We report a 15 year-old Nigerian adolescent male with chronic osteomyelitis caused by an extensively drug-resistant (XDR) Pseudomonas aeruginosa strain of sequence type 773 (ST773) carrying blaNDM-1 and an extended spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae strain. The patient developed neurological side effects in the form of circumoral paresthesia with polymyxin B and asymptomatic elevation of transaminases with aztreonam (used in combination with ceftazidime-avibactam).
KEYWORDS: ESBL-producing Klebsiella pneumoniae, XDR Pseudomonas aeruginosa, cefiderocol, chronic osteomyelitis, pediatric
ABSTRACT
We report a 15 year-old Nigerian adolescent male with chronic osteomyelitis caused by an extensively drug-resistant (XDR) Pseudomonas aeruginosa strain of sequence type 773 (ST773) carrying blaNDM-1 and an extended spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae strain. The patient developed neurological side effects in the form of circumoral paresthesia with polymyxin B and asymptomatic elevation of transaminases with aztreonam (used in combination with ceftazidime-avibactam). Cefiderocol treatment for 14 weeks plus bone implantation resulted in apparent cure and avoided amputation.
CASE PRESENTATION
A 15-year-old Nigerian male suffered a femur fracture after a motor vehicle accident and underwent intramedullary pin placement in Nigeria. Purulent drainage from the surgical site began 1 month after surgery. Surgical debridement was performed, with placement of gentamicin beads. However, the patient had recurrent episodes of wound drainage, treated with a variety of antibiotics (his family was unable to recall details of the treatment). His last episode was 2 months before presentation to our hospital, at which time he was treated with 10 days of intravenous levofloxacin and metronidazole, with improvement of drainage while hospitalized in Nigeria. No information about microbiological diagnosis was available from his previous hospitalizations.
His surgical site wound started draining pus 10 days before presentation. He was prescribed trimethoprim-sulfamethoxazole, but the infection progressed, with wound dehiscence and copious purulent drainage. He presented to our emergency department 10 months after the initial insult, where radiographs showed signs of chronic osteomyelitis of the left femur, with phlegmonous changes extending to the skin. The patient underwent surgery, with removal of the intramedullary nail, debridement of soft tissue, and saucerization of the femoral canal with a 16-mm reamer, irrigator, and aspirator (Depuy Synthes, Paoli, PA). An antibiotic-cement nail containing 2.4 g of tobramycin and 5 g of vancomycin mixed with Simplex cement (Stryker, Kalamazoo, MI) was placed and secured around a 5/32-in. Ilizarov rod during the procedure.
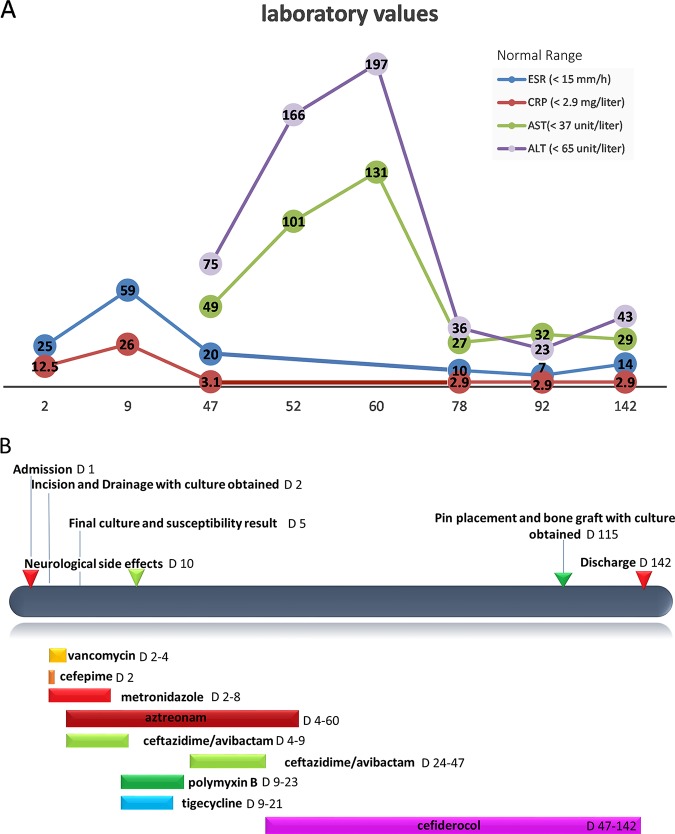
The patient was started on vancomycin, cefepime, and metronidazole after surgery. Surgical-site cultures yielded an extensively drug-resistant (XDR) Pseudomonas aeruginosa strain and an extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae strain. Susceptibilities are shown in (Table 1). Because of a suspicion of the presence of a metallo-β-lactamase (MBL) enzyme in the P. aeruginosa isolate, based on our susceptibility testing, and the high prevalence of metallo-β-lactamases in Nigeria (1), cefepime was switched to the combination of ceftazidime-avibactam (2.5 g intravenously every 8 h [q8h]) plus aztreonam (2 g intravenously q8h); a synergy test was performed and showed no change in the MICs (2). Vancomycin and metronidazole were discontinued. Despite mild symptomatic improvement after 1 week on combination therapy, the patient’s inflammatory markers continued to worsen (Fig. 1).
