed/hcx194 [DOI] [PubMed] [Google Scholar]
- 11. Fauci AS, Haynes BF, Katz P, Wolff SM. Wegener’s granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med 1983;98:76-85. 10.7326/0003-4819-98-1-76 [DOI] [PubMed] [Google Scholar]
- 12. de Groot K, Harper L, Jayne DRW, et al. EUVAS (European Vasculitis Study Group) Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2009;150:670-80. 10.7326/0003-4819-150-10-200905190-00004 [DOI] [PubMed] [Google Scholar]
- 13. Pagnoux C, Carette S, Khalidi NA, et al. French Vasculitis Study Group (FVSG), European Vasculitis Society (EUVAS) and Vasculitis Clinical Research Consortium (VCRC) Comparability of patients with ANCA-associated vasculitis enrolled in clinical trials or in observational cohorts. Clin Exp Rheumatol 2015;33(Suppl 89):77-83. [PMC free article] [PubMed] [Google Scholar]
- 14. Houben E, Groenland SL, van der Heijden JW, Voskuyl AE, Doodeman HJ, Penne EL. Relation between duration of the prodromal phase and renal damage in ANCA-associated vasculitis. BMC Nephrol 2017;18:378. 10.1186/s12882-017-0797-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Booth AD, Almond MK, Burns A, et al. Pan-Thames Renal Research Group Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003;41:776-84. 10.1016/S0272-6386(03)00025-8 [DOI] [PubMed] [Google Scholar]
- 16. West S, Arulkumaran N, Ind PW, Pusey CD. Diffuse alveolar haemorrhage in ANCA-associated vasculitis. Intern Med 2013;52:5-13. 10.2169/internalmedicine.52.8863 [DOI] [PubMed] [Google Scholar]
- 17. Jennette JC, Wilkman AS, Falk RJ. Diagnostic predictive value of ANCA serology. Kidney Int 1998;53:796-8. 10.1038/ki.1998.36 [DOI] [PubMed] [Google Scholar]
- 18. Bossuyt X, Rasmussen N, van Paassen P, et al. A multicentre study to improve clinical interpretation of proteinase-3 and myeloperoxidase anti-neutrophil cytoplasmic antibodies. Rheumatology (Oxford) 2017;56:1533-41. 10.1093/rheumatology/kex170 [DOI] [PubMed] [Google Scholar]
- 19. Houben E, Penne EL, Voskuyl AE, et al. Cardiovascular events in anti-neutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis of observational studies. Rheumatology (Oxford) 2018;57:555-62. 10.1093/rheumatology/kex338 [DOI] [PubMed] [Google Scholar]