TABLE 1.
Susceptibilities of Pseudomonas aeruginosa and Klebsiella pneumoniae isolates reported by the microbiology laboratory
| Antimicrobial | MIC (μg/ml) for: |
|
|---|---|---|
| Pseudomonas aeruginosa | Klebsiella pneumoniae | |
| Amikacin | >32 | <8 |
| Aztreonam | 6 | >32 |
| Aztreonam (synergy) | 6b | NAc |
| Cefepime | >16 | >16 |
| Ciprofloxacin | >2 | >2 |
| Gentamicin | >8 | <1 |
| Meropenem | >8 | <1 |
| Piperacillin-tazobactam | >64 | >64 |
| Tobramycin | >8 | 8 |
| Ceftazidime-avibactam | >256 | 0.38 |
| Ceftolozane-tazobactama | >64 | 1 |
| Tigecyclinea | >4 | 1 |
| Colistin | 0.75 | 0.125 |
| Cefiderocola | 4 | 0.5 |
The MIC results were reported by International Health Management Associates, Inc. (microbiology laboratory, Shionogi Sidero compassionate-use program).
Synergy testing was done by determining MICs of aztreonam by the Etest (1). No change in aztreonam MIC was observed.
NA, not applicable.
FIG 1.
Correlation between changes in inflammatory markers, liver function tests, and changes in antimicrobial therapy. (A) Changes in laboratory values for the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST); (B) antimicrobial regimens used in this patient and correlation with side effects, laboratory abnormalities, and interventions during inpatient days. D, patient day(s).
Ceftazidime-avibactam was replaced by polymyxin B, aztreonam was continued, and tigecycline was added for the K. pneumoniae strain. After starting polymyxin B therapy, the patient experienced facial numbness, blurred vision, tingling sensation in the tongue, and light-headedness. To manage the side effects, the polymyxin B infusion rate was reduced, calcium and ascorbic acid were added based on a previous report (3), and tigecycline was held. However, the patient continued to experience neurological symptoms that led to discontinuation of the polymyxin therapy.
The genome of the P. aeruginosa isolate was sequenced using an Illumina MiSeq platform; genomic DNA was extracted using the Qiagen QIAamp DSP DNA minikit (Qiagen). Genomic libraries were prepared using the Nextera DNA Flex library prep kit, and sequencing was performed on the MiSeq platform (Illumina, Inc.) with 2× 300-bp paired-end reads. Spades v.3.11.1 was used for genome assembly, and data analysis was performed using a custom pipeline, including mlst (https://github.com/tseemann/mlst) v2.10 for in silico multilocus sequence typing with the PubMLST database and Abricate v0.7 for resistance gene screening of contigs with sequences in the Comprehensive Antibiotic Resistance Database (CARD) (4, 5). The genome sequence indicated that the isolate belonged to an international high-risk clone of sequence type 773 (ST773) (which has been associated with the presence of MBLs like VIM and NDM) (6) and carried blaNDM-1. Additional antibiotic resistance determinants included rmtB, aph(3′)-IIb (aminoglycosides), qnrVC1 (quinolones), fosA (fosfomycin), and the intrinsic P. aeruginosa genes coding for the OXA-50 and PDC-2 enzymes. Based on the genomic information and given the patient’s ongoing intolerance to polymyxin B, the antibiotics were switched back to the combination of ceftazidime-avibactam (2.5 g intravenously q8h) plus aztreonam (2 g intravenously q8h) to optimize activity against the metallo-β-lactamase enzyme NDM and maintain activity against the ESBL-producing K. pneumoniae strain (7). However, in the first 2 weeks of restarting this combination, the patient experienced asymptomatic elevation of transaminases (Fig. 1), and X rays showed no signs of bone healing. Concern for drug-related liver effects of aztreonam was raised.
Data availability.
Sequence data for this paper have been deposited under BioSample no. SAMN13562792 and BioProject no. PRJNA595618.
CHALLENGE QUESTION
Given the results of the susceptibility testing, genomic data for the Pseudomonas aeruginosa isolate, and the patient history of antibiotic intolerance, what would be your next step in therapy?
A. Start colistin and continue ceftazidime-avibactam
B. Continue with the same regimen despite the side effects
C. Add ceftolozane-tazobactam to ceftazidime-avibactam
D. Consider alternative therapies, such as cefiderocol compassionate use
E. Restart polymyxin B and continue ceftazidime-avibactam
TREATMENT AND OUTCOME
Due to the lack of treatment options and the risk of requiring amputation if there were further progression of the infection, we made a request to use cefiderocol from Shionogi, Inc., on a compassionate basis (answer D). After Institutional Review Board (IRB) approval from the University of Texas Health Science Center at Houston, the request was granted, with the consent of patient’s mother.
Cefiderocol susceptibility testing of both P. aeruginosa and K. pneumoniae isolates was performed using disc diffusion, and the organisms were reported as susceptible (with zones of inhibition of 18 mm and 27 mm, respectively). Cefiderocol MICs were determined at International Health Management Associates, Inc. (microbiology laboratory, Shionogi Sidero compassionate-use program), and the organisms were reported as susceptible (MICs of 4 and 0.5 μg/ml, respectively). Based on a protocol that was established with Shionogi, Inc., the patient was started on 2 g of cefiderocol every 8 h intravenously administered over 3 h, with creatinine clearance evaluated daily for the first 10 days. The initial therapeutic plan was for 12 weeks in combination with aztreonam. In the first 2 weeks, the patient’s liver function continued to rise, and aztreonam was discontinued. With cefiderocol monotherapy, both his liver function and inflammatory markers normalized. After 7 weeks of therapy, he was found to have intermittent episodes of decreased white cell counts, with the nadir at 1,200/mm3 (normal range, >1,500/mm3), which resolved spontaneously without any changes to the antibiotic regimen. A repeat computed tomography (CT) scan of the femur showed unchanged bone appearance at the site of the fracture without evidence of acute periosteal reaction or erosion to suggest acute osteomyelitis.
Given that there were no signs of bone healing on the CT scan, the decision was made on week 9 to take the patient to the operating room for a bone graft and antibiotic nail exchange. A new antibiotic-cement nail was implanted with 2.4 g of tobramycin and 600 mg of colistin. Bone biopsy specimens were obtained for cultures, and a mixture of allograft chips, autograft from the contralateral femoral shaft, and recombinant human bone morphogenic protein 2 (Medtronic, Minneapolis, MN) were placed to promote bone union. Cultures were sterile, and a histopathology report showed benign bone without associated acute or chronic inflammation. The patient completed four additional weeks of intravenous cefiderocol, for a total of 14 weeks. The patient has remained off antibiotics for 2 months without clinical or radiological evidence of infection and has returned to playing recreational soccer.
The P. aeruginosa ST773 isolate is an extensively drug-resistant strain first isolated from sputum in China in 2006. Since then, this lineage has been reported globally (8). Due to the increasing global burden of XDR Gram-negative bacteria, efforts are ongoing to develop new antibiotics with better potency to combat these bacteria. Cefiderocol (Shionogi & Co. Ltd., Osaka, Japan) is a novel parenteral siderophore cephalosporin that inhibits primarily penicillin-binding protein 3 (PBP3) in Enterobacteriaceae. A unique feature of this antibiotic is the ability to form a chelation complex with iron, which allows active transport of the drug-iron complex into bacterial cells via iron transporters (such as PiuA in P. aeruginosa) (9, 10). Cefiderocol exhibits more-potent in vitro activity than ceftolozane-tazobactam and ceftazidime-avibactam against imipenem-resistant P. aeruginosa, imipenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia isolates (11). Cefiderocol was found to have a lower MIC90 than comparators (amikacin, colistin, ceftolozane-tazobactam, ceftazidime-avibactam, colistin, tigecycline), with in vitro activity against characterized carbapenem-resistant Acinetobacter baumannii complex, Stenotrophomonas maltophilia, Pseudomonas aeruginosa, and Enterobacteriaceae strains (12).
There have been two case reports of the successful use of cefiderocol as compassionate treatment. The first was the use of cefiderocol in addition to meropenem and colistin for 4 weeks in a 78-year-old woman with persistent bacteremia for health care-associated native aortic valve endocarditis due to an XDR P. aeruginosa isolate susceptible in vitro only to colistin. The regimen was successful in terms of clearing blood cultures and allowing surgical intervention and aortic valve replacement (13). In the second case, cefiderocol was used along with linezolid for 14 days in an adult male patient with severe H1N1 influenza complicated by ventilator-associated pneumonia and bloodstream infection caused by and XDR A. baumannii and a carbapenemase-producing K. pneumoniae strain (14). To the best of our knowledge, cefiderocol has not previously been used in osteoarticular infections, and the 14 weeks of treatment is the longest duration of a regimen with this novel antibiotic, recently approved by the FDA. Our experience with a prolonged administration of cefiderocol was notable for a possible hematological toxicity. However, this resolved spontaneously without any dose adjustment. Thus, this antibiotic emerges as a potential therapeutic option for the treatment of complicated infections due to XDR Gram-negative organisms requiring prolonged courses of therapy.
ACKNOWLEDGMENTS
We gratefully acknowledge Shionogi & Co. Ltd., Osaka, Japan, for providing the cefiderocol for compassionate use.
C.A.A. is supported by NIH/NIAID grants R01 AI134637, R21 AI143229, and K24 AI121296, a UTHealth Presidential Award, a University of Texas System STARS Award, and the Texas Medical Center Health Policy Institute Funding Program. W.R.M. is supported by NIH/NIAID grant K08 AI135093. C.A.A. has received grant support from Merck, Inc., Entasis Therapeutics pharmaceuticals, and MeMed Diagnostics. W.R.M. has received grant support from Merck and Entasis Therapeutics and honoraria from Achaogen and Shionogi.
Shionogi & Co. Ltd. reviewed the manuscript but had no role in the conception or drafting of this report. We declare that we have no competing interests, received no funding for this work outside that declared above, and have no conflicts of interest to report.
All authors took part in the care of the patient and drafting of the report.
This Journal section presents a real, challenging case involving a multidrug-resistant organism. The case authors present the rationale for their therapeutic strategy and discuss the impact of mechanisms of resistance on clinical outcome. An expert clinician then provides a commentary on the case.
Footnotes
For the case commentary, see https://doi.org/10.1128/AAC.00059-20.
REFERENCES
- 1.Kazmierczak KM, Rabine S, Hackel M, McLaughlin RE, Biedenbach DJ, Bouchillon SK, Sahm DF, Bradford PA. 2016. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:1067–1078. doi: 10.1128/AAC.02379-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan A, Tran TT, Rios R, Hanson B, Shropshire WC, Sun Z, Diaz L, Dinh AQ, Wanger A, Ostrosky-Zeichner L, Palzkill T, Arias CA, Miller WR. 2019. Extensively drug-resistant Pseudomonas aeruginosa ST309 harboring tandem Guiana extended spectrum β-lactamase enzymes: a newly emerging threat in the United States. Open Forum Infect Dis 6:ofz273. doi: 10.1093/ofid/ofz273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, Kaye D, Mouton JW, Tam VH, Thamlikitkul V, Wunderink RG, Li J, Nation RL, Kaye KS. 2019. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 39:10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. A new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, Lago BA, Dave BM, Pereira S, Sharma AN, Doshi S, Courtot M, Lo R, Williams LE, Frye JG, Elsayegh T, Sardar D, Westman EL, Pawlowski AC, Johnson TA, Brinkman FS, Wright GD, McArthur AG. 2017. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res 45:D566–D573. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kocsis B, Toth A, Gulyas D, Ligeti B, Katona K, Rokusz L, Szabo D. 2019. Acquired qnrVC1 and blaNDM-1 resistance markers in an international high-risk Pseudomonas aeruginosa ST773 clone. J Med Microbiol 68:336–338. doi: 10.1099/jmm.0.000927. [DOI] [PubMed] [Google Scholar]
- 7.Marshall S, Hujer AM, Rojas LJ, Papp-Wallace KM, Humphries RM, Spellberg B, Hujer KM, Marshall EK, Rudin SD, Perez F, Wilson BM, Wasserman RB, Chikowski L, Paterson DL, Vila AJ, van Duin D, Kreiswirth BN, Chambers HF, Fowler VG Jr, Jacobs MR, Pulse ME, Weiss WJ, Bonomo RA. 2017. Can ceftazidime-avibactam and aztreonam overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae? Antimicrob Agents Chemother 61:e02243-16. doi: 10.1128/AAC.02243-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousefi S, Nahaei MR, Farajnia S, Aghazadeh M, Iversen A, Edquist P, Maãtallah M, Giske CG. 2013. A multiresistant clone of Pseudomonas aeruginosa sequence type 773 spreading in a burn unit in Orumieh, Iran. APMIS 121:146–152. doi: 10.1111/j.1600-0463.2012.02948.x. [DOI] [PubMed] [Google Scholar]
- 9.Ito A, Nishikawa T, Matsumoto S, Yoshizawa H, Sato T, Nakamura R, Tsuji M, Yamano Y. 2016. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:7396–7401. doi: 10.1128/AAC.01405-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito A, Sato T, Ota M, Takemura M, Nishikawa T, Toba S, Kohira N, Miyagawa S, Ishibashi N, Matsumoto S, Nakamura R, Tsuji M, Yamano Y. 2017. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against Gram-negative bacteria. Antimicrob Agents Chemother 62:e01454-17. doi: 10.1128/AAC.01454-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsueh SC, Lee YJ, Huang YT, Liao CH, Tsuji M, Hsueh PR. 2018. In vitro activities of cefiderocol, ceftolozane/tazobactam, ceftazidime/avibactam and other comparative drugs against imipenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii, and Stenotrophomonas maltophilia, all associated with bloodstream infections in Taiwan. J Antimicrob Chemother 74:380–386. doi: 10.1093/jac/dky425. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs MR, Abdelhamed AM, Good CE, Rhoads DD, Hujer KM, Hujer AM, Domitrovic TN, Rudin SD, Richter SS, van Duin D, Kreiswirth BN, Greco C, Fouts DE, Bonomo RA. 2018. ARGONAUT-I: activity of cefiderocol (S-649266), a siderophore cephalosporin, against Gram-negative bacteria. Antimicrob Agents Chemother 63:e01801-18. doi: 10.1128/AAC.01801-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edgeworth JD, Merante D, Patel S, Young C, Jones P, Vithlani S, Wyncoll D, Roberts P, Jones A, Den Nagata T, Ariyasu M, Livermore DM, Beale R. 2019. Compassionate use of cefiderocol as adjunctive treatment of native aortic valve endocarditis due to extremely drug-resistant Pseudomonas aeruginosa. Clin Infect Dis 68:1932–1934. doi: 10.1093/cid/ciy963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trecarichi EM, Quirino A, Scaglione V, Longhini F, Garofalo E, Bruni A, Biamonte E, Lionello R, Serapide F, Mazzitelli M, Marascio N, Matera G, Liberto MC, Navalesi P, Torti C, Pisani V, Costa C, Greco G, La Gamba V, Giancotti A, Barreca GS, Peronace C, La Valle O, Cimino G, La Torre P, Gemelli A, Tropea FA, Picicco F, IMAGES Group . 2019. Successful treatment with cefiderocol for compassionate use in a critically ill patient with XDR Acinetobacter baumannii and KPC-producing Klebsiella pneumoniae: a case report. J Antimicrob Chemother 74:3399–3401. doi: 10.1093/jac/dkz318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequence data for this paper have been deposited under BioSample no. SAMN13562792 and BioProject no. PRJNA595618.